
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cell. Infect. Microbiol., 16 August 2023
Sec. Bacteria and Host
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1229298
 Ling Qin1,2,3
Ling Qin1,2,3 Sidan Wang1,2,3
Sidan Wang1,2,3 Zhifen Zheng4,5
Zhifen Zheng4,5 Wenqian Zhang4,5
Wenqian Zhang4,5 Qiang Qu1,2,3
Qiang Qu1,2,3 Jun Li6
Jun Li6 Yurong Tan7
Yurong Tan7 Liming Cao1,2,3*
Liming Cao1,2,3*Nocardiosis is an infectious disease caused by Nocardia that primarily affects immunocompromised hosts. Mycobacterium abscessus is a common opportunistic pathogen that causes disease in humans, including pulmonary and extrapulmonary infection. Nocardia spp. infection is uncommon, and infection with Nocardia wallacei and Mycobacterium abscessus is even rarer. A 59-year-old immunocompetent woman with risk factors for environmental exposure developed nocardiosis and presented to the hospital with a cough, shortness of breath, hemoptysis, and a back abscess. An enhanced computed tomography (CT) of the chest revealed partial destruction of the right lung, as well as consolidation of the right upper lobe. Rare pathogens N. wallacei and Mycobacterium abscessus were detected by metagenomic next-generation sequencing (mNGS) from abscess on the back and lung puncture tissue, respectively. She was treated with a combination of antibiotics and was finally discharged with a good prognosis. In this case, we present a patient who was successfully diagnosed with N. wallacei and Mycobacterium abscessus infection using mNGS. This importance of using mNGS in pathogen detection and the effective use of antibiotics in treating patients with long-term rare infections is highlighted in this report.
Nocardia spp. are aerobic, Gram-positive filamentous branching bacteria with a slow growth rate and a partial acid tolerance. Nocardia spp. are uncommon in dust, decaying vegetation, soil, and aquatic environments(Chen et al., 2014; Wang et al., 2015). Over 80 species of Nocardia have been described, with approximately 30 of them known to cause human disease(King et al., 2009). Nocardiosis primarily affects immunocompromised patients, though immunocompetent individuals may be affected in rare cases. HIV infection, inflammatory bowel disease, chronic lung disease, solid-organ transplantation, autoimmune diseases, corticosteroids use and hematological malignancy are major risk factors for nocardia infection.
Because Nocardia spp. is most commonly transmitted through inhalation, the lung is the most common site of infection (62-86%)(Minero et al., 2009; Haussaire et al., 2017). In approximately 8-31% of patients with invasive nocardiosis, skin involvement can take the form of a single or cluster of pustules, nodules, or deep-seated abscesses, which may also involve the muscles(Coussement et al., 2016; Haussaire et al., 2017). Primary cutaneous nocardiosis can develop in immunocompetent patients as a result of direct microorganism inoculation into the skin as a result of trauma, which is most common in rural agricultural workers(Kanne et al., 2011; Lafont et al., 2020).
Mycobacterium abscessus is an important nontuberculous mycobacterium (NTM) that causes disease in humans, which is a common opportunistic pathogen(Boudehen and Kremer, 2021). Mycobacterium abscessus infections typically affect the skin, soft tissues, and lungs, although they can also occur in other parts of the body. In lung infections, symptoms can include persistent cough, shortness of breath, chest pain, and coughing up blood. The prevalence of M. abscessus infections vary geographically(Hsu et al., 2022). It has been reported to be more common in certain regions, such as the southeastern United States, where hot and humid climates provide favorable conditions for the growth of these bacteria(Dahl et al., 2022).
Direct examination is used to diagnose nocardiosis, which is primarily based on pathogen culture and Gram staining. In contrast, Nocardia requires at least 2-7 days of culture to slow growth and up to 4-6 weeks to develop into visible colonies(Williams et al., 2020). Metagenomic next-generation sequencing (mNGS) is a culture-independent method for detecting infectious pathogens, particularly rare or novel pathogens, and it outperforms traditional diagnostic methods, indicating its potential for use in early diagnosis(Li et al., 2021). Furthermore, mNGS takes only about 24 hours, allowing for rapid pathogen identification in the case of complex infections.
In this case, we present a patient who was successfully diagnosed with N. wallacei and Mycobacterium abscessus infection using mNGS. In the current literature, there are no reported cases of host infection with both bacteria simultaneously. This is the first case of immunocompetent patient with both Nocardia wallacei and Mycobacterium abscessus infection in China, and its clinical features and treatment experience have important value for the use of antibiotics and the treatment of pulmonary infection.
