
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 13 September 2023
Sec. Virus and Host
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1263983
The identification of the first human polyomavirus BK (BKV) has been over half century, The previous epidemiological and phylogenetic studies suggest that BKV prevailed and co-evolved with humans, leading to high seroprevalence all over the world. In general, BKV stays latent and symptomless reactivation in healthy individuals. BKV has been mainly interlinked with BKV-associated nephropathy (BKVAN) in kidney-transplant recipients and hemorrhagic cystitis (HC) in hematopoietic stem cell transplant recipients (HSCTRs). However, the mechanisms underlying BKV latency and reactivation are not fully understood and lack of extensive debate. As Merkel cell polyomavirus (MCV) was identified as a pathogenic agent of malignant cutaneous cancer Merkel cell carcinoma (MCC) since 2008, linking BKV to tumorigenesis of urologic tumors raised concerns in the scientific community. In this review, we mainly focus on advances of mechanisms of BKV latency and reactivation, and BKV-associated diseases or tumorigenesis with systematical review of formerly published papers following the PRISMA guidelines. The potential tumorigenesis of BKV in two major types of cancers, head and neck cancer and urologic cancer, was systematically updated and discussed in depth. Besides, BKV may also play an infectious role contributing to HIV-associated salivary gland disease (HIVSGD) presentation. As more evidence indicates the key role of BKV in potential tumorigenesis, it is important to pay more attention on its etiology and pathogenicity in vitro and in vivo.
BK polyomavirus (BKV), the first human polyomavirus isolated from an immunosuppressed kidney transplant recipient in 1971, is a member of the Polyomaviridae family of double-stranded DNA (dsDNA) viruses. “BK” is named after the initials of this patient (Gardner et al., 1971). In the same year, JC polyomavirus (JCV) was identified from specimens of brain pathology of a patient diagnosed of progressive multifocal leukoencephalopathy (PML) (Padgett et al., 1971). Identification of the first two viruses accelerated understanding of the pathogenicity of human polyomaviruses. For more than three decades, the BKV and JCV were the only well-known polyomaviruses associated with clinical diseases in specific groups of people. With the technological progress of modern molecular methods and next-generation sequencing technique, 13 polyomaviruses were included in the list of human polyomaviruses during the past 20 years (Prezioso et al., 2021) (Zhou et al., 2019). The previous epidemiological and phylogenetic studies suggest that BKV prevailed and co-evolved with humans, leading to its high seroprevalence all over the world (Viscidi et al., 2011; Gaboriaud et al., 2018; Zhou et al., 2019; Zhou et al., 2020). Based on genome sequence diversity, BKV has be divided into six genotypes, in which genotype I is considered as the most frequent worldwide (~80%), followed by genotype IV (15%) (Kotla et al., 2021). BKV is ubiquitous around the globe, with up to 90% of adults being seropositive, and the transmission routes of BKV were speculated through direct contact or fecal-oral transmission during childhood (Krajewski et al., 2020; Furmaga et al., 2021).
In general, BKV infection is self-limited and then remains latent in the urinary tissue for life time among immunocompetent individuals (Furmaga et al., 2021). Nevertheless, in some immunocompromised patients, BKV can reactivate with high level of viral replication. Most commonly, BKV reactivation leads to BKV-associated nephropathy (BKVAN) in some kidney transplant recipients (KTRs). And in some allogeneic hematopoietic stem cell transplant recipients (HSCTRs), the consequences of viral reactivation may be hemorrhagic cystitis (HC) (Krajewski et al., 2020; Laskin et al., 2020). However, the molecular mechanisms of BKV latency and pathogenicity after reactivation are not comprehensively discussed. In addition, BKV was also considered as a potential factor or co-factor of tumorigenesis (Burger-Calderon and Webster-Cyriaque, 2015) (Saber Amoli et al., 2021). And accumulated evidence links BKV to urinary tumors such as prostate and bladder cancer (Mischitelli et al., 2015; Vaezjalali et al., 2018; Villani et al., 2019). In addition, BKV was also linked to HIV-associated salivary gland disease (HIVSGD) in HIV-infected individuals, and HIVSGD is associated with increased lymphoma incidence (Burger-Calderon et al., 2016).
This review will focus on BKV latency, reactivation and the associated diseases, especially concentrate studies on organ-transplant recipients (OTRs), among whom viral reactivation might cause fatal damage. Moreover, the latest studies on the relationship between BKV and different types of cancers will be addressed and discussed.
In general, primary BKV infection occurs during childhood, as studies have shown that 60%-70% of children were seropositive of anti-BKV IgG by the age of 10 (Krajewski et al., 2020; Furmaga et al., 2021). BKV infection is considered to be transmitted through direct human-to-human contact or fecal-oral route, and respiratory route was also speculated to contribute to the high seroprevalence (Furmaga et al., 2021). As most BKV infections are asymptomatic and self-limited, it is not possible to confirm these transmission routes. Serological studies support that primary exposure to BKV occurs during early childhood, and then stay latent in most adult (Viscidi et al., 2011; Prelog et al., 2013; Sroller et al., 2014). The anti-BKV seroprevalence is low in children at their first 6 months with the gradual weakening of protection from maternal antibodies and reaches to 80%-90% among adults worldwide (Viscidi et al., 2011). In the past decades, as the establishment of virus-like particle-based ELISA and multiplex immunoassays, plus new members of human PyVs were constantly identified, more attention was paid to human PyVs and seroprevalence investigations on these viruses were conducted in many countries (Supplementary Table 1) (Stolt et al., 2003; Kean et al., 2009; Nicol et al., 2013; Prelog et al., 2013; Sroller et al., 2014; Zhang et al., 2014; Fukumoto et al., 2015; Laskin et al., 2015; Gaboriaud et al., 2018; Kamminga et al., 2018; Laine et al., 2023). Overall, most sero-epidemiology studies were conducted in developed countries in which BKV, and JCV were the most concerned pathogens. Whereas limited seroprevalence data was from low-income countries, indicating PyV-associated diseases were relatively neglected in the developing world.
In general, BKV, after primary infection, sustains a persistent latent stage in epithelial cells of renal tubules or urothelium for life time under normal conditions (McCaffrey et al., 2021). BKV entry into host cells is mediated via caveolae, entry into the cell is then driven by a caveola-mediated endocytic pathway (Eash et al., 2004). After entering the nucleus, the BKV genome remains episomal in human cells (Gorrill et al., 2006). In recent years, latency mechanism behind viral genome of BKV that enables its coexistence with human hosts aroused more concerns. BKV is a small (diameter 40 nm) non-enveloped icosahedral virus (Furmaga et al., 2021). BKV genome is a double-stranded DNA of approximately 5,000 base pairs (bp) long and comprises three major regions, early viral gene region (EVGR), late viral gene region (LVGR) and noncoding control region (NCCR). The EVGR codes for early T proteins, small t antigen (tAg) and large T antigen (TAg), and LVGR codes for viral capsid proteins VP1, VP2, VP3 and agnoprotein (Agno) (Figure 1A) (Blackard et al., 2020). Based on sequence variation of VP1 gene, BKV has been universally classified into four genotypes (genotype I- IV) (Vaezjalali et al., 2018). As reported, genotype I of BKV are the most frequent around the globe (80%), while genotype IV is mainly reported from countries of Europe and northeastern Asia (Chen et al., 2006) (Hu et al., 2018). In addition to genotyping based on BKV VP1 diversity, two other forms based on NCCR variations were universally accepted for clinical isolates, namely, archetype (ww) and rearranged (rr) variants such as Dunlop strain (Figure 1B) (Henriksen et al., 2015). The BKV archetype contains a 376 bp linear OPQRS block, in which O represents the start of replication and PQRS represents promoters and regulatory regions of EVGR and LVGR (Kotla et al., 2021) (Bethge et al., 2016). Whereas the rearranged variants of BKV occur due to deletion and duplication in the NCCR sequences during reactivation and persistent replication (Figure 1B).
