- 1National Clinical Laboratory on Tuberculosis, Beijing Key Laboratory on Drug-Resistant Tuberculosis, Beijing Chest Hospital, Capital Medical University, Beijing, China
- 2Tuberculosis Department, Beijing Chest Hospital, Capital Medical University, Beijing, China
Non-tuberculous mycobacteria (NTM) are opportunistic pathogens that can infect all body tissues and organs. In particular, the lungs are the most commonly involved organ, with NTM pulmonary diseases causing serious health issues in patients with underlying lung disease. Moreover, NTM infections have been steadily increasing worldwide in recent years. NTM are also naturally resistant to many antibiotics, specifically anti-tuberculosis (anti-TB) drugs. The lack of drugs targeting NTM infections and the increasing drug resistance of NTM have further made treating these mycobacterial diseases extremely difficult. The currently recommended NTM treatments rely on the extended indications of existing drugs, which underlines the difficulties of new antibiotic discovery against NTM. Another challenge is determining which drug combinations are most effective against NTM infection. To a certain extent, anti-NTM drug development depends on using already available antibiotics and compounds. Here, we aimed to review new antibiotics or compounds with good antibacterial activity against NTM, focusing on their mechanisms of action, in vitro and in vivo antibacterial activities.
1 Introduction
Non-tuberculous mycobacteria (NTM) refer to mycobacteria other than Mycobacterium leprae and Mycobacterium tuberculosis complex (MTC). NTM prevalence has demonstrated an increasing global trend in the last few decades (Yu et al., 2016; Brode et al., 2017; Lin et al., 2018; Santin et al., 2018; Cowman et al., 2019). In China, the proportion of NTM isolates among the specimen cultures positive for Mycobacterium species rose from 4.3% in 1979 to 22.9% in 2010 (Zhou et al., 2020). NTM are present in soil and water and are widely dispersed in the natural world. Most of the more than 200 isolated NTM species are non-pathogenic, whereas approximately 30 are clinically relevant. Although the nosocomial spread of M. abscessus (Mab) in cystic fibrosis patients was reported, the precise source of the remaining NTM species infection has no conclusive proof of transmission between people, which is assumed to be acquired via environmental exposure (Bryant et al., 2016; Wu et al., 2018).
The resistance of NTM to existing treatments is increasingly becoming an internationally recognized problem (Griffith et al., 2007; Johansen et al., 2020; Griffith and Daley, 2022). These include the most common clinical cases of Mab and Mycobacterium avium complex (MAC), which account for >90% of all documented NTM pulmonary disease (NTM-PD) cases (Yu et al., 2016).The regimens for Mab infections contain macrolide antibiotics, including clarithromycin (CLA) and azithromycin. However, most patients respond poorly to this class of antibiotics due to the inducible resistance phenotype that occurs during therapy, which is driven by the macrolide-inducible ribosomal methylase encoded by erm (41) (Richard et al., 2020). Mab has recently developed increased resistance to CLA, with reported resistance rates of 14%–38% (Broda et al., 2013; Zhuo et al., 2013; Lee et al., 2014). In addition, Mab also exhibits resistance to azithromycin (resistance rate of 10%), which is relatively lower than that of CLA (Pasipanodya et al., 2017; Hirama et al., 2020). A meta-analysis shows that the estimated pooled treatment success rate for patients with MAC disease was 39% (Xu et al., 2014), similar to the treatment outcomes of extensively drug-resistant tuberculosis (XDR-TB). Macrolides have also been used as the key medication in MAC therapy regimens, with a meta analysis indicating a treatment success rate of 60% (95% confidence interval [CI], 55.1%–64.8%) for macrolide-containing regimens in MAC-PDs (Kwak et al., 2017). Despite the combination strategy of fluoroquinolones, aminoglycosides, and surgical resection, macrolide-resistant MAC lung disease has a poor treatment outcome, resulting in a 1-year all-cause mortality rate of 10% (Park et al., 2019).
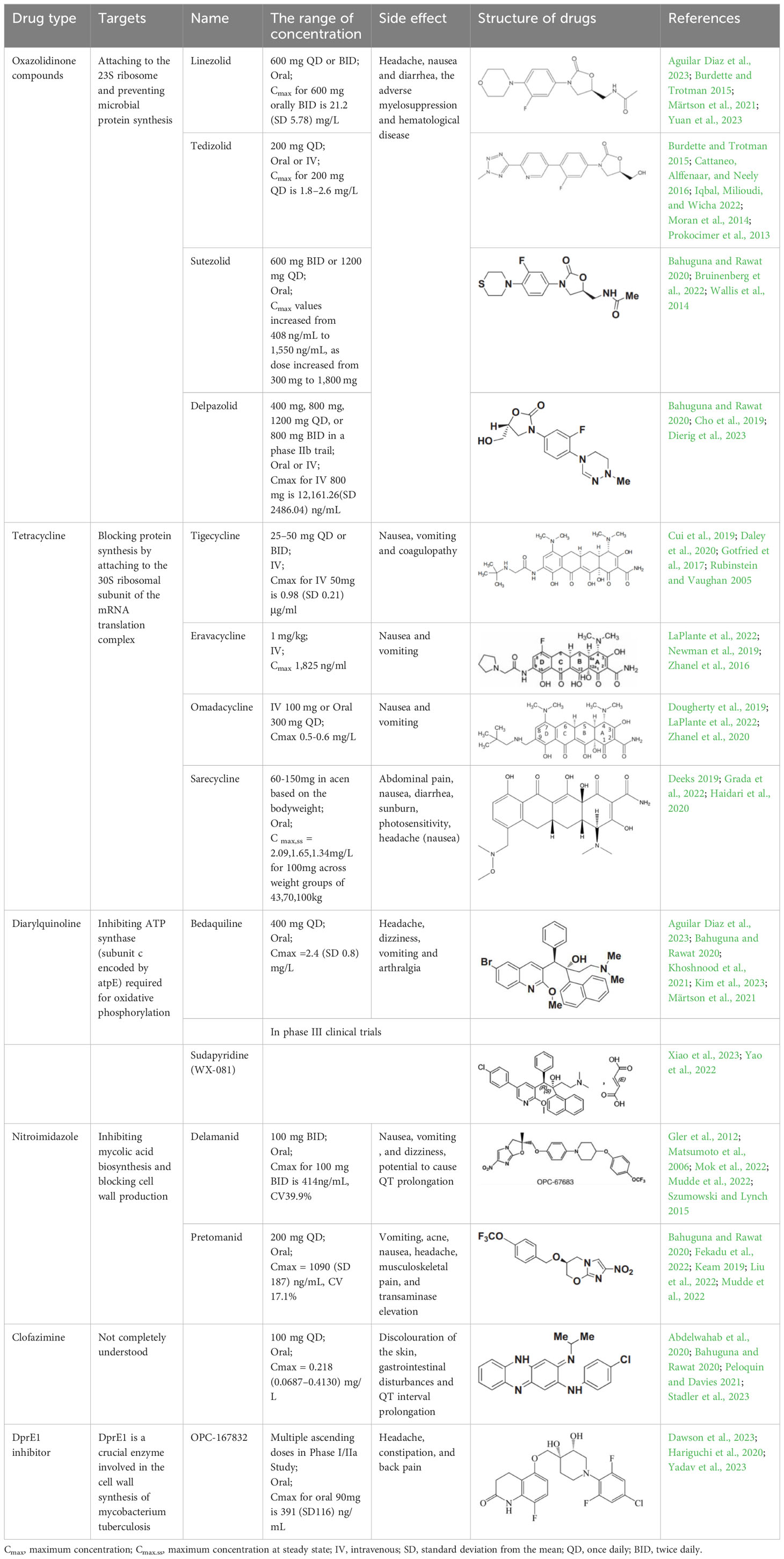
However, no drugs have been specifically developed for the increasingly prevalence of NTM infection worldwide. All pharmaceuticals currently recommended by the American Thoracic Society for NTM therapy regimens are derived from the expanded indications of currently available medications. Over the last 50 years of tuberculosis (TB) research, only bedaquiline (BDQ), delamanid (DLM), and pretomanid are the new drugs that have been approved for commercialization by the US Food and Drug Administration (FDA) due to the challenges in developing novel medications. Therefore, maximizing the anti-NTM activity of presently accessible drugs or antibiotics may be useful for developing anti-NTM medications. In this study, we elaborate on new antibiotics or compounds with potent antimycobacterial activity against NTM. Reported drug type, targets, range of concentration, side effects, and structure are summarized in Table 1 and Figure 1.
2 Oxazolidinone
In vitro and in vivo investigations have shown that oxazolidinones are extremely effective at eliminating M. tuberculosis (Mtb) (Pstragowski et al., 2017; Guo et al., 2021). Their mechanism of action involves attaching to the 23S ribosome, composed of 5S and 23S rRNAs and 36 riboproteins (L1–L36), and blocking the tRNA at the peptidyl transferase center on the ribosomal subunit, thereby preventing microbial protein synthesis (Kadura et al., 2020). The first approved oxazolidinone drug was linezolid (LZD) in 2000. LZD has a wide spectrum of activity against gram-positive bacteria, including methicillin-resistant staphylococci, penicillin-resistant pneumococci, and vancomycin-resistant Enterococcus faecalis, as well as drug-resistant TB (Clemett and Markham, 2000). Subsequently, tedizolid (TZD) was approved by the FDA in 2014 as a novel, effective oxazolidinone precursor drug (Zhanel et al., 2015). Another promising drug is sutezolid (SZD, PNU-100480), a thiomorpholine analog of LZD, showing preliminary evidence of superior efficacy against Mtb and readily detectable bactericidal activity in the sputum and blood (Wallis et al., 2014). Similarly, delpazolid (DZD, LCB01-0371) is a novel oxazolidinone analog that exhibits broad-spectrum anti-gram-positive activity in vitro and in animal infection models as well as effective protection against Mab (Kim et al., 2017).
