
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 16 May 2023
Sec. Intestinal Microbiome
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1191936
This article is part of the Research Topic The interplay of gut-microbiome between infection and inflammation View all 18 articles
 Jingyue Wang1,2†
Jingyue Wang1,2† Xianfeng Zhang3†
Xianfeng Zhang3† Xinyu Yang1,2
Xinyu Yang1,2 Hang Yu2
Hang Yu2 Mengmeng Bu2
Mengmeng Bu2 Jie Fu2
Jie Fu2 Zhengwei Zhang2
Zhengwei Zhang2 Hui Xu2
Hui Xu2 Jiachun Hu2
Jiachun Hu2 Jinyue Lu2
Jinyue Lu2 Haojian Zhang2
Haojian Zhang2 Zhao Zhai2
Zhao Zhai2 Wei Yang1
Wei Yang1 Xiaodan Wu1
Xiaodan Wu1 Yan Wang2*
Yan Wang2* Qian Tong1*
Qian Tong1*Numerous studies have demonstrated that gut microbiota plays an important role in the development and treatment of different cardiovascular diseases, including hypertension, heart failure, myocardial infarction, arrhythmia, and atherosclerosis. Furthermore, evidence from recent studies has shown that gut microbiota contributes to the development of myocarditis. Myocarditis is an inflammatory disease that often results in myocardial damage. Myocarditis is a common cause of sudden cardiac death in young adults. The incidence of myocarditis and its associated dilated cardiomyopathy has been increasing yearly. Myocarditis has gained significant attention on social media due to its association with both COVID-19 and COVID-19 vaccinations. However, the current therapeutic options for myocarditis are limited. In addition, little is known about the potential therapeutic targets of myocarditis. In this study, we review (1) the evidence on the gut-heart axis, (2) the crosslink between gut microbiota and the immune system, (3) the association between myocarditis and the immune system, (4) the impact of gut microbiota and its metabolites on myocarditis, (5) current strategies for modulating gut microbiota, (6) challenges and future directions for targeted gut microbiota in the treatment of myocarditis. The approach of targeting the gut microbiota in myocarditis is still in its infancy, and this is the study to explore the gut microbiota-immune system-myocarditis axis. Our findings are expected to pave the way for the use of gut microbiota as a potential therapeutic target in the treatment of myocarditis.
Myocarditis is an inflammatory disease, characterized by the infiltration of inflammatory cells and deterioration of cardiac function (Richardson et al., 1996; Leuschner et al., 2015). Inflammation of the heart was first described in 1749, and the term myocarditis was coined in 1837. Furthermore, in 1980, the World Health Organization/International Society and Federation of Cardiology Task Force proposed a way of differentiating myocarditis from other myocardial diseases (Richardson et al., 1996). Myocarditis has multifaceted etiology, including infectious causes such as viral, bacterial, fungal, and parasitic infections, and non-infectious causes such as drug, autoimmune, and allergic reactions, amyloidosis, thyrotoxicosis, and genetic predisposition (Wiltshire et al., 2011; Basso, 2022). The clinical manifestations of myocarditis can vary from asymptomatic or subclinical/clinical symptoms to sudden death due to the damage of cardiomyocytes, inflammatory reaction, and myocardial fibrosis. Therefore, myocarditis poses a significant threat to the health and well-being of patients (D’Ambrosio and D’Ambrosio, 2001; Ammirati et al., 2020).
According to a previous study, cases of myocarditis have been associated with coronavirus disease 2019 (COVID-19) and COVID-19 vaccination (Inciardi et al., 2020; Heymans et al., 2022). The underlying mechanisms of myocarditis related to COVID-19 are believed to involve both direct harm caused by the virus itself and cardiac damage resulting from the host’s immune reaction (Siripanthong et al., 2020). Consequently, the management of myocarditis is an attractive area for research (Heymans et al., 2022). The primary management goals of myocarditis include alleviating biventricular load, ensuring adequate systemic and coronary perfusion, and reducing venous congestion. The management goals aim to minimize the risk of multiorgan dysfunction and accelerate recovery, transplantation, or the use of durable assist devices (Basso, 2022). Myocarditis represents a diverse group of diseases with distinct immunophenotypes. Despite extensive research and an improved understanding of the pathogenesis of myocarditis, translating knowledge into effective therapeutic strategies remains a challenge (Heymans et al., 2016). The typical management of myocarditis includes drug therapy, mechanical circulatory support, and management of complications and co-morbidities (Cooper, 2009). In addition, the management of myocarditis also includes vitamins and nutritional support. Drug therapy is the most used treatment modality for myocarditis. However, some drug therapies are ineffective and associated with severe side effects. On the other hand, mechanical circulatory support is a costly intervention and is not suitable for all patients. Moreover, immune modulation is a novel treatment approach that requires further research (Basso, 2022). Some potential strategies being explored include the use of probiotics, prebiotics, and synbiotics to modulate the gut microbiota, as well as fecal microbiota transplantation (FMT) to restore a healthy microbial balance (Schneiderhan et al., 2016; Hu et al., 2019; Wargo, 2020; Fan and Pedersen, 2021). These approaches aim to improve the overall gut health and immune response, which may have a positive impact on myocarditis treatment outcomes. It is important to note that more research is needed to establish the efficacy and safety of these strategies in the context of myocarditis.
The summary of the clinical characteristics and treatment strategies for myocarditis is compiled in Figure 1 (Trachtenberg and Hare, 2017; Błyszczuk and Błyszczuk, 2019; Leone et al., 2019; Cooper, 2021; Basso, 2022; Ammirati et al., 2023). In clinical practice, it is essential to tailor diagnostic or therapeutic approaches to individual patients, taking into account their specific circumstances and conditions. Although myocarditis has been recognized for two centuries, the available therapeutic regimens are limited (Krejci et al., 2016; Spallarossa et al., 2020). Therefore, there is a need for continued research into the pathogenesis and treatment of myocarditis to identify new therapeutic targets and cost-effective agents.
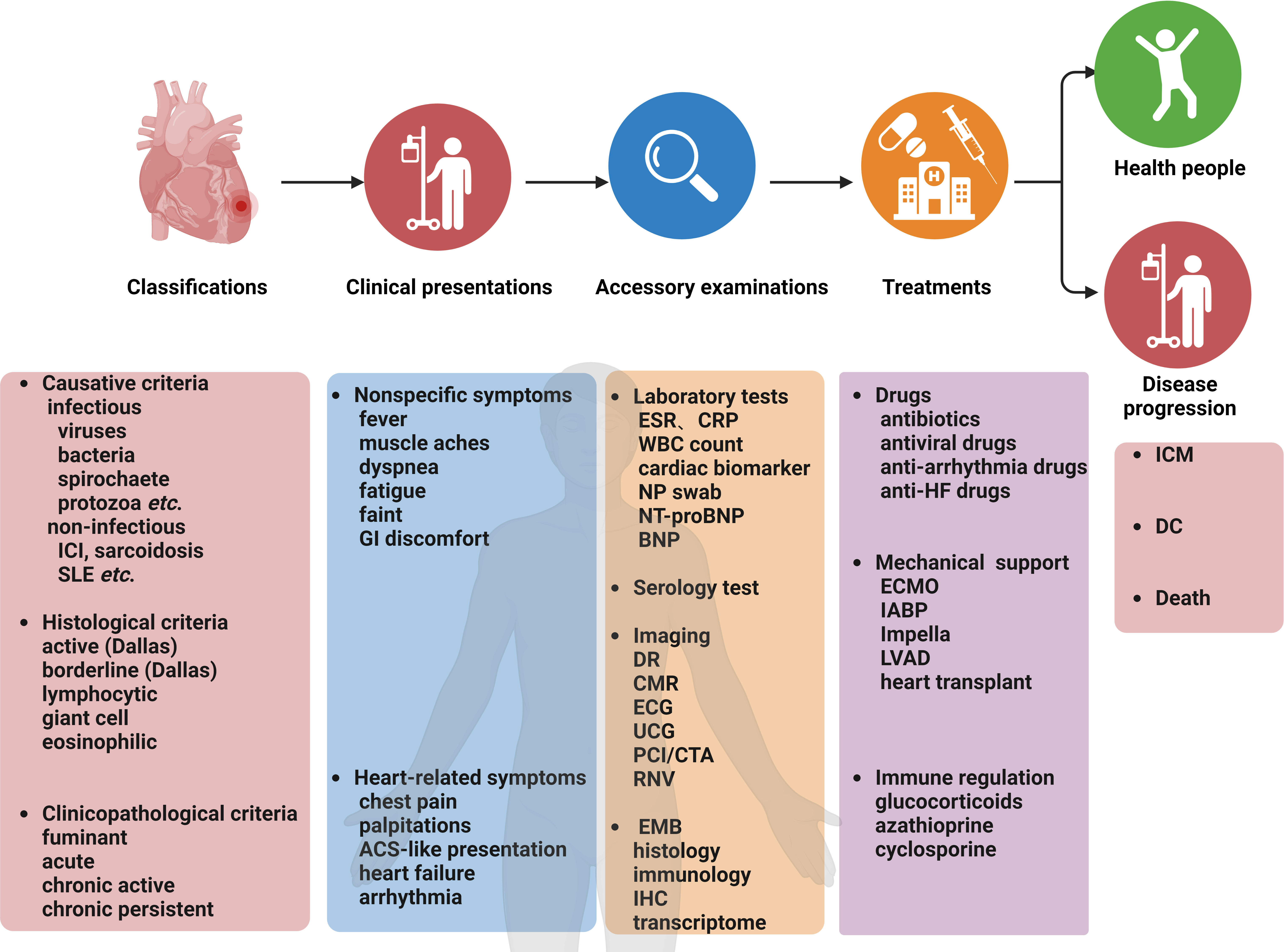
Figure 1 Myocarditis: Classifications, clinical presentations, accessory examinations, treatments, and prognosis. ACS, acute coronary syndrome; CMR, cardiovascular magnetic resonance; CTA, coronary computed tomography angiography; DC, dilated cardiomyopathy; DR, digital radiography; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; EMB, endomyocardial biopsy; FDG-PET, Fluorodeoxyglucose-positron emission tomography; GI, gastrointestinal; HF, heart failure; IABP, intra-aortic balloon pump; ICI, immune checkpoint inhibitors; IHC, immunohistochemistry; ICM, inflammatory cardiomyopathy; LVCD, left ventricular assist device; NP, nasopharyngeal; PCI, percutaneous coronary intervention; RNV, radionuclide ventriculography; SLE, systemic lupus erythematosus; UCG, ultrasound cardiography; WBC, white blood cell.
In recent years, the human gut microbiota has gained significant attention, with metagenomic studies enhancing our understanding of its diverse species and potential applications in health (Gomaa and Gomaa, 2020). The human gut microbiota contains approximately 39 trillion microorganisms and about 150 times more microbial genes (3.3x10^6) than human genes (Collins and Patterson, 2020). The large intestines have the highest microbial density, with about 100 billion bacterial cells per gram of wet stool (Sender et al., 2016). Furthermore, the gut microbiome is highly complex, with its composition varying widely between individuals. The gut microbiome comprises more than 1000 species, including bacteria, archaea, viruses, and fungi (Sekirov et al., 2010). Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria form the most dominant bacterial phyla of the gut microbiome (Yan et al., 2022). Gut bacteria are mainly categorized into symbiotic, opportunistic, and pathogenic bacteria. Symbiotic bacteria interact with each other and with the host in a symbiotic manner. They maintain gut homeostasis and contribute to overall health (Cummings, 1983). The gut microbiome participates in various functions, including nutrient absorption, immune system regulation, and biological antagonism (Adak and Khan, 2019). However, dysbiosis of the gut microbiome has been associated with various diseases (Hou et al., 2022). The composition of the gut microbiome is affected by various factors, including genetics, diet, medication, and environment. Diet is among the most significant factors influencing the gut microbiome (Duda-Chodak et al., 2015; Singh et al., 2017; Klement and Pazienza, 2019). A high-fiber diet and plant-based foods can promote the growth of beneficial bacteria in the gut microbiome, while high-fat and high-sugar diets have been linked to dysbiosis (Koh et al., 2016; Nagai et al., 2016; Hills et al., 2019).
Previous studies have shown that the gut microbiota contributes to the occurrence and development of various cardiovascular diseases (CVDs) (Witkowski et al., 2020), including hypertension (Yang et al., 2018; Verhaar et al., 2020), atherosclerosis (Zhu et al., 2020), heart failure (HF) (Zhang et al., 2021b), arrhythmias (Oniszczuk et al., 2021), and diabetic cardiomyopathy (Bastin and Andreelli, 2020; Yuan et al., 2022). Moreover, recent studies revealed that gut microbiota was also associated with myocarditis (Gil-Cruz et al., 2019; Hu et al., 2019; Mandelbaum et al., 2020; Piccioni et al., 2021; Luo et al., 2022; Ozkan, 2022). Furthermore, gut microbiota modulation through dietary interventions, probiotics, drugs, or fecal microbiota transplantation, has shown promising results in various conditions, including inflammatory bowel disease (Li et al., 2021b), colorectal cancer (Feng et al., 2015; Wong and Yu, 2019; de Souza et al., 2022), liver diseases (Bajaj, 2019; Albillos et al., 2020), obesity (Duca et al., 2013; Gomes et al., 2018), diabetes (Qin et al., 2012; Komaroff, 2017; Hung et al., 2021), arthritis (Scher et al., 2013; Xu et al., 2022), osteoporosis (Lahiri et al., 2019; Di et al., 2021), CVDs (Ko et al., 2019; Bai et al., 2021), and neurological disorders (Varesi et al., 2022).
In recent years, the role of the gut microbiome in health and diseases has attracted significant research attention. However, animal studies and clinical trials evaluating the relationship between myocarditis and gut microbiota are limited (Hu et al., 2019). Therefore, this study reviews (1) the evidence on gut-heart axis, (2) the association between gut microbiota and the immune system, (3) the association between myocarditis and the immune system, (4) the association between gut microbiota and myocarditis, (5) current strategies for modulating gut microbiota, (6) challenges and future directions for targeted gut microbiota in the treatment of myocarditis. This study provides novel ideas for myocarditis treatment and references for future research.
The gut microbiome regulates human health. Recent studies indicate that dysbiosis of the gut microbiota is thought to be cause of most CVDs, including coronary heart disease, hypertension, and heart failure (Lippi et al., 2017; Jia et al., 2019). Gut microbiota dysbiosis can also induce an inflammatory response and affect the metabolism of bile acids (BAs), short-chain fatty acids (SCFAs), trimethylamine-N-oxide (TMAO) and other bioactive molecules, resulting in systemic inflammation and endothelial dysfunction. These changes, in turn, promote the development of atherosclerotic plaques and increase the risk of thrombosis and cardiovascular events (Liu et al., 2020b). The role of the gut-heart axis in cardiovascular health and the relationship between the gut microbiome and CVDs are presented in the subsequent sections and Figure 2.

Figure 2 Gut-Heart axis: The relationship between gut microbiota and cardiovascular diseases. SCFAs, short-chain fatty acids; TMA, trimethylamine; TMAO, TMA-N-oxide.
Coronary heart disease (CHD) is a condition in which the arteries cannot supply adequate oxygenated blood to the heart. Most cases of CHD are caused by the blockage of coronary arteries due to either atherosclerosis, thrombosis, or a combination of both (Ulbricht and Southgate, 1991).
