- 1Department of Microbiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 2Department of Bacteriology, Immunology and Mycology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
- 3Department of Animal Medicine (Infectious Diseases), Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 4Department of Clinical Laboratory Sciences, Applied Medical Sciences College, Najran University, Najran, Saudi Arabia
- 5Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Najran University, Najran, Saudi Arabia
- 6Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 7Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 8Animal and Fish Production Department, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 9Fish and Animal Production Department, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
- 10Al Hadithah General Hospital, Al-Qurayyat, Saudi Arabia
- 11Department of Pharmacy Practice, College of Pharmacy, Al Maarefa University, Riyadh, Saudi Arabia
- 12Medicinal Chemistry Department, Faculty of Pharmacy, Port Said University, Port Said, Egypt
- 13Biology Department, Turabah University College, Taif University, Taif, Saudi Arabia
- 14Department of Microbiology and Immunology, Faculty of Pharmacy, Port Said University, Port Said, Egypt
Introduction: There is an urgent need to develop therapeutic options for biofilm-producing Staphylococcus aureus (S. aureus). Therefore, the renewed interest in essential oils (EOs), especially carvacrol, linalool and eugenol, has attracted the attention of our research group.
Methods: Multidrug resistance and multivirulence profiles in addition to biofilm production of S. aureus strains isolated from cows with mastitis were evaluated using both phenotypic and genotypic methods. The antimicrobial and antibiofilm activities of EOs were tested using both in vitro and molecular docking studies. Moreover, the interactions between commonly used antibiotics and the tested EOs were detected using the checkerboard method.
Results: We found that all our isolates (n= 37) were biofilm methicillin resistant S. aureus (MRSA) producers and 40.5% were vancomycin resistant S. aureus (VRSA). Unfortunately, 73 and 43.2% of the recovered MRSA isolates showed multidrug resistant (MDR) and multivirulence patterns, respectively. The antimicrobial activities of the tested EOs matched with the phenotypic evaluation of the antibiofilm activities and molecular docking studies. Linalool showed the highest antimicrobial and antibiofilm activities, followed by carvacrol and eugenol EOs. Fortunately, synergistic interactions between the investigated EOs and methicillin or vancomycin were detected with fractional inhibitory concentration index (FICI) values ≤ 0.5. Moreover, the antimicrobial resistance patterns of 13 isolates changed to sensitive phenotypes after treatment with any of the investigated EOs. Treatment failure of bovine mastitis with resistant S. aureus can be avoided by combining the investigated EOs with available antimicrobial drugs.
Conclusion: We hope that our findings can be translated into a formulation of new pharmaceutical dosage forms against biofilm-producing S. aureus pathogens.
Introduction
Staphylococcus aureus (S. aureus) is a widely recognized bacterium that can spread to humans and animals resulting in life-threatening illnesses. It is also a major cause of bovine mastitis in cattle, buffalo, sheep and goats (Kløve et al., 2022). In dairy farms, S. aureus mastitis and its produced toxins cause major economic losses including decreased milk production, excessive drug residues contamination and chronic illness leading to deaths (Algammal et al., 2020). The antimicrobial resistance rates among mastitis S. aureus in Egypt are increased during the last few decades (El-Jakee et al., 2011; Ameen et al., 2019). Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA), commonly called superbugs, are among the most significant pathogens that pose major threats to both human and animal health (Shrestha et al., 2021). Unfortunately, most β -lactam antibiotics are ineffective against S. aureus isolates, which harbors mecA/mecC genes (Hiramatsu et al., 2014; Saber et al., 2022). The fact that MRSA may produce biofilms on biotic and abiotic surfaces (Ascioferro et al., 2021) renders the issue even more challenging to be eradicated. It has been known for a considerable time that staphylococcal isolates are the most common causes of infections that are connected with biofilms (Lebeaux et al., 2013). The extracellular matrix, changing metabolic states and growth rate render biofilms more resistant to antibiotics than planktonic organisms (Silva et al., 2021). Notably, MRSA strains biofilm development and multidrug resistant (MDR) profile increase the possibility of chemotherapeutic failure (Abd-El-Hamid et al., 2020).
Moreover, virulence arrays are essential for overcoming the host defense power and increasing bacterial pathogenicity. Most S. aureus mastitis strains are multivirulent and are associated with biofilm production. The biofilm associated protein (BAP) is correlated with the presence of bap gene and ica operon, which control polysaccharide intercellular adhesin synthesis producing an extremely structured multicellular biofilm (Zhang et al., 2018). Moreover, S. aureus enterotoxins (SEs) and hemolysins (Hlα and Hlβ) promote pathogenicity by enhancing the pathogen’s adhesion, colonization and tissue invasion (Puah et al., 2016). Expression of S. aureus virulence genes is controlled via accessory gene regulatory (agr) system, which can be divided into four groups according to agrC and agrD gene sequences; agr I, II, III and IV. Although agr types vary in properties and prevalence according to geography, identifying the dominant type in each region may be beneficial (Javdan et al., 2019).
Intramammary infections caused by biofilm-producing S. aureus are common among cows with chronic mastitis. The incidence of these infections is increasing with poor management practices during milking. Milk is an excellent medium for the growth of bacterial species gaining access to the upper part of the gland with the production of virulence proteins and toxins leading to impairment of the host defense power and inflammation of the mammary gland (Foster and Höök, 1998). Of note, most available antibiotics are inefficient in eradicating these infections. To avoid treatment failure in S. aureus mastitis, new and alternative therapies in combination with available antimicrobial drugs must be formulated. In recent years, there has been growing interest in the utilization of naturally occurring substances such as essential oils (EOs) manufactured from different plant components owing to their biological effects including antioxidant, anti-inflammatory and anticancer (Krifa et al., 2015). Additionally, EOs have been widely reported in the scientific literature as potential antibacterial agents as they are efficient against a wide variety of pathogenic bacteria and yeast (Swamy et al., 2016; Puškárová et al., 2017). The leaves and inflorescence of Origanum vulgare are the main sources for carvacrol essential oil (EO). This plant was used in the ancient alternative medicine as antimicrobial, antidiabetic, anticancer and anti-inflammatory agent (Leyva-Lopez et al., 2017). The monoterpenic phenol such as carvacrol [2-methyl-5-(1-methylethyl) phenol] has significant effects on microbial cell membrane, respiratory metabolism and DNA (Cui et al., 2019). Additionally, it has antivirulence effects on foodborne pathogens, especially S. aureus (Cui et al., 2019). Interestingly, linalool, which has antimicrobial and antifungal properties is the main constituent of Lavandula officinalis and Citrus sinensis EOs (Kasper et al., 2010; Wang et al., 2023). In the same context, eugenol has potential activities against resistant pathogens including bacteria, fungi as well as viral infections. It is found in abundant amounts in clove buds (Syzygium aromaticum), cinnamon bark and leaves (Cinnamomum verum) (Taleuzzaman et al., 2021).
Relating to the abovementioned issues, the purpose of the current study was to investigate the antimicrobial resistance and virulence profiles of MRSA strains causing mastitis and to evaluate the antimicrobial and antibiofilm activities of different natural compounds including carvacrol, linalool, and eugenol against MRSA strains.
Materials and methods
Ethics consideration
All animal care study procedures were conducted in accordance with the guidelines established by the Animal Ethics Review Committee of Suez Canal University (AERC-SCU2023029), Egypt.
Sampling procedures
The current study enrolled 180 milk samples collected under sterile conditions from 180 different cows suffering from clinical mastitis prior to the beginning of antibiotic treatment from various farms in Sharkia and Ismailia Governorates, Egypt; each sample represented one animal. The udder of each animal was palpated before sample collection to check for edema, heat, asymmetry and other abnormalities. Afterwards, the udder and teats were washed and dried and then 70% ethyl alcohol was used to sanitize the udder, teats and tester hands to remove any chance of contamination. When collecting milk samples, the first few strips were discarded to avoid potential contamination from the teat orifice.
