
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 04 April 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1142199
 Lin Zhu1†
Lin Zhu1† Tingting Luo1†
Tingting Luo1† Yining Yuan2
Yining Yuan2 Shu Yang3
Shu Yang3 Chao Niu1
Chao Niu1 Ting Gong1
Ting Gong1 Xueer Wang1
Xueer Wang1 Xiaohong Xie1
Xiaohong Xie1 Jian Luo1
Jian Luo1 Enmei Liu1
Enmei Liu1 Zhou Fu1
Zhou Fu1 Daiyin Tian1*
Daiyin Tian1*Background: Multinational studies have reported that the implementation of nonpharmaceutical interventions (NPIs) to control severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission coincided with the decline of other respiratory viruses, such as influenza viruses and respiratory syncytial virus.
Objective: To investigate the prevalence of common respiratory viruses during the coronavirus disease 2019 (COVID-19) pandemic.
Methods: Respiratory specimens of children with lower respiratory tract infections (LRTIs) hospitalized at the Children’s Hospital of Chongqing Medical University from January 1, 2018 to December 31, 2021 were collected. Seven common pathogens, including respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus A and B (Flu A, Flu B), and parainfluenza virus types 1–3 (PIV1–3), were detected by a multiplex direct immunofluorescence assay (DFA). Demographic data and laboratory test results were analyzed.
Results: 1) A total of 31,113 children with LRTIs were enrolled, including 8141 in 2018, 8681 in 2019, 6252 in 2020, and 8059 in 2021.The overall detection rates decreased in 2020 and 2021 (P < 0.001). The detection rates of RSV, ADV, Flu A, PIV-1, and PIV-3 decreased when NPIs were active from February to August 2020, with Flu A decreasing most predominantly, from 2.7% to 0.3% (P < 0.05). The detection rates of RSV and PIV-1 resurged and even surpassed the historical level of 2018–2019, while Flu A continued decreasing when NPIs were lifted (P < 0.05). 2) Seasonal patterns of Flu A completely disappeared in 2020 and 2021. The Flu B epidemic was observed until October 2021 after a long period of low detection in 2020. RSV decreased sharply after January 2020 and stayed in a nearly dormant state during the next seven months. Nevertheless, the detection rates of RSV were abnormally higher than 10% in the summer of 2021. PIV-3 decreased significantly after the COVID-19 pandemic; however, it atypically surged from August to November 2020.
Conclusion: The NPIs implemented during the COVID-19 pandemic affected the prevalence and seasonal patterns of certain viruses such as RSV, PIV-3, and influenza viruses. We recommend continuous surveillance of the epidemiological and evolutionary dynamics of multiple respiratory pathogens, especially when NPIs are no longer necessary.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread worldwide since December 2019 (Zhu et al., 2020). The impact of the COVID-19 pandemic on public health was catastrophic, with millions of hospitalizations and deaths. In response to the ongoing COVID-19 pandemic, the Chinese government undertook a series of stringent nonpharmaceutical interventions (NPIs) as soon as it outbroke at the end of 2019 in Wuhan, including SARS-CoV-2 detection, home quarantining, contact tracing, mask mandates, regular hand-sanitizing and hand-washing, transient closures of public places, and maintaining social distance. Substantial evidence has shown that such NPIs effectively limited the transmission of SARS-CoV-2 (Lai et al., 2020; Grote et al., 2021; León et al., 2021; Zhao et al., 2021). Moreover, it has also been recorded that in hospitals and private practices, the number of visits for airborne or fecal-oral diseases (common cold, bronchiolitis, seasonal influenza, acute otitis media, and gastroenteritis) decreased significantly during the NPIs implementation period (Pan et al., 2020; Sakamoto et al., 2020).
The positive collateral effect of NPIs in the short term is welcome, as it helps to reduce the additional overload of hospital wards and intensive care units. Two years after the outbreak of the COVID-19 pandemic, public health measures were gradually eased. Business resumed, schools and kindergartens fully reopened in September 2020, and the SARS-CoV-2 vaccination campaign began in 2021. Several studies have reported that strict NPIs affected the transmission and seasonal circulation patterns of respiratory viral infections (Agca et al., 2021; Li et al., 2021; Yeoh et al., 2021; Song et al., 2022), such as influenza viruses and respiratory syncytial virus, and some studies later reported atypical increases in certain viruses after normalization in 2021. There have been increasing concerns that the strict mitigation measures and their subsequent relaxation might have modified the epidemiology of respiratory viruses, which could cause more severe epidemics when NPIs are no longer necessary. In view of these concerns, mastering epidemiological characteristics of common respiratory viruses during the COVID-19 pandemic is vital and essential for regional disease prevention and control.
To investigate the prevalence of common respiratory viruses during the COVID-19 pandemic, we retrospectively analyzed the absolute number and the detection rates of common respiratory viruses in children hospitalized for lower respiratory tract infections (LRTIs) at Children’s Hospital of Chongqing Medical University from January 1, 2020 to December 31, 2021. For comparison, the corresponding data in the same periods of 2018 and 2019 were also utilized.
Patients with LRTIs between January 1, 2018 and December 31, 2021 admitted to the respiratory ward of Children’s Hospital of Chongqing Medical University were enrolled. The inclusion criteria were as follows: 1) children under the age of 18 years hospitalized for LRTIs; 2) available results of multiplex direct immunofluorescence assay (DFA) for respiratory pathogens. The diagnoses of LRTIs included bronchiolitis, bronchitis, and pneumonia. Patients were excluded if they had one of the following situations: 1) children with severe malformations (such as large atrial/ventricular septal defects, bronchopulmonary dysplasia, dextrocardia, and neuromuscular disease); 2) children who received diagnosis of malignant tumors or primary immunodeficiency disease, or who used immunosuppressive drugs during hospitalizations.