The effects of gut microbiota in CHD are due to alterations in their composition and metabolites. One previous study enrolling 29 CHD inpatients and 35 healthy volunteers showed the proportion of phylum Bacteroidetes (56.12%) was lower, whereas that of the phylum Firmicutes was higher (37.06%) in the CHD patients than that of the healthy controls (60.92% and 32.06%, P <0.05) (Cui et al., 2017). Elsewhere, a similar observation was obtained (Emoto et al., 2017). Furthermore, a case-control study revealed that lower Lactobacillus levels are associated with an increased likelihood of severe coronary atherosclerotic lesions and myocardial necrosis as well as a poorer prognosis for patients with the acute coronary syndrome (ACS), particularly those with ST-segment elevation myocardial infarction (Gao et al., 2021). A previous study showed that Faecalibacterium was the dominant microorganism in the healthy control group, whereas Escherichia-Shigella and Enterococcus were enriched in the coronary artery disease group (Zhu et al., 2018). Furthermore, Yang et al. showed that hydroxyurea effectively treated atherosclerosis, reduced serum cholesterol levels, modified the gut microbiota at various levels, and affected cholesterol absorption by decreasing Niemann-Pick C1-like 1 in the epithelial cells of small intestines of apolipoprotein E knockout ApoE(-/-) mice fed on a high-fat diet (Yang et al., 2022b). In addition, the severity of myocardial infarction in rats is associated with intestinal microbial metabolites (Lam et al., 2016). The gut microbiome can also affect lipid metabolism. Certain bacteria in the gut microbiome can metabolize BAs, thereby affecting lipid metabolism. On the other hand, dysbiosis can alter the production of BAs, causing changes in lipid metabolism and increasing the risk of atherosclerosis (Jonsson and Bäckhed, 2017). The gut microbiome can affect the development and progression of atherosclerosis and CHD in various ways. Firstly, inflammatory responses can exacerbate plaque development or cause plaque rupture. Secondly, cholesterol and lipids metabolism by the gut microbiota can influence the development of atherosclerotic plaques. Thirdly, diet and gut microbial metabolites, including TMAO and SCFAs, can have various effects on atherosclerosis (Jonsson and Bäckhed, 2017; Liu et al., 2020a).
Hypertension is one of the most prevalent risk factors for CVDs across the globe. Furthermore, hypertension arises due to a complex interplay of genetic and environmental factors (Li et al., 2021a; Zhou et al., 2021). Globally, hypertension and pre-hypertension account for 8.5 million deaths annually due to stroke, ischemic heart disease, other vascular disorders, and kidney diseases. The prevalence of hypertension among individuals aged 30-79 years doubled from 648 million people to 1278 million people between 1990 and 2019 (Zhou et al., 2021).
Unlike healthy controls, patients with hypertension show microbial diversity and a shift in microbial composition. In addition, the number of species associated with hypertension shows a stronger correlation with disease severity. Li et al. found that pre-hypertensive and hypertensive patients had significantly reduced microbial richness and diversity. They also exhibited distinct metagenomic composition characterized by an overgrowth of disease-associated bacteria and a decrease in healthy-associated bacteria. Additionally, these patients exhibited a Prevotella-dominated gut enterotype and disease-linked microbial function, in contrast to the healthy control group (Li et al., 2017). Surprisingly, the microbiome characteristics of the pre-hypertensive group were similar to those observed in the hypertensive group. In addition, a previous study enrolling 60 patients with primary hypertension and 60 gender-, age-, and body-weight-matched healthy controls revealed that opportunistic pathogenic bacteria such as Klebsiella spp., Streptococcus spp., and Parabacteroides merdae were frequently found in the gut microbiome of hypertensive individuals. In contrast, beneficial bacteria producing SCFAs, such as Roseburia spp. and Faecalibacterium prausnitzii, were more abundant in the control group. Short-chain fatty acids-producing bacteria can modulate blood pressure by promoting vasodilation. In terms of microbial function, the gut microbiome of hypertensive individuals exhibited higher membrane transport, lipopolysaccharide (LPS) biosynthesis, and steroid degradation. However, the healthy controls showed higher metabolism of amino acids, cofactors, and vitamins (Yan et al., 2017). Furthermore, dysbiosis of the gut microbiome in rat models of hypertension can directly influence systolic blood pressure. Therefore, modulation of the gut microbiota can be exploited as a novel therapeutic approach for managing hypertension (Durgan et al., 2016; Adnan et al., 2017). Australian researchers recently published a review article, summarizing the latest research findings on the role of gut microbiota and their metabolic byproducts in host blood pressure regulation mechanisms. Gut microbiota imbalances can affect blood pressure regulation via host gene pathways, vascular function, and the autonomic nervous system. Enteric bacterial metabolites, including SCFAs and indole-3-lactic acid, are beneficial, whereas TMAO is harmful to blood pressure. Regulating gut microbiota through diet or fecal microbiota transplantation (FMT) may serve as a potential therapeutic strategy for blood pressure reduction. Moreover, the article discussed the prospects, challenges, and difficulties of using gut bacteria for blood pressure control in clinical applications (O’Donnell et al., 2023).
Heart failure is a complex clinical syndrome characterized by dyspnea and fatigue due to its inability to efficiently fill or eject blood from the heart. Heart failure can progress to pulmonary or splanchnic congestion and peripheral edema. It can be categorized into four types, including heart failure with preserved ejection fraction (HFpEF), heart failure with mildly reduced ejection fraction (HFmrEF), heart failure with reduced ejection fraction (HFrEF), and heart failure with improved ejection fraction (HFimpEF). HF is a leading cause of morbidity and mortality across the globe. The 2022 guideline offers patient-centric recommendations for healthcare professionals to prevent, diagnose, and manage patients with heart failure (Heidenreich et al., 2022).
Alteration of gut microbiota composition and microbial metabolite shifts, especially those derived from dietary nutrients increase the risk of HF. Tang et al. reported that impaired intestinal barrier function and bowel wall edema in HF patients might promote local and systemic inflammation, as well as bacterial translocation (Tang et al., 2019b). In addition, the inflammation and immune response linked to intestinal barrier impairment and bacterial translocation can exacerbate heart failure (Jia et al., 2019). Moreover, Zhang et al. showed that TMAO was involved in the pathological processes of HF and could act as a marker for identifying patients at risk of disease progression. In addition, they reviewed the gut–TMAO–HF axis as a new target for HF treatment (Zhang et al., 2021b). Romano et al. investigated the relationship between the gut microbiota-derived metabolite phenylacetylgutamine (PAGln) and HF. Clinical and mechanistic analyses reveal a dose-dependent association between circulating PAGln levels and HF presence and severity. PAGln directly promotes HF-relevant phenotypes, including decreased cardiomyocyte sarcomere contraction and elevated B-type natriuretic peptide gene expression in both cultured cardiomyoblasts and murine atrial tissue. The findings suggest that regulating the gut microbiome, particularly PAGln production, may represent a potential therapeutic target for modulating HF (Romano et al., 2023). This together suggests that innovative therapeutic approaches that target gut microbial metabolic pathways or metabolites and modify the gut microbiota composition could be effective in reducing CVDs susceptibility and preventing the progression of HF.
In recent years, numerous studies have investigated gut microbiota as a therapeutic target for CVDs prevention and management. For example, a previous study demonstrated that Bacteroides fragilis could prevent aging-related atrial fibrillation (AF) in rats through regulatory T cells (Tregs) mediated regulation of inflammation. Specifically, Bacteroides fragilis promotes the proliferation and function of Tregs as well as reduces inflammatory responses, thus decreasing the incidence of AF (Zhang et al., 2022). These findings provide novel insights for developing new drugs to prevent or treat AF. Furthermore, a clinical study showed that SCFAs could alleviate the development of AF through G protein-coupled receptor 43/NOD-like receptor family pyrin domain containing 3 (GPR43/NLRP3) signal pathways (Zuo et al., 2022). Moreover, the potential of probiotics, prebiotics, and FMT in modulating gut microbial composition and promoting cardiovascular health has been extensively studied (Oniszczuk et al., 2021). However, further studies are needed to reveal microbial mechanisms with diagnostic and therapeutic implications in CVDs (Walker et al., 2021). Furthermore, a previous study showed that hydroxyurea could prevent diabetic cardiomyopathy by inhibiting inflammation and cell apoptosis (Zhou and Lu, 2022). Inflammation is implicated in CVDs. In addition, dysbiosis has been linked to increased inflammation. A few gut microbiota, particularly Gram-negative bacteria, produce LPS, which activate the immune system and promote inflammatory responses (Yoo et al., 2020).
In summary, the gut-heart axis represents a complex network of interactions involving the gut microbiota, their metabolites, and the cardiovascular system. Myocarditis, an important cardiovascular disease, has gained significant attention in recent years. The emergence of the gut-heart axis concept might imply a potential connection between the gut microbiota and myocarditis. By delving into the role of the gut microbiota in myocarditis, we may discover new opportunities for prevention, diagnosis, and treatment, ultimately enhancing the prognosis and quality of life for those affected.
The immune system is divided into innate and acquired immunity. Innate immunity refers to the immune response present at birth that can produce an effective response to pathogens without previous antigen exposure (Janeway et al., 2001). Components of the innate immune system include: the skin and mucosal barriers, macrophages, natural killer (NK) cells, and the complement system. On the other hand, acquired/adaptive immunity is developed after previous exposure to pathogens or vaccinations. The components of the adaptive immune system include B lymphocytes and T lymphocytes (Janeway et al., 2001). B cells secrete antibodies that can specifically bind to and neutralize pathogens. Furthermore, T cells have different subtypes, including helper T cells and cytotoxic T cells. T cells recognize and attack the surface antigens of infected cells and coordinate the immune response (Parkin et al., 2001). The immune system can help the body to fight infections and can induce inflammation. Cytokines are a class of small proteins with broad biological activity that are synthesized and secreted by immune cells upon stimulation. They maintain the stability of the body’s immune system and regulate the occurrence of pathological processes. Cytokines are classified into pro-inflammatory and anti-inflammatory cytokines based on their effects on inflammation. Anti-inflammatory cytokines include Th2 type cytokines (IL-4, IL-5, IL-10). Pro-inflammatory cytokines include Th1 type cytokines (IFN-γ, IL-2, IL-12p70); IL-1β; IL-6, IL-8, TNF-α; and Th17 type (IL-17) (Fajgenbaum and June, 2020). Understanding the gut microflora, associations among the microbiome and inflammasomes, the immune system, the role of gut microbiota metabolites, and gut permeability may lead to the development of preventive strategies for CVDs (Noor et al., 2021).
Gut microbiota is involved in the regulation of host immunity. For example, Lactobacilli and Bifidobacteria improve the host immune function (Vlasova et al., 2016). Furthermore, the gut microbiota stimulate the training and development of the host immune system and the occurrence of cellular immunity, enabling the host’s innate immune system to distinguish between pathogenic and symbiotic bacteria (Wu and Wu, 2012; Ursell et al., 2014; Yoo et al., 2020). In addition, the gut microbiota colonizes, and proliferates in the intestinal mucosa, forming a layer that protects the host from invasion by foreign pathogens. In addition, gut microbiota can compete with harmful bacteria for nutrients, thereby inhibiting their growth and generating antibacterial substances that suppress the proliferation of pathogens (Belkaid et al., 2014).
Gut microbiota primarily affects the disease process through endogenous metabolites produced by gut microbiota and changes in the composition of gut microbiota. About 70~80% of the human immune cells are found in the gut, and dysbiosis of the gut microbiota is related to alterations in the immune system. Emerging evidence has focused on the role of the gut microbiota in regulating the immune response to viral infections and has shown that the gut microbiota can influence the activity of immune cells, including T cells and dendritic cells (Mizutani et al., 2022). Specifically, some gut microbiota produces metabolites that modulate the activity of immune cells and the production of pro-inflammatory cytokines, thus promoting the development of myocardial inflammation. Figure 3 shows the possible relationship between gut microbiota and their metabolites, immune system, and myocarditis.
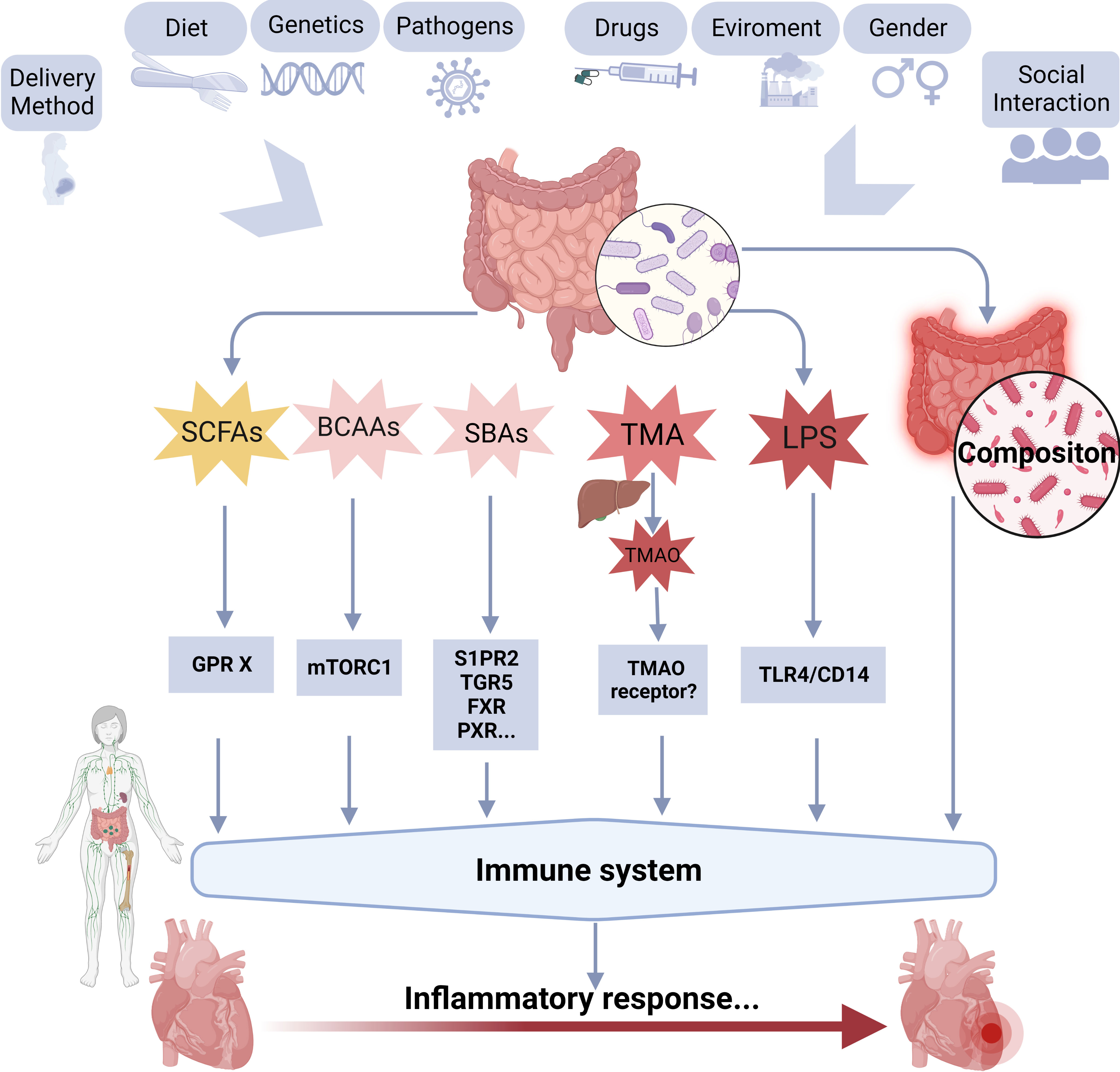
Figure 3 A schematic overview of the possible relationships between gut microbiota, and its metabolites, immune system, and myocarditis. BCAAs, branched-chain amino acids; GPR, G protein-coupled receptor; LPS, lipopolysaccharide; SBAs, secondary bile acids; SCFAs, short-chain fatty acids; TMAO, trimethylamine-N-oxide; TLR4, toll-like receptor 4; FXR, farnesoid X receptor, GPBAR1/TGR5, G protein-coupled bile acid receptor 1; PXR, pregnane X receptor; S1PR2, sphingosine 1-phosphate receptor 2.
