- 1College of Agriculture and Forestry, Linyi University, Linyi, Shandong, China
- 2Animal Zoo Department, Jinan Park Development Service Center, Jinan, Shandong, China
- 3Honghe Hani and Yi Autonmous Prefecture Agriculture and Rural Affairs Bureau, Animal Disease Control Center, Mengzi, Yunnan, China
This present study is the first case of a Porrocaecum angusticolle (P. angusticolle) infection reported in Griffon vulture (Gyps fulvus) in China. This study aimed to identify the nematode species and explore the genetic evolution of worms infecting Gyps fulvus (G.fulvus). Clinical examination revealed several milky white parasites in the stomach and intestinal tract. Polymerase chain reaction and partial 18S gene sequencing analyses identified these worms to be P. angusticolle (SD isolates). Further phylogenetic analyses revealed that they shared the highest genetic identity (99.9%) with a P. angusticolle isolate (EU004820.1) from Germany. Our study is the first report on the identification and characterization of P. angusticolle infecting G.fulvus in China, based on clinical findings and molecular diagnosis. Therefore, our study provides novel insights for the diagnosis of P. angusticolle infections and the prevention of nematode transmission in wild and domestic animals.
1 Introduction
Porrocaecum angusticolle (P. angusticolle) is among the pathogenic nematodes infecting various birds (Mozgovoi, 1953; Digiani and Sutton, 2001; Li et al., 2015) and occasionally mammals (Sprent, 1973; Jian, 1989). To date, approximately 40 Porrocaecum nematode species have been reported. Briefly, P. angusticolle is classified into Eukaryota, Metazoa, Nematoda, Choromadorea, Rhabditida, Ascarididae, and Porrocaecum. Previous studies have reported P. angusticolle infections in Europe, mainly in Italy (Santoro et al., 2010), Portugal (Tomás et al., 2017), the Czech Republic (Kijewska et al., 2002; Guo et al., 2021), Germany (Honisch and Krone, 2008), and Spain (Sanmartín et al., 2004; Santoro et al., 2012). Several birds species have been reported to be infected by P. angusticolle, including Buteo buteo (Kijewska et al., 2002; Santoro et al., 2010; Guo et al., 2021), Strigiformes, Aquila clanga, Aquila chrysaetos, Accipiter gentilis, Accipiter nisus, Aquila pomarine, Aquila rapax, Accipiter striatus, Buteo jamaicensis, Buteo lagopus, Buteo platypterus, Circus aeruginosus, Circus cyaneus, Circaetus gallicus, Elanius caeruleus, Haliaeetus albicilla, Haliastur indus, Milvus milvus, Milvus migrans, Pernis apivorus, Pandion haliaetus (Santoro et al., 2012), Sparrowhawk (Min et al., 2021), Circus aeruginosus (Kijewska et al., 2002), Accipiter gentilis, Accipiter nisus, Buteo lagopus, Falco subbuteo, Milvus migrans, Pandion haliaetus (Honisch and Krone, 2008), Tyto alba, and Strix aluco (Sanmartín et al., 2004). However, limited information exists on P. angusticolle infections in the literature.
Notably, a P. angusticolle infection is typically diagnosed based on clinical symptoms, such as pathological lesions, and molecular diagnosis. To the best of our knowledge, only 16 nucleotide sequences of the P. angusticolle genome have been submitted to the GenBank database, including those of the 18S, 28S, COX, and ITS genes. In the present study, we used primers targeting the 18S gene to perform sequencing of avian samples for the diagnosis of infection and identification of nematode worms. Interestingly, by combining the evaluation of clinical symptoms and molecular identification, we diagnosed, for the first time, a case of P. angusticolle infection of G.fulvus in China.
2 Methods
2.1 Case presentation
In January 2022, we were notified that a male G.fulvus older than 10 years of age had died. Following dissection, we found dozens of milky white parasites in its stomach and intestinal tract, with the size of worms ranging from 7 to 15 cm. We then collected these parasitic worms from the deceased vulture for further studies. We also examined the bird and observed clinical signs of the intestinal tract showing hemorrhagic spots and anabrosis.
