
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 30 August 2023
Sec. Molecular Bacterial Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1237164
This article is part of the Research Topic Bacterial Surface Polymers View all 5 articles
 Elena K. Perry
Elena K. Perry Man-Wah Tan*
Man-Wah Tan*Bacterial biofilms can be found in most environments on our planet, and the human body is no exception. Consisting of microbial cells encased in a matrix of extracellular polymers, biofilms enable bacteria to sequester themselves in favorable niches, while also increasing their ability to resist numerous stresses and survive under hostile circumstances. In recent decades, biofilms have increasingly been recognized as a major contributor to the pathogenesis of chronic infections. However, biofilms also occur in or on certain tissues in healthy individuals, and their constituent species are not restricted to canonical pathogens. In this review, we discuss the evidence for where, when, and what types of biofilms occur in the human body, as well as the diverse ways in which they can impact host health under homeostatic and dysbiotic states.
Bacteria have historically been studied as single-celled planktonic organisms, but outside of the laboratory, most bacteria live in organized multicellular communities embedded in a matrix of extracellular polymers, called biofilms (Flemming and Wuertz, 2019). Biofilms were arguably discovered as early as the 1680s, when Antonie van Leeuwenhoek described the presence of microorganisms and fibrous structures in the “scurf of the teeth” (i.e. dental plaque) (Leeuwenhoek, 1684). Yet it was not until the 1970s that the presence of bacterial biofilms began to be recognized in other sites within the human body (Høiby, 2017), most often through the use of techniques such as electron microscopy and fluorescent in situ hybridization (FISH) coupled with confocal laser scanning microscopy. Over the past few decades, biofilm-containing samples have been acquired from numerous tissues that are classically thought to be sterile, where biofilms are generally associated with infections (Figure 1, Table 1). Biofilms have also been found on tissues that have long been known to harbor commensal microbiota, where the biofilms may or may not be pathogenic (Figure 1, Table 1). In some types of samples, biofilms primarily occur as aggregates suspended in mucus or other host secretions (Figure 2A, Table 1). In others, the biofilms are attached to the tissue itself—typically at a mucosal interface, although there are exceptions (Figure 2B, Table 1). In spite of their morphological differences, suspended aggregates and surface-attached bacterial communities share many functional properties, suggesting that both types of biofilms represent fundamentally similar modes of bacterial existence (Alhede et al., 2011).
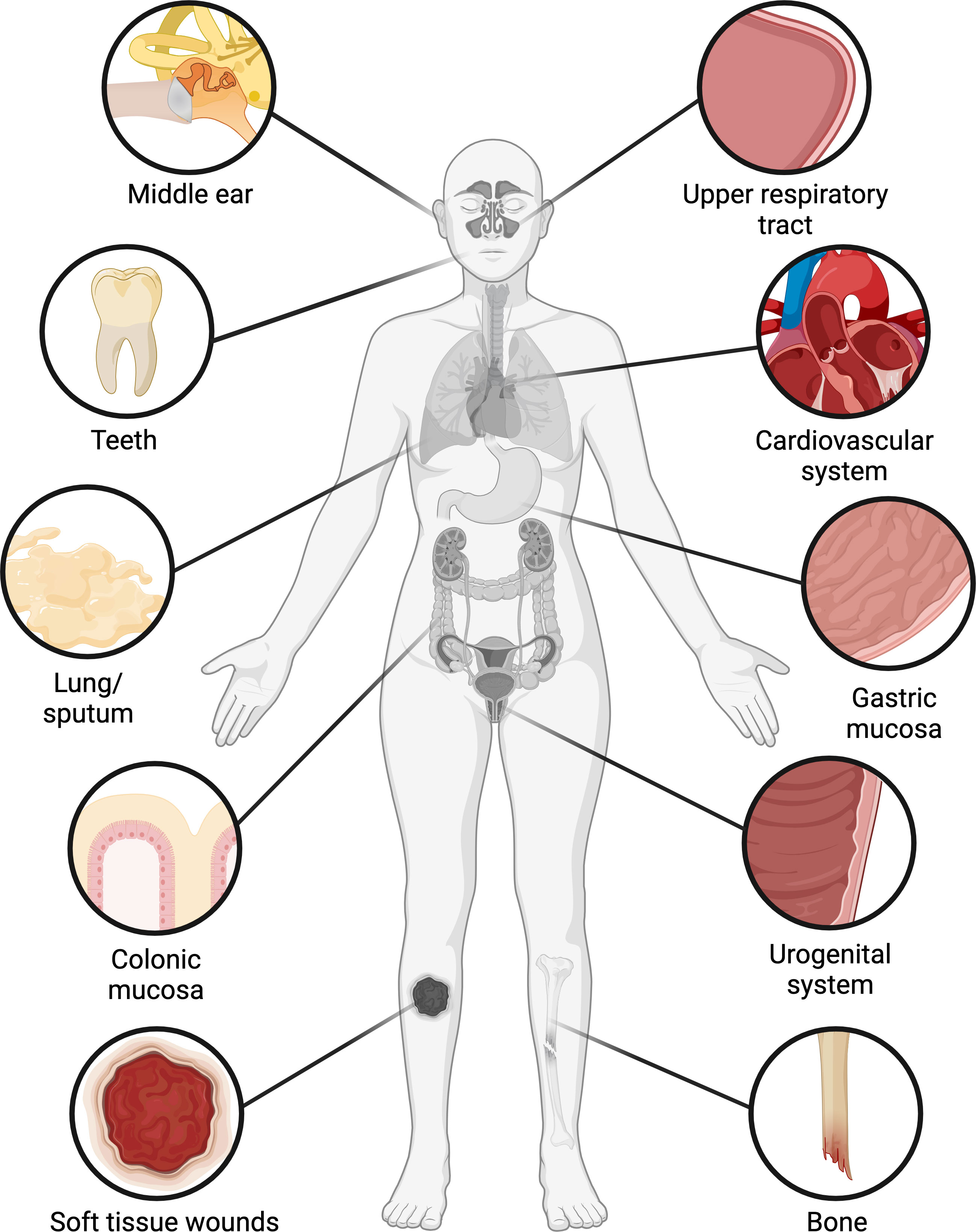
Figure 1 Occurrence of biofilms in the human body. Biofilms have been found in numerous organs and tissues, including the middle ear and upper respiratory tract, oral cavity, cardiovascular system, lung, stomach, colon, urogenital system, bone, and soft tissue wounds. In the oral cavity, colon, and female reproductive tract, biofilms occur with varying frequencies in clinically healthy patients as well as those with infections or underlying conditions, while in other tissues biofilms are generally associated with infections. Figure created with BioRender.com.
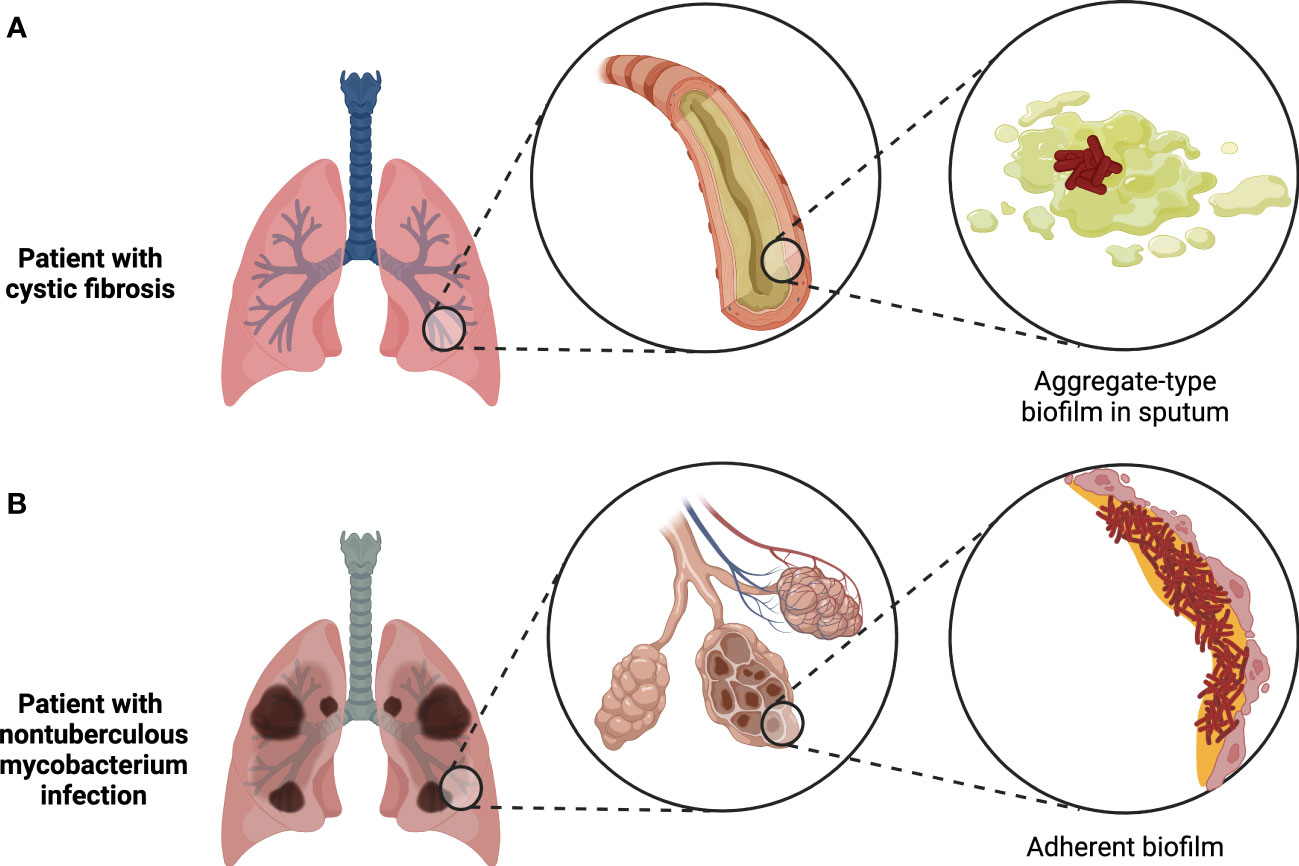
Figure 2 Two major types of biofilms found in the human body. Tissue-associated biofilms generally either take the form of bacterial aggregates suspended in mucus or other host secretions, as in the case of biofilms found in cystic fibrosis patient sputum (A), or adherent biofilms attached to the surface of the tissue, as in the case of biofilms formed by nontuberculous mycobacteria during chronic infections (B). Figure created with BioRender.com.
In this review, we first describe where biofilms have been found in the human body and under what circumstances, as well as which taxa are present; we focus on tissue-associated biofilms rather than biofilms associated with implanted medical devices, which have been recently reviewed elsewhere (Khatoon et al., 2018; Yadav et al., 2020). We then summarize what is known about surface-associated and secreted polymers involved in biofilm formation by human pathogens and commensal bacteria, and how environmental factors influence biofilm development in vivo. We present an overview of the ways in which biofilms have been proposed to impact human health, including supporting evidence. Finally, we discuss key limitations of current knowledge about tissue-associated biofilms and highlight the importance of developing novel experimental models to address major open questions in the field.
One of the longest-recognized sites of infection-associated biofilms is the lower respiratory tract, where the discovery of bacterial aggregates suspended in sputum was first reported in chronically-infected cystic fibrosis (CF) patients 50 years ago (Høiby and Axelsen, 1973; Høiby, 2017). Intriguingly, recent evidence suggests that aggregate-type biofilms in sputum may be common not only in chronic lung infections, as is now well-accepted, but also in acute respiratory infections, where planktonic bacteria have long been assumed to be the dominant form (Kolpen et al., 2022). However, virtually all investigations of biofilms in the lung to date have focused on chronic infections. The most common biofilm-forming pathogen found in CF patients is P. aeruginosa (Bjarnsholt et al., 2009; Williams and Davies, 2012), but aggregates of Staphylococcus or Streptococcus species have also been detected in optically-cleared CF patient sputum samples using genus-specific FISH probes (DePas et al., 2016). In the latter study, Pseudomonas occurred as a bimodal mix of small and large aggregates (<50 µm3 or >1000 µm3, respectively), while Staphylococcus occurred in small to medium-sized aggregates (50-1000 µm3), and Streptococcus primarily existed in large aggregates (>1000 µm3), some of which encased host cells. Besides the taxa that have been definitively detected by FISH in CF sputum biofilms, strains of Burkholderia cepacia complex species isolated from CF patients are frequently proficient at forming biofilms in vitro and may also be capable of forming biofilms in vivo (Cunha et al., 2004); the lack of attempts at direct detection of Burkholderia in CF sputum biofilms by FISH to date may be due to the relatively low prevalence of this clade in CF infections (Salsgiver et al., 2016). The observations published thus far suggest that individual bacterial aggregates in CF sputum tend to be monospecies (Bjarnsholt et al., 2009; DePas et al., 2016), even though patients can be co-infected by multiple species simultaneously and mixed-species biofilms can be formed by common CF pathogens in vitro (Pompilio et al., 2015; Magalhães et al., 2022). In addition, while most biofilms in CF patients are thought to occur in sputum, biofilms of Mycobacterium abscessus have been detected in the interstitial spaces of the alveolar wall in chronically-infected CF patients (Qvist et al., 2015).
Beyond CF patients, lower airway biofilms have been detected in other long-term respiratory infections, such as bronchiectasis and protracted bacterial bronchitis, where the represented species include Haemophilus influenzae, Moraxella catarrhalis, P. aeruginosa, Streptococcus pneumoniae, and Staphylococcus species (Marsh et al., 2022). As with the biofilms found in CF patients, other lung infection-associated biofilms typically occur as suspended aggregates in sputum (Kolpen et al., 2022). However, adherent biofilms may play a role in the pathogenesis of chronic pulmonary infections by nontuberculous mycobacteria, given that M. abscessus biofilms were observed lining a resected lung cavity from an infected patient with chronic obstructive pulmonary disease (Fennelly et al., 2016). Mycobacterium tuberculosis has similarly been proposed to form extracellular biofilms in necrotic lesions and lung cavities (Orme, 2014; Basaraba and Ojha, 2017). Indeed, dense masses and sheets of bacteria that morphologically resemble biofilms have been directly observed in resected lung sections from tuberculosis patients (Nyka, 1963; Nyka and O’Neill, 1970). More recently, clusters of mycobacterial cells in tuberculosis-infected human lungs were shown to be encased by an exopolysaccharide-containing matrix, further supporting their biofilm-like character (Chakraborty et al., 2021). Nevertheless, the overall contribution of biofilm formation to mycobacterial pathogenesis remains poorly understood.
Infection-associated biofilms have also been detected in the middle ear and upper respiratory tract, which are connected by the eustachian tube. In the middle ear, biofilms ranging from microcolonies to large clusters of bacteria have been detected at a high frequency in patients with chronic otitis media, both in aspirate samples and mucosal biopsies, but only very rarely in patients with no known history of ear infection (Hall-Stoodley et al., 2006; Homøe et al., 2009; Lee et al., 2009). Pathogens detected in chronic otitis media-associated biofilms include H. influenzae, M. catarrhalis, S. pneumoniae, Klebsiella pneumoniae, and Staphylococcus aureus, all of which are also common colonizers of the upper respiratory tract. Similar to the biofilms observed in lung sputum samples, discrete biofilms observed in chronic otitis media often appear to be monospecies, even in patients co-infected with multiple pathogens (Hall-Stoodley et al., 2006). Elsewhere in the upper respiratory tract, biofilms have been detected in the sinus mucosa, adenoids, and tonsils (Morris, 2007)—typically in patients with chronic rhinosinusitis or recurrent upper respiratory tract infections, although some studies also reported the presence of putative biofilms in a smaller proportion of patients with non-infectious nasal obstruction (Sanderson et al., 2006; Bezerra et al., 2011).
Outside of the respiratory tract, several studies have reported the presence of bacterial biofilms in chronic or slow-healing soft tissue wounds (Bjarnsholt et al., 2008; James et al., 2008; Kennedy et al., 2010; Fazli et al., 2011; Han et al., 2011; Percival et al., 2015). Wound-colonizing biofilms range in appearance from microcolonies to aggregates to thick, continuous biofilms (Metcalf and Bowler, 2013; Percival et al., 2015). The latter may even be macroscopically visible in some cases (Metcalf and Bowler, 2013), although this remains a point of controversy in the field (White and Cutting, 2012). Biofilms in chronic wounds may be either monospecies or multispecies, with two of the most commonly detected species being P. aeruginosa and S. aureus (Metcalf and Bowler, 2013; Percival et al., 2015). Biofilms have also been found in bone infections (osteomyelitis), which usually follow trauma or occur in tissues that are otherwise compromised, such as through vascular damage (Gristina et al., 1985; Sedghizadeh et al., 2009). Scanning electron microscopy of samples from patients with osteomyelitis of the femur, tibia, or fibula revealed thick, extensive multispecies biofilms coating the surface of the bone, while transmission electron microscopy revealed microcolonies attached to host cells derived from the surrounding tissue (Gristina et al., 1985). The biofilms appeared to be multispecies, due to the presence of different morphotypes such as cocci and rods, and culture results revealed a mixture of species in most patients, including S. aureus, P. aeruginosa, Serratia marcescens, and Bacteroides species (Gristina et al., 1985). Scanning electron microscopy and confocal microscopy similarly revealed dense aggregates of bacteria on the bone surface in patients with diabetic foot osteomyelitis (Johani et al., 2019). Most of the observed biofilms appeared to be multispecies based on the presence of both cocci and rods, and 16S rRNA gene amplicon sequencing revealed that the most abundant genera included Corynebacterium, Finegoldia, Staphylococcus, Streptococcus, Porphyromonas, and Anaerococcus (Johani et al., 2019). Biofilms were also present in patients with osteomyelitis or osteonecrosis of the jawbone, covering large areas of both the internal and external surfaces of the bone (Sedghizadeh et al., 2009). Based on examination of the bacterial morphology by scanning electron microscopy, the biofilms in the jawbone osteomyelitis patients appeared to be predominantly comprised of Actinomyces, a genus commonly found in the oral cavity, while the biofilms in osteonecrosis were multispecies and contained morphotypes consistent with other typical oral bacteria such as Fusobacterium and Streptococcus, in addition to Actinomyces (Sedghizadeh et al., 2009).
In the urogenital system, extracellular-matrix-encased microcolonies of adherent bacteria have been detected in biopsies from male patients with chronic prostatitis (Nickel and Costerton, 1992; Nickel and Costerton, 1993). In addition, Escherichia coli has been shown in a mouse model to form biofilm-like “pods” inside bladder epithelial cells during urinary tract infections (Anderson et al., 2003). Although invasive biopsies are not typically performed for urinary tract infections, analysis of urine from actively infected patients revealed biofilm-like bacterial aggregates and putative intracellular bacterial communities within exfoliated host cells, suggesting that similar mechanisms of pathogenesis also occur in humans (Rosen et al., 2007). Higher up in the urinary tract, biofilms have been observed on the surface and in the interior of kidney stones (Nickel et al., 1985; McLean et al., 1989; Romanova et al., 2015). Typical organisms associated with kidney stone biofilms include P. aeruginosa, E. coli, K. pneumoniae, Proteus mirabilis, Staphylococcus species, and Enterococcus species (Nickel et al., 1985; Romanova et al., 2015). Some of these species possess urease activity (Romanova et al., 2015), which precipitates the formation of specific minerals found in kidney stones (McLean et al., 1989). Moreover, biofilm extracellular matrix components have been proposed to play a role in cementing nascent mineral crystals during kidney stone formation (McLean et al., 1989).
