- 1Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, Yunnan, China
- 2Department of Infectious Disease and Hepatic Disease, The First People’s Hospital of Yunnan Province, Kunming, China
- 3Medical School of Kunming University of Science and Technology, Kunming, China
- 4Kunming Medical University, Kunming, China
Background: Nearly 30%–40% of patients with chronic hepatitis B do not fall into any of the traditional natural history classification and thus are classified as indeterminate. However, it is unclear whether patients in the indeterminate phase (IP) are at a higher risk for hepatocellular carcinoma (HCC) than those in the defined phases (DP) and would benefit from antiviral therapy. We performed a systematic review and meta-analysis of HCC incidence and HBsAg clearance among patients in the IP versus DP.
Methods: We defined the clinical phases as per the AASLD 2018 hepatitis B guidance. We searched PubMed, Embase, Medline, and Web of Science for relevant studies that reported HCC incidence or HBsAg clearance in IP versus DP patients published between January 2007 and March 2023. Annual HCC incidence and HBsAg clearance rates were pooled using a random/common-effects model.
Results: We analyzed data from 14 studies, comprising 7798 IP patients (222 patients developed HCC and 239 achieved HBsAg clearance) and 10,725 DP patients. The pooled annual HCC incidence was 2.54 cases per 1,000 person-years (95% CI, 1.14–4.39) and HBsAg clearance rate was 12.36 cases per 1,000 person-years (95% CI, 10.70–14.13) for the IP patients. IP patients were associated with significantly higher HCC incidence risk (RR = 1.64, 95% CI, 1.34–2.00) and slightly lower annual HBsAg clearance rate (RR = 0.83, 95% CI, 0.70–0.99) than the DP patients. In addition, HBeAg-negative IP patients (2.31%; 95% CI, 0.87–4.45) showed a significantly higher HCC incidence than those who were HBeAg positive (0.00%; 95% CI, 0.00–0.99) (p< 0.001). The Asia-Pacific region IP patients (4.30%; 95% CI, 2.07–7.27) were also associated with a higher HCC incidence versus Europe (0.05%; 95% CI, 0.00–1.39) (p< 0.001). However, there were no significant differences between different strategies (treated vs. untreated: 2.56%; 95% CI, 1.01–4.63 vs. 1.61%; 95% CI, 0.00–5.81, p = 0.09), and heterogeneity was substantial across the studies (I2 = 89%).
Conclusion: The systematic review and meta-analysis showed a high HCC incidence and low HBsAg clearance among patients in the IP, especially for HBeAg-negative patients and the Asian population. We emphasize that future multicenter prospective cohort studies or randomized trials are needed to verify if expanding antiviral therapy for patients in the IP is associated with reduced HCC risk or good treatment outcomes.
Introduction
More than 240 million people worldwide are infected with the chronic hepatitis B (CHB) virus and are at risk for hepatocellular carcinoma (HCC) (Trépo et al., 2014). The natural history of chronic HBV infection includes several defined phases (DP): immune tolerance (IT), HBeAg-positive immune active (IA), inactive hepatitis B surface antigen carriers (IHCs), and HBeAg-negative immune reactivation phase (Gish et al., 2015).
Significantly, emerging data suggest that many CHB patients with increased ALT but low HBV DNA levels, or increased HBV DNA but normal ALT levels, who do not fall into any of the usual immune states are considered to be in the “indeterminate phase (IP)” or “ gray zone” (Hadziyannis, 2007; Yao et al., 2021). It is well accepted within the field that antiviral treatment with either pegylated interferon or a nucleos(t)ide analogue should be offered to CHB patients with liver inflammation and/or moderate-advanced hepatic fibrosis to reduce the progression of liver disease (Yao et al., 2021). The therapeutic goal for new treatments of CHB is to achieve functional cure, that is, hepatitis B surface antigen (HBsAg) clearance (Ning et al., 2019). HBsAg clearance is the desired end point of treatment for CHB patients and is associated with favorable long-term clinical outcomes (Anderson et al., 2021).
Expanding antiviral therapy to benefit more CHB populations and optimizing treatment to reduce the risk of HCC are the two main objectives in current guidelines. However, international guidelines do not recommend antiviral therapy for the treatment of CHB in the IP, mainly due to data being limited on both the risk for HCC and the clinical utility of this indeterminate group. It is unclear whether patients in the IP are at a higher risk for HCC compared to those in the DP and would benefit from antiviral therapy. Therefore, we performed a systematic review and meta-analysis of published literature to evaluate the HCC incidence and HBsAg clearance among patients in IP versus those in DP to better understand this issue.
Methods
This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO): CRD42023404002.
Definitions
We defined the clinical phases as per the AASLD 2018 hepatitis B guidance (Supplementary Table 1) (Terrault et al., 2018). Patients were considered to be in the IP when they did not meet the standard criteria for any of the defined phases. IP patients can be divided into four groups: HBeAg-positive, normal ALT, and HBV DNA ≤ 106 IU/ml (IP-A); HBeAg-positive, elevated ALT, and HBV DNA ≤ 20,000 IU/ml (IP-B); HBeAg-negative, normal ALT, and HBV DNA ≥ 2,000 IU/ml (IP-C); and HBeAg-negative, elevated ALT, and HBV DNA ≤ 2,000 IU/ml (IP-D).
Data sources and search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2015) and searched for studies that reported the incidence of HCC or HBsAg clearance among indeterminate patients. We searched PubMed, Embase, Medline, and Web of Science for articles published in English between 1 January 2007 and 31 March 2023. The entire search strategy is available in Supplemental File 1. In addition, we manually searched the bibliography of the included articles and relevant systematic reviews for additional articles. Study authors were contacted directly if necessary for more details.