Gut bacteria can be classified into six primary phyla based on their genetic and physiological characteristics, including Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, Fusobacteria, Verrucomicrobia, and some other phyla. Firmicutes, Bacteroides, Actinobacteria, and Proteobacteria, account for over 90% of the gut microbiota (Yan et al., 2022). (1) The phylum Firmicutes includes several common gut bacteria, such as Lactobacillus, Streptococcus, and Clostridium. Some Firmicutes species improve gut barrier function and enhance immunity (Rinninella et al., 2019). (2) The phylum Bacteroidetes include several bacteria that produce SCFAs, such as Bacteroides fragilis. Short-chain fatty acids have anti-inflammatory effects on the gut and promote immune homeostasis (Fabersani et al., 2021). (3) The phylum Proteobacteria includes several pathogenic bacteria, including Escherichia coli and Salmonella, which can cause gastrointestinal infections. However, some Proteobacteria, such as Akkermansia muciniphila, have beneficial effects on gut health, including promoting the growth of beneficial bacteria and reducing inflammation (Larsen et al., 2015). (4) The phylum Actinobacteria includes numerous beneficial gut bacteria, such as Bifidobacterium and Collinsella, which modulate the immune system, improve gut barrier function, and reduce inflammation (Barka et al., 2016).
Gut microbiota-dependent metabolites act as a bridge that connects the dynamic equilibrium between the host and the gut microbiota (Schoeler et al., 2019). The gut microbiota generates numerous small-molecule metabolites during microbial food digestion, playing a vital role in communication between host cells and gut bacteria (Ursell et al., 2014). The metabolites could have beneficial or harmful effects. Over the past decade, more than 300 endogenous metabolites of gut bacteria, including SCFAs, BAs, monoamines, biogenic amines, indole derivatives, phenols, vitamins, branched-chain amino acids (BCAAs), and lipids, have been discovered through non-targeted and targeted metabolomic analyses. Among them, extensive studies have been conducted on the primary endogenous metabolites, including, SCFAs, BAs, BCAAs, and TMAO (monoamines). These metabolites play significant roles in various physiopathologic processes. Furthermore, lipopolysaccharides, produced by Gram-negative bacteria, have also attracted attention for their effect on the host through metabolism-independent signaling pathways(Yang et al., 2021). This highlights the importance of understanding the complex interactions between these metabolites and their potential roles in human health and disease (Li et al., 2018b; Wang et al., 2018).Therefore, this section presents the effect of gut microbial metabolites on the host from the perspective of the immune system.
Short-chain fatty acids (SCFAs), or volatile fatty acids, are a class of low-molecular organic fatty acids with approximately two to six carbon atoms. Short-chain fatty acids arise from the fermentation of dietary fiber by microorganisms in the colon (Chen et al., 2020). Short-chain fatty acids mainly include acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, caproic acid, and isocaproic acid. The major metabolic products include SCFAs acetate (C(2)), propionate (C(3)) and butyrate (C(4)) (Schwiertz et al., 2010; Vinolo et al., 2011). The type and amount of SCFAs depend on the composition and fermentative ability of the gut microbiota, digestion time, host-microbe metabolic flux, and the fiber content of the host food (Parada Venegas et al., 2019; Deleu et al., 2021). Short-chain fatty acids are absorbed by the intestine primarily through monocarboxylate transporters 1 (MCT1) and 4 (MCT4) (Kirat et al., 2006; Sivaprakasam et al., 2017). The SCFAs promote cell growth, improve intestinal function, and influence cardiovascular metabolism. In addition, SCFAs act as mediators of the immune response and promote the production of anti-inflammatory cytokines by peripheral blood monocytes (Asarat et al., 2016). Depletion of gut microbiota by antibiotics decreased immune cell composition and impaired repair after myocardial infarction, while supplementation with SCFAs or Lactobacillus probiotics restored these effects. This highlights the importance of gut microbiota-derived SCFAs in modulating pathological outcomes after myocardial infarction and potentially impacting human health and disease as a whole (Tang et al., 2019a).
Short-chain fatty acids regulate the effector functions of CD8+ T cells by activating the G protein-coupled receptor 41(GPR41)(Vinolo et al., 2011). In addition, SCFAs can activate GPR43 or the free fatty acid receptor (FFAR 2) in peripheral adipose tissue, thereby regulating insulin sensitivity, promoting glucagon-like peptide 1 (GLP-1) release from stimulated L cells and regulating inflammation (Priyadarshini et al., 2016). One study proposed that SCFAs exert their effects on leukocytes and endothelial cells via two known mechanisms, i.e., activating GPR41 and GPR43 and inhibiting the histone deacetylase (HDAC) activity (Vinolo et al., 2011). Butyric acid and propionic acid inhibit HDAC activity, LPS-induced secretion of inflammatory mediators by macrophages, and macrophage reactivity, as well as exert anti-inflammation effects in mice (Li et al., 2018a; Yao et al., 2022). In addition, acetic acid induces immunoglobulin A (IgA) production in the intestines of mice and maintains a relatively stable immune system (Mei et al., 2022). However, excessive production of acetic acid can induce colitis (Sharon and Stenson, 1985; Uraz et al., 2013). Furthermore, butyric acid can attenuate inflammation by reducing macrophage adhesion and migration (Smith et al., 1998), hence reducing the production of IL-6 and IL-12, and increasing IL-10 (Park et al., 2022). Moreover, butyric acid and propionic acid produced by the gut microbiota can promote the differentiation of peripheral Tregs and maintain immune homeostasis (Kim et al., 2014; Asarat et al., 2016).
Taken together, SCFAs modulate the immune system and alleviate inflammation.
Bile acids (BAs) promote the emulsification of fats, thus increasing the surface area for pancreatic lipase and improving the solubility of lipids by forming mixed micelles (Amara et al., 2019). Bile acids facilitate the absorption of lipids in the small intestine. Clinical studies and animal experiments have shown that BAs, particularly secondary BAs (SBAs) generated during bacterial metabolism of BAs, can influence intestinal inflammation (Schirmer et al., 2019; Cai et al., 2022). The related gut microbiota mainly comprises Lactic acid bacteria, Enterobacteriaceae, and Enterococci bacteria, which can deconjugate BAs from taurine or glycine to produce deoxycholic acid (DCA) and lithocholic acid (LCA).
Bile acids are important signaling molecules, which regulate host metabolism and energy homeostasis, and affect innate immunity (Hang et al., 2019; Han et al., 2022). One recent study revealed that gut microbiota and LCA could regulate the host immune response by directly altering the balance between Th17 and Tregs (Hang et al., 2019). Previous studies have shown that SBAs could exert pro-inflammatory effects at high concentrations (Hang et al., 2019; Han et al., 2022). Digoxin was identified as the first Th17 cytostatic agent that binds to the retinoic acid-related orphan receptor-gamma-t (RORγT) (Karaś et al., 2018). After digoxin identification, other structurally related cholesterol derivatives have been identified as modulators of RORγT. Bile acids are cholesterol metabolites present in the intestine. Bile acids control Th17 by targeting RORγT activity (Song et al., 2020). BAs can inhibit macrophage function by activating BA receptors and promoting the differentiation of Tregs. The BAs receptors include nuclear receptors such as the farnesoid X receptor (FXR), the G protein-coupled bile acid receptor 1 (GPBAR1/TGR5), pregnane X receptor (PXR), sphingosine 1-phosphate receptor 2 (S1PR2), and other membrane receptors (Wang et al., 2011; Hu et al., 2022; Yao et al., 2022). By activating these receptors, BAs inhibit the overgrowth of intestinal bacteria, thus protecting against visceral infection.
Taken together, BAs may have pro-inflammatory or anti-inflammatory effects, depending on the type and concentration of bile acids.
Branched-chain amino acids (BCAAs) have an aliphatic side chain and a branch (a central carbon atom bound to three or more additional carbon atoms). Leucine, isoleucine, and valine are three naturally occurring proteinogenic BCAAs (Lynch et al., 2014). These amino acids cannot be synthesized in animals. However, they are synthesized in bacteria, plants, and fungi (Neinast et al., 2019). The metabolism of BCAAs involves several complex enzymatic reactions. Furthermore, branched-chain amino acids affect cellular metabolism.
A previous study revealed that dietary supplementation of BCAAs in middle-aged mice is associated with increased mitochondrial formation and bioenergetics as well as reduced ROS production, which could prevent aging and promote survival (D’Antona et al., 2010). Furthermore, BCAAs may inhibit fibrosis by decreasing apoptosis, caspase-3 activity, and oxidative stress in mice (Takegoshi et al., 2017). In addition, BCAAs enhance the immune response of NK cells (Matsumoto et al., 2009) and liver-associated lymphocytes (Kajiwara et al., 1998; Takegoshi et al., 2017). A previous study investigated the potential role of BCAAs in reducing inflammation and improving immune function in athletes and individuals undergoing physical stress. BCAAs serve as signaling molecules. For example, BCAAs can activate the mammalian/mechanistic target of rapamycin complex 1 (mTORC1) (Mann et al., 2021). Previous studies revealed that supplementation of BCAAs reduced inflammation and oxidative stress in athletes, thereby improving their immune function and reducing infection risk (Matsumoto et al., 2009; Kim et al., 2012). However, high plasma levels of BCAAs are associated with inflammation, insulin resistance, and metabolic syndrome (Yoon and Yoon, 2016).
Taken together, the role of BCAAs on inflammation depends on the BCAAs concentration and the individual’s health status.
Trimethylamine-N-Oxide (TMAO) is an intestinal-derived flora-related metabolite synthesized in the liver. It is derived from trimethylamine (TMA), metabolized by the gut microbiota. Some gut microbiota produces trimethylamine lyase, an enzyme that converts dietary choline, betaine, carnitine, and TMA- structured food into TMA (Cho and Caudill, 2017). For example, some bacteria belonging to the phylum Firmicutes, including certain species within the Clostridia class and the Enterococcus genus, as well as the Desulfovibrio genus from the phylum Proteobacteria, can produce enzymes like choline TMA-lyase, which is involved in the generation of trimethylamine. Trimethylamine is transported to the liver via portal circulation, where it is oxidized by flavin monooxygenases 3 (FMO3) to produce TMAO (Liu et al., 2020b).
Trimethylamine-N-Oxide promotes monocyte adhesion, increases macrophage infiltration, and promotes foam cell production. It has been shown that TMAO can inhibit the cellular activity of antioxidant enzymes, including superoxide dismutase (SOD) and catalase (CAT), causing a reduction in the antioxidant activity of cells (He et al., 2021). Similarly, TMAO promotes the production of reactive oxygen species (ROS), thus exacerbating oxidative stress (Querio et al., 2019). Animal studies have shown that TMAO induces vascular inflammation by activating the sirtuin 3 -superoxide dismutase 2- mitochondrial ROS (SIRT3‐SOD2‐mtROS) and stimulating in vitro and in vivo formation of the NLRP3 inflammasome (Chen et al., 2017). In addition, TMAO can induce vascular inflammation by activating the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB) signaling pathways (Seldin et al., 2016). TMAO also activates the inflammatory response by inducing the expression of IL-6, cyclooxygenase-2 (COX-2), endothelial selectin, and intercellular cell adhesion molecule-1 (ICAM-1), which enhances macrophage adhesion through protein kinase C (PKC) and NF-κB signaling pathway. Furthermore, high serum levels of TMAO can increase the production of tumor necrosis factor-α (TNF⁃α) through the NF⁃κB signaling pathway. A recent study demonstrated that silencing of FMO3 was associated with decreased production of TMAO(Schiattarella et al., 2017). TMAO has also been shown to trigger adipose tissue inflammation (Yang et al., 2021).
Taken together, TMAO has pro-inflammatory effects.
Endotoxins/LPS are a complex of lipids and polysaccharides. They are structural components of the outer membrane of Gram-negative bacteria such as Pectinatus. In addition, LPS determines the diversity of bacterial antigens. During bacterial pathogenesis, lipopolysaccharides trigger inflammation by activating the Toll-like receptor 4 (TLR4) in immune cells and other cell types, including adipocytes and hepatocytes (Neves et al., 2013; Schoeler et al., 2019). As activators of innate immune responses, LPS have a non-negligible role in human immune responses (Neves et al., 2013).
Interactions between LPS and Toll-like receptor 4 (TLR4) on surfaces of immune cells such as macrophages and dendritic cells induces a cascade of signaling events that produce of pro-inflammatory cytokines, such as TNF-α, IL-1β, NF-κB, and IL-6. These cytokines play a crucial role in the recruitment and activation of other immune cells, including neutrophils and NK cells, to sites of infection (Medzhitov and Medzhitov, 2007). Besides activating the innate immune system, LPS influences adaptive immune responses. They improve the antigen-presenting abilities of dendritic cells, which are crucial for initiating and shaping adaptive immune responses. The LPS can also promote T cells activation (CD4+ helper and CD8+ cytotoxic T cells) against the pathogen (Hoebe et al., 2004). In addition, LPS is useful in modeling inflammation-related diseases, including sepsis and myocarditis by activating the NF-кB signaling pathway (Wang et al., 2019b).
Gut microbiota-produced metabolites, including SCFAs, BAs, BCAAs, TMAO, and LPS can affect the immune system and contribute to development of inflammatory diseases. Additional studies should focus on elucidating the mechanisms underlying these associations and exploring the potential therapeutic interventions targeting these metabolites.
The role of inflammation in the progression of CVDs has attracted considerable attention. In pathogenesis, IL-1β, IL-6, TNF-α, and interferon-gamma (IFN-γ) are associated with heart inflammation while IL-10, TGFβ, and others are linked to the resolution of inflammation and heart tissue repair. IL-10 mitigates inflammation in the cardiovascular system and exerts protective effects by interacting with SMAD2, p53, HuR, miR-375, and miR-21 pathways (Goswami et al., 2021). Myocarditis is an inflammatory disease that affects myocardium health, and the extent of damage depends on the nature of the pathogen and associated inflammatory responses. Myocarditis is characterized by immune responses specific to the heart and is categorized based on the clinical and histopathological features (Figure 1) (Heymans et al., 2016). Experimental mice models have shown the significance of immune cells in myocarditis development (Swirski et al., 2018). The proinflammatory cytokine, IL-1, is crucial in the development of myocardial inflammation (Cavalli et al., 2016). IL-α activates the ‘inflammasome,’ leading to the infiltration of inflammatory cells, processing and release of active IL-1β (Toldo et al., 2014). IL-1β, a highly studied member of the IL-1 cytokine family, is primarily influenced by the functioning of the NLRP3 inflammasome in inflammation (Abbate et al., 2020). This section focuses on two types of myocarditis: Viral myocarditis and autoimmune myocarditis.
Viral myocarditis is a significant cause of heart failure and dilated cardiomyopathy. Viral infection of the myocardium can lead to myocardial cell necrosis. The pathological and physiological mechanisms of viral myocarditis have been investigated using murine models of enterovirus infection, especially coxsackievirus B3 (CVB3) (Lin et al., 2021). The CVB3 cardiomyophilic strain virus (CVB3m) and the CVB3 non-cardiomyophilic strain virus (CVB3o) are variants of the CVB3 standard strain, which can be adapted for different research objectives to achieve the required pathology of myocarditis in specific tissue types (Błyszczuk, 2019).
