
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 20 November 2023
Sec. Extra-intestinal Microbiome
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1228940
 Yu-Mei Zhou1†
Yu-Mei Zhou1† Jin-Jun Yuan1†
Jin-Jun Yuan1† Yu-Qin Xu1
Yu-Qin Xu1 Yan-Hua Gou1
Yan-Hua Gou1 Yannas Y. X. Zhu1
Yannas Y. X. Zhu1 Chen Chen2
Chen Chen2 Xing-Xian Huang1
Xing-Xian Huang1 Xiao-Ming Ma1
Xiao-Ming Ma1 Min- Pi1*
Min- Pi1* Zhuo-Xin Yang1*
Zhuo-Xin Yang1*Background: There are several clinical and molecular predictors of responses to antidepressant therapy. However, these markers are either too subjective or complex for clinical use. The gut microbiota could provide an easily accessible set of biomarkers to predict therapeutic efficacy, but its value in predicting therapy responses to acupuncture in patients with depression is unknown. Here we analyzed the predictive value of the gut microbiota in patients with postpartum depressive disorder (PPD) treated with acupuncture.
Methods: Seventy-nine PPD patients were enrolled: 55 were treated with acupuncture and 24 did not received any treatment. The 17-item Hamilton depression rating scale (HAMD-17) was used to assess patients at baseline and after eight weeks. Patients receiving acupuncture treatment were divided into an acupuncture-responsive group or non-responsive group according to HAMD-17 scores changes. Baseline fecal samples were obtained from the patients receiving acupuncture and were analyzed by high-throughput 16S ribosomal RNA sequencing to characterize the gut microbiome.
Results: 47.27% patients responded to acupuncture treatment and 12.5% patients with no treatment recovered after 8-week follow-up. There was no significant difference in α-diversity between responders and non-responders. The β-diversity of non-responders was significantly higher than responders. Paraprevotella and Desulfovibrio spp. were significantly enriched in acupuncture responders, and these organisms had an area under the curve of 0.76 and 0.66 for predicting responder patients, respectively.
Conclusions: Paraprevotella and Desulfovibrioare may be useful predictive biomarkers to predict PPD patients likely to respond to acupuncture. Larger studies and validation in independent cohorts are now needed to validate our findings.
Postpartum depressive disorder (PPD) is a common, disabling, but treatable psychiatric condition (Howard et al., 2014). However, without prompt diagnosis and treatment, maternal suicide and infanticide may be extreme outcomes of PPD (Grigoriadis et al., 2017; Netsi et al., 2018). With a global prevalence of 17.22% (Wang et al., 2021), PPD is a significant maternal and family health burden worldwide. While PPD is most commonly treated with antidepressants and psychological therapies, the efficacy of these approaches varies due to high clinical and functional heterogeneity (Consortium, P.D.A.T.C.a.T.P, 2015; Santos et al., 2017). Indeed, antidepressants have been reported to be only ~42% effective (Brown et al., 2021), and psychotherapy only benefits about a third of PPD patients (Huang et al., 2020). Furthermore, antidepressants have side effects (Carvalho et al., 2016), and any adverse events to the baby during lactation must also be considered (Davanzo et al., 2011). Psychotherapy cannot generally be widely used due to its high cost over long periods of time. An increasing number of PPD patients are seeking safe and effective complementary treatments with few side effects.
Acupuncture is safe and effective in pregnant women (Ormsby et al., 2016; Li et al., 2018; Tong et al., 2019; Li et al., 2020). In a study of 31 meta-analyses and 59 randomized controlled trials, acupuncture was shown to be superior to awaiting treatment, control acupuncture (invasive or non-invasive sham control), and antidepressants in terms of reducing the severity of depression (Hamilton, 1960; Li et al., 2020). Another relatively recent meta-analysis highlighted that acupuncture can significantly reduce Hamilton depression rating (HAMD) scores in PPD patients (Li et al., 2019). However, just like other treatments, the efficacy of acupuncture varies between individuals. PPD therapy urgently requires specific biomarkers to predict therapeutic responses to antidepressant treatments, including acupuncture, so that the correct patients can be prescribed the best treatments at the right time.
Several demographic and clinical therapeutic response predictors to traditional antidepressants in PPD have been reported in robust clinical trials including being white/non-Hispanic (Yonkers et al., 2008), having a major depressive episode within four weeks of delivery (Sharp et al., 2010; Hantsoo et al., 2014), concomitant anxiety symptoms (Cohen et al., 2001), an absence of concomitant psychiatric illness (Yonkers et al., 2008), early response to treatment (Cohen et al., 2001), and improvement within one week after initiation of antidepressants (Appleby et al., 1997). Predictors of non-response included a lifetime history of substance use disorder (Yonkers et al., 2008), concomitant anxiety symptoms (Nonacs et al., 2005), and Hispanic or Black ethnicity (Yonkers et al., 2008). However, the generalizability of these predictors is limited by significant methodological variability including a wide range of studied postpartum periods (2–24 months), comorbid diseases (lifetime alcohol abuse, alcohol dependence, drug abuse, drug dependence, or anxiety disorder) (Nonacs et al., 2005; Suri et al., 2005; Misri et al., 2016), and different severities of depression (minor depression or major depression) (Appleby et al., 1997). In recent years, it has been found that the consistency of quantitative electroencephalographic, the default pattern network with different discrete topological structures in the left and right hemispheres and the variance of the global signal are related to the terminal clinical results of antidepressant treatment of MDD (Hunter et al., 2010; Hou et al., 2016; Zhu et al., 2018), however, the acquisition of the above indicators is undoubtedly complicated, and there are few research results on the efficacy prediction of PPD. Furthermore, predictors focus on clinicodemographic factors and there have been few studies on biomarkers– such as genetic and inflammatory markers (Sharma et al., 2020) – to advance the goal of developing objective and clinically acceptable biomarkers that predict treatment outcomes and guide individualized therapy.
There is now mounting evidence supporting a role for the intestinal microbiota in mental health disorders (Rieder et al., 2017; Dubois et al., 2019; Nikolova et al., 2021; McGuinness et al., 2022). This biochemical signaling pathway, also known as the gut-brain axis, is thought to influence cognitive function and mood via neural, metabolic, hormonal, and immune-mediated mechanisms (Foster and McVey Neufeld, 2013). Previous studies (Chung et al., 2019; Zhou et al., 2020; Nikolova et al., 2021) have found differences in the diversity and composition of gut microbial communities between PPD patients and healthy controls. Changes in the intestinal microflora can affect the efficacy of treatment for some diseases (Ma et al., 2019), and intestinal microflora has recently been shown to be a non-invasive diagnostic biomarker for colorectal adenoma and cancer (Liang et al., 2020). In a systematic review, probiotic therapy showed modest benefits in alleviating depressive symptoms in patients with major depressive disorder over four to nine weeks (Alli et al., 2022). Furthermore, Lactobacillus rhamnosus HN001 administered as a probiotic significantly reduced maternal depression and anxiety scores (Slykerman et al., 2017). It is also found that 919 syrup can relieve PPD by regulating the structure and metabolism of intestinal microorganisms and affecting the function of GABA/glutamic acid system in hippocampus (Tian et al., 2021). Additionally, it can be also used to predict responses to cancer immunotherapy in metastatic melanoma patients (Limeta et al., 2020), and dynamic changes in the intestinal microbiota can provide an early prediction of immunotherapy outcomes in patients with hepatocellular carcinoma (Zheng et al., 2019). Recently, some studies have also shown that responses to antipsychotic drugs are related to gut microbiota composition (Schwarz et al., 2018). It is found that the changes of intestinal microbial composition and metabolic function may be related to the response of antidepressants, which provides a potential predictor for the prediction of the curative effect of MDD and can even be used to distinguish MDD from generalized anxiety disorder (Dong et al., 2021; Dong et al., 2022). Therefore, characterizing the nature and impact of the intestinal microbiota on PPD therapy and its value as a biomarker of therapeutic responses would be highly clinically valuable. Acupuncture, as a common complementary alternative therapy, can reduce depressive-like behaviors in chronic unpredictable mild stress (CUMS) rats by regulating intestinal microbes and neurotransmitters (Li et al., 2021). Jiang found that acupuncture can effectively treat all stages of stroke and regulate intestinal flora, thus improving depressive symptoms (Jiang et al., 2023). Therefore, the intestinal microflora may act as clinically relevant biomarkers of therapeutic responses in individuals with mental health diseases, including in those receiving acupuncture.
Here, we first aimed to assess the efficacy of acupuncture in PPD patients. A secondary aim was to identify any differences in the intestinal microbiota in responders and non-responders to acupuncture, with the objective to identify microbiome-based predictors of acupuncture response.