Finally, biofilms have been detected in the circulatory system in the context of infections of the heart (endocarditis) and in atherosclerotic arteries. Infective endocarditis occurs when bacteria—most often S. aureus, Streptococcus species, or Enterococcus species—enter the bloodstream and subsequently attach to the heart valves or inner lining of the heart chambers, typically in patients with congenital valve abnormalities or damaged heart tissue (Cabell et al., 2003; Lerche et al., 2021). During the course of disease progression, irregular masses of tissue form on the heart lining, which are called “vegetations” (Cabell et al., 2003; Lerche et al., 2021). Examination of vegetations by transmission electron microscopy revealed masses of bacteria encased by an extracellular matrix (Marrie et al., 1987). Large biofilm-like clusters of bacteria within infected heart valve tissue have also been identified using FISH and confocal microscopy on biopsies from endocarditis patients (Mallmann et al., 2010). Similarly, in atherosclerotic arteries, FISH performed with a universal bacterial probe revealed the presence of biofilm-like microcolonies of bacteria both within the arterial tissue and between the vascular smooth muscle and the luminal plaque (Lanter et al., 2014; Snow et al., 2016).
In organ systems that are colonized by a commensal microbiota in healthy individuals, such as the female reproductive tract and gastrointestinal tract, the presence of a biofilm is not always correlated with infection or injury. For example, loosely-attached conglomerates of commensal Lactobacillus species, which potentially represent aggregate-type biofilms, can sometimes be found in vaginal biopsies from healthy patients (Swidsinski et al., 2005a). On the other hand, dense biofilms that firmly adhere to the vaginal wall and mainly consist of Gardnerella vaginalis and Fannyhessea vaginae are characteristic of bacterial vaginosis—yet such biofilms are also found in a minority of asymptomatic individuals (Swidsinski et al., 2005a; Hardy et al., 2015). In the gastrointestinal tract, oral biofilms are by far the most extensively studied due to the accessibility of samples and the fact that dental plaque—a form of multispecies biofilm—is ubiquitous even in healthy individuals. The diversity of oral bacteria in humans spans at least several hundred species and nine phyla (Zijnge et al., 2010), and different community compositions have been associated with healthy versus disease states (e.g. periodontitis) (Socransky et al., 1998; Griffen et al., 2012). Nevertheless, the development of dental plaque appears to follow a reproducible general pattern of ecological succession: Streptococcus, Actinomyces, and Veillonella dominate early plaque, followed by an increase in the proportion of Fusobacterium and Porphyromonas, and finally the advent of late colonizers such as Aggregatibacter, which depend on the presence of other species to create a suitable niche (Kolenbrander and London, 1993; Palmer et al., 2003; Al-Ahmad et al., 2007; Periasamy and Kolenbrander, 2010). In addition, the spatial structure of dental plaque is highly organized (Zijnge et al., 2010; Welch et al., 2016), likely reflecting environmental and biochemical gradients in combination with physical and metabolic interactions between the constituent organisms.
Further down in the gastrointestinal tract, extensive biofilms of Helicobacter pylori have been observed on the gastric mucosa of infected patients (Carron et al., 2006; Coticchia et al., 2006). In the ileum and colon, biofilms containing dense masses of bacteria in close contact with the epithelial surface are detectable in a minority of healthy people (6-35% depending on the study) (Swidsinski et al., 2005b; Dejea et al., 2014; Baumgartner et al., 2021). However, in dysbiotic states such as colorectal cancer, inflammatory bowel disease, or irritable bowel syndrome, biofilms are observed more frequently on the intestinal epithelium—sometimes in up to 50% of patients or even higher (Swidsinski et al., 2005b; Dejea et al., 2014; Baumgartner et al., 2021). Biofilms may also represent a reservoir for certain canonical intestinal pathogens; for example, Salmonella spp. form biofilms on gallstones in asymptomatic human carriers (Crawford et al., 2010). Remarkably, while most of the evidence for intestinal biofilms comes from microscopic examination of biopsies, some biofilms may be macroscopically visible during endoscopies (Baumgartner et al., 2021). Such biofilms have been reported in the upper jejunum of patients diagnosed with small intestinal bacterial overgrowth, as well as in the ileum and colon of patients with other disorders (Baumgartner et al., 2021). In accordance with the complexity of the overall gut microbiota, intestinal biofilms are almost invariably polymicrobial, although they tend to be enriched in certain taxa. These taxa vary depending on the study, likely reflecting differences in patient population and/or etiology, but generally include E. coli, Bacteroides species, certain Clostridia such as Ruminococcus gnavus, and/or oral pathogens such as Fusobacterium nucleatum (Swidsinski et al., 2005b; Dejea et al., 2014; Drewes et al., 2017; Baumgartner et al., 2021). Multiple studies have also reported that biofilms are much more common near the ileal-cecal junction and in the ascending colon compared to the transverse colon, descending colon, or rectum, regardless of the patient’s underlying condition (Dejea et al., 2014; Drewes et al., 2017; Baumgartner et al., 2021) (Figure 3). The mechanism driving this skewed longitudinal distribution remains unclear, but presumably is related to physical and/or chemical differences along the length of the colon.
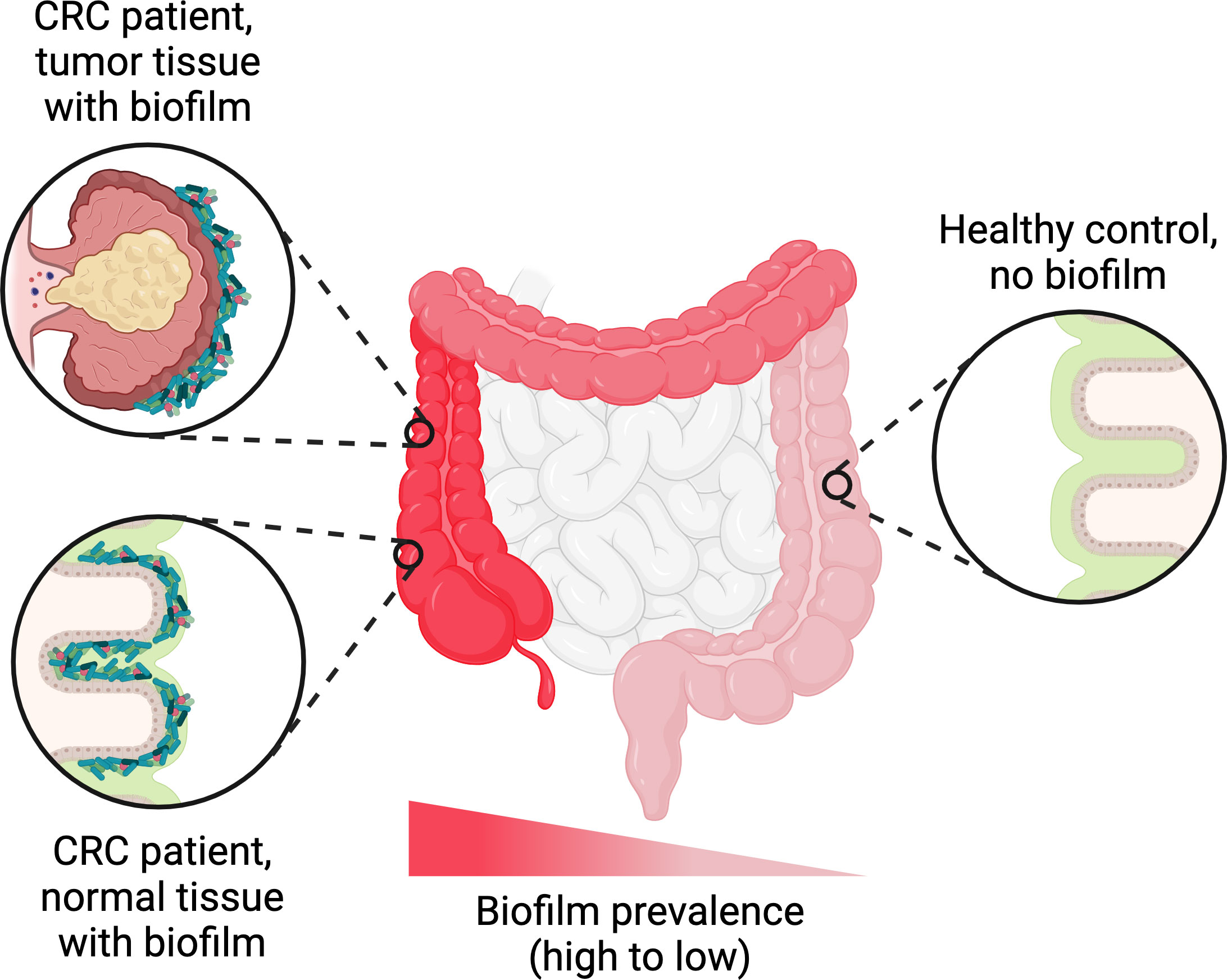
Figure 3 Trends in biofilm occurrence in the distal gastrointestinal tract. Adherent biofilms on the colonic epithelium are found in a higher proportion of patients with conditions such as colorectal cancer (CRC), inflammatory bowel disease, or irritable bowel syndrome compared to healthy controls. In biofilm-positive CRC patients, the biofilms are generally not restricted to the tumor itself and can also be found on normal colonic tissue. Regardless of the patient’s underlying condition, the frequency of biofilm detection follows a decreasing trend along the longitudinal axis of the colon, with higher frequencies in the ascending colon compared to the transverse and descending colon. Figure created with BioRender.com.
While biofilms by definition consist of sessile bacteria, they are not static structures. Rather, they undergo a dynamic and often cyclical process of development and change (Stoodley et al., 2002). Broadly speaking, regardless of the specific form a biofilm takes, the biofilm life cycle encompasses three stages: aggregation and attachment, growth and accumulation, and disaggregation and detachment (Sauer et al., 2022). Each of these steps is influenced both by characteristics intrinsic to the bacteria themselves, such as cell surface architecture, and by extrinsic signals, substrates, and physical forces in their environmental milieu.
The first step in biofilm formation—aggregation and attachment—is largely driven by structures on the bacterial cell surface. The diversity and number of adhesive factors precludes a comprehensive review of every known bacterial adhesin; rather, here we aim to offer a general overview of the types of cell surface structures that have been implicated in adherence to host tissues or the formation of aggregates within the host environment (Table 2). All types of biofilms, including suspended aggregates, involve adhesive interactions between bacterial cells, while tissue-attached biofilms also require adhesins that mediate bacteria-substrate interactions. Some adhesins are thought to predominantly contribute to only one of these types of interactions, but many have been shown to contribute to both. Indeed, experiments that demonstrate the role of an adhesin in attachment to a particular substrate do not necessarily rule out a secondary role in mediating bacterial intercellular adhesion, suggesting that adhesins with dual roles could be even more widespread than is currently evident.
Among the proteinaceous structures involved in bacterial adhesion and aggregation, pili are perhaps the best studied. Sometimes referred to as fimbriae, pili are non-flagellar cell-surface appendages with a modular structure composed of individual protein subunits. Several distinct types of pili have been described thus far, including chaperone–usher pili, type IV pili, type IV secretion pili, type V pili, curli fibers, and sortase-assembled pili (Telford et al., 2006; Hospenthal et al., 2017). Different classes of pili vary in their structure, biochemistry, and biogenesis, but virtually all have been found to play roles in bacterial adhesion except for type IV secretion pili, which instead facilitate conjugative transfer of genetic material between bacterial cells (Hospenthal et al., 2017).
Chaperone-usher pili are so named because of the manner of their biogenesis, which involves chaperone-mediated folding of pilin subunits in the periplasm followed by polymerization and secretion through an “usher” protein (Busch and Waksman, 2012). Pili belonging to this diverse class were the first to be recognized as being involved in bacterial cell adhesion (Duguid et al., 1955; Busch and Waksman, 2012) and are commonly found in Gram-negative pathogens, especially among enteric bacteria (Yen et al., 2002; Zav’yalov et al., 2010). Chaperone-usher pili can either be monoadhesive, meaning that only the subunit at tip of the pilus contains a binding domain that interacts with host tissues or other substrates, or polyadhesive, meaning that each individual subunit is capable of mediating adhesion (Zav’yalov et al., 2010). In both cases, the biochemical properties of the binding domain can confer tropism for specific host tissues. For example, the FimH domain of the monoadhesive type I pilus expressed by uropathogenic E. coli binds to mannosylated uroplakin UP1a and α1β3 integrins on umbrella cells in the bladder epithelium (Eto et al., 2007; Proft and Baker, 2008), while the polyadhesive Saf pilus of Salmonella enterica mediates binding to intestinal epithelial cells (though the host receptor remains unknown) (Zeng et al., 2017). Other chaperone-usher pili likely involved in biofilm formation in vivo include the E. coli common pilus, or ECP, and F1C fimbriae (Wurpel et al., 2013). ECP is ubiquitous across E. coli and has been implicated in adherence to epithelial cells and intestinal colonization by both pathogenic and commensal strains (Lasaro et al., 2009; Avelino et al., 2010). F1C fimbriae are expressed by a much smaller subset of E. coli strains but have been shown to bind specific host glycolipids (Khan et al., 2000), mediate adhesion to endothelial cells in the human kidney and bladder (Virkola et al., 1988), and contribute to persistent intestinal colonization in a mouse model (Lasaro et al., 2009). Notably, while most studies on chaperone-usher pili tropisms have focused on binding to host substrates, some pili in this class may also mediate bacteria-bacteria interactions during biofilm formation through pilus self-association. This has been demonstrated at least for Saf in S. enterica, in which mutations that disrupted pilus self-association also hindered biofilm formation (Zeng et al., 2017). ECP is also likely to be involved in mediating adhesion between bacterial cells, as scanning electron micrographs of E. coli microcolonies revealed complex meshes of intertwined ECP fibers (Garnett et al., 2012).
Type IV pili are the most broadly distributed type of pilus and the only class that has been found in both Gram-negative and Gram-positive bacteria (Pelicic, 2008). Characterized by their particularly thin (5-9 nm diameter) and long (up to several micrometers) morphology (Pelicic, 2008; Hospenthal et al., 2017), type IV pili are thought to be the dominant factor mediating adhesion of P. aeruginosa to human airway epithelial cells (Hahn, 1997), even though they are not always necessary for attachment to abiotic surfaces (Klausen et al., 2003). This adhesion may result from binding of the pilus tip to specific glycolipids (Lee et al., 1994). Beyond P. aeruginosa, type IV pili contribute to localized adherence of enteropathogenic E. coli to host cells (Donnenberg et al., 1992) and epithelial cell adhesion and platelet-mediated biofilm formation by Streptococcus sanguinis (Chen et al., 2019; Martini et al., 2021). Type IV pili expressed by the intestinal bacteria Clostridium perfringens and Clostridioides difficile likewise appear to be involved in biofilm formation at least in vitro (Varga et al., 2008; Maldarelli et al., 2016). As with chaperone-usher pili, type IV pili can mediate bacteria-bacteria interactions and auto-aggregation in addition to interactions with host substrates (Vuopio-Varkila and Schoolnik, 1991; Bordeleau et al., 2015).
Type V pili are unique to bacteria in the class Bacteroidia and have only been defined structurally and biochemically within the last decade (Xu et al., 2016), although their existence has been recognized since the 1980s (Yoshimura et al., 1984). Type V pili expressed by the oral pathogen Porphyromonas gingivalis mediate binding to gingival epithelial cells and human extracellular matrix proteins via a multitude of adhesion epitopes (Hajishengallis, 2007), in addition to promoting coaggregation with Streptococcus gordonii and potentially other oral bacteria (Park et al., 2005). A putative type V pilus also contributes to in vitro biofilm formation by the gut commensal Bacteroides thetaiotaomicron (Mihajlovic et al., 2019). It is therefore tempting to speculate that type V pili could be involved in the formation of the Bacteroides-enriched biofilms observed in some inflammatory bowel disease and colorectal cancer patients (Swidsinski et al., 2005b; Drewes et al., 2017). Variants of this pilus that either promoted or diminished biofilm formation in vitro did not affect colonization of germ-free mice by B. thetaiotaomicron (Mihajlovic et al., 2019). However, its role in adhering to human epithelial cells or mucus has not been directly tested for Bacteroides species, and the possibility remains that type V pili might contribute to co-aggregation with other biofilm-forming bacteria in the gut, or only be essential for adhesion to the intestinal mucosa under certain circumstances.
Other types of pili or fimbriae that can contribute to adhesion to host cells and/or human extracellular matrix include extracellular amyloid fibers called curli that are produced by many strains of E. coli and Salmonella species (Barnhart and Chapman, 2006), the fimbrial protein FadA in F. nucleatum (Han et al., 2005; Fardini et al., 2011), and sortase-assembled pili found in Gram-positive bacteria (Telford et al., 2006). Curli mediates bacterial auto-aggregation and binding to fibronectin, and works synergistically with other bacterially-produced extracellular matrix components to promote adherence to human intestinal epithelial cells (Collinson et al., 1993; Saldaña et al., 2009). A curli-like pilus is also produced by M. tuberculosis during human infection, and mutants lacking this pilus are deficient in laminin binding and in vitro biofilm formation (Alteri et al., 2007; Ramsugit et al., 2013). FadA is a unique amyloid-like fimbrial adhesin found only in Fusobacterium species, which is required for adhesion to human epithelial and endothelial cells (Han et al., 2005; Fardini et al., 2011; Meng et al., 2021). Sortase-assembled pili expressed by Gram-positive oral pathogens can mediate adhesion either to saliva-coated tooth surfaces or host cells, while the pili produced by Streptococcus pneumoniae and Streptococcus agalactiae promote adhesion to lung epithelial cells, and pili produced by Streptococcus pyogenes bind to collagen (Telford et al., 2006; Konto-Ghiorghi et al., 2009). In the case of at least S. agalactiae, the pilus is also essential for adherent biofilm formation on plastic surfaces, but in a manner that is independent of the von Willebrand adhesion domain that mediates epithelial cell adhesion, suggesting that different domains of the pilus confer different surface tropisms (Konto-Ghiorghi et al., 2009). Finally, the sortase-assembled pilus of the probiotic bacterium Lactobacillus rhamnosus GG contains a specific subunit, SpaC, that binds β-galactoside-containing carbohydrate moieties in human intestinal mucus (Kankainen et al., 2009; Nishiyama et al., 2016), in addition to mediating auto-aggregation, collagen binding, and adhesion to intestinal epithelial cells (though the latter could be a result of binding mucins attached to the epithelial cell surface) (Tripathi et al., 2013; Ardita et al., 2014).
Besides pili and fimbriae, numerous other bacterial adhesive factors have been described. Gram-positive cocci such as Staphylococcus, Streptococcus, and Enterococcus spp. express a diverse range of cell-wall anchored proteins involved in intercellular adhesion or adhesion to host extracellular matrix components (Geoghegan and Foster, 2015; Foster, 2019). These include a large family of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) defined by the presence of two adjacent IgG-like folded domains that participate in ligand binding; proteins containing G5-E repeat domains, such as SasG in S. aureus and Aap in Staphylococcus epidermidis, which promote cell aggregation; proteins with legume lectin-like domains, such as SraP in S. aureus, which binds to N-acetylneuraminic acid-containing glycoproteins in saliva and on the surface of mammalian cells; and many others (Geoghegan and Foster, 2015). The tropisms of these adhesins reflect the host environments to which each species is adapted. For example, R. gnavus, which is enriched in some intestinal biofilms, expresses an MSCRAMM, RadA, that binds to intestinal mucins (Maresca et al., 2021), while many oral streptococci and other Gram-positive species produce serine-rich repeat (Srr) proteins and antigen I/II polypeptides that mediate adhesion to saliva-coated surfaces (Nobbs et al., 2011). Surface-associated lipoproteins can also promote adhesion of streptococci to salivary glycoproteins, collagen, or other bacteria (Kolenbrander and London, 1993; Jenkinson, 1994; Bober et al., 2011). Similarly, the intestinal pathogen C. difficile expresses a lipoprotein that promotes its adhesion to intestinal epithelial cells (Kovacs-Simon et al., 2014), although this study did not distinguish between binding directly to the epithelial cells versus binding to surface-associated secreted molecules such as mucins.
