
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 14 June 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1168451
This article is part of the Research Topic Antimicrobial resistance in pediatric infectious diseases: antimicrobial resistance, resistance mechanisms and antimicrobial use View all 18 articles
 Soichiro Kawata1,2,3
Soichiro Kawata1,2,3 Shimpei Morimoto4
Shimpei Morimoto4 Kosuke Kosai5
Kosuke Kosai5 Yasuhide Kawamoto5
Yasuhide Kawamoto5 Yumiko Nakashima1
Yumiko Nakashima1 Yoshitomo Morinaga6
Yoshitomo Morinaga6 Katsunori Yanagihara7
Katsunori Yanagihara7 Lay-Myint Yoshida2
Lay-Myint Yoshida2 Hiroyuki Moriuchi1,8*
Hiroyuki Moriuchi1,8*Background: Extended-spectrum β-lactamase–producing Enterobacterales (ESBL-E) is a great public health concern globally not only in hospitals but also in the community. To our knowledge, there have been few studies on the prevalence of ESBL-E and much less about carbapenem-resistant Enterobacterales (CRE) among children in the community, and there is no such study in Japan despite such situations. This study aimed to clarify their carriage status among Japanese infants in the community by taking the opportunity of the 4-month health checkup.
Methods: This prospective analysis was conducted from April 2020 to March 2021 in Shimabara City, Nagasaki Prefecture, Japan. The research-related items were mailed to all subjects with official documents for the checkup. The fecal samples were obtained from the diaper by guardians beforehand and were collected with the questionnaire and then screened for ESBL-E and CRE by a clinical laboratory company with selective agars followed by identification and confirmation. Only the positive samples were analyzed about resistant genotypes.
Results: One hundred fifty infants aged 4–5 months, over half of the subjects, participated in this study. The overall ESBL-E carriage rate was 19.3% (n = 29), and no CRE carrier was detected among them. All identified ESBL-E were E. coli except for one K. pneumoniae. A significantly higher carriage rate was recorded among the infants born at “Hospital A” (25.0%) than the others (11.3%). Enterobacterales producing CTX-M-9 ± TEM were broadly distributed among the positive samples (65.5%), whereas the CTX-M-1 group was exclusively detected among those from “Hospital A”. Recursive partitioning analysis suggested that delivery facilities might be an important factor for ESBL-E colonization, although the effect could be decreased as they grow. In contrast, no significant effect was observed for other factors such as parent(s) as healthcare worker(s), having a sibling(s), and the mode of delivery.
Conclusion: This study revealed the ESBL-E and CRE carriage status of Japanese infants in the community for the first time, although the setting is somewhat limited. Our findings indicated that environmental factors, especially delivery facilities, influenced ESBL-E colonization among infants aged 4–5 months, implying the need for strengthening countermeasures against antimicrobial resistance at delivery facilities and communities outside the hospitals.
Antimicrobial resistance (AMR) is a serious ongoing problem globally. An estimation from the United Kingdom advocates that AMR in illnesses such as bacterial infections, malaria, tuberculosis, and human immunodeficiency virus will be the first cause of death all around the world in 2050 (O’Neill, 2018). Indeed, patients with urinary tract infections (UTIs) caused by extended-spectrum β-lactamase– producing Enterobacterales (ESBL-E) are not rare on the daily clinical scene, even at pediatric clinics in Japan. In addition, we also experienced an outbreak of carbapenemase-producing Enterobacterales (CPE) and ESBL-E at the neonatal intensive care units (NICUs) in Nagasaki Prefecture, Japan.
ESBL-E has a higher carriage rate in the community than methicillin-resistant Staphylococcus aureus. The global carriage rate of ESBL-E in the community was reported as 16.5% during the period between 1 January 2000 and 13 February 2020. It is increasing continuously from 2.6% (95% CI, 1.6–4.0) in 2003–2005 to 21.1% (95% CI, 15.8%–27.0%) in 2015–2018 and estimated as a 1.5% yearly increase by linear regression analysis (Bezabih et al., 2021). Among six WHO regions (i.e., Southeast Asia, Western Pacific, Africa, Eastern Mediterranean, Americas, and Europe), the Southeast Asian Region had the highest prevalence at 27% (95% CI, 2.9%–51.3%) based on four studies from India, Thailand, and Nepal, whereas the European Region had the lowest at 6.0% (95% CI, 4.6%–7.5%) based on 19 studies.