This was a prospective cohort study approved by the Ethics Committee of Shenzhen Hospital of Traditional Chinese Medicine [K2020-027-01]. The study was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx; ChiCTR2100041687). Patients with PPD were recruited from the Shenzhen Traditional Chinese Medicine Hospital and Shenzhen Maternity & Child Healthcare Hospital (Shenzhen, China). All procedures used in this study conformed to the ethical standards of national and institutional human experimental committees and the Declaration of Helsinki. All subjects supplied written informed consent (Graphic Abstract).
Patients were initially screened for PPD using the Edinburgh Postnatal Depression Scale (EPDS) and then further evaluated using the 17-item Hamilton depression rating scale (HAMD-17) by physicians. All patients were assigned into acupuncture treatment group or no treatment group according to their own preference.
PPD was diagnosed by the evaluating physician according to the Fifth Edition of the Diagnosis and Statistics of Mental Illness (DSM-V) (Battle, 2013; First, 2013). Patients needed to meet five or more of the following symptoms, including at least the first or second symptoms, and the symptoms should have lasted for at least two weeks: (1) low mood and depressive emotion; (2) lack of interest in or loss of enjoyment in activities; (3) significant weight gain or loss; (4) poor sleep, insomnia, or lethargy; (5) psychomotor excitement or retardation; (6) a feeling of fatigue or weakness; (7) a sense that life is worthless, self-accusation, or self-guilt; (8) decline in cognition or difficulty concentrating; and (9) recurrent thoughts of death.
The inclusion criteria were: (1) patients between 20 and 49 years of age; (2) a diagnosis of PPD made by a psychiatrist; (3) illness appearing within a year of delivery; (4) HAMD-17 scores between 7 and 24; and (5) providing informed consent, voluntarily participating in the study, and able to complete the assessment instrument.
Exclusion criteria were: (1) severe psychiatric disorders such as bipolar affective disorder and schizophrenia; (2) mental disorder due to brain diseases or for other reasons, and unable to understand the contents of the questionnaire and cannot be effectively evaluated; (3) pregnancy; (4) patients with a HAMD suicide score >2 points; (5) anyone attempting suicide in the past year; and (6) anyone taking antibiotics or probiotics in the past month.
Patients in the acupuncture group were treated with acupuncture therapy by an acupuncturist with a doctor’s license and at least three years of clinical experience. Before patient enrollment, all acupuncturists participated in standardized operating procedure training, including locating the acupoints and needle manipulation.
The acupoints selected in this study including Baihui (DU20), Yintang (EX-HN3), Zhongwan (RN12), Qihai (RN6), Guanyuan (RN4), Neiguan (PC6), Shenmen (HT7), Hegu (LI4), Sanyinjiao (SP6) and Taichong (LR3). The location of acupoints has been shown in Table 1. When participants were supine, the skin around acupoints were routinely sterilized with 75% alcohol cotton swab, then disposable sterile needles (Product type: HuanQiu, Suzhou, China; 0.3 mm × 40 mm/0.3 mm × 75 mm; C-160630) were inserted into each acupoint to achieve the deqi sensation (a sensation of soreness, numbness, swelling, or radioactivity indicating the effectiveness of acupuncture). Paired alligator clips from the electroacupuncture (EA) apparatus (Hwato brand, Suzhou Medical Appliance Factory) were attached transversely to the needle holders at Baihui (DU20) and Yintang (EX-HN3), Zhongwan (RN12) and Qihai (RN6). The EA stimulation lasted for 30 minutes with a continuous wave of 2Hz and a current intensity of 0.1 to 1 mA depending on the participants comfort level. Acupuncture treatment consisted of 16 sessions, each for 30 minutes, and were administered over 8 weeks.
Patients in the no treatment group didn’t receive any therapy.
The clinical outcome was the response rate. The HAMD is a commonly used scale for clinical evaluation of depressive state (Hamilton, 1960). Depressive symptoms of PPD patients were assessed by HAMD-17 scale (17 items, scored from 0 to 52, higher scores representing more severe the depressive symptoms).
Patients were defined as responders if the HAMD-17 score reduced by ≥50% or the HAMD-17 score was <7 after treatment. Patients were defined as non-responders if the reduction in HAMD-17 score was <50% (Keller, 2003).
Fecal samples of PPD participants in acupuncture treatment group were collected once at baseline and placed in sterile plastic cups, then frozen at −80°C immediately after defecation. The details of fecal sample collection are described elsewhere (Zhou et al., 2019).
DNA was extracted using the MOBIO PowerSoil® DNA Separation Kit according to the manufacturer’s instructions, and stored at −80° in Tris-EDTA buffer solution before microbial MiSeq sequencing. The V4 region of 16S rRNA gene was amplified by PCR with primers 515F (5’-GTGYCAGCMGCCGCGGTAA-3’) and 806R (5’-GGACTACNVGGGTWTCTAAT-3’), along with barcode sequences, as previously described (Zhou et al., 2020). PCR mixtures contained 1 μl of each forward and reverse primer (10μM), 1 μl of template DNA, 4 μl of dNTPs (2.5mM), 5 μl of 10× EasyPfu Buffer, 1 μl of Easy Pfu DNA Polymerase (2.5 U/μl), and 1 μl of double-distilled water in a 50-μl reaction volume. Thermal cycling consisted of an initial denaturation step at 95° for 5min, followed by 30 cycles of denaturation at 94° for 30 s, annealing at 60° for 30 s, and extension at 72° for 40 s, with a final extension step at 72° for 4min. Amplicons were run for each sample on an agarose gel. Expected band size for 515f-806r was ∼300–350 bp. Amplicons were quantified with Quant-iT PicoGreen dsDNA Assay Kit (P11496; Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions. The amplicon library for high-throughput sequencing on the Illumina MiSeq V3 reagent PE150 (300 cycles) platform was combined to an equal amount and subsequently quantified using KAPA Library Quantification Kit (KK4824; Illumina, Inc., San Diego, CA, USA) according to manufacturer’s protocols.
High-throughput sequencing analysis was performed using Quantitative Insights into Microbial Ecology (QIIME) 2.0 according to the manufacturer’s instructions. Raw Illumina read data were deposited into tags, reads belonging to each sample were separated with barcodes, and low-quality reads were removed. The processed tags were clustered into amplicon sequence variants (ASVs) using the commonly used 97% similarity threshold. ASVs were assigned to taxa by matching to the SILVA database. A phylogenetic tree of representative sequences was constructed. α-diversity indices such as evenness, observed species, Shannon, and Faith-PD indices were calculated by Wilcoxon rank sum test. For β-diversity indices, firstly, Wilcoxon rank sum test was used to analyze the inter-group and intra-group differences. The former indicated differences in microbial composition between samples within the same group; the later indicate the differences in microbial composition of pairwise samples from different groups. Secondly, the Bray-Curtis dissimilarity and unweighted unifrac calculated by principal coordinate analyses were used for β-diversity indices. To further identify specific bacteria as biomarkers at the genus level, linear discriminant analysis effect size (LEfSe) was applied through the Huttenhower Lab Galaxy Server (Segata et al., 2011) after taxa summaries were reformatted. LEfSe settings were as previously described (Zhou et al., 2020), and systemic forms with a linear discriminant analysis (LDA) cutoff of 2.0 and a P < 0.05 in the built-in rank sum test were considered statistically significant. Finally, biomarker data (specific bacteria) calculated by LEfSe were further analyzed by receiver operator characteristic (ROC) curve analysis, and area under the curve (AUC) was used to assess the ROC effect. The cut-off value associated with optimal sensitivity and specificity was used to distinguish acupuncture responders and non-responders.
The demographic and clinical outcomes were analyzed using the SPSS 22.0 software (IBM Statistics, Armonk, NY, USA). Normally distributed data were analyzed using Student’s t-test, while non-parametric data were analyzed using the Mann-Whitney U-test with data expressed as medians with interquartile ranges (IQR). Categorical data were compared using the chi-squared test. A P-value < 0.05 was considered statistically significant.
Among 179 patients screened, 88 were enrolled at baseline between March 25 and November 22, 2021. According to patient preference, 60 received acupuncture treatment (acupuncture group) and 28 received no treatment (control group). During the study, nine (10.23%) patients dropped out: five (8.33%) received acupuncture and four (14.29%) had not received acupuncture. Seventy-nine patients completed the eight-week follow-up and assessments (Figure 1).
The baseline demographic and clinical characteristics are shown in Table 2. There were no significant differences in age, body mass index (BMI), number of days postpartum, number of parturitions, duration of disease, delivery mode, family history, and EPDS or HAMD-17 scores between those receiving acupuncture and those not receiving acupuncture at baseline.
47.27% responded to acupuncture treatment in the acupuncture group, and 12.5% patients not receiving treatment recovered after 8-week follow up. This difference was significant (P = 0.003) (Table 3).
