
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell. Infect. Microbiol., 24 October 2023
Sec. Virus and Host
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1256822
This comprehensive review examines the interplay between environmental virology, public health, and sanitation in the unique context of Kenya. The review sheds light on the specific viral threats faced by the country, including waterborne viruses, zoonotic infections, and emerging viral diseases, and their implications for public health. It explores the prevailing public health challenges in Kenya associated with environmental viromics, such as infectious viral diseases, and the rising burden of other infectious particles. The role of sanitation in mitigating viral infections is highlighted, emphasising the importance of clean water supply, proper waste management, and hygienic practises. The review also presents strategies for strengthening environmental virology research in Kenya, including enhancing laboratory capacities and leveraging technological advancements. Furthermore, the policy implications and recommendations derived from the review emphasise the need for multi-sectoral collaboration, evidence-based decision-making, and long-term investments in infrastructure and behaviour change interventions. Implementing these strategies can enhance the understanding of environmental virology, improve public health outcomes, and ensure sustainable sanitation practises in Kenya, ultimately contributing to the well-being of the population and sustainable development.
The intricate interplay among environmental virology, public health, and sanitation assumes a pivotal role in mitigating the formidable challenges encountered by nations globally. In the Kenyan context, a nation grappling with substantial and diverse environmental and public health complexities, elucidating, and enhancing this interplay becomes of paramount significance. The overarching objective of this comprehensive review is to scrutinize the intricate dynamics between environmental virology, public health, and sanitation within Kenya, with a focal point on discerning the specific challenges and prospects within this domain. Kenya, akin to numerous African nations, confronts a spectrum of environmental and public health dilemmas, prominently inclusive of waterborne diseases (Bitanihirwe et al., 2021). The intricacy of the interrelationship is illustrated by Figure 1.
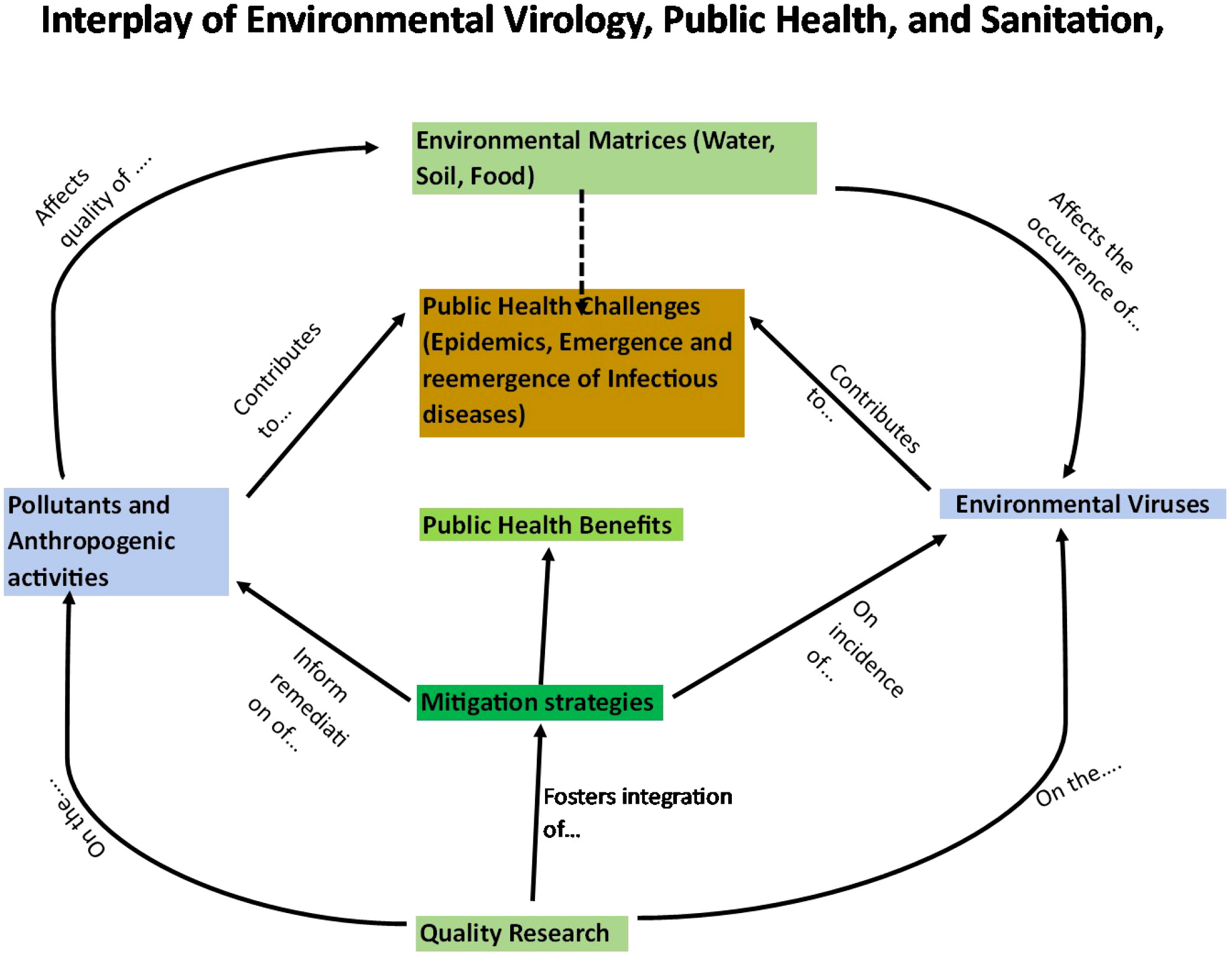
Figure 1 Schematic illustration on the connections among environmental virology, public health and sanitation.