Gram-negative bacteria also produce a variety of non-pilus surface-associated proteins that mediate adhesion and/or aggregation. For example, although the primary role of flagella is usually considered to be motility, the flagella of E. coli and P. aeruginosa also exhibit adhesive interactions with mucins (Lillehoj et al., 2002; Erdem et al., 2007; Troge et al., 2012). In the case of E. coli, not only whole purified flagella but also individual flagellin subunits bound mucins in vitro, suggesting that the entire filament may be capable of mediating such interactions, although the involvement of the flagellar cap protein, FliD, has not been explicitly ruled out (Erdem et al., 2007). In P. aeruginosa, flagellin and the flagellar cap protein have each been reported in different studies to mediate adhesion to mucus (Arora et al., 1998; Lillehoj et al., 2002), with the relative importance of the two components possibly depending on the type of mucins tested. Lectins are another important class of Gram-negative bacterial adhesin, as in Gram-positive bacteria. The surface-associated lectin Fap2 expressed by F. nucleatum mediates enhanced adhesion to colorectal adenocarcinomas through its specificity for the polysaccharide Gal-GalNAc, which is overexpressed by the cancer cells—potentially contributing to the frequent enrichment of F. nucleatum within colorectal-cancer-associated biofilms (Abed et al., 2016). Bacteroides species also express lectin-like adhesins that bind to host-derived glycoconjugates and may contribute to epithelial cell adhesion and/or co-aggregation with other bacterial species (Rogemond and Guinet, 1986; London and Allen, 1990).
Outer membrane proteins can also contribute to adhesion and/or aggregation of Gram-negative bacteria. In many enteric bacteria, including urinary tract pathogens, auto-aggregation that contributes to biofilm formation is mediated at least in part by subgroup of autotransporter proteins, which are a class of outer membrane proteins defined by the presence of a signal peptide, a secreted passenger domain, and translocator domain that forms a pore in the outer membrane (Klemm et al., 2006). Similarly, the autotransporter RadD in F. nucleatum mediates co-aggregation with other species of oral bacteria (Nobbs et al., 2011). Autotransporters expressed by Actinomyces, on the other hand, promote binding to epithelial cells (Nobbs et al., 2011). Other outer membrane proteins involved in adhesion and/or aggregation include the outer membrane protein FomA, which helps F. nucleatum to adhere to saliva-coated surfaces (Nobbs et al., 2011); the outer membrane porin F in P. aeruginosa, which promotes adhesion to human alveolar epithelial cells (Azghani et al., 2002); and the outer membrane protein HBP35 in P. gingivalis promotes co-aggregation with other oral bacteria (Nobbs et al., 2011). In addition, Bacteroides species express a TonB-dependent outer membrane protein that binds to fibronectin (Pauer et al., 2009).
Besides proteinaceous adhesins, certain polysaccharides have been implicated in modulating bacterial aggregation and adhesion. For example, cell-bound N-acetylglucosamine (GlcNAc) polysaccharides produced by E. coli and Staphylococcus species serve as intercellular adhesins that are required for biofilm formation in certain strains (Wang et al., 2004; Itoh et al., 2005; Brady et al., 2008). The genomic locus required for producing such polysaccharides has also been identified in biofilm-forming opportunistic pathogens such as K. pneumoniae and Burkholderia cepacia (Skurnik et al., 2012), and contributes to the structural integrity of biofilms formed by the oral pathogen Aggregatibacter actinomycetemcomitans (Izano et al., 2008b). The exact nature of the molecular interactions underlying the intercellular adhesive function of GlcNAc polysaccharides remains unclear; however, interactions with proteins are likely either not involved or not required, given that cells in E. coli biofilms could be dispersed by hydrolysis of the glycosidic linkages of poly-β-1,6-GlcNAc but not by enzymatic protein digestion (Wang et al., 2004; Itoh et al., 2005). Two other well-known extracellular polysaccharides, Pel and Psl, play strain-specific roles in aggregation of P. aeruginosa and adhesion to surfaces that include host epithelial cells and mucin-coated substrates (Vasseur et al., 2005; Ma et al., 2006; Byrd et al., 2010). In addition, several Streptococcus species synthesize extracellular glucans and fructans from sucrose (Rozen et al., 2001; Banas and Vickerman, 2003). These polysaccharides play essential roles in aggregation of oral bacteria and adherence to tooth surfaces (Rozen et al., 2001; Tamesada et al., 2004; Koo et al., 2010; Matsumoto-Nakano, 2018). Conversely, extracellular polysaccharides can also inhibit biofilm formation when in the form of a capsule, possibly by masking adhesive bacterial cell surface structures (Béchon et al., 2020).
Compared to many of the biofilm-forming species discussed above, considerably less is known about the factors mediating adhesion or aggregation of mycobacterial lung pathogens. Besides the curli-like pilus mentioned in section 3.1.1, one of the few mycobacterial adhesins described to date is a heparin-binding hemagglutinin expressed by M. tuberculosis (Menozzi et al., 1996). This protein promotes mycobacterial auto-aggregation and adhesion to epithelial cells (Menozzi et al., 1996), suggesting a possible role in the formation of the sheet-like putative biofilms seen in some tuberculosis lesions (Nyka and O’Neill, 1970). In addition, the antigen 85 complex of M. tuberculosis binds fibronectin with increasing strength under mechanical stress, which may be important for mycobacterial adhesion under the shear forces experienced in the lung (Viljoen et al., 2020). Which other surface-associated factors are involved in mycobacterial adhesion and aggregation, if any, remains unclear.
Following the initial stages of bacterial aggregation or adhesion to a surface, the secretion of extracellular matrix components plays an important role in biofilm maturation and structural integrity. Biofilm extracellular matrix components have been described from all four classes of biological macromolecules, but most often include polysaccharides, proteins, and extracellular DNA (eDNA).
Numerous extracellular polysaccharides contribute to the biofilm matrix in a taxon-specific manner. In E. coli, colanic acid is not required for surface attachment but is essential for the development of complex three-dimensional biofilm structures in certain strains (Danese et al., 2000). Colanic acid is also required for biofilm formation by S. enterica serovar Typhimurium on human epithelial cells and chicken intestinal tissue, but not on a plastic surface (Ledeboer and Jones, 2005). Besides colanic acid, some strains of E. coli and Salmonella produce cellulose, which works in concert with curli fibers to increase biofilm cohesion, elasticity, and stability (Serra et al., 2013). Still other biofilm-associated high-molecular-weight extracellular polysaccharides produced by enteric bacteria have yet to be named or genetically characterized (Bales et al., 2013).
Biofilms formed by non-enteric bacteria are likewise often rich in extracellular polysaccharides. In an animal model of middle ear infections, sialylated lipooligosaccharides were an indispensable component of the extracellular matrix secreted by H. influenzae (Jurcisek et al., 2005). In oral bacteria, the glucans that promote adhesion to tooth surfaces also play a key role in the development of three-dimensional multicellular structures (Xiao et al., 2012). Similarly, in P. aeruginosa, Psl and Pel polysaccharides not only contribute to the early stages of aggregation and adhesion, but also serve as structural scaffolds in mature biofilms (Colvin et al., 2012). For example, positively-charged Pel can crosslink eDNA through ionic interactions (Jennings et al., 2015). The third major exopolysaccharide produced by P. aeruginosa, alginate, affects the three-dimensional structure of mature biofilms at least in vitro, although it is not required for biofilm formation (Stapper et al., 2004). Overproduction of alginate confers a mucoid phenotype and is common among strains isolated from chronically-infected CF patients, indicating a likely fitness advantage in vivo (Lam et al., 1980; Stapper et al., 2004). The role of alginate within the P. aeruginosa biofilm matrix may be primarily protective rather than structural, as it can scavenge reactive oxygen species, hinder phagocytosis, and inhibit killing by cationic antimicrobial peptides (Limoli et al., 2015). Finally, cellulose (or a cellulose-like polysaccharide) is present in the extracellular matrix of mycobacterial biofilms, including biofilms formed in vitro by nontuberculous mycobacteria and putative in vivo biofilms of M. tuberculosis detected in infected human lungs (Chakraborty et al., 2021). Treatment of nontuberculous mycobacterial biofilms with either cellulase or proteinase K resulted in dispersal of the bacterial cells (Chakraborty et al., 2021), suggesting that cellulose plays an important structural role, possibly in concert with extracellular proteins, as is also the case for enteric bacteria.
The proteinaceous components of biofilm matrices are as diverse as those involved in aggregation and adhesion. Such proteins can contribute structurally as the biofilm matures either by forming amyloids (i.e. aggregates with fibrillar morphology and β-sheet secondary structure) and/or by interacting with other extracellular polymeric substances. For example, amyloid curli fibers produced by enteric bacteria not only interact with cellulose, as mentioned above, but also with eDNA (Gallo et al., 2015; Nicastro et al., 2022). Although less well-recognized than curli, phenol soluble modulins (Psm) produced by S. aureus assemble into amyloid-like fibrils that stabilize the biofilm structure and promote biofilm resistance to enzymatic degradation, surfactant-mediated dispersal, and mechanical stress (Schwartz et al., 2012). S. mutans produces functional amyloids formed by antigen I/II polypeptides and other secreted proteins dependent on sortase-mediated assembly, which promote biofilm formation and are present in dental plaque (Oli et al., 2012). Pseudomonas species can also produce a functional amyloid protein, encoded by the fap operon, that contributes to biofilm mechanical robustness (Zeng et al., 2015) and promotes in vivo aggregation of P. aeruginosa in a rat model of lung infection (Beg et al., 2022). Another extracellular protein that contributes to the structural stability of the P. aeruginosa biofilm matrix is CdrA, which is an adhesin belonging to a two-partner secretion system (Borlee et al., 2010). CdrA binds to the exopolysaccharides Psl and Pel, potentially cross-linking the polysaccharides or tethering them to the bacterial cell surface (Borlee et al., 2010; Reichhardt et al., 2020). Similarly, oral streptococci express a number of proteins that shape the three-dimensional structure of dental plaque biofilms by binding to glucans (Hazlett et al., 1999; Banas and Vickerman, 2003; Matsumoto-Nakano, 2018). The role of Bap, a biofilm-associated protein first discovered in S. aureus, is less clear, but it can form amyloids under certain conditions and is thought to contribute to the biofilm matrix (Cucarella et al., 2001; Taglialegna et al., 2016). Although Bap is not common among clinical isolates of S. aureus, several other Gram-positive and Gram-negative pathogens encode orthologs, ranging from Enterococcus to Salmonella (Cucarella et al., 2001; Toledo-Arana et al., 2001; Latasa et al., 2005). Finally, S. aureus expresses a series of lipoproteins that modulate biofilm porosity by binding to eDNA (Kavanaugh et al., 2019).
eDNA has been recognized as a potential constituent of the in vivo biofilm matrix since at least the 1970s, when analysis of the “slime” produced by several clinical isolates of P. aeruginosa revealed the presence of DNA (Murakawa, 1973). For many years, eDNA in biofilms was thought to be mostly a byproduct of lysed cells with unclear functional significance (Sutherland, 2001). However, a subsequent study revealed that DNase could prevent in vitro biofilm formation by P. aeruginosa, suggesting an important structural or adhesive role (Whitchurch et al., 2002). eDNA has since been shown to be a major component of the extracellular matrix not only for P. aeruginosa but also other bacterial species that form biofilms in vivo, including H. influenzae, S. aureus, and Streptococcus species (Jurcisek and Bakaletz, 2007; Izano et al., 2008a; Nur et al., 2013; Liao et al., 2014; Sugimoto et al., 2018). Depending on the species, eDNA found in biofilms may be released through autolysis and/or secreted in extracellular vesicles (Kadurugamuwa and Beveridge, 1995; Steinberger and Holden, 2005; Liao et al., 2014). Notably, a recent study demonstrated that across several different biofilm-forming human pathogens, the eDNA in both in vitro and in vivo biofilms was present not only as B-DNA—the canonical right-handed helical form—but also as DNase-resistant left-handed Z-DNA as a result of interactions with secreted bacterial DNABII proteins (Buzzo et al., 2021). The proportion of Z-DNA increases as biofilms age, explaining the oft-reported observation that DNase cannot always disperse mature biofilms even though it can disrupt biofilm formation (Buzzo et al., 2021). Thus, Z-DNA plays an important role in biofilm structural stability and resistance to host defenses. DNABII orthologs are ubiquitous in eubacterial genomes (Dey et al., 2017), suggesting that this phenomenon might be broadly applicable across in vivo biofilms that incorporate eDNA.
Besides polymers, the biofilm extracellular matrix can also include outer membrane vesicles (OMVs). OMVs are heterogeneous, self-enclosed bilayered structures that form by blebbing off from the outer membrane of Gram-negative bacteria. OMVs have been observed in dental plaque biofilms (Holliday et al., 2015), and transmission electron microscopy revealed that OMVs were universally present within the extracellular matrix of biofilms formed by P. aeruginosa under multiple growth conditions, as well as in mixed-species biofilms from environmental samples (Schooling and Beveridge, 2006). Thus, OMVs might broadly be part of the biofilm extracellular matrix whenever Gram-negative bacteria are present. OMVs have been shown in vitro to mediate coaggregation of oral bacteria (Kamaguchi and Baba, 1995), and may also perform other biofilm-related functions such as binding aminoglycoside antibiotics and thereby protecting cells in the interior (Schooling and Beveridge, 2006). In addition, OMVs are a major source of lipopolysaccharide within the extracellular matrix and can interact with eDNA via salt-bridging and electrostatic interactions, raising the possibility that they impact biofilm structural and mechanical properties (Schooling and Beveridge, 2006; Schooling et al., 2009).
Finally, while biofilm extracellular matrices characterized in axenic cultures consist exclusively of bacterially-produced components, it is possible that bacteria in vivo may sometimes incorporate host-derived polymers into their biofilm structure. For example, in the lower respiratory tract or gastrointestinal tract, mucins might represent a potential extracellular matrix substrate. In addition, eDNA is released by host polymorphonuclear leukocytes (PMNs) during infections to create Neutrophil Extracellular Traps (NETs) that can ensnare planktonic bacteria. Bacterial DNABII proteins can convert host-derived eDNA around the periphery of in vivo biofilms into Z-DNA (Buzzo et al., 2021), and exogenous DNA can be incorporated into biofilms in vitro (Chiang et al., 2013). Nevertheless, surveys of a small number of clinical samples suggest that host-derived eDNA may not be commonly incorporated into the core structure of in vivo biofilms (Alhede et al., 2020; Buzzo et al., 2021), and no direct evidence has been published to date of mucin incorporation into biofilms as a structural component. More generally, given that it is challenging to comprehensively characterize the biofilm matrix in tissue samples, it remains unclear to what extent the understanding of biofilm extracellular matrix components gained from in vitro studies may translate to the in vivo setting.
The genomic capacity to express surface factors with the right tropisms and extracellular matrix components to provide stability are prerequisites for bacteria to successfully form a biofilm. However, whether a biofilm forms at a particular site in the host also depends on the physiochemical characteristics of the microenvironment. Physical forces that affect biofilm formation have been reviewed in greater depth elsewhere (Renner and Weibel, 2011; Arias and Brito, 2021; Wong et al., 2023), but two major factors that are especially relevant to in vivo environments are fluid flow and viscoelasticity of the host substrate.
Fluid flow and the attendant shear forces are prominent features of the gastrointestinal tract, urinary tract, respiratory tract, and heart chambers. High rates of fluid flow can promote bacterial clearance (Granick et al., 2007; Dawes, 2008), while low shear forces, such as those believed to be present in the abnormally thickened mucus layer of CF patients, can promote bacterial aggregation (Crabbé et al., 2008). Yet the relationship between fluid shear and bacterial clearance is not linear. Local increases in shear rate near a surface can actually trap motile bacteria and promote attachment (Crabbé et al., 2008; Rusconi et al., 2014). This phenomenon results from the competing effects of cell alignment with the direction of flow and the stochasticity of bacterial swimming orientation (Rusconi et al., 2014). Some bacteria have also evolved mechanisms for sensing fluid shear, and in the case of P. aeruginosa, activation of those sensors upregulates the expression of biofilm-related genes (Alsharif et al., 2015; Rodesney et al., 2017; Sanfilippo et al., 2019). Moreover, certain bacterial adhesins exhibit increased adhesive strength in response to tensile forces, as a result of conformational changes (Arias and Brito, 2021). Such adhesins, known as catch-bonds, likely play a role in the ability of S. aureus to colonize heart valves during endocarditis, and the ability of uropathogenic E. coli to colonize the bladder epithelium (Arias and Brito, 2021). Thus, the relationship between shear rate and biofilm formation can be non-intuitive, particularly within the range of rates typical of in vivo environments.
The viscoelastic properties of mucus can also affect the ability of bacteria to establish a biofilm, which is particularly relevant in the respiratory and gastrointestinal tracts. The thickened mucus found in CF lungs behaves more similarly to an elastic solid whereas normal airway mucus is more similar to a viscous liquid (Matsui et al., 2006). Modeling this difference with isotonically concentrated mucus revealed that the thicker mucus promoted the formation of macrocolonies by P. aeruginosa (Matsui et al., 2006). Importantly, such macrocolonies were not formed by a mutant defective for quorum sensing, suggesting that they represented true biofilms and could not simply be explained by physical entrapment of the bacteria (Matsui et al., 2006). Viscoelasticity is also highly relevant in the human distal gastrointestinal tract, where the intestinal epithelium is lined with two mucus layers: a relatively loose outer layer facing the lumen, and a much more compact inner layer adjacent to the epithelium (Johansson et al., 2014). Normally, the high viscosity and/or low porosity of the inner layer prevents the incursion of bacteria even in the absence of flow (Johansson et al., 2014). However, in ulcerative colitis, the inner mucus layer often becomes penetrable to bacteria (Johansson et al., 2014; Post et al., 2019). The reasons for this are not fully understood, but presumably precede the establishment of biofilms adjacent to the epithelium.
Besides physical forces, chemical cues within the host environment can also influence the establishment of a biofilm. For example, the dietary polysaccharide maltodextrin increases the adherence of E. coli to epithelial cells and promotes biofilm formation in a type 1 pilus-dependent manner (Nickerson and McDonald, 2012). Other organic compounds found in the distal gastrointestinal tract that modulate the production of adhesins or extracellular matrix factors by E. coli include N-acetylglucosamine, short-chain fatty acids, and polyamines such as putrescine and spermidine (Rossi et al., 2017). In addition, bile salts or bile acids—detergent-like molecules secreted by the liver to solubilize ingested lipids—induce in vitro biofilm formation by a variety of gut-associated bacteria, from commensal Bacteroides species (Pumbwe et al., 2007; Béchon et al., 2022) to pathogenic E. coli, Salmonella, and Shigella (Faherty et al., 2012; Nickerson et al., 2017; Köseoğlu et al., 2019). The mechanisms underlying this phenomenon do not appear to be conserved across species, ranging from upregulation of a specific autotransporter (in Shigella flexneri) to an unexpected dependency on a constitutively-expressed extracellular DNase (in B. thetaiotaomicron) (Faherty et al., 2012; Béchon et al., 2022). Notably, in a clinical study, patients with detectable intestinal biofilms tended to have higher fecal levels of bile acids, and at least one bile acid was significantly enriched in biofilm-positive biopsies compared to biofilm-negative biopsies (other bile acids were not included in the metabolomics panel) (Baumgartner et al., 2021). Thus, bile acids may play a key role in stimulating biofilm formation by enteric pathogens and members of the gut microbiota in vivo as well as in vitro.