Compared with the HAMD reduction rate between two groups, the results showed reduction rate in acupuncture group was superior than that in control group. This difference was significant (P <0.001) (Table 4).
Compared with baseline, HAMD scores in the control group did not significantly decrease (P = 0.113). However, the HAMD score decreased significantly in patients receiving acupuncture treatment (P <0.001) (Table 5).
There were no significant differences in age, BMI, number of days postpartum, number of parturitions, length of disease, delivery mode, family history, and EPDS or HAMD-17 scores between responders and non-responders (Table 6).
A total of 55 samples from all recruited subjects were sequenced on an Illumina MiSeq sequencer. For downstream analysis, 2259092 qualified reads from 2373462 raw reads were filtered.
We next used different diversity indices (evenness, Faith PD, observed species, Shannon diversity) to assess gut microbial α-diversity. There were no significant differences in diversity between acupuncture responders and non-responders (P = 0.7856, P = 0.4276, P = 0.6679, and P = 0.7208, respectively). However, the gut microbial diversity, as estimated by evenness, Faith PD, observed species, and Shannon diversity, tended to be higher in responders than non-responders (Figure 2).
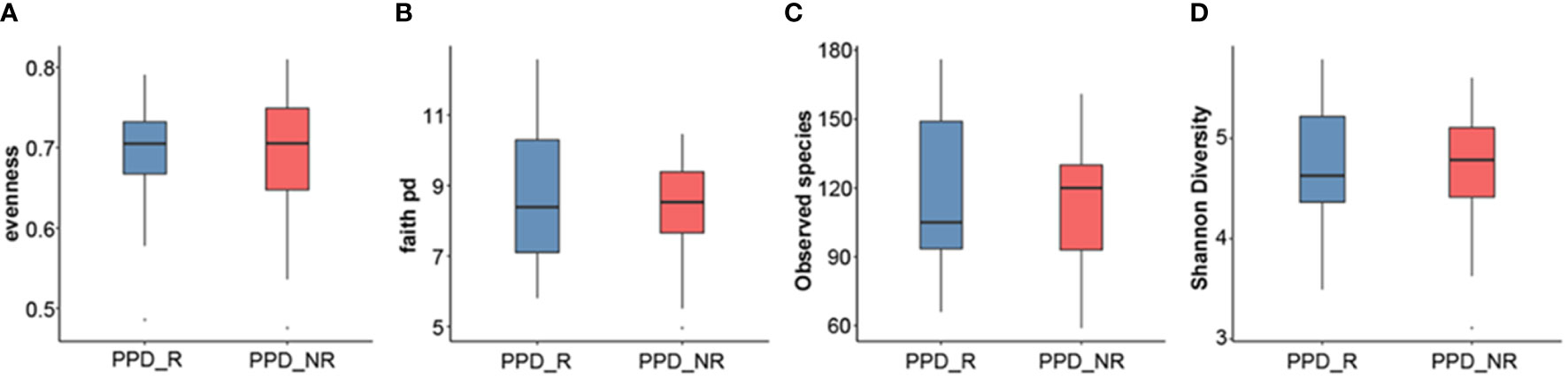
Figure 2 Microbial α-diversity analyses. Evenness index (A), Faith PD (B), observed species (C), Shannon diversity (D). PPD_R, PPD patients who are responsive to acupuncture treatment; PPD_NR, PPD patients who are not responsive to acupuncture treatment.
To better understand differences in overall community composition between the samples, we calculated Bray-Curtis distances and unweighted UniFrac distances, which were both higher in non-responders than responders (P = 0.0065 and P = 5.5e-05) and between groups (P = 0.019 and P = 0.0005) (Figures 3A, C), To further demonstrate differences in species diversity between samples, we applied the Principal Coordination Analysis and non-metric multidimensional scaling (Figures 3B, D). The gut microbial composition was similar between groups, with a tendency to being more centralized in the responder group than in the non-responder group, although this was not statistically significant (P = 0.959 and P = 0.911).
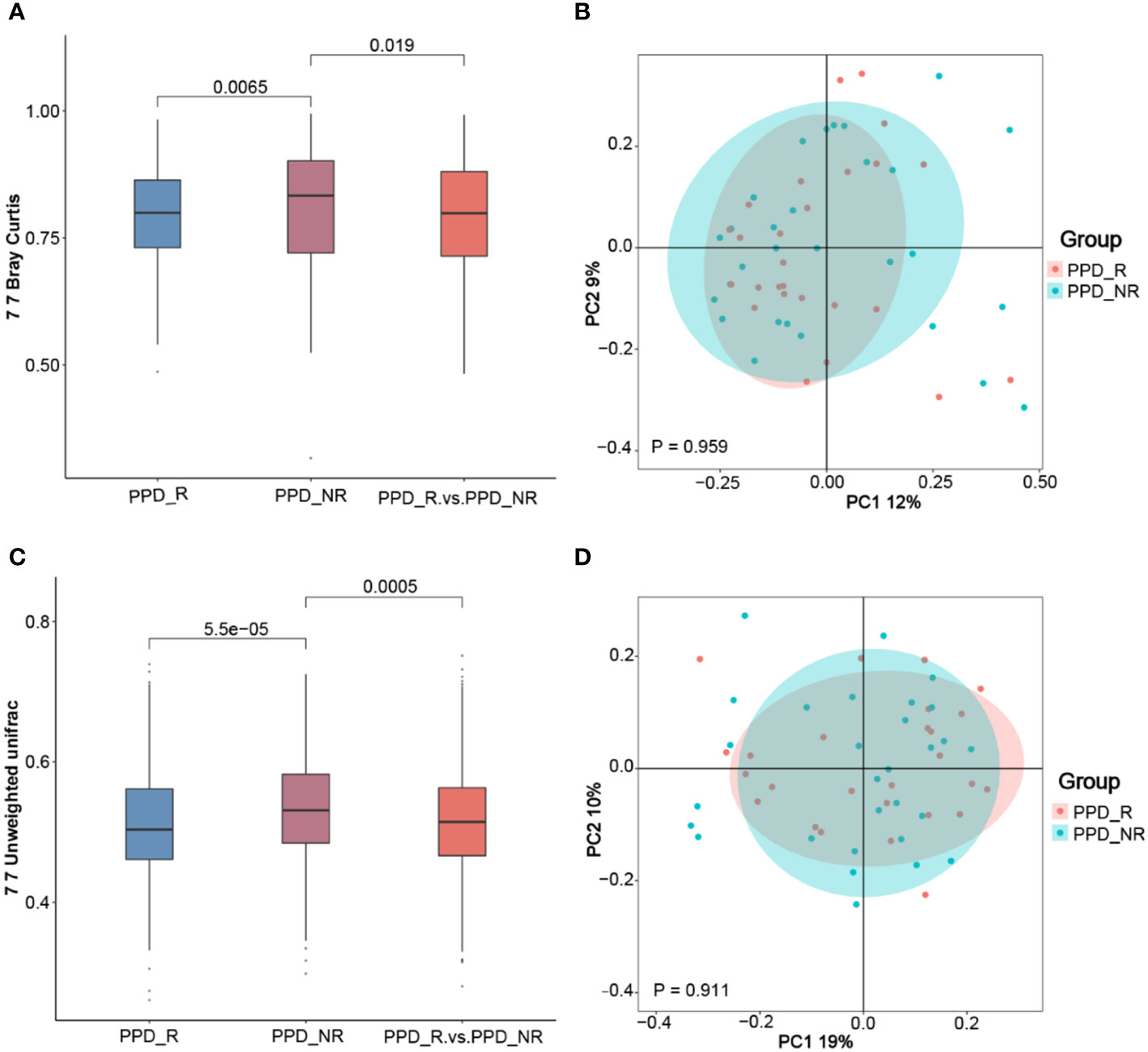
Figure 3 Microbial β-diversity analyses. The Wilcoxon rank-sum test analysis (A) and principal coordinates analysis plots (B) of the fecal microbiomes based on the Bray-Curtis distance; Wilcoxon rank-sum test analysis (C) and principal coordinates analysis plots (D) based on the Unweighted-UniFrac distance metric. PPD_R, PPD patients who are responsive to acupuncture treatment; PPD_NR, PPD patients who are not responsive to acupuncture treatment.
At the phylum level, Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria were the most abundant organisms in the gut microbiota (Figure 4A). The genera of Faecalibacterium, Blautia, Ruminococcaceae, Roseburia, Gemmiger, Megamonas and Bifidobacterium were dominant in the two groups. The 5 genera Faecalibacterium, Ruminococcaceae, Roseburia, Megamonas and Bifidobacterium had higher abundance in the PPD group (9.99, 6.45, 6.09, 4.04, and 3.96%, respectively) as compared to those in the control group (9.42, 4.08, 6.06, 3.87, and 3.28%, respectively). The 2 genera Blautia and Gemmiger had lower abundance in the PPD group (9.51 and 4.16%, Gemmiger) as compared to those in the control group (12.37 and 5.41%, respectively). However, the results didn’t reach significance (all p-values were more than 0.05) (Figure 4B).