Viral entry into the myocardium results in three kinds of responses. The acute phase is characterized by viral entry and replication, the subacute phase is characterized by inflammatory cell infiltration, and the chronic phase is characterized by cardiac remodeling. Myocardial injury includes direct injury mediated by viral infections and indirect injury due to secondary immune responses (Henke et al., 1995). The molecular mechanisms underlying injury were described in detail in a review published in 2016. Targeting these virus-encoded proteases may inhibit viral replication and viral direct damage to the myocardium (Fung et al., 2016). The adaptive immune responses begin after the acute and subacute phases of myocarditis (Lin et al., 2021). Opavsky et al. established gene knockout mice CD4(-/-), CD8(-/-), both co-receptors (CD4(-/-) CD8(-/-)), or T cells receptor beta chain (TCR beta (-/-)) to investigate the impact of T cell subsets on host susceptibility to CVB3 myocarditis. They found that myocarditis severity in the CD4 knockout group, CD4 and CD8 knockout group, and TCR beta knockout group was lighter than that in the CD8 knockout group. Moreover, IFN-γ levels were elevated while TNF-αlevels were suppressed in CD4 and CD8 knockout mice models (Opavsky et al., 1999). Chemokines are a class of small cytokines or signaling proteins secreted by cells. They can induce nearby responsive cells to directionally migrate towards the source of chemokines. A transgenic study involving mice models revealed that CXC chemokine ligand 10 (CXCL10) was upregulated in the early stages of myocardium infection and inhibited viral replication in the early CVB3 infection stages by recruiting NK cells and promoting IFN-γ expressions. However, in the late infection stages, it did not stimulate the antiviral effects to improve the survival rates of mice (Yuan et al., 2009). Male mice with CVB3-induced myocarditis had myocardial infiltrating macrophages expressing increased markers, including inducible nitric oxide synthase, IL-12, TNF-α, and CD16/32, which are associated with classically activated macrophages (M1) (Li et al., 2009).
Mice models are important in studies on autoimmune diseases (Lincez et al., 2011). Experimental autoimmune myocarditis mice models distinguish between autoimmune phases of viral myocarditis from the acute infection phase of CVB in genetically modified mice (Blyszczuk et al., 2008). Disease severity is classified based on the infiltration extent of inflammatory cells during the peak of inflammation. This model can also be used for other types of myocarditis (Błyszczuk, 2019).
Neu et al. reported that autoimmune myocarditis is often indirectly associated with a viral infection. One possible contributing factor is the release or exposure of cardiac myosin after viral-mediated myocyte damage, which induces autoimmune responses and myocardial inflammation (Neu et al., 1987). Pdcd1-/-Ctla4+/- mice spontaneously develop fulminant myocarditis. Therefore, Axelrod et al. performed single-cell sequencing and single-cell TCR sequencing of immune cells infiltrating these myocarditis tissues and found that CD8+ T cells were significantly increased in number and showed clonal expansions. Myocarditis did not develop when CD8+ T cells were removed from these mice. When CD4+ T cells were the only ones to be removed, myocarditis incidences did not change. When CD8+ T cells from Pdcd1-/-Ctla4+/- mice were adoptively transferred to Rag1-/- mice, the recipient mice developed myocarditis after two months. Therefore, CD8+ T cells are highly involved in fulminant myocarditis development (Axelrod et al., 2022). A recent study also found that macrophage migration is a significant histopathological feature of myocarditis, indicating that macrophages are potential therapeutic targets for this disease (Toita et al., 2021).
In previous discussions, we highlighted the strong associations between gut microbiota and the immune system. Furthermore, we elucidated the role of the immune system in myocarditis development. In this section, we assess the relationship between gut microbiota and myocarditis.
It has been demonstrated that FMT, a method used to restore gut microbial homeostasis, can improve myocardial damage in myocarditis mice (Hu et al., 2019). Zhang et al. investigated the significance of GLP-1 receptor agonists in alleviating autoimmune myocarditis by modulating gut microbiota (Zhang Wenyong, 2019). In spontaneous autoimmune myocarditis mice, gut microbiota promotes disease and interacts with MYH6-specific CD4+ T cells. Cruz et al. found that commensal Bacteroides produce an MYH6 mimic, β-galactosidase, which can lead to the activation of cross-reactive Th17 cells, ultimately causing inflammatory cardiomyopathy in individuals with a genetic susceptibility to this condition. Antibiotics reduce inflammation and prevent death in these mice. Acute myocarditis patients have increased anti-Bacteroides IgG and cross-reactive T cell activation (Gil-Cruz et al., 2019). These findings provide a better understanding of the mechanisms underlying the interaction between the microbiome and the immune system in autoimmune diseases. In the meanwhile, these findings also offer fresh insights into potential strategies for preventing and treating inflammatory cardiomyopathy. Additionally, the European Heart Journal reported Cruz et al’s study. The study focused on the largely unknown processes that cause myocarditis, revealing that gut bacteria composition promotes the development of myocarditis and inflammatory cardiomyopathy. These findings provide valuable insights into the role of gut microbiota in myocarditis, paving the way for future research and potential treatments targeting the gut-heart axis (Ozkan, 2022). Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment but can also initiate autoimmune diseases, including cardiomyopathy. Although antibiotics may counteract cardiomyopathy by eliminating cross-reacting bacteria, they could also impede ICI efficacy, as gut bacteria play a crucial role in ICI efficacy. A more targeted approach, including phage therapy, could be considered to specifically eradicate immune-mimicking gut commensals without compromising immunotherapy outcomes (Mandelbaum et al., 2020). Han et al. performed HeLa cellular experiments and revealed that gut microbiota metabolites-BAs can inhibit viral replication and attenuate endoplasmic reticulum stress-induced cell death (Han et al., 2018). Barin et al. revealed that enteric microorganisms play a role in determining the susceptibility of mice to the model of experimental autoimmune myocarditis (EAM) and its sequela, inflammatory dilated cardiomyopathy (Barin et al., 2017). Myopericarditis is an inflammatory heart condition involving the pericardium and myocardium, which has been linked to gut microbiota and its metabolites. Piccioni et al. explored the role of gut microbiota in myopericarditis, particularly in relation to the cardiovascular implications of COVID-19, suggesting that microbiota modulation may be a novel approach for preventing or treating inflammatory cardiomyopathies (Piccioni et al., 2021). One study investigated the causal relationship between gut microbiota, their metabolites, and heart failure and its risk factors using Mendelian randomization analysis. Genetic predictions revealed that with every 1-unit increase in Shigella concentration, the relative risk for myocarditis increases by 38.1%. These findings may guide future microbiome-based interventions in clinical trials (Luo et al., 2022).
In summary, gut microbiota dysbiosis may be implicated in myocarditis pathogenesis. Targeting gut microbiota provides novel clinically relevant strategies for myocarditis treatment. The mechanisms by which gut microbiota promote myocarditis are complex and involve dysregulation of the immune system, inflammation, endothelial dysfunction, and metabolism. By revealing the role of gut microbiota in the development and progression of myocarditis, we can gain insight into the disease’s pathophysiology and devise new therapeutic strategies.
The current strategies for modulating gut microbiota include fecal microbiota transplantation (FMT), live biotherapeutic productions (LBPs), probiotics, prebiotics, symbiotics, dietary interventions, gut microbiota enzyme inhibition, and microbial-drug interactions (Figure 4) (Schneiderhan et al., 2016; Adak and Khan, 2019; Wargo, 2020).
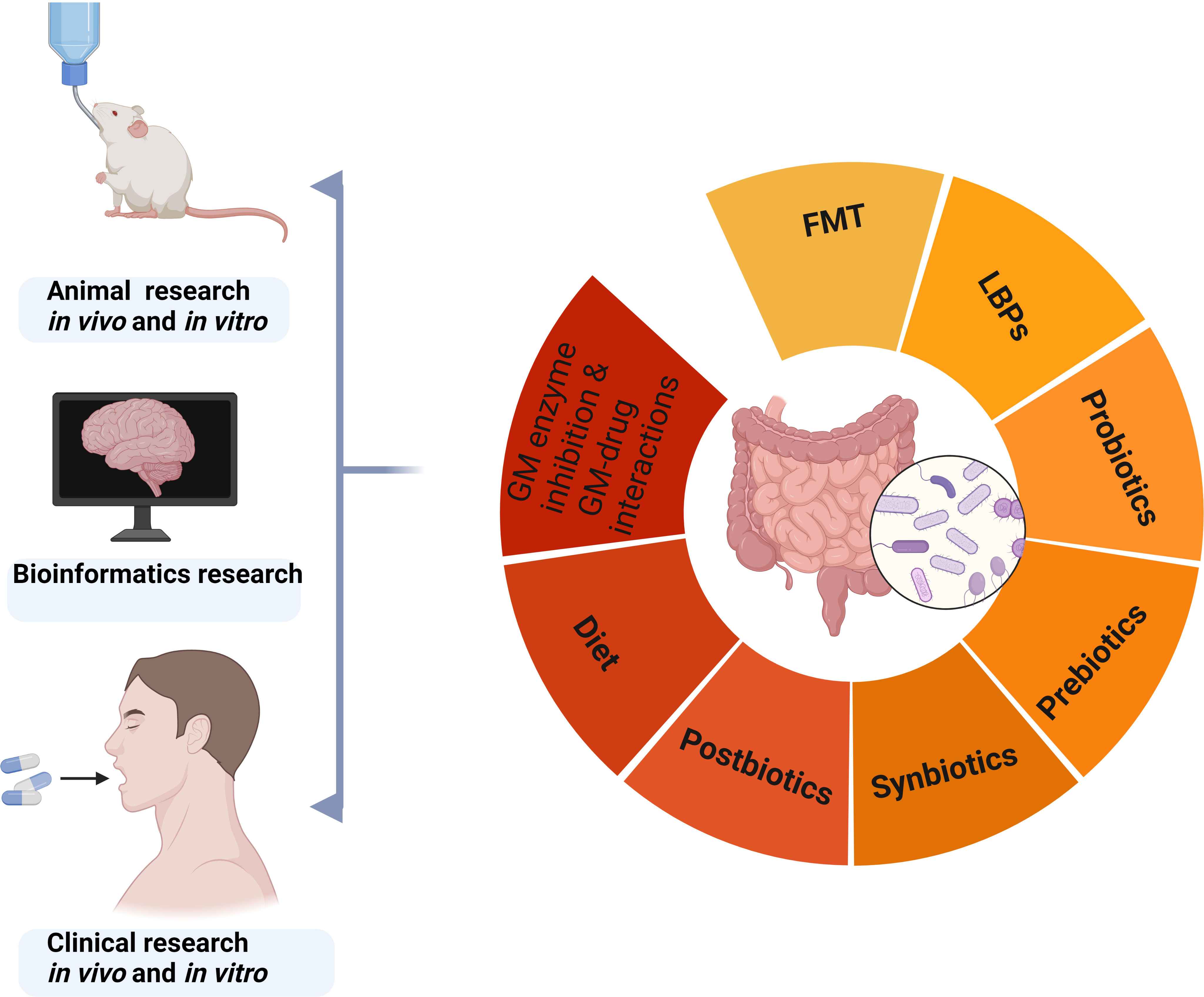
Figure 4 Strategies for regulating gut microbiota based on animal, bioinformatics, and clinical research. GM, gut microbiota; FMT, fecal microbiota transplantation; LBPs, live biotherapeutic productions.
FMT, a natural microbial ecosystem in feces, involves the transfer of fecal material from a healthy donor to a recipient to restore a healthy gut microbiota composition and functions (Wang et al., 2019a). Infant colonization by a specific microbial community, largely originating from the mother, is a natural process that rapidly occurs after birth and is influenced by the delivery mode (Fuentes and de Vos, 2016). As early as the 4th century BC, Chinese medical books recorded the use of fecal preparations to treat gastrointestinal diseases (Wargo, 2020). FMT can effectively treat recurrent Clostridioides difficile infections, and there is a growing interest in the use of FMT in other diseases, including inflammatory bowel disease and metabolic syndromes (Davidovics et al., 2019). The success rate of FMT relies on the composition of the recipient’s microbiome and the interplay between the microbiomes of the donor and recipient at both taxonomic and functional levels (Kazemian et al., 2020). FMT can provide a diverse range of microbial communities which play an important role in restoring functional redundancy of gut microbiota. However, there exist limitations in terms of reproducibility, whereas factors including safety, donor/recipient considerations, dosing, and administration route should be taken into account when considering FMT. Studies on the use of FMT, specifically in the context of myocarditis, are limited (Hu et al., 2019). Further, the safety and efficacy of FMT in myocarditis treatment should be investigated.
1) The LBPs are a type of therapeutic agent comprising many live microorganisms, typically bacteria, ingested or applied to the body for disease prevention and treatment (Charbonneau et al., 2020). They are also referred to as “live biotherapeutics” or “live biotherapeutic agents” (Dhansekaran and Sankaranarayanan, 2021). They differ from probiotics or prebiotics in that they are specifically designed to deliver a therapeutic effect (Dreher-Lesnick et al., 2017; Charbonneau et al., 2020). They are produced under strict manufacturing processes to ensure the viability and efficacy of bacteria when administered to the patient. Some clinical trials are assessing the efficacy of this strategy of regulating gut microbiota in cancer immunotherapy (Wargo, 2020). The potential of LBPs to treat various conditions, including gastrointestinal, metabolic, and immune system disorders is being investigated. A previous review outlined the factors to be considered during the design and development of genetically engineered LBPs to ensure compliance with regulatory standards and gain acceptance from patients (Charbonneau et al., 2020).
2) Antibiotics are drugs that can kill or inhibit the growth of bacteria. Excess or improper use of antibiotics can lead to gut microbiota dysbiosis (Gough, 2022). Probiotic supplementation is a treatment approach that can promote gut microbiota recovery. Probiotics are live microorganisms such as Bifidobacterium, Saccharomyces, Lactic acid bacteria, Lactobacillus acidophilus, Actinomycetes, and Lactobacillus rhamnosus with health benefits upon consumption (Hill et al., 2014). Probiotic supplementation reduces myocardial hypertrophy and heart failure following myocardial infarction in rat models (Gan et al., 2014). Intestinal stem cell regeneration was accelerated by Lactobacillus rhamnosus GG, which promoted colonic barrier recovery in septic mice (Chen et al., 2023). In vitro, anti-inflammatory activities of probiotic supernatants are unique, since they can modulate interleukin 1β (IL-1β), IL-6, TNF-α, and IL-10 production in human macrophages in distinct approaches (De Marco et al., 2018). Probiotic supplementation is considered safe for individuals with functional immune systems (Vallianou et al., 2020). Still, they may induce immune responses, require cold storage, and cannot be used with antibiotics (Suez et al., 2019).
3) Symbiotics are combinations of probiotics and prebiotics with synergistic effects on gut microbiota composition and function. They are developed to address the potential challenges associated with probiotics survival in the gastrointestinal tract (Rioux et al., 2005). Consumption of yogurt and fruits may have combined health benefits due to their potential prebiotic and probiotic effects (Fernandez and Marette, 2017).
1) Prebiotics are nondigestible food ingredients that selectively stimulate the growth and activities of beneficial gut microbiota. They primarily consist of bifidogenic, non-digestible oligosaccharides, such as inulin, its hydrolysis product oligofructose, and (trans) galactooligosaccharides (de Vrese and Schrezenmeir, 2008). Prebiotics include nutritional supplements that promote gut microbiota proliferation. The natural sources of prebiotics include beans, cereals, and soybean among others (Kerry et al., 2018). They may reduce inflammatory responses and improve cardiac functions by regulating gut microbiota. Similar to findings from studies involving adult studies, prebiotic effects in infant nutrition results in significant alterations in gut microbiota composition, with a notable increase in Bifidobacteria levels in fecal matter (Roberfroid et al., 2010).
2) Postbiotics, gaining attention as health-promoting agents, are beneficial compounds produced through the metabolic activities of microorganisms, particularly probiotics. These diverse substances, including cell wall components, SCFAs, and enzymes, have various effects on the host, such as modulating the immune system, improving gut barrier function, and inhibiting pathogenic bacteria growth (Żółkiewicz et al., 2020); Tsilingiri and Rescigno, 2013). As a stable and safe alternative or complement to traditional probiotics, postbiotics show promise in maintaining and improving human health, necessitating additional research to optimize their production and develop effective therapies.
