
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 03 April 2023
Sec. Extra-intestinal Microbiome
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1153894
This article is part of the Research Topic New Techniques in Microbiome Research - Vol II – Host-Microbiome Interactions using ‘meta-omics’ techniques View all 19 articles
The vaginal microbiome is a distinct component of the human microbiome that is colonized by a wide variety of microorganisms. Lactobacilli are the most frequently identified microorganisms in the healthy human vagina. These Gram-positive bacilli can acidify the vaginal microenvironment, inhibit the proliferation of other pathogenic microorganisms, and promote the maintenance of a eubiotic vaginal microbiome. However, a vaginal flora with a reduced proportion or abundance of lactobacilli is associated with various vaginal infections that have been linked to serious health consequences such as infertility, preterm birth, pelvic inflammatory disease, premature rupture of membranes, and miscarriage. Due to their “Generally Recognized as Safe” classification and critical role in vaginal health, probiotic lactobacilli have been widely used as an alternative or adjunct to traditional antibiotic therapy for the treatment of vaginal infections and restoration of the vaginal microbiome. This review focuses on the significant role of probiotic lactobacilli in the vaginal microenvironment and discusses the use of probiotic lactobacilli in the treatment of female vaginal infections in vitro and in vivo.
The vaginal microbiome is now largely recognized as a balanced ecosystem dominated by Lactobacillus species, with notable fluctuation over time and across individuals (Armstrong and Kaul, 2021; Lehtoranta et al., 2022). Under physiological conditions, this Lactobacillus-dominant arrangement has long been regarded as an indicator of vaginal health (Cappello et al., 2023; Shen et al., 2023). The production of lactic acid, biosurfactants, bacteriocin-like chemicals, and hydrogen peroxide (H2O2) by these Gram-positive bacilli, which varies from strain to strain, can acidify the vaginal microenvironment, inhibit the proliferation of other pathogenic microorganisms, and thereby promote the maintenance of a eubiotic vaginal microbiome (Chee et al., 2020; Das et al., 2023). However, a vaginal flora with a reduced proportion or abundance of lactobacilli can lead to vaginal dysbiosis, which is related to vaginal infections such as bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), aerobic vaginitis (AV), human papillomavirus (HPV) infection, human immunodeficiency virus (HIV) infection, herpes simplex virus 2 (HSV-2) infection, and other sexually transmitted infections (STIs) including gonorrhea, trichomoniasis, Chlamydia infection, Mycoplasma infection, and Ureaplasma infection, as well as mixed infections of these diseases (Han et al., 2021).
Owing to their “Generally Recognized as Safe” status, probiotic lactobacilli have been used as alternatives and adjuncts to antibiotics, reducing antimicrobial resistance caused by the overuse of antibiotics in the treatment of illnesses (Aslam et al., 2021; Magalhães et al., 2021; Mei and Li, 2022). In addition, the efficacy of lactobacilli as a prophylactic agent has been demonstrated in long-term administration, although most probiotics are recommended for gastrointestinal use, several are marketed for vaginal health (Lagenaur et al., 2021; Nader-Macías et al., 2021). In recent years, clinical research and development of lactobacilli for the prevention and treatment of vaginal infections in women has advanced rapidly (Lagenaur et al., 2021; Sadahira et al., 2021; Schenk et al., 2021). This paper focuses on the importance of lactobacilli in the vaginal microenvironment and summarizes the use of probiotic lactobacilli in the treatment of several different types of vaginal infections through in vitro and in vivo investigations.
A landmark study conducted by Ravel et al. revealed that the vaginal microbiome of most reproductive-age women is clustered into five microbial community state types (CSTs), four of which are dominated by Lactobacillus (CST I: L.crispatus-dominated CST, CST II: L. gasseri-dominated CST, CST III: L. iners-dominated CST, and CST V: L. jensenii-dominated CST), where CST IV is characterized by a lower level of Lactobacillus and a higher level of anaerobic bacteria (Ravel et al., 2011). Subsequently, Gajer et al. further divided CST IV into two subgroups, CST IV-A and CST IV-B. CST IV-A contains a modest fraction of L. iners as well as anaerobic bacteria, where CST IV-B contains a significant amount of BVAB (Gajer et al., 2012). It is worth noting that vaginal CSTs are changeable owing to menstruation, pregnancy, and sexual activity (Amin et al., 2023). For example, Gardnerella and Ureaplasma are more abundant during the third trimester of a normal pregnancy than during the first and second trimesters, but this change corresponds with a high Lactobacillus abundance (Park et al., 2022; Amin et al., 2023).
Lactobacilli can restore the vaginal microbiome to homeostasis via several mechanisms. Co-aggregation with pathogens may occur when the cell surface of the lactobacilli contains various mucin-binding proteins, fbronectin-binding proteins, and collagen-binding proteins, and these surface proteins can enhance the ability of lactobacilli to adhere to pathogens (Spacova et al., 2020; Dell'Anno et al., 2021; Zawistowska-Rojek et al., 2022). Production of defense metabolites including lactic acid, H2O2, bacteriocin, and biosurfactants may also occur. As fundamental components of vaginal defense, D- and L-lactic acid isomers can maintain the pH of the vaginal environment between 3.5 and 4.5, thereby making it inappropriate for the growth of pathogenic bacteria species (Kerry-Barnard et al., 2022). Most lactobacilli produce both the D- and L-chiral isomers of lactic acid. However, L. iners can only produce L-isomers. It should be noted that D-lactic acid directly affects host tissues by modulating the immune system and gene expression (France et al., 2022). H2O2 is an oxidizing chemical that is hazardous to catalase-negative bacteria, including most anaerobes. It exhibits significant antimicrobial action in vitro, and colonization with lactobacilli that generate H2O2 has been linked to a decreased incidence of BV, preterm delivery, and HIV infection (Bonneville et al., 2021). Bacteriocins are a category of potent, ribosomally produced peptides that are active against Gram-positive and Gram-negative bacteria, as well as some fungi (Mokoena et al., 2021; Darbandi et al., 2022). Biosurfactants are a structurally diverse group of surface-active molecules. They can be extremely important in lowering the ability of pathogens to attach to host cells, which is a prerequisite for the growth of biofilms (Nelson et al., 2020; Jeyanathan et al., 2021; Patel et al., 2021). Another mechanism involves competitive adhesion. Lactobacilli adhesins can cause significant adhesion to the vaginal wall. The strong adhesion of lactobacilli to the vaginal epithelium results in exclusion and rejection of harmful pathogens (Lee et al., 2021; Kawahara et al., 2022). Lactobacilli also exhibit immunomodulatory effects by increasing monocytes and macrophages which play a crucial role in the innate immune response via activation of Toll-like receptors (TLR) and the generation of cytokines (Mitchell et al., 2021). Specifically, lactobacilli and their derivatives can inhibit the expression of various pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1β, IL-2, and tumor necrosis factor (TNF)-α, and promote the production of IL-10, which can prevent systemic and local acute inflammation (Hao et al., 2021; Hu et al., 2022a). Lactobacilli can also affect the maintenance of epithelial cell tight connections. Lactobacilli can accelerate the re-epithelialization of vaginal epithelial cells and increase the production of vascular endothelial growth factor, an essential factor in tissue healing which is recognized as an epithelial cell migration inducer (Albuquerque-Souza et al., 2021).
As mentioned above, lactobacilli in the vagina are thought to be a key defensive mechanism against infection. Consequently, lactobacilli replenishment of the vaginal microbiome is an intriguing strategy for vaginal infection prevention (van de Wijgert and Verwijs, 2020; Mei and Li, 2022). Furthermore, probiotic lactobacilli have been found to be effective in changing the vaginal microbiota and improving individual health (López-Moreno and Aguilera, 2021). There are approximately 170 species of lactobacilli, but only a few such as L. acidophilus, L. brevis, L. crispatus, L. delbrueckii, L. fermentum, L. gasseri, L. helveticus, L. jensenii, L. johnsonii, L. plantarum, L. paracasei, L. reuteri, L. rhamnosus, and L. salivarius have been used to treat vaginal infections (Joseph et al., 2021; Piccioni et al., 2021). L. crispatus and L. rhamnosus are the most often employed species, although L. rhamnosus is less prevalent in the natural vaginal microbiome (Veščičík et al., 2020; Petrova et al., 2021). Furthermore, despite the fact that L. iners is common in the vaginal environment, it has not been used as a probiotic in the vagina. This may be due to its cultural complexity and uncertain importance (Zheng et al., 2021; Novak et al., 2022).
Vaginal infections have been linked to serious health consequences such as infertility, preterm birth, pelvic inflammatory disease (PID), premature rupture of membranes, and miscarriage (Huang et al., 2021; Chen et al., 2021a; Dong et al., 2022). As shown in Figure 1, vaginal infections such as BV, VVC, AV, viral infections, and other STIs are frequently linked to a vaginal flora containing a lower proportion of lactobacilli and a larger number of pathogens, leading to epithelial cell and mucosal damage in the vagina. The emergence and rapid spread of antibiotic-resistant diseases, particularly multidrug-resistant bacteria, has restricted the use of antibiotics throughout the past century (Larsson and Flach, 2022). Hence, the use of probiotic lactobacilli against pathogens has evolved as an alternative or supplementary vaginal infection therapy. In this chapter, we will outline results from in vitro experiments, in vivo animal model investigations, and clinical trials of probiotic lactobacilli in the treatment of various vaginal infections.