Microbiological analysis and characterization of S. aureus isolates
Firstly, S. aureus isolates were isolated onto mannitol salt agar and Baird Parker agar supplemented with an egg yolk–tellurite emulsion (Oxoid, UK). Standard bacteriological procedures were applied to make a preliminary phenotypic identification of S. aureus based on their growth on selective media, β-hemolysis on blood agar and production of golden yellow pigments. Furthermore, microscopical examination of Gram stained films from colonies grown onto mannitol salt agar were observed for the formation of Gram-positive grape-like clusters (Becker et al., 2015; Abd El-Hamid et al., 2019). The recovered isolates were then confirmed to be S. aureus based on their positive reactions for catalase and coagulase tests. The isolates were finally identified using PCR assay to detect nuc gene (Brakstad et al., 1992). All the isolates were preserved frozen in brain heart infusion broth (Oxoid, UK) containing 30% glycerol at - 80°C prior to subsequent detailed analysis.
Detection of biofilm producing S. aureus isolates
In vitro formation of biofilms was phenotypically assessed using two methods; qualitative Congo red agar, CRA (Freeman et al., 1989) and quantitative microtiter plate, MTP (Stepanovic et al., 2000) and genotypically via detection of icaA gene (Ciftci et al., 2009).
Antimicrobial susceptibility testing
In vitro phenotypic antimicrobial susceptibility profiles of all confirmed S. aureus isolates to nine antimicrobial drugs from different groups were assessed on Muller-Hinton agar (Oxoid, UK) using Kirby-Bauer disc diffusion method (Bauer et al., 1966). Standard antibiotic discs (Oxoid, UK) included cefoxitin (CFX), ampicillin (AMP), amoxicillin-clavulanic acid (AMC), erythromycin (E), chloramphenicol (C), sulfamethoxazole-trimethoprim (SXT), ciprofloxacin (CIP), vancomycin (VA) and gentamycin (CN). Clinical and Laboratory Standards Institute (CLSI) interpretation criteria (CLSI, 2020) were used to classify the isolates as either susceptible or resistant depending on the diameter of the inhibition zones surrounding each disc. Using broth microdilution method outlined by CLSI (CLSI, 2020), minimum inhibitory concentrations (MICs) were determined for cefoxitin and vancomycin (Sigma-Aldrich, USA) against all isolates to phenotypically detect MRSA and VRSA, respectively. All isolates showing phenotypic resistance to cefoxitin and vancomycin were subjected to PCR assays for the detection of mecA and vanA genes specific for MRSA and VRSA, respectively as described elsewhere (Kariyama et al., 2000; Larsen et al., 2008). Multidrug resistant isolates were defined as those exhibiting resistance to at least one agent in three or more different classes of antimicrobial agents.
Molecular characterization of virulence genes and agr genotyping
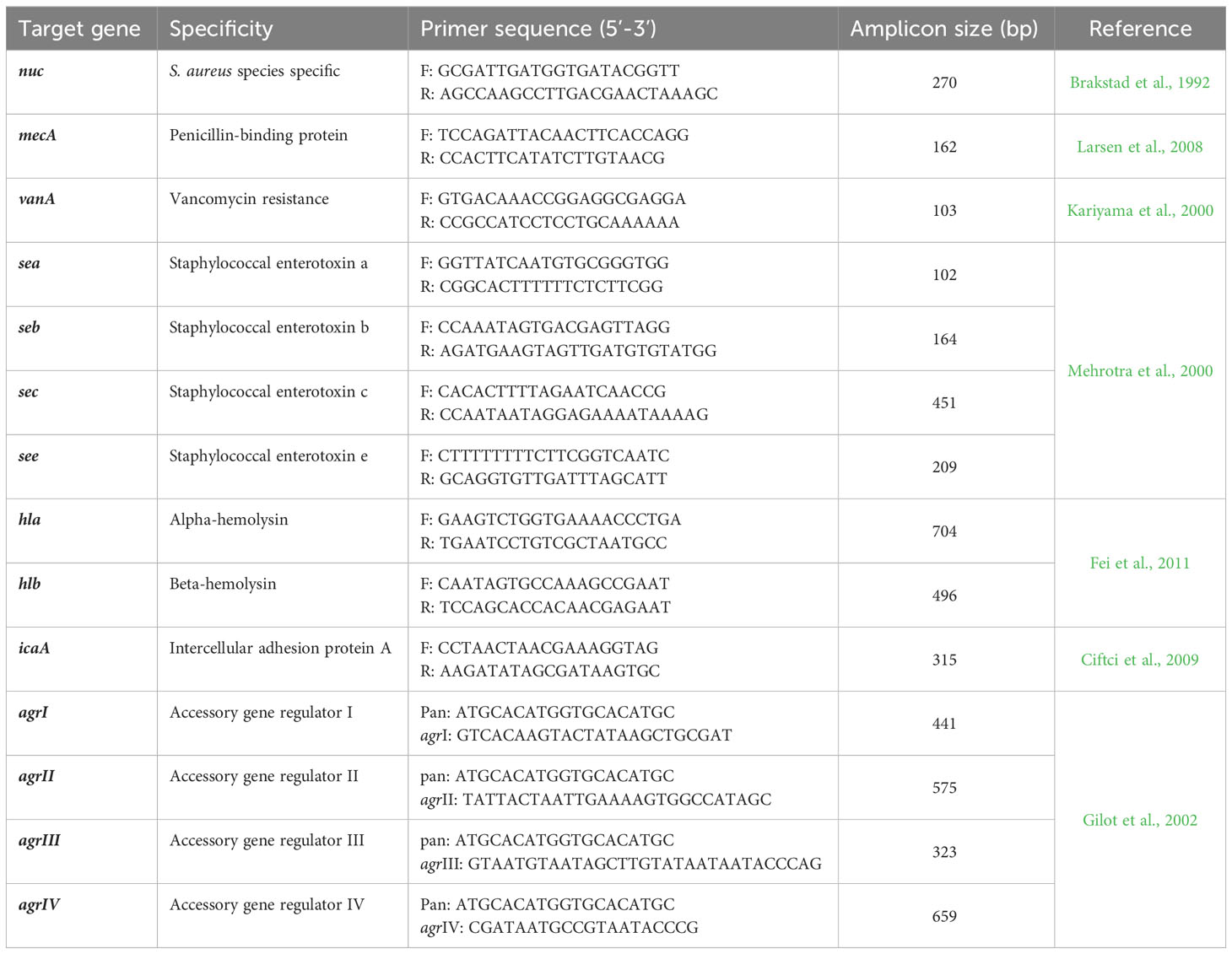
The presence of sea, seb, sec, see, hla and hlb virulence genes in addition to agr types (I–IV) were determined using singleplex and multiplex PCR assays using EmeraldAmp® GT PCR Master Mix (Takara, USA) and specific primers. The primers used for PCR are listed in Table 1. Amplification of target genes was performed as previously stated (Mehrotra et al., 2000; Gilot et al., 2002 and Fei et al., 2011). Controls for each PCR run contained positive (DNA extracted from S. aureus reference strain ATCC25923), negative (DNA extracted from Escherichia coli reference strain ATCC25922) and no template (PCR reaction mixture components without DNA) samples. Electrophoresis of amplified PCR products on a 1.5% agarose gel stained with ethidium bromide (Sigma-Aldrich, USA) allowed for their visualization under ultraviolet light.