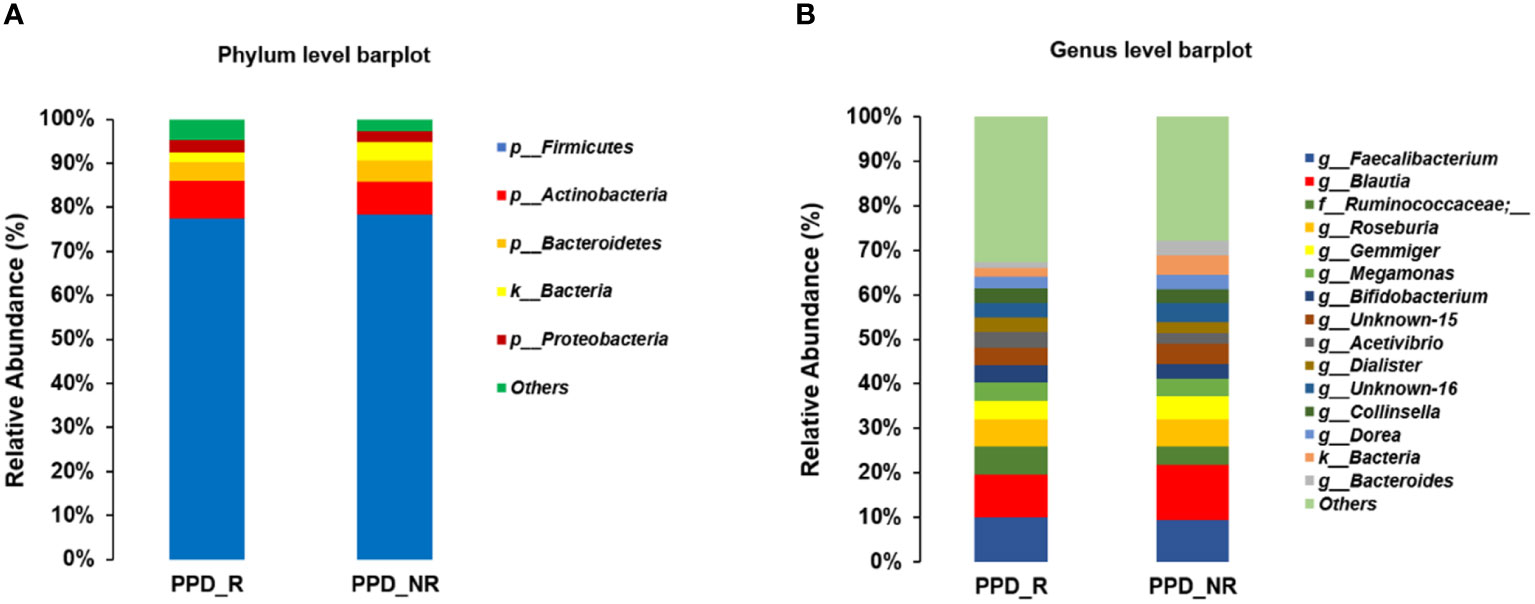
Figure 4 Microbiome composition differences at the phylum (A) and genus (B) levels between the two groups. PPD_R, PPD patients who are responsive to acupuncture treatment; PPD_NR, PPD patients who are not responsive to acupuncture treatment.
LEfSe analysis (p <0.05, LDA > 2) was used to identify specific bacteria associated with acupuncture treatment responses. g_Desulfovibrio, g_Paraprevotella, and Paraprevotella_xylaniphila were enriched in the responder group (Figure 5).
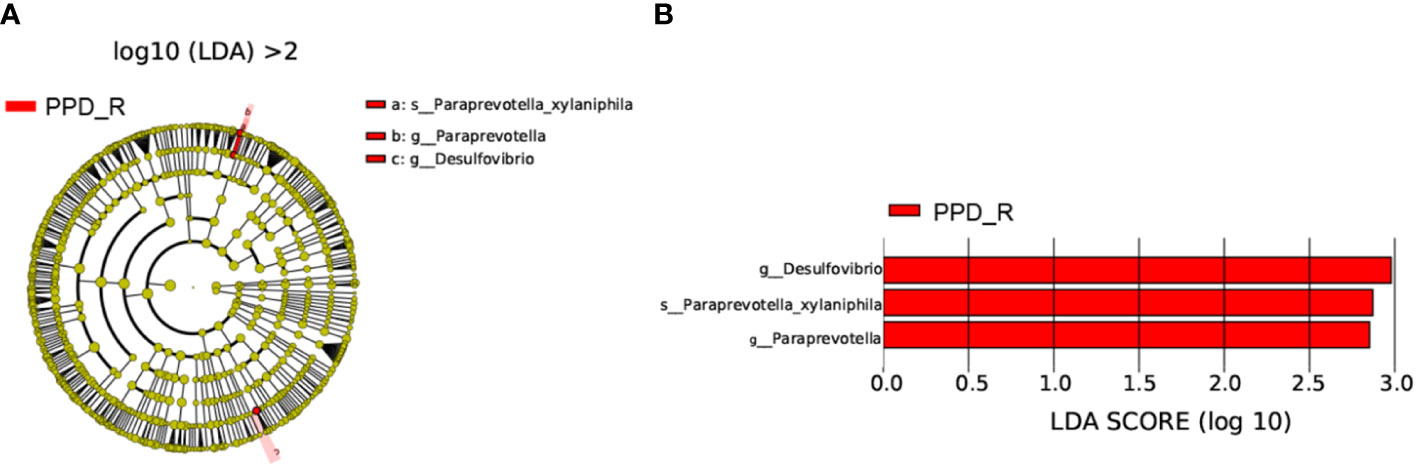
Figure 5 Differences in bacterial taxa between acupuncture responders and non-responders. Cladogram showing the most differentially abundant taxa identified by LEfSe. Red indicates clades enriched in the responder group (A). Comparisons of gut microbiota between acupuncture responders and non-responders (B). Only genera meeting a linear discriminant analysis score threshold >2 are shown. PPD_R, PPD patients who are responsive to acupuncture treatment; PPD_NR, PPD patients who are not responsive to acupuncture treatment.
Having identified these three genera (biomarkers), we performed ROC curve analysis to evaluate their predictive accuracy. The area under the curve (AUC) was 0.76 and 0.66 for g_Paraprevotella and g_Desulfovibrio, respectively. The AUC of combining genera g_Paraprevotella and g_Desulfovibrio was 0.65 (Figure 6).