3) Food plays a vital role in shaping gut microbiota composition and diversity. Dietary interventions, including the Mediterranean diet and Dietary Approaches to Stop Hypertension diet, have beneficial effects on gut microbiota composition and cardiovascular health (Merra et al., 2020; Drapkina et al., 2022). The Mediterranean diet, rich in fruits, vegetables, whole grains, legumes, fish, and olive oil, improves gut microbiota composition, reduces frailty and improves health status (Ghosh et al., 2020). The Dietary Approaches to Stop Hypertension (DASH) diet, rich in fruits, vegetables, whole grains, and low-fat dairy products, reduces blood pressure and improves cardiovascular health (Maifeld et al., 2021). A Western-style diet, rich in saturated fats, salt, and sugar, reduces gut microbiota diversity which increases the prevalence of inflammatory disease (Statovci et al., 2017). Plant-based diets, rich in fibers, are associated with increased gut microbiota diversity and reduced cardiovascular disease risks (Satija and Hu, 2018; Losno et al., 2021).
1) The gut microbiome plays a crucial role in mediating host-environment interactions and exhibits a complex bidirectional relationship with non-antibiotic drugs. This intricate interplay involves the microbiome influencing drug efficacy and toxicity, while simultaneously being affected by the drugs themselves, a phenomenon known as pharmacomicrobiomics (Weersma et al., 2020). We review the relevant research on the interactions between gut microbiota and cardiovascular medications. Silva et al. discovered that statin therapy is a key covariate affecting gut microbiome diversity by analyzing the data from 888 volunteers in the Body Mass Index Spectrum cohort from the MetaCardis project. In patients with ACS under statin medications, the potentially pathogenic bacteria in the gut are reduced, with a better prognosis. Analysis of fecal samples suggests that the gut microbial communities of obese individuals taking cholesterol-lowering statin medications are “healthier” than expected, suggesting that the potential beneficial effects of statins on gut microbiota open up new prospects for disease treatment (Vieira-Silva et al., 2020). Yang et al. discovered a previously unrecognized mechanism in which the human commensal bacteria, Coprococcus comes, catabolizes ester ACE inhibitors in the gut, reducing their antihypertensive effects. The findings revealed that gut microbiota may play a role in the efficacy of antihypertensive medications, which could help explain why some individuals remain resistant to treatment (Yang et al., 2022a). Another study found that liraglutide could treat patients with type 2 diabetes mellitus by targeting the gut microbiota (Shang et al., 2021)
In clinical practice, traditional Chinese medicine (TCM) is orally administered and bidirectionally interacts with the gut microbiota. These interactions have two primary effects: (1) Enhancing effects: TCM modulates gut microbiota composition and metabolism, improving host health while the microbiota enhances TCM bioavailability. (2) Inhibitory effects: Some TCM constituents can weaken gut microbiota metabolic functions, and certain microbes may inhibit TCM absorption and metabolism (Zhu et al., 2023). A recent review revealed that numerous natural molecules (e.g., apigenin, berberine, and quercetin) and plant extracts can effectively alleviate experimental autoimmune myocarditis. Key anti-myocarditis mechanisms include the upregulation of Th1-type cytokines, the elevation of Th2-type cytokines (IL-4 and IL-10), mitigation of oxidative stress, modulation of mitogen-activated protein kinase signaling pathways, and increased sarco-endoplasmic reticulum Ca2+-ATPase levels (Javadi and Sahebkar, 2017). These molecules and extracts can alter the composition and abundance of gut microbiota, suggesting that they hold great potential as treatments that target gut microbiota.
The use of antibiotics can not only fight against pathogenic bacteria, but also affect the intestinal symbiotic flora. Compared to other antibiotics, the gut symbiotic bacteria are more sensitive to macrolides and tetracyclines. Some detoxifying agents protect the gut symbiotic bacteria from antibiotic damage (Maier et al., 2021). Haak et al. found that a one-week course of combined broad-spectrum antibiotics (ciprofloxacin, vancomycin, and metronidazole) has a profound and long-lasting impact on the gut microbiota of healthy humans, causing loss of diversity and shifts in community composition. Although the microbiota showed a remarkable return towards baseline after 8-31 months, the community composition often remained altered, with the long-term consequences remaining largely unknown (Haak et al., 2019).
2) Moreover, Mamic et al. summed up the application of gut microbiome in heart failure and its comorbidities. They highlighted that targeting gut microbial enzymes, which are not found in the host, is a promising approach to overcoming these challenges. The study focuses on the TMAO meta-organismal pathway and suggests that 3,3-dimethy-1-lbutanol, a natural inhibitor of TMA lyases, may be a non-lethal and elegant strategy to target this pathway. In mice fed a high choline diet, administration of 3,3-dimethy-1-lbutanol resulted in decreased circulating TMAO levels, decreased foam cell formation, and fewer atherosclerotic plaques. Targeted inhibition of microbial choline-TMAO conversion was also evaluated in a pressure overload mice model of heart failure, where it improved cardiac remodeling and cardiac function. The study proposes that both pharmacologic modification of the TMAO biosynthetic pathway and targeted dietary interventions may be viable strategies for modulating the pathogenesis and progression of heart failure. However, further human studies are necessary to evaluate the feasibility and efficacy of this approach (Mamic et al., 2021).
Gut microbiota dysbiosis is implicated in the pathogenesis of many diseases. The LBPs, FMT, pre/probiotics, postbiotics, synbiotics, and dietary interventions have the potential for disease prevention or treatment by modulating gut microbiota. The future of promoting overall health and treating diseases by regulating intestinal microorganisms is becoming clearer, and more strategies and methods to regulate gut microbiota are known (Wargo, 2020). The various approaches have shown promising results in animal studies, however, their effectiveness and safety for myocarditis treatment should be investigated further. Elucidating the mechanisms by which gut microbiota contribute to myocarditis pathogenesis may lead to the development of novel therapeutic approaches targeting the gut microbiota.
Myocarditis is a serious inflammatory disease of the heart muscles that can lead to heart failure and sudden cardiac death. The current treatment options for myocarditis are not fully effective, and there is a growing interest to understand the efficacy of targeted gut microbiome therapy (Hu et al., 2019). However, this approach has several challenges, including low efficacy of current treatment methods and the need for personalized treatment (Schneiderhan et al., 2016). Therefore, future research is needed to identify new therapeutic targets. Targeted gut microbiome therapy for myocarditis aims to restore the balance of bacteria in the gut and reducing the production of pro-inflammatory cytokines. However, the complex and diverse nature of the gut microbiome presents challenges in developing targeted therapies, therefore, studies should investigate its roles in disease development to identify effective treatment strategies.
One possible approach for targeted gut microbiome therapy is the use of gut microbiota-related strategies, including probiotics and FMT. Probiotics can reduce inflammation and improve immune functions, which may be beneficial in myocarditis treatment. FMT can help in restoring the balance of bacteria in the gut to reduce inflammation (Hu et al., 2019). Elucidating the mechanisms of enterobacteria in myocarditis will inform the development of enterobacteria-targeting drugs. However, a limited number of studies have investigated the relationship between enterobacteria and myocarditis. Herein, we summarize major studies with regard to myocarditis treatment, which may inspire further research design and direction (Table 1).
The current treatment options for myocarditis are limited, and there is a need for new approaches to improve outcomes. Targeted gut microbiome therapy is a promising approach; however, it is still in the early developmental stages, and more research is necessary to determine its efficacy and safety. Several challenges and limitations to the development of gut microbiota-targeted therapies are defined by alternatives, such as FMT, and LBPs, as well as by the lack of a clear mechanistic understanding of disease pathophysiology. Another challenge is the lack of knowledge about the roles of specific bacteria in myocarditis pathogenesis (Mandelbaum et al., 2020). Although studies have reported that certain bacteria may be involved in the development of this condition, it is still unclear which bacteria are most important and how they interact with the host immune system (Hu et al., 2019). This limits the capacity to develop targeted therapies that can effectively treat myocarditis.
One of the challenges of targeted gut microbiome therapy is the need for personalized treatment. The gut microbiome is highly individualized, and bacterial composition can vary widely from person to person, therefore, a one-size-fits-all approach to treatment is unlikely to be effective. Instead, personalized treatment plans that take into account the specific bacteria present in each patient’s gut microbiome are needed (Wargo, 2020). Precision medicine approaches, such as genomics, metabolomics, and various omics techniques can help in identifying specific bacterial strains that are associated with myocarditis and develop personalized treatment plans based on these findings (Caesar et al., 2021).
Despite the challenges of targeted gut microbiome therapy, it holds great promise in myocarditis treatment. One direction for future research is to identify new targets for therapy based on better understanding of interactions between the gut microbiome and the host immune system. Studies should aim at investigating the etiology, pathogenesis, and gender differences of myocarditis (Tschöpe et al., 2021).
Moreover, it’s possible to apply machine learning and artificial intelligence to analyze gut microbiome data, which can enhance our understanding of the connection between the gut microbiome and myocarditis. By adopting this innovative approach, we can identify previously unknown therapeutic targets and create more personalized treatment plans for individual patients (Loganathan and Priya Doss, 2022).
There is a need for personalized treatment plans based on specific bacteria present in each patient’s gut microbiome (Behrouzi et al., 2019). Targeted gut microbiome therapy has the potential to revolutionize myocarditis treatment and improve disease outcomes. There is a need for large-scale animal and clinical trials to evaluate the safety and efficacy of targeted gut microbiome therapy in myocarditis treatment. Moreover, studies should also explore the optimal timing and duration of treatment and assess the long-term effects of this therapy on patient outcomes.
Myocarditis and inflammatory cardiomyopathy present considerable threats to human life and well-being by causing inflammation and damage to the heart muscle, potentially resulting in severe complications including heart failure, arrhythmias, and even sudden cardiac death. Nonetheless, treatment options for myocarditis remain limited and research efforts face substantial challenges. The gut microbiota is a critical player in the regulation of immune responses and the maintenance of cardiovascular health. Gut microbiota dysbiosis is implicated in myocarditis development and progression, therefore, gut microbiota modulation may have potential therapeutic effects for this disease. Targeting the gut microbiota through interventions such as drugs, probiotics, prebiotics, symbiotics, antibiotics, FMT, and diet represent promising strategies for myocarditis treatment. Additional investigations are essential to understand the underlying mechanisms through which imbalances in gut microbiota promote myocarditis development. The findings will enable the identification of optimal strategies for targeting gut microbiota in the treatment and management of myocarditis.
JW and XZ wrote the first draft of the manuscript. XY, HY, and MB revised the manuscript. ZWZ, ZZ, JF, JH, JL, HZ, ZZ, WY, and XW made the figures and table. YW and QT critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The project was supported by the National Key R&D Program of China through grant 2022YFC3601305 awarded to QT, grant 2022YFA0806400 awarded to YW, and Jilin Province Special Project of Medical and Health Talents through grant 3D5214505428 awarded by QT.
The authors (granted publication licenses) acknowledge the BioRender (www.biorender.com), as figures in this review were created with BioRender platform. The authors also thank Home for researchers editorial team for English language polishing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Żółkiewicz, J., Marzec, A., Ruszczyński, M., Feleszko, W. (2020). Postbiotics-a step beyond pre- and probiotics. Nutrients 12, 2189. doi: 10.3390/nu12082189
Abbate, A., Toldo, S., Marchetti, C., Kron, J., Van Tassell, B. W., Dinarello, C. A., et al. (2020). Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 126, 1260–1280. doi: 10.1161/CIRCRESAHA.120.315937
Adak, A., Khan, M. R. (2019). An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 76, 473–493. doi: 10.1007/s00018-018-2943-4
Adnan, S., Nelson, J. W., Ajami, N. J., Venna, V. R., Petrosino, J. F., Bryan, R. M., et al. (2017). Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genomics 49, 96–104. doi: 10.1152/physiolgenomics.00081.2016
Albillos, A., de Gottardi, A., Rescigno, M. (2020). The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577. doi: 10.1016/j.jhep.2019.10.003
Amara, S., Bourlieu, C., Humbert, L., Rainteau, D., Carrière, F. (2019). Variations in gastrointestinal lipases, pH and bile acid levels with food intake, age and diseases: possible impact on oral lipid-based drug delivery systems. Advanced Drug Delivery Rev. 142, 3–15. doi: 10.1016/j.addr.2019.03.005
Ammirati, E., Frigerio, M., Adler, E. D., Basso, C., Birnie, D. H., Brambatti, M., et al. (2020). Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circ.: Heart Failure 13, e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405
Ammirati, E., Moslehi, J. J., Ammirati, E., Moslehi, J. J. (2023). Diagnosis and treatment of acute myocarditis. JAMA 329, 1098. doi: 10.1001/jama.2023.3371
Asarat, M., Apostolopoulos, V., Vasiljevic, T., Donkor, O., Asarat, M., Apostolopoulos, V., et al. (2016). Short-chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol. Investigations 45, 205–222. doi: 10.3109/08820139.2015.1122613
Axelrod, M. L., Meijers, W. C., Screever, E. M., Qin, J., Carroll, M. G., Sun, X., et al. (2022). T Cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 611, 818–826. doi: 10.1038/s41586-022-05432-3
Bai, H., Gu, R.-J., Chen, L.-Y., Qian, Y., Yu, M.-L., Xu, S.-L., et al. (2021). Electroacupuncture interventions alleviates myocardial ischemia reperfusion injury through regulating gut microbiota in rats. Microvascular Res. 138, 104235. doi: 10.1016/j.mvr.2021.104235
Bajaj, J. S. (2019). Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 235–246. doi: 10.1038/s41575-018-0099-1
Barin, J. G., Talor, M. V., Diny, N. L., Ong, S., Schaub, J. A., Gebremariam, E., et al. (2017). Regulation of autoimmune myocarditis by host responses to the microbiome. Exp. Mol. Pathol. 103, 141–152. doi: 10.1016/j.yexmp.2017.08.003
Barka, E. A., Vatsa, P., Sanchez, L., Gaveau-Vaillant, N., Jacquard, C., Klenk, H.-P., et al. (2016). Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 80, 1–43. doi: 10.1128/MMBR.00019-15
Bastin, M., Andreelli, F. (2020). The gut microbiota and diabetic cardiomyopathy in humans. Diabetes Metab. 46, 197–202. doi: 10.1016/j.diabet.2019.10.003
Behrouzi, A., Nafari, A. H., Siadat, S. D. (2019). The significance of microbiome in personalized medicine. Clin. Transl. Med. 8, 16. doi: 10.1186/s40169-019-0232-y
Belkaid, Y., Hand, T. W., Belkaid, Y., Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. doi: 10.1016/j.cell.2014.03.011
Błyszczuk, P. (2019). Myocarditis in humans and in experimental animal models. Front. Cardiovasc. Med. 6. doi: 10.3389/fcvm.2019.00064
Błyszczuk, P., Błyszczuk, P. (2019). Myocarditis in humans and in experimental animal models. Front. Cardiovasc. Med. 6. doi: 10.3389/fcvm.2019.00064
Blyszczuk, P., Valaperti, A., Eriksson, U., Blyszczuk, P., Valaperti, A., Eriksson, U. (2008). Future therapeutic strategies in inflammatory cardiomyopathy: insights from the experimental autoimmune myocarditis model. CHDDT 8, 313–321. doi: 10.2174/1871529x10808040313
Caesar, L. K., Montaser, R., Keller, N. P., Kelleher, N. L. (2021). Metabolomics and genomics in natural products research: complementary tools for targeting new chemical entities. Nat. Prod Rep. 38, 2041–2065. doi: 10.1039/d1np00036e
Cai, J., Sun, L., Gonzalez, F. J., Cai, J., Sun, L., Gonzalez, F. J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300. doi: 10.1016/j.chom.2022.02.004
Cavalli, G., Pappalardo, F., Mangieri, A., Dinarello, C. A., Dagna, L., Tresoldi, M., et al. (2016). Treating life-threatening myocarditis by blocking interleukin-1*. Crit. Care Med. 44, e751–e754. doi: 10.1097/CCM.0000000000001654
Charbonneau, M. R., Isabella, V. M., Li, N., Kurtz, C. B., Charbonneau, M. R., Isabella, V. M., et al. (2020). Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738. doi: 10.1038/s41467-020-15508-1
Chen, X.-F., Chen, X., Tang, X. (2020). Short-chain fatty acid, acylation and cardiovascular diseases. Clin. Sci. (Lond) 134, 657–676. doi: 10.1042/CS20200128
Chen, L., Li, S., Peng, C., Gui, Q., Li, J., Xu, Z., et al. (2023). Lactobacillus rhamnosus GG promotes recovery of the colon barrier in septic mice through accelerating ISCs regeneration. Nutrients 15, 672. doi: 10.3390/nu15030672
Chen, M., Zhu, X., Ran, L., Lang, H., Yi, L., Mi, M. (2017). Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. JAHA 6, e006347. doi: 10.1161/JAHA.117.006347
Cho, C. E., Caudill, M. A. (2017). Trimethylamine- n -oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol. Metab. 28, 121–130. doi: 10.1016/j.tem.2016.10.005
Collins, S. L., Patterson, A. D. (2020). The gut microbiome: an orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 10, 19–32. doi: 10.1016/j.apsb.2019.12.001
Cooper, L. T. (2021) UpToDate. Available at: https://www.uptodate.cn/contents/clinical-manifestations-and-diagnosis-of-myocarditis-in-adults.