The ESBL-E carriage rate in Japan was reported to be 6.4% among 218 stool specimens obtained from adult volunteers (Luvsansharav et al., 2011) and 7.5% in 67 stool samples acquired from medical students in 2011 (Niki et al., 2011). There have been subsequent reports of ESBL-E carriage rates among adults in Japan, including 4.8% of 4,314 stool specimens obtained from 2,563 food handlers (Nakane et al., 2016), 12.5% of 257 hospitalized patients and 8.5% of 496 workers at 25 meal supply centers in the same community without significant difference (Nakamura et al., 2016), 12.2% of 263 persons who underwent routine medical checkups (Higa et al., 2019), and 9.7% of 547 healthy individuals (Masui et al., 2022), whereas there have been only a few studies on ESBL-E carriage rates among Japanese children, including 12% of 50 inpatients at a pediatric tertiary care hospital (Minami et al., 2012) and 12.5% of 256 pediatric patients with gastroenteritis among 11 hospitals in Mie Prefecture (Nagai et al., 2017). The worldwide prevalence of ESBL-E carriage in children has been reportedly variable, such as 74% of neonates and 59% of children in Ethiopia’s largest tertiary hospital (Desta et al., 2016), 50.4% of hospitalized patients and 11.6% of children in the community in Tanzania (Tellevik et al., 2016), 2.3% of asymptomatic nursery children in Germany (Harries, et al. 2016), and 3.5% of healthy children in the United States (Islam et al., 2018). The ESBL-E carriage rates have shown an increasing trend even among children in the community. The French study showed the rate to be 7.6% of 1886 children aged 6–24 months with a tendency to increase from 2.2% in 2010 to 10.8% in 2016 (Birgy et al., 2016). The study from Sweden reported that 16.8% of 334 children aged 13–45 months were positive in 2016, over a six-fold increase from 2.6% in 2010 (Kaarme et al., 2018).
Meanwhile, relatively few studies have examined the community-acquired carriage of carbapenem-resistant Enterobacterales (CRE). In a review article analyzing 15 studies, the rates of the community-associated or community-onset CRE globally were as various as 0.04%–29% in 10 studies across the world, and five studies did not detect CRE (Kelly et al., 2017). These rates should not be interpreted immediately as the community-acquired carriage rates because most of those reports were not based on the research in the community but on the studies conducted at clinics or estimated from the rates of hospitalized patients on admission. In any case, the results suggest that the CRE threat is not limited to the hospital but is spreading to the community as well. For example, the aforementioned report studying hospitalized patients of all ages in Ethiopia revealed five CRE carriers, all of whom were children. Two of them carried Klebsiella pneumoniae carbapenemase –producing CPE (Desta et al., 2016).
To our knowledge, there are no data about the ESBL-E carriage rate of children in the community in Japan and about the CRE carriage rate of non-hospitalized individuals whether adults or children. We conducted this study to reveal these carriage rates among infants in the community in a rural area and to determine associated factors in this area by analyzing the questionnaires.
This prospective study was performed between April 2020 and March 2021 in Shimabara City, Nagasaki Prefecture, the westernmost part of Japan (Figure S1). This city has an estimated population of 43,556 people with live births of 280 neonates in January 2020 and 43,228 people with 295 neonates in January 2021, according to information from Nagasaki Prefecture (https://www.pref.nagasaki.jp/shared/uploads/2021/05/1622446419.pdf; https://www.pref.nagasaki.jp/shared/uploads/2022/05/1653363988.pdf).
ESBL-E or CRE carriage was defined with the positive results of both screening cultures and identification of the resistant genotypes using the methods described below.
This study was approved by the Human Ethics Committee of Nagasaki University Graduate School of Biomedical Sciences.
To reveal the actual carriage rate of ESBL-E and CRE among healthy children, we took advantage of the opportunity for a 4-month health checkup, which all children were required to have in Japan. All subjects for the checkup received the supporting documentation, the informed consent documents, questionnaires, and kits for stool sample collection with other documents related to the health checkup through the Shimabara Healthcare Center. All infants with the approval of their parents or guardians were included in this study except those who could not take stool samples or those who were regarded as inappropriate for other reasons.
All samples were obtained from the stool on the diaper with two separate swabs including Cary-Blair media for culture named Seed swab γI (Eiken Chemical, Ltd.) at each home mainly by parents following the instruction text or the video created originally and uploaded on YouTube® presented on the supporting documentation beforehand of visiting the health checkup (Figure S2). These stool samples were collected at the Shimabara Healthcare Center on the day of the checkup. If the parents of the participant could not bring the sample but wanted to join the study, then we asked them to take it to the reception for the Pediatric Department at the Shimabara Prefectural Hospital. Every sample was retrieved and screened for ESBL-E and CRE with 48-h aerobic incubation under 35°C in CHROMagar™ ESBL and CHROMagar™ mSuperCARBA (Kanto Chemical Co., Inc.), respectively, at a major Japanese clinical laboratory company. The former yielded colonies of the following colors: mauve red, metallic blue with or without a red halo, and brown with a halo, and the latter yielded those of the following colors: mauve red and metallic blue. Hence, we picked up one colony each from different color groups from these selection agars. Incubation with 5% Trypto-casein Soy Agar was also performed in parallel for each culture to ensure non-contamination and to use for retesting or additional examination. After the bacterial identification and drug susceptibility test using MALDI-TOF MS (matrix-assisted laser desorption/ionization–time-of-flight mass spectrometer) of MALDI Biotyper® microflex® (Bruker) and Dry Plate Eiken (Eiken Chemical, Ltd.) based on the criteria of CLSI M100S-22, respectively, the ESBL production and CRE characteristic were confirmed with the following methods for all screening-positive samples. ESBL-E was identified when Cica Beta Test I was positive and CVA was negative. If Cica Beta Test was inconclusive, then clavulanic acid added double disc synergy test (DDST) test was performed with cefotaxime and cefpodoxime. CRE was identified under the criteria stipulated by the Japanese Infectious Disease Control Law in which the minimum inhibitory concentration (MIC) for meropenem is 2 µg/ml or higher or the MIC for imipenem is 2 µg/ml or higher, and the MIC for cefmetazole is 64 µg/ml or higher.