For example, rotavirus infections stand as a substantial contributor to diarrheal incidence in Kenya, particularly affecting the pediatric demographic (Muendo et al., 2018). Furthermore, Kenya has grappled with outbreaks of other viral diseases such as Rift Valley Fever (Ahmed et al., 2021) and COVID-19, which have exerted profound impacts on public health and the general welfare of the populace. Environmental virology, encompassing the examination of viral pathogens in the environment and their repercussions on human health, serves as the bedrock of this intricate nexus. By investigating the transmission, persistence, and impact of viral infections in the Kenyan context, we endeavor to glean insights into the distinctive challenges and opportunities arising from the nation’s diverse ecosystems, climatic patterns, and demographic dynamics.
Presently, environmental virology research in Kenya predominantly centers on the identification and characterization of viral pathogens within various environmental matrices, including water sources and wastewater. These studies employ molecular techniques such as polymerase chain reaction (PCR) to discern and quantify viral DNA or RNA. Nevertheless, a need persists for more comprehensive inquiries that delve into the enduring viability of viral pathogens under diverse environmental conditions and their potential modes of transmission to humans (van Zyl et al., 2019).
In tandem with environmental factors, socio-economic determinants wield considerable influence over public health outcomes in Kenya. Restricted access to healthcare, education, and resources compounds the burden of diseases, impairing the efficacy of prevention and treatment strategies. Additionally, cultural practices and beliefs can impact health-seeking behaviors and contribute to viral infection transmission. For instance, in specific regions, improper fecal disposal practices such as open defecation may heighten the risk of diseases such as adenoviruses. Tackling these socio-economic factors constitutes a pivotal step toward ameliorating public health in Kenya (Wasonga et al., 2020).
Research demonstrates that access to clean water, effective waste management, and hygienic practices constitute indispensable elements in curtailing the transmission of viral infections. For instance, studies underscore that the enhancement of sanitation infrastructure correlates with a significant reduction in waterborne disease incidence. Furthermore, the promotion of behavior change, exemplified by thorough handwashing with soap, has demonstrated efficacy in mitigating the spread of viral infections. These findings underscore the imperativeness of investing in sanitation measures to safeguard public health and advance sustainable development in Kenya (van Seventer and Hochberg 2017).
In recent years, Kenya has made substantial advancements in fortifying sanitation infrastructure and fostering behavior change. County governments and non-governmental organizations, including UNICEF, have invested in the construction of clean water sources and sanitation facilities in underserved regions, guaranteeing access to potable water and proper waste management (UNICEF, 2023). Moreover, community-based initiatives have been deployed to raise awareness regarding hygiene practices and facilitate behavioral transformations at the individual and household levels. These endeavors have yielded promising outcomes, substantially diminishing the incidence of waterborne diseases and enhancing public health.
This comprehensive review commences by surveying the current landscape of environmental virology research within Kenya, inclusive of viral pathogen identification in the environment and their implications for human health. Subsequently, it delves into the gamut of public health challenges confronting the nation, with a particular emphasis on waterborne diseases and emerging viral outbreaks. The review further expounds on the contemporary quandaries pertaining to environmental and public health virology within Kenya. Ultimately, it culminates by encapsulating the salient challenges and prospects inherent in the interplay among environmental virology, public health, and sanitation within the Kenyan context.