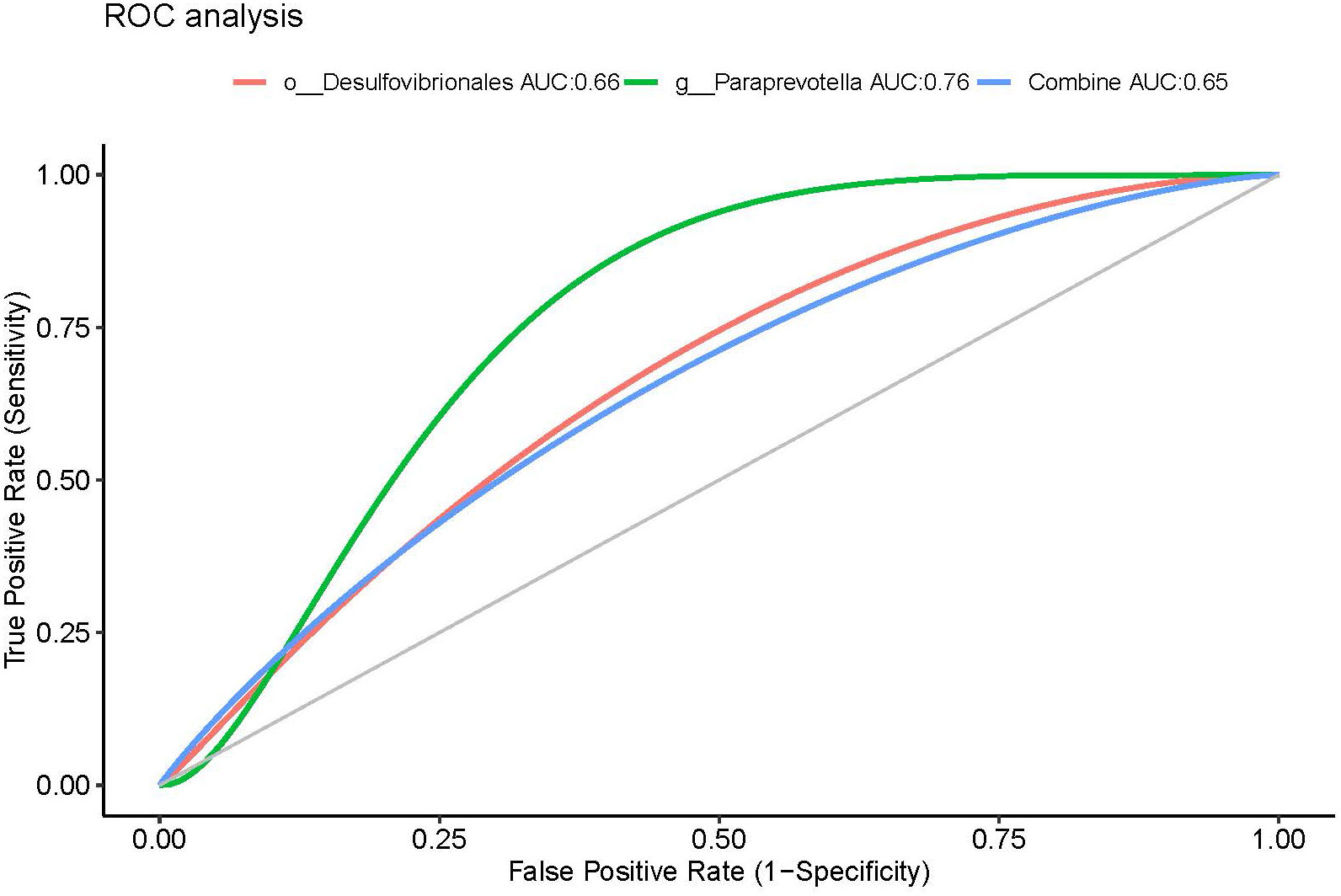
Figure 6 ROC curves using fecal microbiota to distinguish responders from non-responders. AUC, area under the curve; ROC, receiver operating characteristics.
This trial showed that acupuncture alleviated depressive symptoms in patients with PPD over an 8-week treatment period, and 47.27% patients significantly responded to acupuncture treatment. Additionally, based on gut microbiota profiling, we successfully predicted responses to acupuncture and improvements in clinical symptoms in PPD patients after treatment. Desulfovibrio and Paraprevotella were identified as specific predictive genera.
So far, there have been few reports on the predictors of therapeutic effect of PPD. Pinna speculated the neurosteroid biosynthesis and endogenous cannabinoid system might be able to predict antidepressive treatment, but lack of rigorous experimental studies to confirm this idea (Pinna, 2023). Additionally, there are also some limited explanations, such as the influence of running on cortisol, which can also affect the therapeutic effect of PPD in the later stage (Gobinath et al., 2018). These prediction methods are often incomplete and indirect. Therefore, gut microbiota as a predictor is seem to be more objective.
In the present study, our results further confirm the close relationship between the gut microbiota and mental health disorders. It has long been known that the enterotype distribution varies according to depression status, with Bacteroides enteritidis type 2 more prevalent in depressed patients than healthy controls (Valles-Colomer et al., 2019). It has also been shown that intestinal microbiota disorder is a characteristic of major depressive disorder (MDD) patients (Zheng et al., 2016; Zheng et al., 2020). Duan et al. studied treatment responses to escitalopram in a CUMS mouse depression model, comparing changes in metabolic function before and after treatment, and found that treatment responses were related to microbial composition, providing new insights into the mechanisms underlying variable antidepressant efficacy (Duan et al., 2021). Therefore, the flora structure is closely related to the intrinsic pathobiology of depression, suggesting that intestinal microbial biomarkers may be good predictors of antidepressant treatment responses.
Most studies employ a multifaceted approach to characterizing the gut microbiota, usually including measures of both α- and β-diversity. α-diversity is commonly used as a surrogate of community stability and function, which are thought to be beneficial to the host (Shade, 2017). Jiang et al. found that the intestinal microflora α-diversity was higher in antidepressant drug non-responders than responders in MDD patients compared with healthy controls (Jiang et al., 2015). In addition, the α-diversity of the gut microbiota was not significantly different in MDD patients with different treatment responses (Dong et al., 2022). In our study, we found that there were no significant differences in diversity between PPD patients who did and did not response to acupuncture, nor were there differences in the abundance and uniformity of the gut microbiota between the two groups. This mirrors the inconsistent results of previous studies, and the specific reasons underlying these differences need further study.
β-diversity reflects relationships between samples by analyzing the species composition and abundance (Anderson et al., 2011). Our β-diversity analysis showed that responders were significantly separated from non-responders, and the responder group had a more similar species composition. Kelly et al. and Zheng et al. both reported significant differences in β-diversity between individuals with depression and healthy controls (Kelly et al., 2016; Zheng et al., 2016). In the CUMS-induced depression study in mice, the β-diversity was also different between non-responders and responders (Duan et al., 2021), as was the β-diversity in patients who did and did not benefit from anti-programmed cell death protein 1 (PD-1) immunotherapy (Mao et al., 2021). The latter study found that the intestinal microflora affected the spectrum of immunotherapy-related adverse events, with high species diversity and relative abundance perhaps protective against immunotherapy-related adverse events (Mao et al., 2021).
We further analyzed and identified specific genera associated with acupuncture treatment responses. At the genus level, Desulfovibrio and Paraperevotella were enriched in responders, consistent with previous studies reporting a high abundance of Paraprevotella and Desulfovibrio at the genus level in patients with depression (Naseribafrouei et al., 2014; Chen et al., 2018a; Chen et al., 2018b). Desulfovibrio are present in the oral and intestinal tract of approximately 50% of people, where they release hydrogen sulfide as a product of sulfate reduction (Devereux et al., 1990). Hydrogen sulfide is involved in the natural prevention of many digestive tract diseases (Pires et al., 2006), and there is a well-established link between desulfurization bacteria and individual intestinal diseases (Verstreken et al., 2012). For example, Scanlan et al. found significantly more desulfurization bacteria in the feces of colon cancer patients than healthy people (Scanlan et al., 2009), and similarly Rowan et al. found a significantly higher relative abundance of desulfurization bacteria in the intestinal tracts of patients with ulcerative colitis than those of healthy controls (Rowan et al., 2010). Additionally, we discovered that Paraprevotella was enriched in the gut microbiota of patients responding well to acupuncture. Paraprevotella belongs to the Prevotellaceae family, and another family member Prevotella is associated with a healthy plant-based diet and probiotic use (Ley, 2016). Prevotella can also act as an opportunistic pathogen associated with periodontal and dental inflammation, intestinal inflammation, rheumatoid arthritis, and bacterial vaginitis (Arweiler and Netuschil, 2016; Randis and Ratner, 2019; Bertelsen et al., 2021; Jia et al., 2021).
Desulfovibrio and Paraperevotella have different potential pathogenic mechanisms. For example, Desulfovibrio organisms co-cultured with human oral epidermoid carcinoma (KB) cells increased interleukin (IL)-6 production, implicating them in immune responses (Bisson-Boutelliez et al., 2010). Colonization of the intestine with Prevotella leads to metabolic changes in the microbiota that reduce IL-18 production (Iljazovic et al., 2021), thus aggravating intestinal inflammation and possibly leading to systemic autoimmunity. Furthermore, Prevotella can damage intestinal mucosal barrier function by producing sulfatase, which induces and degrades mucus, thus helping itself and other harmful bacteria to access intestinal epithelial cells to generate local inflammation (Wright et al., 2000). In addition, these two genera as Gram-negative bacteria might help to explain the role of microbiota in the development/maintenance of depression. Gram-negative bacteria contain lipopolysaccharides in the outer cell membrane leaflet (Al Bander et al., 2020), and lipopolysaccharides interacts with macrophages and stimulates immune responses through pro-inflammatory cytokine release. Supporting this, increased levels of proinflammatory cytokines including IL-1β and IL-6 and decreased levels of anti-inflammatory cytokines including IL-4 and IL-10 have been detected in people living with depression (Berk et al., 2013; Wong et al., 2016).
An increasing number of studies show that the occurrence and development of depression are closely related to inflammation and immunity (Simmons and Broderick, 2005; Maes et al., 2012; Kelly et al., 2015), Inflammatory cytokines and kynurenine pathway have been found as potential therapeutic targets for PPD, because the increase of plasma IL-6 and IL-8 and the decrease of serotonin, IL-2 and quinolinic acid are related to the severity of depressive symptoms, which increases the risk of PPD (Achtyes et al., 2020). These results indicate that the increased level of some inflammatory biomarkers in PPD patients means that the disease is related to the impaired adaptability of the immune system (Bränn et al., 2020).