Study selection
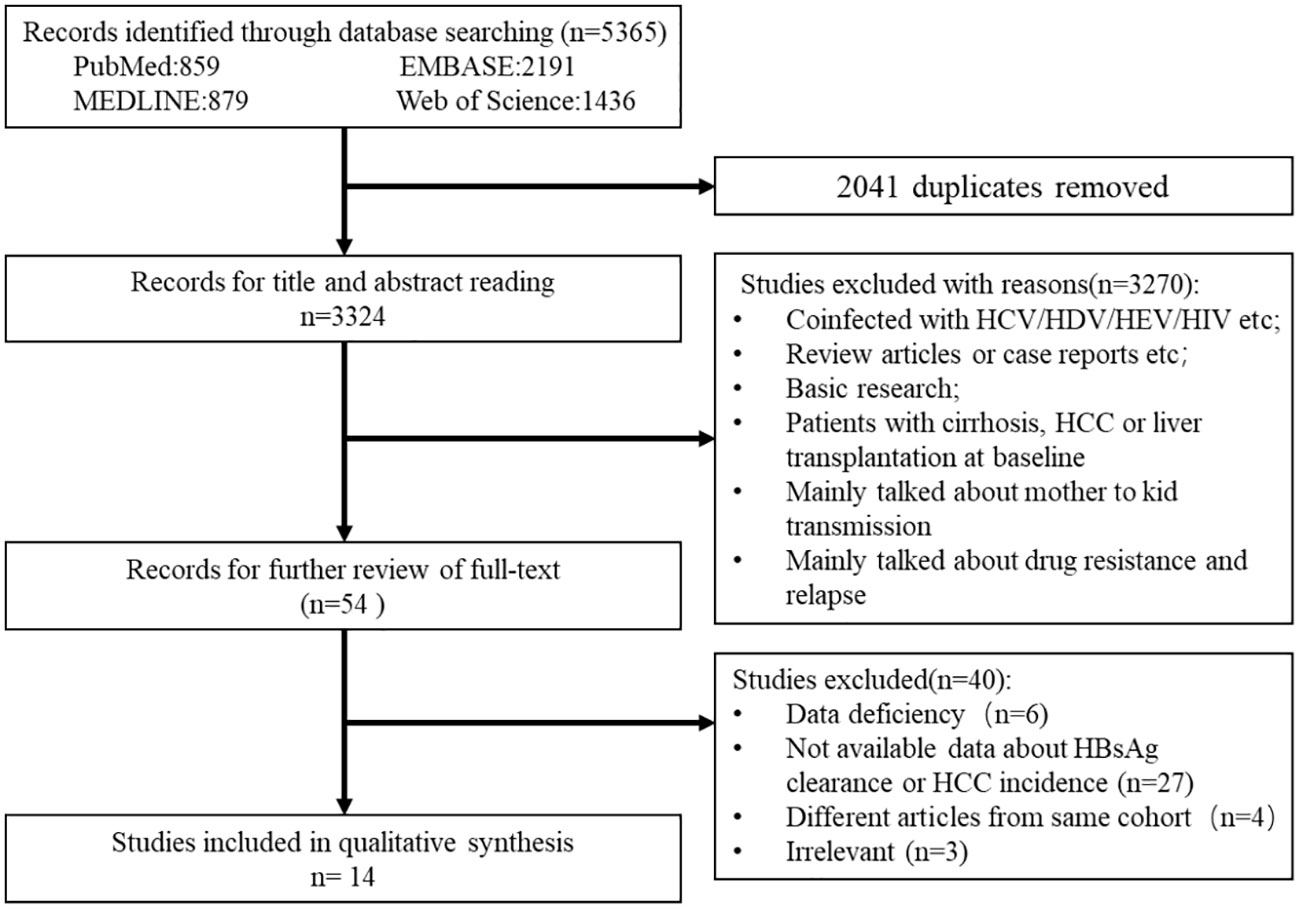
Randomized controlled trials, and prospective and retrospective cohort studies were eligible for inclusion if they met the following criteria: (a) studies with more than 20 indeterminate adult patients without cirrhosis or HCC at baseline and (b) studies with available adequate data on the primary outcomes, including the frequency and rate of HBsAg clearance (whether spontaneously or after antiviral therapy), or HCC incidence, and an average of more than 1-year of follow-up. There were no geographic restrictions.
Case reports, reviews, guidelines, protocols, fundamental research, letters or comments, and reporting data from duplicate studies were all excluded. If there were multiple studies from the same cohort, we only included data from one representative publication, which provided a complete dataset over the longest time frame. Furthermore, we excluded articles that enrolled patients coinfected with HIV, HCV, and/or HDV to reduce the effects of heterogeneity and other factors on the analysis.
Data extraction and quality assessment
Two researchers (ML and TXZ) independently screened, assessed the eligibility of studies, and extracted data with a standard form. The senior consulting investigator (JWG) resolved any disagreement on study inclusion or interpretation of data. Data collected included the first author, publication year, region of study, study design, IP group sample size, DP group sample size, sample sizes of treatment, sex, age, and follow-up years. Outcomes included the number of patients with HBsAg clearance and/or HCC incidence, annual HBsAg clearance rate (per 1,000 person-years), and annual HCC incidence (per 1,000 person-years) in different groups (IP group vs. DP group). For articles that did not report the annual HBsAg clearance rate or HCC incidence, we estimated it by using the following formula:
Two reviewers (YTZ and AMZ) used the Newcastle–Ottawa assessment scale (NOS) (Stang, 2010) to assess the methodological quality and risk of bias in the nonrandomized studies (Supplementary Table 2).