A thorough literature review was conducted to gather relevant scholarly articles, research papers, reports, and case studies related to environmental virology, public health, and sanitation in the Kenyan context. Databases such as PubMed, Google Scholar, and relevant institutional websites were searched using keywords related to the topic. The gathered literature was carefully screened to select research articles and sources that provided significant insights into the interplay of environmental virology, public health, and sanitation in Kenya. The selected data were analysed and synthesised to identify key themes, trends, challenges, and opportunities. The review was organised into sections to provide a logical flow of information. These sections included the introduction, environmental virology in Kenya, public health challenges in Kenya, the role of sanitation in mitigating viral infections, strategies for strengthening environmental virology research, policy implications, and recommendations. Throughout the review, specific attention was given to incorporating the Kenyan perspective. This involved considering the unique environmental, socio-economic, and cultural factors relevant to Kenya’s public health and sanitation landscape. Examples, case studies, and data specific to Kenya were utilised to contextualise the discussions. The review draws upon the expertise and knowledge of researchers familiar with environmental virology, public health, and sanitation. The information provided is based on the existing body of knowledge and research up until the knowledge cutoff date in June 2023.
This section briefly describes the key groups of viruses that are specific to, or important to, aquatic environments with public health ramifications. The list is not exhaustive, and the discussed groups are not endemic to Kenyan settings but are examples drawn from general publications to illustrate the general contexts. The viruses include Enteroviruses, a genus of positive sense ssRNA viruses that belong to the order Picornavirales (Quaranta et al., 2020) They can cause diseases such as poliomyelitis, meningitis, hepatitis, hand-foot-and-mouth disease, and respiratory infections (Aswathyraj et al., 2016). Enteroviruses can be transmitted through the faecal-oral route, respiratory droplets, or contact with contaminated water (Wells and Coyne, 2019). Hepatoviruses: a genus of positive sense ssRNA viruses that belong to the order Picornavirales (Le Gall et al., 2008). They can cause hepatitis A, an acute liver infection that can be spread through the ingestion of contaminated food or water (Jacobsen, 2018). Rotaviruses: a genus of dsRNA viruses that belong to the family Reoviridae. They can cause severe gastroenteritis, especially in children, and can be transmitted through the faecal-oral route or contact with contaminated water (Le Gall et al., 2008).
Adenoviruses: Adenoviruses are a group of viruses that can infect different organs and tissues in humans and animals. Adenoviruses have a double-stranded DNA genome and a non-enveloped icosahedral capsid. [Adenoviruses are one of the groups of enteric viruses, meaning they can be transmitted through the faecal-oral route, but they can also be spread by respiratory droplets or contact with infected surfaces Adenoviruses can cause various diseases, such as respiratory infections, conjunctivitis, gastroenteritis, cystitis, and obesity (Lischka, 2022)].
Noroviruses: Noroviruses are a group of viruses that belong to the family Caliciviridae (Karol et al., 2021). They cause gastroenteritis, an inflammation of the stomach and intestines (Karol et al., 2021). They are also known as the winter vomiting bug or the stomach bug (Sion et al., 2023). Noroviruses have a single-stranded positive-sense RNA genome and a non-enveloped icosahedral capsid (Vinjé et al., 2019). Astroviruses: Astroviruses are a group of viruses that belong to the family Astroviridae and cause gastroenteritis, an inflammation of the stomach and intestines. They are also known as the stomach bug or the star-like virus because of their shape (Vinjé et al., 2019). Astroviruses have a single-stranded positive-sense RNA genome and a non-enveloped icosahedral capsid.
Sapoviruses: Sapoviruses are a type of small, single-stranded RNA virus that belong to the Caliciviridae family. They are known to cause gastroenteritis, an inflammation of the stomach and intestines characterised by symptoms such as diarrhoea, vomiting, abdominal pain, and sometimes fever (Aswathyraj et al., 2016). Sapoviruses are transmitted primarily through the faecal-oral route, which means they can spread through contaminated food, water, or surfaces (Oka et al., 2015).