Only the positive samples were returned and stored in the Microbank™ (Pro Lab Diagnostics, Inc.) at the −70°C deep freezer placed at the Department of Pediatrics, Nagasaki University Hospital.
The following data were obtained from the questionnaires: gender, birth date, sampling date, gestational age, birth weight, mode of delivery, delivery facility, feeding methods within the first week of birth and around 4 months old, hospitalized history (cause of admission, hospital, antibiotic treatment, and length of hospital stay), siblings, ESBL-E or CRE outbreak exposure of siblings, parental occupation (healthcare worker or else), parental history of staying abroad, and parentally history of hospitalization abroad. The birth date was used for calculating age in days at the time of the health checkup.
The genotypes of ESBL were detected with TaKaRa PCR Thermal Cycler Dice® Gradient (TaKaRa Bio, Inc.) by using Cica Geneus® ESBL Genotype Detection KIT2 (Kanto Chemical Co., Inc.) following its instruction manual. DNA extraction was demonstrated with the boiling method using Chelex (Bio-Rad Laboratories, Hercules, CA, USA) following the previous report with minor modifications (Motoshima et al., 2010). The targeted genes are bla CTX-M (-1, -2, -8, -9, and -25 group), bla CTX-M chimera, bla GES (ESBL type), bla TEM, and bla SHV. Each abbreviation indicates the following: CTX-M, cefotaximase-Munich; GES, Guiana extended-spectrum β-lactamase; TEM, temoneira; and SHV, sulfhydryl variable.
All the data obtained from the questionnaires were entered into a Microsoft® Excel® (Microsoft Corporation) worksheet at the venue of the health checkup immediately after the collection, and the parent was asked to fill in if anything remained blank or appeared obscure. JMP® Pro 16.0.0 (SAS Institute, Inc.) was used for every statistical analysis. Fisher’s exact test was used to analyze categorical data, and a t-test was used for continuous data. The two-sided p-values <0.05 were considered statistically significant. Hence, the objectives of the presenting study were exploratory ones, the statistical significance cannot be interpreted that it is valid to reject the corresponding null hypothesis.
In analyses of the association between two binomial variables, the p-values were obtained via Fisher’s exact test and presented to describe the extent of statistical significance of the association. In the analysis, the candidate of the predictor was a continuous variable; instead of Fisher’s exact test, the t-test was used to obtain p-values. To determine associated factors among the background of the study subjects among infants in the community, the odds ratios for ESBL-E and CRE carriages and the confidence intervals were calculated. The 95% confidence intervals of binomial probabilities were obtained using the Wilson score method.
In addition to those, recursive partitioning analysis was conducted to describe the bias of background factors in carriers and non-carriers. The nodes in the tree were generated using the likelihood ratio chi-square. The splitting was stopped with the following criteria: No branching increases the likelihood ratio chi-square in the descendent nodes, the number of individuals allocated into nodes containing positive subjects is less than 10, or it is difficult to interpret the results and make hypotheses.
The research documents and sampling kits were sent to 271 subjects, and 150 infants (55.4%) were eligible to participate in this study except for one infant whose parent had difficulty in informed consent and answering the questionnaire.
The characteristics of the participants are summarized in Table 1. The percentages of preterm (3.3%) and low birth weight infants (5.3%) were lower than those previously reported from Japan (5.7% and 9.4%, respectively) (Isayama, 2019; Mine et al., 2021). Some of the characteristics included too few subjects to assess the impact on ESBL-E carriage, such as “antibiotic treatment during hospitalization”, “sibling’s stay in a relevant ward during the outbreak”, and “parental stay abroad”.
Each participant provided two samples for screening ESBL-E and CRE, and all 300 specimens except for those two from the excluded participant were eligible for testing. ESBL-E was detected from a total of 29 samples (19.3%), whereas no CRE-positive samples were found. Among the ESBL-E positive samples, 28 (96.6%) were Escherichia coli and one was Klebsiella pneumoniae. One K. oxytoca was identified through the screening but excluded because any relevant genotype was detected.