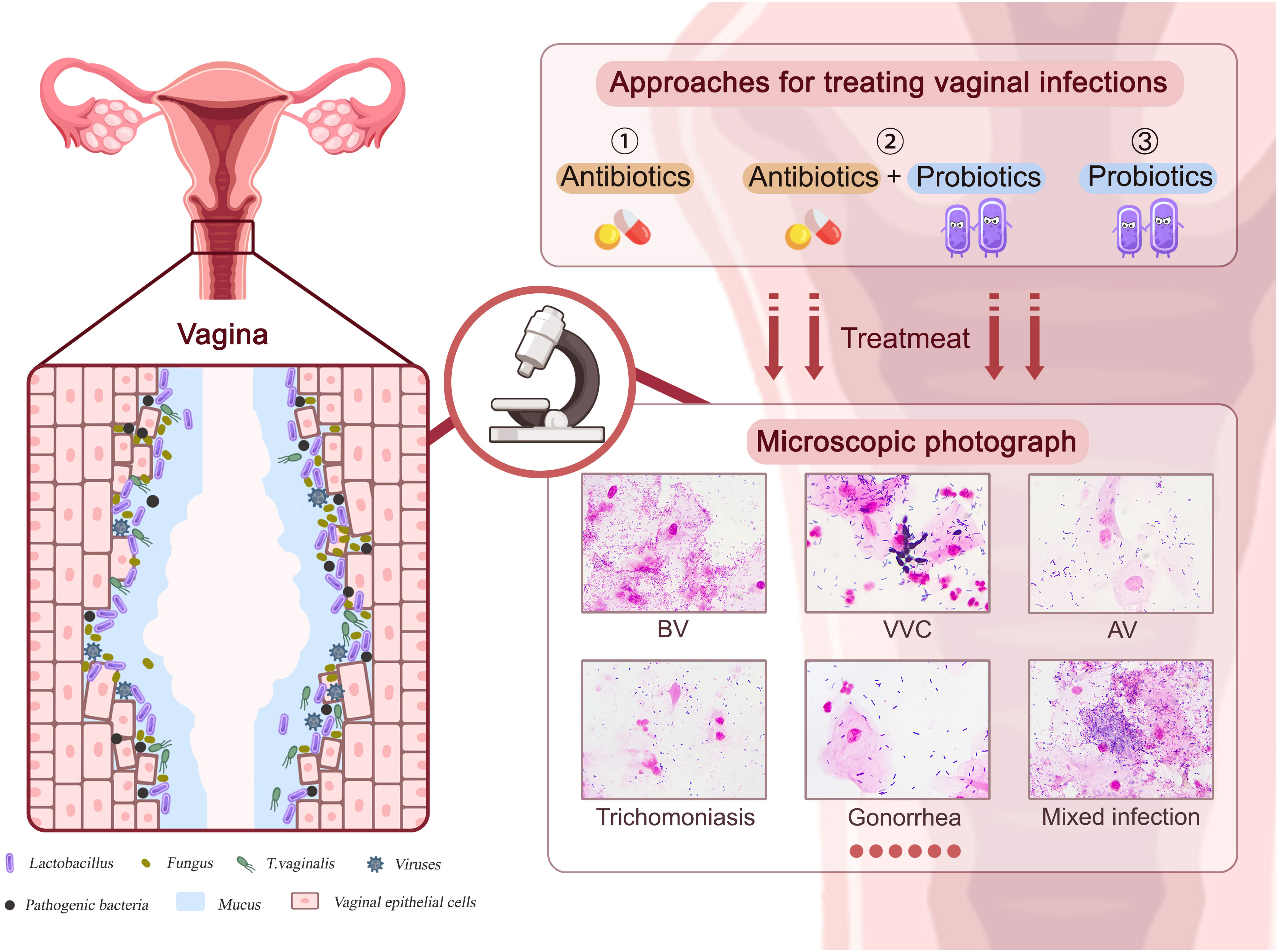
Figure 1 The approaches to treating various vaginal infections. Pathogens associated with BV, VVC, AV, viral infections, and other STIs can cause damage to vaginal epithelial cells and mucosa. Approaches including antibiotics, probiotics, and antibiotics plus probiotics could affect therapeutic efficacy.
BV is one of the most prevalent vaginal diseases in women of reproductive age, and it is characterized by the replacement of beneficial lactobacilli by an anaerobic bacterial overgrowth of Gardnerella vaginalis, Prevotella, Atopobium, and Mobiluncus (Chen et al., 2021b; Abou Chacra et al., 2022). Even though multiple studies have demonstrated a link between BV and the presence of a variety of pathogen genera and species, the significance of these organisms in the etiology and pathophysiology of the illness remains unclear (Hu et al., 2022b). In BV therapy, probiotic lactobacilli have been provided either orally or intravaginally for the maintenance and restoration of a healthy vaginal microbiome (Basavaprabhu et al., 2020).
A considerable number of recent in vitro investigations have shown that lactobacilli exhibit antibacterial activity against BV pathogens. Several Lactobacillus strains isolated from dairy products and the vaginal ecology of healthy women from different countries have antagonistic activity against BV pathogens such as P. bivia, A. vaginae, and G. vaginalis (Happel et al., 2020; Kumherová et al., 2021). More specifically, the acetic acid and lactic acid generated by lactobacilli can alter the morphology of G. vaginalis cells, ultimately causing the cells to shrink or burst (Huang et al., 2022). These two chemicals can also impair the activity of the Na+/K+-ATPase of G. vaginalis, resulting in aberrant ATP metabolism and, eventually, inhibiting G. vaginalis growth and reproduction (Huang et al., 2022). The cell-free supernatants of lactobacilli have also been shown to dramatically reduce G. vaginalis biofilm formation (Qian et al., 2021). Specifically, these probiotics can influence different phases of Gardnerella biofilm development and exhibit the highest inhibitory impact when introduced at the early stage of Gardnerella biofilm formation (He et al., 2021). Lactobacilli can also prevent the adherence of vaginal infection-causing pathogens such as Gardnerella and Mobiluncus to vaginal epithelial cells by increasing the production of the proinflammatory cytokines IL-8 and IL-1β, as well as that of human β-defensin 2, and by decreasing the concentration of secretory leukocyte protease inhibitor (He et al., 2020). Quantitative PCR analysis has shown that L. plantarum can reduce the pathogenicity of G.vaginalis by repressing the expression of the genes related to virulence factors, adhesion, biofilm formation, metabolism, and antimicrobial resistance (Qian et al., 2021).
In vivo studies performed on animal models also confirm the benefits of probiotic lactobacilli on BV infection. L. gasseri can reduce viable G. vaginalis numbers, inhibit sialidase activity, regulate TNF-α and IL-1β expression, and decrease myeloperoxidase activity in experimental mouse models (Zhang et al., 2022). Similarly, in mouse vaginal tissue lysates, a combination of five lactobacilli strains can greatly inhibit G. vaginalis proliferation and considerably lower myeloperoxidase activity, pro-inflammatory cytokine levels (TNF-α, IL-1β, and IL-6), and nitric oxide levels (Choi et al., 2022). In experiments using a Caenorhabditis elegans model, L. plantarum strain P1 has shown potent antibacterial action against G. vaginalis and considerably prolonged the lifespan of C. elegans after exposure to infection agents (Li et al., 2022).
Based on the positive efficacy of lactobacilli in treating BV infection in animal models, clinical trials on the effectiveness of a single strain or combination of probiotics given orally or intravaginally in the treatment of BV infection have been conducted. Previously, L. rhamnosus GR-1 and L. reuteri RC-14, the most thoroughly studied vaginal probiotics, have been administered orally to BV patients and have resulted in a notable improvement in vaginal flora (Martinez et al., 2009; Vujic et al., 2013). Recent clinical trials using these two strains, however, have not achieved ideal outcomes. A randomized controlled trial conducted in Shenzhen, China, was performed to evaluate the effectiveness of metronidazole alone and oral probiotics in addition to antibiotics in the treatment of BV. When L. rhamnosus GR-1 and L. reuteri RC-14 were administered orally for 30 days as an adjunct to metronidazole, no significant difference was seen in the total cure rate (Zhang et al., 2021). One possible cause may be that the L. rhamnosus GR-1 and L. reuteri RC-14 bacteria isolated from the vaginas of Caucasian and African American women might not work in Chinese people. Therefore, ethnicity might be a key factor. Aside from these two well-known Lactobacillus strains, other strains such as L. acidophilus GLA-14, L. crispatus LMG S-29995, L. crispatus DSM32717, L. crispatus DSM32720, L. paracasei LPC-S01, and L. rhamnosus HN001 have also been used orally in clinical investigations (Russo et al., 2019a; Koirala et al., 2020; Reznichenko et al., 2020; Mändar et al., 2023). These trials revealed that oral probiotics can enhance the recovery rate and relieve the symptoms of BV, as well as improve the vaginal microbial profile. As shown in Figure 2, in addition to oral delivery, intravaginal administration is becoming increasingly popular among clinical studies (Marcotte et al., 2019; Baldacci et al., 2020; Cohen et al., 2020; Armstrong et al., 2022; Mändar et al., 2023), possibly because intravaginal administration avoids the passage of lactobacilli into the highly acidic stomach, preventing loss of probiotics and accelerating the onset of effect. Compared to orally administered probiotics, which reach the vagina in approximately seven days, Lactobacillus strains administered intravaginally show effects in two to three days (Mogha and Prajapati, 2016). Most importantly, fueled by the draft guidance of the Food and Drug Administration (FDA) to develop live biotherapeutic products (LBPs), LACTIN-V (including L. crispatus CTV-05) has been developed as an adjuvant therapy and might be the first microbiome-based LBP to prevent the recurrence of BV, a breakthrough in BV treatment (Cohen et al., 2020; Lagenaur et al., 2021; Armstrong et al., 2022).
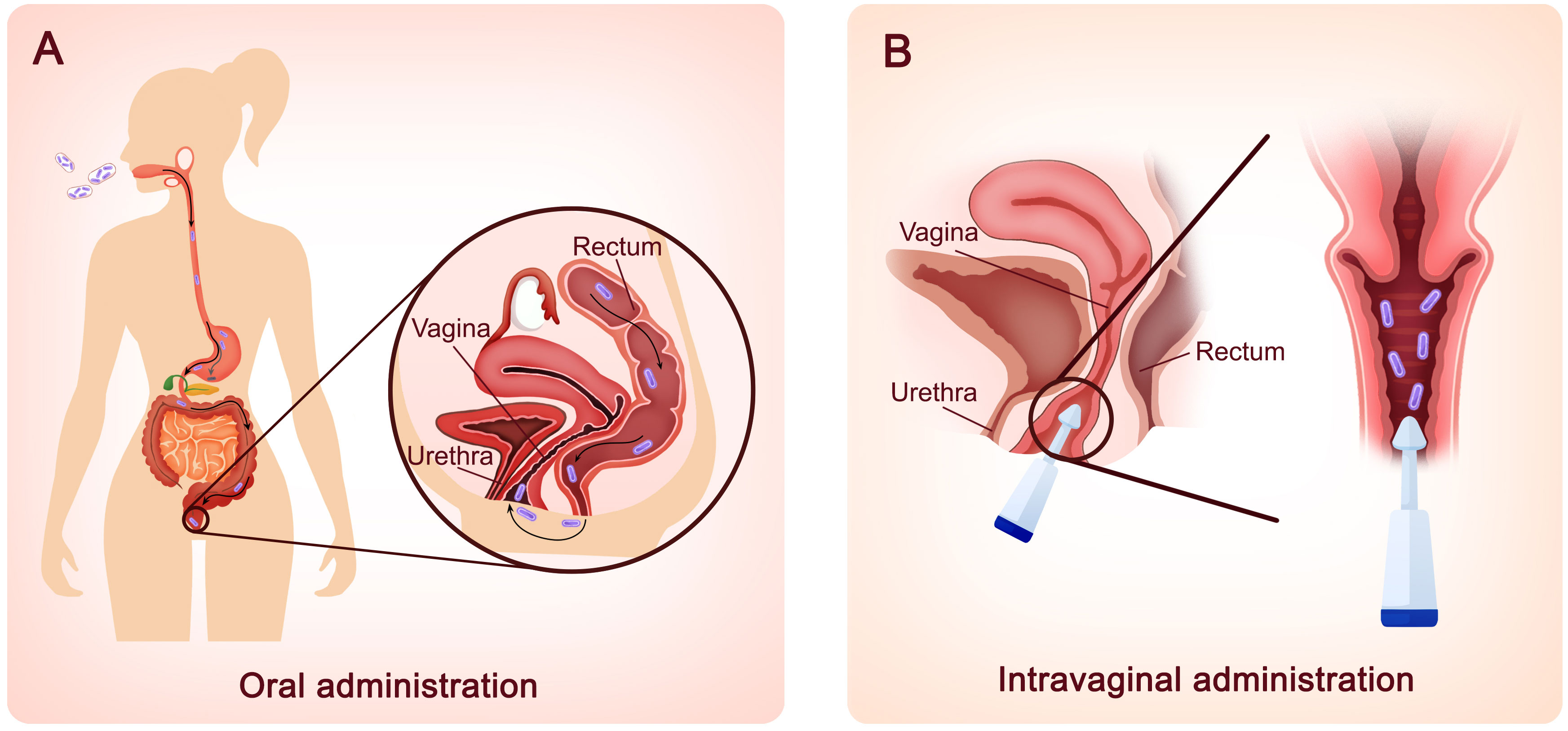
Figure 2 Two different routes for probiotic lactobacilli administration. (A) Oral administration. Probiotic lactobacilli taken orally must travel via the mouth, stomach, intestines, and colon before reaching the vagina via skin contact in the perineum. The probiotics are delivered to the vagina in approximately seven days. (B) Intravaginal administration. Probiotic lactobacilli can also be administered intravaginally using external devices. Within 2–3 days, lactobacillus strains manifest their effects.