Cycloviruses: are an emerging group of viruses that belong to the family Circoviridae and have circular single-stranded DNA genomes. They have been found in a wide range of hosts, such as bats, rodents, birds, insects, and humans (Rosario et al., 2017). Cycloviruses can cause various diseases, such as gastroenteritis, respiratory infections, hepatitis, and neurological disorders (Rosario et al., 2017). However, the pathogenicity and transmission of cycloviruses are not well understood (Dennis et al., 2018).
The summary of select studies from the East African neighbouring countries that have investigated the environmental viral community in the last decade is summarised in Table 1.
These studies give the Kenyan data context for interpretation. The environmental viral community studies are typically dominated by rotaviruses, just like in the Kenyan studies (Kiulia et al., 2010). However, in some cases, a community equally dominated by adenoviruses (types 40 and 41) and enteroviruses (Opanda et al., 2016) was reported. (Haramoto et al., 2018) found that the most abundant class of enteric viruses was different on different types of matrices, indicating that systemic pollution influences community structure and composition. This supports the observation of (Wasonga et al., 2021) and suggests that the matrix with high faecal pollution levels and minerology influences the viral community composition. Even less research has been done on other viral families, like hepatoviruses, as indicators of faecal pollution than on other environmental viruses and bacteria. Hepatitis E was examined in one study in developing nations. Socioeconomic conditions, sanitation standards, access to potable water, and the regional occurrence of zoonotic HEV infections in animals were some of the factors that infection patterns were linked to (Khuroo et al., 2016). With the exception of sapoviruses, caliciviruses have also not been thoroughly studied.
Some significant environmental viral studies have been undertaken in Kenya that present data on the environmental virology of different epidemiological aspects (Table 2). In one study, the frequency of different viral communities’ contamination from diverse matrices was all above 97% (Lambisia et al., 2023).
Studies from other regions of the country support the notion that changing the composition of some viral gene groups is a typical response to pollution, and a similar change in relative abundance has been observed in hydro-chemically contaminated areas of a freshwater matrix (Wasonga et al., 2021). However, because these observations are based on a small number of studies, it is challenging to predict with certainty the changes that will probably be seen in other environmental matrices and settings. The various results of these investigations, however, imply that microbial communities might respond to pollution in various and site-specific ways. To understand these viral communities, a much broader examination of all environmental viruses in Kenyan environmental matrices is needed since there are still relatively few studies on viral communities.
What is certain, though, is that the few studies presented here have shed light on the impending public health issues related to environmental virus contamination. Public health is faced with a wide variety of viral threats, which calls for a deeper comprehension of environmental virology. For instance, waterborne viral infections are a serious problem in Kenya, especially in areas with poor access to clean water and inadequate sanitary facilities (Adelodun et al., 2021). Particularly in vulnerable populations like children and those with weakened immune systems, waterborne viral infections increase the burden of diarrheal diseases. To implement efficient preventive measures and guarantee access to safe drinking water, it is essential to comprehend the origins, modes of transmission, and persistence of these waterborne viruses.
Kenya’s rich biodiversity and close interactions between humans and animals create favourable conditions for zoonotic infections. Diseases such as avian influenza and Rift Valley fever have zoonotic origins and can be transmitted from animals to humans (Sisay et al., 2016). In addition, Kenya, like any other developing country, is vulnerable to emerging viral diseases due to factors such as urbanisation, population growth, and increased international travel. Emerging diseases like the Zika virus, dengue fever, and chikungunya have gained attention in recent years. Knowledge in this area is vital for timely surveillance, early detection, and an effective response to emerging viral outbreaks. The emerging viral diseases have the potential to spread rapidly, leading to increased morbidity and mortality rates. Therefore, understanding the environmental virology aspects of these diseases can guide public health interventions and resource allocation to minimise their impact.
Poor sanitation is associated with open defecation, which can facilitate the spread of enteric viruses and other pathogens. A study found that open defecation decreased from 16.2% in 2003 to 9.9% in 2014, but the burden increased among poor households, especially the poorest. The study also found disparities in access to improved sanitation facilities between different wealth quintiles (Njuguna, 2019).
Water resources are threatened by deforestation, soil erosion, desertification, flooding, pollution, and climate change. These factors can affect the quantity and quality of water for domestic, agricultural, and industrial use, as well as the health of aquatic ecosystems.
A common occurrence in Kenya is diffuse pollution, which can introduce or increase the concentration of viral pathogens in the water sources, putting the public at risk of exposure and infection. For instance, enteric viruses like norovirus and hepatitis A virus can be transported into water sources like rivers, dams, and marine estuaries by urban stormwater. The dynamics and epidemiology of waterborne viral diseases can be affected by diffuse pollution, which can also have an impact on the survival, persistence, and transmission of viral pathogens in water sources.