A 59-year-old woman was presented to our hospital in April 2022 with a 6-year history of coughing, shortness of breath, and a back lump, as well as a 5-year history of hemoptysis that had worsened for 3 months. The cough was paroxysmal, with mucus sputum and pulling pain in the chest and back. Over the preceding 6 years, she had noticed a lump on her right back with mild pain and a clear border. Previously She had previously been admitted to the local hospital and had received anti-infection treatment. With irregular treatment in 6 years, the size of the lump was not fixed. There was no drainage from the abscess, and she had no history of similar skin lesions. In addition to bronchiectasis, respiratory failure, pulmonary heart disease, and pulmonary hypertension, she has chronic obstructive pulmonary disease (COPD). Prior to this presentation, she was taking sulbactam, cefopcrazone, and meropenem in combination with Moxifloxacin for infection control. Over the last three months, the pain in her right chest and back has worsened, and the lump on her back has grown larger. She had been on glucocorticoids for pain relief for the previous month. The patient has no family history of pulmonary and cutaneous infections. In the history of trauma, the patient had a fracture in the distal right radius in 2016, but without particular discomfort after recovery.
When the patient arrived, her vital signs were normal and she was conscious. There was no thoracic deformity. A 7*4 cm lump can be seen on the right back, which is soft and fluctuating, with clear border and pressure pain (Figure 1). The patient’s breath sounds were weakened on the right chest, while vocal conduction and moist rales were emphasized. The cardiovascular and nervous systems performed admirably.
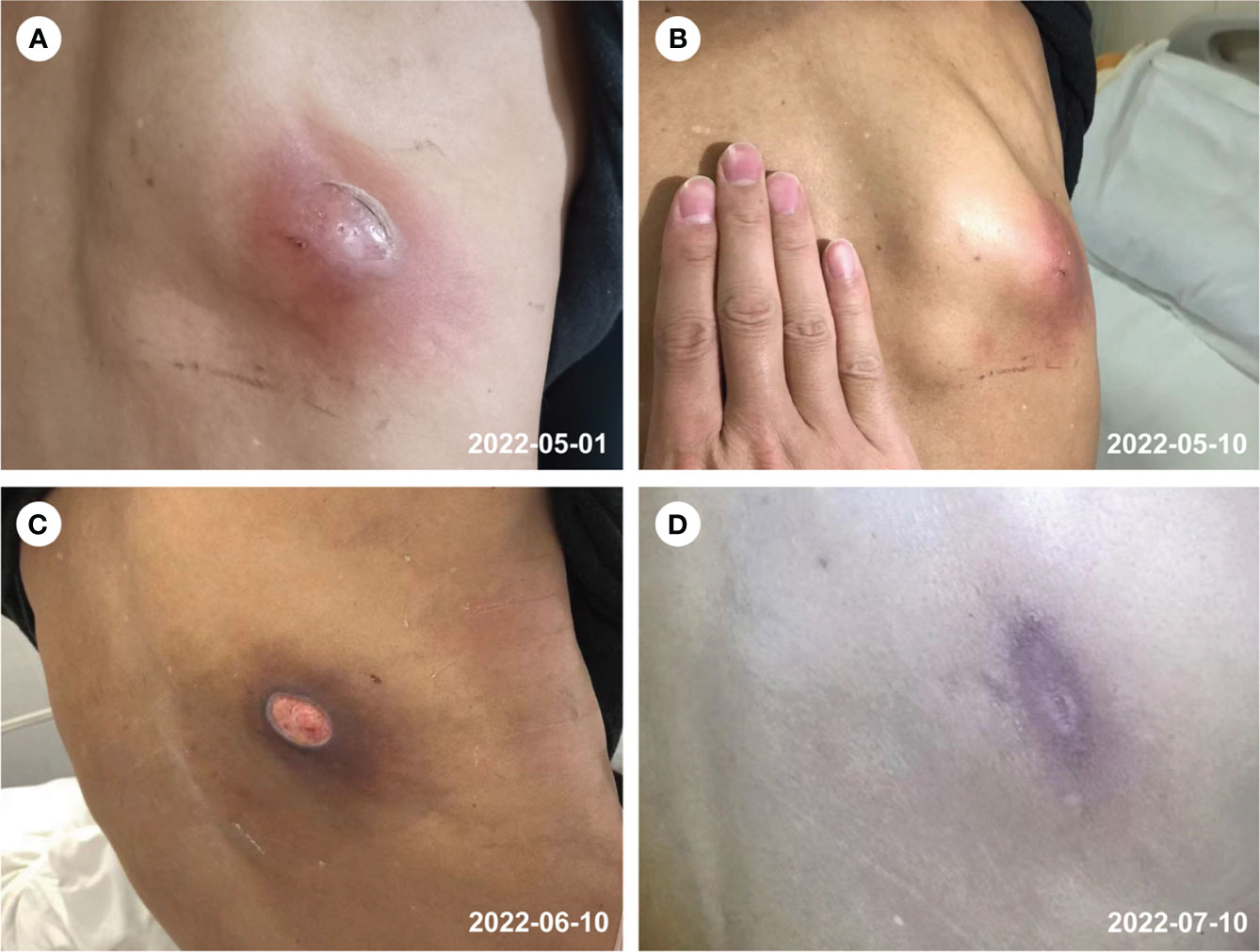
Figure 1 Abscess of the right back (A–D). (A) prior to the treatment, (B) after 2 weeks of treatment, (C) after 6 weeks of treatment, (D) follow-up on 10th July 2022.