VVC is considered the second most common vaginal infection after BV, and it is mainly caused by the opportunist fungus Candida (Willems et al., 2020). According to published research, fluconazole is widely used in the treatment of VVC; however, its function is to inhibit the growth of Candida but not eradicate it (Dunaiski et al., 2022). This can lead to the emergence of fluconazole resistance. As an alternative or adjuvant therapy, probiotic lactobacilli have shown considerable promise in the treatment of VVC (Mohankumar et al., 2022; Sun et al., 2023).
Candida may undergo a morphological change from round-ovoid yeast cells to hyphal mycelial growing organisms, allowing it to thrive as both a commensal colonizer and a pathogen (McKloud et al., 2021). Studies conducted in vitro using vaginal yeast and lactobacilli co-cultures suggest that lactobacilli inhibit the Candida yeast-to-hypha switch, reducing the level in the vagina and competing with Candida for adhesion sites on epithelial receptors, owning to their higher affinity (McKloud et al., 2021; Spaggiari et al., 2022). For example, lactic acid secreted by lactobacilli can inhibite Candida overgrowth and its transition from the commensal yeast form to the pathological hyphal form by modulating Candida protein expression, including agglutinin-like sequence protein (AlS3) and the hypha-associated proteins (HGC1, Ece1, Hwp1and Hyr1) (Chow et al., 2021). Furthermore, inhibiting the development of pathogenic hyphal forms of Candida species by lactobacilli can restrict the capacity of these yeasts to create biofilms (Nelson et al., 2020; McKloud et al., 2021; Vazquez-Munoz et al., 2022). The biosurfactants extracted from lactobacilli such as iturin, lichenysin, and surfactin have the capacity to limit Candida biofilm formation and prevent biomass expansion (Nelson et al., 2020). Lactobacilli can also reduce C. albicans pathogenicity by boosting the local immune system response of human cells by modifying immune cytokines (Andrade et al., 2021; Charlet et al., 2022). In particular, the oleic acid and palmitic acid generated by L. johnsonii can boost the expression of IL-10 and reduce the production of TNF-α, IL-6, and IL-12 in lipopolysaccharide-stimulated macrophages (Charlet et al., 2022). It should be noted that not all Lactobacillus species are advantageous and protective in VVC patients (Kalia et al., 2020). As previously documented, VVC-positive women exhibited an increase in the relative abundance of L. iners. L. iners can induce a marked increase in biofilm formation by C. albicans, enhance hyphal growth, and upregulate expression of the typical hyphae-associated genes hwp1 and ece1 (Sabbatini et al., 2021). This might limit L. iners use in treating vaginal infections.
Recent investigations in animal models have demonstrated the efficacy of oral and intravaginal probiotic therapy in treating VVC. In a mouse experimental model, biosurfactants derived from a vaginal L. crispatus strain can lower viable cell counts of C. albicans and leukocyte influx (De Gregorio et al., 2020). Similarly, in the murine VVC model, prophylactic L. casei/pPG612.1-BLF treatment increased vaginal mucosa IL-17 production, decreased IL-23 levels, and led to reduced Candida burden after 5 days of therapy (Liao et al., 2019).
Notably, several clinical investigations have demonstrated an improvement in the treatment of VVC with or without antibiotic therapy, plus oral or intravaginal probiotic lactobacilli administration. In a randomized, double-blind, placebo-controlled study, eight weeks of probiotic lactobacilli treatment was found to be helpful for pregnant women, particularly in its ability to relieve vulvovaginal symptoms and VVC recurrences, along with decreased emotional and social discomfort (Ang et al., 2022). Similarly, oral or vaginal administration of three L. crispatus strains can lower the combined scores of two of the most important symptoms in VVC patients, the amount of discharge and the level of itching/irritation (Mändar et al., 2023). In addition to lactobacilli alone, the combination of lactobacilli with antibiotics is also an effective therapy for vaginal Candida infection. By improving the composition of vaginal flora and reestablishing vaginal microecology, probiotic lactobacilli vaginal capsules combined with clotrimazole vaginal tablets can enhance the effectiveness of treatments for simple VVC (Zeng et al., 2023). The combination of L. acidophilus GLA-14, L. rhamnosus HN001, and bovine lactoferrin dramatically improved itching and discharge in VVC patients at 3 and 6 months, and throughout the six-month follow-up, the intervention group had considerably fewer recurrences than the placebo group (Russo et al., 2019b).
AV is characterized by a sharply reduced lactobacilli level, high levels of aerobic bacteria, including Streptococcus agalactiae, Staphylococcus aureus, Escherichia coli, and Enterococcus faecalis, and vaginal inflammation (Prasad et al., 2021; Wójkowska-Mach et al., 2021). Antibiotics that target aerobic bacteria such as clindamycin, meclocycline, and kanamycin have been used to treat AV (Ma et al., 2022). However, it is highly improbable that oral use of any of the antibiotics listed above have a long-term beneficial effect on the vaginal environment. Regular and sustained use of probiotic lactobacilli is a safer method in women with AV-associated vaginal flora (Das et al., 2023).
The potential benefits of probiotic lactobacilli have been Investigated in vitro with AV pathogens. Extracellular vesicles, phenyl-lactic acid, bacteriocins, and exopolysaccharides, isolated from lactobacilli display remarkable antioxidant and antiproliferative activity and inhibit S. aureus, E. faecalis, S. agalactiae, and E. coli with noticeable antibiofilm activity (Xiu et al., 2020; Abdul-Rahim et al., 2021; Croatti et al., 2022; Jiang et al., 2022). Studies have shown that lactobacilli can inhibit S. aureus through interfering with the staphylococcal quorum-sensing system agr, a key regulator of virulence genes, and by suppressing the generation of toxic shock syndrome toxin-1 in S. aureus (Li et al., 2011; Singh et al., 2022). Most importantly, as the most prevalent risk pathogen for both early-onset and late-onset newborn sepsis, S. agalactiae viability can also be inhibited by lactobacilli via multiple strategies. Recent studies have shown that supernatants from lactobacilli can inhibit S. agalactiae biofilm formation on endometrial cells (Shiroda et al., 2020; Cajulao and Chen, 2021). Lactobacilli can also lessen the pathogenicity of S. agalactiae by enhancing the local immunological response of human cells. For example, in a model of S. agalactiae-infected primary endometrial epithelial cells, the colonization of lactobacilli can decrease the expression of IL-6, which is elevated in the presence of pathogens, and increase the production of IL-1 (Chenoll et al., 2019).
The potential effectiveness of lactobacilli against AV-associated bacteria has been proven in several studies using animal models. Intravaginally administered L. reuteri have reduced physiological index, vaginal bacterial burden, and histopathological changes in vaginal tissues in E. faecalis infected mice. The probiotic L. reuteri can increase the expression of the anti-inflammatory cytokines Foxp3, interferon-gamma (IFN-γ) and reduce overexpression of the pro-inflammatory cytokines IL-6 and IL-1β caused by E. faecalis infection (Shazadi et al., 2021; Shazadi et al., 2022). Similarly, lactobacilli also have a protective effect against E. coli infection. By the induction of a host IFN-1 response, which in turn increases the production of cathepsin D within lysosomes to kill the pathogen, L. crispatus can alleviate bladder uropathogenic E. coli infection in a mouse model (Song et al., 2022). In mice, serial vaginal inoculation with probiotic L. reuteri gives partial protection against S. agalactiae infections, and this effect is mediated in part by mucosal immunity (Brokaw et al., 2021).
Clinical trials performed to determine whether probiotic lactobacilli can be used to treat AV have demonstrated considerable promise. In a pilot study, pregnant women ingested L. salivarius CECT 9145 daily from week 26 to week 38, and the treatment can reduce the number of S. agalactiae-positive women throughout pregnancy and the number of women with intrapartum antibiotic prophylaxis during birth (Martín et al., 2019). Similarly, in China, vaginal and rectal S. agalactiae-positive pregnant women who consumed two probiotic capsules containing L. rhamnosus and L. reuteri also significantly decreased the species abundance of Enterococcus, Staphylococcus, and Streptococcus in the vaginal flora and improved pregnancy outcomes (Liu et al., 2020). A meta-analysis also found that taking probiotics throughout pregnancy was related to lower GBS recto-vaginal colonization at 35-37 weeks and a healthy perinatal profile (Menichini et al., 2022).
HIV, HPV, and HSV-2 are the three most prevalent sexually transmitted viruses that have a substantial impact on female health. According to the statistics provided by UNAIDS for 2018, there were 36.9 million individuals living with HIV and 1.8 million newly diagnosed cases in 2018 (Baxi et al., 2020). More importantly, young women of reproductive age continue to be one of the most vulnerable and impacted populations, with much higher rates of HIV infection than men of the same age (De Oliveira et al., 2017). HPV can infect the stratified squamous epithelium and stimulate cellular proliferation, resulting in benign hyperplasia or in some cases, cervical cancer following prolonged, unresolved infection (Szymonowicz and Chen, 2020; Alimena et al., 2022). HSV-2 can produce genital herpes in the form of painful sores at the site of viral replication and shedding and has the capacity to penetrate the central nervous system and induce latent dorsal root ganglia infection when in touch with the genital mucosal surface (Chentoufi et al., 2022). Because each of these illnesses raises the chance of developing another STI, the epidemiology of these infections is exceedingly convoluted (Torcia, 2019).