All of the enrolled children were divided into five age groups: under 12 months (0–12 m), 1 to 3 years (1–3 y), 3 to 6 years (3–6 y), 6 to 14 years (6–14 y), and more than 14 years (>14 y). The period between January 1, 2018 and December 31, 2019 was classified as before the COVID-19 pandemic, while the period between January 1, 2020 and December 31, 2021 was classified as during the COVID-19 pandemic. The time span was further subdivided into three phases according to the emergency response level in Chongqing: January 24, 2020 to March 24, 2020 (Phase I, level I–II emergency response), when massive NPIs remained active; March 25, 2020 to August 31, 2020 (Phase II, level III emergency response), when NPIs were gradually relaxed and schools started reopening; September 1, 2020 to December 31, 2021 (Phase III, normalization of the epidemic), when schools fully reopened and social activities resumed. The same phases were also defined based on the corresponding calendar intervals for 2018–2019.
Nasopharyngeal aspirate or bronchoalveolar lavage fluid specimens of the enrolled children were collected by trained nurses after admission and were transported immediately to the clinical laboratory center. Seven common pathogens included respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus A (Flu A), influenza virus B (Flu B), and parainfluenza virus types 1–3 (PIV1–3). A multiplex direct immunofluorescence assay kit (Diagnostic Hybrids, Athens, Ohio, USA) was used for detection and was conducted by professional staff following standard operating procedures (Ye and Liu, 2022).
It should be noted that when a child was hospitalized for LRTIs multiple times (interval less than 1 week), laboratory test results collected for the first hospitalization were included in the analysis. Respiratory specimens of the excluded patients were not included when calculating hospitalization numbers or detection rates.
Demographic data (name, gender, months of age, clinical diagnosis, sampling time, and length of stay) of the patients were obtained from the electronic medical records, and laboratory test results were extracted from the management information system (HIS) of Children’s Hospital of Chongqing Medical University. The study protocol was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University (File No. (2022)196). Written consent was waived because antigen detection of respiratory pathogens was standard and routine for all children admitted to the hospital for LRTIs during the implementation of the study.
The overall detection rates of seven common pathogens, including RSV, ADV, Flu A, Flu B, and PIV1–3, were compared between the periods before and during the COVID-19 pandemic. To determine seasonal patterns of viruses and age-specific differences in viral infections, the trends of virus detection rates over time and the comparison of age-specific detection rates associated with viruses in 2018–2021 were plotted by software R version 4.1.3.
Data extracted from January 1, 2018 to December 31, 2021 were analyzed using IBM SPSS version 25.0. Categorical variables were described as numbers and percentages (n, %), and continuous variables were described as the median and interquartile range (IQR) [M (P25, P75)]. The Kruskal–Wallis test were used for statistical analysis of continuous variables, and Fisher’s exact test or Pearson chi-square test were used for categorical variables. Post-hoc pairwise comparisons were performed with partitions of the χ2 method, and the adjusted test level was α’ = 0.0083. Other tests were based on a two-sided α with statistical significance defined as P < 0.05.
A total of 31,133 children hospitalized with LRTIs were admitted in this study, including 8141 in 2018, 8681 in 2019, 6252 in 2020, and 8059 in 2021. In 2020, the number of hospitalizations for LRTIs decreased by 23.2% and 28.0% compared with 2018 and 2019, respectively. A total of 19,124 (61.4%) cases were male and 12,009 (38.6%) were female. The age of the enrolled children ranged from 17 days to 18 years, and the children’s median (IQR) age was 12.0 (4.8, 34.0) months (Table 1).
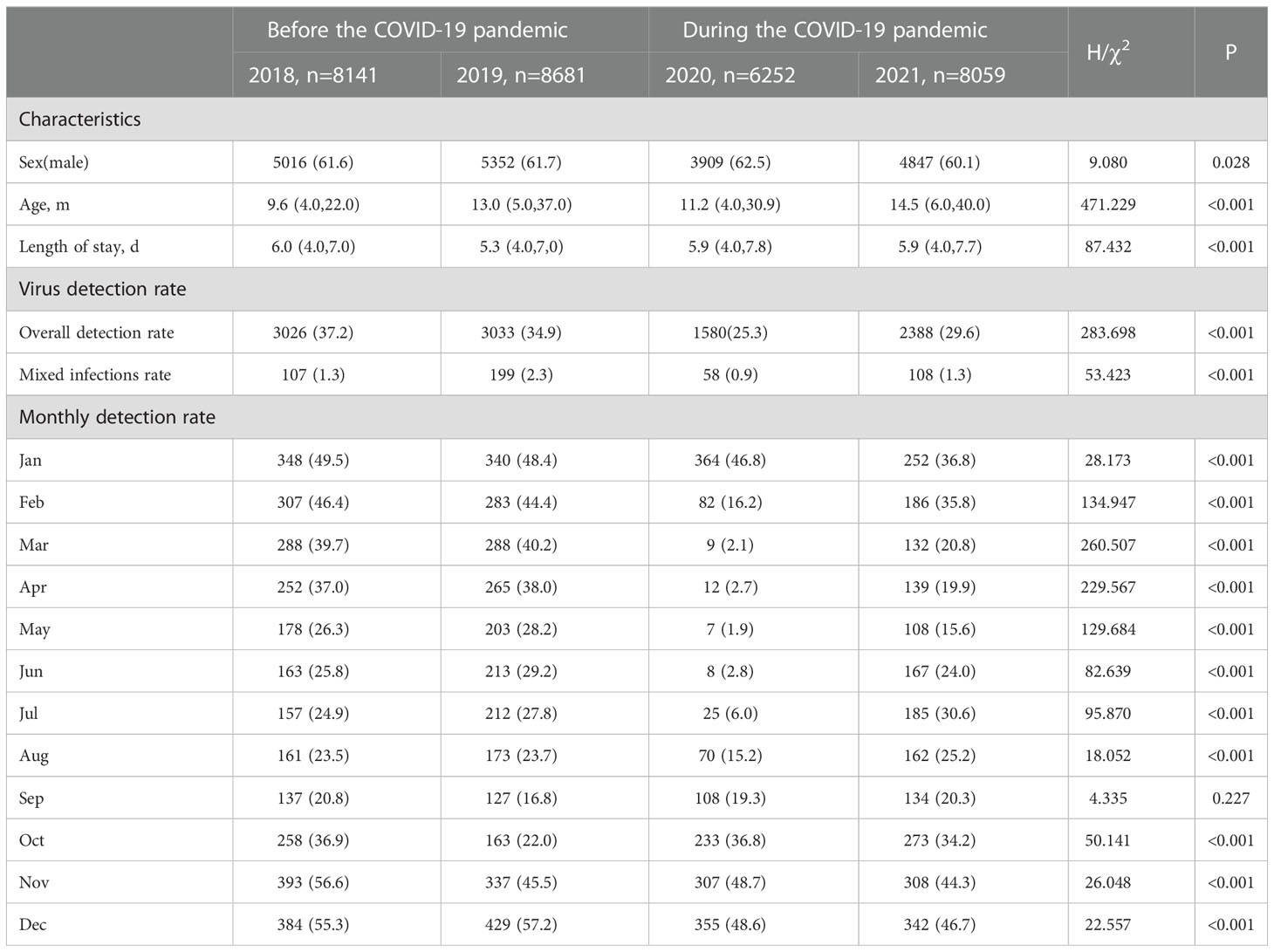
Table 1 Comparison of the characteristics and virus detection rate before and during the COVID-19 pandemic.