The distribution and abundance of hosts and vectors of viral pathogens in water sources can change due to climate change, which can affect the risk and transmission of waterborne viral diseases. For instance, climate change may result in higher temperatures, more precipitation, and greater humidity. These factors may favour the survival of these vectors that transmit viruses. For example, one study demonstrated that orthobunyaviruses like Bunyamwera and Nyando, which can cause febrile illnesses with rash, hemorrhagic fever, and congenital malformations in both people and animals, can be transmitted by mosquito (Koka et al., 2021). Climate change can also increase the frequency and intensity of extreme weather events, such as floods, droughts, and storms, which can damage the infrastructure and facilities for water supply, sanitation, and wastewater treatment. This can result in the discharge or leakage of raw or inadequately treated sewage into water sources, contaminating them with enteric viruses, such as norovirus and hepatitis.
The potential impact of pathogens being transported into drinking water is arguably the most urgent concern regarding viruses in environmental matrices. The primary sources of pathogen contamination are sewage treatment and waste from farm animals. However, some viral pathogens have been detected in native wild animals; for example, rats, mice or birds (Duarte et al., 2019). Although these may not be significant disease reservoirs, it could be argued that pathogens (even in small numbers) could be thought of as a part of the indigenous transient community in environmental matrices due to the pathogens’ presence in native animals and their inevitable migration into waterways.
Monitoring viral pathogens in the environment and spotting potential public health risks require a strong surveillance system as well. To aid in timely detection and response, existing surveillance systems can be strengthened, and early warning mechanisms developed for viral threats. This entails setting up sentinel surveillance sites, incorporating real-time data sharing platforms, and incorporating environmental virology data into already-existing surveillance frameworks. Utilising technological advancements for detection is another crucially important strategy. Environmental virology research has new opportunities thanks to technological advancements. Viral detection, genetic characterization, and environmental monitoring can all be revolutionised by incorporating tools like metagenomic sequencing, remote sensing, and bioinformatics. Utilising these technologies can improve research’s effectiveness and efficiency while revealing important details about the dynamics of viruses, their patterns of transmission, and the environmental factors that affect viral infections in Kenya. A sensory technological revolution is currently happening in environmental monitoring as well as clinical diagnosis, resulting in a paradigm shift in microbiological and other contaminants analysis. This is one area where early warning is crucial to enhancing future research.
Despite significant strides in various aspects of environmental virology, our understanding of native virological communities within Kenyan environmental matrices remains rudimentary. To bridge this knowledge gap, it is imperative that future investigations delve deeper into the factors driving viral diversity and the constituents of these communities. Such endeavors hold global significance, as comprehensive studies in this domain are scarce. A nuanced comprehension of environmental virus diversity and its response to pollution could pinpoint areas necessitating heightened management and monitoring, while also identifying resilient or safeguarded communities amidst anthropogenic pressures. Only by comprehensively grasping the current state of environmental virology can we discern alterations stemming from potential future human impacts.
Among the paramount research priorities, delineating the overarching determinants influencing viral community structures across diverse Kenyan environmental contexts looms large. This foundational insight should serve as a compass guiding applied research endeavors, underpinning initiatives aimed at preserving environmental quality, devising bioremediation strategies, and tracking the transport of viral pathogens. This review has unveiled the spectrum of viral threats confronting Kenya, underscored persistent public health challenges, and elucidated the pivotal role played by sanitation in mitigating viral infections. It is my hope that this synthesis of knowledge paves the way for a more profound understanding of the complex interplay among environmental virology, public health, and sanitation, ultimately contributing to the betterment of both Kenyan and global communities.
MO: Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The Kenya Medical Research Institute (KEMRI) provided support for the scientific and ethical review, which the author gratefully acknowledges. The author appreciates the reviewers’ suggestions, which helped to make this paper better.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adelodun, B., Ajibade, F. O., Ighalo, J. O., Odey, G., Ibrahim, R. G., Kareem, K. Y., et al. (2021). Assessment of socioeconomic inequality based on virus-contaminated water usage in developing countries: A review. Environ. Res. 192, 110309. doi: 10.1016/j.envres.2020.110309
Ahmed, A., Mahmoud, I., Eldigail, M., Elhassan, R. M., Weaver, S. C. (2021). The emergence of rift valley fever in gedaref state urges the need for a cross-border one health strategy and enforcement of the international health regulations. Pathogens 10 (7), 885. doi: 10.3390/pathogens10070885
Aswathyraj, S., Arunkumar, G., Alidjinou, E. K., Hober, D. (2016). Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med. Microbiol. Immunol. 205, 397–407. doi: 10.1007/s00430-016-0465-y. H., A., Mumps, I., A., S.E., 2010. Noroviruses and Sapoviruses. Desk Encyclopedia of.