All four mentioned oxazolidinones demonstrated in vitro antimycobacterial activity against 32 rapidly growing mycobacteria (RGM) reference strains, presenting with MICs of ≤8 μg/ml against most species (Wen et al., 2021). Compared with the other oxazolidinones, TZD had the lowest MIC values for Mab subsp. abscessus and Mab subsp. massiliense, with MIC50 = 1 μg/ml and MIC90 = 2 μg/ml for both subspecies (Brown-Elliott and Wallace, 2017; Wen et al., 2021). DZD also exhibited better antimycobacterial activity (4-fold lower MIC values) than LZD against M. fortuitum isolates. Additionally, all four oxazolidinones showed in vitro antimycobacterial activity against slowly growing mycobacteria (SGM) reference stains, wherein all four drugs had MIC values ≤8 μg/mL against 18 of 20 tested SGM species. Particularly, SZD had the strongest MIC values against M. intracellulare (MIC50 = 2 μg/mL and MIC90 = 4 μg/mL).
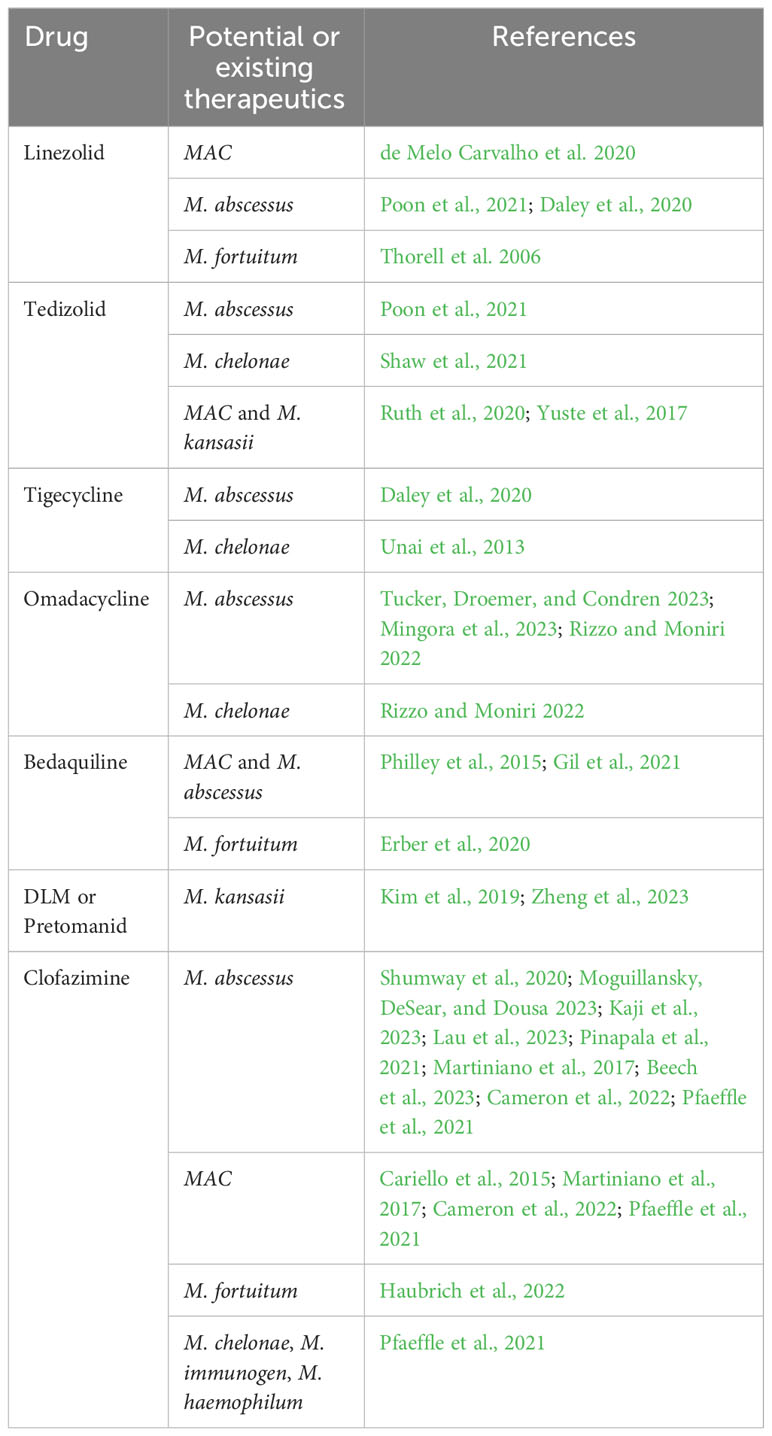
Among these four oxazolidinones, LZD and TZD have been used for the therapy of NTM infection, including MAC, M. abscessus, M. fortuitum and M. kansasii (Table 2). Consistent with in vitro antimycobacterial activity, two of 3 (67%) patients with M. chelonae and M. abscessus were cured or clinically cured with LZD-containing regimens. Seven of 12 (58%) patients with M. chelonae and Mab were cured or clinically cured with TZD- containing regimens (Poon et al., 2021). Potential therapeutics of LZD and TZD and the detailed information of case reports using these two drugs are presented in Table 2 and Table S1.
3 Tetracycline
Tetracyclines have served as cornerstone antibacterial drugs for over 70 years. This class of drugs blocks protein synthesis by attaching to the 30S ribosomal subunit of the mRNA translation complex to inhibit the binding of aminyl tRNA to the mRNA–ribosomal complex (Yadav et al., 2023). Within this tetracycline group, tigecycline (TGC) is the first and only clinically available glycylcycline (Ng and Ngeow, 2022). Additionally, several new antibiotics from the tetracycline class were recently created to address the drawbacks of TGC, i.e., high rate of adverse gastrointestinal effects and mortality (Heaney et al., 2019). Among them, eravacycline (ERC), a synthetic fluorocycline, was authorized by the FDA in 2018. ERC is injected intravenously to treat difficult intra-abdominal infections caused by antibiotic-resistant bacteria (Anonymous, 2018a). In 2018, the FDA approved another drug called omadacycline (OMC) for managing acute bacterial skin and skin structure infections (ABSSI) and community-acquired bacterial pneumonia (CABP). This antibiotic can be administered orally (PO) or intravenously (IV) once per day (Anonymous, 2018b). Sarecycline (SAC), another oral drug, represents the first narrow-spectrum tetracycline-class antibiotic developed for acne treatment (Anonymous, 2018c). Previous studies have demonstrated strong in vitro antimycobacterial activity of TGC against RGM and have recommended using TGC for treating Mab infections according to the current guidelines (Wallace et al., 2002; Lerat et al., 2014; Wallace et al., 2014; Haworth et al., 2017; Daley et al., 2020). Except for SAC, the other tetracyclines (i.e., TGC, OMC, and ERC) had MICs of ≤0.5 μg/ml against 27 RGM reference strains. In particular, ERC generally presented the lowest MICs, with MIC90 values of 0.25 μg/mL, 0.25 μg/mL, and 0.06 μg/mL against the clinical isolates of Mab subsp. abscessus, Mab subsp. massiliense, and M. fortuitum, respectively (Shoen et al., 2019). In the case of TGC and OMC, equivalent in vitro inhibitory activities were found against these isolates, in which the TGC showed MIC90 values that were lower or equal to those of OMC for Mab subsp. abscessus, Mab subsp. massiliense, and M. fortuitum (1 μg/mL, 1 μg/mL, and 0.25 μg/mL versus 1 μg/mL, 2 μg/mL, and 2 μg/mL) (Zhang et al., 2023). Furthermore, the in vitro antimycobacterial activity of ERC was similar to or better than that of CLA (MIC: 0.0625–2 μg/mL for susceptible strains), with CLA being the core antimycobacterial in the Mab treatment regimen (Fujiwara et al., 2021).
Case reported by Frizzell M et al. showed that a patient with M. chelonae infection receiving OMC containing regimen was considered clinically improved (Frizzell et al., 2020). Notably, 44 of 95 (46%) patients with Mab-PD had 1 or more negative cultures, with 17 of 95 (18%) achieving culture conversion (Mingora et al., 2023). Furthermore, the adverse drug effects (ADEs) were relatively mild, 35 patients (29.9%) experienced direct ADEs, nausea/emesis occurring in 21.4% of patients (Table S1). Oral administration, high antimycobacterial activity and relatively low adverse effects make OMC a promising drug for Mab and M. chelonae infection (Table 2).
