
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 25 May 2023
Sec. Antibiotic Resistance and New Antimicrobial drugs
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1181630
 Guojie Shen1†
Guojie Shen1† Li Zhang2†
Li Zhang2† Weina Fan1
Weina Fan1 Haifeng Lv1
Haifeng Lv1 Feifei Wang1
Feifei Wang1 Qingqing Ye1
Qingqing Ye1 Miaozuo Lin3
Miaozuo Lin3 Xia Yu4
Xia Yu4 Hongliu Cai1
Hongliu Cai1 Xiaoliang Wu1*
Xiaoliang Wu1*Background: Multidrug resistance in bacteria is a serious problem in organ transplantations. This study aimed to identify risk factors and establish a predictive model for screening deceased organ donors for multidrug-resistant (MDR) bacteria.
Methods: A retrospective cohort study was conducted at the First Affiliated Hospital of Zhejiang University School of Medicine from July 1, 2019 to December 31, 2022. The univariate and multivariate logistic regression analysis was used to determine independent risk factors associated with MDR bacteria in organ donors. A nomogram was established based on these risk factors. A calibration plot, receiver operating characteristic (ROC) curve, and decision curve analysis (DCA) were used to estimated the model.
Results: In 164 organ donors, the incidence of MDR bacteria in culture was 29.9%. The duration of antibiotic use ≥3 days (odds ratio [OR] 3.78, 95% confidence interval [CI] 1.62–8.81, p=0.002), length of intensive care unit (ICU) stay per day(OR 1.06, 95% CI 1.02–1.11, p=0.005) and neurosurgery (OR 3.31, 95% CI 1.44–7.58, p=0.005) were significant independent predictive factors for MDR bacteria. The nomogram constructed using these three predictors displayed good predictive ability, with an area under the ROC curve value of 0.79. The calibration curve showed a high consistency between the probabilities and observed values. DCA also revealed the potential clinical usefulness of this nomogram.
Conclusions: The duration of antibiotic use ≥3 days, length of ICU stay and neurosurgery are independent risk factors for MDR bacteria in organ donors. The nomogram can be used to monitor MDR bacteria acquisition risk in organ donors.
Organ transplantation is currently considered the best therapeutic option for patients with end-stage organ failure (Pilmis et al., 2023). However, owing to the growing shortage of organs, there is a stark discrepancy between the number of organs available for transplantation and the number of recipients on the waiting list (Lewis et al., 2021). As many patients die each year while waiting to donate organs, nearly 20% of donors are used for organ transplantation to increase the availability of organs for transplantation, even if they are affected by infectious diseases (Len et al., 2008; Nanni Costa et al., 2008).
With the rapid advancement of organ transplantation, donor-derived infections (DDI) have posed a major challenge to the transplant community while saving the lives of many patients with organ failure. A DDI is defined as any infection in the donor that is transmitted to one or more recipients (Sifri and Ison, 2012; Wolfe et al., 2019). It is an important cause of morbidity in solid organ transplant recipients, including vascular anastomotic dehiscence, infection, and death (Nelson et al., 1984; Anesi et al., 2020). Multidrug-resistant (MDR) bacterial infection is a major public health problem of global magnitude and alarming scale and is also a tougher problem in DDI (Rossolini and Mantengoli, 2008; Lewis and Sifri, 2016). When MDR bacterial DDI occur, they can have serious and devastating results, with mortality rates as high as 33-41% (Ison et al., 2013; Lewis and Sifri, 2016).
There is still much controversy as to whether organs from potential donors colonized or infected with MDR organisms should be accepted (Wolfe et al., 2019). The goal of organ transplantation is to minimize the incidence of DDI while maximizing opportunities for transplantation (White et al., 2019). However, screening potential donors for MDR bacterial infections is not always straightforward and generally requires risk stratification, which relies on the potential organ donor’s social and medical disease history, and needs to be confirmed by culture and drug sensitivity results (Nanni Costa et al., 2008; Fishman and Grossi, 2014). The growth and culture of microorganisms take a long time, leading to an underestimation of the incidence of infection and delay in treatment (Fishman et al., 2012). Therefore, it is critical to identify donors with a higher risk of MDR bacterial colonization or infection before transplantation to facilitate targeted monitoring and treatment of recipients. There are few reliable systems for early assessment of MDR bacterial risk in deceased organ donors to date.
This study aimed to identify the risk factors and establish a predictive model suitable for doctors to screen organ donors with MDR bacteria early and thus select appropriate antimicrobial drugs for recipients to reduce the impact of these bacteria.
A retrospective cohort study was performed to investigate adult deceased organ donors (≥18 years of age) who donated at least one solid organ at the First Affiliated Hospital of Zhejiang University School of Medicine from July 1, 2019 to December 31, 2022. Eligible donors were identified by the organ procurement organization (OPO). Specimens were cultured during the donor’s terminal hospitalization using blood, urine, perfusate (OPO cultures), rectal swabs, and respiratory secretions. In vitro antimicrobial susceptibility testing was performed using the Kirby Bauer paper diffusion method, and the minimum inhibitory concentration (MIC) was determined using the agar dilution method (Jiang et al., 2022). Interpretation of the antimicrobial susceptibility test results followed the recommendations of the Clinical and Laboratory Standards Institute (Humphries et al., 2021). This study was approved by the institutional ethics review board of the First Affiliated Hospital of Zhejiang University.
The preoperative clinicopathological characteristics of all the participants were extracted from the hospital’s medical records. The following items were investigated as possible risk factors: age, gender, hypertension, diabetes mellitus, chronic kidney disease, length of stay in intensive care unit (ICU), death mechanism, procedures during terminal hospitalization, use of proton pump inhibitors (PPIs), enteral feeding, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, dialysis, duration of antibiotic use per donor, and used more than one antibiotic. The events and periods considered in the analysis were recorded before collecting positive biological samples.