Therefore, the high expression of these genera in PPD patients may correspond to increased levels of inflammatory biomarkers, and several studies have shown a strong association between persistent inflammatory responses and antidepressant therapy resistance (Carvalho et al., 2013). Electroacupuncture can downregulate inflammatory factors such as IL-6 in the hippocampus of depressed rats, suggesting that electroacupuncture may relieve depression through immune regulation (Guo et al., 2014; Yue et al., 2018). Indeed, α7nAChR is activated by acetylcholine released from cholinergic nerve endings and is a key target for inhibiting pro-inflammatory cytokines release by macrophages (Stakenborg et al., 2017). Acupuncture can reduce inflammatory cytokine production through the vagus nerve by activating α7nAChR (Yang et al., 2021). Acupuncture can also regulate the interaction between the gut microbiota and the brain-gut axis, inhibit proinflammatory cytokine production, alter the number and proportion of the gut microbiota, restore its stability, improve intestinal barrier function, and further adjust body function (Jang et al., 2020; Wang et al., 2020). In acupuncture treatment of PPD, the unique regulation mechanism of immune intestinal flora also played an important role. Finally, we found that acupuncture might inhibit inflammation and improve depression via two pathways: (1) inhibiting the release of inflammatory cytokines by activating the vagus nerve; and (2) regulating the brain-gut axis through the intestinal microflora, some predecessors put forward the same argument previously. (Yang et al., 2022).Therefore, accumulation of Desulfovibrio and Paraperevotella in the intestinal tracts of responsive patients could mediate the immune response induced by acupuncture to better regulate and alleviate depressive symptoms. The conclusion of our research results accords with the above conclusion, which can be understood as that acupuncture has played a better and more sensitive role in the flora of the responders.
The study has several limitations. The sample size of present study was relatively small, and further studies in larger sample sizes are needed to confirm the findings with more advanced analyses methods, such as machine learning methods.
In conclusion, Paraprevotella and Desulfovibrio predicted early responses to antidepressants in patients with PPD receiving acupuncture. These results may help clinicians optimize their management of individual PPD patients in the future. Baseline enrichment and metabolism of Paraprevotella and Desulfovibrio intestinal microbiota in PPD patients were related to treatment outcomes. These findings pave the way for a new approach to personalize and maximize the efficacy of acupuncture treatment in PPD patients and provide potential new and accurate biomarkers for managing PPD patients.
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/, with accession number PRJNA976190.
The studies involving humans were approved by Ethics Committee of Shenzhen Traditional Chinese Medicine Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Y-MZ and J-JY contributed equally. M-P and Z-XY are the corresponding authors. Y-MZ conceived and planned the experiments. Y-MZ and J-JY wrote the manuscript. Y-QX, X-MM, YYXZ, Y-HG, CC and X-XH executed the experiments. M-P and Z-XY contributed to revise the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from National Natural Science Foundation of China (No. 82004470), Shenzhen Traditional Chinese Medicine Hospital “3030 Program” Chinese Medicine Clinical Research Project (G3030202119), Medical Research Foundation of Guangdong Province (B2023099), Natural Science Foundation of Guangdong Province (2019A1515110657) and National Key Research and Development Plan of China (2017YFC1703604).
We thank the Shenzhen Maternity and Child Healthcare Hospital and the Shenzhen Mental Health Center/Shenzhen Kangning Hospital for their expert advice.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SL declared a shared parent affiliation with the authors Y-MZ, J-JY, Y-QX, Y-HG, YYXZ, X-XH, X-MM, M-P, Z-XY to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achtyes, E., Keaton, S. A., Smart, L., Burmeister, A. R., Heilman, P. L., Krzyzanowski, S., et al. (2020). Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 83, 239–247. doi: 10.1016/j.bbi.2019.10.017
Al Bander, Z., Nitert, M. D., Mousa, A., Naderpoor, N. (2020). The gut microbiota and inflammation: an overview. Int. J. Environ. Res. Public Health 17 (20), 7618. doi: 10.3390/ijerph17207618
Alli, S., Gorbovskaya, I., Liu, J., Kolla, N., Brown, L., Müller, D. (2022). The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: A systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 23 (9), 4494. doi: 10.3390/ijms23094494
Anderson, M. J., Crist, T. O., Chase, J. M., Vellend, M., Inouye, B. D., Freestone, A. L., et al. (2011). Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 14 (1), 19–28. doi: 10.1111/j.1461-0248.2010.01552.x
Appleby, L., Warner, R., Whitton, A., Faragher, B. (1997). A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. Bmj 314 (7085), 932–936. doi: 10.1136/bmj.314.7085.932
Arweiler, N. B., Netuschil, L. (2016). The oral microbiota. Adv. Exp. Med. Biol. 902, 45–60. doi: 10.1007/978-3-319-31248-4_4
Battle, D. (2013). Diagnostic and statistical manual of mental disorders (DSM). CoDAS 25 (2), 191–192. doi: 10.1590/s2317-17822013000200017
Berk, M., Williams, L. J., Jacka, F. N., O'Neil, A., Pasco, J. A., Moylan, S., et al. (2013). So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 11, 200. doi: 10.1186/1741-7015-11-200
Bertelsen, A., Elborn, J. S., Schock, B. C. (2021). Microbial interaction: Prevotella spp. reduce P. aeruginosa induced inflammation in cystic fibrosis bronchial epithelial cells. J. Cyst Fibros 20 (4), 682–691. doi: 10.1016/j.jcf.2021.04.012
Bisson-Boutelliez, C., Massin, F., Dumas, D., Miller, N., Lozniewski, A. (2010). Desulfovibrio spp. survive within KB cells and modulate inflammatory responses. Mol. Oral. Microbiol. 25 (3), 226–235. doi: 10.1111/j.2041-1014.2009.00550.x
Bränn, E., Fransson, E., White, R. A., Papadopoulos, F. C., Edvinsson, Å., Kamali-Moghaddam, M., et al. (2020). Inflammatory markers in women with postpartum depressive symptoms. J. Neurosci. Res. 98 (7), 1309–1321. doi: 10.1002/jnr.24312
Brown, J. V. E., Wilson, C. A., Ayre, K., Robertson, L., South, E., Molyneaux, E., et al. (2021). Antidepressant treatment for postnatal depression. Cochrane Database Syst. Rev. 2 (2), Cd013560. doi: 10.1002/14651858.CD013560.pub2
Carvalho, A. F., Sharma, M. S., Brunoni, A. R., Vieta, E., Fava, G. A. (2016). The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: A critical review of the literature. Psychother. Psychosom 85 (5), 270–288. doi: 10.1159/000447034
Carvalho, L., Torre, J., Papadopoulos, A., Poon, L., Juruena, M., Markopoulou, K., et al. (2013). Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J. Affect. Disord. 148 (1), 136–140. doi: 10.1016/j.jad.2012.10.036
Chen, Z., Li, J., Gui, S., Zhou, C., Chen, J., Yang, C., et al. (2018b). Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 29 (5), 417–425. doi: 10.1097/wnr.0000000000000985
Chen, J. J., Zheng, P., Liu, Y. Y., Zhong, X. G., Wang, H. Y., Guo, Y. J., et al. (2018a). Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat 14, 647–655. doi: 10.2147/ndt.S159322
Chung, Y. E., Chen, H. C., Chou, H. L., Chen, I. M., Lee, M. S., Chuang, L. C., et al. (2019). Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 111, 74–82. doi: 10.1016/j.jpsychires.2019.01.016
Cohen, L. S., Viguera, A. C., Bouffard, S. M., Nonacs, R. M., Morabito, C., Collins, M. H., et al. (2001). Venlafaxine in the treatment of postpartum depression. J. Clin. Psychiatry 62 (8), 592–596. doi: 10.4088/jcp.v62n0803
Consortium, P.D.A.T.C.a.T.P (2015). Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry 2 (1), 59–67. doi: 10.1016/s2215-0366(14)00055-8