Table 2 shows the carriage rates according to the background factors, and Figure 1 exhibits the carriage rates according to the delivery facilities. There was no visible difference in the backgrounds between carriers and non-carriers except for their delivery facilities. “Hospital A”, accounting for the largest number of deliveries in the study site, had more than double and significantly higher ESBL-E prevalence than that of the other facilities (22/88 vs. 7/62, 25.0%; 95% CI, 17.1–35.0% vs. 11.3%; 95% CI, 5.6–21.5%; p = 0.039; OR, 2.62; 95% CI, 1.04–6.59).
Recursive partitioning analysis was performed to describe background factor bias in ESBL-E carriers and non-carriers as presented in Figure 2. All factors obtained from the questionnaire except for the sub-answers (i.e., antibiotic usage and the duration of hospital stay) were included in the explanatory variables. To better reflect the actual situation, “delivery facilities” was arranged for “Hospital A” and others, and “occupation” was changed to whether one of the parents is currently or has been in the medical profession within the past 3 years or not and was included in the explanatory variables. The first branch was the “delivery facilities” (“Hospital A” and “The other facilities”) with a significant difference between the nodes as the most major contributors to the tree split (statistical values are the same as described above in 3.3). The descendent nodes created under the node “those from ‘Hospital A’” was the “days of age” with a cutoff of 140 days (15/41 vs. 7/47, 36.6% vs. 14.9%, p = 0.026, OR 3.30; 95% CI, 1.21–8.96). The descendent nodes created under the “those from the other facilities” was “birth weight” with a cutoff of 3,332 g (5/15 vs. 2/47, 33.3% vs. 4.3%; p = 0.007; OR, 11.25; 95% CI, 2.14–57.53). The nodes of “days of age” with a cutoff of 153 days among those older than 140 days from “Hospital A” (7/27 vs. 0/20, 25.9% vs. 0.0%; p = 0.015; OR, ∞; 95% CI, 1.68 –∞) were the only descendent node of above nodes with p < 0.05.
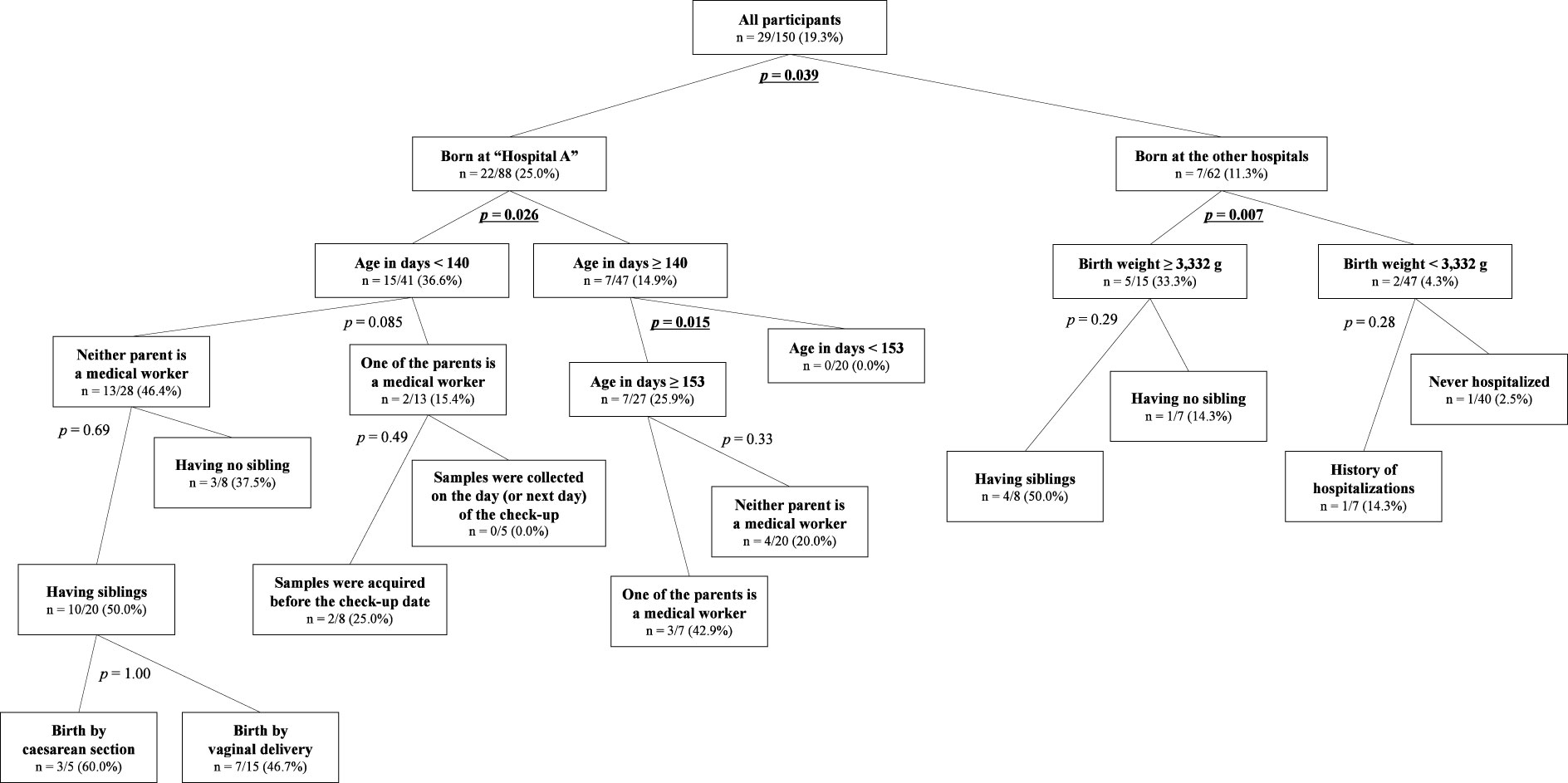
Figure 2 The tree of recursive partitioning analysis about ESBL-E carriage among the participants. The number in each node means the prevalence of ESBL-E carriage [i.e., n = positive/total (carriage rate)]. The p-values shown beside each branch were presented not for rejection of the null hypothesis but for exploratory purposes, and those that are lower than 0.05 are emphasized with bold and are underlined.