Essential oils antibacterial and antibiofilm activities
Carvacrol (98% purity), linalool (97% purity) and eugenol (99% purity) EOs purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) were evaluated for their antibacterial activities against MDR and multivirulent MRSA and VRSA isolates using an agar well diffusion assay (Elmowalid et al., 2022). The stock solutions of EOs were prepared in 10% dimethyl sulfoxide as a diluent, since it is a known universal solvent with no antibacterial activity at this concentration; it was used as a negative control in one well of each tested plate. Positive results were recorded as zones of inhibition of > 7 mm. Subsequently, MIC values of the screened EOs were evaluated via broth microdilution method (Elmowalid et al., 2022). Notably, the effects of investigated EOs at their sub-MIC (0.5 MIC) levels on the biofilms of the tested isolates were further assessed via CRA (Freeman et al., 1989) and MTP (Stepanovic et al., 2000) methods. Two sets were performed, in triplicate, for each isolate in control plates with plain media and in plates with media and sub-MIC levels of the EOs.
Interaction of essential oils with antimicrobials via checkerboard assay
Evaluating the in vitro interaction between EOs and the least effective antimicrobials against MDR and multivirulent MRSA and VRSA isolates was done using a checkerboard technique, in triplicate, adopting the protocol previously detailed (Magi et al., 2015). Assessing the interaction between the investigated antimicrobial compounds was conducted through calculating the fractional inhibitory concentration index (FICI) using the following formula: FICI = MIC of the least effective antimicrobial in combination/MIC of antimicrobial alone + MIC of EO in combination/MIC of EO alone. The obtained FICI values were interpreted as following: synergism; FICI ≤ 0.5, additivity; 0.5 < FICI ≤ 1, indifference; 1 < FICI ≤ 4 and antagonism; FICI > 4.
Molecular docking studies
The molecular docking program MOE 2019 suite was used to investigate the antibiofilm potential of the three EOs against S. aureus (Inc, 2016). The molecular docking was established for Bap of S. aureus. The PerkinElmer ChemOffice Suite 2017 was used to determine and draw the chemical structures of the assessed compounds, which were then available for the molecular docking process (Elmaaty et al., 2021). These compounds were introduced into one database to be downloaded as an MDB extension file. Moreover, the X-ray structure of S. aureus Bap was downloaded from the online RCSB website with PDB entry: 7c7u (Ma et al., 2021). Accordingly, selected protein was prepared for molecular docking as previously discussed (Ma et al., 2021).
Statistical analysis
Significant variations were detected using Chi- square without replication. Typical statistically significant results were identified when the p value was < 0.05. All dendrograms and figures were constructed using the R packages corrplot, heatmap, hmisc, and ggpubr (Galili et al., 2018).
Results
Characterization of biofilm producing S. aureus isolates
Phenotypic analysis of mastitis milk samples revealed 37 staphylococcal isolates (20.6%), which were all confirmed to be S. aureus based on standard conventional bacteriological tests in addition to genetic detection of nuc (S. aureus species-specific) gene. Of note, all recovered isolates were identified phenotypically as biofilm producers depending on growth onto Congo red agar and adherence on MTP and genotypically via their possession for icaA gene.
Antimicrobial susceptibility results
The recovered 37 S. aureus isolates exhibited full resistance to cefoxitin (100%) and high resistance rates were detected against ampicillin (81.1%), followed by erythromycin (67.6%) and gentamycin (62.2%). Meanwhile, higher sensitivity rate was detected against ciprofloxacin (78.4%). There were statistically significant (p < 0.05) variations in the susceptibility patterns among S. aureus isolates against various antimicrobials. All 37 phenotypically cefoxitin resistant S. aureus isolates were positive for mecA gene; thus, molecularly confirmed as MRSA. Basing on phenotypic vancomycin resistance, 15 out of 37 (40.5%) S. aureus isolates were positive for vanA gene being defined as VRSA. Of note, 73% (27/37) of the tested MRSA isolates and all VRSA ones were MDR (Figure 1).
Molecular investigation of virulence genes and agr genotyping
All our isolates were positive for icaA gene (100%), while no isolates were positive for sec gene. Furthermore, sea, seb, see, hla and hlb genes were more prevalent among MRSA isolates (51.3, 35.1, 13.5, 32.4 and 13.5%) than VRSA ones (26.7, 0, 0, 20 and 0%), respectively (Figure 2). Besides, 16 MRSA (43.2%) and 3 VRSA (20%) isolates were multivirulent harboring three or more virulence genes (Figure 2). There were statistically significant (p < 0.05) variations in the occurrence of virulence genes among MRSA and VRSA isolates. Concerning agr genotyping, majority of MRSA (45.9%) and all VRSA (100%) isolates were positive for agrI gene. Furthermore, agrII, agrIII and agrIV genes were more prevalent among MRSA (32.4, 16.2 and 5.4%).
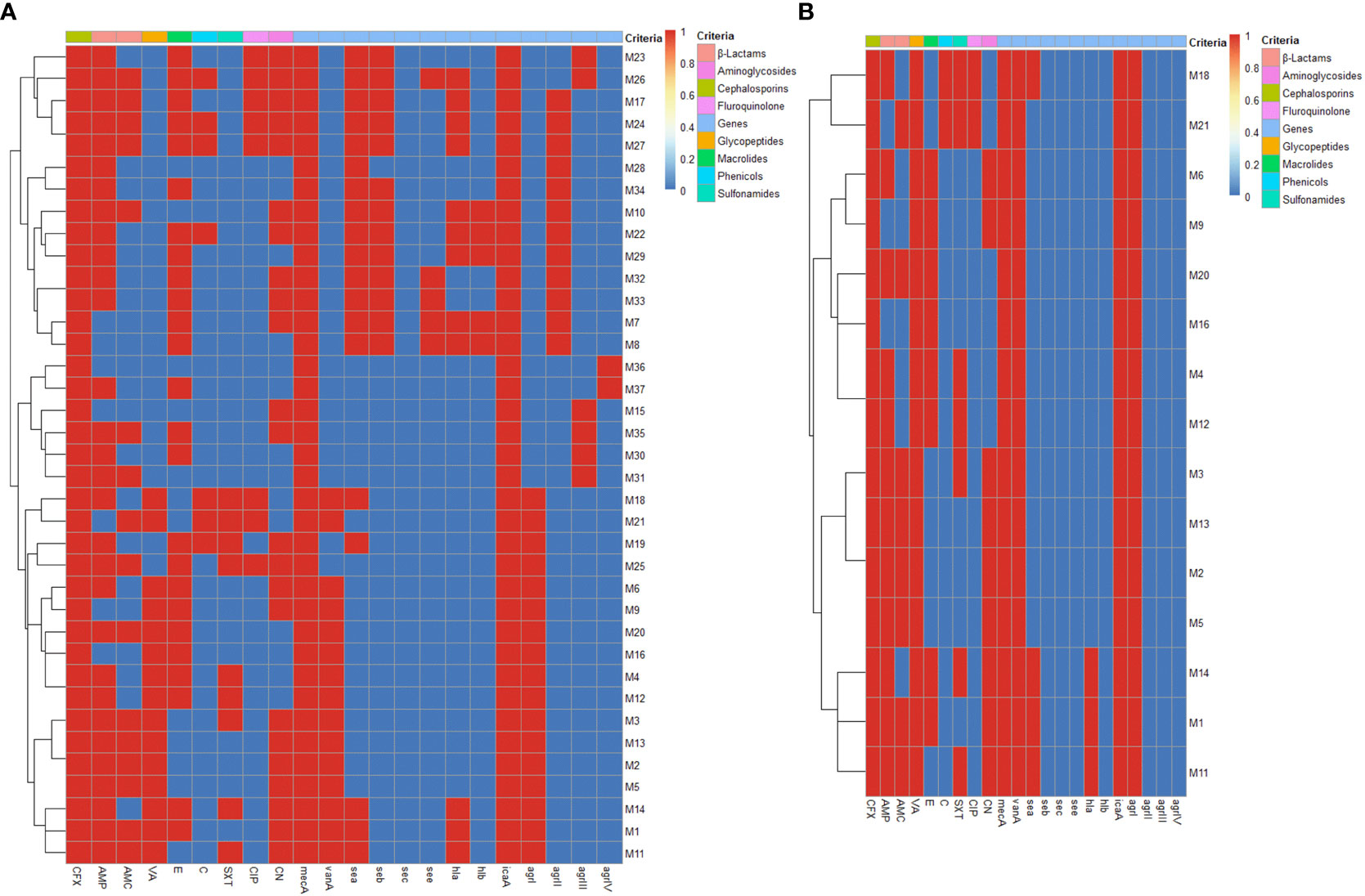
Figure 2 Heat maps illustrating the distribution of phenotypic antimicrobial resistance, mecA, vanA and virulence genes and agr genotypes among MRSA (A) and VRSA (B) isolates. The blue and red colors represent the sensitivity and resistance to certain antimicrobial agent and the absence and presence of mecA, vanA and certain virulence gene and agr genotype, respectively. The recovered isolates are coded on the right of the heat map; M: mastitis milk. CFX: cefoxitin, AMP: ampicillin, AMC: amoxicillin-clavulanic acid, VA: vancomycin, E: erythromycin, C: chloramphenicol, SXT: sulfamethoxazole-trimethoprim, CIP: ciprofloxacin and CN: gentamycin, mecA: methicillin resistance encoding gene, vanA: vancomycin resistance encoding gene, sea: staphylococcal enterotoxin a gene, seb: staphylococcal enterotoxin b gene, sec: staphylococcal enterotoxin c gene, see: staphylococcal enterotoxin e gene, hla: alpha-hemolysin gene, hlb: beta-hemolysin gene, icaA: intercellular adhesion A gene, agr: accessory gene regulator gene.