Statistical analysis
Primary outcomes were the annual HCC incidence and annual HBsAg clearance rates, which were pooled using the random-effects model of DerSimonian and Laird (1986). Because the values of these estimates can be close to 0, we used the Freeman–Tukey Double Arcsine Transformation to stabilize the variance and the Wilson score method to generate 95% confidence intervals (CIs). Heterogeneity across studies was assessed by I2 statistics and Cochran Q test, and a funnel plot and Egger’s test were used to assess any publication bias. Pooled risk ratios (RR) with 95% CIs were calculated to estimate the incidence of HCC and HBsAg clearance rates with the IP group compared with the DP group. All analyses were conducted using the meta package of R software, version 4.3.1. Metarate was used to compute the pooled estimate of both the annual HCC and HBsAg clearance rates, p-values were two-tailed, and p< 0.05 was considered statistically significant.
Results
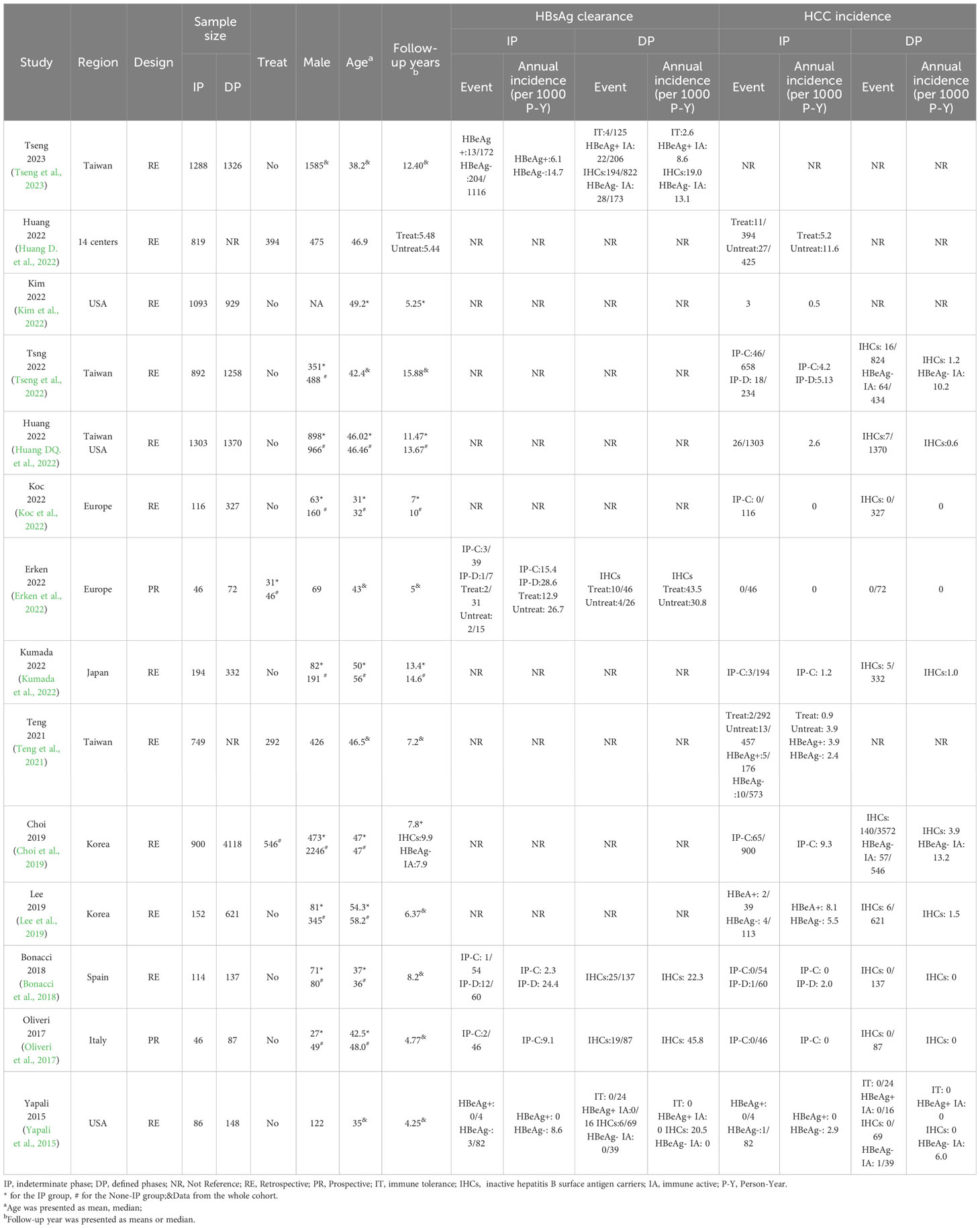
From the electronic and manual search (Figure 1), 3,324 records were identified after removing duplicates and screening the titles and abstracts, and 54 potentially eligible studies were retrieved for full-text reviews. After review, a total of 14 articles (7,798 patients in the IP and 10,725 patients in the DP) were included in the meta-analysis (Yapali et al., 2015; Oliveri et al., 2017; Bonacci et al., 2018; Choi et al., 2019; Lee et al., 2019; Teng et al., 2021; Erken et al., 2022; Huang D. et al., 2022; Huang DQ. et al., 2022; Kim et al., 2022; Koc et al., 2022; Kumada et al., 2022; Tseng et al., 2022; Tseng et al., 2023).
Characteristics of the studies
Table 1 shows the characteristics of included studies and of the participants. CHB patients from 14 countries were included, with the majority from the Asia-Pacific region (seven studies). The mean age of the included studies ranged from 31 to 58.2 years, and 9,248 (49.93%) were men.