Based on its essential role in the female vagina, lactobacilli have shown potential to relieve various viral infections in vitro. By blocking HIV-1-cell receptor connections, extracellular vesicles generated by lactobacilli can protect human cervico-vaginal cells. This inhibition was related to a decrease in viral envelope protein exposure due to the steric hindrance of gp120 (Costantini et al., 2022). In addition to wild-type strains, bioengineered lactobacilli that express anti-HIV molecules, such as human CD4, bn nanobodies, have also demonstrated potent anti-HIV activity and have the potential to be used as a live virucide in the vaginal mucosa of women at high risk for HIV infection (Wei et al., 2019; Kalusche et al., 2020). Lactobacilli also possess antiviral activity against HSV-2, and a vaginal microbiome that is dominated by lactobacilli can produce a considerable decrease in the load of HSV-2 in vaginal epithelial cells (Amerson-Brown et al., 2019). HPV is the most common virus among women, and it was discovered that supernatants of lysates and heat-inactivated lactobacilli can suppress the production of the human papillomavirus genes e6/e7, which are the leading cause of cervical cancer (Hu et al., 2021). Additionally, immune-related pathways in cell models indicate that the L. casei strain can cause the production of large quantities of IL-2, a cytokine with well-established anti-cervical cancer effects (Jacouton et al., 2019a).
In vivo studies in animal models indicate that lactobacilli also possess anti-viral properties. In a humanized mouse model, scientists have engineered Lactobacillus acidophilus to express the HIV-1 receptor human CD4 on the cell surface. The modified strains can block HIV-1 infection locally at the intrarectal site of infection but not systemically, reducing the efficiency of HIV-1 sexual transmission (Wei et al., 2019). Similarly, in animal models, lactobacilli have also been modified to express the granulocyte-macrophage colony-stimulating factor and IL-17A cytokine which can cause cytolytic and proliferative responses in splenocytes that are specific to cervical cancer (Jacouton et al., 2019b; Abdolalipour et al., 2022). Therefore, this may be used as a candidate HPV mucosal vaccine with cross-neutralizing activity against diverse HPV types.
Clinical investigations have been conducted to validate the effects of lactobacilli for the treatment of people afflicted with viruses; nevertheless, the findings are not always positive. In a double-blind placebo-controlled clinical trial, intervention with L. plantarum and Pediococcus acidilactici was found to be safe and to lead to increases in the CD4/CD8 ratio and reductions in sCD14 in HIV-1 infected patients (Blázquez-Bondia et al., 2022). However, using the probiotic Lactobacillus casei Shirota had no discernible impact on immunological activation indicators, NK cells, CD4+ and CD8+ subpopulations, or sCD14 levels in HIV-infected patients receiving suppressive antiretroviral treatment with poor CD4+ recovery over the course of a 12-week period (Tenore et al., 2020). Recent preventive HPV vaccinations have been demonstrated to prevent genital infection with multiple HPV types; however, individuals who were infected with HPV prior to vaccination will likely see little benefit. In patients with cervical intraepithelial neoplasia (CIN) 3, an oral agent that expresses the HPV 16 E7 antigen on the surface of L. casei, is a breakthrough. Studies showed that 70% of patients receiving the optimized dose encountered a pathological down-grade to CIN 2 after 9 weeks of therapy, and 75% of CIN 3 patients were cured in a subsequent phase 2a clinical trial (Ikeda et al., 2019; Park et al., 2019). Comparable to engineered bacteria, long-term treatment with wild Lactobacillus species also demonstrates promising benefits in clinical studies against HPV infection. Long-term use of natural Lactobacillus species can change the CST status and increase HPV clearance in women with dysbiosis and concurrent HPV-infections, and thus may have a beneficial influence on HPV infection management (DI Pierro et al., 2021). Acyclovir is the most common first-line therapy in HSV-2 infected individuals with recurrent genital infections (Sharma et al., 2023). A randomized double-blind controlled trial comparing the effectiveness and safety of the multi-strain probiotic and acyclovir found no significant difference in resolution of episode, viral shedding, lesion healing time, or proportion of discomfort, demonstrating the great promise of probiotic lactobacilli against HSV-2 infection (Mohseni et al., 2018).
Apart from the above-mentioned infections, STIs such as gonorrhea, trichomoniasis, Chlamydia infection, Mycoplasma infection, and Ureaplasma infection can invariably result in a major burden for female patients, even though they are not often fatal (Van Gerwen et al., 2022). These STIs are associated with consequences such as PID, ectopic pregnancy, infertility, seronegative arthropathy, and neurological and cardiovascular disorders (Van Gerwen et al., 2022). STIs during pregnancy can also result in fetal or neonatal mortality, early birth, newborn encephalitis, ocular infections, and pneumonia (Glassford et al., 2020). There is an increasing concern for the level of antibiotic resistance in STIs, which is frequently underestimated by clinicians (Tien et al., 2020; Williams et al., 2021). Urgent demand exists for “antibiotic-free” techniques for the control of STIs. Acting as a significant barrier to pathogens in the promotion of female vaginal health, lactobacilli have a great potential for use against vaginal pathogens (Loeper et al., 2018).
Experiments conducted in vitro established that lactobacilli can protect the lower female genital tract against infection caused by N. gonorrheae. L. crispatus can decrease the adhesion and invasiveness of N. gonorrhoeae through reducing the expression of genes responsible for pro-inflammatory cytokines like TNF-α and CCL20 in N. gonorrhoeae-infected epithelial cells (Płaczkiewicz et al., 2020). Similarly, the cell surface aggregation-promoting factor from L. gasseri can block Trichomonas vaginalis adhesion to human vaginal ectocervical cells in a dose-dependent manner (Phukan et al., 2018; Malfa et al., 2023). The three main intracellular parasites in the female vagina, Mycoplasma, Chlamydia, and Ureaplasma, have also been shown to be inhibited from infecting the vagina by lactate, bacteriocins, and the acidic environment induced by lactobacilli (Parolin et al., 2018; García-Galán et al., 2020; Garza et al., 2021; Chen et al., 2022). Lactobacilli cells can also directly inactivate the extracellular infectious elementary body of C. trachomatis in a concentration-dependent manner, although to a lower extent than their supernatants, by inducing quick and dynamic membrane changes (Parolin et al., 2018).
Several studies indicate that lactobacilli can suppress Chlamydia infectivity in animal models, which provides valuable information for the development of lactobacilli as an additional therapy or vaccine for Chlamydia infection. Female BALB/c mice intravaginally administered with lactobacilli mixtures, but not a single Lactobacillus strain, following genital Chlamydia infection showed dramatically reduced levels of Chlamydia shedding in both the lower vaginal tract and the intestinal tract, decreased production of cytokines such as TNF-α, IFN-γ, and IL-1β in the vagina, and attenuated upper genital tract pathogenicity and inflammation (Chen et al., 2022). An engineered strain of L. plantarum containing the C. trachomatis antigen, also known as heterologous immuno-repeat 2, has the potential to be used as a mucosal booster vaccine to elicit H2-specific IgA responses in the vaginal mucosa, which is relevant to the prevention and treatment of C. trachomatis genital infections in female B6C3F1 mice (Kuczkowska et al., 2017).
To the best of our knowledge, just one clinical experiment has been conducted evaluating the effectiveness of probiotic lactobacilli against vaginal Ureaplasma parvum. Participants in the treatment group of a prospective, monocentric, randomized controlled trial took one sachet a day of a defined probiotic supplement containing four different Lactobacillus strains for a period of four weeks. After the intervention period, there was a significant difference in the relative abundance of U. parvum between the control group (3.52%) and the intervention group (0.77%) (Schenk et al., 2021).
Mixed vaginal infections are characterized by the presence of at least two separate vaginal pathogens at the same time, both of which contribute to an aberrant vaginal environment and result in signs and symptoms (Qi et al., 2021). Even when two different bacteria have been detected in a co-infection, there is still a possibility that one of the infections may not be the source of the vaginal symptoms that are already present (Tumietto et al., 2019). Indeed, it has been documented that the interaction between different pathogens might affect the antibiotic sensitivity of both organisms (Gilbert et al., 2021).
Based on the essential role of lactobacilli in the vaginal microbiome, nearly all probiotic Lactobacillus species can combat distinct vaginal pathogens according to in vitro studies. Two commercially available Lactobacillus strains, L. acidophilus GLA-14 and Lactobacillus rhamnosus HN001, were tested in a co-culture assays against four distinct pathogens that cause both BV and AV (Bertuccini et al., 2017). L. rhamnosus CA15 generated H2O2, organic acid, and lactic acid, and showed a broad spectrum of antagonistic action and enhanced colonization resistance to urinary tract pathogens, such as E. faecalis, E. coli, C. albicans, G. vaginalis, and S. agalactiae (Pino et al., 2022a). Similarly, several Lactobacillus strains isolated from the vaginal ecology of healthy women also showed antagonistic activity against the pathogens that potentially cause BV, VVC, and AV (Kumherová et al.,2021).
Despite few animal studies have examined the effects of probiotic lactobacilli on mixed infections in the vagina, several clinical trials have examined whether probiotics can enhance the efficacy of antimicrobial treatment for co-infections. The oral probiotic product prOVag®, comprised of three Lactobacillus strains, decreased the recurrence rate of BV/AV mixed infections by 51% compared with placebo (Heczko et al., 2015). Adding probiotic lactobacilli to the conventional treatment of TV in patients who also had BV can also significantly raise the cure rates of both TV and BV (Sgibnev and Kremleva, 2020). Participants taking daily oral probiotic lactobacilli capsules can lead to a substantial improvement in the vaginal flora in terms of an increase in lactobacilli and a decrease in enterococci, staphylococci, Gardnerella spp., and Candida spp. in mixed vaginal infected patients (Pino et al., 2022b; Rapisarda et al., 2023).
Over the past decades, a significant amount of knowledge has been gained regarding the vaginal microbiome and how it is related to host health. A considerable amount of development and advancement have been achieved in the use of probiotic lactobacilli in the prevention and treatment of vaginal infections. The primary benefit of probiotic lactobacilli is the recovery of a healthy, natural microbiome in in the vagina, transforming it from a disease-causing, dysbiotic ecosystem to a healthy, symbiotic microbiome. However, despite the well-established safety of probiotics, not all clinical studies have met their effectiveness objectives. There is a great deal of heterogeneity across clinical trials in terms of probiotic strains/combinations used and the target population by ethnicity, age, life stage, and research methodologies. The FDA has not yet authorized any probiotics for the prevention and treatment of female vaginal infections, thus, these probiotics can only be categorized as safe and beneficial dietary supplements (Stavropoulou and Bezirtzoglou, 2020).
In recent years, studies demonstrating the efficiency of fecal microbiota transplants (FMTs) for the treatment of recurrent Clostridioides difficile infections have prompted research into vaginal microbiota transplants as a possible treatment for female vaginal infections (Lev-Sagie et al., 2019). Like FMTs, vaginal samples from healthy donors must be thoroughly tested to rule out infectious microorganisms to prevent severe adverse effects, particularly in immunocompromised patients (Zhang et al., 2018). As opposed to stools, which may be easily obtained from healthy donors, it is difficult to acquire enough vaginal samples from donors due to the small amount of vaginal discharge and the unwillingness of some women to donate. To overcome these restrictions, a platform including transplants of large synthetic microbial consortia, as opposed to individual probiotics or undefined community consortia that may contain infectious pathogens, has been developed. While startup companies like Vedanta Biosciences and the Federation Bio have created artificial microbiome products that are in the commercial development stage, these products are focused more on recurrent Clostridioides difficile infection, cancers, inflammatory bowel disease, and allergies (Seydel, 2021; Dsouza et al., 2022). For the treatment of female vaginal infections, it is possible that the synthesis of diverse microbiomes like the CSTs of healthy women will be achieved. Based on the application of emerging sequencing technologies, a synthetic microbial microbiome may have the potential to function as personalized medicine for people who possess complex and distinctive characteristics.