Inorganic compounds in the host environment also modulate biofilm development and persistence. Iron plays a signaling role in biofilm formation for many species, including P. aeruginosa, E. coli, S. aureus, and S. mutans (Francesca et al., 2004; Banin et al., 2005; Johnson et al., 2005; Musk et al., 2005; Wu and Outten, 2009; Lin et al., 2012). However, the relationship between iron levels and biofilm phenotypes varies across species and is not necessarily monotonic; for example, in P. aeruginosa, iron limitation interferes with biofilm formation, but excess iron can also be inhibitory (Banin et al., 2005; Musk et al., 2005). Oxygen is another widespread regulator of biofilm formation with taxon-specific effects. Uropathogenic E. coli upregulate the expression of type 1 pili in response to oxygen (Floyd et al., 2015), while conversely, S. mutans grows but is unable to form a biofilm in the presence of oxygen (Ahn and Burne, 2007). Hypoxia enhances biofilm formation by S. aureus (Mashruwala et al., 2017), and P. aeruginosa similarly forms larger biofilms when respiring nitrate in the absence of oxygen, compared to aerobic growth with or without nitrate (Yoon et al., 2002)—observations that are highly relevant to the hypoxic, nitrate-replete microenvironment of CF sputum (Grasemann et al., 1998; Worlitzsch et al., 2002). Finally, nitrate itself influences the production of curli and cellulose by uropathogenic E. coli (Martín-Rodríguez et al., 2020) and serves as a cue for the dispersal of Salmonella biofilms (Miller et al., 2022b). Both E. coli and Salmonella encounter nitrate during the course of infection, as human urine contains nitrate (Radomski et al., 1978), and nitrate levels rise in the intestine during inflammation (Rivera-Chávez and Bäumler, 2014).
Biofilms can have numerous impacts on human health depending on their location and composition. Here we review several effects that are supported by experimental evidence or clinical observations. Not all of these effects are mechanistically well-understood, and more may remain to be discovered.
One widely-accepted consequence of biofilm formation during infections is decreased efficacy of antibiotic treatments, which can delay the clearance of pathogens and lead to chronic infections. In fact, although not an apples-to-apples comparison, the antibiotic concentration required to eradicate a biofilm can be more than 1000-fold higher than the minimum inhibitory concentration for the same strain in planktonic form (Ceri et al., 1999). Even very small, actively growing biofilm-like clusters of bacteria containing as few as 150 cells can exhibit reduced antibiotic susceptibility compared to cells growing at lower densities (Connell et al., 2010).
Multiple mechanisms are thought to underlie the decreased antibiotic susceptibility of biofilm-dwelling bacteria. First, electrostatic or other interactions with components of the extracellular matrix can limit the diffusion of certain antibiotics within a biofilm and consequently their ability to access bacteria in the interior. For example, negatively-charged alginate and eDNA can bind positively-charged antibiotics such as aminoglycosides (Hatch and Schiller, 1998; Chiang et al., 2013). Second, the three-dimensional architecture of a biofilm generates gradients of nutrients, pH, terminal electron acceptors, and waste products (Stewart and Franklin, 2008). Consequently, different subpopulations within a biofilm may experience starvation, oxygen limitation, or other physiological stresses, leading to reduced metabolic activity and slower growth. This inherently limits the efficacy of many antibiotics that target the synthesis of cellular components or depend on the membrane potential for uptake into the cell (Tack and Sabath, 1985; Stewart, 2002). Third, such stresses can activate adaptive response pathways that prime bacteria to tolerate subsequent stresses induced by antibiotics (Mulcahy et al., 2008; Nguyen et al., 2011; Bernier et al., 2013; Wilton et al., 2016). Fourth, spontaneous mutation rates may be increased within biofilms as a result of oxidative stress or other factors, leading to de novo acquisition of antibiotic resistance (Driffield et al., 2008; Saint-Ruf et al., 2014).
Importantly, not all of the mechanisms discussed above are necessarily in play for every biofilm. In the study that reported the surprising presence of morphologically similar biofilms in both chronic and acute lower respiratory infections, biofilm-encased bacteria in the acute infections appeared to be more metabolically active than the bacteria in chronic infections, based on the per-cell ribosome content (Kolpen et al., 2022). Although the antibiotic susceptibility of those bacteria was not directly tested, this observation suggests that microenvironmental differences between chronic and acute infections, and not just the presence of a biofilm per se, might play a critical role in modulating antibiotic susceptibility in vivo.
In addition to enhancing bacterial survival during antibiotic treatment, biofilms can also offer protection against the host immune response to infection. The sheer size and dense matrix of a biofilm can present a physical obstacle to engulfment by immune cells, as biofilms that are otherwise impervious to phagocytosis by macrophages become sensitized upon mechanical disruption (Thurlow et al., 2011). Other biofilm immune evasion mechanisms are taxon-specific. For example, P. aeruginosa biofilms respond to the presence of PMNs by secreting rhamnolipids, which stick to the extracellular matrix and induce necrosis in any immune cells that make contact (Alhede et al., 2009). In addition, alginate protects P. aeruginosa biofilms from phagocytosis by macrophages (Leid et al., 2005). Biofilm formation also has a protective effect for Staphylococcus species, but the extent of protection varies depending on the circumstances. One study indicated that fluid shear increases leukocyte penetration of S. aureus biofilms, compared to biofilms grown under static conditions, but the invading leukocytes were nevertheless unable to phagocytose bacteria within the biofilm (Leid et al., 2002). On the other hand, a different study demonstrated that PMNs can penetrate and clear S. aureus biofilms by phagocytosis, although aged biofilms were less susceptible than biofilms that were only a few days old (Günther et al., 2009). S. aureus biofilms can also limit invasion and phagocytosis by macrophages (Thurlow et al., 2011), possibly by inducing macrophage cytotoxicity (Scherr et al., 2015), while the large quantity of poly-N-acetylglucosamine within the Staphylococcus biofilm extracellular matrix serves as a decoy that prevents antibody-mediated killing (Cerca et al., 2006). Extracellular polysaccharides similarly protect S. mutans against killing by neutrophils, an effect that was enhanced for surface-attached bacteria compared to those in suspension (Steinberg et al., 1999).
Interestingly, S. aureus biofilms in vivo decreased the levels of cytokines responsible for immune cell recruitment and activation (Thurlow et al., 2011), and the polysaccharides produced by S. mutans attenuated neutrophil release of reactive oxygen species (Steinberg et al., 1999). Furthermore, in a rabbit ear wound model, infections with predominantly biofilm-associated S. aureus provoked a diminished inflammatory response compared to infections that also contained abundant planktonic bacteria, despite similar levels of viable bacteria in both types of infections (Gurjala et al., 2011). Thus, besides posing a physical obstacle or secreting cytotoxic factors, some bacterial biofilms also dampen the overall magnitude of the immune response via pathways that have not been fully elucidated.
In soft tissue wounds, biofilms delay the healing process. Evidence for this comes not only from the correlation between the chronicity of a wound and the presence of biofilms (James et al., 2008)—which does not indicate the direction of causality—but also from experiments with several animal models of wound healing. For example, in a diabetic mouse model, infection of wounds with pre-formed biofilms of P. aeruginosa delayed complete healing by two to four weeks compared to uninfected controls (Zhao et al., 2012). Similarly, in a non-diabetic mouse model, inoculation of punch wounds with pre-formed Staphylococcus biofilms resulted in slower re-epithelialization (Schierle et al., 2009). Other studies in which biofilms were allowed to develop over time within wounds have further supported the role of biofilms in delayed healing by clarifying the impact of biofilm presence versus simply bacterial presence. In one study, infection of rabbit ear wounds with a biofilm-impaired Pel- and Psl-deficient mutant of P. aeruginosa resulted in improved healing and lower expression of inflammatory cytokines, compared to infection with the wildtype parent strain, even though both strains colonized the wounds to similar levels (Seth et al., 2012). In another study, infection of porcine burn wounds with hyperbiofilm-forming and biofilm-deficient mutants of S. aureus revealed that the hyperbiofilm-forming strain attenuated re-epithelialization compared to the wildtype parent strain (Roy et al., 2020). Moreover, both the hyperbiofilm-former and biofilm-competent wildtype strain induced a significant loss of collagen in granulation tissue compared to the biofilm-deficient mutant (Roy et al., 2020). Multispecies biofilms may have even more severe impacts on wound healing than monospecies biofilms, as inoculation of porcine wounds with a mixed population of biofilm-forming P. aeruginosa and S. aureus uniquely suppressed the expression of a keratinocyte growth factor and further delayed re-epithelialization, compared to inoculation with only one strain or the other (Pastar et al., 2013). Finally, clinical observations suggest that anti-biofilm wound care strategies, such as regular debridement to physically disrupt biofilms, can improve patient outcomes for chronic wounds, further supporting the causal role of biofilms in delayed healing (Wolcott and Rhoads, 2008; Metcalf and Bowler, 2013).
Biofilms in the form of dental plaque are not a marker for disease, but when left unchecked, plaque can undergo dysbiotic shifts that lead to the deterioration of oral health (Frédéric et al., 2018). For example, biofilm-dwelling S. mutans is thought to be one of the major culprits driving the formation of dental caries (Marsh, 2010). Caries form when tooth enamel is eroded by the acid produced by bacterial fermentation of carbohydrates, particularly sucrose (Leme et al., 2006). Sucrose is also a substrate for extracellular matrix production by S. mutans (Banas and Vickerman, 2003), and this three-dimensional matrix in turn facilitates the development of acidic pockets within the plaque biofilm (Xiao et al., 2012).
Like caries, periodontal disease is inextricably linked to plaque biofilms. Early colonizers provide a physical foothold for later colonizers, and the community shifts that occur as plaque matures are associated with the development of gingival inflammation (Kolenbrander et al., 2010; Frédéric et al., 2018). Moreover, the pathogenic potential of oral bacteria is influenced by contact-dependent interspecies communication and signaling by short-range diffusible metabolites, both of which are enhanced within biofilms (Kolenbrander et al., 2010). Mechanical disruption of plaque biofilms is the current standard of care for periodontal disease, and a recent clinical study confirmed that this intervention significantly decreased the abundance of disease-associated species, while taxa that are typically associated with oral health either increased in abundance or remained unchanged (Johnston et al., 2021). However, while it is important for restoring oral health in periodontal patients, mechanical disruption of plaque can also transiently introduce bacteria into the bloodstream and thereby enable biofilms to disseminate to other parts of the body, as in some cases of infective endocarditis (Larsen and Fiehn, 2017).
A relatively recent development in the field of biofilm research is the finding that biofilms in the gastrointestinal tract may promote carcinogenesis. Multiple studies have reported a correlation between colorectal cancer and the incidence of bacterial biofilms on the intestinal epithelium (Dejea et al., 2014; Drewes et al., 2017), and biofilm-positive normal intestinal tissues display markers of a pro-oncogenic state, including increased epithelial cell proliferation and reduced expression or altered localization of E-cadherin (Dejea et al., 2014). In addition, some strains of E. coli and Bacteroides fragilis secrete oncogenic toxins (Wu et al., 2009; Pleguezuelos-Manzano et al., 2020), and such bacteria were enriched in biofilms adhering to pre-cancerous lesions in patients with familial adenomatous polyposis (Dejea et al., 2018). However, perhaps the most direct evidence that biofilms can be pro-oncogenic comes from a study in which microbial consortia from biofilm-positive or biofilm-negative human colonic biopsies were inoculated into three different murine models of carcinogenesis (Tomkovich et al., 2019). In that study, regardless of whether the biofilm-positive biopsies were from colon cancer patients or healthy controls, all biofilm consortia significantly increased the number of colonic tumors that formed over a 12-week period in both germ-free and specific-pathogen-free mice, compared to inocula prepared from biofilm-negative biopsies. Moreover, in contrast to the biofilm-negative biopsy communities, all biofilm consortia at least partially invaded the mucus layer of the distal colon within 12 weeks when inoculated into the germ-free mice, suggesting that certain bacteria are inherently more capable of breaching the mucus barrier (Tomkovich et al., 2019). The biofilm consortia also significantly induced mucosal infiltration by Th17 and other IL-17+ cells, as well as by myeloid cells, in agreement with prior studies indicating a role for IL-17 in tumorigenesis (Wu et al., 2009; Housseau et al., 2016; Tomkovich et al., 2019).
Interestingly, a case-control study in humans revealed a correlation between both the presence and severity of periodontal disease and the incidence of oral cancer (Komlós et al., 2021), suggesting that pathogenic oral biofilms might similarly induce pro-oncogenic changes in surrounding tissues. Consistent with this hypothesis, incubation of oral squamous cell carcinoma cell lines with periodontal pathogens increased cell migration and tumorsphere formation, whereas commensal oral bacteria had no effect (Kamarajan et al., 2020). In addition, H. pylori, which forms biofilms in the gastric mucosa, is a well-known risk factor for gastric cancer. Although a causal link between H. pylori biofilm formation and the initiation or progression of gastric cancer has yet to be definitively demonstrated, strains of H. pylori containing a specific cytotoxin-associated gene pathogenicity island are not only more proficient at forming biofilms in vitro (Wong et al., 2016), but are also associated with increased risk of gastric cancer (Blaser et al., 1995; Huang et al., 2003). Thus, bacterial biofilms on mucosal tissues beyond the distal gastrointestinal tract might more broadly represent a cancer risk factor.
Another potential effect of some biofilms is the development of autoimmune disorders. Such disorders arise in a small percentage of patients following infections with enteric pathogens such as Salmonella, Shigella, and E. coli, all of which can produce amyloid-forming curli as part of their biofilm extracellular matrix (Miller et al., 2022a). Curli fibers bind tightly to eDNA in biofilms, and the resulting curli-DNA complexes potently stimulated the immune system and induced the production of autoantibodies both in a mouse model of lupus and in wildtype mice (Gallo et al., 2015). Similarly, infection of mice with curli-producing invasive Salmonella triggered the production of autoantibodies and induced joint inflammation (Miller et al., 2020). Systemic exposure of immune cells to the curli-DNA complexes appears to be necessary for the stimulation of autoimmunity, as infection with non-invasive curli-producing Salmonella or oral administration of curli-DNA complexes did not have the same effect (Miller et al., 2020). Notably, a recent clinical study reported that flares in lupus patients correlated with increased serum levels of anti-curli/DNA antibodies, and that these episodes also correlated with the presence of bacteria in the patients’ urine, suggesting that similar mechanisms may be at work in humans (Pachucki et al., 2020). Moreover, autoimmune sequelae are also seen in a minority of patients infected with P. aeruginosa, M. tuberculosis, or S. aureus (Miller et al., 2022a). Like enteric bacteria, all of these species produce amyloidogenic proteins that could potentially form complexes with eDNA (see section 3.2).
Although tissue-associated biofilms in humans have primarily been associated with disease states, not all biofilms may have a negative impact on the host. In the oral context, it has been proposed that in healthy dental plaque, commensal bacteria serve as a barrier to colonization by oral pathogens (Marsh, 2010). In support of this hypothesis, certain oral commensal species have been shown to antagonize the growth and/or adhesion of taxa associated with periodontitis, or to interfere with biofilm-formation by S. mutans (Hoogmoed et al., 2008; Tamura et al., 2009; Begić et al., 2023). Colonization resistance has also been studied in the context of the distal gastrointestinal tract. Biofilms formed by commensal enteric bacteria in vitro resist incursion by enteric pathogens (Re et al., 2013), and pre-colonization of mice with specific combinations of commensal E. coli strains prevented subsequent colonization by a pathogenic strain of E. coli (Leatham et al., 2009; Maltby et al., 2013). Pre-colonization with a common probiotic strain was also able to prevent epithelial damage by pathogenic E. coli in human intestinal organoids (Pradhan and Weiss, 2020). Whether biofilm formation is necessary for in vivo intestinal colonization resistance remains unclear. However, the loss of key adhesins involved in biofilm formation interfered with the ability of probiotic E. coli to stably colonize the mouse gut (Lasaro et al., 2009), suggesting that adherence to the mucosal surface might be a prerequisite for sustained beneficial effects, including colonization resistance.
The study of biofilms in human tissues has come a long way since the time of van Leeuwenhoek. Nevertheless, given that much of the existing mechanistic insight into tissue-associated biofilms has been acquired in the context of single-species studies with canonical pathogens, numerous gaps remain in our understanding of where, when, why, and how biofilms form in the human body. Biofilms in vivo are often polymicrobial, yet how interspecies interactions contribute to the course of biofilm development is not well-understood in most cases. In addition, relatively little is known about the regulation and mechanisms of biofilm formation by commensal bacteria, as well as how such biofilms impact health and disease.
Taking the distal gastrointestinal tract as an example, there is no unifying explanation for the observation that biofilms on the intestinal epithelium are more prevalent in various disease states, yet are not always absent in healthy controls. Are the biofilms that are occasionally found in healthy controls precursors to disease, or is there such a thing as a commensal—or even beneficial—biofilm in the gut? And to what extent do biofilms present in disease states actively drive pathology, as opposed to representing a symptom of an underlying disturbance? Similar questions also apply to other organ systems. For example, given that some clinically non-infected patients have putative biofilms in their upper respiratory tract, and that some asymptomatic women have vaginal biofilms composed of species typically associated with disease, are there characteristics that better enable certain people to keep potentially pathogenic biofilms in check? Such characteristics might include variants in genes related to the immune system, or the presence of beneficial bacteria that antagonize pathogenic species. It is also possible that there is a tipping point, perhaps in terms of tissue coverage or length of association, beyond which biofilms are more likely to cause symptoms.
The true prevalence of biofilms in healthy states is also unclear, as biofilm distribution can be patchy and it is impractical to microscopically survey entire organs. While the difference in frequency of detection implies that biofilms are indeed more prevalent in many disease states across different tissue types, it is possible that relatively small, localized biofilms may be more common than is currently appreciated. Because conclusive evidence for biofilms in regions such as the distal gastrointestinal tract, upper respiratory tract, and urogenital tract is based on small biopsies, it is difficult to distinguish between a difference in actual prevalence (i.e. the percentage of people with any biofilm) and a difference in tissue coverage (i.e. the percentage of the tissue surface that is associated with a biofilm). Furthermore, the method of tissue processing can greatly affect the likelihood of detecting a biofilm. For example, when biopsies of the intestinal epithelium are fixed in formalin, adherent biofilms are completely lost; instead, fixation in nonaqueous Carnoy’s solution or Methacarn, which preserve the mucus layer and the biofilm extracellular matrix, appears to be a prerequisite for biofilm detection in this context (Swidsinski et al., 2005b; Dassanayake et al., 2020). It is possible that the prevalence of biofilms on other mucosal tissues may have been underestimated by studies that used standard aqueous fixatives.
Another barrier to understanding the prevalence of tissue-associated biofilms is that some tissues that might harbor biofilms, such as the small intestine, are difficult to access in living patients. Others, such as the urinary tract, are not commonly subjected to biopsies, especially in healthy individuals. With regard to the latter, recent sequencing-based studies on samples obtained by transurethral catheterization or suprapubic aspiration have revealed the presence of a distinct bladder microbiome in healthy people, from which live bacteria can be grown using non-standard culture conditions (Thomas-White et al., 2016). Moreover, experiments performed nearly forty years ago revealed that bacteria obtained from the vagina or distal urethra of healthy women could adhere to human uroepithelial cells in vitro, and some species were able to competitively exclude uropathogenic bacteria (Chan et al., 1984; Chan et al., 1985). Thus, it is not implausible that biofilms might exist in the bladder or urethra beyond the context of urinary tract infections, but the requirement for invasive sampling will hinder the testing of this hypothesis.
Much also remains to be understood about the process of and triggers for biofilm formation within the host environment. Is the formation of biofilms in vivo driven primarily by intrinsic characteristics of their constituent taxa, or are permissive host characteristics and/or environmental triggers also necessary? Most likely, all three factors contribute to some degree, but the extent of their contribution may vary across different body sites. In the lower respiratory tract, the species that are commonly detected in biofilms are also prolific biofilm-formers in vitro, indicating the importance of intrinsic bacterial features. Yet it is clear that host factors and environmental changes, such as the thickening of mucus in CF patients, affect the likelihood of biofilm establishment and persistence, even though not all factors are well-understood. Particularly in the case of factors such as fluid shear that have a non-intuitive, non-linear relationship to biofilm formation, further studies will be required in order to better understand how disease-related changes affect biofilm development by different species and in different contexts.