The annual HCC incidence and subgroup analysis in the IP patients
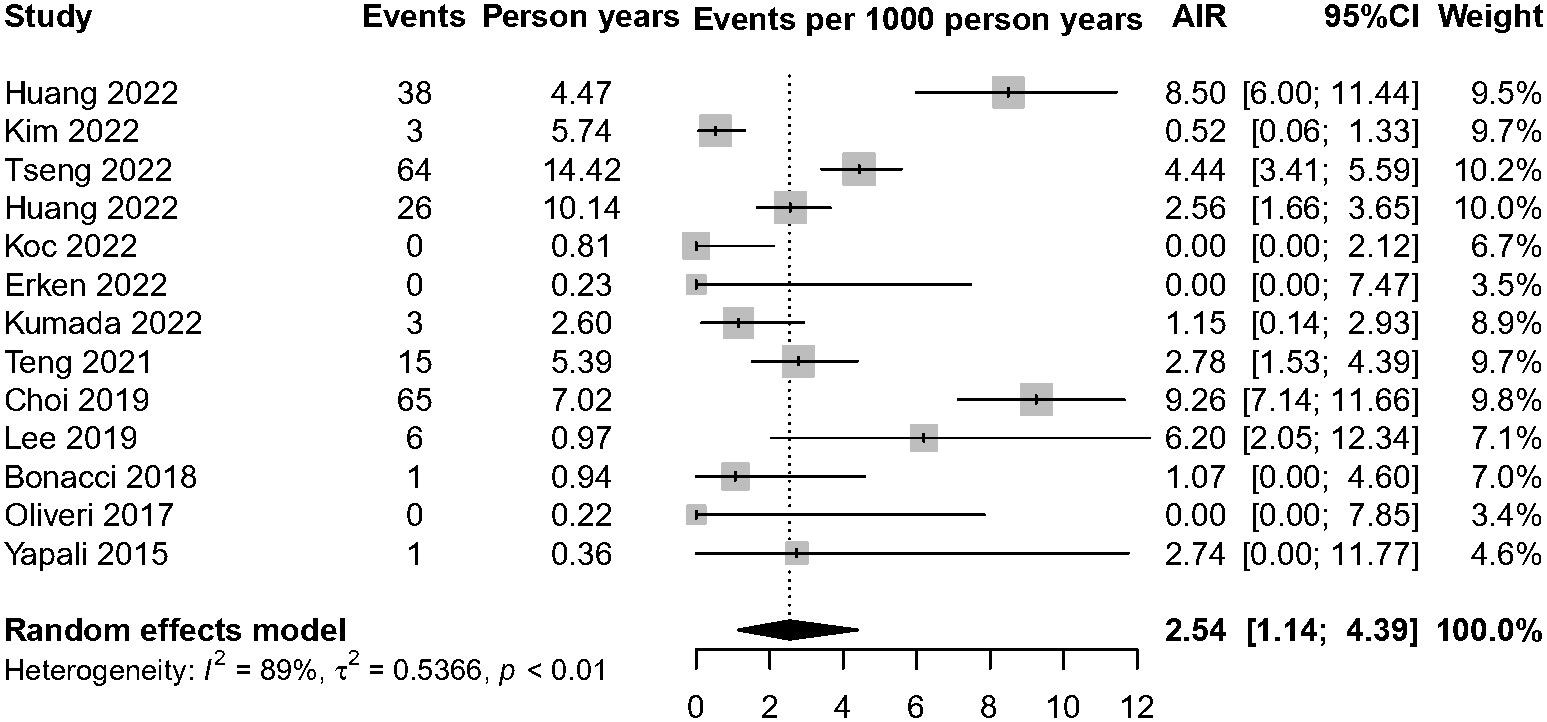
Among 6,510 patients in the IP, 222 patients developed HCC. The annual HCC rates ranged from 0% to 9.26%, with a pooled incidence of 2.54 cases per 1,000 person-years (95% CI, 1.14–4.39, I2 = 89%, random-effects model, Figure 2), and no publication bias as assessed by visual inspection of the funnel plots (Supplementary Figure 1) and results of Egger’s test (p = 0.504). To minimize data heterogeneity among studies, we further performed a subgroup analysis. The pooled annual HCC incidence rates were higher among untreated patients (2.56%, 95% CI, 1.01–4.63) than treated patients (1.61%, 95% CI, 0.00–5.81), although the difference was not statistically significant (p = 0.09) (Supplementary Figure 2). We further analyzed HCC incidence by different HBeAg status and found that HBeAg-negative patients (2.31%; 95% CI, 0.87–4.45) showed higher pooled annual rates of HCC incidence than HBeAg-positive patients (0.00%; 95% CI, 0.00–0.99) (p< 0.001). In addition, we stratified by geographic region, and the results showed Asia-Pacific region (4.30%; 95% CI, 2.07–7.27) also had significantly higher annual rates than Europe (0.05%; 95% CI, 0.00–1.39) (p< 0.001)), but there was no significant difference between Asia-Pacific and America (0.64%; 95% CI, 0.00–4.15) (p = 0.22) (Figure 3).