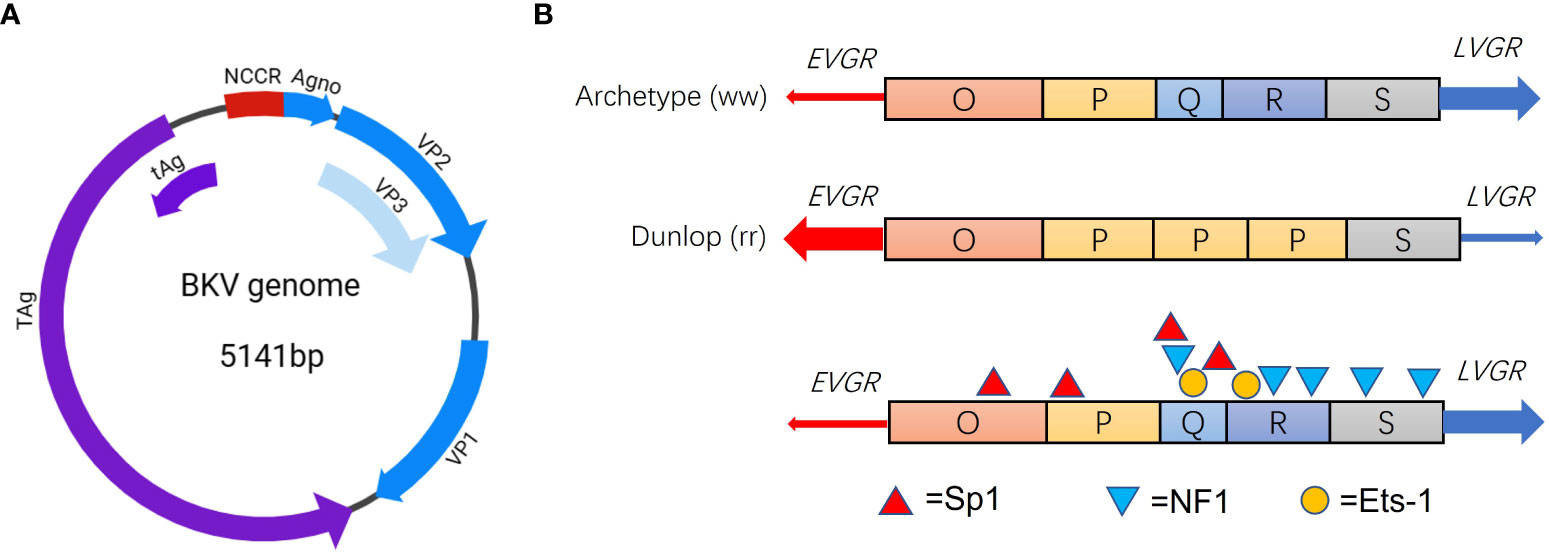
Figure 1 Genome structure of BKV (A) and NCCR blocks of the archetype and Dunlop strain (B). Major TFBS Sp1 (red triangle), NF1 (blue triangle) and Ets-1 (orange circle) in the NCCR blocks of BKV.
On the LVGR, BKV encodes one precursor miRNA complementary to the 3′ coding end of the TAg mRNA (Seo et al., 2008). Broekema and Imperiale found that miRNA plays a key role in limiting replication of archetype BKV by targeting viral early mRNA in an infection model using renal proximal tubule epithelial cells (RPTE), suggesting a self-limiting replication mechanism to remain life-time latency (Broekema and Imperiale, 2013). However, in rearranged NCCR (rr-NCCR) variants, as the miRNA expressed in a low level, early mRNA are expressed in high levels with enhanced early promoter activity (Broekema and Imperiale, 2013). In addition, innate and adaptive immune regulation on virus-host interaction also play an important role for BKV to sustain persistent latency in humans. For instance, the viral miRNA BKV-miR-B1-3p can target the stress-induced ligand ULBP3, a protein recognized by the receptor natural killer group 2, member D (NKG2D). Consequently, BKV-miR-B1-3p downregulated expression level of ULBP3 to evade NKG2D recognition, which leads to NKG2D-mediated elimination (Bauman et al., 2011; Zeng et al., 2019). This immune regulation mechanism of virus-host interaction has been extensively accepted. Recently, miRNA of BKV was clinically used to monitor viral reactivation in blood and urine of KTRs (Demey et al., 2021; Demey et al., 2022).
Besides, a recent study found BKV agnoprotein is able to impair innate immune signaling by disrupting the mitochondrial network and then enhances mitophagy. Specifically, BKV agnoprotein impairs IRF3 nuclear translocation and induces mitochondrial fragmentation. Then the disrupted mitochondria are targeted for SQSTM1/p62 (an autophagy receptor) mitophagy and impair innate immune signaling (Manzetti et al., 2020). Interestingly, a few studies showed the agnoprotein were able to co-localize with lipid‐droplets (LD) in vitro and the predicted α‐helical region ranging from amino acids 22 to 42 of BKV agnoprotein is vital for the localization (Unterstab et al., 2010). Besides, BKV agnoprotein has potentially been involved in disrupting exocytosis (Johannessen et al., 2011), inhibiting viral replication (Gerits et al., 2015) and facilitating the virus egress (Panou et al., 2018).
Previous studies indicated that small t antigen (tAg) of polyomaviruses involves in important pathways regulating viral replication, the innate immune signaling, and transformation for SV40, JCV and Merkel cell polyomavirus (MCV) (Chen et al., 2007; Saribas et al., 2019). However, the regulation mechanism underlying BKV tAg has been less focused. Zou and Imperiale found that BKV tAg downregulated viral DNA replication through similar mechanism of SV40 tAg replacing the B’ regulatory subunit of protein phosphatase 2A (PP2A) to form a complex to promote cell cycle progression (Zou and Imperiale, 2023). More studies elucidating molecular mechanisms of tAg in regulating viral replication are needed.
Recently, Zhao and Imperiale established a novel cell model mimicking viral latency and activation of BKV using a human RPTE cell line expressing human telomerase reverse transcriptase (RPTE-hTERT) (Zhao and Imperiale, 2021). They found that the archetype BKV can persist in vitro for ~100 days with random recombination checked by single-molecule high-throughput sequencing. Eventually, the accumulated recombination events could lead to rr-NCCR that allows higher efficiency of BKV DNA replication (Zhao and Imperiale, 2021). This study provides a useful in vitro model for future studies of viral persistence and reactivation.