We included the following bacteria in our analysis: carbapenem-resistant Enterobacteriaceae (CRE), methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum β-lactamase producing Enterobacteriaceae (ESBL-PE). All other bacteria (such as Pseudomonas species, Acinetobacter species, Enterococcus faecium) were considered MDR bacteria if they showed resistant to at least one agent in three or more antimicrobial categories (Magiorakos et al., 2012; Jiang et al., 2020). The primary outcome of this study was the culture of MDR bacteria during terminal hospitalization or at the time of organ transplantation (OPO culture). Bacterial cultures from any anatomical site or organ procured were also considered. The distinction between infection and colonization by MDR bacteria has not yet been determined. If MDR bacteria were isolated from the same patient on multiple occasions, only the first episode was considered.
Statistical analyses were performed using SPSS (version 22.0; IBM SPSS Statistics, IBM Corporation, Armonk, NY, United States) and R software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were presented as mean ± standard deviation or median and interquartile range (INQ), and Student’s t-test or Wilcoxon test was used for comparisons between groups. Categorical variables are expressed as counts and percentages, and the chi-square test or Fisher’s exact test was used for comparison between groups. Statistical significance was set at a two-tailed p-value of <0.05.
Univariate and multivariate logistic regression analyses were performed to identify the risk factors. Variables with a p-value of <0.1 from univariate analysis was included in a multivariate logistic regression. Effect measures were calculated using odds ratios (OR) and respective 95% confidence intervals (CI). All the selected features were statistically significant and were applied to construct the nomogram prediction model. The “rms” package used to develop the nomogram diagram also uses the R language.
Furthermore, the nomogram performance was assessed using discrimination and calibration. The “pROC” package was used for the ROC curve operation. The area under the curve (AUC) was used to determine the quality of the nomogram. The “rms” package was used to draw and calculate the calibration curves, which were performed by a visual calibration plot comparing the predicted and actual probability of MDR bacteria. The “nricens” package was used for the decision curve analysis (DCA), which is used to determine the clinical practicability of a nomogram based on the net benefit under different threshold probabilities in the deceased organ donors.
In our study, 181 cases with at least one solid organ donated were admitted to the First Affiliated Hospital of Zhejiang University School of Medicine from July 1, 2019 to December 31, 2022. Nine patients with incomplete medical data and eight patients younger than 18 years of age were excluded. A flow diagram of the study design is shown in Figure 1. 138 (84.1%) were male and 26 (15.9%) were female, with a median age of 46 years (INQ 33-54). Common comorbidities included hypertension (n = 43, 26.2%), diabetes mellitus (n = 10, 6.1%), and chronic kidney disease (n = 4, 2.4%). The median length of stay in ICU was 8 days (INQ 5-13). The main death mechanisms were intracranial hemorrhage (71, 43.3%), traumatic brain injury (70, 42.7%), and cerebral infarction (8, 4.9%). Others (15, 9.1%) included asphyxiation (5, 3.0%), drowning (3, 1.8%), drug intoxication (3, 1.8%), pulmonary embolism (2, 1.2%), hypoglycemia (1, 0.6%), and myocardial infarction (1, 0.6%). 49 (29.9%) cases isolated MDR bacteria during terminal donor hospitalization or at the time of organ transplantation. The additional basic patient characteristics are presented in Table 1.
Table 2 lists the classification and percentage of 49 (29.9%) cases that isolated MDR bacteria. Eight MDR bacteria species were cultured in 164 cases. From these cases, gram-negative bacteria were isolated in 42 (25.6%) patients, with a predominance of Acinetobacter baumannii, Klebsiella pneumonia, and Pseudomonas aeruginosa, and gram-positive bacteria from 7 (4.3%) patients were cultured, with Staphylococcus aureus, and Enterococcus faecium as the main bacteria. The predominant sites of infection in first-episode MDR bacteria are respiratory secretions and blood cultures.
Univariate and multivariate logistic analyses were used to identify the potential risk factors. Multivariable analyses demonstrated that the occurrence of MDR bacteria was significantly correlated with The duration of antibiotic use ≥3 days (odds ratio [OR] 3.78, 95% confidence interval [CI] 1.62–8.81, p=0.002), length of ICU stay,days(OR 1.06, 95% CI 1.02–1.11, p=0.005) and neurosurgery (OR 3.31, 95% CI 1.44–7.58, p=0.005). The detailed results of univariate and multivariate analyses are presented in Table 3. Based on regression analysis, a nomogram was constructed to quantitatively predict the risk probability of MDR bacteria in patients with deceased organ donors. Each value of these variables is assigned a score on the point-scale axis. The total score can be easily calculated by summing the individual scores. Figure 2 gave an example to show how the nomogram could be used as a prognostic stratification tool to estimate the probability of MDR bacteria in organ donors.