4 Diarylquinoline
Diarylquinoline antibiotics act by inhibiting ATP synthase (subunit c encoded by atpE) required for oxidative phosphorylation (Kim et al., 2023). BDQ was the first diarylquinoline approved by the FDA in 2012 for treating pulmonary multidrug-resistant TB (MDR-TB) (Cox and Laessig, 2014). Recently, an innovative diarylpyridinated drug called sudapyridine (WX-081), which is formed by substituting the bromoquinoline of BDQ with a 5-phenylpyridine, was applied at the clinical development stage in 2018 and revealed better safety on QTc intervals. This drug has been included in phase III clinical trials as a TB treatment (JYP0081M301) in China since 2022 (Huang et al., 2022).
Based on the similarities of atpE within the genus Mycobacterium, several studies have evaluated the inhibitory activities of BDQ against different NTM species. Our previous study showed that BDQ possessed consistently strong antimycobacterial activity against almost all 18 SGM species tested (all MICs were far below 1 μg/ml), while BDQ also exhibited strong in vitro antimycobacterial activity against the tested RGM reference strains, with most MIC values ≤2 μg/mL (Aguilar-Ayala et al., 2017; Martin et al., 2019; Yu et al., 2019). Considering the bimodal distributions of BDQ MICs, the tentative epidemiological cutoff (ECOFF) values for SGM and RGM were set as 1 μg/mL and 2 μg/mL, respectively (Pang et al., 2017). In a Moscow study, the MIC50 and MIC90 values of BDQ were 0.015 μg/ml and 0.12 μg/ml for M. avium, and 0.007 μg/ml and 0.06 μg/ml for M. intracellulare, respectively. Consequently, the preliminary ECOFF values were defined as 0.12 μg/ml and 0.06 μg/ml for M. avium and M. intracellulare, respectively (Pidot et al., 2021). In South Korea, although the MIC50 and MIC90 values of BDQ were extremely low against Mab subsp. abscessus and Mab subsp. massiliense isolates (MIC50 = 0.062 μg/ml and MIC90 = 0.125 μg/ml), these values were higher than those for MAC (≤0.016 μg/ml) and M. kansasii isolates (≤0.016 μg/ml) (Kim et al., 2019). For sudapyridine, the BDQ analog, the MIC values against most clinical isolates of NTM were at least one dilution higher than the MICs of BDQ for six frequently isolated NTM species (Zhu et al., 2022).
There are several studies utilizing BDQ containing regimens for NTM infection (Philley et al., 2015; Erber et al., 2020; Gil et al., 2021), including Mab, MAC and M. fortuitum (Table S1). A small preliminary report indicated the potential clinical and microbiological activity of BDQ in patients with advanced MAC (n = 6) or Mab (n = 4) lung disease, with 60% of patients (six of 10) demonstrating a microbiologic response (Philley et al., 2015). Due to strong antimycobacterial activity of BDQ, it seems to be used for all pathogenic NTM, especially for refractory Mab and MAC infection (Table 2).
5 Nitroimidazole
Nitroimidazole is a class of novel antimycobacterial agents that eradicate active Mtb by inhibiting mycolic acid biosynthesis and blocking cell wall production (Lewis and Sloan, 2015; Zhang et al., 2019). DLM, a bicyclic nitroimidazole, was initially approved by the European Medicines Agency in 2014 for pulmonary MDR-TB in adult. Pretomanid was the second bicyclic nitroimidazole drug that received its approval in the USA in 2019 for treating adults with pulmonary XDR-TB or treatment-tolerant or non-responsive MDR-TB (Gils et al., 2022).
Our earlier research demonstrated that DLM had highly variable antimycobacterial activity against 19 tested SGM species, with MICs of <0.25 μg/ml in 11 species. In contrast, DLM displayed no significant inhibitory activity against most tested RGM species, with 28 of the 33 tested strains having MICs >32 μg/ml (Yu et al., 2019). Except for a few strains with MICs ≤1 μg/mL, DLM did not possess a strong inhibitory effect against M. intracellulare and M. abscessus. Similarly, Philley JV et al. found that DLM had extremely high MIC90 (>16 μg/ml) values against MAC and Mab complex. Compared to the MICs of DLM for those NTM, relatively low MIC50 (0.25 μg/mL) and MIC90 (1 μg/ml) values were observed against M. kansasii (Kim et al., 2019). Similar to DLM, pretomanid expressed high in vitro antimycobacterial activity against M. kansasii (MIC = 1.71 μg/mL) and moderate antimycobacterial activity against M. xenopi, with an MIC of 3.84 μg/mL (Zheng et al., 2023). Although high inhibitory potency was detected in DLM and pretomanid against M. kansasii in vitro, DLM or pretomanid containing regimens has not been used for the therapy of NTM infection in patients to date. Thus, DLM or pretomanid may be a potential efficacy drug for drug resistant M. kansasii (Table 2).
6 CFZ
CFZ, a traditional hydrophobic riminophenazine, has been prescribed for leprosy management since the 1950s. Although the exact mechanism of CFZ-mediated antimycobacterial activity remains undeciphered, the cell membrane may be the primary target (Cholo et al., 2017; Mirnejad et al., 2018; Stadler et al., 2023). The antimycobacterial activity of this riminophenazine covers a wide range, extending from anti-leprosy to NTM efficacy. Our prior investigation showed that CFZ had good activity against reference strains and clinical isolates of varied SGM species, with MICs well below 1 μg/ml for most strains (Luo et al., 2018). Furthermore, most clinical isolates of Mab and M. fortuitum had MICs >2 μg/ml. However, the ECOFF values for M. kansasii, M. avium and M. intracellulare were defined as 0.5 μg/ml, 1 μg/mL, and 2 μg/ml, respectively, based on their MIC distributions (Luo et al., 2018). In a study of the Mab clinical isolates obtained from patients with cystic fibrosis, 70% of the isolates presented with an MIC of ≤1.5μg/mL (Banaschewski et al., 2019).
CFZ containing regimens has been used for a wide range of NTM infection with about 50% favourable treatment outcomes, including Mab, MAC, M. chelonae and M. haemophilu (Martiniano et al., 2017; Pfaeffle et al., 2021) (Table S1). A prospective cohort study was performed in 36 NTM infection patients treated with CFZ, 22 (58%) out of 36 patients had treatment success, including 12 of 19 (63%) with Mab (Martiniano et al., 2017). Furthermore, a phase II clinical trial of CFZ evaluating its efficacy in MAC-PD treatment is underway (https://clinicaltrials.gov/study/NCT02968212) (Ito et al., 2022).Thus, current data supported CFZ in the treatment of NTM like Mab, MAC and M. chelonae (Table 2).
7 Compound drugs
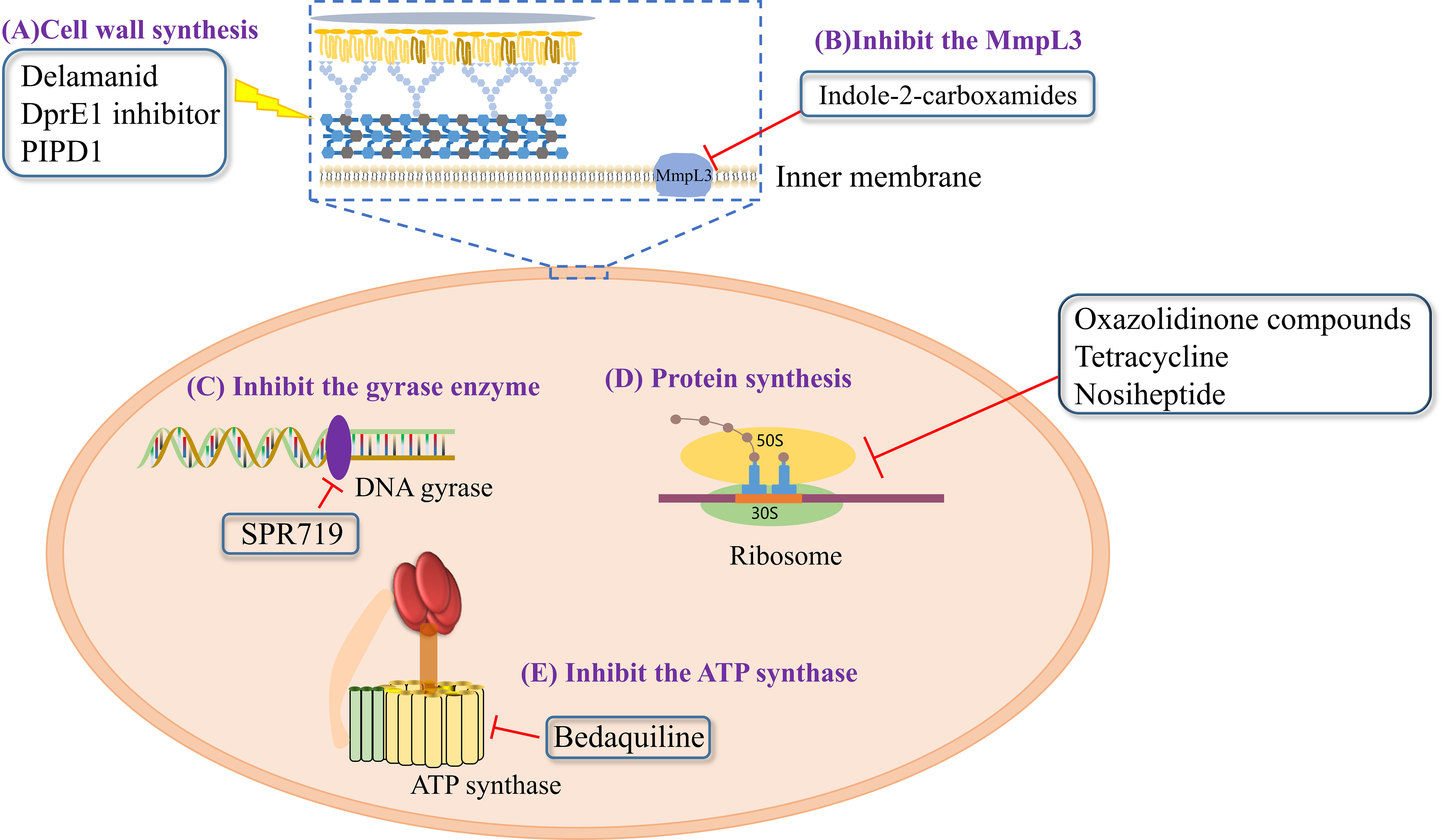
7.1 DprE1 inhibitor
In 2009, the decaprenylphosphoryl-beta-D-ribose oxidase (DprE1) enzyme was identified as a novel anti-TB drug target owing to its crucial role in mycobacteria and its location in the bacterial cell wall (Morrisette et al., 2021; Edwards and Field, 2022). Currently, several DprE1 inhibitors are enrolled in clinical trials, including BTZ-043, macozinone (MCZ, PBTZ169), OPC-167832 and TBA-7371 (Edwards and Field, 2022).There is no in vitro activity of BTZ-043 and TBA-7371 against NTM. PBTZ169 had poor activity against MAC and Mab isolates with MIC90 of >32 μg/mL (Shi et al., 2018). Surprisingly, after 4 weeks treatment in mice, PBTZ169 showed an average 3.33 and 2.29 log10 CFU reductions in the lung against Mab and M. chelonae infection (Zheng et al., 2023). Among these DprE1 inhibitors, OPC-167832 displayed superior efficacy even at low doses in a mouse TB model using as monotherapy or combined treatment with other anti-TB drugs (Robertson et al., 2021; Tasneen et al., 2022). Additionally, other studies have revealed that OPC-167832 harbored substantial activities against Mab in vitro, with MICs ranging from 5.2 μM (2.37 μg/mL) to 15 μM (6.85 μg/mL) (Hariguchi et al., 2020; Sarathy et al., 2022), which made it a promising candidate for Mab infection.