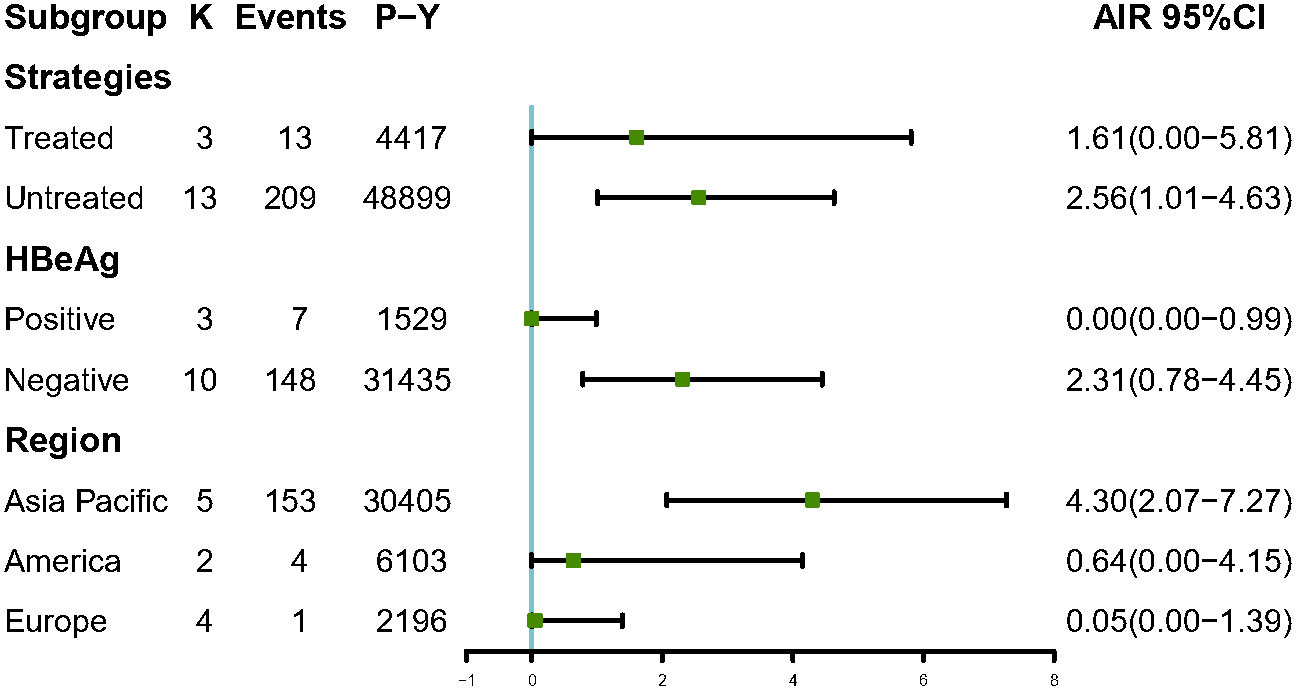
Figure 3 Subgroup analysis of annual HCC incidence by strategies, HBeAg status and region among IP patients. AIR, Annual incidence rate. K, number of studies; P-Y, person-years.
Incidence of HCC among CHB patients in the IP versus DP
Ten studies separately reported the HCC incidence among CHB patients in the IP (n = 3,849) versus the DP (n = 8,470), and the result showed that patients in the IP group were associated with a significantly higher HCC incidence than DP patients; the pooled RR is 1.64 (95% CI, 1.34–2.00, p< 0.001) (Supplementary Figure 3). To evaluate the potential influence of different natural history, subgroup analyses were performed. IP-C patients had a 2.48 times higher annual HCC incidence rate than IHC patients. Similarly, IP-D patients also had significantly higher HCC incidence than IHC patients, and the pooled RR was 4.49 (95% CI, 2.70–7.47, p< 0.001) (Figure 4). Because of the limited number of relevant studies, we did not conduct a subgroup analysis comparing HBeAg-positive IP patients with DP patients.
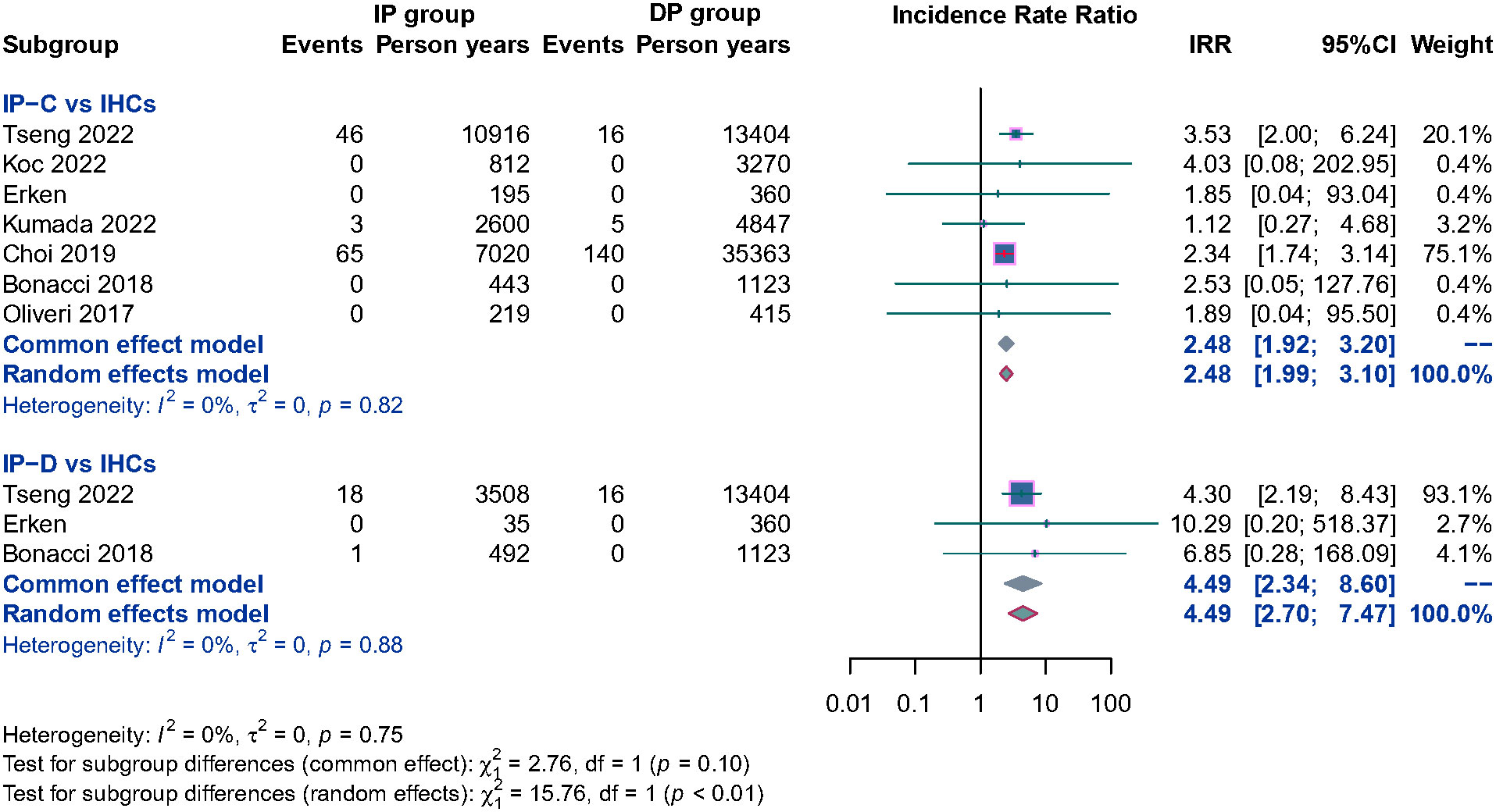
Figure 4 Subgroup analysis of annual HCC incidence among CHB patients in the HBeAg negative IP (IP-C and IP-D) versus IHCs.