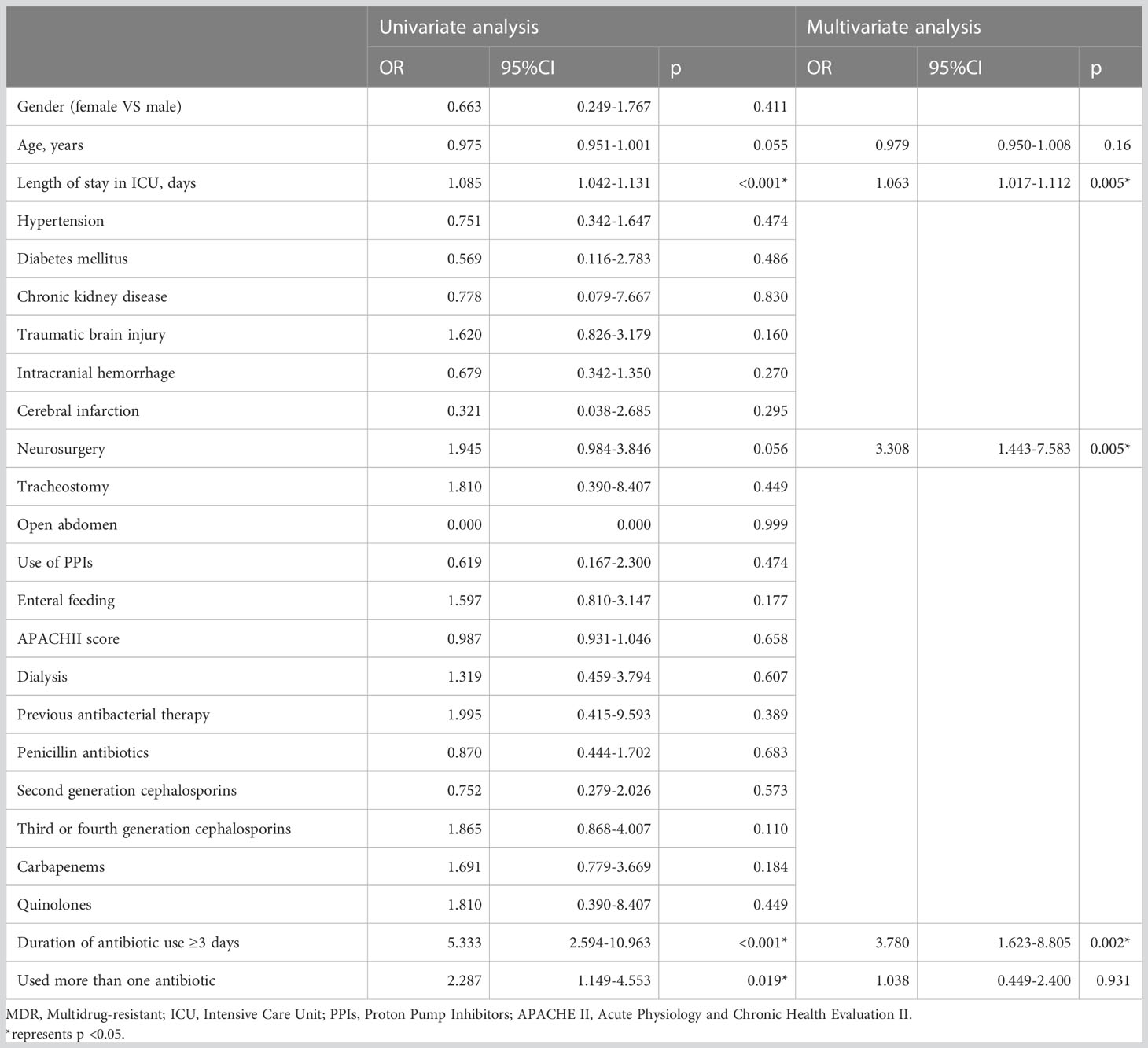
Table 3 Univariate and multivariate logistic regression analysis of the predictors for MDR bacteria in deceased organ donors.
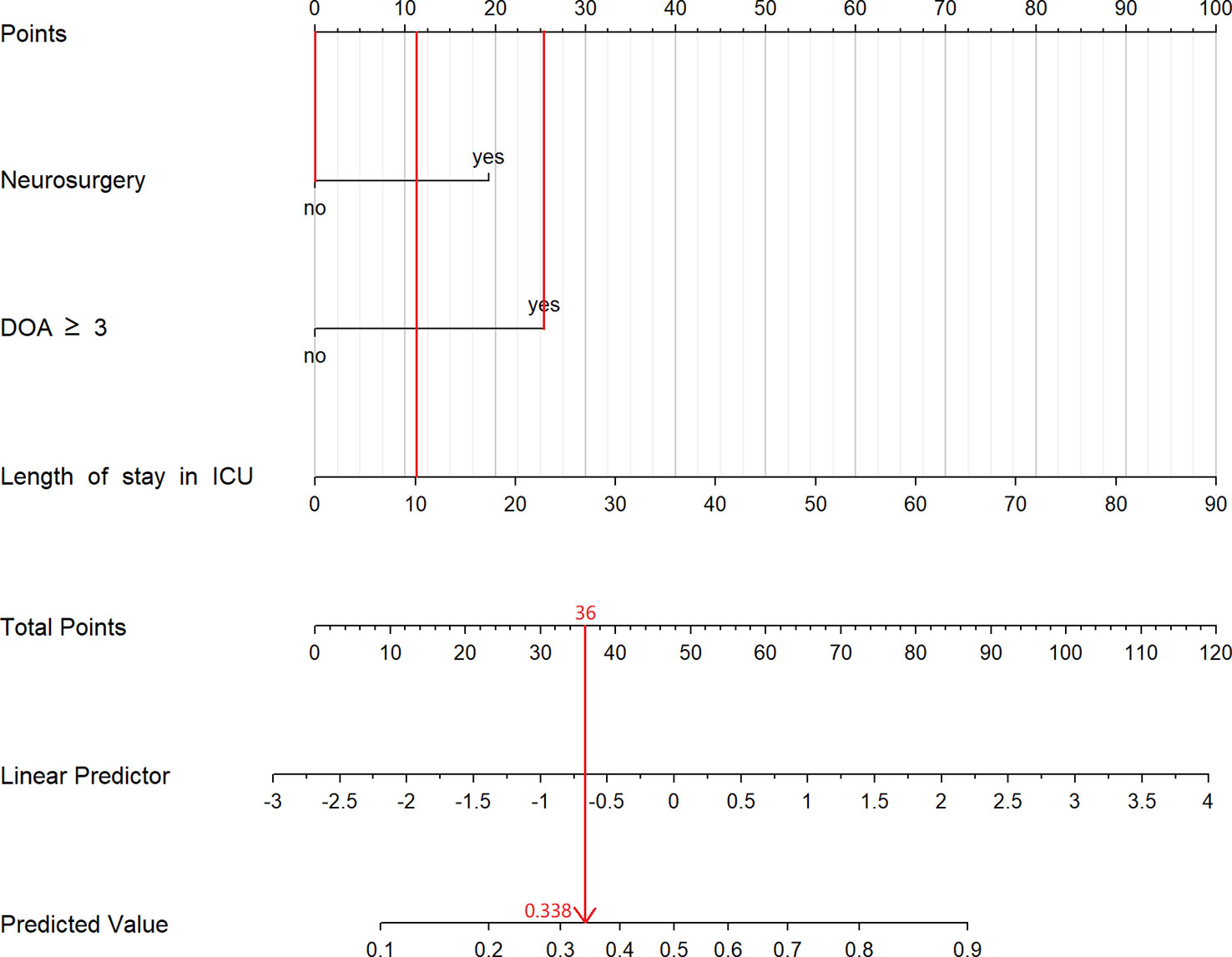
Figure 2 The constructed nomogram for predicting risk of MDR bacteria in organ donors. This patient stayed in ICU for 10 days, used antibiotic more than 3 days and had not experienced neurosurgery. According to the nomogram, we can calculate that the total point for this patient is 36 and its corresponding risk is 33.8%. DOA, duration of antibiotic; ICU, Intensive Care Unit.