7.2 SPR720
SPR720, is a prodrug that is converted to SPR719, is a novel aminobenzimidazole that inhibits the gyrase enzyme by targeting its ATPase subunits (Brown-Elliott et al., 2018; Pennings et al., 2021). Brown-Elliott BA et al. showed that SPR719 had MIC50 values of 0.06–4 μg/mL for 93 RGM isolates, whereas the 41 MAC strains were associated with MIC90 and MIC50 values of ≤2μg/mL and ≤1μg/mL, respectively (Brown-Elliott et al., 2018). Another study of SPR719 demonstrated its activity against clinically relevant mycobacteria in mouse models of M. avium and Mab infections (Talley et al., 2021). Furthermore, a phase I trial showed that a once-daily oral administration of SPR720 (a phosphate prodrug of SPR719) could provide predicted therapeutic exposures of SPR719. Recently, SPR720 was granted the Investigational New Drug status by the FDA as a novel oral agent for pulmonary NTM infections and was recently enlisted in a phase IIa clinical trial for these infections.
7.3 GSK286
GSK286 is a Leucyl–tRNA synthetases (LeuRS) inhibitor with potent in vitro activity against Mtb and a Phase IIa clinical trials for systemic use against tuberculosis is underway (Bouz and Zitko, 2021). GSK286 showed potent antibacterial activity against Mab, with MICs of ≤0.25 μg/mL, yielding a MIC90 of 0.063μg/mL. In contrast, it was not effective against MAC with the MIC50 and MIC90 values were >8μg/mL (Dong et al., 2020).
7.4 Indole-2-carboxamide derivatives
Mycolic acid transporter protein MmpL3 is inhibited by a wide range of structurally unrelated small molecules (Raynaud et al., 2020). Among these MmpL3 inhibitors, indole-2-carboxamides (ICs) block the export of alginate monomycolate to the outer membrane and can significantly inhibit bacterial growth. ICs have been identified as a novel chemical scaffold exhibiting good preclinical efficacy against Mtb and NTM pathogens. Several novel ICs with MIC values of 0.0039–8 µg/mL against NTM have been recently reported (Pandya et al., 2019). Of these, two lead IC compounds (compounds 5 and 25) showed effective bactericidal activity against Mab in vitro (MIC = 0.125 µg/mL) (Pandya et al., 2019). Considering these findings, the chemical inhibition of MmpL3 can be hypothesized to enhance the efficacy of other drugs owing to their crucial role in modulating cell wall structure and composition.
7.5 Piperidinol-based compounds
Piperidinol-based compounds (PIPD1) strongly inhibit the transport of trehalose monomycolate, thereby disrupting the mycolylation of arabinogalactan (Dupont et al., 2016; Pandya et al., 2019). PIPD1 has been reported to possess potent activity against several mycobacterium species, including Mab, M. chelonae and M. smegmatis (MIC <1 μg/mL). In particular, PIPD1 exhibited MICs of 0.125 μg/mL against all 32 Mab strains, while its MBC99 values of 0.125–0.5 µg/mL indicated bactericidal activity (Degiacomi et al., 2019). Additionally, PIPD1 was found to have high levels of antimycobacterial activity in THP-1 macrophages, with decreasing 2 log10 CFU at a concentration of 6 μg/mL (48 × MIC). Moreover, PIPD1 administration (3 μg/mL of PIPD1 for 72 h) in a Mab-infected zebrafish model reduced bacterial load and increased the survival of the infected embryos (Dupont et al., 2016).
In summary, identifying novel anti-NTM drugs is of vital importance in the face of increasing global NTM infections. Anti-TB drugs, such as BDQ and CFZ have shown good in vitro anti-NTM activity and have been proposed for clinical use. Compounds, including OPC-167832, have also shown good in vitro antibacterial activity in clinical trials involving common pathogenic NTM. Therefore, these drugs or compounds have potential for in NTM treatment to improve patient outcomes. Although in vitro and preclinical trials have detected many promising compounds with potential therapeutic effects against NTM infection, clinical trials are urgently required to investigate their efficacy in NTM disease management. In addition, phage therapy for NTM infection also acquired favorable outcomes with exceptional safety profiles and no evidence of phage resistance was observed, which makes it a promising potential therapy. Thus, we believe that the increasing attention on NTM diseases should result in increased efforts on relevant drug discovery necessary to close the gaps in NTM treatment.
Author contributions
YG and XY drafted the manuscript. XY, HH and WN designed the study and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This review was supported by Beijing Public Health Experts Project (grant number G2023-2-002 and G2023-3-004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1243457/full#supplementary-material
References
Abdelwahab, M. T., Wasserman, S., Brust, J. C. M., Gandhi, N. R., Meintjes, G., Everitt, D., et al. (2020). Clofazimine pharmacokinetics in patients with TB: dosing implications. J. Antimicrob. Chemother. 75, 3269–3277. doi: 10.1093/jac/dkaa310
Aguilar-Ayala, D. A., Cnockaert, M., André, E., Andries, K., Gonzalez, Y. M. J. A., Vandamme, P., et al. (2017). In vitro activity of bedaquiline against rapidly growing nontuberculous mycobacteria. J. Med. Microbiol. 66, 1140–1143. doi: 10.1099/jmm.0.000537
Aguilar Diaz, J. M., Abulfathi, A. A., Te Brake, L. H., van Ingen, J., Kuipers, S., Magis-Escurra, C., et al. (2023). New and repurposed drugs for the treatment of active tuberculosis: an update for clinicians. Respiration 102, 83–100. doi: 10.1159/000528274
Anonymous (2018a). XeravaTM (Eravacycline) for Injection (Package Insert) (Watertown, MA: Tetraphase Pharmaceuticals Inc).
Anonymous (2018b). NUZYRA™ (omadacycline) For Injection, For Intravenous (Package Insert) (Boston, MA: Paratek Pharmaceuticals, Inc).
Anonymous (2018c). SEYSARA™ (sarecycline) for Oral (Package Insert) (Madison, MA: Allergan USA, Inc).