Davanzo, R., Copertino, M., De Cunto, A., Minen, F., Amaddeo, A. (2011). Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 6 (2), 89–98. doi: 10.1089/bfm.2010.0019
Devereux, R., He, S. H., Doyle, C. L., Orkland, S., Stahl, D. A., LeGall, J., et al. (1990). Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J. Bacteriol 172 (7), 3609–3619. doi: 10.1128/jb.172.7.3609-3619.1990
Dong, Z., Shen, X., Hao, Y., Li, J., Li, H., Xu, H., et al. (2021). Gut microbiome: A potential indicator for differential diagnosis of major depressive disorder and general anxiety disorder. Front. Psychiatry 12. doi: 10.3389/fpsyt.2021.651536
Dong, Z., Shen, X., Hao, Y., Li, J., Xu, H., Yin, L., et al. (2022). Gut microbiome: A potential indicator for predicting treatment outcomes in major depressive disorder. Front. Neurosci. 16. doi: 10.3389/fnins.2022.813075
Duan, J., Huang, Y., Tan, X., Chai, T., Wu, J., Zhang, H., et al. (2021). Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl. Psychiatry 11 (1), 303. doi: 10.1038/s41398-021-01428-1
Dubois, T., Reynaert, C., Jacques, D., Lepiece, B., Zdanowicz, N. (2019). Role of gut microbiota in the interaction between immunity and psychiatry: a literature review. Psychiatr. Danub 31 (Suppl 3), 381–385. Available at: https://pubmed.ncbi.nlm.nih.gov/31488756/
First, M. B. (2013). Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv Ment. Dis. 201 (9), 727–729. doi: 10.1097/NMD.0b013e3182a2168a
Foster, J. A., McVey Neufeld, K. A. (2013). Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36 (5), 305–312. doi: 10.1016/j.tins.2013.01.005
Gobinath, A. R., Richardson, R. J., Chow, C., Workman, J. L., Lieblich, S. E., Barr, A. M., et al. (2018). Voluntary running influences the efficacy of fluoxetine in a model of postpartum depression. Neuropharmacology 128, 106–118. doi: 10.1016/j.neuropharm.2017.09.017
Grigoriadis, S., Wilton, A. S., Kurdyak, P. A., Rhodes, A. E., VonderPorten, E. H., Levitt, A., et al. (2017). Perinatal suicide in Ontario, Canada: a 15-year population-based study. Cmaj 189 (34), E1085–e1092. doi: 10.1503/cmaj.170088
Guo, T., Guo, Z., Yang, X., Sun, L., Wang, S., Yingge, A., et al. (2014). The alterations of IL-1Beta, IL-6, and TGF-beta levels in hippocampal CA3 region of chronic restraint stress rats after electroacupuncture (EA) pretreatment. Evid Based Complement Alternat Med. 2014, 369158. doi: 10.1155/2014/369158
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 (1), 56–62. doi: 10.1136/jnnp.23.1.56
Hantsoo, L., Ward-O'Brien, D., Czarkowski, K. A., Gueorguieva, R., Price, L. H., Epperson, C. N. (2014). A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacol. (Berl) 231 (5), 939–948. doi: 10.1007/s00213-013-3316-1
Hou, Z., Wang, Z., Jiang, W., Yin, Y., Yue, Y., Zhang, Y., et al. (2016). Divergent topological architecture of the default mode network as a pretreatment predictor of early antidepressant response in major depressive disorder. Sci. Rep. 6, 39243. doi: 10.1038/srep39243
Howard, L. M., Molyneaux, E., Dennis, C. L., Rochat, T., Stein, A., Milgrom, J. (2014). Non-psychotic mental disorders in the perinatal period. Lancet 384 (9956), 1775–1788. doi: 10.1016/s0140-6736(14)61276-9
Huang, R., Yang, D., Lei, B., Yan, C., Tian, Y., Huang, X., et al. (2020). The short- and long-term effectiveness of mother-infant psychotherapy on postpartum depression: A systematic review and meta-analysis. J. Affect. Disord. 260, 670–679. doi: 10.1016/j.jad.2019.09.056
Hunter, A. M., Muthén, B. O., Cook, I. A., Leuchter, A. F. (2010). Antidepressant response trajectories and quantitative electroencephalography (QEEG) biomarkers in major depressive disorder. J. Psychiatr. Res. 44 (2), 90–98. doi: 10.1016/j.jpsychires.2009.06.006
Iljazovic, A., Roy, U., Gálvez, E. J. C., Lesker, T. R., Zhao, B., Gronow, A., et al. (2021). Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 14 (1), 113–124. doi: 10.1038/s41385-020-0296-4
Jang, J. H., Yeom, M. J., Ahn, S., Oh, J. Y., Ji, S., Kim, T. H., et al. (2020). Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson's disease. Brain Behav. Immun. 89, 641–655. doi: 10.1016/j.bbi.2020.08.015
Jia, Y. J., Liao, Y., He, Y. Q., Zheng, M. Q., Tong, X. T., Xue, W. Q., et al. (2021). Association between oral microbiota and cigarette smoking in the chinese population. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.658203
Jiang, H., Deng, S., Zhang, J., Chen, J., Li, B., Zhu, W., et al. (2023). Acupuncture treatment for post-stroke depression: Intestinal microbiota and its role. Front. Neurosci. 17. doi: 10.3389/fnins.2023.1146946
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain behavior Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Keller, M. (2003). Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA 289 (23), 3152–3160. doi: 10.1001/jama.289.23.3152
Kelly, J. R., Borre, Y., C, O. B., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Kelly, J. R., Kennedy, P. J., Cryan, J. F., Dinan, T. G., Clarke, G., Hyland, N. P. (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 9. doi: 10.3389/fncel.2015.00392
Ley, R. E. (2016). Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat. Rev. Gastroenterol. Hepatol. 13 (2), 69–70. doi: 10.1038/nrgastro.2016.4
Li, M., Niu, J., Yan, P., Yao, L., He, W., Wang, M., et al. (2020). The effectiveness and safety of acupuncture for depression: An overview of meta-analyses. Complement Ther. Med. 50, 102202. doi: 10.1016/j.ctim.2019.102202
Li, P., Huang, W., Yan, Y. N., Cheng, W., Liu, S., Huang, Y., et al. (2021). Acupuncture can play an antidepressant role by regulating the intestinal microbes and neurotransmitters in a rat model of depression. Med. Sci. Monit 27, e929027. doi: 10.12659/msm.929027
Li, S., Zhong, W., Peng, W., Jiang, G. (2018). Effectiveness of acupuncture in postpartum depression: a systematic review and meta-analysis. Acupunct Med. 36 (5), 295–301. doi: 10.1136/acupmed-2017-011530
Li, W., Yin, P., Lao, L., Xu, S. (2019). Effectiveness of acupuncture used for the management of postpartum depression: A systematic review and meta-analysis. BioMed. Res. Int. 2019, 6597503. doi: 10.1155/2019/6597503
Liang, J. Q., Li, T., Nakatsu, G., Chen, Y. X., Yau, T. O., Chu, E., et al. (2020). A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 69 (7), 1248–1257. doi: 10.1136/gutjnl-2019-318532
Limeta, A., Ji, B., Levin, M., Gatto, F., Nielsen, J. (2020). Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight 5 (23), e140940. doi: 10.1172/jci.insight.140940
Ma, Q., Li, Y., Li, P., Wang, M., Wang, J., Tang, Z., et al. (2019). Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie 117, 109138. doi: 10.1016/j.biopha.2019.109138
Maes, M., Kubera, M., Leunis, J. C., Berk, M. (2012). Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 141 (1), 55–62. doi: 10.1016/j.jad.2012.02.023
Mao, J., Wang, D., Long, J., Yang, X., Lin, J., Song, Y., et al. (2021). Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. immunotherapy Cancer 9 (12), e003334. doi: 10.1136/jitc-2021-003334
McGuinness, A. J., Davis, J. A., Dawson, S. L., Loughman, A., Collier, F., O'Hely, M., et al. (2022). A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27 (4), 1920–1935. doi: 10.1038/s41380-022-01456-3
Misri, S., Swift, E., Abizadeh, J., Shankar, R. (2016). Overcoming functional impairment in postpartum depressed or anxious women: a pilot trial of desvenlafaxine with flexible dosing. Ther. Adv. Psychopharmacol. 6 (4), 269–276. doi: 10.1177/2045125316656297
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linløkken, A., Wilson, R., et al. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 26 (8), 1155–1162. doi: 10.1111/nmo.12378
Netsi, E., Pearson, R. M., Murray, L., Cooper, P., Craske, M. G., Stein, A. (2018). Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry 75 (3), 247–253. doi: 10.1001/jamapsychiatry.2017.4363
Nikolova, V. L., Hall, M. R. B., Hall, L. J., Cleare, A. J., Stone, J. M., Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 78 (12), 1343–1354. doi: 10.1001/jamapsychiatry.2021.2573