2.2 Polymerase chain reaction analysis
We washed each parasite with double distilled water three times for 5 min each time. Subsequently, we added 20 μL of proteinase K (Vazymy, Nanjing, China) in each worm sample, vortexed for mixed the sample, and incubated it at 56°C (Constant temperature) for at least 3 h. We then subjected the digested parasite suspension to DNA extraction using the FastPure Cell/Tissue DNA Isolation Mini Kit (Vazymy). Each PCR amplification system included 12.5 µL Taq DNA Polymerase Mix (Transgen Co., Beijing, China), 10.5 µL ddH2O, forward and reverse primers (100 pmol/µL, 0.5 µL each), and 1 µL template DNA in a total volume of 25 µL. We also used double distilled water instead of template DNA as a blank control. We used 3 pairs of primers (NC1-NC3) designed in previous studies to amplify the small subunit DNA segment (18S), cytochrome oxidase I (COX1), and internal transcribed spacer (ITS) genes (Folmer et al., 1994; Gasser et al., 1999; Floyd et al., 2005). Three primer pairs followed as: 18S(NC1F), 5′-CGCGAATAGCTCATTACAACAGC-3′ and 18S(NC1R), 5′-GGGCGGTATCTGATC GCC-3′; COX1(NC2F), 5′-GTAGGTGAACCTGCGG AAGGATCATT-3′ and COX1(NC2R), 5′-TTAGTTTCTTTTCCTCCGCT-3′; ITS(NC3F), 5′-GGTCAACAAATCATAAAGATATTGG-3′ and ITS(NC3R), 5′-TAAACTTCAGGGTGAC CAAAAAATCA-3′. The PCR amplification conditions were as follows: pre-denaturation at 95°C for 5 min; 35 cycles of denaturation at 95°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 60 s; and a final extension at 72°C for 10 min.
2.3 Gene sequence and analysis
Following recovery and purification using the Agarose Gel DNA Extraction Kit, PCR products were sequenced by Biosune Biotechnology Co., Ltd. We analyzed the obtained sequencing results using the MEGA and DNAstar software and compared the identified sequences of 18S, COX1, and ITS genes of P. angusticolle with those of other nematodes. First, we used the GenBank and PubMed online websites to perform a comparative analysis of the sequences of highly homologous geographical parasite strains, especially their 18S sequences. In addition, using the DNAstar MegAlign Pro with the Clustral W algorithm, we analyzed the distance and divergence of these sequences. Subsequently, we used the Kimura 2-parameter model and maximum likelihood method in MEGA 6.0 software to draw the phylogenetic tree.
3 Results and discussion
Decreased immune function following parasite infection might be one of the potential risk factors leading to the death of G.fulvus birds (Figure 1) (Guivier et al., 2017; Lima and Lodoen, 2019). In the present study, we report the first case of an identified P. angusticolle infection of G.fulvus, confirmed by clinical findings and molecular diagnosis (Cabezón et al., 2011; Darwich et al., 2012; Chakarov and Blanco, 2021). Notably, we identified adult worms in the stomach and intestinal tract of the bird instead of nematode eggs (Figure 2). Previous studies have reported the occurrence of approximately 40 nematode species worldwide (Li and Scholz, 2019), including Porrocaecum semiteres (Syrota and Kharchenko, 2015), Porrocaecum ensicaudatum (Kijewska et al., 2002), Porrocaecum aridae, Porrocaecum crissum deslong, Porrocaecum praelongum, Porrocaecum reticulatum (Li et al., 2015), Contracaecum multipapillatum (Navone et al., 2000; Valles-Vega et al., 2017), Contracaecum micropapillatum, Contracaecum bancrofti, Contracaecum variegatum, Contracaecum eudyptulae, and Contracaecum ogmorhini (Shamsi et al., 2009). To date, the complete lifecycle of most Porrocaecum and Contracaecum species remains unclear. Only a few species have been reported to include intermediate hosts, such as earthworms (Moravec, 1971), insectivores (Portolés et al., 2004), and fish (Moravec, 2009). Under suitable environmental temperatures at the range of 22–32°C, eggs are hatched into active larvae, which enter the earthworm, developing into invasive larvae and cysts after 2 months. Following feeding of the terminal host on an infected worm-containing carcass, the invasive larvae in the worm develop into adults in the small intestine of the terminal host over 3 weeks. For example, in the case of C. multipapillatum, the first intermediate host is the cyclops, while the second intermediate host is fish (Moravec, 2009).