Bahuguna, A., Rawat, D. S. (2020). An overview of new antitubercular drugs, drug candidates, and their targets. Med. Res. Rev. 40, 263–292. doi: 10.1002/med.21602
Banaschewski, B., Verma, D., Pennings, L. J., Zimmerman, M., Ye, Q., Gadawa, J., et al. (2019). Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J. Cyst Fibros 18, 714–720. doi: 10.1016/j.jcf.2019.05.013
Beech, A. J., Weinberg, S. E., Mortimer, A. E., Lynch, F., Bedford, J., Calisti, G. (2023). Mycobacterium abscessus skin and soft tissue infection following autologous fat grafting in Kurdistan treated with an antibiotic combination including Imipenem-Relebactam and Rifabutin. J. Clin. Tuberc Other Mycobact Dis. 32, 100381. doi: 10.1016/j.jctube.2023.100381
Bouz, G., Zitko, J. (2021). Inhibitors of aminoacyl-tRNA synthetases as antimycobacterial compounds: An up-to-date review. Bioorg Chem. 110, 104806. doi: 10.1016/j.bioorg.2021.104806
Broda, A., Jebbari, H., Beaton, K., Mitchell, S., Drobniewski, F. (2013). Comparative drug resistance of Mycobacterium abscessus and M. chelonae isolates from patients with and without cystic fibrosis in the United Kingdom. J. Clin. Microbiol. 51, 217–223. doi: 10.1128/JCM.02260-12
Brode, S. K., Marchand-Austin, A., Jamieson, F. B., Marras, T. K. (2017). Pulmonary versus nonpulmonary nontuberculous mycobacteria, ontario, Canada. Emerg. Infect. Dis. 23, 1898–1901. doi: 10.3201/eid2311.170959
Brown-Elliott, B. A., Rubio, A., Wallace, R. J. (2018). In vitro susceptibility testing of a novel benzimidazole, SPR719, against nontuberculous mycobacteria. Antimicrob. Agents Chemother. 62 (11), e01503–18. doi: 10.1128/AAC.01503-18
Brown-Elliott, B. A., Wallace, R. J., Jr. (2017). In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria. J. Clin. Microbiol. 55, 1747–1754. doi: 10.1128/JCM.00274-17
Bruinenberg, P., Nedelman, J., Yang, T. J., Pappas, F., Everitt, D. (2022). Single ascending-dose study to evaluate the safety, tolerability, and pharmacokinetics of sutezolid in healthy adult subjects. Antimicrob. Agents Chemother. 66, e0210821. doi: 10.1128/aac.02108-21
Bryant, J. M., Grogono, D. M., Rodriguez-Rincon, D., Everall, I., Brown, K. P., Moreno, P., et al. (2016). Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354, 751–757. doi: 10.1126/science.aaf8156
Burdette, SD, Trotman, R.. (2015). Tedizolid: The First Once-Daily Oxazolidinone Class Antibiotic. Clin Infect Dis. 61(8): 1315–21. doi: 10.1093/cid/civ501
Cameron, L. H., Peloquin, C. A., Hiatt, P., Mann, M., Starke, J. R., Faircloth, J., et al. (2022). Administration and monitoring of clofazimine for NTM infections in children with and without cystic fibrosis. J. Cyst Fibros 21, 348–352. doi: 10.1016/j.jcf.2021.08.010
Cariello, P. F., Kwak, E. J., Abdel-Massih, R. C., Silveira, F. P. (2015). Safety and tolerability of clofazimine as salvage therapy for atypical mycobacterial infection in solid organ transplant recipients. Transpl Infect. Dis. 17, 111–118. doi: 10.1111/tid.12340
Cattaneo, D., Alffenaar, J. W., Neely, M. (2016). Drug monitoring and individual dose optimization of antimicrobial drugs: oxazolidinones. Expert Opin. Drug Metab. Toxicol. 12, 533–544. doi: 10.1517/17425255.2016.1166204
Cho, Y. S., Lim, H. S., Han, S., Yoon, S. K., Kim, H., Cho, Y. L., et al. (2019). Single-dose intravenous safety, tolerability, and pharmacokinetics and absolute bioavailability of LCB01-0371. Clin. Ther. 41, 92–106. doi: 10.1016/j.clinthera.2018.11.009
Cholo, M. C., Mothiba, M. T., Fourie, B., Anderson, R. (2017). Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J. Antimicrob. Chemother. 72, 338–353. doi: 10.1093/jac/dkw426
Clemett, D., Markham, A. (2000). Linezolid. Drugs 59, 815–827. doi: 10.2165/00003495-200059040-00007
Cowman, S., van Ingen, J., Griffith, D. E., Loebinger, M. R. (2019). Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 54 (1), 1900250. doi: 10.1183/13993003.00250-2019
Cox, E., Laessig, K. (2014). FDA approval of bedaquiline–the benefit-risk balance for drug-resistant tuberculosis. N Engl. J. Med. 371, 689–691. doi: 10.1056/NEJMp1314385
Cui, N., Cai, H., Li, Z., Lu, Y., Wang, G., Lu, A. (2019). Tigecycline-induced coagulopathy: a literature review. Int. J. Clin. Pharm. 41, 1408–1413. doi: 10.1007/s11096-019-00912-5
Daley, C. L., Iaccarino, J. M., Lange, C., Cambau, E., Wallace, R. J., Andrejak, C., et al. (2020). Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin. Infect. Dis. 71, 905–913. doi: 10.1093/cid/ciaa1125
Dawson, R., Diacon, A. H., Narunsky, K., De Jager, V. R., Stinson, K. W., Zhang, X., et al. (2023). Phase I single ascending dose and food effect study in healthy adults and phase I/IIa multiple ascending dose study in patients with pulmonary tuberculosis to assess pharmacokinetics, bactericidal activity, tolerability, and safety of OPC-167832. Antimicrob. Agents Chemother. 67, e0147722. doi: 10.1128/aac.01477-22
Deeks, E. D. (2019). Sarecycline: first global approval. Drugs 79, 325–329. doi: 10.1007/s40265-019-1053-4
Degiacomi, G., Sammartino, J. C., Chiarelli, L. R., Riabova, O., Makarov, V., Pasca, M. R. (2019). Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients. Int. J. Mol. Sci. 20 (23), 5868. doi: 10.3390/ijms20235868
de Melo Carvalho, R., Nunes, A. L., Sa, R., Ramos, I., Valente, C., Saraiva da Cunha, J. (2020). Mycobacterium chimaera disseminated infection. J. Med. cases 11, 35–36. doi: 10.14740/jmc3420
Dierig, A., Hoelscher, M., Schultz, S., Hoffmann, L., Jarchow-MacDonald, A., Svensson, E. M., et al. (2023). A phase IIb, open-label, randomized controlled dose ranging multi-centre trial to evaluate the safety, tolerability, pharmacokinetics and exposure-response relationship of different doses of delpazolid in combination with bedaquiline delamanid moxifloxacin in adult subjects with newly diagnosed, uncomplicated, smear-positive, drug-sensitive pulmonary tuberculosis. Trials 24, 382. doi: 10.1186/s13063-023-07354-5
Dong, W., Li, S., Wen, S., Jing, W., Shi, J., Ma, Y., et al. (2020). In vitro susceptibility testing of GSK656 against mycobacterium species. Antimicrob. Agents Chemother. 64 (2), e01577–19. doi: 10.1128/AAC.01577-19
Dougherty, J. A., Sucher, A. J., Chahine, E. B., Shihadeh, K. C. (2019). Omadacycline: A new tetracycline antibiotic. Ann. Pharmacother. 53, 486–500. doi: 10.1177/1060028018818094
Dupont, C., Viljoen, A., Dubar, F., Blaise, M., Bernut, A., Pawlik, A., et al. (2016). A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol. Microbiol. 101, 515–529. doi: 10.1111/mmi.13406
Edwards, B. D., Field, S. K. (2022). The struggle to end a millennia-long pandemic: novel candidate and repurposed drugs for the treatment of tuberculosis. Drugs 82, 1695–1715. doi: 10.1007/s40265-022-01817-w
Erber, J., Weidlich, S., Tschaikowsky, T., Rothe, K., Schmid, R. M., Schneider, J., et al. (2020). Successful bedaquiline-containing antimycobacterial treatment in post-traumatic skin and soft-tissue infection by Mycobacterium fortuitum complex: a case report. BMC Infect. Dis. 20, 365. doi: 10.1186/s12879-020-05075-7
Fekadu, G., Tolossa, T., Turi, E., Bekele, F., Fetensa, G. (2022). Pretomanid development and its clinical roles in treating tuberculosis. J. Glob Antimicrob. Resist. 31, 175–184. doi: 10.1016/j.jgar.2022.09.001
Frizzell, M., Carr, E., Brust, K. (2020). Omadacycline for treatment of Mycobacterium chelonae skin infection. Proc. (Bayl Univ Med. Cent) 33, 610–611. doi: 10.1080/08998280.2020.1792748
Fujiwara, K., Uesugi, F., Furuuchi, K., Tanaka, Y., Yoshiyama, T., Saotome, M., et al. (2021). Minimum Inhibitory Concentrations before and after Antibacterial Treatment in Patients with Mycobacterium abscessus Pulmonary Disease. Microbiol. Spectr. 9, e0192821. doi: 10.1128/Spectrum.01928-21