In 10,027/31,133 (32.2%) respiratory specimens of the enrolled children, one or more viruses were detected: 3026/8141 (37.2%) in 2018, 3033/8681 (34.9%) in 2019, 1588/6252 (25.3%) in 2020, and 2388/8059 (29.6%) in 2021. The overall detection rates in 2020 and 2021 were significantly lower than in prior years (P < 0.001). In the present study, two or more viruses were detected in 472 respiratory aspirates (107 in 2018, 199 in 2019, 58 in 2020, and 108 in 2021). Mixed infection rates were significantly different over four years (χ2 = 53.423, P < 0.05), with the rate in 2020 being lower than that in 2019 (χ2 = 40.107, P < 0.001). Monthly detection rates declined sharply from February to August 2020. Less than 10% of respiratory aspirates of the hospitalized children with LRTIs were infected by one or more viruses from March to July 2020 (Table 1).
Overall, the detection rates of ADV, Flu A, and PIV-3 decreased during the COVID-19 pandemic (P < 0.05), while that of PIV-1 increased (P < 0.05). The detection rate of Flu A decreased most predominantly, from 2.7% to 0.3%. There were no significant differences in the detection rates of RSV, Flu B, and PIV-2 (P > 0.05). More specifically, dramatic reductions in the detection rates were seen for RSV, Flu A, and PIV-3 in Phase I, when NPIs were actively maintained (P < 0.05). The detection rates of RSV and PIV-3 continued decreasing despite partial relaxation of the mass NPIs in Phase II (P < 0.05). The detection rates of PIV-1 and ADV also lowered in Phase II (P < 0.05). Unlike Flu A, RSV and PIV-1 resurged in Phase III and even surpassed the historical level of 2018–2019 when NPIs were largely lifted in Chongqing, including full school reopening and resuming of social activities (P < 0.05). The detection rates of Flu B and PIV-2 were not significantly different in Phase I and Phase II compared with the pre-pandemic level (P > 0.05) (Figure 1 and Table 2).
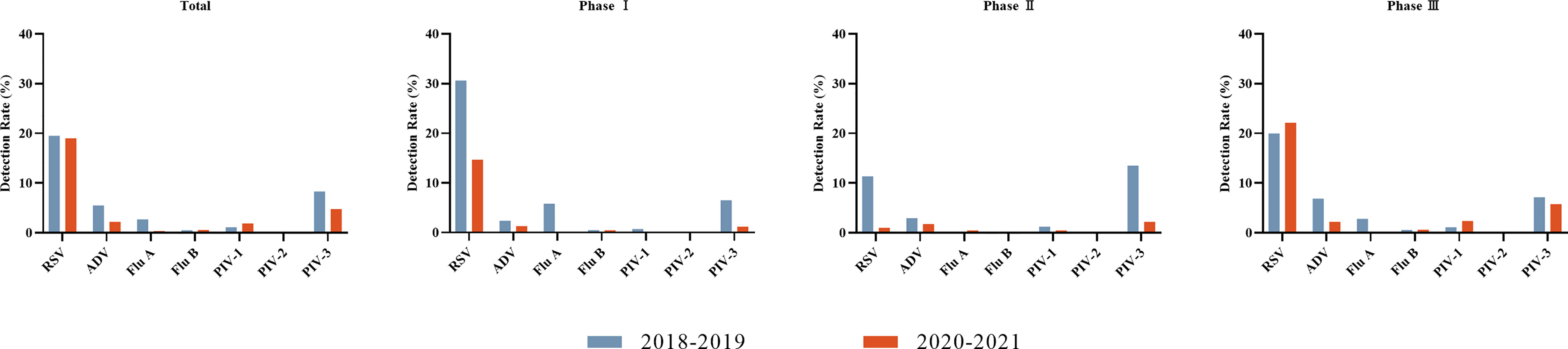
Figure 1 Detection rates of viruses before the COVID-19 pandemic (2018–2019) and during the COVID-19 pandemic (2020–2021) for each of three phases. Seven respiratory viruses were included: respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus A and B (Flu A, Flu B), and parainfluenza virus types 1–3 (PIV1–3).
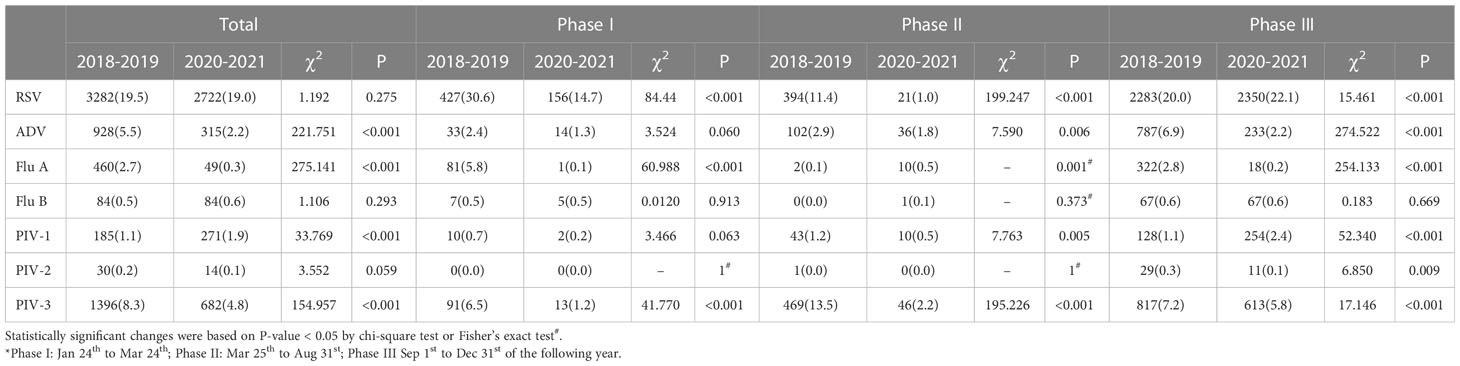
Table 2 Detection rates of seven viruses before the COVID-19 pandemic (2018–2019) and during the COVID-19 pandemic (2020–2021) for each of three phases.