Intrinsic bacterial characteristics likely also contribute to biofilm formation in the distal gastrointestinal tract, given that biofilm communities transplanted from human biopsies can invade the distal colonic mucus layer in mice (Tomkovich et al., 2019). Importantly, however, that experiment was performed in germ-free mice, which at baseline have a more penetrable inner mucus layer than conventional mice (Johansson et al., 2015). In this respect, germ-free mice bear more resemblance to ulcerative colitis patients than to healthy humans (Johansson et al., 2014), raising the possibility that defects in the mucus structure are also a predisposing factor for biofilm formation. The repeated observation that epithelial biofilms are more commonly detected in the proximal colon compared to the distal colon further suggests that environmental triggers are likely to be important, yet the influence of extrinsic factors on regulation of biofilm formation remains almost entirely unknown for many of the taxa that have been detected in intestinal biofilms. Even in the case of known environmental triggers, such as bile acids, the underlying signal transduction pathways have yet to be elucidated. In addition, basic information is lacking about the range of fluid shear rates within the human intestinal environment and the influence of solid-induced shear forces generated by fecal contents (Hinman et al., 2021).
Finally, with the exception of oral biofilms, little information is available about the dynamics of biofilm formation and persistence in vivo, due to the invasive nature of sample collection for most tissues. The ability to conduct time-course studies in situ in the oral context has revealed reproducible trends in community composition over time. Does ecological succession similarly occur in other tissue-associated multispecies biofilms, such as those found in the intestine or chronic wounds, and if so, are there generalizable principles? Relatedly, how persistent are biofilms, both in health and in disease, and when, why, and how do they disperse? Drivers and mechanisms of biofilm dispersal have been studied in vitro and reviewed elsewhere (Rumbaugh and Sauer, 2020), but direct observations in vivo are challenging for both technical and practical reasons.
Given the difficulty of accessing most tissues for in situ biofilm studies, addressing the questions outlined above will require the development of advanced experimental systems that model the human tissue environment as accurately as possible. The most common methods for studying biofilms, such as microtiter plate assays or flow chambers (Azeredo et al., 2017), fail to capture important aspects of the host environment, which can severely affect the human relevance of study outcomes (Bjarnsholt et al., 2013). For example, lactoferrin, a host-derived innate defense molecule, is effective at preventing P. aeruginosa biofilm formation on abiotic surfaces in well-oxygenated flow chambers (Singh et al., 2002), but culturing P. aeruginosa in concentrated mucus from human airways revealed a complete lack of efficacy of lactoferrin in preventing the formation of suspended aggregate-type biofilms resembling those found in CF patients (Matsui et al., 2006). Furthermore, while animal models can enable biofilms to be studied in a more holistic in vivo context, bacterial tropisms can be host-specific, and common model organisms such as mice exhibit significant differences with humans in terms of anatomy, diet, bile acid composition, native microbiota, respiratory secretions, and so on (Benahmed et al., 2014; Nguyen et al., 2015).
Although no model system is perfect, two organ systems for which relatively advanced in vitro models are already available are the respiratory tract and the gastrointestinal tract. With regard to the respiratory tract, different aspects of the CF environment have been modeled individually, such by using artificial sputum media to mimic the nutritional composition of CF sputum (Fung et al., 2010), or culturing bacteria in agar suspensions to study the effects of aggregation and metabolic gradients on antibiotic susceptibility (Spero and Newman, 2018). More recently, an ex vivo pig lung model for infections of CF bronchioles showed promise in overcoming many of the limitations of simpler in vitro models and mouse models (Harrington et al., 2020). Progress has also been made towards modeling respiratory infections of non-CF patients, such as through the use of differentiated primary human airway cells cultured at an air-liquid interface (Hasan et al., 2018). As for the gastrointestinal tract, complex in vitro models have been developed that simulate many aspects of the oral environment, including saliva, fluid flow, and surfaces that resemble natural tooth enamel, although challenges remain in modeling bacterial interactions with gingival tissue (Luo et al., 2022). Primary-tissue-derived models of the human gastric mucosa and intestinal epithelium have also taken shape over the last decade, including three-dimensional organoids that largely recapitulate the cell-type diversity of the native tissue; apical-out organoids in which the orientation of the polarized epithelium is inverted for easier experimental access; two-dimensional organoid-derived monolayers; and organoid-derived microfluidic systems (Sato et al., 2011; Fujii et al., 2018; Co et al., 2019; Kim et al., 2019; Sontheimer-Phelps et al., 2020). Such models are increasingly used for the study of host-microbe interactions, and some have demonstrated the capacity to support co-cultures for several days (Jalili-Firoozinezhad et al., 2019; Shin et al., 2019). Microfluidic systems are particularly attractive because they enable the incorporation of shear forces. However, further iterations may be required to establish the utility of organoid-derived models for studying biofilm interactions with the host epithelium (Puschhof et al., 2021).
It is an exciting time to be studying the impact of biofilms on human health and disease. A significant body of evidence has accumulated regarding the existence of biofilms in diverse tissues. For many of the most common biofilm-forming species, reductionist studies have led to a detailed understanding of the molecular factors involved in biofilm development and persistence. Moreover, intriguing correlations between biofilm presence and multiple disease states hint that developing biofilm-targeting drugs could impact the treatment and prevention of not only chronic infections, as is now well-accepted, but also other intractable conditions ranging from inflammatory bowel disease to cancer. While many questions remain to be explored, the advent of complex in vitro experimental models will provide researchers with new tools to untangle how the host environment affects biofilm phenotypes, and how specific biofilm components trigger host responses. This in turn will improve our ability to detect and manipulate tissue-associated biofilms in situ, with potentially wide-ranging implications for human health.
EP wrote the article with input from M-WT. All authors contributed to the article and approved the submitted version.
This study received funding from Genentech, Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author EP and M-WT were employed by Genentech, Inc.
This study received funding from Genentech, Inc. The funder had the following involvement with the study: employer of the authors.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abed, J., Emgård, J. E. M., Zamir, G., Faroja, M., Almogy, G., Grenov, A., et al. (2016). Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed gal-galNAc. Cell Host Microbe 20, 215–225. doi: 10.1016/j.chom.2016.07.006
Ahn, S.-J., Burne, R. A. (2007). Effects of oxygen on biofilm formation and the atlA autolysin of Streptococcus mutans. J. Bacteriol 189, 6293–6302. doi: 10.1128/jb.00546-07
Al-Ahmad, A., Wunder, A., Auschill, T. M., Follo, M., Braun, G., Hellwig, E., et al. (2007). The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 56, 681–687. doi: 10.1099/jmm.0.47094-0
Alhede, M., Alhede, M., Qvortrup, K., Kragh, K. N., Jensen, P.Ø., Stewart, P. S., et al. (2020). The origin of extracellular DNA in bacterial biofilm infections in vivo. Pathog. Dis. 78, ftaa018. doi: 10.1093/femspd/ftaa018
Alhede, M., Bjarnsholt, T., Jensen, P.Ø., Phipps, R. K., Moser, C., Christophersen, L., et al. (2009). Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155, 3500–3508. doi: 10.1099/mic.0.031443-0
Alhede, M., Kragh, K. N., Qvortrup, K., Allesen-Holm, M., Gennip, M., Christensen, L. D., et al. (2011). Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PloS One 6, e27943. doi: 10.1371/journal.pone.0027943
Alsharif, G., Ahmad, S., Islam, M. S., Shah, R., Busby, S. J., Krachler, A. M. (2015). Host attachment and fluid shear are integrated into a mechanical signal regulating virulence in Escherichia coli O157:H7. Proc. Natl. Acad. Sci. 112, 5503–5508. doi: 10.1073/pnas.1422986112
Alteri, C. J., Xicohténcatl-Cortes, J., Hess, S., Caballero-Olín, G., Girón, J. A., Friedman, R. L. (2007). Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. 104, 5145–5150. doi: 10.1073/pnas.0602304104
Anderson, G. G., Palermo, J. J., Schilling, J. D., Roth, R., Heuser, J., Hultgren, S. J. (2003). Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107. doi: 10.1126/science.1084550
Ardita, C. S., Mercante, J. W., Kwon, Y. M., Luo, L., Crawford, M. E., Powell, D. N., et al. (2014). Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl. Environ. Microbiol. 80, 5068–5077. doi: 10.1128/aem.01039-14
Arias, S. L., Brito, I. L. (2021). Biophysical determinants of biofilm formation in the gut. Curr. Opin. BioMed. Eng. 18, 100275. doi: 10.1016/j.cobme.2021.100275
Arora, S. K., Ritchings, B. W., Almira, E. C., Lory, S., Ramphal, R. (1998). The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect. Immun. 66, 1000–1007. doi: 10.1128/iai.66.3.1000-1007.1998
Avelino, F., Saldaña, Z., Islam, S., Monteiro-Neto, V., Dall’Agnol, M., Eslava, C. A., et al. (2010). The majority of enteroaggregative Escherichia coli strains produce the E. coli common pilus when adhering to cultured epithelial cells. Int. J. Med. Microbiol. 300, 440–448. doi: 10.1016/j.ijmm.2010.02.002
Azeredo, J., Azevedo, N. F., Briandet, R., Cerca, N., Coenye, T., Costa, A. R., et al. (2017). Critical review on biofilm methods. Crit. Rev. Microbiol. 43, 313–351. doi: 10.1080/1040841x.2016.1208146
Azghani, A. O., Idell, S., Bains, M., Hancock, R. E. W. (2002). Pseudomonas aeruginosa outer membrane protein F is an adhesin in bacterial binding to lung epithelial cells in culture. Microb. Pathogenesis 33, 109–114. doi: 10.1006/mpat.2002.0514
Bales, P. M., Renke, E. M., May, S. L., Shen, Y., Nelson, D. C. (2013). Purification and characterization of biofilm-associated EPS exopolysaccharides from ESKAPE organisms and other pathogens. PloS One 8, e67950. doi: 10.1371/journal.pone.0067950
Banas, J. A., Vickerman, M. M. (2003). Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral. Biol. M 14, 89–99. doi: 10.1177/154411130301400203
Banin, E., Vasil, M. L., Greenberg, E. P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. 102, 11076–11081. doi: 10.1073/pnas.0504266102
Barnhart, M. M., Chapman, M. R. (2006). Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147. doi: 10.1146/annurev.micro.60.080805.142106
Basaraba, R. J., Ojha, A. K. (2017). Mycobacterial biofilms: Revisiting tuberculosis bacilli in extracellular necrotizing lesions. Microbiol. Spectr. 5, TBTB2-0024-2016. doi: 10.1128/microbiolspec.tbtb2-0024-2016
Baumgartner, M., Lang, M., Holley, H., Crepaz, D., Hausmann, B., Pjevac, P., et al. (2021). Mucosal biofilms are an endoscopic feature of irritable bowel syndrome and ulcerative colitis. Gastroenterology 161, 1245–1256.e20. doi: 10.1053/j.gastro.2021.06.024
Béchon, N., Mihajlovic, J., Lopes, A.-A., Vendrell-Fernández, S., Deschamps, J., Briandet, R., et al. (2022). Bacteroides thetaiotaomicron uses a widespread extracellular DNase to promote bile-dependent biofilm formation. Proc. Natl. Acad. Sci. 119, e2111228119. doi: 10.1073/pnas.2111228119
Béchon, N., Mihajlovic, J., Vendrell-Fernández, S., Chain, F., Langella, P., Beloin, C., et al. (2020). Capsular polysaccharide cross-regulation modulates Bacteroides thetaiotaomicron biofilm formation. Mbio 11, e00729–e00720. doi: 10.1128/mbio.00729-20
Beg, A. Z., Rashid, F., Talat, A., Haseen, M. A., Raza, N., Akhtar, K., et al. (2022). Functional amyloids in Pseudomonas aeruginosa are essential for the proteome modulation that leads to pathoadaptation in pulmonary niches. Microbiol. Spectr. 11, e03071–e03022. doi: 10.1128/spectrum.03071-22
Begić, G., Badovinac, I. J., Karleuša, L., Kralik, K., Peloza, O. C., Kuiš, D., et al. (2023). Streptococcus salivarius as an important factor in dental biofilm homeostasis: influence on Streptococcus mutans and Aggregatibacter actinomycetemcomitans in mixed biofilm. Int. J. Mol. Sci. 24, 7249. doi: 10.3390/ijms24087249
Benahmed, M. A., Elbayed, K., Daubeuf, F., Santelmo, N., Frossard, N., Namer, I. J. (2014). NMR HRMAS spectroscopy of lung biopsy samples: Comparison study between human, pig, rat, and mouse metabolomics. Magn. Reson. Med. 71, 35–43. doi: 10.1002/mrm.24658
Bernier, S. P., Lebeaux, D., DeFrancesco, A. S., Valomon, A., Soubigou, G., Coppée, J.-Y., et al. (2013). Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PloS Genet. 9, e1003144. doi: 10.1371/journal.pgen.1003144
Bezerra, T. F. P., Padua, F. G., de, M., Gebrim, E. M. M. S., Saldiva, P. H. N., Voegels, R. L. (2011). Biofilms in chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg. 144, 612–616. doi: 10.1177/0194599811399536
Bjarnsholt, T., Alhede, M., Alhede, M., Eickhardt-Sørensen, S. R., Moser, C., Kühl, M., et al. (2013). The in vivo biofilm. Trends Microbiol. 21, 466–474. doi: 10.1016/j.tim.2013.06.002
Bjarnsholt, T., Jensen, P.Ø., Fiandaca, M. J., Pedersen, J., Hansen, C. R., Andersen, C. B., et al. (2009). Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 44, 547–558. doi: 10.1002/ppul.21011
Bjarnsholt, T., Kirketerp-Møller, K., Jensen, P.Ø., Madsen, K. G., Phipps, R., Krogfelt, K., et al. (2008). Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regener. 16, 2–10. doi: 10.1111/j.1524-475x.2007.00283.x
Blaser, M. J., Perez-Perez, G. I., Kleanthous, H., Cover, T. L., Peek, R. M., Chyou, P. H., et al. (1995). Infection with Helicobacter pylori strains possessing CagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 55, 2111–2115.
Bober, M., Mörgelin, M., Olin, A. I., Pawel-Rammingen, U.v., Collin, M. (2011). The membrane bound LRR lipoprotein Slr, and the cell wall-anchored M1 protein from Streptococcus pyogenes both interact with type I collagen. PloS One 6, e20345. doi: 10.1371/journal.pone.0020345
Bordeleau, E., Purcell, E. B., Lafontaine, D. A., Fortier, L.-C., Tamayo, R., Burrus, V. (2015). Cyclic di-GMP riboswitch-regulated type IV pili contribute to aggregation of Clostridium difficile. J. Bacteriol. 197, 819–832. doi: 10.1128/jb.02340-14
Borlee, B. R., Goldman, A. D., Murakami, K., Samudrala, R., Wozniak, D. J., Parsek, M. R. (2010). Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 75, 827–842. doi: 10.1111/j.1365-2958.2009.06991.x
Brady, R. A., Leid, J. G., Calhoun, J. H., Costerton, J. W., Shirtliff, M. E. (2008). Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 52, 13–22. doi: 10.1111/j.1574-695x.2007.00357.x
Busch, A., Waksman, G. (2012). Chaperone–usher pathways: diversity and pilus assembly mechanism. Philos. Trans. R. Soc. B Biol. Sci. 367, 1112–1122. doi: 10.1098/rstb.2011.0206
Buzzo, J. R., Devaraj, A., Gloag, E. S., Jurcisek, J. A., Robledo-Avila, F., Kesler, T., et al. (2021). Z-form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell 184, 5740–5758.e17. doi: 10.1016/j.cell.2021.10.010
Byrd, M. S., Pang, B., Mishra, M., Swords, W. E., Wozniak, D. J. (2010). The Pseudomonas aeruginosa exopolysaccharide Psl facilitates surface adherence and NF-κB activation in A549 cells. Mbio 1, e00140–e00110. doi: 10.1128/mbio.00140-10
Cabell, C. H., Abrutyn, E., Karchmer, A. W. (2003). Bacterial endocarditis. Circulation 107, e185–e187. doi: 10.1161/01.cir.0000071082.36561.f1
Carron, M. A., Tran, V. R., Sugawa, C., Coticchia, J. M. (2006). Identification of Helicobacter pylori biofilms in human gastric mucosa. J. Gastrointest Surg. 10, 712–717. doi: 10.1016/j.gassur.2005.10.019
Cerca, N., Jefferson, K. K., Oliveira, R., Pier, G. B., Azeredo, J. (2006). Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect. Immun. 74, 4849–4855. doi: 10.1128/iai.00230-06
Ceri, H., Olson, M. E., Stremick, C., Read, R. R., Morck, D., Buret, A. (1999). The calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37, 1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999
Chakraborty, P., Bajeli, S., Kaushal, D., Radotra, B. D., Kumar, A. (2021). Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat. Commun. 12, 1606. doi: 10.1038/s41467-021-21748-6
Chan, R. C. Y., Bruce, A. W., Reid, G. (1984). Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of Gram-negative uropathogens by competitive exclusion. J. Urol. 131, 596–601. doi: 10.1016/s0022-5347(17)50512-1
Chan, R. C., Reid, G., Irvin, R. T., Bruce, A. W., Costerton, J. W. (1985). Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect. Immun. 47, 84–89. doi: 10.1128/iai.47.1.84-89.1985
Chen, Y.-Y. M., Chiang, Y.-C., Tseng, T.-Y., Wu, H.-Y., Chen, Y.-Y., Wu, C.-H., et al. (2019). Molecular and functional analysis of the type IV pilus gene cluster in Streptococcus sanguinis SK36. Appl. Environ. Microb. 85, e02788-18. doi: 10.1128/aem.02788-18
Chiang, W.-C., Nilsson, M., Jensen, P.Ø., Høiby, N., Nielsen, T. E., Givskov, M., et al. (2013). Extracellular DNA shields against aminoglycosides in Pseudomonas aerguinosa biofilms. Antimicrob. Agents Ch 57, 2352–2361. doi: 10.1128/aac.00001-13
Co, J. Y., Margalef-Català, M., Li, X., Mah, A. T., Kuo, C. J., Monack, D. M., et al. (2019). Controlling epithelial polarity: A human enteroid model for host-pathogen interactions. Cell Rep. 26, 2509–2520.e4. doi: 10.1016/j.celrep.2019.01.108