Taken together, there are several potential molecular or immune mechanisms connected to BKV latency in association with NCCR, miRNA, Agno and tAg regulation as summarized in Figure 2.
Latent infection of BKV might last for life-time for most immunocompetent individuals. However, BVK predominantly reactivates and causes diseases in immunocompromised population, particularly the kidney transplant recipients (KTRs), hematopoietic stem cell transplantation (HSCT) recipients and HIV/AIDS patients (Nankivell et al., 2017; Pan et al., 2018; Hirsch and Randhawa, 2019; Raupp et al., 2019; Wunderink et al., 2019) (Laskin et al., 2020). Interestingly, over 80% of immunocompetent adults are seropositive for BKV as previously reported (Supplementary Table 1), but BKVAN occurs almost exclusively in KTRs, which raises concerns on the underlying pathogenesis mechanisms and attracts mounting concern as number of worldwide kidney transplants increases. BKVAN is clinically confirmed by tissue biopsy, and then clinical management through immunosuppression modulation is essential to balance exacerbation of the disease and acute rejection (Chantziantoniou et al., 2016). It was reported that about 60% of KTRs have detectable BK viruria, and up to 10% of the patients develop BKVAN (Babel et al., 2011; Chen et al., 2020). 15%-50% of the BKVAN patients will progress to graft loss within 2-3 years in the absence of proper intervention (Leeaphorn et al., 2020). Evidence indicated that the development of BKVAN was linked to immunosuppressive regimens for KTRs (Leeaphorn et al., 2020; Cheung and Tang, 2022). It has been found that patients taking more powerful immunosuppressive drugs, such as mycophenolate and tacrolimus, are more likely to develop BKVAN (Mehrvar et al., 2021). Therefore, frequent monitoring on viral load of BKV in the first year after transplantation, and timely adjustments or rational reduction of immunosuppression are highly recommended (Dalianis et al., 2019). However, reduction of immunosuppression using immunosuppressant drugs has a double-edged sword effect on clinical outcomes with increasing risk of acute rejection (Cheung and Tang, 2022).
Generally, high viral load of BKV (>104 copies/ml plasma or >107 copies/ml urine) are considered as indications of viral reactivation (Marinic et al., 2014; Ambalathingal et al., 2017). With reactivation, BKV potentially disrupt cell-cycle regulation and innate immune response, then significantly increase the level of viral replication, leading to cell necrosis and flaking. The exfoliated cells with viral inclusions in urine specimens are termed “decoy cells” as they are easily misdiagnosed as cancer cells (Chantziantoniou et al., 2016). Decoy cells (DC) that can be detected in the fresh urine sediment using microscopy are used to prompt the early signs of BKV activation (Poloni et al., 2016). Although DC can be identified on urine cytology, but it’s positive predictive value for BKVAN diagnosis is low (Chen et al., 2020) (Hirsch et al., 2002). Moreover, many of KTRs with positive urinary DC did not develop BKVAN (Huang et al., 2013). Therefore, optimizing diagnostic methods of accurate identification of BKV-infected DC is valuable for clinical diagnosis and treatment decisions.
It is widely recognized that both rr-NCCR of BKV and immunosuppression of the host jointly promote the development of BKVAN. There are many kinds of transcription factor binding sites (TFBS) in NCCR of archetype BKV, sequence variability of the TFBS may contribute to viral activation, replication, and pathogenesis (Blackard et al., 2020). In order to compare functional differences of rr-NCCR, Olsen et al. reconstructed Dunlop strain of BKV by replacing the NCCR from 12 BKV isolates of urine or renal biopsy specimen in Vero cells, and observed impressive difference of replication efficiency in RPTEs, indicating that sequence variability of NCCR has impact on replication efficiency of BKV (Olsen et al., 2009).
BKV strains exhibit higher-level genetic diversity in the NCCR than that in protein coding regions. Forms of rr-NCCR are commonly identified from persons with BKV-associated diseases (Cubitt, 2006). It is found that BKV isolates with rr-NCCR replicate much more efficiently than archetype BKV, indicating NCCR rearrangements regulate bidirectional gene expression levels (Gosert et al., 2008). An in vitro study finds that deletion or duplication in different blocks of NCCR may lead to elevated or decreased viral genome replication, indicating that rearrangements of NCCR contribute to regulate viral protein expression (Helle et al., 2017). The rr-NCCR caused by block deletion or duplication will alter the distribution and composition of TFBS, which in turn regulates the viral gene expression (Bethge et al., 2016) (Bethge et al., 2015). Bethge et al. identified Sp1 site in NCCR as a key regulator of gene expression of EVGR and LVGR (Bethge et al., 2015). And the in vitro experiments suggest that transcription factors Ets1, NF-1 and Sp1 (Figure 1B) determine the strength toward early or late gene expression (Bethge et al., 2015). This study provides new evidence on how different composition of TFBS regulate early and late gene expression of BKV and contribute to viral replication. Therefore, the potential anti-viral therapeutic strategies based on specific transcription factors will be promising for patients.
High seroprevalence of human polyomaviruses indicate its ubiquity in nature. The major human tissues harboring BKV are in urinary system such as the kidney and bladder (Krajewski et al., 2020). It is a complex network in pathogen-host interactions, which cause the diverse clinical outcomes of BKV infection in humans (Babel et al., 2011) (Nankivell et al., 2017). Anyway, BKV-associated diseases were mainly linked to host immune dysfunction in transplant recipients taking immunosuppressant drugs, patients undergoing cancer treatment and HIV/AIDS patients with immunodeficiency (Broekema et al., 2010; Mitterhofer et al., 2014). The most common clinical outcome due to BKV reactivation in transplant recipients are BKVAN, which can lead to graft loss in up to 60% of affected patients (Babel et al., 2011). Other than that, BKV was also considered as a potential factor or co-factor of tumorigenesis and diseases that were much less discussed in the scientific community. Among the known human oncogenic viruses, human papillomavirus (HPV) has similar genomic structure with BKV, offering an excellent reference model to understand the potential mechanisms of BKV-induced tumorigenesis (Papadimitriou et al., 2016). Similar with HPVs, BKV, as its key tumorigenesis mechanism, has an TAg-mediated disruption of the tumor suppressor genes p53 and pRb, which consequently leads to dysregulation of cell cycling and apoptosis (Trave and Zanier, 2016) (Storey et al., 1998; Narisawa-Saito and Kiyono, 2007).