A ROC curve was used to evaluate the discriminatory capacity of the predictive model. For the predictive model, the pooled AUC of the nomogram was 0.79 (95%CI: 0.71-0.87), indicating a moderately good performance (Figures 3A, B). A calibration plot was developed to determine the predictive capacity of the nomograms using 500 bootstrap re-samples. Figure 4 illustrates that the predictive model and validation set closely adhere to the reference line. DCA demonstrated that this model could increase the net benefits and show a wide range of threshold probabilities (Figure 5).
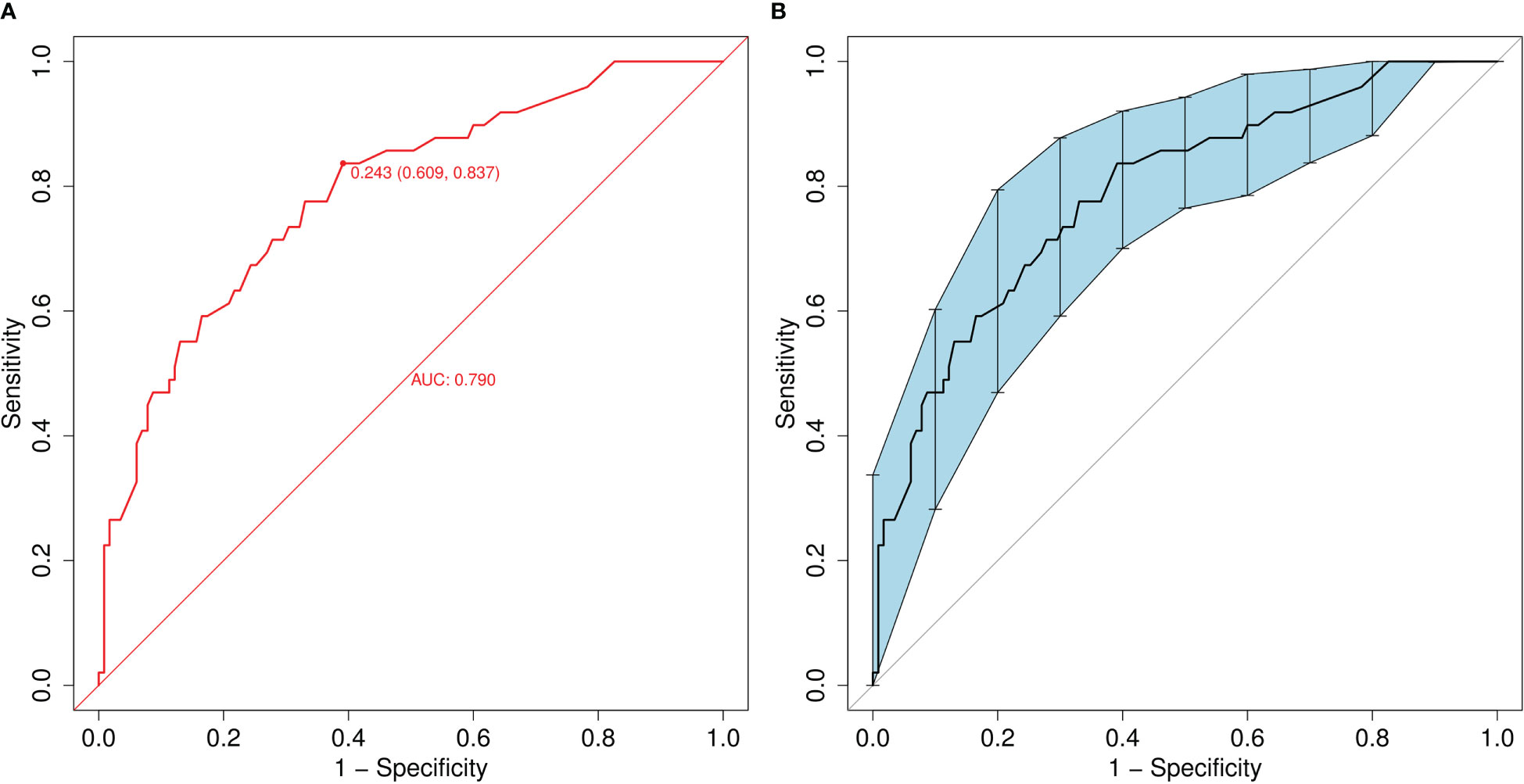
Figure 3 (A) Receiver operating characteristic (ROC) curve of the nomogram model for predicting the risk of MDR bacteria. The y-axis represents the true positive rate of the risk prediction, and the x-axis represents the false positive rate of the risk prediction. (B) ROC of the predictive MDR bacteria risk nomogram using 200 bootstrap re-samples.