The hemoglobin concentration was 83 g/L, the HCT was 27.1%, and the erythrocyte sedimentation rate (ESR) was 120mm/h, according blood tests. The tumor markers were all negative. An enhanced computed tomography (CT) of the chest revealed a right shift of the mediastinum and partial destruction of the right lung, as well as consolidation of the right upper lobe (Figure 2). An ultrasound of the heart revealed an enlarged right ventricle and pulmonary hypertension. The SPAP was calculated to be 69 mmHg. Magnetic resonance imaging (MRI) of the brain showed high signals in the paraventricular white matter, which could be vasogenic.
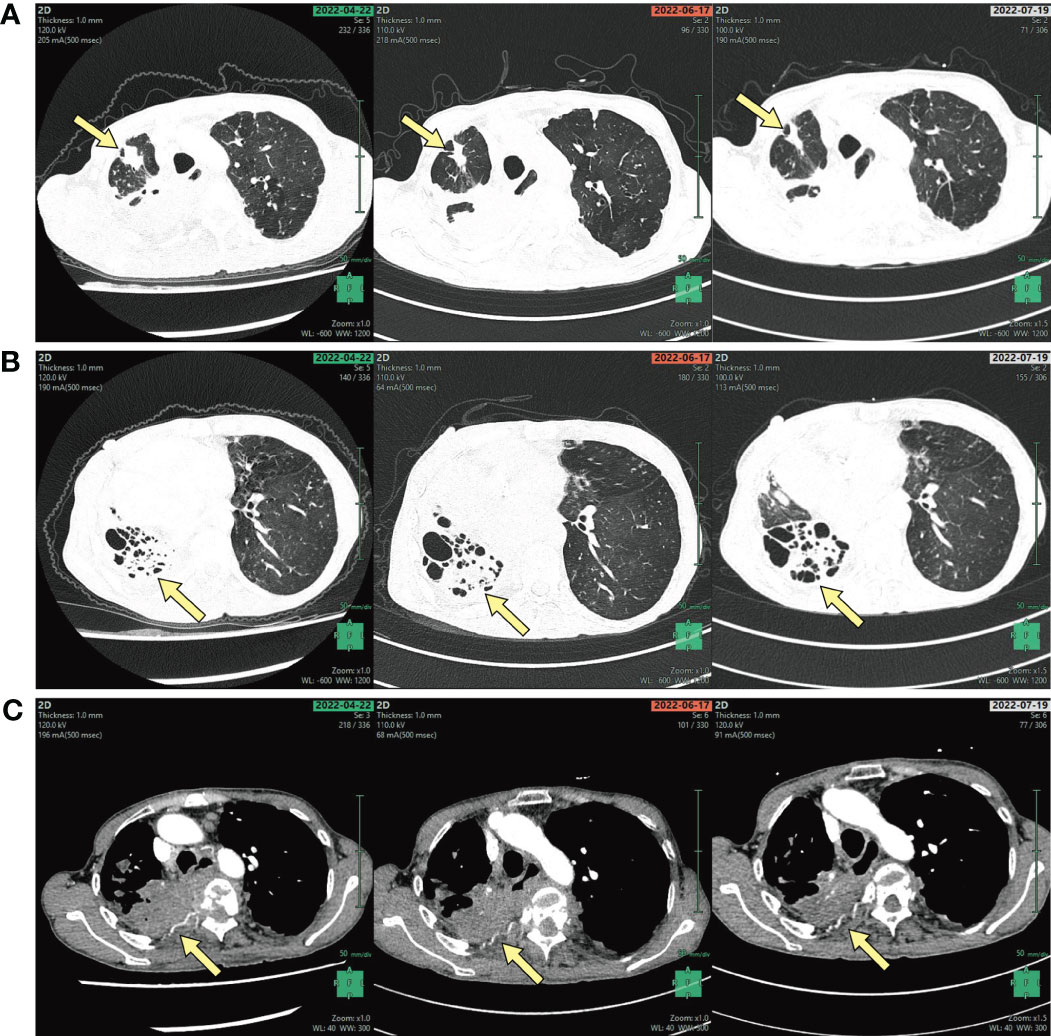
Figure 2 (A) CT of the chest shows a right upper lung nodule, (B) destruction of the right lung, (C) the bone destruction of the right ribs (arrows, A–C).