PL drafted the manuscript for publication. PL and XC prepared the draft of Figures. YL provided the microscope images of Figure 1. XC and RL reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Science and Technology Project of Jinan Municipal Health Commission (2020-4-71 and 2022-1-37).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdolalipour, E., Mahooti, M., Gorji, A., Ghaemi, A. (2022). Synergistic therapeutic effects of probiotic Lactobacillus casei TD-2 consumption on GM-CSF-induced immune responses in a murine model of cervical cancer. Nutr. Cancer. 74, 372–382. doi: 10.1080/01635581.2020.1865419
Abdul-Rahim, O., Wu, Q., Price, T. K., Pistone, G., Diebel, K., Bugni, T. S., et al. (2021). Phenyl-lactic acid is an active ingredient in bactericidal supernatants of Lactobacillus crispatus. J. Bacteriol. 203, e0036021. doi: 10.1128/JB.00360-21
Abou Chacra, L., Fenollar, F., Diop, K. (2022). Bacterial vaginosis: What do we currently know? Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.672429
Albuquerque-Souza, E., Ishikawa, K. H., Amado, P. P., Nicoli, J. R., Holzhausen, M., Mayer, M. P. A. (2021). Probiotics improve re-epithelialization of scratches infected by porphyromonas gingivalis through up-regulating CXCL8-CXCR1/CXCR2 axis. Anaerobe. 72, 102458. doi: 10.1016/j.anaerobe.2021.102458
Alimena, S., Davis, J., Fichorova, R. N., Feldman, S. (2022). The vaginal microbiome: A complex milieu affecting risk of human papillomavirus persistence and cervical cancer. Curr. Probl Cancer. 46, 100877. doi: 10.1016/j.currproblcancer.2022.100877
Amerson-Brown, M. H., Miller, A. L., Maxwell, C. A., White, M. M., Vincent, K. L., Bourne, N., et al. (2019). Cultivated human vaginal microbiome communities impact zika and herpes simplex virus replication in ex vivo vaginal mucosal cultures. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03340
Amin, M. E., Azab, M., Hanora, A., Atwa, K., Shabayek, S. (2023). Compositional changes in the vaginal bacterial microbiome of healthy pregnant women across the three gestational trimesters in ismailia, Egypt. Microorganisms. 11, 139. doi: 10.3390/microorganisms11010139
Andrade, J. C., Kumar, S., Kumar, A., Černáková, L., Rodrigues, C. F. (2021). Application of probiotics in candidiasis management. Crit. Rev. Food Sci. Nutr. 22, 1–16. doi: 10.1080/10408398.2021.1926905
Ang, X. Y., Chung, F. Y., Lee, B. K., Azhar, S. N. A., Sany, S., Roslan, N. S., et al. (2022). Lactobacilli reduce recurrences of vaginal candidiasis in pregnant women: A randomized, double-blind, placebo-controlled study. J. Appl. Microbiol. 132, 3168–3180. doi: 10.1111/jam.15158
Armstrong, E., Hemmerling, A., Miller, S., Burke, K. E., Newmann, S. J., Morris, S. R., et al. (2022). Sustained effect of LACTIN-V (Lactobacillus crispatus CTV-05) on genital immunology following standard bacterial vaginosis treatment: Results from a randomised, placebo-controlled trial. Lancet Microbe 3, e435–e442. doi: 10.1016/S2666-5247(22)00043-X
Armstrong, E., Kaul, R. (2021). Beyond bacterial vaginosis: vaginal lactobacilli and HIV risk. Microbiome. 9, 239. doi: 10.1186/s40168-021-01183-x
Aslam, B., Khurshid, M., Arshad, M. I., Muzammil, S., Rasool, M., Yasmeen, N., et al. (2021). Antibiotic resistance: one health one world outlook. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.771510
Baldacci, F., Baldacci, M., Bertini, M. (2020). Lactobacillus rhamnosus BMX 54 + lactose, a symbiotic long-lasting vaginal approach to improve women's health. Int. J. Womens Health 12, 1099–1104. doi: 10.2147/IJWH.S259311
Basavaprabhu, H. N., Sonu, K. S., Prabha, R. (2020). Mechanistic insights into the action of probiotics against bacterial vaginosis and its mediated preterm birth: An overview. Microb. Pathog. 141, 104029. doi: 10.1016/j.micpath.2020.104029
Baxi, K., Sawarkar, S., Momin, M., Patel, V., Fernandes, T. (2020). Vaginal siRNA delivery: Overview on novel delivery approaches. Drug Delivery Transl. Res. 10, 962–974. doi: 10.1007/s13346-020-00741-4
Bertuccini, L., Russo, R., Iosi, F., Superti, F. (2017). Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 30, 163–167. doi: 10.1177/0394632017697987
Blázquez-Bondia, C., Parera, M., Català-Moll, F., Casadellà, M., Elizalde-Torrent, A., Aguiló, M., et al. (2022). Probiotic effects on immunity and microbiome in HIV-1 discordant patients. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1066036
Bonneville, L., Maia, V., Barroso, I., Martínez-Suárez, J. V., Brito, L. (2021). Lactobacillus plantarum in dual-species biofilms with Listeria monocytogenes enhanced the anti-Listeria activity of a commercial disinfectant based on hydrogen peroxide and peracetic acid. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.631627
Brokaw, A., Furuta, A., Dacanay, M., Rajagopal, L., Adams Waldorf, K. M. (2021). Bacterial and host determinants of group b streptococcal vaginal colonization and ascending infection in pregnancy. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.720789
Cajulao, J. M., Chen, L. (2021). Lactobacillus rhamnosus reduces the cytotoxic effects of group b streptococcus on HeLa cells. Microb. Pathog. 161, 105271. doi: 10.1016/j.micpath.2021.105271
Cappello, C., Acin-Albiac, M., Pinto, D., Polo, A., Filannino, P., Rinaldi, F., et al. (2023). Do nomadic lactobacilli fit as potential vaginal probiotics? the answer lies in a successful selective multi-step and scoring approach. Microb. Cell Fact. 22, 27. doi: 10.1186/s12934-023-02030-4
Charlet, R., Le Danvic, C., Sendid, B., Nagnan-Le Meillour, P., Jawhara, S. (2022). Oleic acid and palmitic acid from Bacteroides thetaiotaomicron and Lactobacillus johnsonii exhibit anti-inflammatory and antifungal properties. Microorganisms. 10, 1803. doi: 10.3390/microorganisms10091803
Chee, W. J. Y., Chew, S. Y., Than, L. T. L. (2020). Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 19, 203. doi: 10.1186/s12934-020-01464-4
Chen, X., Lu, Y., Chen, T., Li, R. (2021b). The female vaginal microbiome in health and bacterial vaginosis. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.631972
Chen, H., Min, S., Wang, L., Zhao, L., Luo, F., Lei, W., et al. (2022). Lactobacillus modulates Chlamydia infectivity and genital tract pathology in vitro and in vivo. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.877223
Chen, T., Xia, C., Hu, H., Wang, H., Tan, B., Tian, P., et al. (2021a). Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents. 57, 106277. doi: 10.1016/j.ijantimicag.2021.106277
Chenoll, E., Moreno, I., Sánchez, M., Garcia-Grau, I., Silva, Á., González-Monfort, M., et al. (2019). Selection of new probiotics for endometrial health. Front. Cell Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00114
Chentoufi, A. A., Dhanushkodi, N. R., Srivastava, R., Prakash, S., Coulon, P. A., Zayou, L., et al. (2022). Combinatorial herpes simplex vaccine strategies: from bedside to bench and back. Front. Immunol. 13. doi: 10.3389/fimmu.2022.849515
Choi, S. I., Won, G., Kim, Y., Kang, C. H., Kim, G. H. (2022). Lactobacilli strain mixture alleviates bacterial vaginosis through antibacterial and antagonistic activity in Gardnerella vaginalis-infected C57BL/6 mice. Microorganisms. 10, 471. doi: 10.3390/microorganisms10020471
Chow, E. W. L., Pang, L. M., Wang, Y. (2021). From Jekyll to Hyde: The yeast-hyphal transition of Candida albicans. Pathogens. 10, 859. doi: 10.3390/pathogens10070859
Cohen, C. R., Wierzbicki, M. R., French, A. L., Morris, S., Newmann, S., Reno, H., et al. (2020). Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl. J. Med. 382, 1906–1915. doi: 10.1056/NEJMoa1915254
Costantini, P. E., Vanpouille, C., Firrincieli, A., Cappelletti, M., Margolis, L., Ñahui Palomino, R. A. (2022). Extracellular vesicles generated by gram-positive bacteria protect human tissues ex vivo from HIV-1 infection. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.822882
Croatti, V., Parolin, C., Giordani, B., Foschi, C., Fedi, S., Vitali, B. (2022). Lactobacilli extracellular vesicles: Potential postbiotics to support the vaginal microbiota homeostasis. Microb. Cell Fact. 21, 237. doi: 10.1186/s12934-022-01963-6
Darbandi, A., Asadi, A., Mahdizade Ari, M., Ohadi, E., Talebi, M., Halaj Zadeh, M., et al. (2022). Bacteriocins: properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36, e24093. doi: 10.1002/jcla.24093
Das, S., Bhattacharjee, M. J., Mukherjee, A. K., Khan, M. R. (2023). Recent advances in understanding of multifaceted changes in the vaginal microenvironment: Implications in vaginal health and therapeutics. Crit. Rev. Microbiol. 21, 1–27. doi: 10.1080/1040841X.2022.2049696
De Gregorio, P. R., Parolin, C., Abruzzo, A., Luppi, B., Protti, M., Mercolini, L., et al. (2020). Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb. Cell Fact. 19, 133. doi: 10.1186/s12934-020-01390-5
Dell'Anno, M., Giromini, C., Reggi, S., Cavalleri, M., Moscatelli, A., Onelli, E., et al. (2021). Evaluation of adhesive characteristics of l. plantarum and L. reuteri isolated from Weaned piglets. Microorganisms. 9, 1587. doi: 10.3390/microorganisms9081587
De Oliveira, T., Kharsany, A. B., Gräf, T., Cawood, C., Khanyile, D., Grobler, A., et al. (2017). Transmission networks and risk of HIV infection in KwaZulu-natal, south Africa: A community-wide phylogenetic study. Lancet HIV. 4, e41–e50. doi: 10.1016/S2352-3018(16)30186-2
DI Pierro, F., Criscuolo, A. A., Dei Giudici, A., Senatori, R., Sesti, F., Ciotti, M., et al. (2021). Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: Results of a preliminary, uncontrolled, open trial. Minerva Obstet Gynecol. 73, 621–631. doi: 10.23736/S2724-606X.21.04752-7