Cui, L., Zhao, T., Hu, H., Zhang, W., Hua, X. (2017). Association study of gut flora in coronary heart disease through high-throughput sequencing. BioMed. Res. Int. 2017, 1–10. doi: 10.1155/2017/3796359
Cummings, J. H. (1983). FERMENTATION IN THE HUMAN LARGE INTESTINE: EVIDENCE AND IMPLICATIONS FOR HEALTH. Lancet 321, 1206–1209. doi: 10.1016/S0140-6736(83)92478-9
D’Ambrosio, A., D’Ambrosio, A. (2001). The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 85, 499–504. doi: 10.1136/heart.85.5.499
D’Antona, G., Ragni, M., Cardile, A., Tedesco, L., Dossena, M., Bruttini, F., et al. (2010). Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 12, 362–372. doi: 10.1016/j.cmet.2010.08.016
Davidovics, Z. H., Michail, S., Nicholson, M. R., Kociolek, L. K., Pai, N., Hansen, R., et al. (2019). Fecal microbiota transplantation for recurrent clostridium difficile infection and other conditions in children: a joint position paper from the north American society for pediatric gastroenterology, hepatology, and nutrition and the European society for pediatric gastroenterology, hepatology, and nutrition. J. Pediatr. Gastroenterol. Nutr. 68, 130–143. doi: 10.1097/MPG.0000000000002205
Deleu, S., Machiels, K., Raes, J., Verbeke, K., Vermeire, S., Deleu, S., et al. (2021). Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine 66, 103293. doi: 10.1016/j.ebiom.2021.103293
De Marco, S., Sichetti, M., Muradyan, D., Piccioni, M., Traina, G., Pagiotti, R., et al. (2018). Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evidence-Based Complementary Altern. Med. 2018, e1756308. doi: 10.1155/2018/1756308
de Souza, J. B., Brelaz-de-Castro, M. C. A., Cavalcanti, I. M. F. (2022). Strategies for the treatment of colorectal cancer caused by gut microbiota. Life Sci. 290, 120202. doi: 10.1016/j.lfs.2021.120202
de Vrese, M., Schrezenmeir, J. (2008). Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 111, 1–66. doi: 10.1007/10_2008_097
Dhansekaran, D., Sankaranarayanan, A. (2021) Advances in probiotics microorganisms in food and health. Available at: https://www.elsevier.com/books/advances-in-probiotics/dhansekaran/978-0-12-822909-5 (Accessed January 6, 2023).
Di, D., Li, C., Dai, Y., Wei, M., Wang, S., Song, W., et al. (2021). Integrative analysis of LGR5/6 gene variants, gut microbiota composition and osteoporosis risk in elderly population. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.765008
Drapkina, O. M., Yafarova, A. A., Kaburova, A. N., Kiselev, A. R. (2022). Targeting gut microbiota as a novel strategy for prevention and treatment of hypertension, atrial fibrillation and heart failure: current knowledge and future perspectives. Biomedicines 10, 2019. doi: 10.3390/biomedicines10082019
Dreher-Lesnick, S. M., Stibitz, S., Carlson, P. E., Jr., Dreher-Lesnick, S. M., Stibitz, S., Carlson, P. E., Jr. (2017). U.S. regulatory considerations for development of live biotherapeutic products as drugs. Microbiol. Spectr. (2017) 5 (5). doi: 10.1128/microbiolspec.BAD-0017-2017
Duca, F. A., Sakar, Y., Covasa, M. (2013). The modulatory role of high fat feeding on gastrointestinal signals in obesity. J. Nutr. Biochem. 24, 1663–1677. doi: 10.1016/j.jnutbio.2013.05.005
Duda-Chodak, A., Tarko, T., Satora, P., Sroka, P. (2015). Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur. J. Nutr. 54, 325–341. doi: 10.1007/s00394-015-0852-y
Durgan, D. J., Ganesh, B. P., Cope, J. L., Ajami, N. J., Phillips, S. C., Petrosino, J. F., et al. (2016). Role of the gut microbiome in obstructive sleep apnea–induced hypertension. Hypertension 67, 469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672
Emoto, T., Yamashita, T., Kobayashi, T., Sasaki, N., Hirota, Y., Hayashi, T., et al. (2017). Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels 32, 39–46. doi: 10.1007/s00380-016-0841-y
Fabersani, E., Portune, K., Campillo, I., López-Almela, I., la Paz, S. M., Romaní-Pérez, M., et al. (2021). Bacteroides uniformis CECT 7771 alleviates inflammation within the gut-adipose tissue axis involving TLR5 signaling in obese mice. Sci. Rep. 11, 11788. doi: 10.1038/s41598-021-90888-y
Fajgenbaum, D. C., June, C. H. (2020). Cytokine storm. N Engl. J. Med. 383, 2255–2273. doi: 10.1056/NEJMra2026131
Fan, Y., Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi: 10.1038/s41579-020-0433-9
Feng, Q., Liang, S., Jia, H., Stadlmayr, A., Tang, L., Lan, Z., et al. (2015). Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 6, 6528. doi: 10.1038/ncomms7528
Fernandez, M. A., Marette, A. (2017). Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv. Nutr. 8, 155S–164S. doi: 10.3945/an.115.011114
Fuentes, S., de Vos, W. M. (2016). How to manipulate the microbiota: fecal microbiota transplantation. Adv. Exp. Med. Biol. 902, 143–153. doi: 10.1007/978-3-319-31248-4_10
Fung, G., Luo, H., Qiu, Y., Yang, D., McManus, B., Fung, G., et al. (2016). Myocarditis. Circ. Res. 118, 496–514. doi: 10.1161/CIRCRESAHA.115.306573
Gan, X. T., Ettinger, G., Huang, C. X., Burton, J. P., Haist, J. V., Rajapurohitam, V., et al. (2014). Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ.: Heart Failure 7, 491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978
Gao, J., Wang, J., Zhao, L.-L., Yao, T.-T., Chen, Y., Ma, J., et al. (2021). Gut lactobacillus level is a predictive marker for coronary atherosclerotic lesions progress and prognosis in patients with acute coronary syndrome. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.687827
Ghosh, T. S., Rampelli, S., Jeffery, I. B., Santoro, A., Neto, M., Capri, M., et al. (2020). Mediterranean Diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 69, 1218–1228. doi: 10.1136/gutjnl-2019-319654
Gil-Cruz, C., Perez-Shibayama, C., De Martin, A., Ronchi, F., van der Borght, K., Niederer, R., et al. (2019). Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 366, 881–886. doi: 10.1126/science.aav3487
Gomaa, E. Z., Gomaa, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek 113, 2019–2040. doi: 10.1007/s10482-020-01474-7
Gomes, A. C., Hoffmann, C., Mota, J. F. (2018). The human gut microbiota: metabolism and perspective in obesity. Gut Microbes 9, 308–325. doi: 10.1080/19490976.2018.1465157
Goswami, S. K., Ranjan, P., Dutta, R. K., Verma, S. K., Goswami, S. K., Ranjan, P., et al. (2021). Management of inflammation in cardiovascular diseases. Pharmacol. Res. 173, 105912. doi: 10.1016/j.phrs.2021.105912
Gough, E. K. (2022). The impact of mass drug administration of antibiotics on the gut microbiota of target populations. Infect. Dis. Poverty 11, 76. doi: 10.1186/s40249-022-00999-5
Haak, B. W., Lankelma, J. M., Hugenholtz, F., Belzer, C., de Vos, W. M., Wiersinga, W. J. (2019). Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 74, 782–786. doi: 10.1093/jac/dky471
Han, Q., Huang, X., Yan, F., Yin, J., Xiao, Y. (2022). The role of gut microbiota in the skeletal muscle development and fat deposition in pigs. Antibiotics 11, 793. doi: 10.3390/antibiotics11060793
Han, J., Jeong, H. I., Park, C., Yoon, J., Ko, J., Nam, S.-J., et al. (2018). Cholic acid attenuates ER stress-induced cell death in coxsackievirus-B3 infection. J. Microbiol. Biotechnol. 28, 109–114. doi: 10.4014/jmb.1708.08009
Hang, S., Paik, D., Yao, L., Kim, E., Trinath, J., Lu, J., et al. (2019). Bile acid metabolites control TH17 and treg cell differentiation. Nature 576, 143–148. doi: 10.1038/s41586-019-1785-z
He, Z., Kwek, E., Hao, W., Zhu, H., Liu, J., Ma, K. Y., et al. (2021). Hawthorn fruit extract reduced trimethylamine-n-oxide (TMAO)-exacerbated atherogenesis in mice via anti-inflammation and anti-oxidation. Nutr. Metab. (Lond) 18, 6. doi: 10.1186/s12986-020-00535-y
Heidenreich, P. A., Bozkurt, B., Aguilar, D., Allen, L. A., Byun, J. J., Colvin, M. M., et al. (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American college of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145, e876–e894. doi: 10.1161/CIR.0000000000001062
Henke, A., Huber, S., Stelzner, A., Whitton, J. L., Henke, A., Huber, S., et al. (1995). The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 69, 6720–6728. doi: 10.1128/JVI.69.11.6720-6728.1995
Heymans, S., Cooper, L. T., Heymans, S., Cooper, L. T. (2022). Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat. Rev. Cardiol. 19, 75–77. doi: 10.1038/s41569-021-00662-w
Heymans, S., Eriksson, U., Lehtonen, J., Cooper, L. T. (2016). The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J. Am. Coll. Cardiol. 68, 2348–2364. doi: 10.1016/j.jacc.2016.09.937
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hills, R., Pontefract, B., Mishcon, H., Black, C., Sutton, S., Theberge, C. (2019). Gut microbiome: profound implications for diet and disease. Nutrients 11, 1613. doi: 10.3390/nu11071613
Hoebe, K., Beutler, B., Hoebe, K., Beutler, B. (2004). LPS, dsRNA and the interferon bridge to adaptive immune responses: trif, tram, and other TIR adaptor proteins. J. Endotoxin Res. 10, 130–136. doi: 10.1177/09680519040100021001
Hou, K., Wu, Z.-X., Chen, X.-Y., Wang, J.-Q., Zhang, D., Xiao, C., et al. (2022). Microbiota in health and diseases. Sig. Transduct Target Ther. (2022) 7 (1), 135. doi: 10.1038/s41392-022-00974-4
Hu, T., Wu, Q., Yao, Q., Jiang, K., Yu, J., Tang, Q. (2022). Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 81, 101706. doi: 10.1016/j.arr.2022.101706
Hu, X.-F., Zhang, W.-Y., Wen, Q., Chen, W.-J., Wang, Z.-M., Chen, J., et al. (2019). Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol. Res. 139, 412–421. doi: 10.1016/j.phrs.2018.11.042
Hung, W.-C., Hung, W.-W., Tsai, H.-J., Chang, C.-C., Chiu, Y.-W., Hwang, S.-J., et al. (2021). The association of targeted gut microbiota with body composition in type 2 diabetes mellitus. Int. J. Med. Sci. 18, 511–519. doi: 10.7150/ijms.51164
Inciardi, R. M., Lupi, L., Zaccone, G., Italia, L., Raffo, M., Tomasoni, D., et al. (2020). Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 819. doi: 10.1001/jamacardio.2020.1096
Janeway, C. A., Travers, P., Walport, M., Shlomchik, M. J. (2001). Immunobiology: the immune system in health and disease. 5th ed (New York: Garland Science).