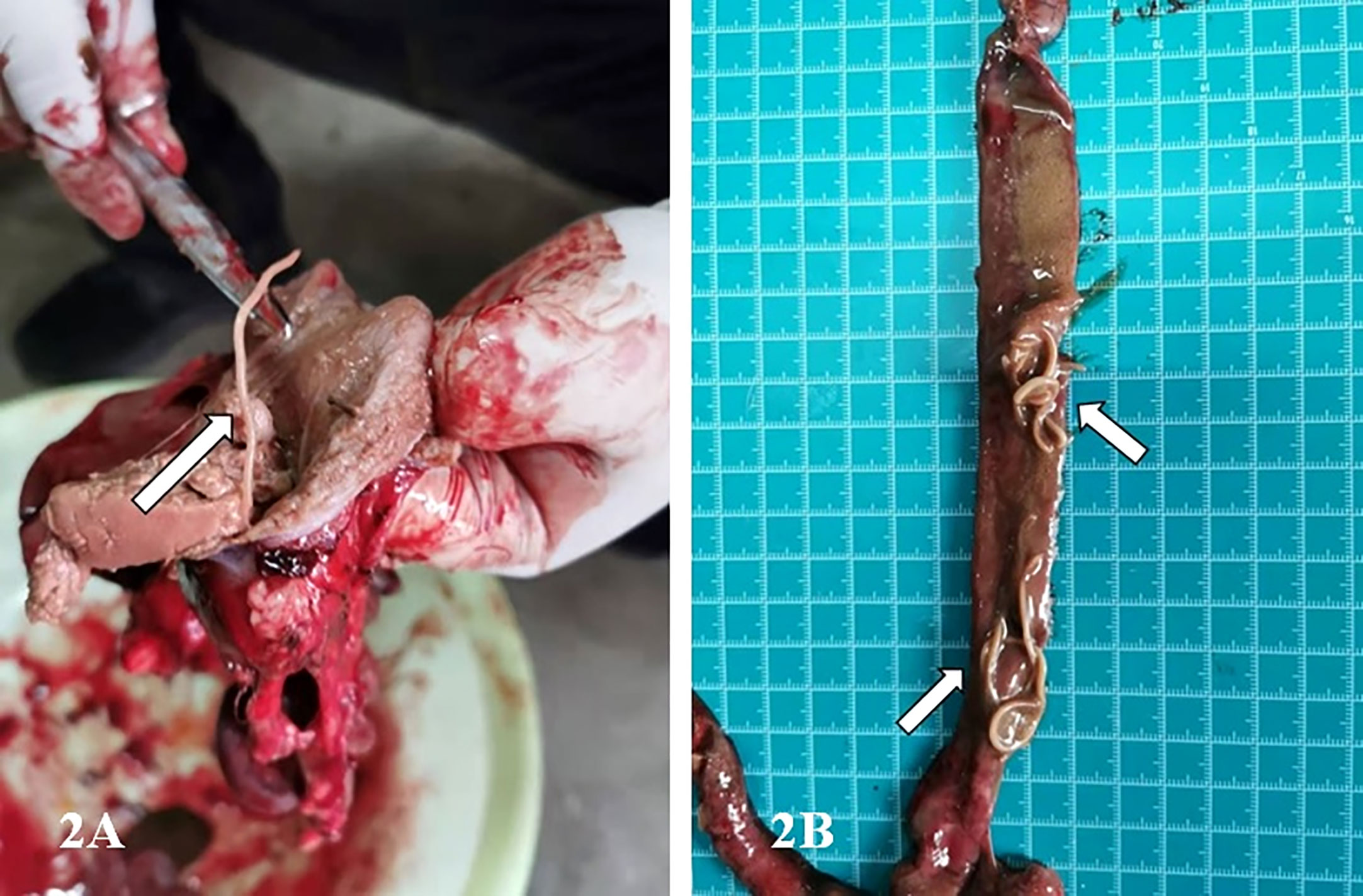
Figure 2 Images showing the infected stomach and intestinal tract of the G.fulvus bird. (A) One milky white parasite in the stomach of G.fulvus; (B) Dozens of milky white worms in the intestinal tract of G.fulvus.