The seasonal dynamics of common respiratory viruses was distorted during the COVID-19 pandemic. Detection of all seven viruses declined quickly after the outbreak on January 24, 2020, when a Level I public health emergency response was implemented in Chongqing. RSV usually peaks in winter and declines by early spring, as shown before the COVID-19 pandemic. However, the RSV detection rates decreased sharply from 35.99% in January 2020 to 12.82% in February 2020, and stayed in a nearly dormant state during the next seven months, without RSV-infected cases in April 2020. The monthly positive rate of RSV increased quickly to 16.43% in October 2020, peaked in winter, and then gradually decreased to 10.85% (75/691) in May 2021. However, the detection rates did not decrease continuously but increased again in June 2021 (12.23%, 85/695) and stayed abnormally high (over 10%) until September 2021.
The influenza virus is a pathogen with apparent seasonality patterns that shows high incidence in winter and spring (Wang et al., 2016). However, there was a sudden decline in Flu A and Flu B in January 2020, and nearly no influenza viruses were detected during the next several months of 2020. Seasonal patterns of Flu A completely disappeared in the winter of 2020 and 2021, but Flu B gradually rose until September 2021 and then peaked in October and November after a long period of very low detection.
PIV-3 circulated most frequently from late spring to summer in 2018 and 2019. However, no PIV-3 was detected from April to June 2020. However, the detection rates of PIV-3 atypically increased from August to November 2020, with a delay of about 5 months, and then fell before rising in June 2021. PIV-1 and PIV-2 were normally sporadically detected during the four years analyzed.
The detection rate of ADV in 2019 was extremely high all year round, peaking in summer. Infection caused by ADV can occur throughout the year; it mainly occurs in winter and spring in northern China, and is common in spring and summer in southern China (Ji et al., 2021). In 2019, the detection rate of ADV was high all year round in Chongqing. ADV peaked between June and August in 2020 and 2021, similar to 2018 and 2019 (Figure 2; Supplementary Figure).
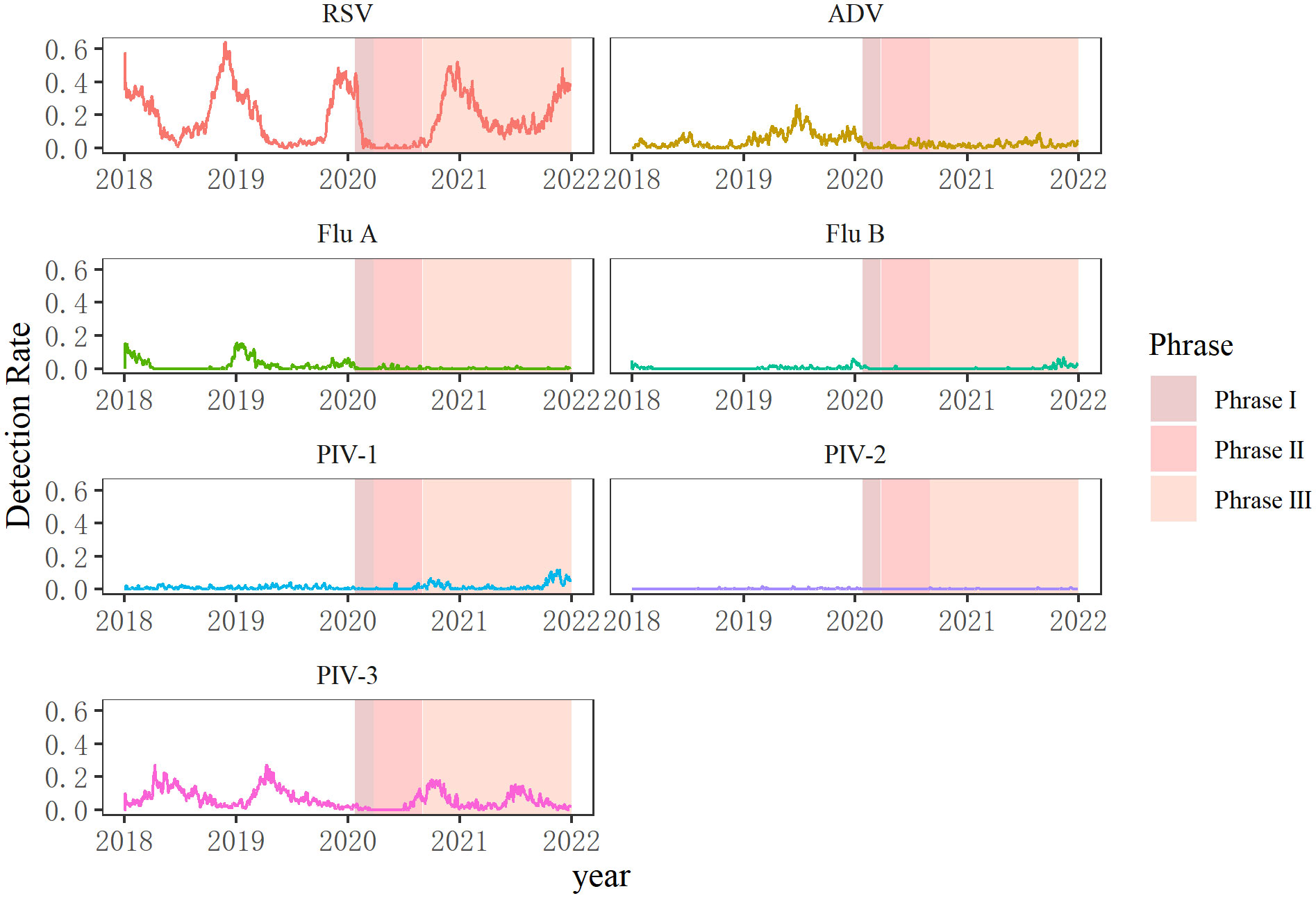
Figure 2 Seasonal patterns of viruses before the COVID-19 pandemic (2018–2019) and during the COVID-19 pandemic (2020–2021). Seven respiratory viruses were included: respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus A and B (Flu A, Flu B), and parainfluenza virus types 1–3 (PIV1–3).