Bitanihirwe, B., Ssewanyana, D., Ddumba-Nyanzi, I. (2021). Pacing forward in the face of fragility: lessons from african institutions and governments' response to public health emergencies. Front. Public Health 9, 714812. doi: 10.3389/fpubh.2021.714812
Bwogi, J., Karamagi, C., Byarugaba, D. K., Tushabe, P., Kiguli, S., Namuwulya, P., et al. (2023). Co-surveillance of rotaviruses in humans and domestic animals in Central Uganda reveals circulation of wide genotype diversity in the animals. Viruses 15 (3), 738. doi: 10.3390/v15030738
Bwogi, J., Malamba, S., Kigozi, B., Namuwulya, P., Tushabe, P., Kiguli, S., et al. (2016). The epidemiology of rotavirus disease in under-five-year-old children hospitalized with acute diarrhea in Central Uganda, 2012-2013. Arch Virol. 161 (4), 999–1003. doi: 10.1007/s00705-015-2742-2
Dennis, T. P.W., Flynn, P. J., de Souza, W. M., Singer, J. B., Moreau, C. S., Wilson, S. J., et al. (2018). Insights into circovirus host range from the genomic fossil record. J. Virol. 92 (16), e00145-18. doi: 10.1128/JVI.00145-18
Duarte, M. A., Silva, J. M. F., Brito, C. R., Teixeira, D. S., Melo, F. L., Ribeiro, B. M., et al. (2019). Faecal virome analysis of wild animals from Brazil. Viruses 11 (9), 803. doi: 10.3390/v11090803
Endale, A., Michlmayr, D., Abegaz, W. E., Asebe, G., Larrick, J. W., Medhin, G., et al (2023). Community-based sero-prevalence of chikungunya and yellow fever in the South Omo Valley of Southern Ethiopia. PloS Negl. Trop. Dis. 14 (9), e0008549. doi: 10.1371/journal.pntd.0008549
Haramoto, E., Kitajima, M., Hata, A., Torrey, J. R., Masago, Y., Sano, D., et al (2018). A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 135, 168–186. doi: 10.1016/j.watres.2018.02.004
Jacobsen, K. H. (2018). Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb. Perspect. Med. 8 (10), a031716. doi: 10.1101/cshperspect.a031716
Karol, L., Agnieszka, T., Aleksandra, P., Pawel, G., Tomasz, D., Ja, W., et al. (2021). Eosinophilic gastroenteritis and graft‐versus‐host disease induced by transmission of N. with fecal microbiota transplant, 2021. Transpl. Infect. Dis. 23.
Katukiza, A. Y., Temanu, H., Chung, J. W., Foppen, J. W., Lens, P. N. (2013). Genomic copy concentrations of selected waterborne viruses in a slum environment in Kampala, Uganda. J. Water Health 11 (2), 358–370. doi: 10.2166/wh.2013.184
Khuroo, M. S., Khuroo, M. S., Khuroo, N. S. (2016). Transmission of Hepatitis E Virus in Developing Countries. Viruses 8 (9), 253. doi: 10.3390/v8090253
Kiulia, N. M., Netshikweta, R., Page, N. A., Van Zyl, W. B., Kiraithe, M. M., Nyachieo, A., et al. (2010). The detection of enteric viruses in selected urban and rural river water and sewage in Kenya, with special reference to rotaviruses. J. Appl. Microbiol. 109 (3), 818–828. doi: 10.1111/j.1365-2672.2010.04710.x
Koka, H., Lutomiah, J., Langat, S., Koskei, E., Nyunja, A., Mutisya, J., et al. (2021). Evidence of circulation of Orthobunyaviruses in diverse mosquito species in Kwale County, Kenya. Virol. J. 18 (1), 204. doi: 10.1186/s12985-021-01670-5
Lambisia, A. W., Makori, T. O., Mutunga, M., Cheruiyot, R., Murunga, N., Quick, J., et al. (2023). Genomic epidemiology of human adenovirus F40 and F41 in coastal Kenya: A retrospective hospital-based surveillance study, (2013–2022). Virus Evol. 9 (1), vead023. doi: 10.1093/ve/vead023
Le Gall, O., Christian, P., Fauquet, C. M., King, A. M., Knowles, N. J., Nakashima, N., et al. (2008). Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch Virol. 153 (4), 715–727. doi: 10.1007/s00705-008-0041-x