In vitro antibacterial and antibiofilm activities of the tested essential oils
The antibacterial potentials of carvacrol, linalool, and eugenol EOs were investigated against MDR and multivirulent MRSA strains. Considering the zones of inhibition and MIC values, these natural compounds exhibited excellent antibacterial efficacy against all investigated isolates with relevant inhibition zones’ diameters ranging from 20 to 37 mm and MIC values of 0.5 - 8 µg/mL. In contrast to eugenol, linalool EO showed the highest antimicrobial activities with mean inhibition zones’ diameters of 24 ± 0.5 mm and MIC values between 0.5 and 2 µg/mL.
Regarding the antibiofilm activities of carvacrol, linalool, and eugenol EOs, pronounced effects were noticed against the examined isolates. Linalool EO showed the highest antibiofilm activities, followed by carvacrol then eugenol (p < 0.5). This was evidenced by prominent reduction in the capacity of all tested biofilm producing isolates post exposure to the screened EOs comparing with the untreated ones with inhibitory capacity percentages fluctuating from 98.90 to 99.96%.
Assessing interaction between antimicrobials and essential oils
Owing to the full resistance of tested MRSA and VRSA isolates to cefoxitin and vancomycin, respectively, the activities of both antibiotics were examined in combination with the screened EOs. The results of checkerboard assay exhibited noteworthy synergistic interactions between these antibiotics and the investigated EOs against all MRSA and VRSA isolates with FIC values ≤ 0.5. Fortunately, the antimicrobial resistance patterns of 13 isolates; 10 MRSA and 3 VRSA changed to cefoxitin and vancomycin sensitive phenotypes upon treating with any of the investigating EOs, respectively.
Molecular docking results
The molecular docking study was carried out to evaluate the potential of the three tested EOs against S. aureus biofilms getting far deep understanding and further insights about their antibiofilm activities. According to molecular docking results, linalool showed the highest binding capacity, followed by carvacrol then eugenol. Linalool could make a stable complex with a binding energy of -6.00 Kcal/mol at root mean square deviation (RMSD) value of 1.32 Å. It was disclosed that the hydroxyl group of linalool could form H bond with GLN506 at a distance of 3.15 Å. Additionally, the terminal methyl group at position 8 of linalool could form H-pi with TYR366 at a distance of 3.78 Å as depicted in Table 2 and Figure 3. Besides, it was shown that carvacrol could make a stable complex with Bap with a binding energy of -5.59 Kcal/mol at an RMSD value of 1.09 Å. It was found that the phenyl moiety of carvacrol could form pi-H bond with ARG738 at a distance of 3.73 Å (Table 2 and Figure 3). Moreover, eugenol was able to form a stable complex with a binding energy of -5.44 Kcal/mol at an RMSD value of 0.64 Å. It was revealed that the phenyl ring of eugenol could form pi-H bond with ARG738 at a distance of 4.62 Å as represented in Table 2 and Figure 3.
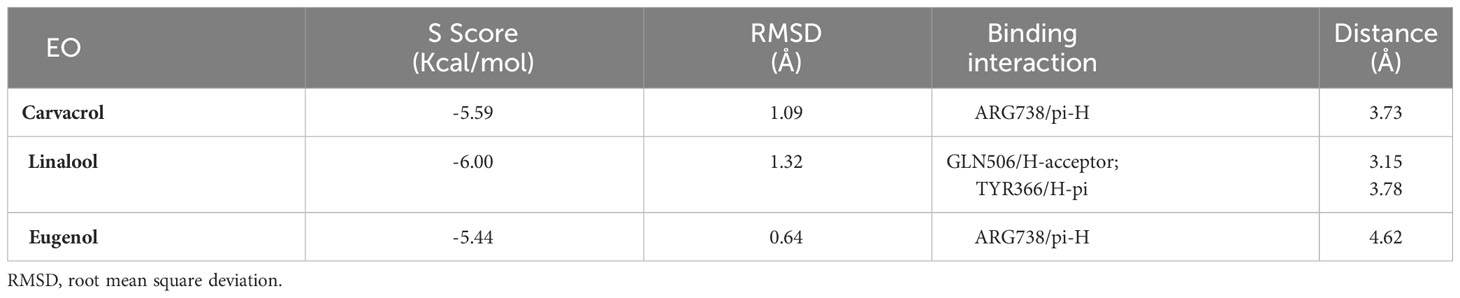
Table 2 Ligand-protein complex binding energy, RMSD and binding interactions of carvacrol, linalool and eugenol EOs with S. aureus biofilm associated protein.