The overall positive rates reached the peak in children aged 0–12 months, with the peaks of 45.4% in 2018, 42.4% in 2019, 30.5% in 2020, and 38.8% in 2021, and declined with the rising age of the enrolled children. Comparison of age-specific detection rates of seven respiratory viruses in the four years showed that the overall detection rates were greatly reduced among all age groups in 2020 (P < 0.001), with most dramatic declines observed in children aged 0–12 months and 1–3 years. The detection rate of Flu A decreased remarkably, and only sporadic cases were detected in all age groups. In 2021, Flu A continued decreasing, while the number of cases with RSV infection among 1–3-year-old children was higher than in the previous three years (P < 0.001). Other viruses such as ADV, Flu B, PIV-1, and PIV-3 gradually resumed in 2021 and followed similar patterns as their counterparts in 2018 and 2019 (Figure 3; Supplementary Table).
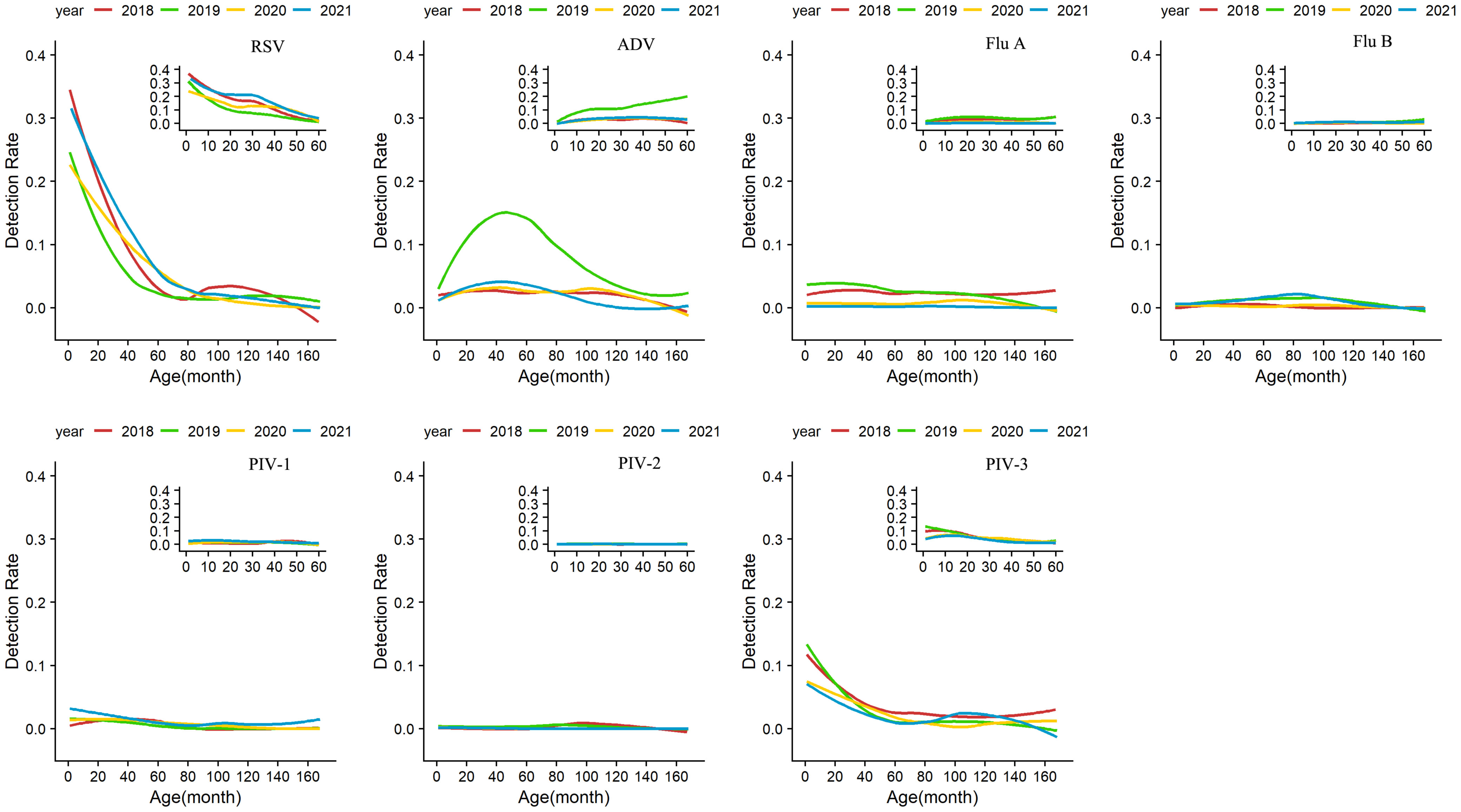
Figure 3 Age-specific detection rates of viruses from January 1, 2018 to December 31, 2021. Seven respiratory viruses were included: respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus A and B (Flu A, Flu B), and parainfluenza virus types 1–3 (PIV1–3).