Collinson, S. K., Doig, P. C., Doran, J. L., Clouthier, S., Trust, T. J., Kay, W. W. (1993). Thin, aggregative fimbriae mediate binding of Salmonella enteritidis to fibronectin. J. Bacteriol 175, 12–18. doi: 10.1128/jb.175.1.12-18.1993
Colvin, K. M., Irie, Y., Tart, C. S., Urbano, R., Whitney, J. C., Ryder, C., et al. (2012). The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 14, 1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x
Connell, J. L., Wessel, A. K., Parsek, M. R., Ellington, A. D., Whiteley, M., Shear, J. B. (2010). Probing prokaryotic social behaviors with bacterial “Lobster traps”. mBio 1, e00202–e00210. doi: 10.1128/mbio.00202-10
Coticchia, J. M., Sugawa, C., Tran, V. R., Gurrola, J., Kowalski, E., Carron, M. A. (2006). Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer disease. J. Gastrointest Surg. 10, 883–889. doi: 10.1016/j.gassur.2005.12.009
Crabbé, A., Boever, P. D., Houdt, R. V., Moors, H., Mergeay, M., Cornelis, P. (2008). Use of the rotating wall vessel technology to study the effect of shear stress on growth behaviour of Pseudomonas aeruginosa PA01. Environ. Microbiol. 10, 2098–2110. doi: 10.1111/j.1462-2920.2008.01631.x
Crawford, R. W., Rosales-Reyes, R., Ramírez-Aguilar, M., de la, L., Chapa-Azuela, O., Alpuche-Aranda, C., et al. (2010). Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. 107, 4353–4358. doi: 10.1073/pnas.1000862107
Cucarella, C., Solano, C., Valle, J., Amorena, B., Lasa, I., Penadés, J. R. (2001). Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol 183, 2888–2896. doi: 10.1128/jb.183.9.2888-2896.2001
Cunha, M. V., Sousa, S. A., Leitã;o, J. H., Moreira, L. M., Videira, P. A., Sá-Correia, I. (2004). Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and in persistence of respiratory infections. J. Clin. Microbiol. 42, 3052–3058. doi: 10.1128/jcm.42.7.3052-3058.2004
Danese, P. N., Pratt, L. A., Kolter, R. (2000). Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol 182, 3593–3596. doi: 10.1128/jb.182.12.3593-3596.2000
Dassanayake, R. P., Falkenberg, S. M., Stasko, J. A., Shircliff, A. L., Lippolis, J. D., Briggs, R. E. (2020). Identification of a reliable fixative solution to preserve the complex architecture of bacterial biofilms for scanning electron microscopy evaluation. PloS One 15, e0233973. doi: 10.1371/journal.pone.0233973
Dawes, C. (2008). Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 139, 18S–24S. doi: 10.14219/jada.archive.2008.0351
Dejea, C. M., Fathi, P., Craig, J. M., Boleij, A., Taddese, R., Geis, A. L., et al. (2018). Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597. doi: 10.1126/science.aah3648
Dejea, C. M., Wick, E. C., Hechenbleikner, E. M., White, J. R., Welch, J. L. M., Rossetti, B. J., et al. (2014). Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. 111, 18321–18326. doi: 10.1073/pnas.1406199111
DePas, W. H., Starwalt-Lee, R., Sambeek, L. V., Kumar, S. R., Gradinaru, V., Newman, D. K. (2016). Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. mBio 7, e00796–e00716. doi: 10.1128/mbio.00796-16
Dey, D., Nagaraja, V., Ramakumar, S. (2017). Structural and evolutionary analyses reveal determinants of DNA binding specificities of nucleoid-associated proteins HU and IHF. Mol. Phylogenet Evol. 107, 356–366. doi: 10.1016/j.ympev.2016.11.014
Donnenberg, M. S., Girón, J. A., Nataro, J. P., Kaper, J. B. (1992). A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6, 3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x
Drewes, J. L., White, J. R., Dejea, C. M., Fathi, P., Iyadorai, T., Vadivelu, J., et al. (2017). High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes 3, 34. doi: 10.1038/s41522-017-0040-3
Driffield, K., Miller, K., Bostock, J. M., O’Neill, A. J., Chopra, I. (2008). Increased mutability of Pseudomonas aeruginosa in biofilms. J. Antimicrob. Chemoth 61, 1053–1056. doi: 10.1093/jac/dkn044
Duguid, J. P., Smith, I. W., Dempster, G., Edmunds, P. N. (1955). Non-flagellar filamentous appendages (“fimbriæ”) and hæmagglutinating activity in Bacterium coli. J. Pathol. Bacteriol 70, 335–348. doi: 10.1002/path.1700700210
Erdem, A. L., Avelino, F., Xicohtencatl-Cortes, J., Girón, J. A. (2007). Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J. Bacteriol 189, 7426–7435. doi: 10.1128/jb.00464-07
Eto, D. S., Jones, T. A., Sundsbak, J. L., Mulvey, M. A. (2007). Integrin-mediated host cell invasion by type 1–piliated uropathogenic Escherichia coli. PloS Pathog. 3, e100. doi: 10.1371/journal.ppat.0030100
Faherty, C. S., Redman, J. C., Rasko, D. A., Barry, E. M., Nataro, J. P. (2012). Shigella flexneri effectors OspE1 and OspE2 mediate induced adherence to the colonic epithelium following bile salts exposure. Mol. Microbiol. 85, 107–121. doi: 10.1111/j.1365-2958.2012.08092.x
Fardini, Y., Wang, X., Témoin, S., Nithianantham, S., Lee, D., Shoham, M., et al. (2011). Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 82, 1468–1480. doi: 10.1111/j.1365-2958.2011.07905.x
Fazli, M., Bjarnsholt, T., Kirketerp-Møller, K., Jørgensen, A., Andersen, C. B., Givskov, M., et al. (2011). Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds. Wound Repair Regener. 19, 387–391. doi: 10.1111/j.1524-475x.2011.00681.x
Fennelly, K. P., Ojano-Dirain, C., Yang, Q., Liu, L., Lu, L., Progulske-Fox, A., et al. (2016). Biofilm formation by Mycobacterium abscessus in a lung cavity. Am. J. Resp. Crit. Care 193, 692–693. doi: 10.1164/rccm.201508-1586im
Flemming, H.-C., Wuertz, S. (2019). Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 17, 247–260. doi: 10.1038/s41579-019-0158-9
Floyd, K. A., Moore, J. L., Eberly, A. R., Good, J. A. D., Shaffer, C. L., Zaver, H., et al. (2015). Adhesive fiber stratification in uropathogenic Escherichia coli biofilms unveils oxygen-mediated control of type 1 pili. PloS Pathog. 11, e1004697. doi: 10.1371/journal.ppat.1004697
Foster, T. J. (2019). The MSCRAMM family of cell-wall-anchored surface proteins of Gram-positive cocci. Trends Microbiol. 27, 927–941. doi: 10.1016/j.tim.2019.06.007
Francesca, B., Ajello, M., Bosso, P., Morea, C., Andrea, P., Giovanni, A., et al. (2004). Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans. Biometals 17, 271–278. doi: 10.1023/b:biom.0000027704.53859.d3
Frédéric, L. J., Michel, B., Selena, T. (2018). Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials 11, 1802. doi: 10.3390/ma11101802
Fujii, M., Matano, M., Toshimitsu, K., Takano, A., Mikami, Y., Nishikori, S., et al. (2018). Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 23, 787–793.e6. doi: 10.1016/j.stem.2018.11.016
Fung, C., Naughton, S., Turnbull, L., Tingpej, P., Rose, B., Arthur, J., et al. (2010). Gene expression of Pseudomonas aeruginosa in a mucin-containing synthetic growth medium mimicking cystic fibrosis lung sputum. J. Med. Microbiol. 59, 1089–1100. doi: 10.1099/jmm.0.019984-0
Gallo, P. M., Rapsinski, G. J., Wilson, R. P., Oppong, G. O., Sriram, U., Goulian, M., et al. (2015). Amyloid-DNA composites of bacterial biofilms stimulate autoimmunity. Immunity 42, 1171–1184. doi: 10.1016/j.immuni.2015.06.002
Garnett, J. A., Martínez-Santos, V. I., Saldaña, Z., Pape, T., Hawthorne, W., Chan, J., et al. (2012). Structural insights into the biogenesis and biofilm formation by the Escherichia coli common pilus. Proc. Natl. Acad. Sci. 109, 3950–3955. doi: 10.1073/pnas.1106733109
Geoghegan, J. A., Foster, T. J. (2015). “Cell wall-anchored surface proteins of Staphylococcus aureus: many proteins, multiple functions,” in Current Topics in Microbiology and Immunology, ed. Bagnoli, F., Rappuoli, R., Grandi, G. (Cham, Switzerland: Springer) 409, 95–120. doi: 10.1007/82_2015_5002
Granick, M. S., Tenenhaus, M., Knox, K. R., Ulm, J. P. (2007). Comparison of wound irrigation and tangential hydrodissection in bacterial clearance of contaminated wounds: results of a randomized, controlled clinical study. Ostomy Wound Manage. 53, 64–6, 68–70, 72.
Grasemann, H., Ioannidis, I., Tomkiewicz, R. P., Groot, H., de, Rubin, B. K., Ratjen, F. (1998). Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child 78, 49. doi: 10.1136/adc.78.1.49
Griffen, A. L., Beall, C. J., Campbell, J. H., Firestone, N. D., Kumar, P. S., Yang, Z. K., et al. (2012). Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. Isme J. 6, 1176–1185. doi: 10.1038/ismej.2011.191
Gristina, A. G., Oga, M., Webb, L. X., Hobgood, C. D. (1985). Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science 228, 990–993. doi: 10.1126/science.4001933
Günther, F., Wabnitz, G. H., Stroh, P., Prior, B., Obst, U., Samstag, Y., et al. (2009). Host defence against Staphylococcus aureus biofilms infection: Phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Mol. Immunol. 46, 1805–1813. doi: 10.1016/j.molimm.2009.01.020
Gurjala, A. N., Geringer, M. R., Seth, A. K., Hong, S. J., Smeltzer, M. S., Galiano, R. D., et al. (2011). Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regener. 19, 400–410. doi: 10.1111/j.1524-475x.2011.00690.x
Hahn, H. P. (1997). The type 4 pilus is the major virulence-associated adhesin of Pseudomonas aerguinosa – a review. Gene 192, 99–108. doi: 10.1016/s0378-1119(97)00116-9
Hajishengallis, G. (2007). Peptide mapping of a functionally versatile fimbrial adhesin from Porphyromonas gingivalis. Int. J. Pept. Res. Ther. 13, 533–546. doi: 10.1007/s10989-007-9084-1
Hall-Stoodley, L., Hu, F. Z., Gieseke, A., Nistico, L., Nguyen, D., Hayes, J., et al. (2006). Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. Jama 296, 202–211. doi: 10.1001/jama.296.2.202
Han, Y. W., Ikegami, A., Rajanna, C., Kawsar, H. I., Zhou, Y., Li, M., et al. (2005). Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol 187, 5330–5340. doi: 10.1128/jb.187.15.5330-5340.2005
Han, A., Zenilman, J. M., Melendez, J. H., Shirtliff, M. E., Agostinho, A., James, G., et al. (2011). Multifaceted approach for characterizing microbial parameters of chronic wounds. Wound Repair Regener. 19, 532–541. doi: 10.1111/j.1524-475x.2011.00720.x
Hardy, L., Jespers, V., Dahchour, N., Mwambarangwe, L., Musengamana, V., Vaneechoutte, M., et al. (2015). Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One 10, e0136658. doi: 10.1371/journal.pone.0136658
Harrington, N. E., Sweeney, E., Harrison, F. (2020). Building a better biofilm - Formation of in vivo-like biofilm structures by Pseudomonas aeruginosa in a porcine model of cystic fibrosis lung infection. Biofilm 2, 100024. doi: 10.1016/j.bioflm.2020.100024
Hasan, S., Sebo, P., Osicka, R. (2018). A guide to polarized airway epithelial models for studies of host–pathogen interactions. FEBS J. 285, 4343–4358. doi: 10.1111/febs.14582
Hatch, R. A., Schiller, N. L. (1998). Alginate Lyase Promotes Diffusion of Aminoglycosides through the Extracellular Polysaccharide of Mucoid Pseudomonas aeruginosa. Antimicrob. Agents Ch 42, 974–977. doi: 10.1128/aac.42.4.974
Hazlett, K. R. O., Mazurkiewicz, J. E., Banas, J. A. (1999). Inactivation of the gbpA gene of Streptococcus mutans alters structural and functional aspects of plaque biofilm which are compensated by recombination of the gtfB and gtfC genes. Infect. Immun. 67, 3909–3914. doi: 10.1128/iai.67.8.3909-3914.1999
Hinman, S. S., Huling, J., Wang, Y., Wang, H., Bretherton, R. C., DeForest, C. A., et al. (2021). Magnetically-propelled fecal surrogates for modeling the impact of solid-induced shear forces on primary colonic epithelial cells. Biomaterials 276, 121059. doi: 10.1016/j.biomaterials.2021.121059
Høiby, N. (2017). A short history of microbial biofilms and biofilm infections. Apmis 125, 272–275. doi: 10.1111/apm.12686
Høiby, N., Axelsen, N. H. (1973). Identification and quantification of precipitns against Pseudomonas aeruginosa in patients with cystic fibrosis by means of cross immunoelectrophoresis with intermediate gel. Acta Pathologica Microbiol. Scand. Sect B Microbiol. Immunol. 81B, 298–308. doi: 10.1111/j.1699-0463.1973.tb02207.x
Holliday, R., Preshaw, P. M., Bowen, L., Jakubovics, N. S. (2015). The ultrastructure of subgingival dental plaque, revealed by high-resolution field emission scanning electron microscopy. BDJ Open 1, 15003. doi: 10.1038/bdjopen.2015.3
Homøe, P., Bjarnsholt, T., Wessman, M., Sørensen, H. C. F., Johansen, H. K. (2009). Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur. Arch. Oto-rhino-l 266, 1533–1538. doi: 10.1007/s00405-009-0940-9
Hoogmoed, C. G. V., Geertsema-doornbusch, G. I., Teughels, W., Quirynen, M., Busscher, H. J., Mei, H.C.V.d. (2008). Reduction of periodontal pathogens adhesion by antagonistic strains. Oral. Microbiol. Immun. 23, 43–48. doi: 10.1111/j.1399-302x.2007.00388.x
Hospenthal, M. K., Costa, T. R. D., Waksman, G. (2017). A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 15, 365–379. doi: 10.1038/nrmicro.2017.40
Housseau, F., Wu, S., Wick, E. C., Fan, H., Wu, X., Llosa, N. J., et al. (2016). Redundant innate and adaptive sources of IL17 production drive colon tumorigenesis. Cancer Res. 76, 2115–2124. doi: 10.1158/0008-5472.can-15-0749
Huang, J. Q., Zheng, G. F., Sumanac, K., Irvine, E. J., Hunt, R. H. (2003). Meta-analysis of the relationship between CagA seropositivity and gastric cancer. Gastroenterology 125, 1636–1644. doi: 10.1053/j.gastro.2003.08.033
Itoh, Y., Wang, X., Hinnebusch, B. J., Preston, J. F., Romeo, T. (2005). Depolymerization of β-1,6- N -acetyl- d -glucosamine disrupts the integrity of diverse bacterial biofilms. J. Bacteriol 187, 382–387. doi: 10.1128/jb.187.1.382-387.2005
Izano, E. A., Amarante, M. A., Kher, W. B., Kaplan, J. B. (2008a). Differential roles of poly- N -acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microb. 74, 470–476. doi: 10.1128/aem.02073-07
Izano, E. A., Sadovskaya, I., Wang, H., Vinogradov, E., Ragunath, C., Ramasubbu, N., et al. (2008b). Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb. Pathogenesis 44, 52–60. doi: 10.1016/j.micpath.2007.08.004
Jalili-Firoozinezhad, S., Gazzaniga, F. S., Calamari, E. L., Camacho, D. M., Fadel, C. W., Bein, A., et al. (2019). A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. BioMed. Eng. 3, 520–531. doi: 10.1038/s41551-019-0397-0
James, G. A., Swogger, E., Wolcott, R., Pulcini, E., deLancey, Secor, P., Sestrich, J., et al. (2008). Biofilms in chronic wounds. Wound Repair Regener. 16, 37–44. doi: 10.1111/j.1524-475x.2007.00321.x
Jenkinson, H. F. (1994). Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121, 133–140. doi: 10.1111/j.1574-6968.1994.tb07089.x
Jennings, L. K., Storek, K. M., Ledvina, H. E., Coulon, C., Marmont, L. S., Sadovskaya, I., et al. (2015). Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. 112, 11353–11358. doi: 10.1073/pnas.1503058112
Johani, K., Fritz, B. G., Bjarnsholt, T., Lipsky, B. A., Jensen, S. O., Yang, M., et al. (2019). Understanding the microbiome of diabetic foot osteomyelitis: insights from molecular and microscopic approaches. Clin. Microbiol. Infect. 25, 332–339. doi: 10.1016/j.cmi.2018.04.036
Johansson, M. E. V., Gustafsson, J. K., Holmén-Larsson, J., Jabbar, K. S., Xia, L., Xu, H., et al. (2014). Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291. doi: 10.1136/gutjnl-2012-303207
Johansson, M. E. V., Jakobsson, H. E., Holmén-Larsson, J., Schütte, A., Ermund, A., Rodríguez-Piñeiro, A. M., et al. (2015). Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592. doi: 10.1016/j.chom.2015.10.007
Johnson, M., Cockayne, A., Williams, P. H., Morrissey, J. A. (2005). Iron-responsive regulation of biofilm formation in Staphylococcus aureus involves Fur-dependent and Fur-independent mechanisms. J. Bacteriol 187, 8211–8215. doi: 10.1128/jb.187.23.8211-8215.2005
Johnston, W., Rosier, B. T., Artacho, A., Paterson, M., Piela, K., Delaney, C., et al. (2021). Mechanical biofilm disruption causes microbial and immunological shifts in periodontitis patients. Sci. Rep-uk 11, 9796. doi: 10.1038/s41598-021-89002-z
Jurcisek, J. A., Bakaletz, L. O. (2007). Biofilms formed by Nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J. Bacteriol 189, 3868–3875. doi: 10.1128/jb.01935-06
Jurcisek, J., Greiner, L., Watanabe, H., Zaleski, A., Apicella, M. A., Bakaletz, L. O. (2005). Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 73, 3210–3218. doi: 10.1128/iai.73.6.3210-3218.2005
Kadurugamuwa, J. L., Beveridge, T. J. (1995). Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol 177, 3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995
Kamaguchi, A., Baba, H. (1995). Porphyromonas gingivalis vesicles promote the coaggregation of mutans streptococci and early colonizers. Jpn. J. Oral. Biol. 37, 335–339. doi: 10.2330/joralbiosci1965.37.335
Kamarajan, P., Ateia, I., Shin, J. M., Fenno, J. C., Le, C., Zhan, L., et al. (2020). Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PloS Pathog. 16, e1008881. doi: 10.1371/journal.ppat.1008881
Kankainen, M., Paulin, L., Tynkkynen, S., Ossowski, I., von, Reunanen, J., Partanen, P., et al. (2009). Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc. Natl. Acad. Sci. 106, 17193–17198. doi: 10.1073/pnas.0908876106
Kavanaugh, J. S., Flack, C. E., Lister, J., Ricker, E. B., Ibberson, C. B., Jenul, C., et al. (2019). Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio 10, e01137–e01119. doi: 10.1128/mbio.01137-19
Kennedy, P., Brammah, S., Wills, E. (2010). Burns, biofilm and a new appraisal of burn wound sepsis. Burns 36, 49–56. doi: 10.1016/j.burns.2009.02.017
Khan, A. S., Kniep, B., Oelschlaeger, T. A., Die, I. V., Korhonen, T., Hacker, J. (2000). Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect. Immun. 68, 3541–3547. doi: 10.1128/iai.68.6.3541-3547.2000
Khatoon, Z., McTiernan, C. D., Suuronen, E. J., Mah, T.-F., Alarcon, E. I. (2018). Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4, e01067. doi: 10.1016/j.heliyon.2018.e01067
Kim, R., Attayek, P. J., Wang, Y., Furtado, K. L., Tamayo, R., Sims, C. E., et al. (2019). An in vitro intestinal platform with a self-sustaining oxygen gradient to study the human gut/microbiome interface. Biofabrication 12, 015006. doi: 10.1088/1758-5090/ab446e
Klausen, M., Heydorn, A., Ragas, P., Lambertsen, L., Aaes-Jørgensen, A., Molin, S., et al. (2003). Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48, 1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x