Previous studies have mainly focused on the morphological and genetic characterization of cases of P. angusticolle infections in B. buteo, Strigiformes, A. clanga, A. chrysaetos, and other birds (Kijewska et al., 2002; Santoro et al., 2010; Santoro et al., 2012; Tomás et al., 2017; Guo et al., 2021). Therefore, information on the lifecycle of P. angusticolle is still lacking. In these previous studies, adult P. angusticolle worms were typically found in the superficial layer of the stomach and intestinal tract. Interestingly, both Porrocaecum larvae and adult worms can drill into the mucous membrane of the gastric wall of the glandular stomach to produce hemorrhagic spots, bruises, and erosive ulcers, which affect the growth and development of birds, and can lead to death in cases of severe infection (Mozgovoi, 1953; Guo et al., 2021).
In the present study, genetic analyses revealed that the partial sequence of the identified 18S rRNA fragment of P. angusticolle in our study (Figure 2) exhibited the highest phylogenetic identity (99.9%) with that of an isolate from Germany (Germany: EU004820.1). Subsequently, we analyzed the phylogenetic relations of our P. angusticolle strain to that of other Porrocaecum species, such as P. reticulatum (China HB: MF072700.1), P. depressum (USA: U94379.1), Porrocaecum sp. (Italy: MT141136.1), and P. streperae (USA: EF180074.1), and found that they ranged from 99.6 to 99.9% (Figure 3). The generated phylogenetic tree further confirmed the evolutionary relationship between Porrocaecum and other nematode species (Figure 3). In conclusion, in our study, we performed 18S gene analysis to identify and characterize a P. angusticolle infection in G.fulvus in China. We also examined the COX and ITS genes; however, limited information on the phylogenetic relationships of these genes is available in PubMed and GenBank.
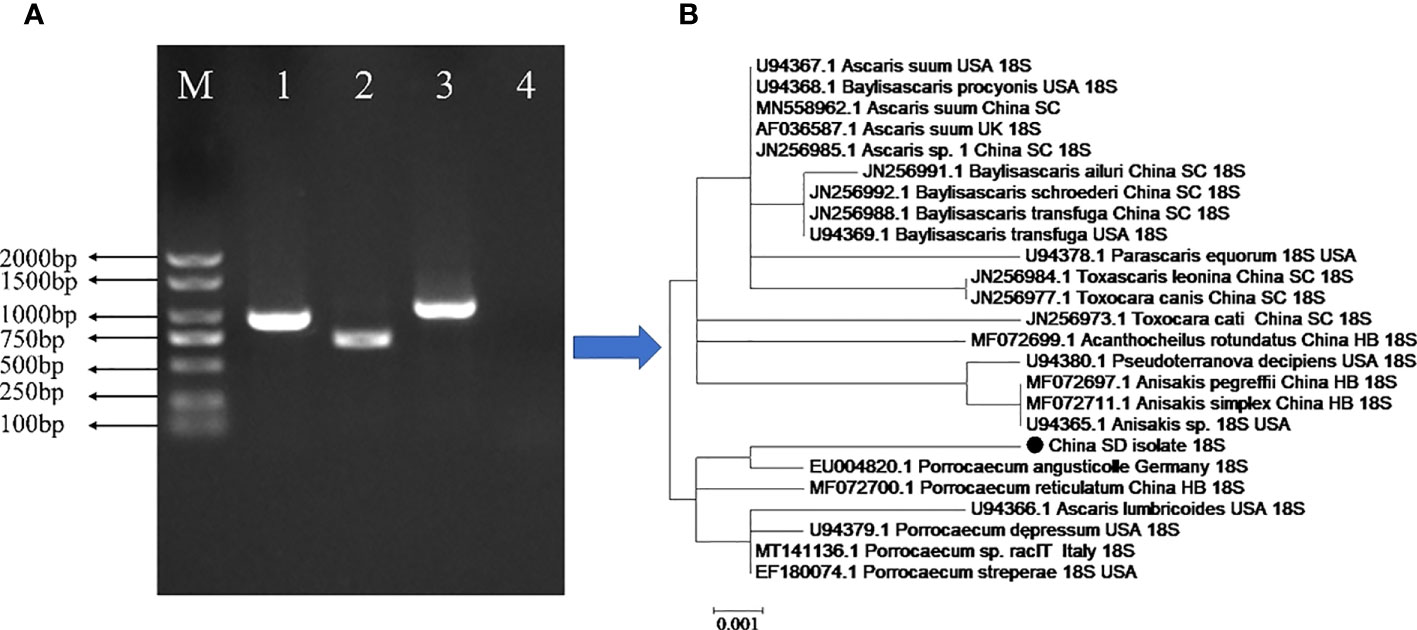
Figure 3 Genetic characterization of the parasite infecting the stomach and intestinal tract of the G.fulvus bird. (A) PCR amplification of the 18S, COX1, and ITS genes of P. angusticolle. M: Marker; Lane 1:18S gene; Lane 2: COX1 gene; Lane3: ITS gene; Lane 4: Blank control. (B) Phylogenetic tree construction of the P. angusticolle 18S gene with high homology of 24 geographical nematode isolates.