Chongqing, located in the southwest of China, swiftly applied the strictest NPIs from January to May 2020 after the outbreak of a novel virus with a high basic reproductive number (R0) in Wuhan (Salzberger et al., 2021). Under the circumstances, the total number of children hospitalized for LRTIs in 2020 decreased significantly. The overall detection rate in 2020 was much lower than in 2018 and 2019 (25.3% vs. 37.2% & 34.9%). More specifically, we found that the detection rates of ADV, Flu A, and PIV-3 decreased when NPIs were active from February to August 2020, with Flu A decreasing most predominantly, from 2.7% to 0.3%. Although the overall detection rates of RSV were similar to before, RSV decreased significantly after the outbreak. By continuous surveillance, we also found that the detection rates of RSV and PIV-1 resurged after NPIs lifting and even surpassed the historical level of 2018–2019. Similar results were also described by reports from Italy, South Korea, Hongkong, Shenzhen, Hangzhou, and Shanghai (Liu et al., 2021; Park et al., 2021; Chiu et al., 2022; Nenna et al., 2022; Wang et al., 2022; Ye and Liu, 2022). Multiple factors could contribute to the phenomenon. On the one hand, ADV, Flu A, PIV-1, PIV-3, and RSV are transmitted by contact, droplets, and fomites. Mitigation practices such as wearing a mask, hand disinfecting, physical distancing, home quarantine, and cessation of global travel might have played a protective role in the transmission at the early stage of the pandemic, but have become less stringent in 2021. On the other hand, viral evolution, new viral strains, or mutations might have occurred to circumvent NPIs for the COVID-19 pandemic.
Significant decreases in viral infection in all age groups were reported in 2020 compared with the previous two years, which was particularly notable in children aged 0–12 months and 1–3 years. This may be due to the lower exposure to viruses caused by various NPIs in Chongqing. For example, employees worked at home and held meetings online, and therefore children had less contact with anyone other than household members during the COVID-19 pandemic. Our results might help illustrate the role of household transmission of respiratory viruses from older siblings and adults.
RSV is the most important viral pathogen causing acute LRTIs in children under the age of 5 years (Shi et al., 2017). In this study, RSV was the most frequently detected virus among children younger than 6 years old with LRTIs. The detection rate of RSV sharply dropped after an outbreak in January 2020 and stayed in a nearly dormant state during the next seven months. However, the detection rates from June to September of 2021 remained abnormally high (over 10%), as reported in Shanghai and Beijing (Jia et al., 2022; Jiang et al., 2022). We also found higher proportions of RSV-infected children aged 1–3 years in 2021. Multinational studies have also reported atypical seasonal characteristics of RSV during the pandemic (Casalegno et al., 2021; Ohnishi and Kawano, 2021; Weinberger Opek et al., 2021; Mohebi et al., 2022). In a recent study from Australia, researchers reported up to a 98% reduction in RSV cases and unexpected outbreaks since September 2020 as physical restrictions relaxed, with increased age distribution (median age, 18.4 months vs. 7.3–12.5 months in 2012–2019) (Foley et al., 2021). There was a rapid increase in RSV cases in New Zealand, and the surveillance numbers after a partial relaxation of the strict border closure policy in April 2021 were five times higher than the 2015–2019 peak average. It was also shown that the number of infected children aged 0–4 years was three times higher than the peak average in 2015–2019 (Hatter et al., 2021). South Korea reported an outbreak of RSV in November 2021 after more than 1-year zero detection, when public measures were lifted and the SARS-Cov-2 vaccination was implemented. However, no differences in median age were reported (Kim et al., 2022). Factors contributing to this usual summer peak are complicated, and relaxation of NPIs has likely played a predominant role. Moreover, this out-of-season upsurge might also be related to the emergence of new viral genetic lineage strains. Original research conducted in Taiwan, a province of China, reported that the delay of RSV outbreak in 2020 was caused by two novel RSV ON1.1 variants (Lee et al., 2022). A study in Argentina also showed that the delayed RSV upsurge was related to new viral variants (Dolores et al., 2022). Epidemiological data in Beijing revealed abnormal RSV prevalence in the summer of 2021 along with the shift of subtypes from B to A (Jiang et al., 2022).
The influenza virus is another important pathogen for LRTIs and has caused severe disease burdens worldwide (Li et al., 2022). However, we observed almost no Flu A and Flu B since the outbreak in late January 2020. Low detection of influenza viruses persisted even after full school reopening, work resuming, and distance shortening in 2021. A model predicted that future influenza viruses rebound would occur after the resumption of social activities, due to the low exposure and an overall increase of susceptibility in society during the pandemic (Baker et al., 2020). However, a lower-than-usual magnitude was also observed in the winter of 2021. A multicenter study showed that the 2020–2021 seasonal influenza epidemics were delayed, shorter, and less intense, and that school closings, canceling of public events, and restricting internal movements seemed to reduce transmissions (Davis et al., 2022). NPIs applied during COVID-19 may have played a vital role in the process. Furthermore, negative interactions between Flu A and rhinovirus (RV) may have also played a role (Piret and Boivin, 2022). It has been reported that RV increased sharply after the reopening of schools in 2020, exceeding the same period of previous years (Poole et al., 2020; Redlberger-Fritz et al., 2021; Takashita et al., 2021). However, we did not analyze epidemiological information of RV in this report. It is necessary to continue monitoring RV and influenza viruses in our following project. Influenza virus vaccination was not a convincing factor. Under the impact of the COVID-19 pandemic, the willingness to receive influenza vaccines has been increasing to avoid double infections of SARS-CoV-2 and influenza virus (Hou et al., 2021). Even so, the influenza virus vaccination coverage in China, 9.4% among the general population (Cohen et al., 2021), was very low compared with other countries and far from the herd immunity threshold (Fine et al., 2011).
Previous studies have shown that PIV-3 circulates most frequently from late spring to summer, and its detection rate in winter is low (Li et al., 2019). However, there was a very low prevalence from January to July 2020, but a sharp increase from August to November, when schools reopened and NPIs relaxation started, with a delay of about 5 months. Out-of-season trends of PIV-3 were reported in other studies as well (Liu et al., 2021; Takashita et al., 2021; Kim et al., 2022; Ye and Liu, 2022). However, the mechanism behind this atypical pattern is not yet clear. Lifted travel restrictions, reopening of educational institutions, and resumption of socioeconomic activities may all have triggered this unusual epidemic. These results also indicate that the healthcare system may need to prepare for future atypical prevalence of common respiratory viruses such as influenza viruses.
This study has several limitations. First, this was a single-center study, and we only collected data on patients with LRTIs, who were more likely to be severely affected, with a selection bias. Multicenter research trials and the inclusion of larger patient cohorts in the future will provide help for the control, prevention, and treatment of respiratory virus infections. Second, this was a retrospective analysis that only surveyed a small proportion of viral respiratory antigens by direct immunofluorescence assay; thus, further epidemiological surveillance of more pathogens by molecular assay and their circulating subtypes will help reveal the more-detailed epidemiological information. Third, this study was conducted in 2021, when strict NPIs were continued in Chongqing with varying stringency according to the number of local and imported cases of SARS-CoV-2 infections, and we did not observe the large-scale out-of-season outbreaks as reported abroad. More multicenter epidemiological studies are needed when mitigation measures are completely lifted.