The annual HBsAg clearance among CHB patients in the IP versus DP
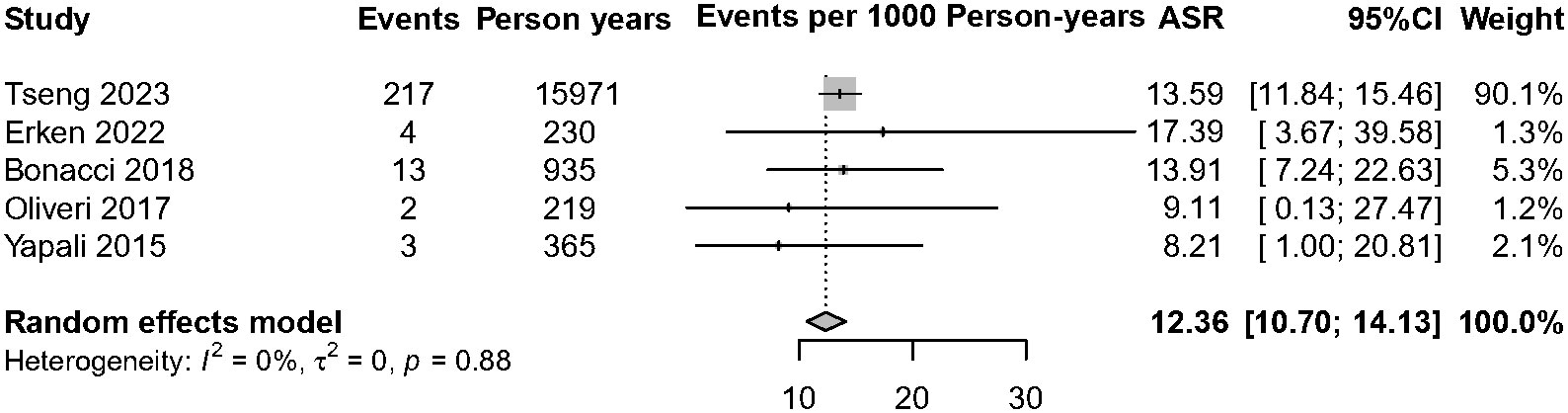
Currently, the existing literature is sparse on HBsAg clearance of IP patients. Among the five studies with 1,580 IP patients on HBsAg clearance, over 98% of patients (n = 1,549) have no antiviral treatment. A total of 239 patients achieved HBsAg clearance, and the annual HBsAg clearance pooled incidence was 12.36 cases per 1,000 person-years (95% CI, 10.70–14.13, I2 = 0%, common-effect model, Figure 5). We further analyzed the data of patients who achieved HBsAg clearance in IP versus DP patients (n = 1,770). The result showed that IP patients were associated with a lower annual HBsAg clearance rate compared with the DP patients (RR = 0.83, 95% CI, 0.70–0.99, p = 0.03) (Supplementary Figure 4).
Discussion
In this meta-analysis of 14 studies, which included 7,798 IP patients, a total of 222 patients developed HCC, and 239 patients achieved HBsAg clearance, resulting in a pooled annual HCC incidence of 2.54 cases per 1,000 person-years (95% CI, 1.14–4.39) and HBsAg clearance rates of 12.36 cases per 1000 person-years (95% CI, 10.70–14.13). In addition, we found that IP patients were associated with significantly higher HCC incidence risk (RR = 1.64, 95% CI, 1.34–2.00) and slightly lower annual HBsAg clearance rates (RR = 0.83, 95% CI, 0.70–0.99) than the DP patients; expanding antiviral therapy might be beneficial in patients in this phase.
The HCC pooled annual incidence rates ranged from 0% to 9.26% across the studies due to different strategies (antiviral treatment or observation), HBeAg status, or regions. By subgroup analyses, we found that IP patients who were HBeAg negative at baseline from the Asia-Pacific region had a higher risk of developed HCC. The reasons may partly be because HBV infection often occurs in young people, and patients usually have a long disease course by the CHB stage in Asian populations. Another possible reason is the differences in the geographic distribution of HBV genotypes. Genotype A is most frequent in North America and Africa, genotypes B and C are the dominant viruses throughout East Asia, and genotype D is most common in Southern Europe and India (Tong and Revill, 2016). Analysis of patients by HBV genotypes was not performed because of a lack of primary data, with most included studies not reporting HBV genotype information. Notably, a retrospective study by Huang et al. (2023) showed that antiviral therapy reduces HCC risk by 70% among IP CHB patients. In this meta-analysis, the pooled annual HCC incidence rates were higher among untreated patients (2.56%, 95% CI, 1.01–4.63) than treated ones (1.61%, 95% CI, 0.00–5.81), but the difference was not statistically significant (p = 0.09). However, the severe heterogeneity of our results requires that they be interpreted with caution.