The G.fulvus is a bird species belonging to the Accipitridae family of vultures. It inhabits a wide range of habitats, reaching up to 2500 m above sea level, such as rocky alpine and plateau areas, grasslands, and scrub and semi-desert areas (Davidović et al., 2020; Pirastru et al., 2021). Owing to its excellent night vision, G.fulvus forages for dead animals during the night while feeding on wild animals such as goats, deer, and gazelles in the daytime, relying on its sensitive smell to locate decaying animal carcasses (Marin et al., 2014; Sevilla et al., 2020). It is widely distributed throughout Europe, the Middle East, and North Africa, as well as in India and the Himalayas. However, it is most common in countries bordering the Mediterranean Sea, with the largest population number detected in Spain, accounting for three-quarters of the European population (Davidović et al., 2022). To the best of our knowledge, this study is the first report on the identification and genetic characterization of P. angusticolle infection in G.fulvus in China.
Although this study is the first report of P. angusticolle infection in G.fulvus, it has some limitations. First, although we combined PCR methods, gene sequencing, and clinical factors to diagnose the P. angusticolle infection, there are no completely set diagnostic criteria for P. angusticolle. In addition, we did not record enough clinical pictures and symptoms to support our diagnosis, and few studies have reported P. angusticolle infection in birds.
Nevertheless, our study extends the current geographical distribution and host species of P. angusticolle, confirming the spread and genetic evolution of this nematode in Asia and highlighting the importance of the molecular diagnosis of P. angusticolle infections in domestic and wild animals.
Data availability statement
The Genebank accession ID: OQ216840,which can be found below: https://www.ncbi.nlm.nih.gov/genbank/.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this Brief Research Report.
Author contributions
GL designed the study and drafted the manuscript. QL, WZ and XS collected the animal specimens and supported the experiment. All persons who have made substantial contributions to the work are reported in the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (No.31502057) and Linyi University High-level Talent Funding Support (No. Z6122016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cabezón, O., García-Bocanegra, I., Molina-López, R., Marco, I., Blanco, J. M., Höfle, U., et al. (2011). Seropositivity and risk factors associated with Toxoplasma gondii infection in wild birds from Spain. PloS One 6, e29549. doi: 10.1371/journal.pone.0029549
Chakarov, N., Blanco, G. (2021). Blood parasites in sympatric vultures: role of nesting habits and effects on body condition. Int. J. Environ. Res. Public Health 18, 2431. doi: 10.3390/ijerph18052431
Darwich, L., Cabezón, O., Echeverria, I., Pabón, M., Marco, I., Molina-López, R., et al. (2012). Presence of Toxoplasma gondii and Neospora caninum DNA in the brain of wild birds. Vet. Parasitol. 183, 377–381. doi: 10.1016/j.vetpar.2011.07.024
Davidović, S., Jelić, M., Marinković, S., Mihajlović, M., Tanasić, V., Hribšek, I., et al. (2020). Genetic diversity of the Griffon vulture population in Serbia and its importance for conservation efforts in the balkans. Sci. Rep. 10, 20394. doi: 10.1038/s41598-020-77342-1
Davidović, S., Marinković, S., Kukobat, M., Mihajlović, M., Tanasić, V., Hribšek, I., et al. (2022). Genetic diversity analysis of mitochondrial cyt b gene, phylogeny and phylogeography of protected. Life (Basel). 12, 164. doi: 10.3390/life12020164