Klemm, P., Vejborg, R. M., Sherlock, O. (2006). Self-associating autotransporters, SAATs: Functional and structural similarities. Int. J. Med. Microbiol. 296, 187–195. doi: 10.1016/j.ijmm.2005.10.002
Kolenbrander, P. E., London, J. (1993). Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol 175, 3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993
Kolenbrander, P. E., Palmer, R. J., Periasamy, S., Jakubovics, N. S. (2010). Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 8, 471–480. doi: 10.1038/nrmicro2381
Kolpen, M., Kragh, K. N., Enciso, J. B., Faurholt-Jepsen, D., Lindegaard, B., Egelund, G. B., et al. (2022). Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 77, 1015–1022. doi: 10.1136/thoraxjnl-2021-217576
Komlós, G., Csurgay, K., Horváth, F., Pelyhe, L., Németh, Z. (2021). Periodontitis as a risk for oral cancer: a case–control study. BMC Oral. Health 21, 640. doi: 10.1186/s12903-021-01998-y
Konto-Ghiorghi, Y., Mairey, E., Mallet, A., Duménil, G., Caliot, E., Trieu-Cuot, P., et al. (2009). Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PloS Pathog. 5, e1000422. doi: 10.1371/journal.ppat.1000422
Koo, H., Xiao, J., Klein, M. I., Jeon, J. G. (2010). Exopolysaccharides Produced by Streptococcus mutans Glucosyltransferases Modulate the Establishment of Microcolonies within Multispecies Biofilms. J. Bacteriol 192, 3024–3032. doi: 10.1128/jb.01649-09
Köseoğlu, V. K., Hall, C. P., Rodríguez-López, E. M., Agaisse, H. (2019). The autotransporter IcsA promotes Shigella flexneri biofilm formation in the presence of bile salts. Infect. Immun. 87, e00861-18. doi: 10.1128/iai.00861-18
Kovacs-Simon, A., Leuzzi, R., Kasendra, M., Minton, N., Titball, R. W., Michell, S. L. (2014). Lipoprotein CD0873 is a novel adhesin of Clostridium difficile. J. Infect. Dis. 210, 274–284. doi: 10.1093/infdis/jiu070
Lam, J., Chan, R., Lam, K., Costerton, J. W. (1980). Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28, 546–556. doi: 10.1128/iai.28.2.546-556.1980
Lanter, B. B., Sauer, K., Davies, D. G. (2014). Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. mBio 5, e01206–e01214. doi: 10.1128/mbio.01206-14
Larsen, T., Fiehn, N. (2017). Dental biofilm infections – an update. APMIS 125, 376–384. doi: 10.1111/apm.12688
Lasaro, M. A., Salinger, N., Zhang, J., Wang, Y., Zhong, Z., Goulian, M., et al. (2009). F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl. Environ. Microb. 75, 246–251. doi: 10.1128/aem.01144-08
Latasa, C., Roux, A., Toledo-Arana, A., Ghigo, J., Gamazo, C., Penadés, J. R., et al. (2005). BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol. Microbiol. 58, 1322–1339. doi: 10.1111/j.1365-2958.2005.04907.x
Leatham, M. P., Banerjee, S., Autieri, S. M., Mercado-Lubo, R., Conway, T., Cohen, P. S. (2009). Precolonized Human Commensal Escherichia coli Strains Serve as a Barrier to E. coli O157:H7 Growth in the Streptomycin-Treated Mouse Intestine. Infect. Immun. 77, 2876–2886. doi: 10.1128/iai.00059-09
Ledeboer, N. A., Jones, B. D. (2005). Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar typhimurium on HEp-2 cells and chicken intestinal epithelium. J. Bacteriol 187, 3214–3226. doi: 10.1128/jb.187.9.3214-3226.2005
Lee, M. R., Pawlowski, K. S., Luong, A., Furze, A. D., Roland, P. S. (2009). Biofilm presence in humans with chronic suppurative otitis media. Otolaryngol. - Head Neck Surg. 141, 567–571. doi: 10.1016/j.otohns.2009.08.010
Lee, K. K., Sheth, H. B., Wong, W. Y., Sherburne, R., Paranchych, W., Hodges, R. S., et al. (1994). The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11, 705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x
Leeuwenhoek, A. (1684). An abstract of a letter from Mr. Anthony Leevvenhoeck at Delft, dated Sep. 17. 1683. Containing some microscopical observations, about animals in the scurf of the teeth, the substance call'd worms in the nose, the cuticula consisting of scales. Phil. Trans. R. Soc. 14, 568–574. doi: 10.1098/rstl.1684.0030
Leid, J. G., Shirtliff, M. E., Costerton, J. W., Stoodley, P. (2002). Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 70, 6339–6345. doi: 10.1128/iai.70.11.6339-6345.2002
Leid, J. G., Willson, C. J., Shirtliff, M. E., Hassett, D. J., Parsek, M. R., Jeffers, A. K. (2005). The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-γ-mediated macrophage killing. J. Immunol. 175, 7512–7518. doi: 10.4049/jimmunol.175.11.7512
Leme, A. F. P., Koo, H., Bellato, C. M., Bedi, G., Cury, J. A. (2006). The role of sucrose in cariogenic dental biofilm formation—New insight. J. Dent. Res. 85, 878–887. doi: 10.1177/154405910608501002
Lerche, C. J., Schwartz, F., Theut, M., Fosbøl, E. L., Iversen, K., Bundgaard, H., et al. (2021). Anti-biofilm approach in infective endocarditis exposes new treatment strategies for improved outcome. Front. Cell Dev. Biol. 9. doi: 10.3389/fcell.2021.643335
Liao, S., Klein, M. I., Heim, K. P., Fan, Y., Bitoun, J. P., Ahn, S.-J., et al. (2014). Streptococcus mutans extracellular DNA Is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol 196, 2355–2366. doi: 10.1128/jb.01493-14
Lillehoj, E. P., Kim, B. T., Kim, K. C. (2002). Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol-lung C 282, L751–L756. doi: 10.1152/ajplung.00383.2001
Limoli, D. H., Jones, C. J., Wozniak, D. J. (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 3, MB-0011-2014. doi: 10.1128/microbiolspec.mb-0011-2014
Lin, M.-H., Shu, J.-C., Huang, H.-Y., Cheng, Y.-C. (2012). Involvement of iron in biofilm formation by Staphylococcus aureus. PloS One 7, e34388. doi: 10.1371/journal.pone.0034388
London, J., Allen, J. (1990). Purification and characterization of a Bacteroides loeschei adhesin that interacts with procaryotic and eucaryotic cells. J. Bacteriol 172, 2527–2534. doi: 10.1128/jb.172.5.2527-2534.1990
Luo, T. L., Vanek, M. E., Gonzalez-Cabezas, C., Marrs, C. F., Foxman, B., Rickard, A. H. (2022). In vitro model systems for exploring oral biofilms: From single-species populations to complex multi-species communities. J. Appl. Microbiol. 132, 855–871. doi: 10.1111/jam.15200
Ma, L., Jackson, K. D., Landry, R. M., Parsek, M. R., Wozniak, D. J. (2006). Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol 188, 8213–8221. doi: 10.1128/jb.01202-06
Magalhães, A. P., França, A., Pereira, M. O., Cerca, N. (2022). Unveiling co-infection in cystic fibrosis airways: transcriptomic analysis of Pseudomonas aeruginosa and Staphylococcus aureus dual-species biofilms. Front. Genet. 13. doi: 10.3389/fgene.2022.883199
Maldarelli, G. A., Piepenbrink, K. H., Scott, A. J., Freiberg, J. A., Song, Y., Achermann, Y., et al. (2016). Type IV pili promote early biofilm formation by Clostridium difficile. Pathog. Dis. 74, ftw061. doi: 10.1093/femspd/ftw061
Mallmann, C., Siemoneit, S., Schmiedel, D., Petrich, A., Gescher, D. M., Halle, E., et al. (2010). Fluorescence in situ hybridization to improve the diagnosis of endocarditis: a pilot study. Clin. Microbiol. Infect. 16, 767–773. doi: 10.1111/j.1469-0691.2009.02936.x
Maltby, R., Leatham-Jensen, M. P., Gibson, T., Cohen, P. S., Conway, T. (2013). Nutritional Basis for Colonization Resistance by Human Commensal Escherichia coli Strains HS and Nissle 1917 against E. coli O157:H7 in the Mouse Intestine. PloS One 8, e53957. doi: 10.1371/journal.pone.0053957
Maresca, M., Alatou, R., Pujol, A., Nicoletti, C., Perrier, J., Giardina, T., et al. (2021). RadA, a MSCRAMM adhesin of the dominant symbiote ruminococcus gnavus E1, binds human immunoglobulins and intestinal mucins. Biomol 11, 1613. doi: 10.3390/biom11111613
Marrie, T. J., Cooper, J. H., Costerton, J. W. (1987). Ultrastructure of cardiac bacterial vegetations on native valves with emphasis on alterations in bacterial morphology following antibiotic treatment. Can. J. Cardiol. 3, 275–280.
Marsh, P. D. (2010). Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N Am. 54, 441–454. doi: 10.1016/j.cden.2010.03.002
Marsh, R. L., Binks, M. J., Smith-Vaughan, H. C., Janka, M., Clark, S., Richmond, P., et al. (2022). Prevalence and subtyping of biofilms present in bronchoalveolar lavage from children with protracted bacterial bronchitis or non-cystic fibrosis bronchiectasis: a cross-sectional study. Lancet Microbe 3, e215–e223. doi: 10.1016/s2666-5247(21)00300-1
Martini, A. M., Moricz, B. S., Woods, L. J., Jones, B. D. (2021). Type IV pili of Streptococcus sanguinis contribute to pathogenesis in experimental infective endocarditis. Microbiol. Spectr. 9, e01752–e01721. doi: 10.1128/spectrum.01752-21
Martín-Rodríguez, A. J., Rhen, M., Melican, K., Richter-Dahlfors, A. (2020). Nitrate metabolism modulates biosynthesis of biofilm components in uropathogenic Escherichia coli and acts as a fitness factor during experimental urinary tract infection. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00026
Mashruwala, A. A., Guchte, A.v. de, Boyd, J. M. (2017). Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus. Elife 6, e23845. doi: 10.7554/elife.23845
Matsui, H., Wagner, V. E., Hill, D. B., Schwab, U. E., Rogers, T. D., Button, B., et al. (2006). A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. 103, 18131–18136. doi: 10.1073/pnas.0606428103
Matsumoto-Nakano, M. (2018). Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent. Sci. Rev. 54, 22–29. doi: 10.1016/j.jdsr.2017.08.002
McLean, R. J. C., Nickel, J. C., Beveridge, T. J., Costerton, J. W. (1989). Observations of the ultrastructure of infected kidney stones. J. Med. Microbiol. 29, 1–7. doi: 10.1099/00222615-29-1-1
Meng, Q., Gao, Q., Mehrazarin, S., Tangwanichgapong, K., Wang, Y., Huang, Y., et al. (2021). Fusobacterium nucleatum secretes amyloid-like FadA to enhance pathogenicity. EMBO Rep. 22, e52891. doi: 10.15252/embr.202152891
Menozzi, F. D., Rouse, J. H., Alavi, M., Laude-Sharp, M., Muller, J., Bischoff, R., et al. (1996). Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 184, 993–1001. doi: 10.1084/jem.184.3.993
Metcalf, D. G., Bowler, P. G. (2013). Biofilm delays wound healing: A review of the evidence. Burn Trauma 1, 5–12. doi: 10.4103/2321-3868.113329
Mihajlovic, J., Bechon, N., Ivanova, C., Chain, F., Almeida, A., Langella, P., et al. (2019). A putative type V pilus contributes to Bacteroides thetaiotaomicron biofilm formation capacity. J. Bacteriol 201, e00650-18. doi: 10.1128/jb.00650-18
Miller, A. L., Anda, J., Wong, G. C. L., Tükel, Ç. (2022a). Amyloid-containing biofilms and autoimmunity. Curr. Opin. Struc Biol. 75, 102435. doi: 10.1016/j.sbi.2022.102435
Miller, A. L., Nicastro, L. K., Bessho, S., Grando, K., White, A. P., Zhang, Y., et al. (2022b). Nitrate Is an Environmental Cue in the Gut for Salmonella enterica Serovar Typhimurium Biofilm Dispersal through Curli Repression and Flagellum Activation via Cyclic-di-GMP Signaling. Mbio 13, e02886–e02821. doi: 10.1128/mbio.02886-21
Miller, A. L., Pasternak, J. A., Medeiros, N. J., Nicastro, L. K., Tursi, S. A., Hansen, E. G., et al. (2020). In vivo synthesis of bacterial amyloid curli contributes to joint inflammation during S. Typhimurium infection. PloS Pathog. 16, e1008591. doi: 10.1371/journal.ppat.1008591
Morris, D. P. (2007). Bacterial biofilm in upper respiratory tract infections. Curr. Infect. Dis. Rep. 9, 186–192. doi: 10.1007/s11908-007-0030-3
Mulcahy, H., Charron-Mazenod, L., Lewenza, S. (2008). Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PloS Pathog. 4, e1000213. doi: 10.1371/journal.ppat.1000213
Murakawa, T. (1973). Slime production by Pseudomonas aeruginosa. Jpn J. Microbiol. 17, 513–520. doi: 10.1111/j.1348-0421.1973.tb00937.x
Musk, D. J., Banko, D. A., Hergenrother, P. J. (2005). Iron salts perturb biofilm formation and disrupt existing biofilms of Pseudomonas aeruginosa. Chem. Biol. 12, 789–796. doi: 10.1016/j.chembiol.2005.05.007
Nguyen, D., Joshi-Datar, A., Lepine, F., Bauerle, E., Olakanmi, O., Beer, K., et al. (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986. doi: 10.1126/science.1211037
Nguyen, T. L. A., Vieira-Silva, S., Liston, A., Raes, J. (2015). How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8, 1–16. doi: 10.1242/dmm.017400
Nicastro, L. K., Anda, J., de, Jain, N., Grando, K. C. M., Miller, A. L., Bessho, S., et al. (2022). Assembly of ordered DNA-curli fibril complexes during Salmonella biofilm formation correlates with strengths of the type I interferon and autoimmune responses. PloS Pathog. 18, e1010742. doi: 10.1371/journal.ppat.1010742
Nickel, J. C., Costerton, J. W. (1992). Coagulase-negative staphylococcus in chronic prostatitis. J. Urol. 147, 398–400. doi: 10.1016/s0022-5347(17)37247-6
Nickel, J. C., Costerton, J. W. (1993). Bacterial localization in antibiotic-refractory chronic bacterial prostatitis. Prostate 23, 107–114. doi: 10.1002/pros.2990230204
Nickel, J. C., Emtage, J., Costerton, J. W. (1985). Ultrastructural microbial ecology of infection-induced urinary stones. J. Urol. 133, 622–627. doi: 10.1016/s0022-5347(17)49116-6
Nickerson, K. P., Chanin, R. B., Sistrunk, J. R., Rasko, D. A., Fink, P. J., Barry, E. M., et al. (2017). Analysis of Shigella flexneri resistance, biofilm formation, and transcriptional profile in response to bile salts. Infect. Immun. 85, e01067-16. doi: 10.1128/iai.01067-16
Nickerson, K. P., McDonald, C. (2012). Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PloS One 7, e52132. doi: 10.1371/journal.pone.0052132
Nishiyama, K., Ueno, S., Sugiyama, M., Yamamoto, Y., Mukai, T. (2016). Lactobacillus rhamnosus GG SpaC pilin subunit binds to the carbohydrate moieties of intestinal glycoconjugates. Anim. Sci. J. 87, 809–815. doi: 10.1111/asj.12491
Nobbs, A. H., Jenkinson, H. F., Jakubovics, N. S. (2011). Stick to your gums. J. Dent. Res. 90, 1271–1278. doi: 10.1177/0022034511399096
Nur, A., Hirota, K., Yumoto, H., Hirao, K., Liu, D., Takahashi, K., et al. (2013). Effects of extracellular DNA and DNA-binding protein on the development of a Streptococcus intermedius biofilm. J. Appl. Microbiol. 115, 260–270. doi: 10.1111/jam.12202
Nyka, W. (1963). Studies on Mycobacterium tuberculosis in lesions of the human lung. A new method of staining tubercle bacilli in tissue sections. Am. Rev. Respir. Dis. 88, 670–679. doi: 10.1164/arrd.1963.88.5.670
Nyka, W., O’Neill, E. F. (1970). A new approach to the study of non-acid-fast mycobacteria. Ann. Ny Acad. Sci. 174, 862–871. doi: 10.1111/j.1749-6632.1970.tb45605.x
Oli, M. W., Otoo, H. N., Crowley, P. J., Heim, K. P., Nascimento, M. M., Ramsook, C. B., et al. (2012). Functional amyloid formation by Streptococcus mutans. Microbiology 158, 2903–2916. doi: 10.1099/mic.0.060855-0
Orme, I. M. (2014). A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis 94, 8–14. doi: 10.1016/j.tube.2013.07.004
Pachucki, R. J., Corradetti, C., Kohler, L., Ghadiali, J., Gallo, P. M., Nicastro, L., et al. (2020). Persistent bacteriuria and antibodies recognizing curli/eDNA complexes from Escherichia coli are linked to flares in systemic lupus erythematosus. Arthritis Rheumatol 72, 1872–1881. doi: 10.1002/art.41400
Palmer, R. J., Gordon, S. M., Cisar, J. O., Kolenbrander, P. E. (2003). Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol 185, 3400–3409. doi: 10.1128/jb.185.11.3400-3409.2003
Park, Y., Simionato, M. R., Sekiya, K., Murakami, Y., James, D., Chen, W., et al. (2005). Short Fimbriae of Porphyromonas gingivalis and Their Role in Coadhesion with Streptococcus gordonii. Infect. Immun. 73, 3983–3989. doi: 10.1128/iai.73.7.3983-3989.2005
Pastar, I., Nusbaum, A. G., Gil, J., Patel, S. B., Chen, J., Valdes, J., et al. (2013). Interactions of Methicillin Resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in Polymicrobial Wound Infection. PloS One 8, e56846. doi: 10.1371/journal.pone.0056846
Pauer, H., Ferreira, E., de, O., Santos-Filho, J. D., Portela, M. B., Zingali, R. B., et al. (2009). A TonB-dependent outer membrane protein as a Bacteroides fragilis fibronectin-binding molecule. FEMS Immunol. Med. Microbiol. 55, 388–395. doi: 10.1111/j.1574-695x.2009.00532.x
Pelicic, V. (2008). Type IV pili: e pluribus unum? Mol. Microbiol. 68, 827–837. doi: 10.1111/j.1365-2958.2008.06197.x
Percival, S. L., McCarty, S. M., Lipsky, B. (2015). Biofilms and wounds: An overview of the evidence. Adv. Wound Care 4, 373–381. doi: 10.1089/wound.2014.0557
Periasamy, S., Kolenbrander, P. E. (2010). Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J. Bacteriol 192, 2965–2972. doi: 10.1128/jb.01631-09
Pleguezuelos-Manzano, C., Puschhof, J., Huber, A. R., Hoeck, A., Wood, H. M., Nomburg, J., et al. (2020). Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273. doi: 10.1038/s41586-020-2080-8
Pompilio, A., Crocetta, V., Nicola, S. D., Verginelli, F., Fiscarelli, E., Bonaventura, G. D. (2015). Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00951
Post, S., van der, Jabbar, K. S., Birchenough, G., Arike, L., Akhtar, N., Sjovall, H., et al. (2019). Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 68, 2142. doi: 10.1136/gutjnl-2018-317571
Pradhan, S., Weiss, A. A. (2020). Probiotic properties of Escherichia coli Nissle in human intestinal organoids. Mbio 11, e01470–e01420. doi: 10.1128/mbio.01470-20
Proft, T., Baker, E. N. (2008). Pili in Gram-negative and Gram-positive bacteria — structure, assembly and their role in disease. Cell Mol. Life Sci. 66, 613. doi: 10.1007/s00018-008-8477-4
Pumbwe, L., Skilbeck, C. A., Nakano, V., Avila-Campos, M. J., Piazza, R. M. F., Wexler, H. M. (2007). Bile salts enhance bacterial co-aggregation, bacterial-intestinal epithelial cell adhesion, biofilm formation and antimicrobial resistance of Bacteroides fragilis. Microb. Pathogenesis 43, 78–87. doi: 10.1016/j.micpath.2007.04.002
Puschhof, J., Pleguezuelos-Manzano, C., Martinez-Silgado, A., Akkerman, N., Saftien, A., Boot, C., et al. (2021). Intestinal organoid cocultures with microbes. Nat. Protoc. 16, 4633–4649. doi: 10.1038/s41596-021-00589-z
Qvist, T., Eickhardt, S., Kragh, K. N., Andersen, C. B., Iversen, M., Høiby, N., et al. (2015). Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 46, 1823–1826. doi: 10.1183/13993003.01102-2015