Tissue samples from the back abscess and lung samples were sent for pathological examination by metagenomic next-generation sequencing (mNGS) (Beijing Genomics Institute (BGI)-Wuhan, China). The mNGS was performed in BGI-Wuhan exactly following the previous protocol, including DNA extraction, libraries construction and sequencing. High-quality sequencing data were generated by removing low-quality reads, followed by computational subtraction of human host sequences mapped to the human reference genome (hg19) using Burrows-Wheeler Alignment. The remaining data were classified by simultaneously aligning to Pathogens metagenomics Database (PMDB), consisting of bacteria, fungi, viruses and parasites.
Samples from the back abscess and the lung tissue identified the pathogenic pathogen infection. In the back abscess, acid-fast staining, X-pert, and PPD (tuberculin pure protein derivative) test were all negative, while mNGS pathological examination showed N. wallacei from the tissue samples, and mNGS of the blood sample also identified the same result Regarding the pulmonary infection, sputum, lung tissue, and blood sample were used to identify M. abscessus infection. The aerobic culture and swab of sputum revealed moderate G+ coccus and mild G+ bacillus. The mNGS of lung samples showed M. abscessus infection. The lungs’ pathology revealed fibrous tissue proliferation but no evidence of malignancy. In this case, blood and abscess samples were collected from patients on April 29, 2022 and May 11, 2022, respectively, for mNGS detection (Table 1). After treatment, the RPM (reads per million sequences) of N. wallacei in abscess decreased from 795.12 to 239.10 by NGS.
Based on the pathogen detection and clinical symptoms, she was diagnosed with pulmonary infection caused by Mycobacterium abscessus and cutaneous nocardiosis caused by N. wallacei. Before the pathogenic pathogen was confirmed, she was started on Piperacillin and Tazobactam 4.5g every 8 hours for 9 days for empiric anti-infection treatment. After the diagnosis, her treatment was changed to Imipenem 1.0g ivgtt, Clarithromycin 0.5g po, Doxycycline 0.1g po every 12 hours to suppress Mycobacterium abscessus, and TMP-SMX 0.96g po every 6 hours to suppress N. wallacei. Twelve days after treatment, her anti-infection therapy was changed to a combination of SMX, Doxycycline, Clarithromycin, and Cefoxitin. which is recommended as a long-term treatment. The patient’s condition has improved with her back abscess significantly become smaller as a result of targeted medications, and she has returned to the local hospital with the treatment plan for further observation. A follow-up CT of the chest two months later revealed that most of the right lung lesion had been absorbed miraculously, and her clinical symptoms had improved with a favorable prognosis.
We present a case of a patient who was successfully diagnosed with N. wallacei and Mycobacterium abscessus infection using mNGS. This is the first case of infection with both N. wallacei and Mycobacterium abscessus in an immunocompetent patient in China. Although inhalation is the most common clinical manifestation of nocardiosis, skin contact with the bacteria can result in extrapulmonary dissemination. In this case, the patient has a cutaneous N. wallacei infection as well as a pulmonary Mycobacterium abscessus infection.
Infections caused by Nocardia spp. are becoming more common, but the infection caused by N. wallacei was limited. The first human infection of N. wallacei was reported in the United States in 1979. At that time, it was identified as the drug pattern IV of N. asteroides(Conville et al., 2008). In 2008, Conville et al. proposed N. wallacei as a new species designation to the drug pattern IV, which was recognized with the name N. wallacei(Conville et al., 2008). In China, the first N. wallacei infection reported in an immunocompetent patient with pulmonary nocardiosis and without disseminated infection. There have been additional reports of disseminated infection in the United States, France, and Mexico(Cassir et al., 2013; Cooper et al., 2014; Welsh et al., 2018).