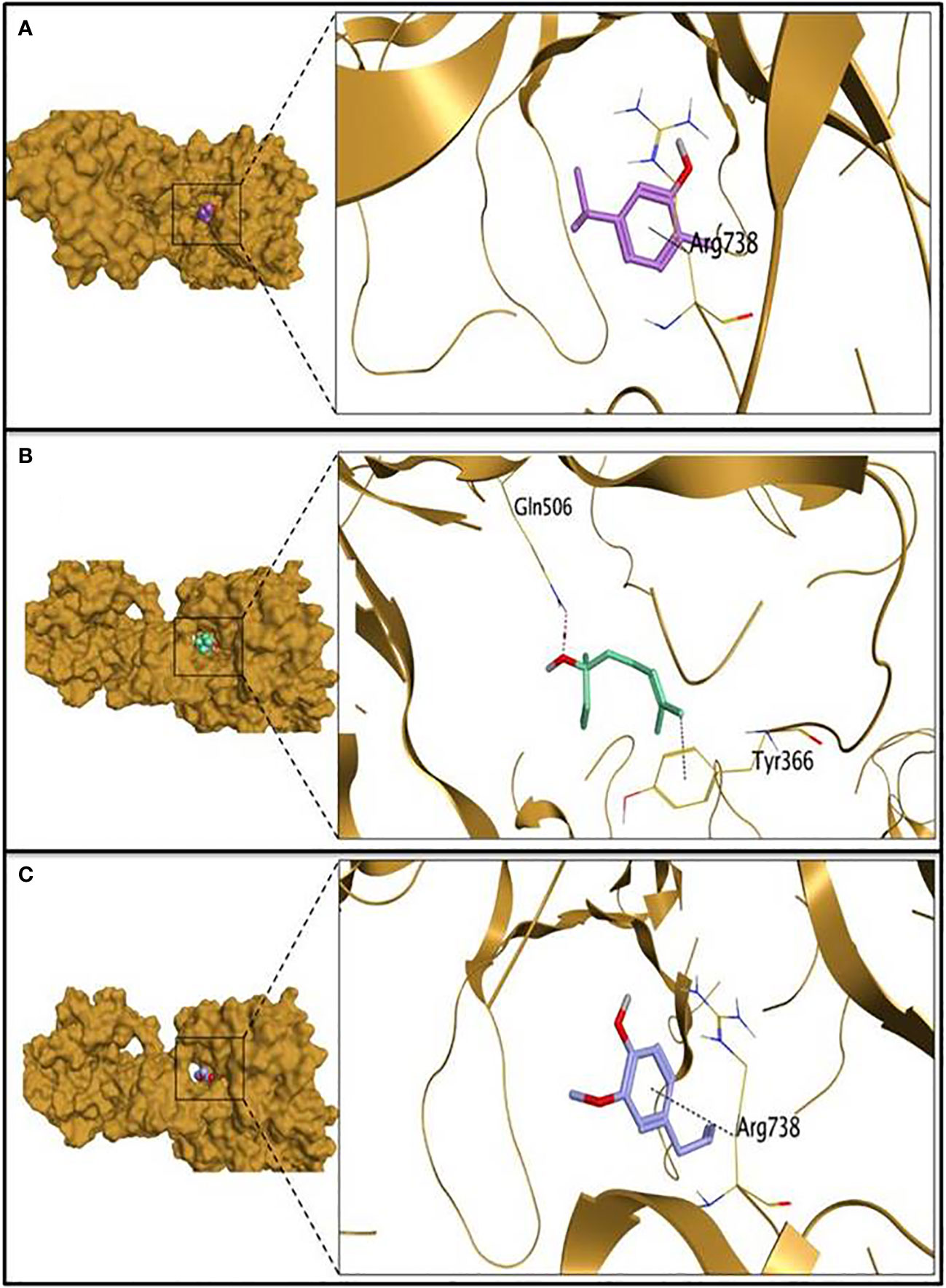
Figure 3 The 3D protein positioning and 3D binding interactions of the tested essential oils with S. aureus biofilm associated protein; binding of carvacrol (A), linalool (B) and eugenol (C) with PDB entry of 7c7u.
Discussion
Bovine mastitis as one of the most important dairy cattle diseases affecting mammary tissue may be chronic, clinical or subclinical leading to great economic losses. Of note, serious zoonotic diseases were always associated with different form of mastitis (Hoe and Ruegg, 2005). Moreover, mastitis shares in the wide spread of antimicrobial resistance globally (Beyene et al., 2017). The resistant biofilm producing S. aureus is the most common cause of bovine mastitis; therefore, new therapeutic options including complementary and alternative therapies are urgently required (Ghaly et al., 2021; Mosallam et al., 2021; Elfaky et al., 2022; Ghaly et al., 2023). So, we aimed to find successful antimicrobial protocols to prevent the wide spreading of mastitis that is particularly associated with biofilm producing S. aureus in the endemic area.
In this study, we recorded relative higher prevalence of bovine mastitis with S. aureus infections (20.6%). Other studies announced lower prevalence rates of S. aureus (16.1, 10 and 3%) among mastitic cows (Schukken et al., 2009; Tenhagen et al., 2009; Beyene et al., 2017). The variations in the prevalence rates of S. aureus among mastitic cows in this study and other previous studies may be attributed to differences in the standard hygienic practices applied in different countries (Getaneh and Gebremedhin, 2017). Therefore, proper control practices should be directed to prevent the wide spreading of bovine mastitis through segregations or selective culling of infected animals in some cases, which did not respond to any type of treatment protocols side by side with proper milking procedures (Ruegg, 2017). Interestingly, poor prognosis is always associated with the bacterial pathogens with multivirulence arrays (Ammar et al., 2020). Biofilm production is one of the main causes of antimicrobial resistance and it is a leading trait for increasing the sharpness and frequency of bovine mastitis treatment failure. Biofilm producing S. aureus is the causative agent of severe mastitis cases that respond very slowly to treatment (Szweda et al., 2012; Abd-El-Hamid et al., 2020). In this study, all our S. aureus isolates were phenotypically and genotypically identified as biofilm producers. The phenotypic identification of biofilm producers matched with the genetic detection of icaA gene among all S. aureus isolates. Previously, almost all S. aureus isolates causing bovine mastitis were able to produce biofilms (de Castro Melo et al., 2013; Bendary et al., 2016; Notcovich et al., 2018).
Of note, all our isolates were identified as MRSA, which showed complete resistance to cefoxitin and harbored mecA gene. Unfortunately, VRSA were detected among our isolates with a relative higher prevalence rate (40.5%). Convergently, previous reports announced that MRSA were the most prevalent recovered strains among bovine mastitis (Holmes and Zadoks, 2011; Krukowski et al., 2020). Moreover, 73% (27/37) of the tested MRSA isolates and all VRSA ones were MDR. Of note, the antimicrobial resistance and the wide spreading of MDR isolates were increasingly noticeable among bovine mastitis cases worldwide (Hoque et al., 2018; Salauddin et al., 2020). The antimicrobial resistance is a global multifaceted phenomenon and the increasing rates of this problem may be attributed to several factors including the inappropriate use of antibiotics, especially in veterinary fields as growth enhancers, self-medication and the poor application of antimicrobial stewardship programs (Prestinaci et al., 2015).
Surprisingly, most of our isolates were multivirulent harboring three or more virulence genes, which may compound the severity of the diseases. Therefore, bovine mastitis associated with biofilm producing MRSA showing MDR patterns is a common crisis globally. Therefore, there is an urgent need for additional efforts and researches to address this issue. Poor prognosis is always associated with biofilm producing MRSA strains owing to the extreme resistance of microbial cells in biofilms. For that, finding new alternatives are important research approaches, which have attracted the interest of many researchers. One of innovative approaches to treat S. aureus biofilm-related infections was evaluated in previous studies using non-antibiotics drugs (Kiedrowski and Horswill, 2011; Richter et al., 2017). Some detergent such as cis-2-decanoic acid could disperse staphylococcal biofilms (Davies and Marques, 2009). Moreover, EOs such as carvacrol, linalool and eugenol have been used in food industry owing to their preservative potency against foodborne pathogens. In this report, we evaluated the antimicrobial and antibiofilm activities of these EOs against S. aureus, the predominant contagious pathogens causing bovine mastitis and we found great anti-MRSA activities of the investigated EOs, especially linalool. This finding goes parallel with the published results for linalool antimicrobial and antibiofilm activities (Bagamboula et al., 2004; Aelenei et al., 2019). The cell membrane, especially mesosomes and cell wall integrity are the targeting sites of linalool (Gao et al., 2019). Interestingly, the synergistic interactions between the investigated EOs and other antimicrobial drugs were announced in this study. Several authors stated that treatment failure owing to antimicrobial resistance could be solved via using combinations of the available antibiotics and other natural compound such as EOs (Lahmar et al., 2017; Aelenei et al., 2019; Özel et al., 2022). These EOs could increase the rates of antimicrobial susceptibility and reversal of antimicrobial resistance. The antibiotic actions could be rescued when used in combination with EOs. In accordance with our results, the resistances of MRSA to β-lactam antibiotics were highly reduced in the presence of EOs (Lahmar et al., 2017). Although the exact synergistic interactions between antibiotics and EOs have still not been exactly clarified, several authors used time-kill curve analysis to confirm the efficacy of these combinations (Aleksic et al., 2014; Abd El-Hamid et al., 2022).