Radomski, J. L., Palmiri, C., Hearn, W. L. (1978). Concentrations of nitrate in normal human urine and the effect of nitrate ingestion. Toxicol. Appl. Pharm. 45, 63–68. doi: 10.1016/0041-008x(78)90028-5
Ramsugit, S., Guma, S., Pillay, B., Jain, P., Larsen, M. H., Danaviah, S., et al. (2013). Pili contribute to biofilm formation in vitro in Mycobacterium tuberculosis. Antonie Van Leeuwenhoek 104, 725–735. doi: 10.1007/s10482-013-9981-6
Re, S. D., Valle, J., Charbonnel, N., Beloin, C., Latour-Lambert, P., Faure, P., et al. (2013). Identification of commensal Escherichia coli genes involved in biofilm resistance to pathogen colonization. PloS One 8, e61628. doi: 10.1371/journal.pone.0061628
Reichhardt, C., Jacobs, H. M., Matwichuk, M., Wong, C., Wozniak, D. J., Parsek, M. R. (2020). The Versatile Pseudomonas aeruginosa Biofilm Matrix Protein CdrA Promotes Aggregation through Different Extracellular Exopolysaccharide Interactions. J. Bacteriol 202, e00216–e00220. doi: 10.1128/jb.00216-20
Renner, L. D., Weibel, D. B. (2011). Physicochemical regulation of biofilm formation. Mrs Bull. 36, 347–355. doi: 10.1557/mrs.2011.65
Rivera-Chávez, F., Bäumler, A. J. (2014). The pyromaniac inside you: Salmonella metabolism in the host gut. Annu. Rev. Microbiol. 69, 1–18. doi: 10.1146/annurev-micro-091014-104108
Rodesney, C. A., ROman, B., Dhamani, N., Cooley, B. J., Katira, P., Touhami, A., et al. (2017). Mechanosensing of shear by Pseudomonas aeruginosa leads to increased levels of the cyclic-di-GMP signal initiating biofilm development. Proc. Natl. Acad. Sci. 114, 5906–5911. doi: 10.1073/pnas.1703255114
Rogemond, V., Guinet, R. M. (1986). Lectinlike adhesins in the Bacteroides fragilis group. Infect. Immun. 53, 99–102. doi: 10.1128/iai.53.1.99-102.1986
Romanova, Y., Mulabaev, N. S., Tolordava, E. R., Seregin, A. V., Seregin, I. V., Alexeeva, N. V., et al. (2015). Microbial communities on kidney stones. Mol. Genet. Microbiol. Virol. 30, 78–84. doi: 10.3103/s089141681502007x
Rosen, D. A., Hooton, T. M., Stamm, W. E., Humphrey, P. A., Hultgren, S. J. (2007). Detection of intracellular bacterial communities in human urinary tract infection. PloS Med. 4, e329. doi: 10.1371/journal.pmed.0040329
Rossi, E., Cimdins, A., Lüthje, P., Brauner, A., Sjöling, Å., Landini, P., et al. (2017). “It’s a gut feeling” – Escherichia coli biofilm formation in the gastrointestinal tract environment. Crit. Rev. Microbiol. 44, 1–30. doi: 10.1080/1040841x.2017.1303660
Roy, S., Santra, S., Das, A., Dixith, S., Sinha, M., Ghatak, S., et al. (2020). Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 271, 1174–1185. doi: 10.1097/sla.0000000000003053
Rozen, R., Bachrach, G., Bronshteyn, M., Gedalia, I., Steinberg, D. (2001). The role of fructans on dental biofilm formation by Streptococcus sobrinus, Streptococcus mutans, Streptococcus gordonii and Actinomyces viscosus. FEMS Microbiol. Lett. 195, 205–210. doi: 10.1111/j.1574-6968.2001.tb10522.x
Rumbaugh, K. P., Sauer, K. (2020). Biofilm dispersion. Nat. Rev. Microbiol. 18, 571–586. doi: 10.1038/s41579-020-0385-0
Rusconi, R., Guasto, J. S., Stocker, R. (2014). Bacterial transport suppressed by fluid shear. Nat. Phys. 10, 212–217. doi: 10.1038/nphys2883
Saint-Ruf, C., Garfa-Traoré, M., Collin, V., Cordier, C., Franceschi, C., Matic, I. (2014). Massive diversification in aging colonies of Escherichia coli. J. Bacteriol 196, 3059–3073. doi: 10.1128/jb.01421-13
Saldaña, Z., Xicohtencatl-Cortes, J., Avelino, F., Phillips, A. D., Kaper, J. B., Puente, J. L., et al. (2009). Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ. Microbiol. 11, 992–1006. doi: 10.1111/j.1462-2920.2008.01824.x
Salsgiver, E. L., Fink, A. K., Knapp, E. A., LiPuma, J. J., Olivier, K. N., Marshall, B. C., et al. (2016). Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 149, 390–400. doi: 10.1378/chest.15-0676
Sanderson, A. R., Leid, J. G., Hunsaker, D. (2006). Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope 116, 1121–1126. doi: 10.1097/01.mlg.0000221954.05467.54
Sanfilippo, J. E., Lorestani, A., Koch, M. D., Bratton, B. P., Siryaporn, A., Stone, H. A., et al. (2019). Microfluidic-based transcriptomics reveal force-independent bacterial rheosensing. Nat. Microbiol. 4, 1274–1281. doi: 10.1038/s41564-019-0455-0
Sato, T., Stange, D. E., Ferrante, M., Vries, R. G. J., Es, J.H.v., Brink, S.v. d., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772. doi: 10.1053/j.gastro.2011.07.050
Sauer, K., Stoodley, P., Goeres, D. M., Hall-Stoodley, L., Burmølle, M., Stewart, P. S., et al. (2022). The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 20, 608–620. doi: 10.1038/s41579-022-00767-0
Scherr, T. D., Hanke, M. L., Huang, O., James, D. B. A., Horswill, A. R., Bayles, K. W., et al. (2015). Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. Mbio 6, e01021–e01015. doi: 10.1128/mbio.01021-15
Schierle, C. F., Garza, M. D., Mustoe, T. A., Galiano, R. D. (2009). Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regener. 17, 354–359. doi: 10.1111/j.1524-475x.2009.00489.x
Schooling, S. R., Beveridge, T. J. (2006). Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957. doi: 10.1128/jb.00257-06
Schooling, S. R., Hubley, A., Beveridge, T. J. (2009). Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol. 191, 4097–4102. doi: 10.1128/jb.00717-08
Schwartz, K., Syed, A. K., Stephenson, R. E., Rickard, A. H., Boles, B. R. (2012). Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PloS Pathog. 8, e1002744. doi: 10.1371/journal.ppat.1002744
Sedghizadeh, P. P., Kumar, S. K. S., Gorur, A., Schaudinn, C., Shuler, C. F., Costerton, J. W. (2009). Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J. Am. Dent. Assoc. 140, 1259–1265. doi: 10.14219/jada.archive.2009.0049
Serra, D. O., Richter, A. M., Hengge, R. (2013). Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol 195, 5540–5554. doi: 10.1128/jb.00946-13
Seth, A. K., Geringer, M. R., Galiano, R. D., Leung, K. P., Mustoe, T. A., Hong, S. J. (2012). Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. J. Am. Coll. Surgeons 215, 388–399. doi: 10.1016/j.jamcollsurg.2012.05.028
Shin, W., Wu, A., Massidda, M. W., Foster, C., Thomas, N., Lee, D.-W., et al. (2019). A robust longitudinal co-culture of obligate anaerobic gut microbiome with human intestinal epithelium in an anoxic-oxic interface-on-a-chip. Front. Bioeng Biotechnol. 7. doi: 10.3389/fbioe.2019.00013
Singh, P. K., Parsek, M. R., Greenberg, E. P., Welsh, M. J. (2002). A component of innate immunity prevents bacterial biofilm development. Nature 417, 552–555. doi: 10.1038/417552a
Skurnik, D., Davis, M. R., Benedetti, D., Moravec, K. L., Cywes-Bentley, C., Roux, D., et al. (2012). Targeting pan-resistant bacteria with antibodies to a broadly conserved surface polysaccharide expressed during infection. J. Infect. Dis. 205, 1709–1718. doi: 10.1093/infdis/jis254
Snow, D. E., Everett, J., Mayer, G., Cox, S. B., Miller, B., Rumbaugh, K., et al. (2016). The presence of biofilm structures in atherosclerotic plaques of arteries from legs amputated as a complication of diabetic foot ulcers. J. Wound Care 25, S16–S22. doi: 10.12968/jowc.2016.25.sup2.s16
Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C., Kent, R. L. (1998). Microbial complexes in subgingival plaque. J. Clin. Periodontol 25, 134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x
Sontheimer-Phelps, A., Chou, D. B., Tovaglieri, A., Ferrante, T. C., Duckworth, T., Fadel, C., et al. (2020). Human colon-on-a-chip enables continuous in vitro analysis of colon mucus layer accumulation and physiology. Cell Mol. Gastroenterol. Hepatol. 9, 507–526. doi: 10.1016/j.jcmgh.2019.11.008
Spero, M. A., Newman, D. K. (2018). Chlorate specifically targets oxidant-starved, antibiotic-tolerant populations of Pseudomonas aeruginosa biofilms. mBio 9, e01400–e01418. doi: 10.1128/mbio.01400-18
Stapper, A. P., Narasimhan, G., Ohman, D. E., Barakat, J., Hentzer, M., Molin, S., et al. (2004). Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J. Med. Microbiol. 53, 679–690. doi: 10.1099/jmm.0.45539-0
Steinberg, D., Poran, S., Shapira, L. (1999). The effect of extracellular polysaccharides from Streptococcus mutans on the bactericidal activity of human neutrophils. Arch. Oral. Biol. 44, 437–444. doi: 10.1016/s0003-9969(99)00014-x
Steinberger, R. E., Holden, P. A. (2005). Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl. Environ. Microb. 71, 5404–5410. doi: 10.1128/aem.71.9.5404-5410.2005
Stewart, P. S. (2002). Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292, 107–113. doi: 10.1078/1438-4221-00196
Stewart, P. S., Franklin, M. J. (2008). Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6, 199–210. doi: 10.1038/nrmicro1838
Stoodley, P., Sauer, K., Davies, D. G., Costerton, J. W. (2002). Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209. doi: 10.1146/annurev.micro.56.012302.160705
Sugimoto, S., Sato, F., Miyakawa, R., Chiba, A., Onodera, S., Hori, S., et al. (2018). Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep-uk 8, 2254. doi: 10.1038/s41598-018-20485-z
Sutherland, I. W. (2001). The biofilm matrix – an immobilized but dynamic microbial environment. Trends Microbiol. 9, 222–227. doi: 10.1016/s0966-842x(01)02012-1
Swidsinski, A., Mendling, W., Loening-Baucke, V., Ladhoff, A., Swidsinski, S., Hale, L. P., et al. (2005a). Adherent biofilms in bacterial vaginosis. Obstetrics Gynecol 106, 1013–1023. doi: 10.1097/01.aog.0000183594.45524.d2
Swidsinski, A., Weber, J., Loening-Baucke, V., Hale, L. P., Lochs, H. (2005b). Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 43, 3380–3389. doi: 10.1128/jcm.43.7.3380-3389.2005
Tack, K. J., Sabath, L. D. (1985). Increased minimum inhibitory concentrations with anaerobiasis for tobramycin, gentamicin, and amikacin, compared to latamoxef, piperacillin, chloramphenicol, and clindamycin. Chemotherapy 31, 204–210. doi: 10.1159/000238337
Taglialegna, A., Navarro, S., Ventura, S., Garnett, J. A., Matthews, S., Penades, J. R., et al. (2016). Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PloS Pathog. 12, e1005711. doi: 10.1371/journal.ppat.1005711
Tamesada, M., Kawabata, S., Fujiwara, T., Hamada, S. (2004). Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J. Dent. Res. 83, 874–879. doi: 10.1177/154405910408301110
Tamura, S., Yonezawa, H., Motegi, M., Nakao, R., Yoneda, S., Watanabe, H., et al. (2009). Inhibiting effects of Streptococcus salivarius on competence-stimulating peptide-dependent biofilm formation by Streptococcus mutans. Oral. Microbiol. Immun. 24, 152–161. doi: 10.1111/j.1399-302x.2008.00489.x
Telford, J. L., Barocchi, M. A., Margarit, I., Rappuoli, R., Grandi, G. (2006). Pili in Gram-positive pathogens. Nat. Rev. Microbiol. 4, 509–519. doi: 10.1038/nrmicro1443
Thomas-White, K., Brady, M., Wolfe, A. J., Mueller, E. R. (2016). The bladder is not sterile: history and current discoveries on the urinary microbiome. Curr. Bladder Dysfunct Rep. 11, 18–24. doi: 10.1007/s11884-016-0345-8
Thurlow, L. R., Hanke, M. L., Fritz, T., Angle, A., Aldrich, A., Williams, S. H., et al. (2011). Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in Vivo. J. Immunol. 186, 6585–6596. doi: 10.4049/jimmunol.1002794
Toledo-Arana, A., Valle, J., Solano, C., Arrizubieta, M. J., Cucarella, C., Lamata, M., et al. (2001). The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microb. 67, 4538–4545. doi: 10.1128/aem.67.10.4538-4545.2001
Tomkovich, S., Dejea, C. M., Winglee, K., Drewes, J. L., Chung, L., Housseau, F., et al. (2019). Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J. Clin. Invest. 129, 1699–1712. doi: 10.1172/jci124196
Tripathi, P., Beaussart, A., Alsteens, D., Dupres, V., Claes, I., Ossowski, I.v., et al. (2013). Adhesion and nanomechanics of pili from the probiotic Lactobacillus rhamnosus GG. ACS Nano 7, 3685–3697. doi: 10.1021/nn400705u
Troge, A., Scheppach, W., Schroeder, B. O., Rund, S. A., Heuner, K., Wehkamp, J., et al. (2012). More than a marine propeller – the flagellum of the probiotic Escherichia coli strain Nissle 1917 is the major adhesin mediating binding to human mucus. Int. J. Med. Microbiol. 302, 304–314. doi: 10.1016/j.ijmm.2012.09.004
Varga, J. J., Therit, B., Melville, S. B. (2008). Type IV pili and the CcpA protein are needed for maximal biofilm formation by the Gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 76, 4944–4951. doi: 10.1128/iai.00692-08
Vasseur, P., Vallet-Gely, I., Soscia, C., Genin, S., Filloux, A. (2005). The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 151, 985–997. doi: 10.1099/mic.0.27410-0
Viljoen, A., Alsteens, D., Dufrêne, Y. (2020). Mechanical forces between mycobacterial antigen 85 complex and fibronectin. Cells 9, 716. doi: 10.3390/cells9030716
Virkola, R., Westerlund, B., Holthöfer, H., Parkkinen, J., Kekomäki, M., Korhonen, T. K. (1988). Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect. Immun. 56, 2615–2622. doi: 10.1128/iai.56.10.2615-2622.1988
Vuopio-Varkila, J., Schoolnik, G. K. (1991). Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J. Exp. Med. 174, 1167–1177. doi: 10.1084/jem.174.5.1167
Wang, X., Preston, J. F., Romeo, T. (2004). The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol 186, 2724–2734. doi: 10.1128/jb.186.9.2724-2734.2004
Welch, J. L. M., Rossetti, B. J., Rieken, C. W., Dewhirst, F. E., Borisy, G. G. (2016). Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. 113, E791–E800. doi: 10.1073/pnas.1522149113
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C., Mattick, J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science 295, 1487–1487. doi: 10.1126/science.295.5559.1487
White, R. J., Cutting, K. F. (2012). Letters. J. Wound Care 21, 140–141. doi: 10.12968/jowc.2012.21.3.140
Williams, H. D., Davies, J. C. (2012). Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax 67, 465. doi: 10.1136/thoraxjnl-2011-201498
Wilton, M., Charron-Mazenod, L., Moore, R., Lewenza, S. (2016). Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Ch 60, 544–553. doi: 10.1128/aac.01650-15
Wolcott, R. D., Rhoads, D. D. (2008). A study of biofilm-based wound management in subjects with critical limb ischaemia. J. Wound Care 17, 145–155. doi: 10.12968/jowc.2008.17.4.28835
Wong, L. L., Mugunthan, S., Kundukad, B., Ho, J. C. S., Rice, S. A., Hinks, J., et al. (2023). Microbial biofilms are shaped by the constant dialogue between biological and physical forces in the extracellular matrix. Environ. Microbiol. 25, 199–208. doi: 10.1111/1462-2920.16306
Wong, E. H. J., Ng, C. G., Chua, E. G., Tay, A. C. Y., Peters, F., Marshall, B. J., et al. (2016). Comparative genomics revealed multiple Helicobacter pylori genes associated with biofilm formation in vitro. PloS One 11, e0166835. doi: 10.1371/journal.pone.0166835
Worlitzsch, D., Tarran, R., Ulrich, M., Schwab, U., Cekici, A., Meyer, K. C., et al. (2002). Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109, 317–325. doi: 10.1172/jci0213870
Wu, Y., Outten, F. W. (2009). IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J. Bacteriol 191, 1248–1257. doi: 10.1128/jb.01086-08
Wu, S., Rhee, K.-J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H.-R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022. doi: 10.1038/nm.2015
Wurpel, D. J., Beatson, S. A., Totsika, M., Petty, N. K., Schembri, M. A. (2013). Chaperone-usher fimbriae of Escherichia coli. PloS One 8, e52835. doi: 10.1371/journal.pone.0052835
Xiao, J., Klein, M. I., Falsetta, M. L., Lu, B., Delahunty, C. M., Yates, J. R., et al. (2012). The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PloS Pathog. 8, e1002623. doi: 10.1371/journal.ppat.1002623
Xu, Q., Shoji, M., Shibata, S., Naito, M., Sato, K., Elsliger, M.-A., et al. (2016). A distinct type of pilus from the human microbiome. Cell 165, 690–703. doi: 10.1016/j.cell.2016.03.016
Yadav, M. K., Vidal, J. E., Song, J.-J. (2020). “Chapter 2 - Microbial biofilms on medical indwelling devices,” in New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms, ed. Yadav, M. K., Singh, B. P. (Amsterdam, The Netherlands: Elsevier), 15–28.
Yen, M.-R., Peabody, C. R., Partovi, S. M., Zhai, Y., Tseng, Y.-H., Saier, M. H. (2002). Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim. Et Biophys. Acta Bba - Biomembr 1562, 6–31. doi: 10.1016/s0005-2736(02)00359-0
Yoon, S. S., Hennigan, R. F., Hilliard, G. M., Ochsner, U. A., Parvatiyar, K., Kamani, M. C., et al. (2002). Pseudomonas aeruginosa anaerobic respiration in biofilms relationships to cystic fibrosis pathogenesis. Dev. Cell 3, 593–603. doi: 10.1016/s1534-5807(02)00295-2
Yoshimura, F., Takahashi, K., Nodasaka, Y., Suzuki, T. (1984). Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J. Bacteriol 160, 949–957. doi: 10.1128/jb.160.3.949-957.1984
Zav’yalov, V., Zavialov, A., Zav’yalova, G., Korpela, T. (2010). Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: structure and function from a clinical standpoint. FEMS Microbiol. Rev. 34, 317–378. doi: 10.1111/j.1574-6976.2009.00201.x
Zeng, G., Vad, B. S., Dueholm, M. S., Christiansen, G., Nilsson, M., Tolker-Nielsen, T., et al. (2015). Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.01099
Zeng, L., Zhang, L., Wang, P., Meng, G. (2017). Structural basis of host recognition and biofilm formation by Salmonella Saf pili. eLife 6, e28619. doi: 10.7554/elife.28619
Zhao, G., Usui, M. L., Underwood, R. A., Singh, P. K., James, G. A., Stewart, P. S., et al. (2012). Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair Regener. 20, 342–352. doi: 10.1111/j.1524-475x.2012.00793.x
Keywords: biofilm, aggregate, chronic infection, microbiome, adhesin, extracellular matrix, antibiotic tolerance, carcinogenesis
Citation: Perry EK and Tan M-W (2023) Bacterial biofilms in the human body: prevalence and impacts on health and disease. Front. Cell. Infect. Microbiol. 13:1237164. doi: 10.3389/fcimb.2023.1237164
Received: 08 June 2023; Accepted: 11 August 2023;
Published: 30 August 2023.
Edited by:
Seána Duggan, University of Exeter, United KingdomReviewed by:
Paweł Krzyżek, Wroclaw Medical University, PolandCopyright © 2023 Perry and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man-Wah Tan, dGFuLm1hbi13YWhAZ2VuZS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.