In this study, we enrolled more than 30,000 hospitalized children diagnosed with LRTIs with a time span covering two years before and during the epidemic to explore the prevalence of the respiratory viruses. We summarized that the NPIs implemented during the COVID-19 pandemic had great impacts on the prevalence of common respiratory viruses and seasonal patterns of certain viruses such as RSV, PIV-3, and influenza viruses. Clinicians should be aware that seasonal respiratory viruses might not exhibit typical circulation patterns and be cautious of a possible unusual rebound and unexpected resurgence of seasonal respiratory viruses, such as influenza viruses, when sanitary restrictions are fully lifted. Therefore, we suggest continuous surveillance of the epidemiological and evolutionary dynamics of multiple respiratory pathogens, especially when major public policies are launched.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study protocol was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University (File No. (2022)196). Written consent was waived because antigen detection of respiratory pathogens is standard and routine for all children admitted for LRTIs in the hospital while the study was implemented.
DT designed the project. Material preparation and data collection were performed by LZ, TL, and XW. Data was analyzed by SY, YY, TL, and LZ. This program was guided by ZF, EL, DT, JL, and XX. Manuscript drafted by LZ. TG, and CN revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant: 81870012) and Program for Youth Innovation in Future Medicine, Chongqing Medical University (Number: W0063).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1142199/full#supplementary-material
Agca, H., Akalin, H., Saglik, I., Hacimustafaoglu, M., Celebi, S., Ener, B. (2021). Changing epidemiology of influenza and other respiratory viruses in the first year of COVID-19 pandemic. J. Infection Public Health 14 (9), 1186–1190. doi: 10.1016/j.jiph.2021.08.004
Baker, R. E., Park, S. W., Yang, W., Vecchi, G. A., Metcalf, C. J. E., Grenfell, B. T. (2020). The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc. Natl. Acad. Sci. United States America 117 (48), 30547–30553. doi: 10.1073/pnas.2013182117
Casalegno, J. S., Ploin, D., Cantais, A., Masson, E., Bard, E., Valette, M., et al (2021) Characteristics of the delayed respiratory syncytial virus epidemic 2020/2021, rhône Loire, France Euro surveillance: Bull. Europeen sur les maladies transmissibles = Eur. Communicable Dis. Bull. 26, 29 doi: 10.2807/1560-7917.Es.2021.26.29.2100630
Chiu, S. S., Cowling, B. J., Peiris, J. S. M., Chan, E. L. Y., Wong, W. H. S., Lee, K. P. (2022). Effects of nonpharmaceutical COVID-19 interventions on pediatric hospitalizations for other respiratory virus infections, Hong Kong. Emerging Infect. Dis. 28 (1), 62–68. doi: 10.3201/eid2801.211099
Cohen, R., Ashman, M., Taha, M. K., Varon, E., Angoulvant, F., Levy, C., et al. (2021). Pediatric infectious disease group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 51 (5), 418–423. doi: 10.1016/j.idnow.2021.05.004
Davis, W. W., Mott, J. A., Olsen, S. J. (2022). The role of non-pharmaceutical interventions on influenza circulation during the COVID-19 pandemic in nine tropical Asian countries. Influenza Other Respir. Viruses 16 (3), 568–576. doi: 10.1111/irv.12953
Dolores, A., Stephanie, G., Mercedes, S., N. J., Érica, G., Mistchenko, A. S., et al. (2022). RSV Reemergence in Argentina since the SARS-CoV-2 pandemic. J. Clin. Virol. 149, 105126. doi: 10.1016/j.jcv.2022.105126
Fine, P., Eames, K., Heymann, D. L. (2011). Herd immunity": a rough guide. Clin. Infect. Dis. 52 (7), 911–916. doi: 10.1093/cid/cir007
Foley, D. A., Yeoh, D. K., Minney-Smith, C. A., Martin, A. C., Mace, A. O., Sikazwe, C. T., et al. (2021). The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin. Infect. diseases: an Off. Publ. Infect. Dis. Soc. America 73 (9), e2829–e2830. doi: 10.1093/cid/ciaa1906
Grote, U., Arvand, M., Brinkwirth, S., Brunke, M., Buchholz, U., Eckmanns, T., et al. (2021). Measures to cope with the COVID-19 pandemic in Germany: nonpharmaceutical and pharmaceutical interventions. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 64 (4), 435–445. doi: 10.1007/s00103-021-03306-z
Hatter, L., Eathorne, A., Hills, T., Bruce, P., Beasley, R. (2021). Respiratory syncytial virus: paying the immunity debt with interest. Lancet Child Adolesc. Health 5 (12), e44–e45. doi: 10.1016/s2352-4642(21)00333-3
Hou, Z., Song, S., Du, F., Shi, L., Zhang, D., Lin, L., et al. (2021). The influence of the COVID-19 epidemic on prevention and vaccination behaviors among Chinese children and adolescents: Cross-sectional online survey study. JMIR Public Health Surveillance 7 (5), e26372. doi: 10.2196/26372
Ji, L., Chen, L., Xu, D., Wu, X. (2021). Molecular typing and epidemiologic profiles of human metapneumovirus infection among children with severe acute respiratory infection in huzhou, China. Mol. Biol. Rep. 48 (12), 7697–7702. doi: 10.1007/s11033-021-06776-1
Jia, R., Lu, L., Su, L., Lin, Z., Gao, D., Lv, H., et al. (2022). Resurgence of respiratory syncytial virus infection during COVID-19 pandemic among children in shanghai, China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.938372