Nocardia wallacei and Mycobacterium abscessus are both opportunistic pathogenic bacteria that can cause infections, particularly in immunocompromised individuals(Boudehen and Kremer, 2021). Patients with weakened immune system, such as those with diabetes, ulcerative colitis, cirrhosis, HIV infection, and stem cell or solid organ transplant recipients, are more likely to contract N. wallacei infections (Puing et al., 2021). The effects of these infections in immunocompromised patients can be significant and potentially severe. In both Nocardia wallacei and Mycobacterium abscessus infections, the immunocompromised state of the patient can contribute to increased susceptibility, more severe infections, and a higher risk of complications. However, our case shows infection of N. wallecei and M. abscessus in an immunocompetent patient, making this the first instance of these two pathogens co-infecting. The three-day cultivation results confirmed the mNGS results (Figure 3).
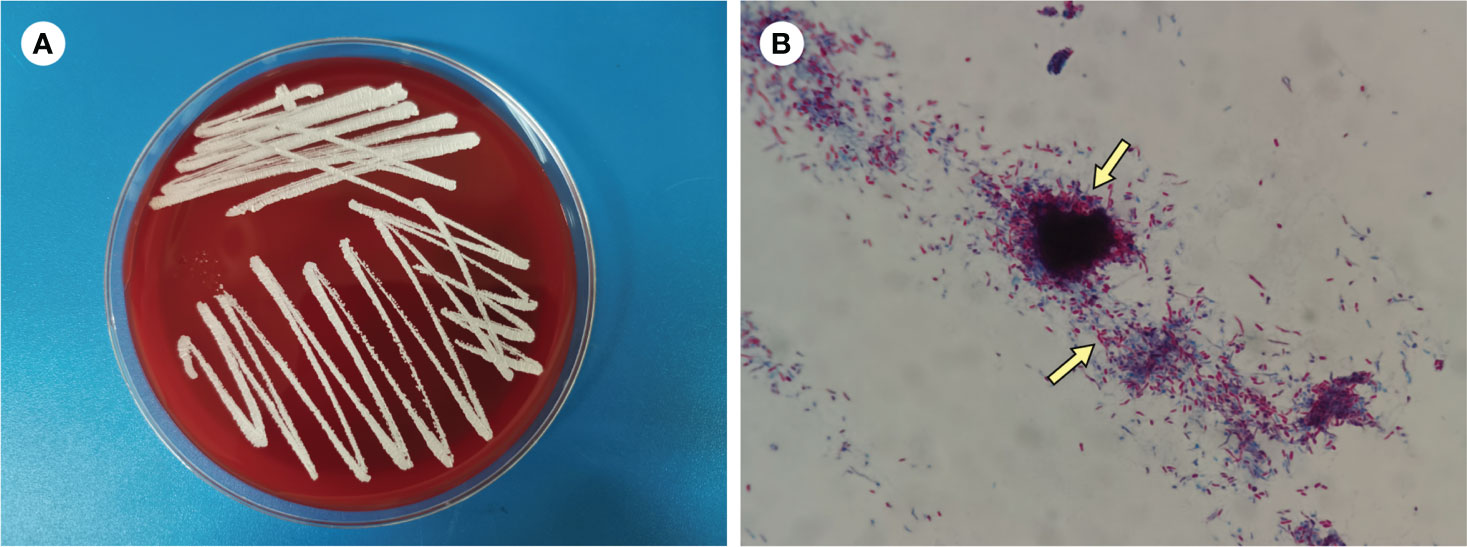
Figure 3 (A) Culture on blood agar plate for 3 days (B) Modified acid-fast staining of BALF (×1000).