Gil, E., Sweeney, N., Barrett, V., Morris-Jones, S., Miller, R. F., Johnston, V. J., et al. (2021). Bedaquiline as treatment for disseminated nontuberculous mycobacteria infection in 2 patients co-infected with HIV. Emerg. Infect. Dis. 27, 944–948. doi: 10.3201/eid2703.202359
Gils, T., Lynen, L., de Jong, B. C., Van Deun, A., Decroo, T. (2022). Pretomanid for tuberculosis: a systematic review. Clin. Microbiol. Infect. 28, 31–42. doi: 10.1016/j.cmi.2021.08.007
Gler, M. T., Skripconoka, V., Sanchez-Garavito, E., Xiao, H., Cabrera-Rivero, J. L., Vargas-Vasquez, D. E., et al. (2012). Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl. J. Med. 366, 2151–2160. doi: 10.1056/NEJMoa1112433
Gotfried, M. H., Horn, K., Garrity-Ryan, L., Villano, S., Tzanis, E., Chitra, S., et al. (2017). Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob. Agents Chemother. 61 (9), e01135–17. doi: 10.1128/AAC.01135-17
Grada, A., Del Rosso, J. Q., Graber, E., Bunick, C. G., Stein Gold, L., Moore, A. Y., et al. (2022). Sarecycline treatment for acne vulgaris: Rationale for weight-based dosing and limited impact of food intake on clinical efficacy. Dermatol. Ther. 35, e15275. doi: 10.1111/dth.15275
Griffith, D. E., Aksamit, T., Brown-Elliott, B. A., Catanzaro, A., Daley, C., Gordin, F., et al. (2007). An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175, 367–416. doi: 10.1164/rccm.200604-571ST
Griffith, D. E., Daley, C. L. (2022). Treatment of mycobacterium abscessus pulmonary disease. Chest 161, 64–75. doi: 10.1016/j.chest.2021.07.035
Guo, S., Wang, B., Fu, L., Chen, X., Zhang, W., Huang, H., et al. (2021). In Vitro and In Vivo Activity of Oxazolidinone Candidate OTB-658 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 65, e0097421. doi: 10.1128/AAC.00974-21
Haidari, W., Bruinsma, R., Cardenas-de la Garza, J. A., Feldman, S. R. (2020). Sarecycline review. Ann. Pharmacother. 54, 164–170. doi: 10.1177/1060028019873111
Hariguchi, N., Chen, X., Hayashi, Y., Kawano, Y., Fujiwara, M., Matsuba, M., et al. (2020). OPC-167832, a novel carbostyril derivative with potent antituberculosis activity as a dprE1 inhibitor. Antimicrob. Agents Chemother. 64 (6), e02020–19. doi: 10.1128/AAC.02020-19
Haubrich, K., Mammen, C., Sekirov, I., Mitchell, H. (2022). Mycobacterium fortuitum peritoneal dialysis-related peritonitis in a child: A case report and review of the literature. J. Assoc. Med. Microbiol. Infect. Dis. Can. 7, 125–130. doi: 10.3138/jammi-2021-0029
Haworth, C. S., Banks, J., Capstick, T., Fisher, A. J., Gorsuch, T., Laurenson, I. F., et al. (2017). British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72, ii1–ii64. doi: 10.1136/thoraxjnl-2017-210929
Heaney, M., Mahoney, M. V., Gallagher, J. C. (2019). Eravacycline: the tetracyclines strike back. Ann. Pharmacother. 53, 1124–1135. doi: 10.1177/1060028019850173
Hirama, T., Singer, L. G., Brode, S. K., Marras, T. K., Husain, S. (2020). Outcomes of a peri- and postoperative management protocol for non-TB mycobacteria in lung transplant recipients. Chest 158, 523–528. doi: 10.1016/j.chest.2020.01.056
Huang, Z., Luo, W., Xu, D., Guo, F., Yang, M., Zhu, Y., et al. (2022). Discovery and preclinical profile of sudapyridine (WX-081), a novel anti-tuberculosis agent. Bioorg Med. Chem. Lett. 71, 128824. doi: 10.1016/j.bmcl.2022.128824
Iqbal, K., Milioudi, A., Wicha, S. G. (2022). Pharmacokinetics and pharmacodynamics of tedizolid. Clin. Pharmacokinet. 61, 489–503. doi: 10.1007/s40262-021-01099-7
Ito, M., Koga, Y., Hachisu, Y., Murata, K., Sunaga, N., Maeno, T., et al. (2022). Treatment strategies with alternative treatment options for patients with Mycobacterium avium complex pulmonary disease. Respir. Investig. 60, 613–624. doi: 10.1016/j.resinv.2022.05.006
Johansen, M. D., Herrmann, J. L., Kremer, L. (2020). Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 18, 392–407. doi: 10.1038/s41579-020-0331-1
Kadura, S., King, N., Nakhoul, M., Zhu, H., Theron, G., Köser, C. U., et al. (2020). Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J. Antimicrob. Chemother. 75, 2031–2043. doi: 10.1093/jac/dkaa136
Kaji, M., Namkoong, H., Nagao, G., Azekawa, S., Nakagawara, K., Tanaka, H., et al. (2023). Nasopharyngeal mycobacterium abscessus infection: A case report and literature review. Infect. Drug Resist. 16, 3955–3963. doi: 10.2147/IDR.S415197
Keam, S. J. (2019). Pretomanid: first approval. Drugs 79, 1797–1803. doi: 10.1007/s40265-019-01207-9
Khoshnood, S., Goudarzi, M., Taki, E., Darbandi, A., Kouhsari, E., Heidary, M., et al. (2021). Bedaquiline: Current status and future perspectives. J. Glob Antimicrob. Resist. 25, 48–59. doi: 10.1016/j.jgar.2021.02.017
Kim, T. S., Choe, J. H., Kim, Y. J., Yang, C. S., Kwon, H. J., Jeong, J., et al. (2017). Activity of LCB01-0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob. Agents Chemother. 61 (9), e02752-16. doi: 10.1128/AAC.02752-16
Kim, D. H., Jhun, B. W., Moon, S. M., Kim, S. Y., Jeon, K., Kwon, O. J., et al. (2019). In vitro activity of bedaquiline and delamanid against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob. Agents Chemother. 63 (8), e00665-19. doi: 10.1128/AAC.00665-19
Kim, D. H., Kim, S. Y., Huh, H. J., Lee, N. Y., Koh, W. J., Jhun, B. W. (2023). In Vitro Activity of Rifamycin Derivatives against Nontuberculous Mycobacteria, including Macrolide-/Amikacin-Resistant Clinical Isolates. Antimicrob. Agents Chemother. 65 (5), e02611-20. doi: 10.1128/AAC.02611-20
Kwak, N., Park, J., Kim, E., Lee, C. H., Han, S. K., Yim, J. J. (2017). Treatment outcomes of mycobacterium avium complex lung disease: A systematic review and meta-analysis. Clin. Infect. Dis. 65, 1077–1084. doi: 10.1093/cid/cix517
LaPlante, K. L., Dhand, A., Wright, K., Lauterio, M. (2022). Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann. Med. 54, 1686–1700. doi: 10.1080/07853890.2022.2085881
Lau, R. J., Lackey, T. G., Samedi, V., Fink, D. S. (2023). Nontuberculous mycobacterial infection of larynx and cervical trachea. Ann. Otol. Rhinol. Laryngol. 132 (11), 1487–1492. doi: 10.1177/00034894231161871:34894231161871
Lee, S. H., Yoo, H. K., Kim, S. H., Koh, W. J., Kim, C. K., Park, Y. K., et al. (2014). The drug resistance profile of Mycobacterium abscessus group strains from Korea. Ann. Lab. Med. 34, 31–37. doi: 10.3343/alm.2014.34.1.31
Lerat, I., Cambau, E., Roth Dit Bettoni, R., Gaillard, J. L., Jarlier, V., Truffot, C., et al. (2014). In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J. Infect. Dis. 209, 905–912. doi: 10.1093/infdis/jit614
Lewis, J. M., Sloan, D. J. (2015). The role of delamanid in the treatment of drug-resistant tuberculosis. Ther. Clin. Risk Manag 11, 779–791. doi: 10.2147/TCRM.S71076
Lin, C., Russell, C., Soll, B., Chow, D., Bamrah, S., Brostrom, R., et al. (2018). Increasing prevalence of nontuberculous mycobacteria in respiratory specimens from US-affiliated pacific island jurisdictions(1). Emerg. Infect. Dis. 24, 485–491. doi: 10.3201/eid2403.171301
Liu, Y., Tan, Y., Wei, G., Lu, Z., Liu, Y., Yang, B., et al. (2022). Safety and pharmacokinetic profile of pretomanid in healthy Chinese adults: Results of a phase I single dose escalation study. Pulm Pharmacol. Ther. 73-74, 102132. doi: 10.1016/j.pupt.2022.102132
Luo, J., Yu, X., Jiang, G., Fu, Y., Huo, F., Ma, Y., et al. (2018). In vitro activity of clofazimine against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob. Agents Chemother. 62 (7), e00072-18. doi: 10.1128/AAC.00072-18
Martin, A., Godino, I. T., Aguilar-Ayala, D. A., Mathys, V., Lounis, N., Villalobos, H. R. (2019). In vitro activity of bedaquiline against slow-growing nontuberculous mycobacteria. J. Med. Microbiol. 68, 1137–1139. doi: 10.1099/jmm.0.001025
Martiniano, S. L., Wagner, B. D., Levin, A., Nick, J. A., Sagel, S. D., Daley, C. L. (2017). Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 152, 800–809. doi: 10.1016/j.chest.2017.04.175