The genotypes of ESBL were detected for all 30 positive samples except for one of K. oxytoca (Table 3). The CTX-M-9 group was the most predominant genotype accounting for 16 E. coli isolates, followed by the CTX-M-1 group with the TEM gene (n = 7), CTX-M-9 group with TEM gene (n = 3), CTX-M-1 group (n = 1), and TEM gene (n = 1). The only K. pneumoniae isolate harbored SHV with the TEM gene.
In addition, as shown in Table 4 and Figure S3, a total of eight CTX-M-1 group E. coli were dominantly detected in the participants born at “Hospital A” with statistical significance compared with those from the other facilities (8/88 vs. 0/62, 9.1% vs. 0.0%; p = 0.021; OR, ∞; 95% CI, 1.58–∞) and of 11 TEM E. coli as well (10/88 vs. 1/62, 11.4% vs. 1.6%; p = 0.027; OR, 7.82; 95% CI, 1.24–48.37), whereas CTX-M-9 group isolates, which accounted for 19 (65.5%) of the 29 ESBL positives, were widely distributed regardless of the delivery facilities (12/88 vs. 7/62, 13.6% vs. 11.3%; p = 0.81; OR, 1.24; 95% CI, 0.47–3.26).
To the best of our knowledge, this is the first study about the ESBL-E carriage situation among infants in the community of Japan, as well as about the CRE carriage for non-hospitalized people in Japan although no CRE carrier was detected. In the present study, the ESBL-E carriage rate of infants aged 4 to 5 months in Shimabara city was 19.3%. The infants born at “Hospital A” had a significantly high carriage rate of 25.0% compared with 11.3% in those born at other delivery facilities. The overall prevalence was >1.5-fold higher than those from the previous studies among healthy adults or pediatric samples from hospitals in Japan (Luvsansharav et al., 2011; Niki et al., 2011; Minami et al., 2012; Nakamura et al., 2016; Nakane et al., 2016; Nagai et al., 2017; Higa et al., 2019; Masui et al., 2022); however, it is worthy to note that the ESBL-E prevalence at delivery facilities other than “Hospital A” was comparable to those from the previous studies.
The fecal carriage of ESBL-E can cause UTIs. The annual proportion of ESBL-E among pediatric UTIs in Japan has been reported to be 3.6%–21.1% (Nagata et al., 2015; Yanai et al., 2019; Sakata et al., 2021; Okumiya et al., 2022), except for a study from Shimane Prefecture reporting over 50% prevalence (Horie et al., 2019). These data should not be immediately interpreted as the ESBL-E carriage rate in the community because the specimens obtained at hospitals tend to show higher carriage rates (Bezabih et al., 2022). However, clinicians should be aware of the ESBL-E prevalence among patients with UTIs at their hospitals for appropriate treatment strategies. For example, in high-prevalence hospitals, intravenous administration of cefmetazole or flomoxef can be selected as the initial therapy for febrile UTI at hospitals with high ESBL-E prevalence in Japan (Matsumura et al., 2015; Fukuchi et al., 2016). Moreover, the escalation to meropenem without waiting for culture results may not be overtreatment, depending on the clinical course of UTI and the prevalence of ESBL-E because 5%–10% of UTIs with gram-negative rods are complicated by bacteremia (Schnadower et al., 2010; Sakata et al., 2021).
Several studies have shown that the younger the child, the higher the carriage rate (Tellevik et al., 2016; Cheng et al., 2022), and the results of our present study suggest that children aged around 4 months may be susceptible to environmental factors, especially the delivery facilities in which they were born. The recursive partitioning analysis indicated that the birth facility significantly contributed to ESBL-E carriage among our study population. Because previous studies have not included “delivery facility” in the survey items, our finding needs to be examined by future studies. Interestingly, further splitting suggested a slightly decreasing trend in the carriage rate over time, and other factors such as the occupation of their parents as medical workers, having siblings, or the mode of delivery did not show statistical significance for the ESBL-E carriage status, although these might be the results of the limitations about our study subjects. We are planning to conduct a similar study on the ESBL-E carriage for the same population when they became age 3 shortly, which will hopefully reveal changes in the carriage status.