It may be difficult to diagnose such patients with pulmonary and extrapulmonary infection. Before the pathogens were identified, she was given anti-infection treatments several times, but she did not respond well. Conventional specimen evaluation and primary diagnostic methods, such as bacterial culture of abscess punctual fluid, sputum culture and Acid-Fast Bacteria (AFB) Staining, were all negative. Pathological examination of lung tissue samples revealed no evidence of malignancy. Although molecular biology with amplification and sequencing of one or two genes among rrs, hsp65, secA1 and soda (Margalit et al., 2021)is the gold standard for Nocardia species identification, it is limited by detection time and equipment availability. The mNGS, an emerging diagnostic method, has excelled in the rapid clinical diagnosis of a variety of diseases. When compared to traditional serology and culture methods, it has a shorter turnaround time, higher accuracy, and better diagnostic performance(Miao et al., 2018). Our findings suggested that mNGS could provide a method of monitoring disease progression and therapeutic efficacy. The successful diagnosis of rare pathogen infection using mNGS in this case suggests a more effective and straightforward way to identify rare pathogen infection, which contributes to the successful anti-infection treatment.
N. wallacei is expected to be toxic to sulfonamide antibiotics, particularly trimethoprim-sulfamethoxazole (TMP-SMX). TMP-SMX remains the gold standard, with active ingredients include imipenem, linezolid, ceftriaxone, and fluoroquinolones(Derungs et al., 2021). Amikacin, imipenem, or meropenem should be given in addition to TMP-SMX in immunocompromised and disseminated infections caused by N. wallacei(Bryant et al., 2021). Local abscesses, on the other hand, are typically treated with incision, drainage, and surgery to remove necrotic tissue(Weng et al., 2020). For Mycobacterium abscessus infection, susceptibility-based treatment for macrolides and amikacin is preferred over empiric therapy for Mycobacterium abscessus infection(Nick et al., 2022), and its active drugs include clarithromycin, azithromycin, imipenem, cefoxitin, and tigecycline based on in vitro susceptibility test results. The 2020 NTM Guideline recommended a multidrug treatment regimen containing at least 3 active antibiotics to treat its pulmonary infection(Daley et al., 2020). Antibiotic susceptibility should be tested prior to treatment and antibiotic combination therapy should be chosen based on the susceptibility test results(Griffith and Daley, 2022).
To treat the Norcadosis and Mycobacterium infection, a combination of TMP-SMX and imipenem, clarithromycin, and doxycycline was used for a total of 12 days. Her clinical improvement was linked to the phage treatment. After the initial period of infection treatment, we switched to a combination of TMP-SMX, Doxycycline, Clarithromycin, and Cefoxitin, which is recommended as a long-term treatment. Her follow-up exams revealed that the abscess on her back had been absorbed, and her pulmonary lesions had significantly improved.
Because Nocardiosis has a high mortality rate and a high rate of misdiagnosis(Soueges et al., 2022), early diagnosis and treatment are critical, especially infection with other pathogens. In our case, the patient began empiric antibiotic treatment a year before the pathogen was identified, but it had little effect. Her pre-existing medical conditions may worsen as a result of the delay in administering proper antimicrobials. The application of mNGS in pathogen detection contributes significantly to the case’s successful diagnosis and treatment. The treatment of N. wallacei infection requires early and accurate pathogen diagnosis of Nocardiosis.
Nocardia wallacei and Mycobacterium abscessus are both opportunistic pathogens. Only one third of infections caused by Nocardia wallacei happen in immunocompetent patients, while hosts infected by Mycobacterium abscessus are mostly immunocompromised(Johansen et al., 2020; Sur et al., 2022). Infections caused by these two bacteria alone are rare in immunocompetent patients, and the simultaneous infection is even rarer(Wei et al., 2021). This case is the first successfully treated infection with N. wallacei and M. abscessus in China. The treatment process and medication regimen have important value for the treatment of rare pathogen infection, especially the treatment of these infection in immunocompetent patients.
This is the first case of N. wallacei and M. abscessus simultaneous infection in an immunocompetent patient in China. mNGS technology was used to confirm the pathogens, and the patient was discharged with her condition improved after timely and proper anti-infection treatment. This case shows that mNGS has a significant advantage in the detection of rare and mixed infections. In this case, treating a rare disseminated infection successfully can help clinicians deal with rare infections by preventing underdiagnosis and misdiagnosis.
The datasets presented in this study are deposited in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/PRJNA982594.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
LQ and LC conceived the study. LQ drafted the first manuscript. SW, QQ, JL and YT followed the patients during the diagnostic and therapeutic path. ZZ and WZ provided data analysis and interpretation for the mNGS detection. All authors contributed to the article and approved the submitted version.