Conclusion
Multidrug resistance and multivirulence were the common phenotypes among MRSA strains incriminated in bovine mastitis. Despite the great difficulties to control and eradicate these phenotypes with common used antimicrobial drugs, EOs, especially linalool as proven in this study give us the bright hope to increase the therapeutic options and the possibility of treatment success. Therefore, we recommended using combination therapies between the available antibiotics and the natural compounds such as EOs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Collection of milk samples in this study was approved by the Animal Ethics Review Committee of Suez Canal University (AERCSCU2023029), Egypt.
Author contributions
MA: Methodology, Software, Writing – review & editing. RE: Investigation, Formal Analysis, Writing – review & editing. MBa: Data curation, Project administration, Writing – review & editing. MA: Software, Visualization, Writing – review & editing. AS: Data curation, Formal Analysis, Writing – review & editing. KA: Funding acquisition, Data curation, Writing – review & editing. FS: Supervision, Writing – review & editing. AM: Supervision, Formal Analysis, Writing – review & editing. NA: Conceptualization, Validation, Writing – review & editing. MG: Resources, Supervision, Writing – review & editing. AE: Methodology, Software, Writing – review & editing. HA: Formal Analysis, Writing – review & editing. MBe: Methodology, Writing – original draft, Writing – review & editing.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project Number PNURSP2023R318, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Moreover, this work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT4,029]
Acknowledgments
The authors would like to appreciate Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also, the authors acknowledge the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT4,029] for support this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Hamid, M. I., Awad, N. F. S., Hashem, Y. M., Abdel-Rahman, M. A., Abdelaziz, A. M., Mohammed, I. A., et al. (2019). In vitro evaluation of various antimicrobials against field Mycoplasma gallisepticum and Mycoplasma synoviae isolates in Egypt. Poultry Sci. 98, 6281–6288. doi: 10.3382/ps/pez576
Abd-El-Hamid, M. I., El-Naenaeey, E. I., Hegazy, W. A., Mosbah, R. A., Nassar, M. S., Bakhrebah, M. A., et al. (2020). Promising antibiofilm agents: Recent breakthrough against biofilm producing methicillin-resistant Staphylococcus aureus. Antibiotics 9, 667. doi: 10.3390/antibiotics9100667
Abd El-Hamid, M. I., Sewid, A. H., Samir, M., Hegazy, W. H., Bahnass, M. M., Mosbah, R. A., et al. (2022). Clonal diversity and epidemiological characteristics of ST239-MRSA strains. Front. Cell. infection Microbiol. 12, 782045. doi: 10.3389/fcimb.2022.782045
Aelenei, P., Rimbu, C. M., Guguianu, E., Dimitriu, G., Aprotosoaie, A. C., Brebu, M., et al. (2019). Coriander essential oil and linalool - interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 68, 156–164. doi: 10.1111/lam.13100
Aleksic, V., Mimica-Dukic, N., Simin, N., Nedeljkovic, N. S., Knezevic, P. (2014). Synergistic effect of Myrtus communis L. essential oils and conventional antibiotics against multi-drug resistant Acinetobacter baumannii wound isolates. Phytomed. Int. J. phytother. phytopharmacol. 21, 1666–1674. doi: 10.1016/j.phymed.2014.08.013
Algammal, A. M., Enany, M. E., El-Tarabili, R. M., Ghobashy, M. O. I., Helmy, Y. A. (2020). Prevalence, antimicrobial resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens 9, 362. doi: 10.3390/pathogens9050362
Ameen, F., Reda, S. A., El Shatoury, S., Riad, E. M., Enany, M. E., Alarfaj, A. A. (2019). Prevalence of antibiotic resistant mastitis pathogens in dairy cows in Egypt and potential biological control agents produced from plant endophytic actinobacteria. Saudi J. Biol. Sci. 26, 1492–1498. doi: 10.1016/j.sjbs.2019.09.008
Ammar, A. M., El-Naenaeey, E. Y., El-Malt, R. M. S., El-Gedawy, A. A., Khalifa, E., Elnahriry, S. S., et al. (2020). Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Animals 11, 3. doi: 10.3390/ani11010003
Ascioferro, S., Carbone, D., Parrino, B., Pecoraro, C., Giovannetti, E., Cirrincione, G., et al. (2021). Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 16, 65–80. doi: 10.1002/cmdc.202000677
Bagamboula, C., Uyttendaele, M., Debevere, J. (2004). Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 21, 33–42. doi: 10.1016/s0740-0020(03)00046-7
Bauer, A. W., Kirby, W. M., Sherris, J. C., Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496. doi: 10.1093/ajcp/45.4_ts.493
Becker, K., Skov, R. L., Von Eiff, C. (2015). Staphylococcus, micrococcus, and other catalase-positive cocci. In Jorgensen, JH, Carroll, KC, Funke, G, Pfaller, MA (ed), Manual of clinical microbiology, 11th ed (Washington, DC: ASM Press), p. 354–382. doi: 10.1128/9781555817381.CH21
Bendary, M. M., Solyman, S. M., Azab, M. M., Mahmoud, N. F., Hanora, A. M. (2016). Characterization of methicillin resistant Staphylococcus aureus isolated from human and animal samples in Egypt. Cell. Mol. Biol. (Noisy-le-Grand France) 62, 94–100. doi: 10.14715/cmb/2016.62.2.16
Beyene, T., Hayishe, H., Gizaw, F., Beyi, A. F., Abunna, F., Mammo, B., et al. (2017). Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Res. Notes 10, 171. doi: 10.1186/s13104-017-2487-y
Brakstad, O. G., Aasbakk, K., Maeland, J. A. (1992). Detection of S. aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30, 1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992
Ciftci, A., Findik, A., Onuk, A., Savasan, S. (2009). Detection of methicillin resistance and slime factor production of Staphylococus aureus in bovine mastitis. Braz. J. Microbiol. 40, 254–261. doi: 10.1590/S1517-83822009000200009
Clinical and Laboratory Standards Institute (CLSI) (2020). “Performance standards for antimicrobial susceptibility testing,” in CLSI supplement M100, 30th-ed (Pennsylvania, USA: Wayne).
Cui, H., Zhang, C., Li, C., Lin, L. (2019). Antibacterial mechanism of oregano essential oil. Ind. Crops Prod 139, 111498. doi: 10.1016/j.indcrop.2019.111498
Davies, D. G., Marques, C. N. (2009). A fatty acid messengeris responsible for inducing dispersion in microbial biofilms. J. Bacteriol 191, 1393–1403. doi: 10.1128/JB.01214-08
de Castro Melo, P., Ferreira, L. M., Filho, A. N., Zafalon, L. F., Vicente, H. I., de Souza, V. (2013). Comparison of methods for the detection of biofilm formation by Staphylococcus aureus isolated from bovine subclinical mastitis. Braz. J. Microbiol. 44, 119–124. doi: 10.1590/S1517-83822013005000031
Elfaky, M. A., Abdel-Hamid, M. I., Khalifa, E., Alshareef, W. A., Mosbah, R. A., Elazab, S. T., et al. (2022). Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 106 (4), 1691–1703. doi: 10.1007/s00253-022-11781-w
El-Jakee, J., Atta, N. S., Samy, A., Bakry, M., Elgabry, E., Kandil, M. M., et al. (2011). Antimicrobial resistance in clinical isolates of Staphylococcus aureus from bovine and human sources in Egypt. Glob. Vet. 7, 581–586. https://scholar.cu.edu.eg/sites/default/files/jakee/files/antimicrobial_resistance_in_clinical_isolates_of.pdf
Elmaaty, A. A., Darwish, M. K., Khattab, M., Elhady, S. S., Salah, M., Hamed, M. A. (2021). In a search for potential drug candidates for combating COVID-19: computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J. Biomolec. Structure Dynam. 40, 8866–8893. doi: 10.1080/07391102.2021.1918256.