Javadi, B., Sahebkar, A. (2017). Natural products with anti-inflammatory and immunomodulatory activities against autoimmune myocarditis. Pharmacol. Res. 124, 34–42. doi: 10.1016/j.phrs.2017.07.022
Jia, Q., Li, H., Zhou, H., Zhang, X., Zhang, A., Xie, Y., et al. (2019). Role and effective therapeutic target of gut microbiota in heart failure. Cardiovasc. Ther. 2019, 1–10. doi: 10.1155/2019/5164298
Jonsson, A. L., Bäckhed, F. (2017). Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 14, 79–87. doi: 10.1038/nrcardio.2016.183
Kajiwara, K., Okuno, M., Kobayashi, T., Honma, N., Maki, T., Kato, M., et al. (1998). Oral supplementation with branched-chain amino acids improves survival rate of rats with carbon tetrachloride-induced liver cirrhosis. Dig. Dis. Sci. 43, 1572–1579. doi: 10.1023/A:1018831302578
Karaś, K., Sałkowska, A., Sobalska-Kwapis, M., Walczak-Drzewiecka, A., Strapagiel, D., Dastych, J., et al. (2018). Digoxin, an overlooked agonist of RORγ/RORγT. Front. Pharmacol. 9. doi: 10.3389/fphar.2018.01460
Kazemian, N., Ramezankhani, M., Sehgal, A., Khalid, F. M., Kalkhoran, A. H. Z., Narayan, A., et al. (2020). The trans-kingdom battle between donor and recipient gut microbiome influences fecal microbiota transplantation outcome. Sci. Rep. 10, 18349. doi: 10.1038/s41598-020-75162-x
Kerry, R. G., Patra, J. K., Gouda, S., Park, Y., Shin, H.-S., Das, G. (2018). Benefaction of probiotics for human health: a review. J. Food Drug Anal. 26, 927–939. doi: 10.1016/j.jfda.2018.01.002
Kim, C. H., Park, J., Kim, M. (2014). Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 14, 277–288. doi: 10.4110/in.2014.14.6.277
Kim, H. K., Suzuki, T., Saito, K., Yoshida, H., Kobayashi, H., Kato, H., et al. (2012). Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J. Am. Geriatr. Soc. 60, 16–23. doi: 10.1111/j.1532-5415.2011.03776.x
Kirat, D., Masuoka, J., Hayashi, H., Iwano, H., Yokota, H., Taniyama, H., et al. (2006). Monocarboxylate transporter 1 (MCT1) plays a direct role in short-chain fatty acids absorption in caprine rumen. J. Physiol. 576, 635–647. doi: 10.1113/jphysiol.2006.115931
Klement, R., Pazienza, V. (2019). Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina 55, 84. doi: 10.3390/medicina55040084
Ko, C.-Y., Hu, A.-K., Chou, D., Huang, L.-M., Su, H.-Z., Yan, F.-R., et al. (2019). Analysis of oral microbiota in patients with obstructive sleep apnea-associated hypertension. Hypertens. Res. 42, 1692–1700. doi: 10.1038/s41440-019-0260-4
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Komaroff, A. L. (2017). The microbiome and risk for obesity and diabetes. JAMA 317, 355. doi: 10.1001/jama.2016.20099
Krejci, J., Mlejnek, D., Sochorova, D., Nemec, P., Krejci, J., Mlejnek, D., et al. (2016). Inflammatory cardiomyopathy: a current view on the pathophysiology, diagnosis, and treatment. BioMed. Res. Int. 2016, 1–11. doi: 10.1155/2016/4087632
Lahiri, S., Kim, H., Garcia-Perez, I., Reza, M. M., Martin, K. A., Kundu, P., et al. (2019). The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 11, eaan5662. doi: 10.1126/scitranslmed.aan5662
Lam, V., Su, J., Hsu, A., Gross, G. J., Salzman, N. H., Baker, J. E. (2016). Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PloS One 11, e0160840. doi: 10.1371/journal.pone.0160840
Larsen, J. M., Musavian, H. S., Butt, T. M., Ingvorsen, C., Thysen, A. H., Brix, S., et al. (2015). Chronic obstructive pulmonary disease and asthma-associated proteobacteria, but not commensalPrevotellaspp., promote toll-like receptor 2-independent lung inflammation and pathology. Immunology 144, 333–342. doi: 10.1111/imm.12376
Lee, Y.-G., Park, J.-H., Jeon, E.-S., Kim, J.-H., Lim, B.-K., Lee, Y.-G., et al. (2016). Fructus amomi cardamomi extract inhibits coxsackievirus-B3 induced myocarditis in a murine myocarditis model. J. Microbiol. Biotechnol. 26, 2012–2018. doi: 10.4014/jmb.1605.05056
Leone, O., Pieroni, M., Rapezzi, C., Olivotto, I. (2019). The spectrum of myocarditis: from pathology to the clinics. Virchows Arch. 475, 279–301. doi: 10.1007/s00428-019-02615-8
Leuschner, F., Courties, G., Dutta, P., Mortensen, L. J., Gorbatov, R., Sena, B., et al. (2015). Silencing of CCR2 in myocarditis. Eur. Heart J. 36, 1478–1488. doi: 10.1093/eurheartj/ehu225
Li, Z., Quan, G., Jiang, X., Yang, Y., Ding, X., Zhang, D., et al. (2018b). Effects of metabolites derived from gut microbiota and hosts on pathogens. Front. Cell. Infect. Microbiol. 8. doi: 10.3389/fcimb.2018.00314
Li, M., van Esch, B. C. A. M., Henricks, P. A. J., Folkerts, G., Garssen, J. (2018a). The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 9. doi: 10.3389/fphar.2018.00533
Li, Y., Xia, S., Jiang, X., Feng, C., Gong, S., Ma, J., et al. (2021b). Gut microbiota and diarrhea: an updated review. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.625210
Li, K., Xu, W., Guo, Q., Jiang, Z., Wang, P., Yue, Y., et al. (2009). Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ. Res. 105, 353–364. doi: 10.1161/CIRCRESAHA.109.195230
Li, M., Yan, K., Wei, L., Yang, J., Lu, C., Xiong, F., et al. (2015). Zinc finger antiviral protein inhibits coxsackievirus B3 virus replication and protects against viral myocarditis. Antiviral Res. 123, 50–61. doi: 10.1016/j.antiviral.2015.09.001
Li, J., Yang, X., Zhou, X., Cai, J. (2021a). The role and mechanism of intestinal flora in blood pressure regulation and hypertension development. Antioxid. Redox Signaling 34, 811–830. doi: 10.1089/ars.2020.8104
Li, J., Zhao, F., Wang, Y., Chen, J., Tao, J., Tian, G., et al. (2017). Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14. doi: 10.1186/s40168-016-0222-x
Lin, X., Sun, Y., Jing, Z. C., Xu, X. Q. (2021). Improving the diagnostic and therapeutic efficacy for myocarditis and inflammatory cardiomyopathy through deepened understanding on the pathogenesis of the diseases. Zhonghua xin xue guan bing za zhi 49, 6–11. doi: 10.3760/cma.j.cn112148-20201202-00953
Lincez, P. J., Walic, M., Horwitz, M. S., Lincez, P. J., Walic, M., Horwitz, M. S. (2011). The key players of coxsackievirus-induced myocarditis. IntechOpen 12, 243–270. doi: 10.5772/20932
Lippi, G., Danese, E., Mattiuzzi, C., Favaloro, E. J. (2017). The intriguing link between the intestinal microbiota and cardiovascular disease. Semin. Thromb. Hemost. 43, 609–613. doi: 10.1055/s-0036-1597903
Liu, Y., Dai, M., Liu, Y., Dai, M. (2020b). Trimethylamine n-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflammation 2020, 1–15. doi: 10.1155/2020/4634172
Liu, H., Li, M., Song, Y., Xu, W., Liu, H., Li, M., et al. (2018). TRIM21 restricts coxsackievirus B3 replication, cardiac and pancreatic injury via interacting with MAVS and positively regulating IRF3-mediated type-I interferon production. Front. Immunol. 9. doi: 10.3389/fimmu.2018.02479
Liu, H., Zhuang, J., Tang, P., Li, J., Xiong, X., Deng, H., et al. (2020a). The role of the gut microbiota in coronary heart disease. Curr. Atheroscler Rep. 22, 77. doi: 10.1007/s11883-020-00892-2
Loganathan, T., Priya Doss, C. G. (2022). The influence of machine learning technologies in gut microbiome research and cancer studies – A review. Life Sci. 311, 121118. doi: 10.1016/j.lfs.2022.121118
Losno, E. A., Sieferle, K., Perez-Cueto, F. J. A., Ritz, C. (2021). Vegan diet and the gut microbiota composition in healthy adults. Nutrients 13, 2402. doi: 10.3390/nu13072402
Luo, Q., Hu, Y., Chen, X., Luo, Y., Chen, J., Wang, H. (2022). Effects of gut microbiota and metabolites on heart failure and its risk factors: a two-sample mendelian randomization study. Front. Nutr. 9. doi: 10.3389/fnut.2022.899746
Lynch, C. J., Adams, S. H., Lynch, C. J., Adams, S. H. (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736. doi: 10.1038/nrendo.2014.171
Maier, L., Goemans, C. V., Wirbel, J., Kuhn, M., Eberl, C., Pruteanu, M., et al. (2021). Unravelling the collateral damage of antibiotics on gut bacteria. Nature 599, 120–124. doi: 10.1038/s41586-021-03986-2
Maifeld, A., Bartolomaeus, H., Löber, U., Avery, E. G., Steckhan, N., Markó, L., et al. (2021). Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat. Commun. 12, 1970. doi: 10.1038/s41467-021-22097-0
Mamic, P., Chaikijurajai, T., Tang, W. H. W., Mamic, P., Chaikijurajai, T., Tang, W. H. W. (2021). Gut microbiome - a potential mediator of pathogenesis in heart failure and its comorbidities: state-of-the-art review. J. Mol. Cell. Cardiol. 152, 105–117. doi: 10.1016/j.yjmcc.2020.12.001
Mandelbaum, N., Gefen, T., Geva-Zatorsky, N. (2020). Gut bacteria–not for the faint of heart. Cell Host Microbe 27, 1–3. doi: 10.1016/j.chom.2019.12.011
Mann, G., Mora, S., Madu, G., Adegoke, O. A. J. (2021). Branched-chain amino acids: catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front. Physiol. 12. doi: 10.3389/fphys.2021.702826
Matsumoto, K., Koba, T., Hamada, K., Sakurai, M., Higuchi, T., Miyata, H. (2009). Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J. Sports Med. Phys. Fitness 49, 424–431. doi: 10.2519/jospt.2009.0415
Medzhitov, R., Medzhitov, R. (2007). Recognition of microorganisms and activation of the immune response. Nature 449, 819–826. doi: 10.1038/nature06246
Mei, L., Chen, Y., Wang, J., Lu, J., Zhao, J., Zhang, H., et al. (2022). Lactobacillus fermentum stimulates intestinal secretion of immunoglobulin a in an individual-specific manner. Foods 11, 1229. doi: 10.3390/foods11091229
Merra, G., Noce, A., Marrone, G., Cintoni, M., Tarsitano, M. G., Capacci, A., et al. (2020). Influence of Mediterranean diet on human gut microbiota. Nutrients 13, 7. doi: 10.3390/nu13010007
Mizutani, T., Ishizaka, A., Koga, M., Tsutsumi, T., Yotsuyanagi, H. (2022). Role of microbiota in viral infections and pathological progression. Viruses 14, 950. doi: 10.3390/v14050950
Nagai, M., Obata, Y., Takahashi, D., Hase, K. (2016). Fine-tuning of the mucosal barrier and metabolic systems using the diet-microbial metabolite axis. Int. Immunopharmacol. 37, 79–86. doi: 10.1016/j.intimp.2016.04.001
Neinast, M., Murashige, D., Arany, Z. (2019). Branched chain amino acids. Annu. Rev. Physiol. 81, 139–164. doi: 10.1146/annurev-physiol-020518-114455
Neu, N., Beisel, K. W., Traystman, M. D., Rose, N. R., Craig, S. W. (1987). Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3-induced myocarditis. J. Immunol. 138, 2488–2492. doi: 10.4049/jimmunol.138.8.2488
Neves, A. L., Coelho, J., Couto, L., Leite-Moreira, A., Roncon-Albuquerque, R., Neves, A. L., et al. (2013). Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 51, R51–R64. doi: 10.1530/JME-13-0079
Nie, N., Li, Z., Li, W., Huang, X., Jiang, Z., Shen, Y., et al. (2023). Myricetin ameliorates experimental autoimmune myocarditis in mice by modulating immune response and inhibiting MCP-1 expression. Eur. J. Pharmacol. 942, 175549. doi: 10.1016/j.ejphar.2023.175549
Noor, R., Naz, A., Maniha, S. M., Tabassum, N., Tabassum, T., Tabassum, T., et al. (2021). Microorganisms and cardiovascular diseases: importance of gut bacteria. Landmark 26, 22. doi: 10.52586/4921
O’Donnell, J. A., Zheng, T., Meric, G., Marques, F. Z. (2023). The gut microbiome and hypertension. Nat. Rev. Nephrol. 19, 153–167. doi: 10.1038/s41581-022-00654-0
Oniszczuk, A., Oniszczuk, T., Gancarz, M., Szymańska, J., Oniszczuk, A., Oniszczuk, T., et al. (2021). Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 26, 1172. doi: 10.3390/molecules26041172
Opavsky, M. A., Penninger, J., Aitken, K., Wen, W.-H., Dawood, F., Mak, T., et al. (1999). Susceptibility to myocarditis is dependent on the response of αβ T lymphocytes to coxsackieviral infection. Circ. Res. 85, 551–558. doi: 10.1161/01.res.85.6.551
Ozkan, J. (2022). What’s your gut telling you? how researchers are investigating the link between myocarditis and intestinal microbiota. Eur. Heart J. 43, 100–102. doi: 10.1093/eurheartj/ehab396
Parada Venegas, D., de la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10. doi: 10.3389/fimmu.2019.00277
Park, Y., Kim, T., Ham, J., Choi, J., Lee, H., Yeon, Y. J., et al. (2022). Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 15, 832–843. doi: 10.1111/1751-7915.13795
Parkin, J., Cohen, B., Parkin, J., Cohen, B. (2001). An overview of the immune system. Lancet 357, 1777–1789. doi: 10.1016/S0140-6736(00)04904-7
Piccioni, A., Saviano, A., Cicchinelli, S., Franza, L., Rosa, F., Zanza, C., et al. (2021). Microbiota and myopericarditis: the new frontier in the car-diological field to prevent or treat inflammatory cardiomyo-pathies in COVID-19 outbreak. Biomedicines 9, 1234. doi: 10.3390/biomedicines9091234
Priyadarshini, M., Wicksteed, B., Schiltz, G. E., Gilchrist, A., Layden, B. T., Priyadarshini, M., et al. (2016). SCFA receptors in pancreatic β cells: novel diabetes targets? Trends Endocrinol. Metab. 27, 653–664. doi: 10.1016/j.tem.2016.03.011
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Querio, G., Antoniotti, S., Levi, R., Gallo, M. P. (2019). Trimethylamine n-oxide does not impact viability, ROS production, and mitochondrial membrane potential of adult rat cardiomyocytes. IJMS 20, 3045. doi: 10.3390/ijms20123045
Richardson, P., McKenna, W., Bristow, M., Maisch, B., Mautner, B., O’Connell, J., et al. (1996). Report of the 1995 world health Organization/International society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation 93, 841–842. doi: 10.1161/01.cir.93.5.841
Rinninella, E., Raoul, P., Cintoni, M., Franceschi, F., Miggiano, G., Gasbarrini, A., et al. (2019). What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms 7, 14. doi: 10.3390/microorganisms7010014
Rioux, K. P., Madsen, K. L., Fedorak, R. N. (2005). The role of enteric microflora in inflammatory bowel disease: human and animal studies with probiotics and prebiotics. Gastroenterol. Clin. North Am. 34, 465–482, ix. doi: 10.1016/j.gtc.2005.05.005
Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., et al. (2010). Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104 (Suppl 2), S1–63. doi: 10.1017/S0007114510003363
Romano, K. A., Nemet, I., Prasad Saha, P., Haghikia, A., Li, X. S., Mohan, M. L., et al. (2023). Gut microbiota-generated phenylacetylglutamine and heart failure. Circ.: Heart Failure 16, e009972. doi: 10.1161/CIRCHEARTFAILURE.122.009972
Satija, A., Hu, F. B. (2018). Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 28, 437–441. doi: 10.1016/j.tcm.2018.02.004
Scher, J. U., Sczesnak, A., Longman, R. S., Segata, N., Ubeda, C., Bielski, C., et al. (2013). Expansion of intestinal prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2, e01202. doi: 10.7554/eLife.01202
Schiattarella, G. G., Sannino, A., Toscano, E., Giugliano, G., Gargiulo, G., Franzone, A., et al. (2017). Gut microbe-generated metabolite trimethylamine-n-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur. Heart J. 38, 2948–2956. doi: 10.1093/eurheartj/ehx342
Schirmer, M., Garner, A., Vlamakis, H., Xavier, R. J. (2019). Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 17, 497–511. doi: 10.1038/s41579-019-0213-6
Schneiderhan, J., Master-Hunter, T., Locke, A. (2016). Targeting gut flora to treat and prevent disease. J. Fam. Pract. 65, 34–38.