BKV builds persistent latent infection in the genitourinary system of humans for life time (McCaffrey et al., 2021). As a member of human polyomaviruses, BKV shares similar genome structures with oncogenic virus MCV that causes a malignant cutaneous cancer Merkel cell carcinoma (MCC) (Helle et al., 2017). BKV was linked as a potential etiological agent of urologic diseases or tumors such as prostate and bladder cancer. As development of high throughput sequencing, studies with the deep sequencing technology have recently begun to understand the frequency and potential networks of BKV-associated tumorigenesis (Starrett and Buck, 2019). Two former studies of comprehensive molecular characterization of bladder cancers on the basis of deep sequencing technique observed that gene of BKV was integrated into the genome in 1 of 413 bladder tumors (Robertson et al., 2017) (Cancer Genome Atlas Research N, 2014). The findings suggest low incidence of BKV gene integration into bladder tumor genome in the immunocompetent individuals. However, recent observations have revealed that KTRs who develop BKV viremia or BKVAN have about 11-fold risk of bladder cancer incidence in comparison to KTRs without signs of BKV reactivation (Liu et al., 2017) (Gupta et al., 2018). These findings specifically implicate that BKV reactivation is the precondition of bladder cancerogenesis in immunosuppressed transplant recipients.
To our knowledge, prostate cancer is a popular urinary tumor in the elderly of developed countries and disrupted p53 networks is thought to be the major pathways for prostate cancer incidence (Mischitelli et al., 2015). TAg of BKV is responsible for viral transformation, evidence suggests that molecular mechanisms of BKV tumorigenesis is linked to TAg-mediated p53 inhibition (Harris et al., 1996). Recently, a case-control study conducted by Gorish and colleagues observed that BKV TAg was identified among 30% (n=55) tissue specimens of prostate cancer patients but only in 7% (n=55) of the controls’ specimens (P=0.002 and Odd ratio= 5.7), suggesting that BKV is a potently associated with higher risk of prostate cancer (Gorish et al., 2019).
Head and neck cancers are a heterogeneous group of tumors representing the 6th-7th most popular types of cancers around the world (Mody et al., 2021). Approximately 90% of head and neck cancers belong to squamous cell carcinomas (HNSCCs). And the activated virus infections are one of major causative agents (Polz et al., 2015) (Kitamura et al., 2023).
A recent study assessed the prevalence of BKV in Iranian patients with brain malignancies, and found TAg sequences of BKV were detected in 26 out of 58 (44.8%) brain tumor tissues, indicating the possible pathogenic interlink between BKV persistence and central nervous system (Saber Amoli et al., 2021). Another study investigated the correlation of BKV and the development of papillary thyroid carcinoma (PTC) in Iranian PTC patients. Among 1057 PTC samples including 645 paraffin-embedded and 412 fresh biopsy samples, 48.3% were positive for the BKV DNA with mean viral load of 0.5×104 copies/cell. Besides, TAg RNA expression was relatively higher in fresh biopsy samples (Tarharoudi et al., 2022). Polz and colleague analyzed the presence of BKV in paraffin-embedded sections of oral squamous cell carcinomas (OSCC), and they observed that BKV nucleic acid was detected in 18.5% (n=92) of OSCC patients but much lower detection rate (3.3%) from the controls (Polz et al., 2015). However, reports of BKV-associated head and neck cancer are still too limited to determine its etiological interlink with head and neck cancer. After all, BKV is ubiquitous and life-long latent in humans.
After primary infection, BKV mainly disseminates and predominantly colonizes into sites of kidney and urinary tracts (Imperiale, 2000). Interestingly, BKV DNA was also detected from saliva of HIV-infected individuals and HIV-negative controls, which raised concerns of its pathogenicity in people living with HIV (Jeffers and Webster-Cyriaque, 2011). HIV-associated salivary gland disease (HIVSGD) is one of the most common salivary gland-associated complications among HIV-infected population (Jeffers and Webster-Cyriaque, 2011; Burger-Calderon et al., 2016). Generally, HIVSGD presents xerostomia or/and diffused swelling. The incidence of HIVSGD could reach up to 48% in HIV-positive individuals in underdeveloped countries (McArthur et al., 2000). According to Patton’s data observed during 1995-2008, HIV/AIDS patients were much more likely to develop HIVSGD in the era of protease inhibitor therapy (Patton et al., 2000). Although HIVSGD is generally thought to be a benign lesion, some of them could progress to malignant lymphoma under certain conditions like HIV infection (Ellis, 2007). Notably, lymphomas account for a large portion of major salivary gland malignancies, in which salivary gland lymphomas occur in about 75–80% parotid gland, 5–20% submandibular gland, and less than 5% small sublingual salivary glands (Barnes et al., 1998). With accumulated evidence interlinking head and neck cancers with BKV, question about whether BKV may contribute as co-factorial role to tumorigenesis.
Since Jeffers et al. detected significantly higher BKV viral loads in the saliva of patients diagnosed with HIVSGD as compared to HIV negative patients, evidence linking BKV to HIVSGD has augmented (Jeffers et al., 2009). The BKV NCCR rearrangement derived from block duplications and/or deletions commonly occurs in immunocompromised patients with BKV reactivation. Burger-Calderon et al. found over 90% of the BKV NCCRs in HIVSGD carried a block arrangement form “OPQPQQS” in throatwash samples of the immunosuppressed individuals (Burger-Calderon et al., 2016). It has been reported that rearrangements of NCCR potently enhanced viral transformation and host-cell permissiveness (Gosert et al., 2008) (Burger-Calderon et al., 2016). Taken together, BKV may play an infectious role contributing to HIVSGD presentation. However, future studies will have to address the pathogenicity of BKV in vitro and in vivo specifically.
BKV is a ubiquitous agent causing latent infection in over 80% adults around the world. Most concern on this virus is BKVAN in KTRs, among whom up to 60% eventually progress to graft loss in the first 2-3 years in the absence of proper intervention. Thus, clinical recommendations on management of BKV infection include: a) frequently monitoring of viral load of BKV in blood is highly recommended the first 12 months after kidney transplantation; b) in order to the risk for BKVAN incidence, reducing the use of immunosuppressants is recommended (Dalianis et al., 2019). Generally, the BKV has a self-limiting regulating mechanism in association with miRNA that targets early mRNA to limit archetype BKV replication. Theoretically, BKV is latent in archetype among most immunocompetent individuals unless there is a sustained weakening of the immune system caused by HIV infection or kidney transplant. The activation of BKV is usually accompanied by block rearrangement of NCCR in which TFBS deletion or insertion will regulate EVGR and LVGR expression. Therefore, NCCR sequence could be an important indicator to evaluate the BKV activation. Although human RPTE cell are the major harboring sites for its life-long latency, BKV has tropism for normal human brain tissue, human salivary gland cells and pancreatic cells in vitro, which indicates BKV association with various tumors in non-genitourinary tissues (Elsner and Dorries, 1992; Jeffers et al., 2009).
Approximately 12% of human cancers are related to viruses such as Epstein-Barr virus (EBV), human papillomavirus (HPV), hepatitis B virus (HBV), hepatitis C virus (HCV), Kaposi’s sarcoma herpesvirus (KSHV) and MCV (White et al., 2014). The accumulated evidence in the past two decades suggests that BKV may cause urologic tumors and head and neck cancers as well, posing new challenges to the immunosuppressed individuals in the absence specific anti-viral drugs or vaccines. Altogether, figuring out the pathogenic mechanisms causing BKV-associated diseases and potential tumorigenesis is important, and the constant improvement of rapid clinical diagnosis for BKV activation is needed.