Digiani, M. C., Sutton, C. A. (2001). New reports and a redescription of Porrocaecum heteropterum (Diesing 1851) (Ascarididae), a rare nematode parasitic in south American threskiornithid birds. Syst. Parasitol. 49, 1–6. doi: 10.1023/a:1010730611828
Floyd, R. M., Rogers, A. D., Lambshead, P. J. D., Smith, C. R. (2005). Nematode-specific PCR primers for the 18S small subunit r RNA gene. Mol. Ecol. Notes. 5, 611–612. doi: 10.1111/j.1471-8286.2005.01009.x
Folmer, O., Black, M., Hoeh, W., Lutz, R., Vrijenhoek, R. (1994). DNA Primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Gasser, R. B., Rossi, L., Zhu, X. (1999). Identification of nematodirus species (Nematoda: molineidae) from wild ruminants in Italy using ribosomal DNA markers. Int. J. Parasitol. 29, 1809–1817. doi: 10.1016/s0020-7519(99)00123-x
Guivier, E., Lippens, C., Faivre, B., Sorci, G. (2017). Plastic and micro-evolutionary responses of a nematode to the host immune environment. Exp. Parasitol. 181, 14–22. doi: 10.1016/j.exppara.2017.07.002
Guo, N., Sitko, J., Chen, H. X., Li, L. (2021). Morphological and genetic characterization of Porrocaecum angusticolle (Molin 1860) (Nematoda: ascaridomorpha) from the common buzzard buteo buteo (Linnaeus) (Accipitriformes: accipitridae) in Czech republic. Parasitol. Int. 83, 102365. doi: 10.1016/j.parint.2021.102365
Honisch, M., Krone, O. (2008). Phylogenetic relationships of spiruromorpha from birds of prey based on 18S rDNA. J. Helminthol. 82, 129–133. doi: 10.1017/S0022149X08912359
Jian, S. C. (1989). Description of a new species of porrocaecum from dog-badger (Ascaridata: anisakidae), Sichuan. J. Zool. (Chinese Journal) 8, 4–6.
Kijewska, A., Rokicki, J., Sitko, J., Wegrzyn, G. (2002). Ascaridoidea: a simple DNA assay for identification of 11 species infecting marine and freshwater fish, mammals, and fish-eating birds. Exp. Parasitol. 101, 35–39. doi: 10.1016/s0014-4894(02)00031-0
Li, L., Guo, Y. N., Zhang, L. P. (2015). Porrocaecum parvum n. sp. and P. reticulatum (Linstow 1899) (Nematoda: ascaridoidea) from birds in China. Syst. Parasitol. 92, 141–149. doi: 10.1007/s11230-015-9593-9
Li, L., Scholz, T. (2019). Redescription of Porrocaecum semiteres (Zeder 1800) (Nematoda: ascaridida) from the song thrush turdus philomelos (Passeriformes: turdidae). Acta Parasitol. 64, 1–6. doi: 10.2478/s11686-018-00001-z
Lima, T. S., Lodoen, M. B. (2019). Mechanisms of human innate immune evasion by Toxoplasma gondii. front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00103
Marin, C., Palomeque, M. D., Marco-Jiménez, F., Vega, S. (2014). Wild Griffon vultures (Gyps fulvus) as a source of salmonella and campylobacter in Eastern Spain. PloS One 9, e94191. doi: 10.1371/journal.pone.0094191
Min, X., Gao, Z., Lin, Y., Lu, C. H. (2021). Annual long-distance migration strategies and home range of Chinese sparrowhawk (Accipiter soloensis) from south China. Animals. (Basel). 11, 2237. doi: 10.3390/ani11082237
Moravec, F. (1971). A new natural intermediate host of the nematode Porrocaecum semiteres (Zeder 1800). Folia Parasitol. (Praha). 18, 26.
Moravec, F. (2009). Experimental studies on the development of contracaecum rudolphii (Nematoda: anisakidae) in copepod and fish paratenic hosts. Folia Parasitol. (Praha). 56, 185–193. doi: 10.14411/fp.2009.023
Mozgovoi, A. A. (1953). “Ascaridata of animals and man and the diseases caused by them,” in Osnovy nematologii, vol. 2 . Ed. Skrjabin, K. I. (Moscow: Izdatel’stvo Akademii Nauk SSSR), 617.