Elmowalid, G. E., Ahmad, A. M., El-Hamid, M. A., Ibrahim, D., Wahdan, A., El Oksh, A. A., et al. (2022). Nigella sativa extract potentially inhibited methicillin resistant Staphylococcus aureus induced infection in rabbits: potential immunomodulatory and growth promoting properties. Animals 12, 2635. doi: 10.3390/ani12192635
Fei, W., Hongjun, Y., Hong-bin, H., Changfa, W., Yundong, G., Qifeng, Z., et al. (2011). Study on the hemolysin phenotype and the genetype distribution of Staphylococcus aureus caused bovine mastitis in Shandong dairy farms. Intern. J. Appl. Res. Vet. Med. 9, 2011. http://www.jarvm.com/articles/Vol9Iss4/Yang.pdf?fbclid=IwAR3nVIAQLitkQVr3OmuYgCmfaYSim2p0FdlqMEoVVWAlmS4g2K7KexKShBU.
Foster, T. J., Höök, M. (1998). Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6, 484–488. doi: 10.1016/S0966-842X(98)01400-0
Freeman, D. J., Falkiner, F. R., Keane, C. T. (1989). New method for detecting slime production by coagulase-negative staphylococci. J. Clin. Pathol. 42, 872–874. doi: 10.1136/jcp.42.8.872
Galili, T., O’callaghan, A., Sidi, J., Sievert, C. (2018). Heatmaply: an R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 34, 1600–1602. doi: 10.1093/bioinformatics/btx657
Gao, Z., Van Nostrand, J. D., Zhou, J., Zhong, W., Chen, K., Guo, J. (2019). Anti-listeria activities of linalool and its mechanism revealed by comparative transcriptome analysis. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02947
Getaneh, A. M., Gebremedhin, E. Z. (2017). Meta-analysis of the prevalence of mastitis and associated risk factors in dairy cattle in Ethiopia. Trop. Anim. Health Prod 49, 697–705. doi: 10.1007/s11250-017-1246-3
Ghaly, M. F., Albalawi, M. A., Bendary, M. M., Shahin, A., Shaheen, M. A., Abu Eleneen, A. F., et al. (2023). Tamarindus indica extract as a promising antimicrobial and antivirulence therapy. Antibiotics 12, 464. doi: 10.3390/antibiotics12030464
Ghaly, M. F., Nasr, Z. M., Abousaty, A. I., Seadawy, H. G., Shaheen, M. A. A., Albogami, S., et al. (2021). Alternative and complementary therapies against foodborne salmonella infections. Antibiotics 10, 1453. doi: 10.3390/antibiotics10121453
Gilot, P., Lina, G., Cochard, T., Poutrel, B. (2002). Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of S. aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 40, 4060–4067. doi: 10.1128/JCM.40.11.4060-4067.2002
Hiramatsu, K., Katayama, Y., Matsuo, M., Sasaki, T., Morimoto, Y., Sekiguchi, A., et al. (2014). Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother. 20, 593–601. doi: 10.1016/j.jiac.2014.08.001
Hoe, F. G., Ruegg, P. L. (2005). Relationship between antimicrobial susceptibility of clinical mastitis pathogens and treatment outcome in cows. J. Am. Veterinary Med. Assoc. 227, 1461–1468. doi: 10.2460/javma.2005.227.1461
Holmes, M. A., Zadoks, R. N. (2011). Methicillin resistant S. aureus in human and bovine mastitis. J. mammary gland Biol. neoplasia 16, 373–382. doi: 10.1007/s10911-011-9237-x
Hoque, M. N., Das, Z. C., Rahman, A. M., Haider, M. G., Islam, M. A. (2018). Molecular characterization of Staphylococcus aureus strains in bovine mastitis milk in Bangladesh. Int. J. veterinary Sci. Med. 6, 53–60. doi: 10.1016/j.ijvsm.2018.03.008
Inc, C.C.G (2016). Molecular operating environment (MOE) (1010 Sherbooke St. West, Suite# 910, Montreal: Chemical Computing Group Inc).
Javdan, S., Narimani, T., Shahini Shams Abadi, M., Gholipour, A. (2019). Agr typing of Staphylococcus aureus species isolated from clinical samples in training hospitals of Isfahan and Shahrekord. BMC Res. Notes 12, 363. doi: 10.1186/s13104-019-4396-8
Kariyama, R., Mitsuhata, R., Chow, J. W., Clewell, D. B., Kumon, H. (2000). Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J. Clin. Microbiol. 38, 3092–3095. doi: 10.1128/JCM.38.8.3092-3095.2000
Kasper, S., Gastpar, M., Müller, W. E., Volz, H. P., Möller, H. J., Dienel, A., et al. (2010). Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of 'subsyndromal' anxiety disorder: a randomized, double-blind, placebo controlled trial. Int. Clin. Psychopharmacol. 25, 277–287. doi: 10.1097/YIC.0b013e32833b3242
Kiedrowski, M. R., Horswill, A. R. (2011). New approaches for treating staphylococcal biofilm infections. Ann. New York Acad. Sci. 1241, 104–121. doi: 10.1111/j.1749-6632.2011.06281.x
Kløve, D. C., Jensen, V. F., Astrup, L. B. (2022). First finding of a methicillin-resistant Staphylococcus aureus (MRSA) t304/ST6 from bovine clinical mastitis. Antibiotics 11, 1393. doi: 10.3390/antibiotics11101393
Krifa, M., El Mekdad, H., Bentouati, N., Pizzi, A., Ghedira, K., Hammami, M., et al. (2015). Immunomodulatory and anticancer effects of Pituranthos tortuosus essential oil. Tumor Biol. 36, 5165–5170. doi: 10.1007/s13277-015-3170-3
Krukowski, H., Bakuła, Z., Iskra, M., Olender, A., Bis-Wencel, H., Jagielski, T. (2020). The first outbreak of methicillin-resistant Staphylococcus aureus in dairy cattle in Poland with evidence of on-farm and intrahousehold transmission. J. dairy Sci. 103, 10577–10584. doi: 10.3168/jds.2020-18291
Lahmar, A., Bedoui, A., Mokdad-Bzeouich, I., Dhaouifi, Z., Kalboussi, Z., Cheraif, I., et al. (2017). Reversal of resistance in bacteria underlies synergistic effect of essential oils with conventional antibiotics. Microbial. pathogenesis 106, 50–59. doi: 10.1016/j.micpath.2016.10.018
Larsen, A. R., Stegger, M., Sørum, M. (2008). Spa typing directly from a mecA, spa and pvl multiplex PCR assay-a cost-effective improvement for methicillin-resistant Staphylococcus aureus surveillance. Clin. Microbiol. Infect. 14, 611–614. doi: 10.1111/j.1469-0691.2008.01995.x
Lebeaux, D., Chauhan, A., Rendueles, O., Beloin, C. (2013). rom in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2, 288–356. doi: 10.3390/pathogens2020288
Leyva-Lopez, N., Gutierrez-Grijalva, E. P., Vazquez-Olivo, G., Heredia, J. B. (2017). Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules 22, 989. doi: 10.3390/molecules22060989
Ma, J., Cheng, X., Xu, Z., Zhang, Y., Valle, J., Fan, S., et al. (2021). Structural mechanism for modulation of functional amyloid and biofilm formation by Staphylococcal Bap protein switch. EMBO J. 40, e107500. doi: 10.15252/embj.2020107500