XZ: Conceptualization, Supervision, Writing – original draft, Writing – review and editing. CZ: Data curation, Investigation, Resources, Writing – review and editing. HL: Data curation, Investigation, Resources, Writing – review and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1263983/full#supplementary-material
BKVAN, BKV-associated nephropathy; HC, Hemorrhagic cystitis; PML, Progressive multifocal leukoencephalopathy; HIVSGD, HIV-associated salivary gland disease; KTRs, Kidney transplant recipients; HSCT, Hematopoietic stem cell transplantation; TFBS, Transcription factor binding sites; EVGR, Early viral gene region; LVGR, Late viral gene region.
Ambalathingal, G. R., Francis, R. S., Smyth, M. J., Smith, C., Khanna, R. (2017). BK polyomavirus: clinical aspects, immune regulation, and emerging therapies. Clin. Microbiol. Rev. 30 (2), 503–528. doi: 10.1128/CMR.00074-16
Babel, N., Volk, H. D., Reinke, P. (2011). BK polyomavirus infection and nephropathy: the virus-immune system interplay. Nat. Rev. Nephrol. 7 (7), 399–406. doi: 10.1038/nrneph.2011.59
Barnes, L., Myers, E. N., Prokopakis, E. P. (1998). Primary Malignant lymphoma of the parotid gland. Arch. Otolaryngol Head Neck Surg. 124 (5), 573–577. doi: 10.1001/archotol.124.5.573
Bauman, Y., Nachmani, D., Vitenshtein, A., Tsukerman, P., Drayman, N., Stern-Ginossar, N., et al. (2011). An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe 9 (2), 93–102. doi: 10.1016/j.chom.2011.01.008
Bethge, T., Ajuh, E., Hirsch, H. H. (2016). Imperfect symmetry of sp1 and core promoter sequences regulates early and late virus gene expression of the bidirectional BK polyomavirus noncoding control region. J. Virol. 90 (22), 10083–10101. doi: 10.1128/JVI.01008-16
Bethge, T., Hachemi, H. A., Manzetti, J., Gosert, R., Schaffner, W., Hirsch, H. H. (2015). Sp1 sites in the noncoding control region of BK polyomavirus are key regulators of bidirectional viral early and late gene expression. J. Virol. 89 (6), 3396–3411. doi: 10.1128/JVI.03625-14
Blackard, J. T., Davies, S. M., Laskin, B. L. (2020). BK polyomavirus diversity-Why viral variation matters. Rev. Med. Virol. 30 (4), e2102. doi: 10.1002/rmv.2102
Broekema, N. M., Abend, J. R., Bennett, S. M., Butel, J. S., Vanchiere, J. A., Imperiale, M. J. (2010). A system for the analysis of BKV non-coding control regions: application to clinical isolates from an HIV/AIDS patient. Virology 407 (2), 368–373. doi: 10.1016/j.virol.2010.08.032
Broekema, N. M., Imperiale, M. J. (2013). miRNA regulation of BK polyomavirus replication during early infection. Proc. Natl. Acad. Sci. U.S.A. 110 (20), 8200–8205. doi: 10.1073/pnas.1301907110
Burger-Calderon, R., Ramsey, K. J., Dolittle-Hall, J. M., Seaman, W. T., Jeffers-Francis, L. K., Tesfu, D., et al. (2016). Distinct BK polyomavirus non-coding control region (NCCR) variants in oral fluids of HIV- associated Salivary Gland Disease patients. Virology 493, 255–266. doi: 10.1016/j.virol.2016.03.020
Burger-Calderon, R., Webster-Cyriaque, J. (2015). Human BK polyomavirus-the potential for head and neck malignancy and disease. Cancers (Basel) 7 (3), 1244–1270. doi: 10.3390/cancers7030835
Cancer Genome Atlas Research N (2014). Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507 (7492), 315–322. doi: 10.1038/nature12965
Chantziantoniou, N., Joudeh, A. A., Hamed, R. M. A., Al-Abbadi, M. A. (2016). Significance, cytomorphology of decoy cells in polyomavirus-associated nephropathy: Review of clinical, histopathological, and virological correlates with commentary. J. Am. Soc. Cytopathol 5 (2), 71–85. doi: 10.1016/j.jasc.2015.11.004
Chen, X. T., Chen, W. F., Hou, X. T., Yang, S. C., Yang, H. F., Li, J., et al. (2020). Non-invasive urinary sediment double-immunostaining predicts BK polyomavirus associated-nephropathy in kidney transplant recipients. Ann. Transl. Med. 8 (5), 235. doi: 10.21037/atm.2020.01.15
Chen, Y., Xu, Y., Bao, Q., Xing, Y., Li, Z., Lin, Z., et al. (2007). Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 14 (6), 527–534. doi: 10.1038/nsmb1254
Chen, Q., Zheng, H. Y., Zhong, S., Ikegaya, H., He, H. X., Wei, W., et al. (2006). : Subtype IV of the BK polyomavirus is prevalent in East Asia. Arch. Virol. 151 (12), 2419–2429. doi: 10.1007/s00705-006-0814-z
Cheung, C. Y., Tang, S. C. W. (2022). Personalized immunosuppression after kidney transplantation. Nephrol. (Carlton) 27 (6), 475–483. doi: 10.1111/nep.14035
Cubitt, C. L. (2006). Molecular genetics of the BK virus. Adv. Exp. Med. Biol. 577, 85–95. doi: 10.1007/0-387-32957-9_6
Dalianis, T., Eriksson, B. M., Felldin, M., Friman, V., Hammarin, A. L., Herthelius, M., et al. (2019). Management of BK-virus infection - Swedish recommendations. Infect. Dis. (Lond) 51 (7), 479–484. doi: 10.1080/23744235.2019.1595130
Demey, B., Bentz, M., Descamps, V., Morel, V., Francois, C., Castelain, S., et al. (2022). BK Polyomavirus bkv-miR-B1-5p: A Stable Micro-RNA to Monitor Active Viral Replication after Kidney Transplantation. Int. J. Mol. Sci. 23 (13):7240. doi: 10.3390/ijms23137240
Demey, B., Descamps, V., Presne, C., Helle, F., Francois, C., Duverlie, G., et al. (2021). BK polyomavirus micro-RNAs: time course and clinical relevance in kidney transplant recipients. Viruses 13 (2):352. doi: 10.3390/v13020351
Eash, S., Querbes, W., Atwood, W. J. (2004). Infection of vero cells by BK virus is dependent on caveolae. J. Virol. 78 (21), 11583–11590. doi: 10.1128/JVI.78.21.11583-11590.2004
Ellis, G. L. (2007). Lymphoid lesions of salivary glands: Malignant and benign. Med. Oral. Patol Oral. Cir Bucal 12 (7), E479–E485.