Lischka, P. (2022). “Antiviral therapy of adenovirus infections,” in New drug development for known and emerging viruses. Eds. Rübsamen-Schaeff, H., Buschmann, H.. (New Jersey, USA: Wiley‐VCH GmbH). doi: 10.1002/9783527810697.ch11
Mattioli, M. C., Boehm, A. B., Davis, J., Harris, A. R., Mrisho, M., Pickering, A. J. (2014). Enteric pathogens in stored drinking water and on caregiver's hands in Tanzanian households with and without reported cases of child diarrhea. PloS One 9 (1), e84939. doi: 10.1371/journal.pone.0084939
Moyo, S. J., Hanevik, K., Blomberg, B., Kommedal, O., Nordbø, S. A., Maselle, S., et al. (2014). Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania a case control study. BMC Infect. Dis. 14, 666. doi: 10.1186/s12879-014-0666-1
Muendo, C., Laving, A., Kumar, R., Osano, B., Egondi, T., Njuguna, P. (2018). Prevalence of rotavirus infection among children with acute diarrhoea after rotavirus vaccine introduction in Kenya, a hospital cross-sectional study. BMC Pediatr. 18 (1), 323. doi: 10.1186/s12887-018-1291-8
Mwasi, S. L., Wallace, D. B., Jennifer, R. V., Omballa, V., Alice, O., Samuel, K., et al. (2019). Molecular characterization of human enteroviruses detected in children under five years old in Kenya 2009 - 2015. Afr. J. Health Sci. 32 (2), 1022–9272.
Nakawesi, J. S., Wobudeya, E., Ndeezi, G., Mworozi, E. A., Tumwine, J. K. (2010). Prevalence and factors associated with rotavirus infection among children admitted with acute diarrhea in Uganda. BMC Pediatr. 10, 69. doi: 10.1186/1471-2431-10-69
Njuguna, J. (2019). Progress in sanitation among poor households in Kenya: evidence from demographic and health surveys. BMC Public Health. 19 (1), 135. doi: 10.1186/s12889-019-6459-0
O'Brien, E., Nakyazze, J., Wu, H., Kiwanuka, N., Cunningham, W., Kaneene, J. B., et al. (2017). Viral diversity and abundance in polluted waters in Kampala, Uganda. Water Res. 127, 41–49. doi: 10.1016/j.watres.2017.09.063
Ochieng, C., Lutomiah, J., Makio, A., Koka, H., Chepkorir, E., Yalwala, S., et al. (2013). Mosquito-borne arbovirus surveillance at selected sites in diverse ecological zones of Kenya; 2007 – 2012. Virol. J. 10, 140. doi: 10.1186/1743-422X-10-140
Oka, T., Wang, Q., Katayama, K., Saif, L. J. (2015). Comprehensive review of human sapoviruses. Clin. Microbiol. Rev. 28 (1), 32–53. doi: 10.1128/CMR.00011-14
Opanda, S. M., Wamunyokoli, F., Khamadi, S., Coldren, R., Bulimo, W. D. (2016). Genotyping of enteroviruses isolated in Kenya from pediatric patients using partial VP1 region. Springerplus 5, 158. doi: 10.1186/s40064-016-1834-0
Quaranta, P., Lottini, G., Chesi, G., Contrafatto, F., Russotto, R., Macera, L., et al. (2020). DDX3 inhibitors show antiviral activity against positive-sense single-stranded RNA viruses but not against negative-sense single-stranded RNA viruses: the coxsackie B model. Antiviral Res. 178, 104750. doi: 10.1016/j.antiviral.2020.104750
Rosario, K., Breitbart, M., Harrach, B., Segalés, J., Delwart, E., Biagini, P., et al (2017). Revisiting the taxonomy of the family Circoviridae: establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch Virol 162, 1447–1463. doi: 10.1007/s00705-017-3247-y
Sion, E., Ab-Rahim, S., Muhamad, M. (2023). Trends on Human Norovirus Virus-like Particles (HuNoV-VLPs) and Strategies for the Construction of Infectious Viral Clones toward In Vitro Replication. Life 13, 1447. doi: 10.3390/life13071447