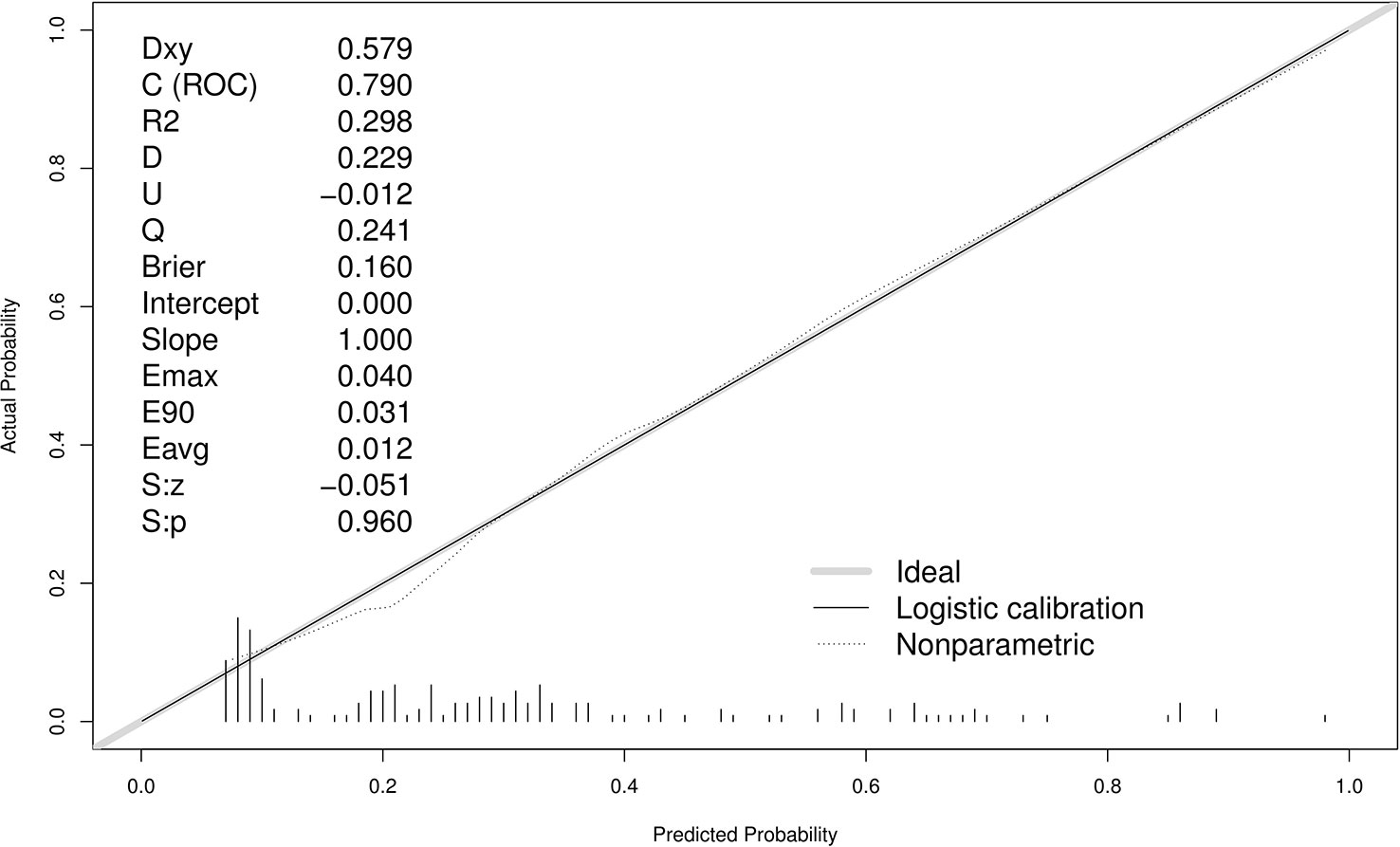
Figure 4 Calibration curves of the predictive MDR bacteria risk nomogram using 500 bootstrap re-samples. The y-axis represents actual diagnosed cases of MDR bacteria, and the x-axis represents the predicted risk of MDR bacteria. The diagonal dotted line represents a perfect prediction by an ideal model, while the solid line represents the performance of the data.