Dong, Z., Fan, C., Hou, W., Rui, C., Wang, X., Fan, Y., et al. (2022). Vaginal exposure to Candida albicans during early gestation results in adverse pregnancy outcomes via inhibiting placental development. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.816161
Dsouza, M., Menon, R., Crossette, E., Bhattarai, S. K., Schneider, J., Kim, Y. G., et al. (2022). Colonization of the live biotherapeutic product VE303 and modulation of the microbiota and metabolites in healthy volunteers. Cell Host Microbe 30, 583–598.e8. doi: 10.1016/j.chom.2022.03.016
Dunaiski, C. M., Kock, M. M., Jung, H., Peters, R. P. H. (2022). Importance of Candida infection and fluconazole resistance in women with vaginal discharge syndrome in Namibia. Antimicrob. Resist. Infect. Control. 11, 104. doi: 10.1186/s13756-022-01143-6
France, M., Alizadeh, M., Brown, S., Ma, B., Ravel, J. (2022). Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 7, 367–378. doi: 10.1038/s41564-022-01083-2
Gajer, P., Brotman, R. M., Bai, G., Sakamoto, J., Schütte, U. M., Zhong, X., et al. (2012). Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra52. doi: 10.1126/scitranslmed.3003605
García-Galán, A., de la Fe, C., Gomis, J., Bataller, E., Sánchez, A., Quereda, J. J., et al. (2020). The addition of lactobacillus spp. negatively affects Mycoplasma bovis viability in bovine cervical mucus. BMC Vet. Res. 16, 251. doi: 10.1186/s12917-020-02454-9
Garza, J., Gandhi, K., Choi, S., Sanchez, A., Ventolini, G. (2021). Cytokine profiles and Lactobacillus species presence in pre-menopausal subjects with genital Mycoplasma genitalium or Ureaplasma urealyticum colonization. Womens Health (Lond). 17, 17455065211009181. doi: 10.1177/17455065211009181
Gilbert, N. M., Foster, L. R., Cao, B., Yin, Y., Mysorekar, I. U., Lewis, A. L. (2021). Gardnerella vaginalis promotes group b streptococcus vaginal colonization, enabling ascending uteroplacental infection in pregnant mice. Am. J. Obstet Gynecol. 224, 530.e1–530.e17. doi: 10.1016/j.ajog.2020.11.032
Glassford, M., Davis, M., Rivas, S. (2020). Sexually transmitted infections in pregnancy: An update for primary care providers. Nurs. Clin. North Am. 55, 403–416. doi: 10.1016/j.cnur.2020.06.004
Han, Y., Liu, Z., Chen, T. (2021). Role of vaginal microbiota dysbiosis in gynecological diseases and the potential interventions. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.643422
Hao, H., Zhang, X., Tong, L., Liu, Q., Liang, X., Bu, Y., et al. (2021). Effect of extracellular vesicles derived from Lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front. Immunol. 12. doi: 10.3389/fimmu.2021.777147
Happel, A. U., Kullin, B., Gamieldien, H., Wentzel, N., Zauchenberger, C. Z., Jaspan, H. B., et al. (2020). Exploring potential of vaginal Lactobacillus isolates from south African women for enhancing treatment for bacterial vaginosis. PloS Pathog. 16, e1008559. doi: 10.1371/journal.ppat.1008559
He, Y., Na, R., Niu, X., Xiao, B., Yang, H. (2021). Lactobacillus rhamnosus and Lactobacillus casei affect various stages of Gardnerella species biofilm formation. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.568178
He, Y., Niu, X., Wang, B., Na, R., Xiao, B., Yang, H. (2020). Evaluation of the inhibitory effects of Lactobacillus gasseri and Lactobacillus crispatus on the adhesion of seven common lower genital tract infection-causing pathogens to vaginal epithelial cells. Front. Med. (Lausanne). 7. doi: 10.3389/fmed.2020.00284
Heczko, P. B., Tomusiak, A., Adamski, P., Jakimiuk, A. J., Stefański, G., Mikołajczyk-Cichońska, A., et al (2015). Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebo-controlled trial. BMC Womens Health 15, 115.
Hu, J., Deng, F., Zhao, B., Lin, Z., Sun, Q., Yang, X., et al. (2022b). Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via toll-like receptor 2 signaling. Microbiome. 10, 38. doi: 10.1186/s40168-022-01227-w
Hu, S., Hao, Y., Zhang, X., Yang, Y., Liu, M., Wang, N., et al. (2021). Lacticaseibacillus casei LH23 suppressed HPV gene expression and inhibited cervical cancer cells. Probiotics Antimicrob. Proteins. doi: 10.1007/s12602-021-09848-7
Hu, J., Wu, Y., Quan, L., Yang, W., Lang, J., Tian, G., et al. (2022a). Research of cervical microbiota alterations with human papillomavirus infection status and women age in sanmenxia area of China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1004664
Huang, Y. P., Shi, J. Y., Luo, S. C., Xu, S. Y., Zhang, J. D., Molnár, I., et al. (2022). Antimicrobial substances and mechanisms of Lactobacillus rhamnosus against Gardnerella vaginalis. Probiotics Antimicrob. Proteins. doi: 10.1007/s12602-022-10019-5
Huang, J., Zheng, L., Su, Y., Wang, F., Kong, H., Chang, Y., et al. (2021). Effects of group b streptococcus infection on vaginal micro-ecology and pregnancy outcomes of pregnant women in late pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 267, 274–279. doi: 10.1016/j.ejogrb.2021.11.419
Ikeda, Y., Uemura, Y., Asai-Sato, M., Nakao, T., Nakajima, T., Iwata, T., et al. (2019). Safety and efficacy of mucosal immunotherapy using human papillomavirus (HPV) type 16 E7-expressing Lactobacillus-based vaccine for the treatment of high-grade squamous intraepithelial lesion (HSIL): the study protocol of a randomized placebo-controlled clinical trial (MILACLE study). Jpn J. Clin. Oncol. 49, 877–880. doi: 10.1093/jjco/hyz095
Jacouton, E., Michel, M. L., Torres-Maravilla, E., Chain, F., Langella, P., Bermúdez-Humarán, L. G. (2019a). Elucidating the immune-related mechanisms by which probiotic strain Lactobacillus casei BL23 displays anti-tumoral properties. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03281
Jacouton, E., Torres Maravilla, E., Boucard, A. S., Pouderous, N., Pessoa Vilela, A. P., Naas, I., et al. (2019b). Anti-tumoral effects of recombinant Lactococcus lactis strain secreting IL-17A cytokine. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03355
Jeyanathan, A., Ramalhete, R., Blunn, G., Gibbs, H., Pumilia, C. A., Meckmongkol, T. (2021). Lactobacillus cell-free supernatant as a novel bioagent and biosurfactant against Pseudomonas aeruginosa in the prevention and treatment of orthopedic implant infection. J. BioMed. Mater Res. B Appl. Biomater. 109, 1634–1643. doi: 10.1002/jbm.b.34821
Jiang, Y. H., Xin, W. G., Yang, L. Y., Ying, J. P., Zhao, Z. S., Lin, L. B., et al. (2022). A novel bacteriocin against Staphylococcus aureus from Lactobacillus paracasei isolated from yunnan traditional fermented yogurt: purification, antibacterial characterization, and antibiofilm activity. J. Dairy Sci. 105, 2094–2107. doi: 10.3168/jds.2021-21126
Joseph, R. J., Ser, H. L., Kuai, Y. H., Tan, L. T., Arasoo, V. J. T., Letchumanan, V., et al. (2021). Finding a balance in the vaginal microbiome: How do we treat and prevent the occurrence of bacterial vaginosis? Antibiotics (Basel). 10, 719. doi: 10.3390/antibiotics10060719
Kalia, N., Singh, J., Kaur, M. (2020). Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 19, 5. doi: 10.1186/s12941-020-0347-4
Kalusche, S., Vanshylla, K., Kleipass, F., Gruell, H., Müller, B., Zeng, Z., et al. (2020). Lactobacilli expressing broadly neutralizing nanobodies against HIV-1 as potential vectors for HIV-1 prophylaxis? Vaccines (Basel). 8, 758. doi: 10.3390/vaccines8040758
Kawahara, T., Shimizu, I., Tanaka, Y., Tobita, K., Tomokiyo, M., Watanabe, I. (2022). Lactobacillus crispatus strain KT-11 s-layer protein inhibits rotavirus infection. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.783879
Kerry-Barnard, S., Zhou, L., Phillips, L., Furegato, M., Witney, A. A., Sadiq, S. T., et al. (2022). Vaginal microbiota in ethnically diverse young women who did or did not develop pelvic inflammatory disease: Community-based prospective study. Sex. Transm. Infect. 0, 1–7. doi: 10.1136/sextrans-2021-055260
Koirala, R., Gargari, G., Arioli, S., Taverniti, V., Fiore, W., Grossi, E., et al. (2020). Effect of oral consumption of capsules containing Lactobacillus paracasei LPC-S01 on the vaginal microbiota of healthy adult women: A randomized, placebo-controlled, double-blind crossover study. FEMS Microbiol. Ecol. 96, fiaa084. doi: 10.1093/femsec/fiaa084
Kuczkowska, K., Myrbråten, I., Øverland, L., Eijsink, V. G. H., Follmann, F., Mathiesen, G., et al. (2017). Lactobacillus plantarum producing a Chlamydia trachomatis antigen induces a specific IgA response after mucosal booster immunization. PloS One 12, e0176401. doi: 10.1371/journal.pone.0176401
Kumherová, M., Veselá, K., Kosová, M., Mašata, J., Horáčková, Š., Šmidrkal, J. (2021). Novel potential probiotic lactobacilli for prevention and treatment of vulvovaginal infections. Probiotics Antimicrob. Proteins. 13, 163–172. doi: 10.1007/s12602-020-09675-2
Lagenaur, L. A., Hemmerling, A., Chiu, C., Miller, S., Lee, P. P., Cohen, C. R., et al. (2021). Connecting the dots: Translating the vaginal microbiome into a drug. J. Infect. Dis. 223, S296–S306. doi: 10.1093/infdis/jiaa676
Larsson, D. G. J., Flach, C. F. (2022). Antibiotic resistance in the environment. Nat. Rev. Microbiol. 20, 257–269. doi: 10.1038/s41579-021-00649-x
Lee, D., Im, J., Park, D. H., Jeong, S., Park, M., Yoon, S., et al. (2021). Lactobacillus plantarum lipoteichoic acids possess strain-specific regulatory effects on the biofilm formation of dental pathogenic bacteria. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.758161
Lehtoranta, L., Ala-Jaakkola, R., Laitila, A., Maukonen, J. (2022). Healthy vaginal microbiota and influence of probiotics across the female life span. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.819958