Elsner, C., Dorries, K. (1992). Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology 191, 72–80. doi: 10.1016/0042-6822(92)90167-N
Fukumoto, H., Li, T. C., Kataoka, M., Hasegawa, H., Wakita, T., Saeki, H., et al. (2015). Seroprevalence of trichodysplasia spinulosa-associated polyomavirus in Japan. J. Clin. Virol. 65, 76–82. doi: 10.1016/j.jcv.2015.02.014
Furmaga, J., Kowalczyk, M., Zapolski, T., Furmaga, O., Krakowski, L., Rudzki, G., et al. (2021). BK polyomavirus-biology, genomic variation and diagnosis. Viruses 13 (8):1502. doi: 10.3390/v13081502
Gaboriaud, P., Ferte, M., Arnold, F., Leblond, V., Nicol, J., Debare, H., et al. (2018). Age-specific seroprevalence of human polyomavirus 12 and Saint Louis and New Jersey polyomaviruses. Emerg. Microbes Infect. 7 (1), 22. doi: 10.1038/s41426-018-0026-0
Gardner, S. D., Field, A. M., Coleman, D. V., Hulme, B. (1971). New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1 (7712), 1253–1257. doi: 10.1016/s0140-6736(71)91776-4
Gerits, N., Johannessen, M., Tummler, C., Walquist, M., Kostenko, S., Snapkov, I., et al. (2015). Agnoprotein of polyomavirus BK interacts with proliferating cell nuclear antigen and inhibits DNA replication. Virol. J. 12, 7. doi: 10.1186/s12985-014-0220-1
Gorish, B. M. T., Ournasseir, M. E. H., Shammat, I. M. (2019). A correlation study of BK Polyoma Virus infection and prostate Cancer among Sudanese patients - immunofluorescence and molecular based case-control study. Infect. Agent Cancer 14, 25. doi: 10.1186/s13027-019-0244-7
Gorrill, T., Feliciano, M., Mukerjee, R., Sawaya, B. E., Khalili, K., White, M. K. (2006). Activation of early gene transcription in polyomavirus BK by human immunodeficiency virus type 1 Tat. J. Gen. Virol. 87 (6), 1557–1566. doi: 10.1099/vir.0.81569-0
Gosert, R., Rinaldo, C. H., Funk, G. A., Egli, A., Ramos, E., Drachenberg, C. B., et al. (2008). Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J. Exp. Med. 205 (4), 841–852. doi: 10.1084/jem.20072097
Gupta, G., Kuppachi, S., Kalil, R. S., Buck, C. B., Lynch, C. F., Engels, E. A. (2018). Treatment for presumed BK polyomavirus nephropathy and risk of urinary tract cancers among kidney transplant recipients in the United States. Am. J. Transplant. 18 (1), 245–252. doi: 10.1111/ajt.14530
Harris, K. F., Christensen, J. B., Imperiale, M. J. (1996). BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J. Virol. 70 (4), 2378–2386. doi: 10.1128/jvi.70.4.2378-2386.1996
Helle, F., Brochot, E., Handala, L., Martin, E., Castelain, S., Francois, C., et al. (2017). Biology of the BKPyV: an update. Viruses 9 (11):327. doi: 10.3390/v9110327
Henriksen, S., Mittelholzer, C., Gosert, R., Hirsch, H. H., Rinaldo, C. H. (2015). Human BK polyomavirus plasmid pBKV (34-2) (Dunlop) contains mutations not found in the originally published sequences. Genome Announc 3 (2):e00046-15. doi: 10.1128/genomeA.00046-15
Hirsch, H. H., Knowles, W., Dickenmann, M., Passweg, J., Klimkait, T., Mihatsch, M. J., et al. (2002). Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl. J. Med. 347 (7), 488–496. doi: 10.1056/NEJMoa020439
Hirsch, H. H., Randhawa, P. S. (2019). Practice ASTIDCo: BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 33 (9), e13528. doi: 10.1111/ctr.13528
Hu, C., Huang, Y., Su, J., Wang, M., Zhou, Q., Zhu, B. (2018). The prevalence and isolated subtypes of BK polyomavirus reactivation among patients infected with human immunodeficiency virus-1 in southeastern China. Arch. Virol. 163 (6), 1463–1468. doi: 10.1007/s00705-018-3724-y
Huang, G., Chen, W. F., Wang, C. X., Fei, J. G., Deng, S. X., Qiu, J., et al. (2013). Noninvasive tool for the diagnosis of polyomavirus BK-associated nephropathy in renal transplant recipients. Diagn. Microbiol. Infect. Dis. 75 (3), 292–297. doi: 10.1016/j.diagmicrobio.2012.11.012
Imperiale, M. J. (2000). The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology 267 (1), 1–7. doi: 10.1006/viro.1999.0092
Jeffers, L. K., Madden, V., Webster-Cyriaque, J. (2009). BK virus has tropism for human salivary gland cells in vitro: implications for transmission. Virology 394 (2), 183–193. doi: 10.1016/j.virol.2009.07.022
Jeffers, L., Webster-Cyriaque, J. Y. (2011). Viruses and salivary gland disease (SGD): lessons from HIV SGD. Adv. Dent. Res. 23 (1), 79–83. doi: 10.1177/0022034510396882
Johannessen, M., Walquist, M., Gerits, N., Dragset, M., Spang, A., Moens, U. (2011). BKV agnoprotein interacts with alpha-soluble N-ethylmaleimide-sensitive fusion attachment protein, and negatively influences transport of VSVG-EGFP. PloS One 6 (9), e24489. doi: 10.1371/journal.pone.0024489
Kamminga, S., van der Meijden, E., Wunderink, H. F., Touze, A., Zaaijer, H. L., Feltkamp, M. C. W. (2018). Development and evaluation of a broad bead-based multiplex immunoassay to measure igG seroreactivity against human polyomaviruses. J. Clin. Microbiol. 56 (4):e01566-17. doi: 10.1128/JCM.01566-17
Kean, J. M., Rao, S., Wang, M., Garcea, R. L. (2009). Seroepidemiology of human polyomaviruses. PloS Pathog. 5 (3), e1000363. doi: 10.1371/journal.ppat.1000363
Kitamura, N., Hashida, Y., Higuchi, T., Ohno, S., Sento, S., Sasabe, E., et al. (2023). Detection of Merkel cell polyomavirus in multiple primary oral squamous cell carcinomas. Odontology. doi: 10.1007/s10266-023-00807-y
Kotla, S. K., Kadambi, P. V., Hendricks, A. R., Rojas, R. (2021). BK polyomavirus-pathogen, paradigm and puzzle. Nephrol. Dial Transplant. 36 (4), 587–593. doi: 10.1093/ndt/gfz273
Krajewski, W., Kaminska, D., Poterek, A., Malkiewicz, B., Klak, J., Zdrojowy, R., et al. (2020). Pathogenicity of BK virus on the urinary system. Cent Eur. J. Urol 73 (1), 94–103. doi: 10.5173/ceju.2020.0034
Laine, H. K., Waterboer, T., Syrjanen, K., Grenman, S., Louvanto, K., Syrjanen, S. (2023). Seroprevalence of polyomaviruses BK and JC in Finnish women and their spouses followed-up for three years. Sci. Rep. 13 (1), 879. doi: 10.1038/s41598-023-27850-7