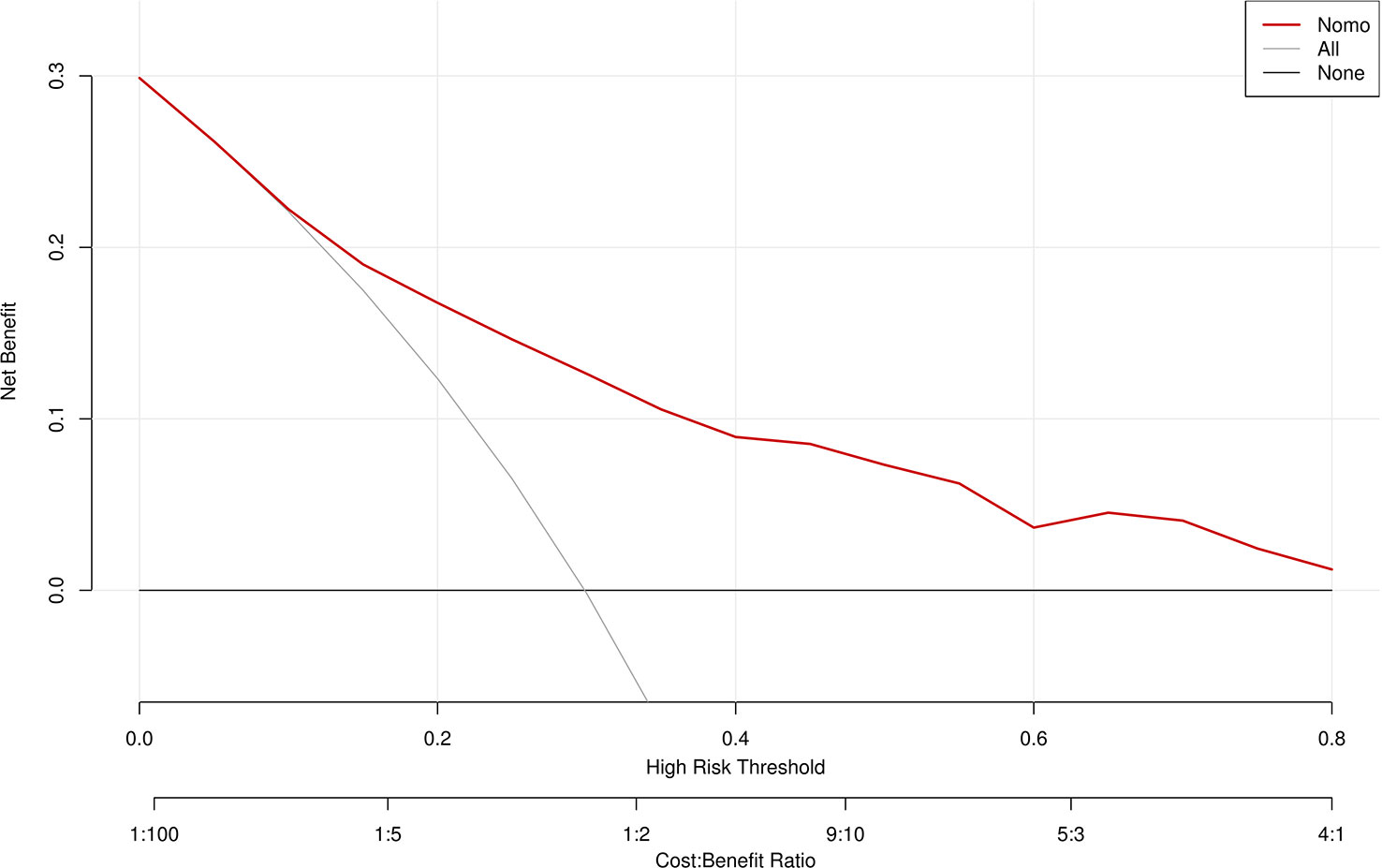
Figure 5 Decision curve analysis for the MDR bacteria risk nomogram. The y-axis measures the net benefit. The thick solid line represents the assumption that all patients have no MDR bacteria, the thin solid line represents the assumption that all patients have MDR bacteria, and the dotted line represents the risk nomogram.
This retrospective cohort study found that the duration of antibiotic use ≥3 days, length of ICU stay and neurosurgery were risk factors for MDR bacterial infection or colonization in deceased organ donors. Based on previous studies, we collected as many predictors as possible associated with MDR bacteria. Deceased organ donors were almost always admitted to the ICU, experienced mechanical ventilation, indwelling central venous catheters, nasogastric tubes, urinary catheters; other possible risk factors, such as enteral nutrition, use of PPIs and antibiotics, surgery, age, gender, etc., were considered (Augustin et al., 2010; Vasudevan et al., 2014; Yamamoto et al., 2017). In this process, various confounding factors must be considered as variables. We determined that multivariate logistic regression analysis provides a good solution to this problem. A nomogram incorporating these three prognostic factors to predict the incidence of MDR bacteria was established and evaluated using ROC curve, calibration, and DCA, providing good predictive accuracy and discriminative ability.
Deceased organ donors may be accompanied by hemodynamic instability and a higher risk of infection, thus extending the duration of antibiotic administration. This has led to the overuse of antibiotics, which may be critical to the development of bacterial resistance (Christaki et al., 2020). In univariate and multivariate logistic analyses, we found a consistent association between the duration of antibiotic use ≥3 days and the emergence of MDR bacteria. This is consistent with previous findings that reducing the duration of antibiotic use leads to a lower incidence of MDR bacteria (Tacconelli et al., 2014; van Langeveld et al., 2017). A randomized trail conducted by Singh et al. revealed no difference in mortality or ICU length of stay for 3 days of empirical antibiotic therapy compared to those who received longer antibiotic therapy (Singh et al., 2000).Shorter course of antibiotic therapy leads to fewer subsequent superinfections attributed to antibiotic-resistant pathogens (Chastre et al., 2006).Thus, continuous monitoring of the duration of antibiotic use may provide a key warning indicator for predicting the increased incidence of drug-resistant bacteria.
In addition, our study found that potential donors had a terminal hospital stay of 8 days (INQ 5–13), which was also longer than that in other studies (Ye et al., 2017; Anesi et al., 2020). Previous studies have shown an association between a prolonged ICU stay and the occurrence of MDR bacteria (Chavada et al., 2018). Our study obtained similar result from the univariate and multivariate analysis. Therefore, minimizing the inappropriate use of antibiotics and shortening the hospitalization time of potential organ donors are important to reduce the incidence of MDR bacteria.
In our study, recent neurosurgery was also a risk factor for the development of MDR bacteria, and a few studies have included data on antimicrobial resistance in the neurocritical care population (Abulhasan et al., 2020). Surgery, an invasive procedure, may lead to an increased risk of bacterial infection; other studies have reported similar results (Mariappan et al., 2017; Lin et al., 2022). Agarwal et al. studied 330 neurosurgical patients with healthcare-associated infections and reported an overall incidence of infection of 6.67%; however, all isolates (100%) were MDR (Agarwal et al., 2017). This may be related to changes in sensory organ function in potential donors and the long-term use of medical devices.
The advantage of our study is that we identified the risk factors and established the first risk prediction model for the onset of MDR bacteria in deceased organ donors in China. The nomogram we constructed enabled healthcare professionals to easily calculate the MDR bacterial acquisition risk for potential donors, allowing them to assess changes in their risk frequently. Therefore, it will facilitate the early detection of patients with high-risk MDR bacterial colonization or infection so recipients can develop individualized strategies in the perioperative period to contain the emergence and spread of MDR strains and reduce their impact.