Furthermore, we found that IP patients, especially IP-C and IP-D patients, were associated with significantly higher HCC incidence risk than the IHCs who were HBeAg negative, had lower HBV DNA levels, and had quantitative HBsAg (Cao et al., 2017). In addition, owing to the limited number of relevant studies, we did not conduct a subgroup analysis comparing HBeAg-positive IP patients versus DP patients.
CHB patients spontaneously achieving HBsAg clearance is a rare event. Of note, over 98% of IP patients had no antiviral treatment among the included five studies in this meta-analysis, a total of 237 patients spontaneously achieved HBsAg clearance among 1,580 IP patients, the annual HBsAg clearance pooled incidence was 12.36 cases per 1,000 person-years (95% CI, 10.70–14.13), and a high rate of HBsAg spontaneous clearance was observed in IP patients, probably because 88.86% of patients (1,404/1,580) were HBeAg negative at baseline, which was associated with a high rate of HBsAg clearance. Nevertheless, the pooled RR was 0.89 among CHB patients in the IP versus DP; the differences still reached statistical significance (p = 0.03).
This study has some limitations. First, there are different definitions of normal ALT among different international guidelines. The ALT cutoff is 35 U/L for male individuals and 25 U/L for female individuals defined by the AASLD 2018 guideline (Terrault et al., 2018); however, the upper limit of normal ALT is 40 U/L based on EASL and APASL guidelines (Sarin et al., 2016; Lampertico et al., 2017). In general, the IP was defined according to the AASLD 2018 guidance in this meta-analysis, but because the existing literature lacks relevant IP studies, we did not strictly follow the criteria of normal ALT defined by the AASLD 2018 guideline. Second, owing to the limited number of relevant HBeAg-positive studies and the fact that the sample sizes in available studies are usually small, the results of this meta-analysis mainly reflect in HBeAg-negative IP patients of HCC incidence/HBsAg clearance. Third, because current international guidelines do not recommend treatment for IP patients, few prospective cohort studies or RCT studies have been conducted to investigate post-treatment HBsAg clearance and long-term HCC incidence. Fourth, some subgroup analyses in our study included a relatively small number of studies and subjects, causing inaccurate estimation. Therefore, the results of this meta-analysis have some inherent heterogeneities.
To the best of our knowledge, this is the first systematic study with a large sample size to analyze the annual HCC incidence and HBsAg clearance rates for CHB patients in the IP. In short, this meta-analysis shows that IP patients were associated with significantly higher HCC incidence risk and slightly lower annual HBsAg clearance rates than the DP patients. We emphasize that future multicenter prospective cohort studies or randomized trials are needed to verify if expanding antiviral therapy for patients in the IP is associated with reduced HCC risk or good treatment outcomes.
Author contributions
ML, JG, and XX conceived and designed the protocol and study. ML and TZ identified studies to be screened. YZ and A-MZ identified studies for eligibility, extracted data, and assessed the methodological quality of the included studies. ML performed the analysis with assistance from TZ and YZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Yunnan (Kunming) Zhang Wenhong export workstation (YSZJGZZ-2020051) and Kunming University of Science of Technology & the First People’s Hospital of Yunnan Province Joint Special Project on Medical Research (KUST-KH2022022Y).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1226755/full#supplementary-material
References
Anderson, R. T., Choi, H., Lenz, O., Peters, M. G., Janssen, H., Mishra, P., et al. (2021). Association between seroclearance of hepatitis B surface antigen and long-term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 19, 463–472. doi: 10.1016/j.cgh.2020.05.041
Bonacci, M., Lens, S., Marino, Z., Londono, M. C., Rodriguez-Tajes, S., Mas, A., et al. (2018). Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. ALIMENTARY. Pharmacol. Ther. 47, 1397–1408. doi: 10.1111/apt.14613
Cao, Z., Liu, Y., Ma, L., Lu, J., Jin, Y., Ren, S., et al. (2017). A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha. Hepatology 66, 1058–1066. doi: 10.1002/hep.29213
Choi, G. H., Kim, G. A., Choi, J., Han, S., Lim, Y. S. (2019). High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment. Pharmacol. Ther. 50, 215–226. doi: 10.1111/apt.15311
DerSimonian, R., Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials. 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Erken, R., Loukachov, V. V., de Niet, A., Jansen, L., Stelma, F., Helder, J. T., et al. (2022). A Prospective Five-Year Follow-up After peg-Interferon Plus Nucleotide Analogue Treatment or no Treatment in HBeAg Negative Chronic Hepatitis B Patients. J. Clin. Exp. Hepatol. 12, 735–744. doi: 10.1016/j.jceh.2021.12.011
Gish, R. G., Given, B. D., Lai, C. L., Locarnini, S. A., Lau, J. Y., Lewis, D. L., et al. (2015). Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 121, 47–58. doi: 10.1016/j.antiviral.2015.06.008