Nonacs, R. M., Soares, C. N., Viguera, A. C., Pearson, K., Poitras, J. R., Cohen, L. S. (2005). Bupropion SR for the treatment of postpartum depression: a pilot study. Int. J. Neuropsychopharmacol. 8 (3), 445–449. doi: 10.1017/s1461145705005079
Ormsby, S. M., Smith, C. A., Dahlen, H. G., Hay, P. J., Lind, J. M. (2016). Evaluation of an antenatal acupuncture intervention as an adjunct therapy for antenatal depression (AcuAnteDep): study protocol for a pragmatic randomised controlled trial. Trials 17, 93. doi: 10.1186/s13063-016-1204-9
Pinna, G. (2023). Biomarkers and treatments for mood disorders encompassing the neurosteroid and endocannabinoid systems. J. Neuroendocrinol 35 (2), e13226. doi: 10.1111/jne.13226
Pires, R. H., Venceslau, S. S., Morais, F., Teixeira, M., Xavier, A. V., Pereira, I. A. (2006). Characterization of the Desulfovibrio desulfuricans ATCC 27774 DsrMKJOP complex–a membrane-bound redox complex involved in the sulfate respiratory pathway. Biochemistry 45 (1), 249–262. doi: 10.1021/bi0515265
Randis, T. M., Ratner, A. J. (2019). Gardnerella and prevotella: co-conspirators in the pathogenesis of bacterial vaginosis. J. Infect. Dis. 220 (7), 1085–1088. doi: 10.1093/infdis/jiy705
Rieder, R., Wisniewski, P. J., Alderman, B. L., Campbell, S. C. (2017). Microbes and mental health: A review. Brain Behav. Immun. 66, 9–17. doi: 10.1016/j.bbi.2017.01.016
Rowan, F., Docherty, N. G., Murphy, M., Murphy, B., Calvin Coffey, J., O'Connell, P. R. (2010). Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon Rectum 53 (11), 1530–1536. doi: 10.1007/DCR.0b013e3181f1e620
Santos, H., Jr., Tan, X., Salomon, R. (2017). Heterogeneity in perinatal depression: how far have we come? A systematic review. Arch. Womens Ment. Health 20 (1), 11–23. doi: 10.1007/s00737-016-0691-8
Scanlan, P. D., Shanahan, F., Marchesi, J. R. (2009). Culture-independent analysis of desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol. Ecol. 69 (2), 213–221. doi: 10.1111/j.1574-6941.2009.00709.x
Schwarz, E., Maukonen, J., Hyytiäinen, T., Kieseppä, T., Orešič, M., Sabunciyan, S., et al. (2018). Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 192, 398–403. doi: 10.1016/j.schres.2017.04.017
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12 (6), R60. doi: 10.1186/gb-2011-12-6-r60
Shade, A. (2017). Diversity is the question, not the answer. Isme J. 11 (1), 1–6. doi: 10.1038/ismej.2016.118
Sharma, V., Khan, M., Baczynski, C., Boate, I. (2020). Predictors of response to antidepressants in women with postpartum depression: a systematic review. Arch. Womens Ment. Health 23 (5), 613–623. doi: 10.1007/s00737-020-01044-w
Sharp, D. J., Chew-Graham, C., Tylee, A., Lewis, G., Howard, L., Anderson, I., et al. (2010). A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technol. Assess. 14 (43), 1–153. doi: 10.3310/hta14430
Simmons, D. A., Broderick, P. A. (2005). Cytokines, stressors, and clinical depression: augmented adaptation responses underlie depression pathogenesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29 (5), 793–807. doi: 10.1016/j.pnpbp.2005.03.009
Slykerman, R., Hood, F., Wickens, K., Thompson, J., Barthow, C., Murphy, R., et al. (2017). Effect of lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: A randomised double-blind placebo-controlled trial. EBioMedicine 24, 159–165. doi: 10.1016/j.ebiom.2017.09.013
Stakenborg, N., Gomez-Pinilla, P. J., Boeckxstaens, G. E. (2017). Postoperative ileus: pathophysiology, current therapeutic approaches. Handb. Exp. Pharmacol. 239, 39–57. doi: 10.1007/164_2016_108
Suri, R., Burt, V. K., Altshuler, L. L. (2005). Nefazodone for the treatment of postpartum depression. Arch. Womens Ment. Health 8 (1), 55–56. doi: 10.1007/s00737-005-0071-2
Tian, X. Y., Xing, J. W., Zheng, Q. Q., Gao, P. F. (2021). 919 syrup alleviates postpartum depression by modulating the structure and metabolism of gut microbes and affecting the function of the hippocampal GABA/glutamate system. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.694443
Tong, P., Dong, L. P., Yang, Y., Shi, Y. H., Sun, T., Bo, P. (2019). Traditional Chinese acupuncture and postpartum depression: A systematic review and meta-analysis. J. Chin. Med. Assoc. 82 (9), 719–726. doi: 10.1097/jcma.0000000000000140
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4 (4), 623–632. doi: 10.1038/s41564-018-0337-x
Verstreken, I., Laleman, W., Wauters, G., Verhaegen, J. (2012). Desulfovibrio desulfuricans bacteremia in an immunocompromised host with a liver graft and ulcerative colitis. J. Clin. Microbiol. 50 (1), 199–201. doi: 10.1128/jcm.00987-11
Wang, L., An, J., Song, S., Mei, M., Li, W., Ding, F., et al. (2020). Electroacupuncture preserves intestinal barrier integrity through modulating the gut microbiota in DSS-induced chronic colitis. Life Sci. 261, 118473. doi: 10.1016/j.lfs.2020.118473
Wang, Z., Liu, J., Shuai, H., Cai, Z., Fu, X., Liu, Y., et al. (2021). Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 11 (1), 543. doi: 10.1038/s41398-021-01663-6
Wong, M. L., Inserra, A., Lewis, M. D., Mastronardi, C. A., Leong, L., Choo, J., et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21 (6), 797–805. doi: 10.1038/mp.2016.46
Wright, D. P., Rosendale, D. I., Robertson, A. M. (2000). Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett. 190 (1), 73–79. doi: 10.1111/j.1574-6968.2000.tb09265.x
Yang, N. N., Lin, L. L., Li, Y. J., Li, H. P., Cao, Y., Tan, C. X., et al. (2022). Potential mechanisms and clinical effectiveness of acupuncture in depression. Curr. Neuropharmacol 20 (4), 738–750. doi: 10.2174/1570159x19666210609162809
Yang, N. N., Yang, J. W., Ye, Y., Huang, J., Wang, L., Wang, Y., et al. (2021). Electroacupuncture ameliorates intestinal inflammation by activating α7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics 11 (9), 4078–4089. doi: 10.7150/thno.52574
Yonkers, K. A., Lin, H., Howell, H. B., Heath, A. C., Cohen, L. S. (2008). Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J. Clin. Psychiatry 69 (4), 659–665. doi: 10.4088/jcp.v69n0420
Yue, N., Li, B., Yang, L., Han, Q. Q., Huang, H. J., Wang, Y. L., et al. (2018). Electro-acupuncture alleviates chronic unpredictable stress-induced depressive- and anxiety-like behavior and hippocampal neuroinflammation in rat model of depression. Front. Mol. Neurosci. 11. doi: 10.3389/fnmol.2018.00149
Zheng, P., Yang, J., Li, Y., Wu, J., Liang, W., Yin, B., et al. (2020). Gut microbial signatures can discriminate unipolar from bipolar depression. Adv. Sci. (Weinh) 7 (7), 1902862. doi: 10.1002/advs.201902862
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatry 21 (6), 786–796. doi: 10.1038/mp.2016.44
Zheng, Y., Wang, T., Tu, X., Huang, Y., Zhang, H., Tan, D., et al. (2019). Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 7 (1), 193. doi: 10.1186/s40425-019-0650-9
Zhou, Y., Chen, C., Yu, H., Yang, Z. (2020). Fecal microbiota changes in patients with postpartum depressive disorder. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.567268
Zhou, Y., Yu, H., Guo, Y., Chen, C., Huang, X., Gou, Y., et al. (2019). Efficacy of acupuncture versus sham acupuncture for postpartum depression disorder: Study protocol for a randomized controlled trial. Eur. J. Integr. Med. 31, 100982. doi: 10.1016/j.eujim.2019.100982
Keywords: fecal microbiota, postpartum depressive disorder, acupuncture, the gut-brain axis, predictive biomarker, Paraprevotella, Desulfovibrio
Citation: Zhou Y-M, Yuan J-J, Xu Y-Q, Gou Y-H, Zhu YYX, Chen C, Huang X-X, Ma X-M, Pi M and Yang Z-X (2023) Fecal microbiota as a predictor of acupuncture responses in patients with postpartum depressive disorder. Front. Cell. Infect. Microbiol. 13:1228940. doi: 10.3389/fcimb.2023.1228940
Received: 26 May 2023; Accepted: 31 October 2023;
Published: 20 November 2023.
Edited by:
Xin Xu, Sichuan University, ChinaReviewed by:
Delong Zhang, South China Normal University, ChinaCopyright © 2023 Zhou, Yuan, Xu, Gou, Zhu, Chen, Huang, Ma, Pi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuo-Xin Yang, MDAxMTg4QGd6dWNtLmVkdS5jbg==; Min- Pi, cG0wMzA1QGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.