Our study has several limitations. First, This was a retrospective study and the sample size of this study was limited; the risk prediction model has not been validated with external data, and its generalization performance is unclear. Second, other risk factors have not been identified. Deceased donors are necessarily admitted to the ICU with indwelling tracheal intubation, urinary catheters, nasogastric tubes, central venous catheters, and mechanical ventilation, making none of these factors available as covariates included in the analysis. Finally, these data were collected from a single center. Our results may not be extrapolated to all regions because MDR bacterial epidemiology depends on local conditions and shows a high degree of variation between countries and regions.
Our study developed the first predictive tool to identify the risk of MDR bacteria in deceased organ donors using three factors: the duration of antibiotic use ≥3 days, length of ICU stay and neurosurgery. Targeted antibiotic therapy is required for organ donors while avoiding the overuse of antibiotics. The nomogram is easy to apply and can help clinicians select the appropriate empirical antibiotic therapies for recipients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by clinical research ethics committee of the First Affliated Hospital, College of Medicine, Zhejiang University.(No. 2022-705). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XW had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study design: GS and HC. Data collection: HL, FW, WF, QY and ML. Statistical analysis: GS and XY. Manuscript draft: GS and LZ. Manuscript revised: XW. All authors contributed to the article and approved the submitted version.
This study was supported by the Basic Public Welfare Research Program of Zhejiang Province (Grant No. LGF20H150007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1181630/full#supplementary-material
APACHE II, Acute Physiology and Chronic Health Evaluation II; AUC, Area under the curve; CI, Confidence interval; CRE , Carbapenem-resistant Enterobacteriaceae; DCA, Decision curve analysis; DDI, Donor-derived infection; DOA, Duration of antibiotic; ESBL-PE, Extended-spectrum β-lactamase producing Enterobacteriaceae; GN, Gram-negative; GP, Gram-positive;ICU, Intensive Care Unit; INQ, Interquartile range; MDR, Multidrug-resistant; MIC, Minimum inhibitory concentration; MRSA, Methicillin-resistant Staphylococcus aureus; OPO, Organ procurement organization; OR, Odds ratio; PPIs, Proton pump inhibitors; ROC, Receiver operating characteristic.
Abulhasan, Y. B., Abdullah, A. A., Shetty, S. A., Ramadan, M. A., Yousef, W., Mokaddas, E. M. (2020). Health care-associated infections in a neurocritical care unit of a developing country. Neurocrit Care 32 (3), 836–846. doi: 10.1007/s12028-019-00856-8
Agarwal, R., Mohapatra, S., Rath, G. P., Kapil, A. (2017). Active surveillance of health care associated infections in neurosurgical patients. J. Clin. Diagn. Res. 11 (7), DC01–DC04. doi: 10.7860/JCDR/2017/26681.10146
Anesi, J. A., Han, J. H., Lautenbach, E., Lee, D. H., Clauss, H., Climaco, A., et al. (2020). Impact of deceased donor multidrug-resistant bacterial organisms on organ utilization. Am. J. Transplant. 20 (9), 2559–2566. doi: 10.1111/ajt.15830
Augustin, P., Kermarrec, N., Muller-Serieys, C., Lasocki, S., Chosidow, D., Marmuse, J. P., et al. (2010). Risk factors for multidrug resistant bacteria and optimization of empirical antibiotic therapy in postoperative peritonitis. Crit. Care 14 (1), R20. doi: 10.1186/cc8877
Chastre, J., Luyt, C. E., Combes, A., Trouillet, J. L. (2006). Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator-associated pneumonia to decrease resistance in the intensive care unit. Clin. Infect. Dis. 43 Suppl 2, S75–S81. doi: 10.1086/504483
Chavada, R., Tong, D., Maley, M. (2018). In-hospital surgery as a risk factor for onset of AmpC-producing escherichia coli blood stream infections. Diseases 6 (3), 71. doi: 10.3390/diseases6030071
Christaki, E., Marcou, M., Tofarides, A. (2020). Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J. Mol. Evol. 88 (1), 26–40. doi: 10.1007/s00239-019-09914-3
Fishman, J. A., Greenwald, M. A., Grossi, P. A. (2012). Transmission of infection with human allografts: essential considerations in donor screening. Clin. Infect. Dis. 55 (5), 720–727. doi: 10.1093/cid/cis519
Fishman, J. A., Grossi, P. A. (2014). Donor-derived infection–the challenge for transplant safety. Nat. Rev. Nephrol. 10 (11), 663–672. doi: 10.1038/nrneph.2014.159
Humphries, R., Bobenchik, A. M., Hindler, J. A., Schuetz, A. N. (2021). Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. J. Clin. Microbiol. 59 (12), e0021321. doi: 10.1128/JCM.00213-21
Ison, M. G., Grossi, P., Practice ASTIDCo (2013). Donor-derived infections in solid organ transplantation. Am. J. Transplant. 13 Suppl 4, 22–30. doi: 10.1111/ajt.12095
Jiang, A. M., Shi, X., Liu, N., Gao, H., Ren, M. D., Zheng, X. Q., et al. (2020). Nosocomial infections due to multidrug-resistant bacteria in cancer patients: a six-year retrospective study of an oncology center in Western China. BMC Infect. Dis. 20 (1), 452. doi: 10.1186/s12879-020-05181-6