In the present study, the CTX-M-9 group was broadly detected among infants born at various delivery facilities, whereas the CTX-M-1 group was exclusively detected among those born at “Hospital A” with statistical significance, strongly suggesting environmental differences among birth facilities and the possibility of CTX-M-9 spreading in the community outside hospitals. In Japan, these two genotypes are common even in non-clinical specimens (Masui et al., 2022; Sekizuka et al., 2022). In particular, the CTX-M-9 group became dominant over the CTX-M-2 group, which was the most prevalent in the early 2000s (Suzuki et al., 2009). These results emphasize the need for action against AMR not only in hospitals but also in the community and birth facilities.
There are several limitations to our study. First, unlike other studies conducted in medical institutions, the samples were collected by the parent, who might technically be less reliable. To address this issue, we have not only provided an instruction manual simply explaining precautions for specimen collection but also directly confirmed that a sufficient amount of stool adhered to the swabs at the time of collection before handing them over to a clinical laboratory company. Contamination from the environment, such as the surface of diapers, is not expected to significantly affect test results, as the number of bacteria in the stool should be overwhelmingly larger.
Second, parents have not been tested for their carriage status for two reasons: first, performing it would make them hesitate to participate, and, second, submission of plural specimens would increase the risk of sample mix-up.
Third, the results obtained in our study are only from infants aged around 4 to 5 months in a rural city in Japan; therefore, the external validity of the results remains to be assessed. In addition, it is impractical to model the mechanisms of harboring ESBL-E and the network of background factors, thus, we performed the recursive partitioning analysis but not multivariable regression analyses. Future studies in Japan are awaited including the similar survey that we are planning to perform at the age of three as mentioned above.
Finally, because of the influence of the COVID-19 pandemic, the health checkup in May 2020 was canceled and allocated to another month’s checkup schedule. Hence, the ages in months of some participants were slightly older than 4 months. However, the data still reflect the prevalence of carriage during early infancy in Shimabara City and showed a slightly decreasing trend in the carriage rate over time as a by-product.
In conclusion, our study revealed the ESBL-E carriage rate as 19.3% and no CRE carriage among infants aged 4 to 5 months in a rural city in Japan. This is the first report on the ESBL-E carriage rates in children in the Japanese community and the CRE carriage rate in any non-hospitalized individuals in Japan. The ESBL-E carriage rate appeared to be associated with delivery facilities: The infants born at “Hospital A” had a higher carriage rate (25.0%) than those born at other facilities (11.3%) and CTX-M-1 was detected only in those from “Hospital A” among the positive samples. Our results suggested that infants aged 4 to 5 months may be more prone to acquire ESBL-E due to environmental factors, especially their delivery facilities. These findings highlighted the importance of AMR countermeasures not only in hospitals but also in the community and maternity facilities.
A part of this study was presented at the 10th Asian Congress of Pediatric Infectious Diseases (Seoul, October 2022).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Human Ethics Committee of Nagasaki University Graduate School of Biomedical Sciences. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Study design: SK, SM, YN, YM, KY, and HM. Data collection: SK. Data interpretation: SK, SM, KK, and HM. Genotype identification: YK and KK. Statistical analysis: SK and SM. All authors contributed to the initial draft of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the budgets from The Morinaga Foundation for Health and Nutrition and the Funds donated by the three cities (Shimabara, Minami-Shimabara, and Unzen) in the Shimabara Peninsula (20320539).