Magi, G., Marini, E., Facinelli, B. (2015). Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 6, 165. doi: 10.3389/fmicb.2015.00165
Mehrotra, M., Wang, G., Johnson, W. M. (2000). Multiplex PCR for detection of genes for S. aureus enterotoxins, exfoliative toxins, toxic shock syndrome Toxin 1 and methicillin resistance. J. Clin. Microbiol. 38, 1032–1035. doi: 10.1128/JCM.38.3.1032-1035.2000
Mosallam, F. M., Helmy, E. A., Bendary, M. M., El-Batal, I. A. (2021). Potency of a novel synthesized Ag- eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 36, 1524–1537. doi: 10.1557/s43578-021-00226-1
Notcovich, S., DeNicolo, G., Flint, S. H., Williamson, N. B., Gedye, K., Grinberg, A., et al. (2018). Biofilm-forming potential of Staphylococcus aureus isolated from clinical mastitis cases in New Zealand. Veterinary Sci. 5, 8. doi: 10.3390/vetsci5010008
Özel, Y., Yılmaz, U., Ünlü, M., Vardar Ünlü, G. (2022). Antibacterial activity and synergistic interaction of various essential oil components and antibiotics. Mikrobiyoloji bulteni 56, 95–102. doi: 10.5578/mb.20229908
Prestinaci, F., Pezzotti, P., Pantosti, A. (2015). Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Global Health 109, 309–318. doi: 10.1179/2047773215Y.0000000030
Puah, S., Chua, K., Tan, J. (2016). Virulence factors and antibiotic susceptibility of Staphylococcus aureus isolates in ready-to-eat foods: detection of S. aureus contamination and a high prevalence of virulence genes. Int. J. Environ. Res. 13, 199. doi: 10.3390/ijerph13020199
Puškárová, A., Bučková, M., Kraková, L., Pangallo, D., Kozics, K. (2017). The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 7, 8211. doi: 10.1038/s41598-017-08673-9
Richter, K., Van den Driessche, F., Coenye, T. (2017). Innovative approaches to treat Staphylococcus aureus biofilm-related infections. Essays Biochem. 61, 61–70. doi: 10.1042/EBC20160056
Ruegg, P. L. (2017). A 100-Year Review: Mastitis detection, management, and prevention. J. dairy Sci. 100, 10381–10397. doi: 10.3168/jds.2017-13023
Saber, T., Samir, M., El-Mekkawy, R. M., Ariny, E., El-Sayed, S. R., Enan, G., et al. (2022). Methicillin- and vancomycin-resistant Staphylococcus aureus from humans and ready-to-eat meat: characterization of antimicrobial resistance and biofilm formation ability. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.735494
Salauddin, M., Akter, M. R., Hossain, M. K., Nazir, K. H., Noreddin, A., El Zowalaty, M. E. (2020). Molecular detection of multidrug resistant Staphylococcus aureus isolated from bovine mastitis milk in Bangladesh. Veterinary Sci. 7, 36. doi: 10.3390/vetsci7020036
Schukken, Y. H., Gonzalez, R. N., Tikofsky, L. L., Schulte, H. F., Santisteban, C. G., Welcome, F. L., et al. (2009). CNS mastitis: nothing to worry about? Vet. Microbiol. 134, 9–14. doi: 10.1016/j.vetmic.2008.09.014
Shrestha, A., Bhattarai, R. K., Luitel, H., Karki, S., Basnet, H. B. (2021). Prevalence of methicillin-resistant Staphylococcus aureus and pattern of antimicrobial resistance in mastitis milk of cattle in Chitwan, Nepal. BMC Vet. Res. 17, 239. doi: 10.1186/s12917-021-02942-6
Silva, V., Almeida, L., Gaio, V., Cerca, N., Manageiro, V., Caniça, M., et al. (2021). Biofilm formation of multidrug-resistant MRSA strains isolated from different types of human infections. Pathogens 30, 970. doi: 10.3390/pathogens10080970
Stepanovic, S., Vukovic, D., Dakic, I., Savie, B., Svabic-Vlahovic, M. A. (2000). modified microtiter plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40, 175–179. doi: 10.1016/S0167-7012(00)00122-6
Swamy, M. K., Akhtar, M. S., Sinniah, U. R. (2016). Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evidence-based. Complementary Altern. Med. 2016, 1–21. doi: 10.1155/2016/3012462
Szweda, P., Schielmann, M., Milewski, S., Frankowska, A., Jakubczak, A. (2012). Biofilm production and presence of ica and bap genes in Staphylococcus aureus strains isolated from cows with mastitis in the Eastern Poland. Pol. J. Microbiol. 61, 65–69. doi: 10.33073/pjm-2012-009
Taleuzzaman, M., Jain, P., Verma, R., Iqbal, Z., Mirza, M. A. (2021). Eugenol as a potential drug candidate: A review. Curr. Top. Med. Chem. 21, 1804–1815. doi: 10.2174/1568026621666210701141433
Tenhagen, B. A., Hansen, I., Reinecke, A., Heuwieser, W. (2009). Prevalence of pathogens in milk samples of dairy cows with clinical mastitis and in heifers at first parturition. J. Dairy Res. 76, 179–187. doi: 10.1017/S0022029908003786
Wang, Q., Wang, X., Huang, L., Cheng, Y., Ren, L., Yang, H., et al. (2023). Promoter characterization of a citrus linalool synthase gene mediating interspecific variation in resistance to a bacterial pathogen. BMC Plant Biol. 23, 405. doi: 10.1186/s12870-023-04413-6
Keywords: carvacrol, linalool, eugenol, MDR, MRSA, antibiofilm
Citation: Abd El-Hamid MI, El-Tarabili RM, Bahnass MM, Alshahrani MA, Saif A, Alwutayd KM, Safhi FA, Mansour AT, Alblwi NAN, Ghoneim MM, Elmaaty AA, Al-harthi HF and Bendary MM (2023) Partnering essential oils with antibiotics: proven therapies against bovine Staphylococcus aureus mastitis. Front. Cell. Infect. Microbiol. 13:1265027. doi: 10.3389/fcimb.2023.1265027
Received: 21 July 2023; Accepted: 28 August 2023;
Published: 15 September 2023.
Edited by:
Mahmoud Abdelkhalek Elfaky, King Abdulaziz University, Saudi ArabiaReviewed by:
Uğur Parin, Adnan Menderes University, TürkiyePoonam Mudgil, Western Sydney University, Australia
Copyright © 2023 Abd El-Hamid, El-Tarabili, Bahnass, Alshahrani, Saif, Alwutayd, Safhi, Mansour, Alblwi, Ghoneim, Elmaaty, Al-harthi and Bendary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmoud M. Bendary, bWljcm9fYmVuZGFyeUB5YWhvby5jb20=
 Marwa I. Abd El-Hamid
Marwa I. Abd El-Hamid Reham M. El-Tarabili
Reham M. El-Tarabili Mosa M. Bahnass
Mosa M. Bahnass Mohammed Abdulrahman Alshahrani5
Mohammed Abdulrahman Alshahrani5 Khairiah Mubarak Alwutayd
Khairiah Mubarak Alwutayd Abdallah Tageldein Mansour
Abdallah Tageldein Mansour Mohammed M. Ghoneim
Mohammed M. Ghoneim Mahmoud M. Bendary
Mahmoud M. Bendary