Lev-Sagie, A., Goldman-Wohl, D., Cohen, Y., Dori-Bachash, M., Leshem, A., Mor, U., et al. (2019). Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25, 1500–1504. doi: 10.1038/s41591-019-0600-6
Li, Y., Jiang, Y., Lessing, D. J., Chu, W. (2022). Exploring of probiotic potential vaginal lactobacillus isolates from healthy women against Gardnerella vaginalis and Caenorhabditis elegans model testing. J. Appl. Microbiol. 133, 1392–1401. doi: 10.1111/jam.15643
Li, J., Wang, W., Xu, S. X., Magarvey, N. A., McCormick, J. K. (2011). Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. U.S.A. 108, 3360–3365. doi: 10.1073/pnas.1017431108
Liao, H., Liu, S., Wang, H., Su, H., Liu, Z. (2019). Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol. 19, 7. doi: 10.1186/s12866-018-1370-x
Liu, Y., Huang, Y., Cai, W., Li, D., Zheng, W., Xiao, Y., et al. (2020). Effect of oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on vaginal group b streptococcus colonization and vaginal microbiome in late pregnancy. Nan Fang Yi Ke Da Xue Xue Bao. 40, 1753–1759. doi: 10.12122/j.issn.1673-4254.2020.12.09
Loeper, N., Graspeuntner, S., Rupp, J. (2018). Microbiota changes impact on sexually transmitted infections and the development of pelvic inflammatory disease. Microbes Infect. 20, 505–511. doi: 10.1016/j.micinf.2018.02.003
López-Moreno, A., Aguilera, M. (2021). Vaginal probiotics for reproductive health and related dysbiosis: systematic review and meta-analysis. J. Clin. Med. 10, 1461. doi: 10.3390/jcm10071461
Ma, X., Wu, M., Wang, C., Li, H., Fan, A., Wang, Y., et al. (2022). The pathogenesis of prevalent aerobic bacteria in aerobic vaginitis and adverse pregnancy outcomes: a narrative review. Reprod. Health 19, 21. doi: 10.1186/s12978-021-01292-8
Magalhães, C., Lima, M., Trieu-Cuot, P., Ferreira, P. (2021). To give or not to give antibiotics is not the only question. Lancet Infect. Dis. 21, e191–e201. doi: 10.1016/S1473-3099(20)30602-2
Malfa, P., Brambilla, L., Giardina, S., Masciarelli, M., Squarzanti, D. F., Carlomagno, F., et al. (2023). Evaluation of antimicrobial, antiadhesive and co-aggregation activity of a multi-strain probiotic composition against different urogenital pathogens. Int. J. Mol. Sci. 24, 1323. doi: 10.3390/ijms24021323
Mändar, R., Sõerunurk, G., Štšepetova, J., Smidt, I., Rööp, T., Kõljalg, S., et al. (2023). Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: a randomised double-blind placebo controlled clinical trial. Benef Microbes 1, 1–10. doi: 10.3920/BM2022.0091
Marcotte, H., Larsson, P. G., Andersen, K. K., Zuo, F., Mikkelsen, L. S., Brandsborg, E., et al. (2019). An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infect. Dis. 19, 824. doi: 10.1186/s12879-019-4425-1
Martín, V., Cárdenas, N., Ocaña, S., Marín, M., Arroyo, R., Beltrán, D., et al. (2019). Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients. 11, 810. doi: 10.3390/nu11040810
Martinez, R. C., Franceschini, S. A., Patta, M. C., Quintana, S. M., Candido, R. C., Ferreira, J. C., et al. (2009). Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbiol. 48, 269–274. doi: 10.1111/j.1472-765X.2008.02477.x
McKloud, E., Delaney, C., Sherry, L., Kean, R., Williams, S., Metcalfe, R., et al. (2021). Recurrent vulvovaginal candidiasis: A dynamic interkingdom biofilm disease of Candida and Lactobacillus. mSystems. 6, e0062221. doi: 10.1128/mSystems.00622-21
Mei, Z., Li, D. (2022). The role of probiotics in vaginal health. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.963868
Menichini, D., Chiossi, G., Monari, F., De Seta, F., Facchinetti, F. (2022). Supplementation of probiotics in pregnant women targeting group b streptococcus colonization: A systematic review and meta-analysis. Nutrients. 14, 4520. doi: 10.3390/nu14214520
Mitchell, C. M., Ma, N., Mitchell, A. J., Wu, M. C., Valint, D. J., Proll, S., et al. (2021). Association between postmenopausal vulvovaginal discomfort, vaginal microbiota, and mucosal inflammation. Am. J. Obstet. Gynecol. 225, 159.e1–159.e15. doi: 10.1016/j.ajog.2021.02.034
Mogha, K. V., Prajapati, J. B. (2016). Probiotics for treating bacterial vaginosis. Rev. Med. Microbiol. 27, 87–94. doi: 10.1097/mrm.0000000000000080
Mohankumar, B., Shandil, R. K., Narayanan, S., Krishnan, U. M. (2022). Vaginosis: advances in new therapeutic development and microbiome restoration. Microb. Pathog. 168, 105606. doi: 10.1016/j.micpath.2022.105606
Mohseni, A. H., Taghinezhad-S, S., Keyvani, H., Ghobadi, N. (2018). Comparison of acyclovir and multistrain Lactobacillus brevis in women with recurrent genital herpes infections: A double-blind, randomized, controlled study. Probiotics Antimicrob. Proteins. 10, 740–747. doi: 10.1007/s12602-017-9320-8
Mokoena, M. P., Omatola, C. A., Olaniran, A. O. (2021). Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens. Molecules. 26, 7055. doi: 10.3390/molecules26227055
Nader-Macías, M. E. F., De Gregorio, P. R., Silva, J. A. (2021). Probiotic Lactobacilli in formulas and hygiene products for the health of the urogenital tract. Pharmacol. Res. Perspect. 9, e00787. doi: 10.1002/prp2.787
Nelson, J., El-Gendy, A. O., Mansy, M. S., Ramadan, M. A., Aziz, R. K. (2020). The biosurfactants iturin, lichenysin and surfactin, from vaginally isolated lactobacilli, prevent biofilm formation by pathogenic Candida. FEMS Microbiol. Lett. 367, fnaa126. doi: 10.1093/femsle/fnaa126
Novak, J., Ravel, J., Ma, B., Ferreira, C. S. T., Tristão, A. D. R., Silva, M. G., et al. (2022). Characteristics associated with Lactobacillus iners-dominated vaginal microbiota. Sex. Transm. Infect. 98, 353–359. doi: 10.1136/sextrans-2020-054824
Park, Y. C., Ouh, Y. T., Sung, M. H., Park, H. G., Kim, T. J., Cho, C. H., et al. (2019). A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol Oncol. 30, e88. doi: 10.3802/jgo.2019.30.e88
Park, S., You, Y. A., Kim, Y. H., Kwon, E., Ansari, A., Kim, S. M. (2022). Ureaplasma and Prevotella colonization with Lactobacillus abundance during pregnancy facilitates term birth. Sci. Rep. 12, 10148. doi: 10.1038/s41598-022-13871-1
Parolin, C., Frisco, G., Foschi, C., Giordani, B., Salvo, M., Vitali, B., et al. (2018). Lactobacillus crispatus BC5 interferes with Chlamydia trachomatis infectivity through integrin modulation in cervical cells. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02630
Patel, M., Siddiqui, A. J., Hamadou, W. S., Surti, M., Awadelkareem, A. M., Ashraf, S. A. (2021). Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from Lactobacillus rhamnosus with its physicochemical and functional properties. Antibiotics (Basel). 10, 1546. doi: 10.3390/antibiotics10121546
Petrova, M. I., Reid, G., Ter Haar, J. A. (2021). Lacticaseibacillus rhamnosus GR-1, a.k.a. lactobacillus rhamnosus GR-1: Past and future perspectives. Trends Microbiol. 29, 747–761. doi: 10.1016/j.tim.2021.03.010
Phukan, N., Brooks, A. E. S., Simoes-Barbosa, A. (2018). A cell surface aggregation-promoting factor from Lactobacillus gasseri contributes to inhibition of Trichomonas vaginalis adhesion to human vaginal ectocervical cells. Infect. Immun. 86, e00907–e00917. doi: 10.1128/IAI.00907-17
Piccioni, A., Franza, L., Vaccaro, V., Saviano, A., Zanza, C., Candelli, M., et al. (2021). Microbiota and probiotics: The role of Limosilactobacillus reuteri in diverticulitis. Medicina (Kaunas). 57, 802. doi: 10.3390/medicina57080802
Pino, A., Rapisarda, A. M. C., Vaccalluzzo, A., Sanfilippo, R. R., Coman, M. M., Grimaldi, R. L., et al. (2022b). Oral intake of the commercial probiotic blend synbio® for the management of vaginal dysbiosis. J. Clin. Med. 12, 27. doi: 10.3390/jcm12010027
Pino, A., Vaccalluzzo, A., Caggia, C., Balzaretti, S., Vanella, L., Sorrenti, V., et al. (2022a). Lacticaseibacillus rhamnosus CA15 (DSM 33960) as a candidate probiotic strain for human health. Nutrients. 14, 4902. doi: 10.3390/nu14224902
Płaczkiewicz, J., Chmiel, P., Malinowska, E., Bącal, P., Kwiatek, A. (2020). Lactobacillus crispatus and its enolase and glutamine synthetase influence interactions between Neisseria gonorrhoeae and human epithelial cells. J. Microbiol. 58, 405–414. doi: 10.1007/s12275-020-9505-9
Prasad, D., Parween, S., Kumari, K., Singh, N. (2021). Prevalence, etiology, and associated symptoms of vaginal discharge during pregnancy in women seen in a tertiary care hospital in bihar. Cureus. 13, e12700. doi: 10.7759/cureus.12700
Qi, W., Li, H., Wang, C., Li, H., Zhang, B., Dong, M., et al. (2021). Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.759795
Qian, Z., Zhu, H., Zhao, D., Yang, P., Gao, F., Lu, C., et al. (2021). Probiotic lactobacillus sp. strains inhibit growth, adhesion, biofilm formation, and gene expression of bacterial vaginosis-inducing Gardnerella vaginalis. Microorganisms. 9, 728. doi: 10.3390/microorganisms9040728
Rapisarda, A. M. C., Pino, A., Grimaldi, R. L., Caggia, C., Randazzo, C. L., Cianci, A. (2023). Lacticaseibacillus rhamnosus CA15 (DSM 33960) strain as a new driver in restoring the normal vaginal microbiota: A randomized, double-blind, placebo-controlled clinical trial. Front. Surg. 9. doi: 10.3389/fsurg.2022.1075612
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108 Suppl 1, 4680–4687. doi: 10.1073/pnas.1002611107
Reznichenko, H., Henyk, N., Maliuk, V., Khyzhnyak, T., Tynna, Y., Filipiuk, I., et al. (2020). Oral intake of Lactobacilli can be helpful in symptomatic bacterial vaginosis: A randomized clinical study. J. Low Genit Tract Dis. 24, 284–289. doi: 10.1097/LGT.0000000000000518