Schoeler, M., Caesar, R., Schoeler, M., Caesar, R. (2019). Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 20, 461–472. doi: 10.1007/s11154-019-09512-0
Schwiertz, A., Taras, D., Schäfer, K., Beijer, S., Bos, N. A., Donus, C., et al. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195. doi: 10.1038/oby.2009.167
Sekirov, I., Russell, S. L., Antunes, L. C. M., Finlay, B. B., Sekirov, I., Russell, S. L., et al. (2010). Gut microbiota in health and disease. Physiol. Rev. 90, 859–904. doi: 10.1152/physrev.00045.2009
Seldin, M. M., Meng, Y., Qi, H., Zhu, W., Wang, Z., Hazen, S. L., et al. (2016). Trimethylamine n-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. JAHA 5, e002767. doi: 10.1161/JAHA.115.002767
Sender, R., Fuchs, S., Milo, R., Sender, R., Fuchs, S., Milo, R. (2016). Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340. doi: 10.1016/j.cell.2016.01.013
Shang, J., Liu, F., Zhang, B., Dong, K., Lu, M., Jiang, R., et al. (2021). Liraglutide-induced structural modulation of the gut microbiota in patients with type 2 diabetes mellitus. PeerJ 9, e11128. doi: 10.7717/peerj.11128
Sharon, P., Stenson, W. F. (1985). Metabolism of arachidonic acid in acetic acid colitis in rats. Gastroenterology 88, 55–63. doi: 10.1016/S0016-5085(85)80132-3
Singh, R. K., Chang, H.-W., Yan, D., Lee, K. M., Ucmak, D., Wong, K., et al. (2017). Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15, 73. doi: 10.1186/s12967-017-1175-y
Siripanthong, B., Nazarian, S., Muser, D., Deo, R., Santangeli, P., Khanji, M. Y., et al. (2020). Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 17, 1463–1471. doi: 10.1016/j.hrthm.2020.05.001
Sivaprakasam, S., Bhutia, Y. D., Yang, S., Ganapathy, V. (2017). Short-chain fatty acid transporters: role in colonic homeostasis. Compr. Physiol. 8, 299–314. doi: 10.1002/cphy.c170014
Smith, J. G., Yokoyama, W. H., German, J. B., Smith, J. G., Yokoyama, W. H., German, J. B. (1998). Butyric acid from the diet: actions at the level of gene expression. Crit. Rev. Food Sci. Nutr. 38, 259–297. doi: 10.1080/10408699891274200
Song, X., Sun, X., Oh, S. F., Wu, M., Zhang, Y., Zheng, W., et al. (2020). Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415. doi: 10.1038/s41586-019-1865-0
Spallarossa, P., Sarocchi, M., Tini, G., Arboscello, E., Toma, M., Ameri, P., et al. (2020). How to monitor cardiac complications of immune checkpoint inhibitor therapy. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.00972
Statovci, D., Aguilera, M., MacSharry, J., Melgar, S. (2017). The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 8. doi: 10.3389/fimmu.2017.00838
Suez, J., Zmora, N., Segal, E., Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25 (5), 716–729. doi: 10.1038/s41591-019-0439-x
Swirski, F. K., Nahrendorf, M., Swirski, F. K., Nahrendorf, M. (2018). Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 18, 733–744. doi: 10.1038/s41577-018-0065-8
Takegoshi, K., Honda, M., Okada, H., Takabatake, R., Matsuzawa-Nagata, N., Campbell, J. S., et al. (2017). Branched-chain amino acids prevent hepatic fibrosis and development of hepatocellular carcinoma in a non-alcoholic steatohepatitis mouse model. Oncotarget 8, 18191–18205. doi: 10.18632/oncotarget.15304
Tang, T. W. H., Chen, H.-C., Chen, C.-Y., Yen, C. Y. T., Lin, C.-J., Prajnamitra, R. P., et al. (2019a). Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 139, 647–659. doi: 10.1161/CIRCULATIONAHA.118.035235
Tang, W. H. W., Li, D. Y., Hazen, S. L., Tang, W. H. W., Li, D. Y., Hazen, S. L. (2019b). Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 16, 137–154. doi: 10.1038/s41569-018-0108-7
Toita, R., Kawano, T., Murata, M., Kang, J.-H., Toita, R., Kawano, T., et al. (2021). Bioinspired macrophage-targeted anti-inflammatory nanomedicine: a therapeutic option for the treatment of myocarditis. Mater. Sci. Eng.: C 131, 112492. doi: 10.1016/j.msec.2021.112492
Toldo, S., Kannan, H., Bussani, R., Anzini, M., Sonnino, C., Sinagra, G., et al. (2014). Formation of the inflammasome in acute myocarditis. Int. J. Cardiol. 171, e119–e121. doi: 10.1016/j.ijcard.2013.12.137
Trachtenberg, B. H., Hare, J. M. (2017). Inflammatory cardiomyopathic syndromes. Circ. Res. 121, 803–818. doi: 10.1161/CIRCRESAHA.117.310221
Tschöpe, C., Ammirati, E., Bozkurt, B., Caforio, A. L. P., Cooper, L. T., Felix, S. B., et al. (2021). Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat. Rev. Cardiol. 18, 169–193. doi: 10.1038/s41569-020-00435-x
Tsilingiri, K., Rescigno, M. (2013). Postbiotics: what else? Benef. Microbes 4, 101–107. doi: 10.3920/BM2012.0046
Ulbricht, T. L. V., Southgate, D. A. T. (1991). Coronary heart disease: seven dietary factors. Lancet 338, 985–992. doi: 10.1016/0140-6736(91)91846-m
Uraz, S., Tahan, G., Aytekin, H., Tahan, V. (2013). N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scand. J. Clin. Lab. Invest. 73, 61–66. doi: 10.3109/00365513.2012.734859
Ursell, L. K., Haiser, H. J., Van Treuren, W., Garg, N., Reddivari, L., Vanamala, J., et al. (2014). The intestinal metabolome: an intersection between microbiota and host. Gastroenterology 146, 1470–1476. doi: 10.1053/j.gastro.2014.03.001
Vallianou, N., Stratigou, T., Christodoulatos, G. S., Tsigalou, C., Dalamaga, M. (2020). Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr. Obes. Rep. 9, 179–192. doi: 10.1007/s13679-020-00379-w
Varesi, A., Pierella, E., Romeo, M., Piccini, G. B., Alfano, C., Bjørklund, G., et al. (2022). The potential role of gut microbiota in alzheimer’s disease: from diagnosis to treatment. Nutrients 14, 668. doi: 10.3390/nu14030668
Verhaar, B. J. H., Prodan, A., Nieuwdorp, M., Muller, M. (2020). Gut microbiota in hypertension and atherosclerosis: a review. Nutrients 12, 2982. doi: 10.3390/nu12102982
Vieira-Silva, S., Falony, G., Belda, E., Nielsen, T., Aron-Wisnewsky, J., Chakaroun, R., et al. (2020). Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315. doi: 10.1038/s41586-020-2269-x
Vinolo, M. A. R., Rodrigues, H. G., Nachbar, R. T., Curi, R., Vinolo, M. A. R., Rodrigues, H. G., et al. (2011). Regulation of inflammation by short chain fatty acids. Nutrients 3, 858–876. doi: 10.3390/nu3100858
Vlasova, A. N., Kandasamy, S., Chattha, K. S., Rajashekara, G., Saif, L. J. (2016). Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 172, 72–84. doi: 10.1016/j.vetimm.2016.01.003
Walker, R. L., Vlamakis, H., Lee, J. W. J., Besse, L. A., Xanthakis, V., Vasan, R. S., et al. (2021). Population study of the gut microbiome: associations with diet, lifestyle, and cardiometabolic disease. Genome Med. 13, 188. doi: 10.1186/s13073-021-01007-5
Wang, Y.-D., Chen, W.-D., Yu, D., Forman, B. M., Huang, W., Wang, Y.-D., et al. (2011). The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated b cells (NF-κB) in mice. Hepatology 54, 1421–1432. doi: 10.1002/hep.24525
Wang, J.-W., Kuo, C.-H., Kuo, F.-C., Wang, Y.-K., Hsu, W.-H., Yu, F.-J., et al. (2019a). Fecal microbiota transplantation: review and update. J. Formosan Med. Assoc. 118, S23–S31. doi: 10.1016/j.jfma.2018.08.011
Wang, R., Li, D., Ouyang, J., Tian, X., Zhao, Y., Peng, X., et al. (2019b). Leonurine alleviates LPS-induced myocarditis through suppressing the NF-кB signaling pathway. Toxicology 422, 1–13. doi: 10.1016/j.tox.2019.04.011
Wang, Z., Zhao, Y., Wang, Z., Zhao, Y. (2018). Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 9, 416–431. doi: 10.1007/s13238-018-0549-0
Weersma, R. K., Zhernakova, A., Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut 69, 1510–1519. doi: 10.1136/gutjnl-2019-320204
Wiltshire, S. A., Leiva-Torres, G. A., Vidal, S. M., Wiltshire, S. A., Leiva-Torres, G. A., Vidal, S. M. (2011). Quantitative trait locus analysis, pathway analysis, and consomic mapping show genetic variants of Tnni3k, Fpgt, or H28 control susceptibility to viral myocarditis. J. Immunol. 186, 6398–6405. doi: 10.4049/jimmunol.1100159
Witkowski, M., Weeks, T. L., Hazen, S. L., Witkowski, M., Weeks, T. L., Hazen, S. L. (2020). Gut microbiota and cardiovascular disease. Circ. Res. 127, 553–570. doi: 10.1161/CIRCRESAHA.120.316242
Wong, S. H., Yu, J. (2019). Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 16, 690–704. doi: 10.1038/s41575-019-0209-8
Wu, H.-J., Wu, E. (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3, 4–14. doi: 10.4161/gmic.19320
Xu, H., Pan, L.-B., Yu, H., Han, P., Fu, J., Zhang, Z.-W., et al. (2022). Gut microbiota-derived metabolites in inflammatory diseases based on targeted metabolomics. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.919181
Xue, B., Li, H., Guo, M., Wang, J., Xu, Y., Zou, X., et al. (2018). TRIM21 promotes innate immune response to RNA viral infection through Lys27-linked polyubiquitination of MAVS. J. Virol. 92, e00321-18. doi: 10.1128/JVI.00321-18
Yan, Q., Cai, L., Guo, W. (2022). New advances in improving bone health based on specific gut microbiota. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.821429
Yan, Q., Gu, Y., Li, X., Yang, W., Jia, L., Chen, C., et al. (2017). Alterations of the gut microbiome in hypertension. Front. Cell. Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00381
Yang, T., Mei, X., Tackie-Yarboi, E., Akere, M. T., Kyoung, J., Mell, B., et al. (2022a). Identification of a gut commensal that compromises the blood pressure-lowering effect of ester angiotensin-converting enzyme inhibitors. Hypertension 79, 1591–1601. doi: 10.1161/HYPERTENSIONAHA.121.18711
Yang, T., Richards, E. M., Pepine, C. J., Raizada, M. K., Yang, T., Richards, E. M., et al. (2018). The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456. doi: 10.1038/s41581-018-0018-2
Yang, X.-Y., Yu, H., Fu, J., Guo, H.-H., Han, P., Ma, S.-R., et al. (2022b). Hydroxyurea ameliorates atherosclerosis in ApoE-/- mice by potentially modulating niemann-pick C1-like 1 protein through the gut microbiota. Theranostics 12, 7775–7787. doi: 10.7150/thno.76805
Yang, X., Zhang, X., Yang, W., Yu, H., He, Q., Xu, H., et al. (2021). Gut microbiota in adipose tissue dysfunction induced cardiovascular disease: role as a metabolic organ. Front. Endocrinol. 12. doi: 10.3389/fendo.2021.749125
Yao, Y., Cai, X., Fei, W., Ye, Y., Zhao, M., Zheng, C. (2022). The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 62, 1–12. doi: 10.1080/10408398.2020.1854675
Yoo, J., Groer, M., Dutra, S., Sarkar, A., McSkimming, D. (2020). Gut microbiota and immune system interactions. Microorganisms 8, 1587. doi: 10.3390/microorganisms8101587
Yoon, M.-S., Yoon, M.-S. (2016). The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 8, 405. doi: 10.3390/nu8070405
Yuan, S., Cai, Z., Luan, X., Wang, H., Zhong, Y., Deng, L., et al. (2022). Gut microbiota: a new therapeutic target for diabetic cardiomyopathy. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.963672
Yuan, J., Liu, Z., Lim, T., Zhang, H., He, J., Walker, E., et al. (2009). CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ. Res. 104, 628–638. doi: 10.1161/CIRCRESAHA.108.192179
Zhang, Y., Cai, S., Ding, X., Lu, C., Wu, R., Wu, H., et al. (2021a). MicroRNA-30a-5p silencing polarizes macrophages toward M2 phenotype to alleviate cardiac injury following viral myocarditis by targeting SOCS1. Am. J. Physiol.-Heart Circulatory Physiol. 320, H1348–H1360. doi: 10.1152/ajpheart.00431.2020
Zhang, Q.-M., Song, W.-Q., Li, Y.-J., Qian, J., Zhai, A.-X., Wu, J., et al. (2012). Over-expression of mitochondrial antiviral signaling protein inhibits coxsackievirus B3 infection by enhancing type-I interferons production. Virol. J. 9, 312. doi: 10.1186/1743-422X-9-312
Zhang, Y., Sun, D., Zhao, X., Luo, Y., Yu, H., Zhou, Y., et al. (2022). Bacteroides fragilis prevents aging-related atrial fibrillation in rats via regulatory T cells-mediated regulation of inflammation. Pharmacol. Res. 177, 106141. doi: 10.1016/j.phrs.2022.106141
Zhang, Y., Wang, Y., Ke, B., Du, J., Zhang, Y., Wang, Y., et al. (2021b). TMAO: how gut microbiota contributes to heart failure. Trans. Res. 228, 109–125. doi: 10.1016/j.trsl.2020.08.007
Zhang Wenyong, C. L. (2019). The role and mechanism of GLP-1 analogue liraglutide in autoimmune myocarditis (Huazhong University of Science and Technology). Doctoral dissertation. doi: 10.27157/d.cnki.ghzku.2019.005078
Zhao, Y., Yan, K., Wang, Y., Cai, J., Wei, L., Li, S., et al. (2020). Lithium chloride confers protection against viral myocarditis via suppression of coxsackievirus B3 virus replication. Microb. Pathogenesis 144, 104169. doi: 10.1016/j.micpath.2020.104169
Zhou, B., Carrillo-Larco, R. M., Danaei, G., Riley, L. M., Paciorek, C. J., Stevens, G. A., et al. (2021). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398, 957–980. doi: 10.1016/S0140-6736(21)01330-1
Zhou, Y., Lu, Q. (2022). Hydroxyurea protects against diabetic cardiomyopathy by inhibiting inflammation and apoptosis. Biomed. Pharmacother. 153, 113291. doi: 10.1016/j.biopha.2022.113291
Zhu, Q., Gao, R., Zhang, Y., Pan, D., Zhu, Y., Zhang, X., et al. (2018). Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genomics 50, 893–903. doi: 10.1152/physiolgenomics.00070.2018
Zhu, Y., Li, Q., Jiang, H., Zhu, Y., Li, Q., Jiang, H. (2020). Gut microbiota in atherosclerosis: focus on trimethylamine n-oxide. APMIS 128, 353–366. doi: 10.1111/apm.13038
Zhu, L.-R., Li, S.-S., Zheng, W.-Q., Ni, W.-J., Cai, M., Liu, H.-P. (2023). Targeted modulation of gut microbiota by traditional Chinese medicine and natural products for liver disease therapy. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1086078
Keywords: myocarditis, gut microbiota, metabolites, immune system, treatment
Citation: Wang J, Zhang X, Yang X, Yu H, Bu M, Fu J, Zhang Z, Xu H, Hu J, Lu J, Zhang H, Zhai Z, Yang W, Wu X, Wang Y and Tong Q (2023) Revitalizing myocarditis treatment through gut microbiota modulation: unveiling a promising therapeutic avenue. Front. Cell. Infect. Microbiol. 13:1191936. doi: 10.3389/fcimb.2023.1191936
Received: 22 March 2023; Accepted: 24 April 2023;
Published: 16 May 2023.
Edited by:
Piyush Baindara, University of Missouri, United StatesReviewed by:
Manpreet Kaur, Cactus Communications Pvt. Ltd., IndiaCopyright © 2023 Wang, Zhang, Yang, Yu, Bu, Fu, Zhang, Xu, Hu, Lu, Zhang, Zhai, Yang, Wu, Wang and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Tong, dG9uZ3FpYW5Aamx1LmVkdS5jbg==; Yan Wang, d2FuZ3lhbkBpbW0uYWMuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.