We are thankful to the patient for her cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Boudehen, Y.-M., Kremer, L. (2021). Mycobacterium abscessus. Trends Microbiol. 29 (10), 951–952. doi: 10.1016/j.tim.2021.06.006
Bryant, J. M., Brown, K. P., Burbaud, S., Everall, I., Belardinelli, J. M., Rodriguez-Rincon, D., et al. (2021). Stepwise pathogenic evolution of Mycobacterium abscessus. Science 372 (6541), eabb8699. doi: 10.1126/science.abb8699
Cassir, N., Million, M., Noudel, R., Drancourt, M., Brouqui, P. (2013). Sulfonamide resistance in a disseminated infection caused by Nocardia wallacei: a case report. J. Med. Case Rep. 7 (1), 1–4. doi: 10.1186/1752-1947-7-103
Chen, J., Zhou, H., Xu, P., Zhang, P., Ma, S., Zhou, J. (2014). Clinical and radiographic characteristics of pulmonary nocardiosis: clues to earlier diagnosis. PloS One 9 (3), e90724. doi: 10.1371/journal.pone.0090724
Conville, P. S., Brown, J. M., Steigerwalt, A. G., Brown-Elliott, B. A., Witebsky, F. G. (2008). Nocardia wallacei sp. nov. and Nocardia blacklockiae sp. nov., human pathogens and members of the “Nocardia transvalensis Complex”. J. Clin. Microbiol. 46 (4), 1178–1184. doi: 10.1128/JCM.00994-11
Cooper, C. J., Said, S., Popp, M., Alkhateeb, H., Rodriguez, C., Aguilar, M. P., et al. (2014). A complicated case of an immunocompetent patient with disseminated nocardiosis. Infect. Dis. Rep. 6 (1), 5327. doi: 10.4081/idr.2014.5327
Coussement, J., Lebeaux, D., van Delden, C., Guillot, H, Freund, R, Marbus, S, et al. (2016). Nocardia infection in solid organ transplant recipients: a multicenter European case-control study. Rev. Infect. Dis. 63 (3), 338–345. doi: 10.1093/cid/ciw241
Dahl, V. N., Mølhave, M., Fløe, A., van Ingen, J., Schön, T., Lillebaek, T., et al. (2022). Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int. J. Infect. Dis. 125, 120–131. doi: 10.1093/cid/ciaa241
Daley, C. L., Iaccarino, J. M., Lange, C., Cambau, E., Wallace, R. J., Jr., Andrejak, C., et al. (2020). Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin. Infect. Dis. 71 (4), e1–e36. doi: 10.1093/cid/ciaa241
Derungs, T., Leo, F., Loddenkemper, C., Schneider, T. (2021). Treatment of disseminated nocardiosis: A host–pathogen approach with adjuvant interferon gamma. Lancet Infect. Dis. 21 (10), e334–e340. doi: 10.1016/S1473-3099(20)30920-8
Griffith, D. E., Daley, C. L. (2022). Treatment of Mycobacterium abscessus pulmonary disease. Chest 161 (1), 64–75. doi: 10.1164/rccm.200604-571ST
Haussaire, D., Fournier, P.-E., Djiguiba, K., Moal, V., Legris, T., Purgus, R., et al. (2017). Nocardiosis in the south of France over a 10-years period 2004–2014. Int. J. Infect. Dis. 57, 13–20. doi: 10.1016/j.ijid.2017.01.005
Hsu, J.-Y., Cheng, A., Ku, C.-C., Chen, Y.-C., Wang, J.-T., Hsieh, T.-W., et al. (2022). Mycobacterium abscessus and Mycobacterium massiliense exhibit distinct host and organ specificity: a cross-sectional study. Int. J. Infect. Dis. 116, 21–26. doi: 10.1016/j.ijid.2021.12.348
Johansen, M. D., Herrmann, J.-L., Kremer, L. (2020). Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 18 (7), 392–407. doi: 10.1038/s41579-020-0331-1
Kanne, J. P., Yandow, D. R., Mohammed, T.-L. H., Meyer, C. A. (2011). CT findings of pulmonary nocardiosis. Am. J. Roentgenology 197 (2), W266–W272. doi: 10.2214/AJR.10.6208
King, A. S., Castro, J. G., Dow, G. C. (2009). Nocardia farcinica lung abscess presenting in the context of advanced HIV infection: Spontaneous resolution in response to highly active antiretroviral therapy alone. Can. J. Infect. Dis. Med. Microbiol. 20 (3), e103–e106. doi: 10.1155/2009/181750