Russo, R., Karadja, E., De Seta, F. (2019a). Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef Microbes 10, 19–26. doi: 10.3920/BM2018.0075
Russo, R., Superti, F., Karadja, E., De Seta, F. (2019b). Randomised clinical trial in women with recurrent vulvovaginal candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses. 62, 328–335. doi: 10.1111/myc.12883
Sabbatini, S., Visconti, S., Gentili, M., Lusenti, E., Nunzi, E., Ronchetti, S., et al. (2021). Lactobacillus iners cell-free supernatant enhances biofilm formation and hyphal/pseudohyphal growth by Candida albicans vaginal isolates. Microorganisms. 9, 2577. doi: 10.3390/microorganisms9122577
Sadahira, T., Wada, K., Araki, M., Mitsuhata, R., Yamamoto, M., Maruyama, Y., et al. (2021). Efficacy of Lactobacillus vaginal suppositories for the prevention of recurrent cystitis: A phase II clinical trial. Int. J. Urol. 28, 1026–1031. doi: 10.1111/iju.14636
Schenk, M., Grumet, L., Sternat, J., Reinschissler, N., Weiss, G. (2021). Effect of probiotics on vaginal Ureaplasma parvum in women suffering from unexplained infertility. Reprod. BioMed. Online. 43, 503–514. doi: 10.1016/j.rbmo.2021.06.004
Seydel, C. (2021). Federation bio: from single strains to a purely synthetic microbiome. Nat. Biotechnol. doi: 10.1038/d41587-021-00009-3
Sgibnev, A., Kremleva, E. (2020). Probiotics in addition to metronidazole for treatment Trichomonas vaginalis in the presence of BV: A randomized, placebo-controlled, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 39, 345–351. doi: 10.1007/s10096-019-03731-8
Sharma, D., Sharma, S., Akojwar, N., Dondulkar, A., Yenorkar, N., Pandita, D., et al. (2023). An insight into current treatment strategies, their limitations, and ongoing developments in vaccine technologies against herpes simplex infections. Vaccines (Basel). 11, 206. doi: 10.3390/vaccines11020206
Shazadi, K., Ahmad, S. Z., Ahmad, S. S., Arshad, N. (2021). In vivo prophylactic efficacy of Lactobacillus reuteri MT180537 against aerobic vaginitis. Microb. Pathog. 160, 105197. doi: 10.1016/j.micpath.2021.105197
Shazadi, K., Arshad, N., Ambreen, H. S., Riaz, A., Mehreen, A. (2022). In vivo studies could not confirm in vitro prophylactic synergism between moringa essential oil and Lactobacillus reuteri (MT180537). Braz. J. Biol. 84, e254513. doi: 10.1590/1519-6984.254513
Shen, X., Xu, L., Zhang, Z., Yang, Y., Li, P., Ma, T., et al. (2023). Postbiotic gel relieves clinical symptoms of bacterial vaginitis by regulating the vaginal microbiota. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1114364
Shiroda, M., Aronoff, D. M., Gaddy, J. A., Manning, S. D. (2020). The impact of Lactobacillus on group b streptococcal interactions with cells of the extraplacental membranes. Microb. Pathog. 148, 104463. doi: 10.1016/j.micpath.2020.104463
Singh, R. P., Shadan, A., Ma, Y. (2022). Biotechnological applications of probiotics: A multifarious weapon to disease and metabolic abnormality. Probiotics Antimicrob. Proteins. 14, 1184–1210. doi: 10.1007/s12602-022-09992-8
Song, C. H., Kim, Y. H., Naskar, M., Hayes, B. W., Abraham, M. A., Noh, J. H., et al. (2022). Lactobacillus crispatus limits bladder uropathogenic e. coli infection by triggering Host type I Interferon response. Proc. Natl. Acad. Sci. U.S.A. 119, e2117904119. doi: 10.1073/pnas.2117904119
Spacova, I., O'Neill, C., Lebee, R. S. (2020). Lacticaseibacillus rhamnosus GG inhibits infection of human keratinocytes by staphylococcus aureus through mechanisms involving cell surface molecules and pH reduction. Benef Microbes 11, 703–715. doi: 10.3920/BM2020.0075
Spaggiari, L., Sala, A., Ardizzoni, A., De Seta, F., Singh, D. K., Gacser, A., et al. (2022). Lactobacillus acidophilus, l. plantarum, L. rhamnosus, and L. reuteri cell-free supernatants inhibit Candida parapsilosis pathogenic potential upon infection of vaginal epithelial cells monolayer and in a transwell coculture system in vitro. Microbiol. Spectr. 10, e0269621. doi: 10.1128/spectrum.02696-21
Stavropoulou, E., Bezirtzoglou, E. (2020). Probiotics in medicine: A long debate. Front. Immunol. 11. doi: 10.3389/fimmu.2020.02192
Sun, Z., Ge, X., Qiu, B., Xiang, Z., Jiang, C., Wu, J., et al. (2023). Vulvovaginal candidiasis and vaginal microflora interaction: Microflora changes and probiotic therapy. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1123026
Szymonowicz, K. A., Chen, J. (2020). Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 17, 864–878. doi: 10.20892/j.issn.2095-3941.2020.0370
Tenore, S. B., Avelino-Silva, V. I., Costa, P. R., Franco, L. M., Sabino, E. C., Kalil, J., et al. (2020). Immune effects of Lactobacillus casei shirota in treated HIV-infected patients with poor CD4+ T-cell recovery. AIDS. 34, 381–389. doi: 10.1097/QAD.0000000000002420
Tien, V., Punjabi, C., Holubar, M. K. (2020). Antimicrobial resistance in sexually transmitted infections. J. Travel Med. 27, taz101. doi: 10.1093/jtm/taz101
Torcia, M. G. (2019). Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int. J. Mol. Sci. 20, 266. doi: 10.3390/ijms20020266
Tumietto, F., Posteraro, B., Sanguinetti, M. (2019). Looking for appropriateness in the cure of mixed vaginitis: The role of fenticonazole as an empiric treatment. Future Microbiol. 14, 1349–1355. doi: 10.2217/fmb-2019-0189
van de Wijgert, J., Verwijs, M. C. (2020). Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG. 127, 287–299. doi: 10.1111/1471-0528.15870
Van Gerwen, O. T., Muzny, C. A., Marrazzo, J. M. (2022). Sexually transmitted infections and female reproductive health. Nat. Microbiol. 7, 1116–1126. doi: 10.1038/s41564-022-01177-x
Vazquez-Munoz, R., Thompson, A., Russell, J. T., Sobue, T., Zhou, Y., Dongari-Bagtzoglou, A. (2022). Insights from the Lactobacillus johnsonii genome suggest the production of metabolites with antibiofilm activity against the pathobiont Candida albicans. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.853762
Veščičík, P., Kacerovská Musilová, I., Stráník, J., Šěpán, M., Kacerovský, M. (2020). Lactobacillus crispatus dominant vaginal microbita in pregnancy. Ceska Gynekol. 85, 67–70.
Vujic, G., Jajac Knez, A., Despot Stefanovic, V., Kuzmic Vrbanovic, V. (2013). Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur. J. Obstet Gynecol Reprod. Biol. 168, 75–79. doi: 10.1016/j.ejogrb.2012.12.031
Wei, W., Wiggins, J., Hu, D., Vrbanac, V., Bowder, D., Mellon, M., et al. (2019). Blocking HIV-1 infection by chromosomal integrative expression of human CD4 on the surface of Lactobacillus acidophilus ATCC 4356. J. Virol. 93, e01830–e01818. doi: 10.1128/JVI.01830-18
Willems, H. M. E., Ahmed, S. S., Liu, J., Xu, Z., Peters, B. M. (2020). Vulvovaginal candidiasis: A current understanding and burning questions. J. Fungi (Basel). 6, 27. doi: 10.3390/jof6010027
Williams, E., Fairley, C. K., Williamson, D. (2021). Novel strategies for prevention and treatment of antimicrobial resistance in sexually-transmitted infections. Curr. Opin. Infect. Dis. 34, 591–598. doi: 10.1097/QCO.0000000000000793
Wójkowska-Mach, J., Pomorska-Wesołowska, M., Romanik, M., Romaniszyn, D. (2021). Prevalence and antimicrobial susceptibility profiles of microorganisms associated with lower reproductive tract infections in women from southern Poland-retrospective laboratory-based study. Int. J. Environ. Res. Public Health 18, 335. doi: 10.3390/ijerph18010335
Xiu, L., Sheng, S., Hu, Z., Liu, Y., Li, J., Zhang, H., et al. (2020). Exopolysaccharides from Lactobacillus kiferi as adjuvant enhanced the immuno-protective against Staphylococcus aureus infection. Int. J. Biol. Macromol. 161, 10–23. doi: 10.1016/j.ijbiomac.2020.06.005
Zawistowska-Rojek, A., Kośmider, A., Stępień, K., Tyski, S. (2022). Adhesion and aggregation properties of Lactobacillaceae strains as protection ways against enteropathogenic bacteria. Arch. Microbiol. 204, 285. doi: 10.1007/s00203-022-02889-8
Zeng, X., An, R., Li, H., Zhang, Y. (2023). Improved treatment of vulvovaginal candidiasis with clotrimazole plus probiotic lacidophilin vaginal capsules: A prospective, real-world study. Med. (Baltimore). 102, e32664. doi: 10.1097/MD.0000000000032664
Zhang, Q., Cheng, Q., Cui, S., Zhao, J., Chen, W., Zhang, H. (2022). Inhibitory effect of Lactobacillus gasseri CCFM1201 on Gardnerella vaginalis in mice with bacterial vaginosis. Arch. Microbiol. 204, 315. doi: 10.1007/s00203-022-02896-9
Zhang, F., Cui, B., He, X., Nie, Y., Wu, K., Fan, D., et al. (2018). Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell. 9, 462–473. doi: 10.1007/s13238-018-0541-8
Zhang, Y., Lyu, J., Ge, L., Huang, L., Peng, Z., Liang, Y., et al. (2021). Probiotic Lacticaseibacillus rhamnosus GR-1 and Limosilactobacillus reuteri RC-14 as an adjunctive treatment for bacterial vaginosis do not increase the cure rate in a Chinese cohort: A prospective, parallel-group, randomized, controlled study. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.669901
Keywords: lactobacilli, vaginal microbiome, probiotic, vaginal infections, treatment
Citation: Liu P, Lu Y, Li R and Chen X (2023) Use of probiotic lactobacilli in the treatment of vaginal infections: In vitro and in vivo investigations. Front. Cell. Infect. Microbiol. 13:1153894. doi: 10.3389/fcimb.2023.1153894
Received: 01 February 2023; Accepted: 23 March 2023;
Published: 03 April 2023.
Edited by:
Carlo Contini, University of Ferrara, ItalyReviewed by:
Jose Eleuterio Junior, Federal University of Ceara, BrazilCopyright © 2023 Liu, Lu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongguo Li, cm9uZ2d1b2xpX3NmeUAxNjMuY29t; Xiaodi Chen, Y2hlbnhpYW9kaTIzNTJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.