The authors are grateful to the parents who consented for their infants to participate in the study despite the busy childcare. We would also like to acknowledge the great contribution of the staff of Shimabara Healthcare Center in the preparation and dispatch of documents and collaboration on each day of 4-month health checkups.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1168451/full#supplementary-material
Bezabih, Y. M., Bezabih, A., Dion, M., Batard, E., Teka, S., Obole, A., et al. (2022). Comparison of the global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli between healthcare and community settings: a systematic review and meta-analysis. JAC Antimicrob. Resist. 4 (3), dlac048. doi: 10.1093/jacamr/dlac048
Bezabih, Y. M., Sabiiti, W., Alamneh, E., Bezabih, A., Peterson, G. M., Bezabhe, W. M., et al. (2021). The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J. Antimicrob. Chemother. 76 (1), 22–29. doi: 10.1093/jac/dkaa399
Birgy, A., Levy, C., Bidet, P., Thollot, F., Derkx, V., Béchet, S., et al. (2016). ESBL-producing Escherichia coli ST131 versus non-ST131: evolution and risk factors of carriage among French children in the community between 2010 and 2015. J. Antimicrob. Chemother. 71 (10), 2949–2956. doi: 10.1093/jac/dkw219
Cheng, M. F., Ho, P. Y., Wang, J. L., Tseng, F. C., Chang, J. T., Huang, I. F., et al. (2022). Prevalence and household risk factors for fecal carriage of ESBL-producing, sequence type 131, and extraintestinal pathogenic Escherichia coli among children in southern Taiwan. J. Microbiol. Immunol. Infect. 55 (4), 695–707. doi: 10.1016/j.jmii.2022.04.001
Desta, K., Woldeamanuel, Y., Azazh, A., Mohammad, H., Desalegn, D., Shimelis, D. (2016). High gastrointestinal colonization rate with extended-spectrum β-Lactamase-Producing Enterobacteriaceae in hospitalized patients: emergence of carbapenemase-producing K. pneumoniae in Ethiopia. PloS One 11 (8), e0161685. doi: 10.1371/journal.pone.0161685
Fukuchi, T., Iwata, K., Kobayashi, S., Nakamura, T., Ohji, G. (2016). Cefmetazole for bacteremia caused by ESBL-producing enterobacteriaceae comparing with carbapenems. BMC Infect. Dis. 16 (1), 427. doi: 10.1186/s12879-016-1770-1
Harries, M., Dreesman, J., Rettenbacher-Riefler, S., Mertens, E. (2016). Faecal carriage of extended-spectrum β-lactamase-producing enterobacteriaceae and shiga toxin-producing Escherichia coli in asymptomatic nursery children in lower Saxony (Germany), 2014. Epidemiol. Infect. 144 (16), 3540–3548. doi: 10.1017/S0950268816001837
Higa, S., Sarassari, R., Hamamoto, K., Yakabi, Y., Higa, K., Koja, Y., et al. (2019). Characterization of CTX-m type ESBL-producing Enterobacteriaceae isolated from asymptomatic healthy individuals who live in a community of the Okinawa prefecture, Japan. J. Infect. Chemother. 25 (4), 314–317. doi: 10.1016/j.jiac.2018.09.005
Horie, A., Nariai, A., Katou, F., Abe, Y., Saito, Y., Koike, D., et al. (2019). Increased community-acquired upper urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in children and the efficacy of flomoxef and cefmetazole. Clin. Exp. Nephrol. 23 (11), 1306–1314. doi: 10.1007/s10157-019-01775-w
Isayama, T. (2019). The clinical management and outcomes of extremely preterm infants in Japan: past, present, and future. Transl. Pediatr. 8 (3), 199–211. doi: 10.21037/tp.2019.07.10
Islam, S., Selvarangan, R., Kanwar, N., McHenry, R., Chappell, J. D., Halasa, N., et al. (2018). Intestinal carriage of third-generation cephalosporin-resistant and extended-spectrum β-Lactamase-Producing Enterobacteriaceae in healthy US children. J. Pediatr. Infect. Dis. Soc 7 (3), 234–240. doi: 10.1093/jpids/pix045
Kaarme, J., Riedel, H., Schaal, W., Yin, H., Nevéus, T., Melhus, Å. (2018). Rapid increase in carriage rates of Enterobacteriaceae producing extended-spectrum β-lactamases in healthy preschool children, Sweden. Emerg. Infect. Dis. 24 (10), 1874–1881. doi: 10.3201/eid2410.171842
Kelly, A. M., Mathema, B., Larson, E. L. (2017). Carbapenem-resistant enterobacteriaceae in the community: a scoping review. Int. J. Antimicrob. Agents 50 (2), 127–134. doi: 10.1016/j.ijantimicag.2017.03.012
Luvsansharav, U. O., Hirai, I., Niki, M., Nakata, A., Yoshinaga, A., Moriyama, T., et al. (2011). Prevalence of fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae among healthy adult people in Japan. J. Infect. Chemother. 17 (5), 722–725. doi: 10.1007/s10156-011-0225-2
Masui, T., Nakano, R., Nakano, A., Saito, K., Suzuki, Y., Kakuta, N., et al. (2022). Predominance of CTX-M-9 group among ESBL-producing Escherichia coli isolated from healthy individuals in Japan. Microb. Drug Resist. 28 (3), 355–360. doi: 10.1089/mdr.2021.0062
Matsumura, Y., Yamamoto, M., Nagao, M., Komori, T., Fujita, N., Hayashi, A. (2015). Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob. Agents Chemother. 59 (9), 5107–5113. doi: 10.1128/AAC.00701-15
Minami, K., Shoji, Y., Kasai, M., Ogiso, Y., Nakamura, T., Kawakami, Y., et al. (2012). Proportion of rectal carriage of extended-spectrum β-lactamase producing enterobacteriaceae in the inpatients of a pediatric tertiary care hospital in Japan. Jpn. J. Infect. Dis. 65 (6), 548–550. doi: 10.7883/yoken.65.548
Mine, T., Tsuboi, S., Fukushima, F. (2021). Twenty-year trends of low birth weight in Japan: a joinpoint regression analysis of data from 2000 to 2019. Front. Reprod. Health 3. doi: 10.3389/frph.2021.772575
Motoshima, M., Yanagihara, K., Morinaga, Y., Matsuda, J., Sugahara, K., Yamada, Y. (2010). Genetic diagnosis of community-acquired MRSA: a multiplex real-time PCR method for staphylococcal cassette chromosome mec typing and detecting toxin genes. Tohoku J. Exp. Med. 220 (2), 165–170. doi: 10.1620/tjem.220.165
Nagai, Y., Kusuhara, H., Kobayashi, A., Akachi, S. (2017). Prevalence of extended-spectrum β-lactamase (ESBL)-producing isolates from pediatric patients with gastroenteritis. Ann. Rep. Mie Pref. Hlth. Environ. Res. Inst. 19, 34–40.