Märtson, A. G., Burch, G., Ghimire, S., Alffenaar, J. C., Peloquin, C. A. (2021). Therapeutic drug monitoring in patients with tuberculosis and concurrent medical problems. Expert Opin. Drug Metab. Toxicol. 17, 23–39. doi: 10.1080/17425255.2021.1836158
Matsumoto, M., Hashizume, H., Tomishige, T., Kawasaki, M., Tsubouchi, H., Sasaki, H., et al. (2006). OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PloS Med. 3, e466. doi: 10.1371/journal.pmed.0030466
Mingora, C. M., Bullington, W., Faasuamalie, P. E., Levin, A., Porter, G., Stadnick, R., et al. (2023). Long-term safety and tolerability of omadacycline for the treatment of mycobacterium abscessus infections. Open Forum Infect. Dis. 10, ofad335. doi: 10.1093/ofid/ofad335
Mirnejad, R., Asadi, A., Khoshnood, S., Mirzaei, H., Heidary, M., Fattorini, L., et al. (2018). Clofazimine: A useful antibiotic for drug-resistant tuberculosis. BioMed. Pharmacother. 105, 1353–1359. doi: 10.1016/j.biopha.2018.06.023
Moguillansky, N., DeSear, K., Dousa, K. M. (2023). A 40-year-old female with mycobacterium abscessus successfully treated with a dual beta-lactam combination. Cureus 15, e40993. doi: 10.7759/cureus.40993
Mok, J., Lee, M., Kim, D. K., Kim, J. S., Jhun, B. W., Jo, K. W., et al. (2022). 9 months of delamanid, linezolid, levofloxacin, and pyrazinamide versus conventional therapy for treatment of fluoroquinolone-sensitive multidrug-resistant tuberculosis (MDR-END): a multicentre, randomised, open-label phase 2/3 non-inferiority trial in South Korea. Lancet 400, 1522–1530. doi: 10.1016/S0140-6736(22)01883-9
Moran, G. J., Fang, E., Corey, G. R., Das, A. F., De Anda, C., Prokocimer, P. (2014). Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 14, 696–705. doi: 10.1016/S1473-3099(14)70737-6
Morrisette, T., Alosaimy, S., Philley, J. V., Wadle, C., Howard, C., Webb, A. J., et al. (2021). Preliminary, real-world, multicenter experience with omadacycline for mycobacterium abscessus infections. Open Forum Infect. Dis. 8, ofab002. doi: 10.1093/ofid/ofab002
Mudde, S. E., Upton, A. M., Lenaerts, A., Bax, H. I., De Steenwinkel, J. E. M. (2022). Delamanid or pretomanid? A Solomonic judgement! J. Antimicrob. Chemother. 77, 880–902. doi: 10.1093/jac/dkab505
Newman, J. V., Zhou, J., Izmailyan, S., Tsai, L. (2019). Mass balance and drug interaction potential of intravenous eravacycline administered to healthy subjects. Antimicrob. Agents Chemother. 63 (3), e01810-18. doi: 10.1128/AAC.01810-18
Ng, H. F., Ngeow, Y. F. (2022). Genetic determinants of tigecycline resistance in mycobacteroides abscessus. Antibiotics (Basel) 11 (5), 572. doi: 10.3390/antibiotics11050572
Pandya, A. N., Prathipati, P. K., Hegde, P., Li, W., Graham, K. F., Mandal, S., et al. (2019). Indole-2-Carboxamides Are Active against Mycobacterium abscessus in a Mouse Model of Acute Infection. Antimicrob. Agents Chemother. 63 (3), e02245-18. doi: 10.1128/AAC.02245-18
Pang, Y., Zheng, H., Tan, Y., Song, Y., Zhao, Y. (2017). In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob. Agents Chemother. 61 (5), e02627-16. doi: 10.1128/AAC.02627-16
Park, Y., Lee, E. H., Jung, I., Park, G., Kang, Y. A. (2019). Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium avium complex pulmonary disease: a systematic review and meta-analysis. Respir. Res. 20, 286. doi: 10.1186/s12931-019-1258-9
Pasipanodya, J. G., Ogbonna, D., Ferro, B. E., Magombedze, G., Srivastava, S., Deshpande, D., et al. (2017). Systematic review and meta-analyses of the effect of chemotherapy on pulmonary mycobacterium abscessus outcomes and disease recurrence. Antimicrob. Agents Chemother. 61 (11), e01206–17. doi: 10.1128/AAC.01206-17
Peloquin, C. A., Davies, G. R. (2021). The treatment of tuberculosis. Clin. Pharmacol. Ther. 110, 1455–1466. doi: 10.1002/cpt.2261
Pennings, L. J., Ruth, M. M., Wertheim, H., van Ingen, J. (2021). The benzimidazole SPR719 shows promising concentration-dependent activity and synergy against nontuberculous mycobacteria. Antimicrob. Agents Chemother. 65 (4), e02469-20. doi: 10.1128/AAC.02469-20
Pfaeffle, H. O. I., Alameer, R. M., Marshall, M. H., Houpt, E. R., Albon, D. P., Heysell, S. K. (2021). Clofazimine for treatment of multidrug-resistant non-tuberculous mycobacteria. Pulm Pharmacol. Ther. 70, 102058. doi: 10.1016/j.pupt.2021.102058
Philley, J. V., Wallace, R. J., Jr., Benwill, J. L., Taskar, V., Brown-Elliott, B. A., Thakkar, F., et al. (2015). Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 148, 499–506. doi: 10.1378/chest.14-2764
Pidot, S. J., Porter, J. L., Lister, T., Stinear, T. P. (2021). In vitro activity of SPR719 against Mycobacterium ulcerans, Mycobacterium marinum and Mycobacterium chimaera. PloS Negl. Trop. Dis. 15, e0009636. doi: 10.1371/journal.pntd.0009636
Pinapala, A., Koh, L. J., Ng, K. H., Tambyah, P. A., Yap, H. K. (2021). Clofazimine in Mycobacterium abscessus peritonitis: A pediatric case report. Perit Dial Int. 41, 104–109. doi: 10.1177/0896860820909702
Poon, Y. K., La Hoz, R. M., Hynan, L. S., Sanders, J., Monogue, M. L. (2021). Tedizolid vs linezolid for the treatment of nontuberculous mycobacteria infections in solid organ transplant recipients. Open Forum Infect. Dis. 8, ofab093. doi: 10.1093/ofid/ofab093
Prokocimer, P., De Anda, C., Fang, E., Mehra, P., Das, A. (2013). Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. Jama 309, 559–569. doi: 10.1001/jama.2013.241
Pstragowski, M., Zbrzezna, M., Bujalska-Zadrozny, M. (2017). ADVANCES IN PHARMACOTHERAPY OF TUBERCULOSIS. Acta Pol. Pharm. 74, 3–11.
Raynaud, C., Daher, W., Roquet-Banères, F., Johansen, M. D., Stec, J., Onajole, O. K., et al. (2020). Synergistic Interactions of Indole-2-Carboxamides and β-Lactam Antibiotics against Mycobacterium abscessus. Antimicrob. Agents Chemother. 64 (5), e02548-19. doi: 10.1128/AAC.02548-19
Richard, M., Gutiérrez, A. V., Kremer, L. (2020). Dissecting erm(41)-Mediated Macrolide-Inducible Resistance in Mycobacterium abscessus. Antimicrob. Agents Chemother. 64 (2), e01879-19. doi: 10.1128/AAC.01879-19
Rizzo, A. R., Moniri, N. H. (2022). Omadacycline for management of Mycobacterium abscessus infections: a review of its effectiveness, place in therapy, and considerations for use. BMC Infect. Dis. 22, 874. doi: 10.1186/s12879-022-07857-7
Robertson, G. T., Ramey, M. E., Massoudi, L. M., Carter, C. L., Zimmerman, M., Kaya, F., et al. (2021). Comparative analysis of pharmacodynamics in the C3HeB/feJ mouse tuberculosis model for dprE1 inhibitors TBA-7371, PBTZ169, and OPC-167832. Antimicrob. Agents Chemother. 65, e0058321. doi: 10.1128/AAC.00583-21
Rubinstein, E., Vaughan, D. (2005). Tigecycline: a novel glycylcycline. Drugs 65, 1317–1336. doi: 10.2165/00003495-200565100-00002
Ruth, M. M., Koeken, V., Pennings, L. J., Svensson, E. M., Wertheim, H. F. L., Hoefsloot, W., et al. (2020). Is there a role for tedizolid in the treatment of non-tuberculous mycobacterial disease? J. Antimicrob. Chemother. 75, 609–617. doi: 10.1093/jac/dkz511
Santin, M., Barrabeig, I., Malchair, P., Gonzalez-Luquero, L., Benitez, M. A., Sabria, J., et al. (2018). Pulmonary infections with nontuberculous mycobacteria, catalonia, Spain, 1994-2014. Emerg. Infect. Dis. 24, 1091–1094. doi: 10.3201/eid2406.172095
Sarathy, J. P., Zimmerman, M. D., Gengenbacher, M., Dartois, V., Dick, T. (2022). Mycobacterium tuberculosis DprE1 Inhibitor OPC-167832 Is Active against Mycobacterium abscessus In Vitro. Antimicrob. Agents Chemother. 66, e0123722. doi: 10.1128/aac.01237-22
Shaw, T. D., Smyth, M., Turner, G., Hunter, M. (2021). Prolonged tedizolid use in cutaneous non-tuberculous mycobacterial infection. J. Clin. Tuberc Other Mycobact Dis. 24, 100261. doi: 10.1016/j.jctube.2021.100261
Shi, J., Lu, J., Wen, S., Zong, Z., Huo, F., Luo, J., et al. (2018). In vitro activity of PBTZ169 against multiple mycobacterium species. Antimicrob. Agents Chemother. 62 (11), e01314-18. doi: 10.1128/AAC.01314-18