Navone, G. T., Etchegoin, J. A., Cremonte, F. (2000). Contracaecum multipapillatum (Nematoda: anisakidae) from egretta alba (Aves: ardeidae) and comments on other species of this genus in Argentina. J. Parasitol. 86, 807–810. doi: 10.1645/0022-3395(2000)086[0807:CMNAFE]2.0.CO;2
Pirastru, M., Mereu, P., Manca, L., Bebbere, D., Naitana, S., Leoni, G. G. (2021). Anthropogenic drivers leading to population decline and genetic preservation of the Eurasian griffon vulture (Gyps fulvus). Life (Basel). 11, 1038. doi: 10.3390/life11101038
Portolés, E., Granel, P., Esteban, J. G., Cabaret, J. (2004). Helminth associations in white-toothed shrews Crocidura russula (Insectivora: soricidae) from the albufera natural park, Spain. J. Parasitol. 90, 572–578. doi: 10.1645/GE-3211
Sanmartín, M. L., Alvarez, F., Barreiro, G., Leiro, J. (2004). Helminth fauna of falconiform and strigiform birds of prey in Galicia, Northwest Spain. Parasitol. Res. 92, 255–263. doi: 10.1007/s00436-003-1042-z
Santoro, M., Kinsella, J. M., Galiero, G., degli Uberti, B., Aznar, F. J. (2012). Helminth community structure in birds of prey (Accipitriformes and falconiformes) in southern Italy. J. Parasitol. 98, 22–29. doi: 10.1645/GE-2924.1
Santoro, M., Tripepi, M., Kinsella, J. M., Panebianco, A., Mattiucci, S. (2010). Helminth infestation in birds of prey (Accipitriformes and falconiformes) in southern Italy. Vet. J. 186, 119–122. doi: 10.1016/j.tvjl.2009.07.001
Sevilla, E., Marín, C., Delgado-Blas, J. F., González-Zorn, B., Vega, S., Kuijper, E., et al. (2020). Wild griffon vultures (Gyps fulvus) fed at supplementary feeding stations: potential carriers of pig pathogens and pig-derived antimicrobial resistance? Transbound Emerg. Dis. 67, 1295–1305. doi: 10.1111/tbed.13470
Shamsi, S., Norman, R., Gasser, R., Beveridge, I. (2009). Redescription and genetic characterization of selected contracaecum spp. (Nematoda: anisakidae) from various hosts in Australia. Parasitol. Res. 104, 1507–1525. doi: 10.1007/s00436-009-1357-5
Sprent, J. F. (1973). The ascaridoid nematodes of rodents with a redescription of Porrocaecum ratti. Parasitology 66, 367–380. doi: 10.1017/s0031182000045959
Syrota, Y. Y., Kharchenko, V. O. (2015). Analysis of study comprehensiveness for nematode fauna of hydrophilic birds in Ukrainian polissya. Ann. Parasitol. 61, 165–174. doi: 10.17420/ap6103.03
Tomás, A., Rebelo, M. T., Fonseca, I. P. (2017). Occurrence of helminth parasites in the gastrointestinal tract of wild birds from wildlife rehabilitation and investigation centre of ria Formosa in southern Portugal. Vet. Parasitol. Reg. Stud. Rep. 8, 13–20. doi: 10.1016/j.vprsr.2016.12.008
Valles-Vega, I., Molina-Fernández, D., Benítez, R., Hernández-Trujillo, S., Adroher, F. J. (2017). Early development and life cycle of contracaecum multipapillatum s.l. from a brown pelican Pelecanus occidentalis in the gulf of California, Mexico. Dis. Aquat. Organ. 125, 167–178. doi: 10.3354/dao03147
Keywords: Porrocaecum angusticolle (P. angusticolle), Griffon vulture (Gyps fulvus), diagnose, PCR, China
Citation: Liu G, Liu Q, Zhang W and Shen X (2023) First reported Porrocaecum angusticolle infection in Griffon vulture (Gyps fulvus) in China. Front. Cell. Infect. Microbiol. 13:1181999. doi: 10.3389/fcimb.2023.1181999
Received: 08 March 2023; Accepted: 12 June 2023;
Published: 11 July 2023.
Edited by:
Shoaib Ashraf, Harvard Medical School, United StatesReviewed by:
Panat Anuracpreeda, Mahidol University, ThailandBenjamin Cull, University of Minnesota Twin Cities, United States
Copyright © 2023 Liu, Liu, Zhang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gongzhen Liu, Z29uZ3poZW5saXVAMTI2LmNvbQ==; Qing Liu, bGl1cWluZzA2MzFAMTYzLmNvbQ==
 Gongzhen Liu
Gongzhen Liu Qing Liu2*
Qing Liu2*