Shioda, K., Cosmas, L., Audi, A., Gregoricus, N., Vinjé, J., Parashar, U. D., et al. (2016). Population-based incidence rates of diarrheal disease associated with norovirus, sapovirus, and astrovirus in Kenya. PloS One 11 (4), e0145943. doi: 10.1371/journal.pone.0145943
Sisay, Z., Djikeng, A., Berhe, N., Belay, G., Abegaz, W. E., Wang, Q. H., et al (2010). First detection and molecular characterization of sapoviruses and noroviruses with zoonotic potential in swine in Ethiopia. Arch Virol. 161 (10), 2739–2747. doi: 10.1007/s00705-016-2974-9
Sisay, Z., Djikeng, A., Berhe, N., Belay, G., Abegaz, W. E., Wang, Q. H., et al. (2016). First detection and molecular characterization of sapoviruses and noroviruses with zoonotic potential in swine in Ethiopia. Arch. Virol. 161 (10), 2739–2747. doi: 10.1007/s00705-016-2974-9
Ukuli, Q. A., Erima, B., Mubiru, A., Atim, G., Tugume, T., Kibuuka, H., et al. (2023). Molecular characterisation of human adenoviruses associated with respiratory infections in Uganda. BMC Infect. Dis. 23 (1), 435. doi: 10.1186/s12879-023-08403-9
UNICEF (2023) Water, Sanitation and Hygiene (UNICEF Kenya). Available at: https://www.unicef.org/Kenya/water-sanitation-and-hygiene (Accessed 9.26.23).
van Seventer, J. M., Hochberg, N. S. (2017). Principles of infectious diseases transmission diagnosis prevention and control. Int. J. Public. Health 2017, 22–39. doi: 10.1016/B978-0-12-803678-5.00516-6
van Zyl, W. B., Zhou N, A., Wolfaardt, M., Matsapola, P. N., Ngwana, F. B., Symonds, E. M., et al. (2019). Detection of potentially pathogenic enteric viruses in environmental samples from Kenya using the bag-mediated filtration system. Water Sci. Technol. Water Supply 19 (6), 1668–1676. doi: 10.2166/ws.2019.046
Vinjé, J., Estes, M. K., Esteves, P., Green, K. Y., Katayama, K., Knowles, N. J., et al. (2019). ICTV Virus Taxonomy Profile: Caliciviridae. J Gen Virol. 100 (11), 1469–1470. doi: 10.1099/jgv.0.001332
Wainaina, E., Otieno, C. A., Kamau, J., Nyachieo, A., Lowther, S. A. (2020). Norovirus infections and knowledge, attitudes and practices in food safety among food handlers in an informal urban settlement, Kenya 2017. BMC Public Health 20 (1), 474. doi: 10.1186/s12889-020-8401-x
Wasonga, M., Maingi, J., Omwoyo, O. (2020). Occurrence of enteric viruses in surface water and the relationship with changes in season and physical water quality dynamics. Adv. Virol. 2020, 9062041. doi: 10.1155/2020/9062041
Wasonga, M. O., Maingi, J., Omwoyo, O. (2021). Effects of Contamination of Freshwater Habitat With Common Heavy Metals and Anions on the Prevalence of Human Adenoviruses and Enteroviruses. Front. Public Health 8, 603217. doi: 10.3389/fpubh.2020.603217
Wells, A. I., Coyne, C. B. (2019). Enteroviruses: a gut-wrenching game of entry, detection, and evasion. Viruses. 11 (5), 460. doi: 10.3390/v11050460
Keywords: viral threats, public health challenges, sanitation, waste management, environment, waterborne diseases
Citation: Opere MW (2023) Analysing the interplay of environmental virology, public health, and sanitation: a comprehensive review from a Kenyan perspective. Front. Cell. Infect. Microbiol. 13:1256822. doi: 10.3389/fcimb.2023.1256822
Received: 11 July 2023; Accepted: 06 October 2023;
Published: 24 October 2023.
Edited by:
Sayed F. Abdelwahab, Minia University, EgyptReviewed by:
Abdennaceur Hassen, Centre de Recherches et des Technologies des Eaux, TunisiaCopyright © 2023 Opere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Wasonga Opere, bW9wZXJlQHRtdS5hYy5rZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.