Shoen, C., Benaroch, D., Sklaney, M., Cynamon, M. (2019). In vitro activities of omadacycline against rapidly growing mycobacteria. Antimicrob. Agents Chemother. 63 (5), e02522-18. doi: 10.1128/AAC.02522-18
Shumway, C., Aggarwal, S., Park, S. T., Wade, M., Kedhar, S. (2020). Complicated case of Mycobacterium abscessus conjunctivitis in Sjögren's syndrome. Am. J. Ophthalmol. Case Rep. 19, 100765. doi: 10.1016/j.ajoc.2020.100765
Stadler, J. A. M., Maartens, G., Meintjes, G., Wasserman, S. (2023). Clofazimine for the treatment of tuberculosis. Front. Pharmacol. 14, 1100488. doi: 10.3389/fphar.2023.1100488
Szumowski, J. D., Lynch, J. B. (2015). Profile of delamanid for the treatment of multidrug-resistant tuberculosis. Drug Des. Devel Ther. 9, 677–682. doi: 10.2147/DDDT.S60923
Talley, A. K., Thurston, A., Moore, G., Gupta, V. K., Satterfield, M., Manyak, E., et al. (2021). First-in-human evaluation of the safety, tolerability, and pharmacokinetics of SPR720, a novel oral bacterial DNA gyrase (GyrB) inhibitor for mycobacterial infections. Antimicrob. Agents Chemother. 65, e0120821. doi: 10.1128/AAC.01208-21
Tasneen, R., Garcia, A., Converse, P. J., Zimmerman, M. D., Dartois, V., Kurbatova, E., et al. (2022). Novel regimens of bedaquiline-pyrazinamide combined with moxifloxacin, rifabutin, delamanid and/or OPC-167832 in murine tuberculosis models. Antimicrob. Agents Chemother. 66, e0239821. doi: 10.1128/aac.02398-21
Thorell, E. A., Sharma, M., Jackson, M. A., Selvarangan, R., Woods, G. M. (2006). Disseminated nontuberculous mycobacterial infections in sickle cell anemia patients. J. Pediatr. Hematol. Oncol. 28, 678–681. doi: 10.1097/01.mph.0000243646.59111.28
Tucker, M. K., Droemer, L., Condren, M. (2023). Use of omadacycline as a component of mycobacterium abscessus eradication in an adolescent with cystic fibrosis. J. Pediatr. Pharmacol. Ther. 28, 172–176. doi: 10.5863/1551-6776-28.2.172
Unai, S., Miessau, J., Karbowski, P., Bajwa, G., Hirose, H. (2013). Sternal wound infection caused by Mycobacterium chelonae. J. Card Surg. 28, 687–692. doi: 10.1111/jocs.12194
Wallace, R. J., Jr., Brown-Elliott, B. A., Crist, C. J., Mann, L., Wilson, R. W. (2002). Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob. Agents Chemother. 46, 3164–3167. doi: 10.1128/AAC.46.10.3164-3167.2002
Wallace, R. J., Jr., Dukart, G., Brown-Elliott, B. A., Griffith, D. E., Scerpella, E. G., Marshall, B. (2014). Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J. Antimicrob. Chemother. 69, 1945–1953. doi: 10.1093/jac/dku062
Wallis, R. S., Dawson, R., Friedrich, S. O., Venter, A., Paige, D., Zhu, T., et al. (2014). Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PloS One 9, e94462. doi: 10.1371/journal.pone.0094462
Wen, S., Gao, X., Zhao, W., Huo, F., Jiang, G., Dong, L., et al. (2021). Comparison of the in vitro activity of linezolid, tedizolid, sutezolid, and delpazolid against rapidly growing mycobacteria isolated in Beijing, China. Int. J. Infect. Dis. 109, 253–260. doi: 10.1016/j.ijid.2021.06.055
Wu, M. L., Aziz, D. B., Dartois, V., Dick, T. (2018). NTM drug discovery: status, gaps and the way forward. Drug Discovery Today 23, 1502–1519. doi: 10.1016/j.drudis.2018.04.001
Xiao, H., Yu, X., Shang, Y., Ren, R., Xue, Y., Dong, L., et al. (2023). In vitro and intracellular antibacterial activity of sudapyridine (WX-081) against tuberculosis. Infect. Drug Resist. 16, 217–224. doi: 10.2147/IDR.S390187
Xu, H. B., Jiang, R. H., Li, L. (2014). Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 33, 347–358. doi: 10.1007/s10096-013-1962-1
Yadav, S., Soni, A., Tanwar, O., Bhadane, R., Besra, G. S., Kawathekar, N. (2023). DprE1 inhibitors: enduring aspirations for future antituberculosis drug discovery. ChemMedChem. 18 (16), e202300099. doi: 10.1002/cmdc.202300099:e202300099
Yao, R., Wang, B., Fu, L., Li, L., You, K., Li, Y. G., et al. (2022). Sudapyridine (WX-081), a Novel Compound against Mycobacterium tuberculosis. Microbiol. Spectr. 10, e0247721. doi: 10.1128/spectrum.02477-21
Yuan, S, Shen, DD, Bai, YR, Zhang, M, Zhou, T, Sun, C, et al. (2023). Oxazolidinone: A promising scaffold for the development of antibacterial drugs. Eur J Med Chem. 250, 115239. doi: 10.1016/j.ejmech.2023.115239
Yu, X., Gao, X., Li, C., Luo, J., Wen, S., Zhang, T., et al. (2019). In vitro activities of bedaquiline and delamanid against nontuberculous mycobacteria isolated in beijing, China. Antimicrob. Agents Chemother. 63 (8), e00031-19. doi: 10.1128/AAC.00031-19
Yu, X., Liu, P., Liu, G., Zhao, L., Hu, Y., Wei, G., et al. (2016). The prevalence of non-tuberculous mycobacterial infections in mainland China: Systematic review and meta-analysis. J. Infect. 73, 558–567. doi: 10.1016/j.jinf.2016.08.020
Yuste, J. R., Bertó, J., Del Pozo, J. L., Leiva, J. (2017). Prolonged use of tedizolid in a pulmonary non-tuberculous mycobacterial infection after linezolid-induced toxicity. J. Antimicrob. Chemother. 72, 625–628. doi: 10.1093/jac/dkw484
Zhanel, G. G., Cheung, D., Adam, H., Zelenitsky, S., Golden, A., Schweizer, F., et al. (2016). Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 76, 567–588. doi: 10.1007/s40265-016-0545-8
Zhanel, G. G., Esquivel, J., Zelenitsky, S., Lawrence, C. K., Adam, H. J., Golden, A., et al. (2020). Omadacycline: A novel oral and intravenous aminomethylcycline antibiotic agent. Drugs 80, 285–313. doi: 10.1007/s40265-020-01257-4
Zhanel, G. G., Love, R., Adam, H., Golden, A., Zelenitsky, S., Schweizer, F., et al. (2015). Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens. Drugs 75, 253–270. doi: 10.1007/s40265-015-0352-7
Zhang, J., Ba, Y., Wang, S., Yang, H., Hou, X., Xu, Z. (2019). Nitroimidazole-containing compounds and their antibacterial and antitubercular activities. Eur. J. Med. Chem. 179, 376–388. doi: 10.1016/j.ejmech.2019.06.068
Zhang, T., Du, J., Dong, L., Wang, F., Zhao, L., Jia, J., et al. (2023). In vitro antimicrobial activities of tigecycline, eravacycline, omadacycline, and sarecycline against rapidly growing mycobacteria. Microbiol. Spectr. 11, e0323822. doi: 10.1128/spectrum.03238-22
Zheng, L., Qi, X., Zhang, W., Wang, H., Fu, L., Wang, B., et al. (2023). Efficacy of PBTZ169 and pretomanid against Mycobacterium avium, Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum in BALB/c mice models. Front. Cell Infect. Microbiol. 13, 1115530. doi: 10.3389/fcimb.2023.1115530
Zhou, L., Xu, D., Liu, H., Wan, K., Wang, R., Yang, Z. (2020). Trends in the prevalence and antibiotic resistance of non-tuberculous mycobacteria in mainland China, 2000-2019: systematic review and meta-analysis. Front. Public Health 8, 295. doi: 10.3389/fpubh.2020.00295
Zhu, R., Shang, Y., Chen, S., Xiao, H., Ren, R., Wang, F., et al. (2022). In vitro activity of the sudapyridine (WX-081) against non-tuberculous mycobacteria isolated in Beijing, China. Microbiol. Spectr. 10, e0137222. doi: 10.1128/spectrum.01372-22
Keywords: NTM (nontuberculous mycobacteria), antibiotic, inhibitor, NTM pulmonary disease, treatment
Citation: Gu Y, Nie W, Huang H and Yu X (2023) Non-tuberculous mycobacterial disease: progress and advances in the development of novel candidate and repurposed drugs. Front. Cell. Infect. Microbiol. 13:1243457. doi: 10.3389/fcimb.2023.1243457
Received: 20 June 2023; Accepted: 11 September 2023;
Published: 02 October 2023.
Edited by:
Zichen Yang, Xinqiao Hospital, ChinaReviewed by:
Abhishek Mishra, Houston Methodist Research Institute, United StatesMartin I. Voskuil, University of Colorado Denver, United States
Copyright © 2023 Gu, Nie, Huang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Yu, eXV4aWFAbWFpbC5jY211LmVkdS5jbg==; Hairong Huang, aHVhbmdoYWlyb25nQHRiMTIzLm9yZw==; Wenjuan Nie, d2VuanVhbi5uaWVAb3V0bG9vay5jb20=
 Yuzhen Gu1
Yuzhen Gu1 Hairong Huang
Hairong Huang Xia Yu
Xia Yu