Lafont, E., Conan, P.-L., Rodriguez-Nava, V., Lebeaux, D. (2020). Invasive nocardiosis: disease presentation, diagnosis and treatment–old questions, new answers? Infection Drug Res. 13, 4601–4613. doi: 10.2147/IDR.S249761
Li, N., Cai, Q., Miao, Q., Song, Z., Fang, Y., Hu, B. (2021). High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods 5 (1), 2000792. doi: 10.1002/smtd.202000792
Margalit, I., Lebeaux, D., Tishler, O., Goldberg, E., Bishara, J., Yahav, D., et al. (2021). How do I manage nocardiosis? Clin. Microbiol. Infection 27 (4), 550–558. doi: 10.1016/j.cmi.2020.12.019
Miao, Q., Ma, Y., Wang, Q., Pan, J., Zhang, Y., Jin, W., et al. (2018). Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin. Infect. Dis. 67 (suppl_2), S231–S240. doi: 10.1093/cid/ciy693
Minero, M. V., Marín, M., Cercenado, E., Rabadán, P. M., Bouza, E., Muñoz, P. (2009). Nocardiosis at the turn of the century. Medicine 88 (4), 250–261. doi: 10.1097/MD.0b013e3181afa1c8
Nick, J. A., Dedrick, R. M., Gray, A. L., Vladar, E. K., Smith, B. E., Freeman, K. G., et al. (2022). Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 185 (11), 1860–1874, e1812. doi: 10.1016/j.cell.2022.04.024
Puing, A. G., Epstein, D. J., Banaei, N., Subramanian, A. K., Liu, A. Y. (2021). Nocardiosis in immunocompromised patients on alternative pneumocystis prophylaxis. Emerging Infect. Dis. 27 (10), 2734. doi: 10.3201/eid2710.210620
Soueges, S., Bouiller, K., Botelho-Nevers, E., Gagneux-Brunon, A., Chirouze, C., Rodriguez-Nava, V., et al. (2022). Prognosis and factors associated with disseminated nocardiosis: a ten-year multicenter study. J. Infection 85 (2), 130–136. doi: 10.3201/eid2710.210620
Sur, S., Patra, T., Karmakar, M., Banerjee, A. (2022). Mycobacterium abscessus: insights from a bioinformatic perspective. Crit. Rev. Microbiol. 49 (4), 499–514. doi: 10.1080/1040841X.2022.2082268
Wang, H.-K., Sheng, W.-H., Hung, C.-C., Chen, Y.-C., Lee, M.-H., Lin, W. S., et al. (2015). Clinical characteristics, microbiology, and outcomes for patients with lung and disseminated nocardiosis in a tertiary hospital. J. Formosan Med. Assoc. 114 (8), 742–749. doi: 10.1016/j.jfma.2013.07.017
Wei, M., Xu, X., Yang, J., Wang, P., Liu, Y., Wang, S., et al. (2021). MLSA phylogeny and antimicrobial susceptibility of clinical Nocardia isolates: a multicenter retrospective study in China. BMC Microbiol. 21 (1), 1–11. doi: 10.1186/s12866-021-02412-x
Welsh, O., Salinas-Carmona, M. C., Brown-Elliott, B. A., Smith, T., Cardenas-De La Garza, J. A., Wallace, J. R. J. (2018). Disseminated actinomycetoma due to Nocardia wallacei. Int. J. Dermatol. 57 (5), 580–582. doi: 10.1111/ijd.13909
Weng, Y.-W., Huang, C.-K., Sy, C.-L., Wu, K.-S., Tsai, H.-C., Lee, S. S.-J. (2020). Treatment for Mycobacterium abscessus complex–lung disease. J. Formosan Med. Assoc. 119, S58–S66. doi: 10.1016/j.jfma.2020.05.028
Keywords: Nocardia wallacei, cutaneous Nocardiosis, Mycobacterium abscessus, mNGS, mixed infection
Citation: Qin L, Wang S, Zheng Z, Zhang W, Qu Q, Li J, Tan Y and Cao L (2023) A complicated infection by cutaneous Nocardia wallacei and pulmonary Mycobacterium abscessus in a Chinese immunocompetent patient: a case report. Front. Cell. Infect. Microbiol. 13:1229298. doi: 10.3389/fcimb.2023.1229298
Received: 26 May 2023; Accepted: 28 July 2023;
Published: 16 August 2023.
Edited by:
Michael Marceau, Université Lille Nord de France, FranceReviewed by:
Yolanda González Hernández, National Institute for Respiratory Diseases, MexicoCopyright © 2023 Qin, Wang, Zheng, Zhang, Qu, Li, Tan and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Cao, Y2xtaW5nQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.