Nagata, H., Furuse, A., Irie, S., Kanou, K., Kawasaki, T., Sawada, T., et al. (2015). Clinical comparison between childhood upper urinary tract infection due to extended- and non-extended-spectrum β-lactamase-producing bacteria. J. Jpn. Soc Pediatr. Renal Failure 28 (1), 37–42.
Nakamura, A., Komatsu, M., Noguchi, N., Ohno, Y., Hashimoto, E., Matsutani, H., et al. (2016). Analysis of molecular epidemiologic characteristics of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli colonizing feces in hospital patients and community dwellers in a Japanese city. J. Infect. Chemother. 22 (2), 102–107. doi: 10.1016/j.jiac.2015.11.001
Nakane, K., Kawamura, K., Goto, K., Arakawa, Y. (2016). Long-term colonization by blaCTX-M-Harboring Escherichia coli in healthy Japanese people engaged in food handling. Appl. Environ. Microbiol. 82 (6), 1818–1827. doi: 10.1128/AEM.02929-15
Niki, M., Hirai, I., Yamamoto, Y. (2011). Prevalence of ESBL-producing Enterobacteriaceae in medical students. Jpn. J. Environ. Infect. 26 (3), 154–156. doi: 10.4058/jsei.26.154
Okumiya, K., Kuga, S., Fujimoto, T. (2022). Pathogenic organisms and drug resistance in community-acquired upper urinary tract infections in children. Jpn. J. Pediatr. 75 (5), 811–816.
O’Neill, J. (2018). Tackling drug-resistant infections globally: final report and recommendations. 2016 (UK: HM Government and Welcome Trust). Available at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.771510/full.
Sakata, Y., Oishi, T., Miyata, I., Kitano, T., Mitsui, K., Inoue, T., et al. (2021). Analysis of appropriate treatment for children with urinary tract infections when considering the detection of ESBL-producing bacteria. J. Pediatr. Infect. Dis. Immunol. 33 (4), 333–342.
Schnadower, D., Kuppermann, N., Macias, C. G., Freedman, S. B., Baskin, M. N., Ishimine, P., et al. (2010). Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics 126 (6), 1074–1083. doi: 10.1542/peds.2010-0479
Sekizuka, T., Tanaka, R., Hashino, M., Yatsu, K., Kuroda, M. (2022). Comprehensive genome and plasmidome analysis of antimicrobial resistant bacteria in wastewater treatment plant effluent of Tokyo. Antibiotics (Basel) 11 (10), 1283. doi: 10.3390/antibiotics11101283
Suzuki, S., Shibata, N., Yamane, K., Wachino, J., Ito, K., Arakawa, Y. (2009). Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J. Antimicrob. Chemother. 63 (1), 72–79. doi: 10.1093/jac/dkn463
Tellevik, M. G., Blomberg, B., Kommedal, Ø., Maselle, S. Y., Langeland, N., Moyo, S. J. (2016). High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae among children in dar es salaam, Tanzania. PloS One 11 (12), e0168024. doi: 10.1371/journal.pone.0168024
Keywords: extended-spectrum beta-lactamase-producing Enterobacterales, carbapenem-resistant Enterobacterales, antimicrobial resistance, antimicrobial stewardship, infants, fecal carriage, Escherichia coli, obstetrics facilities
Citation: Kawata S, Morimoto S, Kosai K, Kawamoto Y, Nakashima Y, Morinaga Y, Yanagihara K, Yoshida L-M and Moriuchi H (2023) The fecal carriage rate of extended-spectrum β-lactamase–producing or carbapenem-resistant Enterobacterales among Japanese infants in the community at the 4-month health examination in a rural city. Front. Cell. Infect. Microbiol. 13:1168451. doi: 10.3389/fcimb.2023.1168451
Received: 17 February 2023; Accepted: 23 May 2023;
Published: 14 June 2023.
Edited by:
Yonghong Yang, Capital Medical University, ChinaReviewed by:
Toyotaka Sato, Hokkaido University, JapanCopyright © 2023 Kawata, Morimoto, Kosai, Kawamoto, Nakashima, Morinaga, Yanagihara, Yoshida and Moriuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyuki Moriuchi, aGlyb21vcmlAbmFnYXNha2ktdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.