Jiang, A., Shi, X., Zheng, H., Liu, N., Chen, S., Gao, H., et al. (2022). Establishment and validation of a nomogram to predict the in-hospital death risk of nosocomial infections in cancer patients. Antimicrob. Resist. Infect. Control 11 (1), 29. doi: 10.1186/s13756-022-01073-3
Len, O., Gavaldà, J., Blanes, M., Montejo, M., San Juan, R., Moreno, A., et al. (2008). Donor infection and transmission to the recipient of a solid allograft. Am. J. Transplant. 8 (11), 2420–2425. doi: 10.1111/j.1600-6143.2008.02397.x
Lewis, A., Koukoura, A., Tsianos, G. I., Gargavanis, A. A., Nielsen, A. A., Vassiliadis, E. (2021). Organ donation in the US and Europe: the supply vs demand imbalance. Transplant. Rev. (Orlando) 35 (2), 100585. doi: 10.1016/j.trre.2020.100585
Lewis, J. D., Sifri, C. D. (2016). Multidrug-resistant bacterial donor-derived infections in solid organ transplantation. Curr. Infect. Dis. Rep. 18 (6), 18. doi: 10.1007/s11908-016-0526-9
Lin, B. Y., Liu, J. T., Jin, F. L. (2022). Risk factors for the colonization or infection of carbapenem-resistant enterobacteriaceae in children: a meta analysis. Zhongguo Dang Dai Er Ke Za Zhi 24 (1), 96–101. doi: 10.7499/j.issn.1008-8830.2109025
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 (3), 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Mariappan, S., Sekar, U., Kamalanathan, A. (2017). Carbapenemase-producing enterobacteriaceae: risk factors for infection and impact of resistance on outcomes. Int. J. Appl. Basic Med. Res. 7 (1), 32–39. doi: 10.4103/2229-516X.198520
Nanni Costa, A., Grossi, P., Gianelli Castiglione, A., Grigioni, W. F. (2008). Italian Transplant research n. quality and safety in the Italian donor evaluation process. Transplantation 85 (8 Suppl), S52–S56. doi: 10.1097/TP.0b013e31816c2f05
Nelson, P. W., Delmonico, F. L., Tolkoff-Rubin, N. E., Cosimi, A. B., Fang, L. S., Russell, P. S., et al. (1984). Unsuspected donor pseudomonas infection causing arterial disruption after renal transplantation. Transplantation 37 (3), 313–314. doi: 10.1097/00007890-198403000-00020
Pilmis, B., Weiss, E., Scemla, A., Le Monnier, A., Grossi, P. A., Slavin, M. A., et al. (2023). Multidrug-resistant enterobacterales infections in abdominal solid organ transplantation. Clin. Microbiol. Infect. 29 (1), 38–43. doi: 10.1016/j.cmi.2022.06.005
Rossolini, G. M., Mantengoli, E. (2008). Antimicrobial resistance in Europe and its potential impact on empirical therapy. Clin. Microbiol. Infect. 14 Suppl 6, 2–8. doi: 10.1111/j.1469-0691.2008.02126.x
Sifri, C. D., Ison, M. G. (2012). Highly resistant bacteria and donor-derived infections: treading in uncharted territory. Transpl Infect. Dis. 14 (3), 223–228. doi: 10.1111/j.1399-3062.2012.00752.x
Singh, N., Rogers, P., Atwood, C. W., Wagener, M. M., Yu, V. L. (2000). Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. a proposed solution for indiscriminate antibiotic prescription. Am. J. Respir. Crit. Care Med. 162 (2 Pt 1), 505–511. doi: 10.1164/ajrccm.162.2.9909095
Tacconelli, E., Cataldo, M. A., Dancer, S. J., De Angelis, G., Falcone, M., Frank, U., et al. (2014). ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 20 Suppl 1, 1–55. doi: 10.1111/1469-0691.12427
van Langeveld, I., Gagnon, R. C., Conrad, P. F., Gamelli, R. L., Martin, B., Choudhry, M. A., et al. (2017). Multiple-drug resistance in burn patients: a retrospective study on the impact of antibiotic resistance on survival and length of stay. J. Burn Care Res. 38 (2), 99–105. doi: 10.1097/BCR.0000000000000479
Vasudevan, A., Mukhopadhyay, A., Li, J., Yuen, E. G., Tambyah, P. A. (2014). A prediction tool for nosocomial multi-drug resistant gram-negative bacilli infections in critically ill patients - prospective observational study. BMC Infect. Dis. 14, 615. doi: 10.1186/s12879-014-0615-z
White, S. L., Rawlinson, W., Boan, P., Sheppeard, V., Wong, G., Waller, K., et al. (2019). Infectious disease transmission in solid organ transplantation: donor evaluation, recipient risk, and outcomes of transmission. Transplant. Direct 5 (1), e416. doi: 10.1097/TXD.0000000000000852
Wolfe, C. R., Ison, M. G., Practice ASTIDCo (2019). Donor-derived infections: guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 33 (9), e13547. doi: 10.1111/ctr.13547
Yamamoto, N., Asada, R., Kawahara, R., Hagiya, H., Akeda, Y., Shanmugakani, R. K., et al. (2017). Prevalence of, and risk factors for, carriage of carbapenem-resistant enterobacteriaceae among hospitalized patients in Japan. J. Hosp Infect. 97 (3), 212–217. doi: 10.1016/j.jhin.2017.07.015
Keywords: multidrug-resistant bacteria, transplantation, organ donors, prediction model, nomogram
Citation: Shen G, Zhang L, Fan W, Lv H, Wang F, Ye Q, Lin M, Yu X, Cai H and Wu X (2023) Establishment of a risk prediction model for multidrug-resistant bacteria in deceased organ donors: a retrospective cohort study in China. Front. Cell. Infect. Microbiol. 13:1181630. doi: 10.3389/fcimb.2023.1181630
Received: 07 March 2023; Accepted: 15 May 2023;
Published: 25 May 2023.
Edited by:
Zichen Yang, Xinqiao Hospital, ChinaReviewed by:
Yu Yao, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaCopyright © 2023 Shen, Zhang, Fan, Lv, Wang, Ye, Lin, Yu, Cai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoliang Wu, OTM2d3hsQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.