Laskin, B. L., Denburg, M. R., Furth, S. L., Moatz, T., Altrich, M., Kleiboeker, S., et al. (2020). The natural history of BK polyomavirus and the host immune response after stem cell transplantation. Clin. Infect. Dis. 71 (12), 3044–3054. doi: 10.1093/cid/ciz1194
Laskin, B. L., Sullivan, K. E., Hester, J., Goebel, J., Davies, S. M., Jodele, S. (2015). Antibodies to BK virus in children prior to allogeneic hematopoietic cell transplant. Pediatr. Blood Cancer 62 (9), 1670–1673. doi: 10.1002/pbc.25536
Leeaphorn, N., Thongprayoon, C., Chon, W. J., Cummings, L. S., Mao, M. A., Cheungpasitporn, W. (2020). Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Am. J. Transplant. 20 (5), 1334–1340. doi: 10.1111/ajt.15723
Liu, S., Chaudhry, M. R., Berrebi, A. A., Papadimitriou, J. C., Drachenberg, C. B., Haririan, A., et al. (2017). Polyomavirus replication and smoking are independent risk factors for bladder cancer after renal transplantation. Transplantation 101 (6), 1488–1494. doi: 10.1097/TP.0000000000001260
Manzetti, J., Weissbach, F. H., Graf, F. E., Unterstab, G., Wernli, M., Hopfer, H., et al. (2020). BK polyomavirus evades innate immune sensing by disrupting the mitochondrial network and promotes mitophagy. iScience 23 (7), 101257. doi: 10.1016/j.isci.2020.101257
Marinic, K., Sinchi, J., Gomez, M., Diaz, R., Grillo, S., Habegger-de Sorrentino, A. (2014). Monitoring of BK virus in transplant patients of the renal unit of the Perrando Hospital, Chaco, Argentina. Nefrologia 34 (6), 799–800. doi: 10.3265/Nefrologia.pre2014.Jul.12657
McArthur, C. P., Subtil-DeOliveira, A., Palmer, D., Fiorella, R. M., Gustafson, S., Tira, D., et al. (2000). Characteristics of salivary diffuse infiltrative lymphocytosis syndrome in West Africa. Arch. Pathol. Lab. Med. 124 (12), 1773–1779. doi: 10.5858/2000-124-1773-COSDIL
McCaffrey, J., Bhute, V. J., Shenoy, M. (2021). BK virus infection and outcome following kidney transplantation in childhood. Sci. Rep. 11 (1), 2468. doi: 10.1038/s41598-021-82160-0
Mehrvar, A., Naderi, A., Mehrvar, N., Nourian, M. (2021). Successful treatment with intravenous and intravascular cidofovir for BK virus-associated hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation: A case report. Case Rep. Oncol. 14 (2), 892–895. doi: 10.1159/000516269
Mischitelli, M., Bellizzi, A., Anzivino, E., Rodio, D. M., Sciarra, A., Gentile, V., et al. (2015). Results, questions, perspectives of a study on human Polyomavirus BK and molecular actors in prostate cancer development. Cancer Genomics Proteomics 12 (2), 57–65.
Mitterhofer, A. P., Tinti, F., Pietropaolo, V., Umbro, I., Anzivino, E., Bellizzi, A., et al. (2014). Role of BK virus infection in end-stage renal disease patients waiting for kidney transplantation–viral replication dynamics from pre- to post-transplant. Clin. Transplant. 28 (3), 299–306. doi: 10.1111/ctr.12312
Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I., Saba, N. F. (2021). Head and neck cancer. Lancet 398 (10318), 2289–2299. doi: 10.1016/S0140-6736(21)01550-6
Nankivell, B. J., Renthawa, J., Sharma, R. N., Kable, K., O’Connell, P. J., Chapman, J. R. (2017). BK virus nephropathy: histological evolution by sequential pathology. Am. J. Transplant 17, 2065–2077. doi: 10.1111/ajt.14292
Narisawa-Saito, M., Kiyono, T. (2007). Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 98 (10), 1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x
Nicol, J. T., Robinot, R., Carpentier, A., Carandina, G., Mazzoni, E., Tognon, M., et al. (2013). Age-specific seroprevalences of merkel cell polyomavirus, human polyomaviruses 6, 7, and 9, and trichodysplasia spinulosa-associated polyomavirus. Clin. Vaccine Immunol. 20 (3), 363–368. doi: 10.1128/CVI.00438-12
Olsen, G. H., Hirsch, H. H., Rinaldo, C. H. (2009). Functional analysis of polyomavirus BK non-coding control region quasispecies from kidney transplant recipients. J. Med. Virol. 81 (11), 1959–1967. doi: 10.1002/jmv.21605
Padgett, B. L., Walker, D. L., ZuRhein, G. M., Eckroade, R. J., Dessel, B. H. (1971). Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1 (7712), 1257–1260. doi: 10.1016/s0140-6736(71)91777-6
Pan, L., Lyu, Z., Adam, B., Zeng, G., Wang, Z., Huang, Y., et al. (2018). Polyomavirus BK nephropathy-associated transcriptomic signatures: A critical reevaluation. Transplant. Direct 4 (2), e339. doi: 10.1097/TXD.0000000000000752
Panou, M. M., Prescott, E. L., Hurdiss, D. L., Swinscoe, G., Hollinshead, M., Caller, L. G., et al. (2018). Agnoprotein is an essential egress factor during BK polyomavirus infection. Int. J. Mol. Sci. 19 (3):902. doi: 10.3390/ijms19030902
Papadimitriou, J. C., Randhawa, P., Rinaldo, C. H., Drachenberg, C. B., Alexiev, B., Hirsch, H. H. (2016). BK polyomavirus infection and renourinary tumorigenesis. Am. J. Transplant. 16 (2), 398–406. doi: 10.1111/ajt.13550
Patton, L. L., McKaig, R., Strauss, R., Rogers, D., Eron, J. J., Jr. (2000). Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 89 (3), 299–304. doi: 10.1016/S1079-2104(00)70092-8
Poloni, J. A., Pinto, G. G., Giordani, M. S., Keitel, E., Inocente, N., Voegeli, C. F., et al. (2016). Bright field microscopy to detect decoy cells due to BK virus infection in the fresh and unstained urine sediment in kidney allograft recipients. J. Clin. Lab. Anal. 30 (6), 1044–1050. doi: 10.1002/jcla.21978
Polz, D., Morshed, K., Stec, A., Podsiadlo, L., Polz-Dacewicz, M. (2015). Do polyomavirus hominis strains BK and JC play a role in oral squamous cell carcinoma? Ann. Agric. Environ. Med. 22 (1), 106–109. doi: 10.5604/12321966.1141378
Prelog, M., Egli, A., Zlamy, M., Hirsch, H. H. (2013). JC and BK polyomavirus-specific immunoglobulin G responses in patients thymectomized in early childhood. J. Clin. Virol. 58 (3), 553–558. doi: 10.1016/j.jcv.2013.08.035