Hadziyannis, S. J. (2007). Unrevealing the natural course of the so-called “inactive HBsAg or HBV carrier state”. Hepatol. Int. 1, 281–284. doi: 10.1007/s12072-007-9004-7
Huang, D. Q., Li, X., Le, M. H., Le, A. K., Yeo, Y. H., Trinh, H. N., et al. (2022). Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin. Gastroenterol. Hepatol. 20, 1803–1812. doi: 10.1016/j.cgh.2021.01.019
Huang, D., Tran, A. K., Yeh, M. L., Tsai, P. C., Chuang, W. L., Huang, C. F., et al. (2022). Antiviral therapy reduces hepatocellular carcinoma risk among hbv patients in the indeterminate phase. Hepatology 76, S34–S35. doi: 10.1002/hep.32697
Huang, D. Q., Tran, A., Yeh, M. L., Yasuda, S., Tsai, P. C., Huang, C. F., et al. (2023). Antiviral therapy substantially reduces hepatocellular carcinoma risk in chronic Hepatitis B patients in the indeterminate phase. Hepatology. doi: 10.1097/HEP.0000000000000459
Kim, H., Yu, X., Kramer, J. R., Lee, T. H., El-Serag, H. B., Kanwal, F. (2022). Prognosis and clinical correlates of outcomes in U.S. patients with indeterminate phase chronic hepatitis b virus infection. Gastroenterology 162, 1139. doi: 10.1016/S0016-5085(22)63417-6
Koc, ÖM, Verbeek, J., Koek, G. H., Bielen, R., Busschots, D., Gamil, M., et al. (2022). A long-term study of liver-related events in Caucasian hepatitis B patients with normal ALT values and high viremia. Acta Gastroenterol. Belg. 85, 56–61. doi: 10.51821/85.1.9160
Kumada, T., Toyoda, H., Yasuda, S., Ito, T., Tanaka, J. (2022). Mortality of inactive hepatitis B virus carriers in Japan is similar to that of the general population. Hepatol. Res. 52, 81–92. doi: 10.1111/hepr.13723
Lampertico, P., Agarwal, K., Berg, T., Buti, M., Janssen, H. L. A., Papatheodoridis, G., et al. (2017). EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 67, 370–398. doi: 10.1016/j.jhep.2017.03.021
Lee, H. W., Kim, S. U., Baatarkhuu, O., Park, J. Y., Kim, D. Y., Ahn, S. H., et al. (2019). Progression of untreated minimally active chronic HBV infection compared to inactive infection. Clin. Gastroenterol. Hepatol. 17, 2808–2810. doi: 10.1016/j.cgh.2019.01.002
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. doi: 10.1186/2046-4053-4-1
Ning, Q., Wu, D., Wang, G. Q., Ren, H., Gao, Z. L., Hu, P., et al. (2019). Roadmap to functional cure of chronic hepatitis B: An expert consensus. J. Viral Hepat. 26, 1146–1155. doi: 10.1111/jvh.13126
Oliveri, F., Surace, L., Cavallone, D., Colombatto, P., Ricco, G., Salvati, N., et al. (2017). Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis B virus infection: Benign course towards HBsAg clearance. Liver. Int. 37, 1622–1631. doi: 10.1111/liv.13416
Sarin, S. K., Kumar, M., Lau, G. K., Abbas, Z., Chan, H. L., Chen, C. J., et al. (2016). Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10, 1–98. doi: 10.1007/s12072-015-9675-4
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Teng, W., Chang, T. T., Yang, H. I., Peng, C. Y., Su, C. W., Su, T. H., et al. (2021). Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines. Hepatol. Int. 15, 1421–1430. doi: 10.1007/s12072-021-10263-x
Terrault, N. A., Lok, A., McMahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., et al. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67, 1560–1599. doi: 10.1002/hep.29800
Tong, S., Revill, P. (2016). Overview of hepatitis B viral replication and genetic variability. J. Hepatol. 64, S4–S16. doi: 10.1016/j.jhep.2016.01.027
Trépo, C., Chan, H. L., Lok, A. (2014). Hepatitis B virus infection. Lancet 384, 2053–2063. doi: 10.1016/S0140-6736(14)60220-8
Tseng, T. C., Chiang, C., Liu, C. J., Hong, C. M., Su, T. H., Yang, H. C., et al. (2023). Low hepatitis B core-related antigen levels correlate higher spontaneous seroclearance of hepatitis B surface antigen in chronic hepatitis B patients with high hepatitis B surface antigen levels. Gastroenterology 164, 669–679. doi: 10.1053/j.gastro.2023.01.005
Tseng, T. C., Hosaka, T., Liu, C. J., Suzuki, F., Hong, C. M., Kumada, H., et al. (2022). Hepatitis B core-related antigen stratifies the risk of liver cancer in HBeAg-negative patients with indeterminate phase. Am. J. Gastroenterol. 117, 748–757. doi: 10.14309/ajg.0000000000001691
Yao, K. F., Liu, J. C., Wang, J., Yan, X. M., Xia, J., Yang, Y., et al. (2021). Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J. OF. Viral HEPATITIS. 28, 1025–1033. doi: 10.1111/jvh.13511
Keywords: chronic hepatitis B, indeterminate phase, HBsAg, clearance, HCC, meta-analysis
Citation: Liu M, Zhao T, Zhang Y, Zhang A-M, Geng J and Xia X (2023) The incidence of hepatocellular carcinoma and clearance of hepatitis B surface for CHB patients in the indeterminate phase: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 13:1226755. doi: 10.3389/fcimb.2023.1226755
Received: 28 May 2023; Accepted: 21 August 2023;
Published: 12 September 2023.
Edited by:
Terry Cheuk-Fung Yip, The Chinese University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Wenjun Wang, Xi’an Jiaotong University, ChinaValentina Cossiga, University of Naples Federico II, Italy
Copyright © 2023 Liu, Zhao, Zhang, Zhang, Geng and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiawei Geng, Z2VuZ19lZHUyMDIzQDE2My5jb20=; Xueshan Xia, b2xpdmVyeGlhMjAwMEBhbGl5dW4uY29t
†These authors have contributed equally to this work
 Min Liu1,2†
Min Liu1,2† A-Mei Zhang
A-Mei Zhang Jiawei Geng
Jiawei Geng