Prezioso, C., Van Ghelue, M., Pietropaolo, V., Moens, U. (2021). Detection of Quebec polyomavirus DNA in samples from different patient groups. Microorganisms 9 (5):1082. doi: 10.3390/microorganisms9051082
Raupp, F. V. V., Meinerz, G., da Silva, C. K., Bianco, P. C. D., Goldani, J. C., Pegas, K. L., et al. (2019). BK Polyomavirus-associated nephropathy managed by screening policy in a real-life setting. Transpl Infect. Dis. 22(1):e13213. doi: 10.1111/tid.13213
Robertson, A. G., Kim, J., Al-Ahmadie, H., Bellmunt, J., Guo, G., Cherniack, A. D., et al. (2017). Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171 (3), 540–556 e525. doi: 10.1016/j.cell.2017.09.007
Saber Amoli, S., Zebardast, A., Keyvani, H., Yahyapour, Y., Ghodsi, S. M., Maniati, M., et al. (2021). Prevalence and viral load determination of BK polyomavirus among Iranian patients with brain tumors. Caspian J. Intern. Med. 12 (2), 173–179. doi: 10.22088/cjim.12.2.173
Saribas, A. S., Coric, P., Bouaziz, S., Safak, M. (2019). Expression of novel proteins by polyomaviruses and recent advances in the structural and functional features of agnoprotein of JC virus, BK virus, and simian virus 40. J. Cell Physiol. 234 (6), 8295–8315. doi: 10.1002/jcp.27715
Seo, G. J., Fink, L. H., O’Hara, B., Atwood, W. J., Sullivan, C. S. (2008). Evolutionarily conserved function of a viral microRNA. J. Virol. 82 (20), 9823–9828. doi: 10.1128/JVI.01144-08
Sroller, V., Hamsikova, E., Ludvikova, V., Vochozkova, P., Kojzarova, M., Fraiberk, M., et al. (2014). Seroprevalence rates of BKV, JCV, and MCPyV polyomaviruses in the general Czech Republic population. J. Med. Virol. 86 (9), 1560–1568. doi: 10.1002/jmv.23841
Starrett, G. J., Buck, C. B. (2019). The case for BK polyomavirus as a cause of bladder cancer. Curr. Opin. Virol. 39, 8–15. doi: 10.1016/j.coviro.2019.06.009
Stolt, A., Sasnauskas, K., Koskela, P., Lehtinen, M., Dillner, J. (2003). Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 84 (Pt 6), 1499–1504. doi: 10.1099/vir.0.18842-0
Storey, A., Thomas, M., Kalita, A., Harwood, C., Gardiol, D., Mantovani, F., et al. (1998). Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 393 (6682), 229–234. doi: 10.1038/30400
Tarharoudi, R., Sari, S., Sakhaee, F., Vaziri, F., Rahimi Jamnani, F., Siadat, S. D., et al. (2022). BK polyomavirus in Iranian patients with papillary thyroid cancer: Is it a major future challenge? J. Med. Virol. 94 (12), 6023–6027. doi: 10.1002/jmv.28047
Trave, G., Zanier, K. (2016). HPV-mediated inactivation of tumor suppressor p53. Cell Cycle 15 (17), 2231–2232. doi: 10.1080/15384101.2016.1191257
Unterstab, G., Gosert, R., Leuenberger, D., Lorentz, P., Rinaldo, C. H., Hirsch, H. H. (2010). The polyomavirus BK agnoprotein co-localizes with lipid droplets. Virology 399 (2), 322–331. doi: 10.1016/j.virol.2010.01.011
Vaezjalali, M., Azimi, H., Hosseini, S. M., Taghavi, A., Goudarzi, H. (2018). Different strains of BK polyomavirus: VP1 sequences in a group of Iranian prostate cancer patients. Urol J. 15 (2), 44–48. doi: 10.22037/uj.v0i0.3833
Villani, S., Gagliano, N., Procacci, P., Sartori, P., Comar, M., Provenzano, M., et al. (2019). Characterization of an in vitro model to study the possible role of polyomavirus BK in prostate cancer. J. Cell Physiol. 234 (7), 11912–11922. doi: 10.1002/jcp.27871
Viscidi, R. P., Rollison, D. E., Sondak, V. K., Silver, B., Messina, J. L., Giuliano, A. R., et al. (2011). Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus. Clin. Vaccine Immunol. 18 (10), 1737–1743. doi: 10.1128/CVI.05175-11
White, M. K., Pagano, J. S., Khalili, K. (2014). Viruses and human cancers: a long road of discovery of molecular paradigms. Clin. Microbiol. Rev. 27 (3), 463–481. doi: 10.1128/CMR.00124-13
Wunderink, H. F., De Brouwer, C. S., Gard, L., De Fijter, J. W., Kroes, A. C. M., Rotmans, J. I., et al. (2019). Source and relevance of the BK polyomavirus genotype for infection after kidney transplantation. Open Forum Infect. Dis. 6 (3), ofz078. doi: 10.1093/ofid/ofz078
Zeng, G., Wang, Z., Huang, Y., Abedin, Z., Liu, Y., Randhawa, P. (2019). Cellular and viral miRNA expression in polyomavirus BK infection. Transpl Infect. Dis. 21 (5), e13159. doi: 10.1111/tid.13159
Zhang, C., Liu, F., He, Z., Deng, Q., Pan, Y., Liu, Y., et al. (2014). Seroprevalence of Merkel cell polyomavirus in the general rural population of Anyang, China. PloS One 9 (9), e106430. doi: 10.1371/journal.pone.0106430
Zhao, L., Imperiale, M. J. (2021). A cell culture model of BK polyomavirus persistence, genome recombination, and reactivation. mBio 12 (5), e0235621. doi: 10.1128/mBio.02356-21
Zhou, X., Bai, H., Kataoka, M., Ito, M., Muramatsu, M., Suzuki, T., et al. (2019). Characterization of the self-assembly of New Jersey polyomavirus VP1 into virus-like particles and the virus seroprevalence in Japan. Sci. Rep. 9 (1), 13085. doi: 10.1038/s41598-019-49541-y
Zhou, X., Nakashima, K., Ito, M., Zhang, X., Sakai, S., Feng, C., et al. (2020). Prevalence and viral loads of polyomaviruses BKPyV, JCPyV, MCPyV, TSPyV and NJPyV and hepatitis viruses HBV, HCV and HEV in HIV-infected patients in China. Sci. Rep. 10 (1), 17066. doi: 10.1038/s41598-020-74244-0
Keywords: BK polyomavirus, latency, BKV-associated nephropathy (BKVAN), reactivation, tumorigenesis
Citation: Zhou X, Zhu C and Li H (2023) BK polyomavirus: latency, reactivation, diseases and tumorigenesis. Front. Cell. Infect. Microbiol. 13:1263983. doi: 10.3389/fcimb.2023.1263983
Received: 20 July 2023; Accepted: 29 August 2023;
Published: 13 September 2023.
Edited by:
Yongfen Xu, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Mingzhe Guo, University of Nevada, Reno, United StatesCopyright © 2023 Zhou, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianfeng Zhou, bmNjZGN6eGZAMTI2LmNvbQ==; Hui Li, bmNjZGN5amJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.