Jiang, M. L., Xu, Y. P., Wu, H., Zhu, R. N., Sun, Y., Chen, D. M., et al. (2022). Changes in endemic patterns of respiratory syncytial virus infection in pediatric patients under the pressure of nonpharmaceutical interventions for COVID-19 in Beijing, China. J. Med. Virol. 95 (1), e28411. doi: 10.1002/jmv.28411
Kim, Y. K., Song, S. H., Ahn, B., Lee, J. K., Choi, J. H., Choi, S. H., et al. (2022). Shift in clinical epidemiology of human parainfluenza virus type 3 and respiratory syncytial virus b infections in Korean children before and during the COVID-19 pandemic: A multicenter retrospective study. J. Korean Med. Sci. 37 (28), e215. doi: 10.3346/jkms.2022.37.e215
Lai, S., Ruktanonchai, N. W., Zhou, L., Prosper, O., Luo, W., Floyd, J. R., et al. (2020). Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature 585 (7825), 410–413. doi: 10.1038/s41586-020-2293-x
Lee, C. Y., Wu, T. H., Fang, Y. P., Chang, J. C., Wang, H. C., Lin, S. J., et al. (2022). Delayed respiratory syncytial virus outbreak in 2020 in Taiwan was correlated with two novel RSV-a genotype ON1 variants. Influenza Other Respir. Viruses 16 (3), 511–520. doi: 10.1111/irv.12951
León, T. M., Vargo, J., Pan, E. S., Jain, S., Shete, P. B. (2021). Nonpharmaceutical interventions remain essential to reducing coronavirus disease 2019 burden even in a well-vaccinated society: A modeling study. Open Forum Infect. Dis. 8 (9), ofab415. doi: 10.1093/ofid/ofab415
Li, Y., Reeves, R. M., Wang, X., Bassat, Q., Brooks, W. A., Cohen, C., et al. (2019). Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Global Health 7 (8), e1031–e1045. doi: 10.1016/s2214-109x(19)30264-5
Li, Y., Wang, X., Blau, D. M., Caballero, M. T., Feikin, D. R., Gill, C. J., et al. (2022). Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 399 (10340), 2047–2064. doi: 10.1016/s0140-6736(22)00478-0
Li, L., Wang, H., Liu, A., Wang, R., Zhi, T., Zheng, Y., et al. (2021). Comparison of 11 respiratory pathogens among hospitalized children before and during the COVID-19 epidemic in shenzhen, China. Virol. J. 18 (1), 202. doi: 10.1186/s12985-021-01669-y
Liu, P., Xu, M., Cao, L., Su, L., Lu, L., Dong, N., et al. (2021). Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol. J. 18 (1), 159. doi: 10.1186/s12985-021-01627-8
Mohebi, L., Karami, H., Mirsalehi, N., Ardestani, N. H., Yavarian, J., Mard-Soltani, M., et al. (2022). A delayed resurgence of respiratory syncytial virus (RSV) during the COVID-19 pandemic: An unpredictable outbreak in a small proportion of children in the southwest of Iran, April 2022. J. Med. Virol. 94 (12), 5802–5807. doi: 10.1002/jmv.28065
Nenna, R., Matera, L., Pierangeli, A., Oliveto, G., Viscido, A., Petrarca, L., et al(2022)First COVID-19 lockdown resulted in most respiratory viruses disappearing among hospitalised children, with the exception of rhinoviruses. Acta Paediatrica 111 (7), 1399–1403 doi: 10.1111/apa.16326
Ohnishi, T., Kawano, Y. (2021). Resurgence of respiratory syncytial virus infection during an atypical season in Japan. J. Pediatr. Infect. Dis. Soc. 10 (10), 982–983. doi: 10.1093/jpids/piab065
Pan, A., Liu, L., Wang, C., Guo, H., Hao, X., Wang, Q., et al. (2020). Association of public health interventions with the epidemiology of the COVID-19 outbreak in wuhan, China. Jama 323 (19), 1915–1923. doi: 10.1001/jama.2020.6130
Park, J. Y., Kim, H. I., Kim, J. H., Park, S., Hwang, Y. I., Jang, S. H., et al. (2021). Changes in respiratory virus infection trends during the COVID-19 pandemic in south Korea: the effectiveness of public health measures. Korean J. Intern. Med. 36 (5), 1157–1168. doi: 10.3904/kjim.2021.026
Piret, J., Boivin, G. (2022). Viral interference between respiratory viruses. Emerging Infect. Dis. 28 (2), 273–281. doi: 10.3201/eid2802.211727
Poole, S., Brendish, N. J., Tanner, A. R., Clark, T. W. (2020). Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Respir. Med. 8 (12), e92–e93. doi: 10.1016/s2213-2600(20)30502-6
Redlberger-Fritz, M., Kundi, M., Aberle, S. W., Puchhammer-Stöckl, E. (2021). Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J. Clin. Virol. 137, 104795. doi: 10.1016/j.jcv.2021.104795
Sakamoto, H., Ishikane, M., Ueda, P. (2020). Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. Jama 323 (19), 1969–1971. doi: 10.1001/jama.2020.6173
Salzberger, B., Buder, F., Lampl, B., Ehrenstein, B., Hitzenbichler, F., Holzmann, T., et al. (2021). Epidemiology of SARS-CoV-2. Infection 49 (2), 233–239. doi: 10.1007/s15010-020-01531-3
Shi, T., McAllister, D. A., O'Brien, K. L., Simoes, E. A. F., Madhi, S. A., Gessner, B. D., et al (2017). Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390 (10098), 946–958 doi: 10.1016/s0140-6736(17)30938-8
Song, X., Delaney, M., Shah, R. K., Campos, J. M., Wessel, D. L., DeBiasi, R. L. (2022). Common seasonal respiratory viral infections in children before and during the coronavirus disease 2019 (COVID-19) pandemic. Infection Control Hosp. Epidemiol. 43 (10), 1454–1458. doi: 10.1017/ice.2021.430
Takashita, E., Kawakami, C., Momoki, T., Saikusa, M., Shimizu, K., Ozawa, H., et al. (2021). Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza other Respir. Viruses 15 (4), 488–494. doi: 10.1111/irv.12854
Wang, D., Chen, L., Ding, Y., Zhang, J., Hua, J., Geng, Q., et al. (2016). Viral etiology of medically attended influenza-like illnesses in children less than five years old in suzhou, China 2011-2014. J. Med. Virol. 88 (8), 1334–1340. doi: 10.1002/jmv.24480
Wang, H., Zheng, Y., de Jonge, M. I., Wang, R., Verhagen, L. M., Chen, Y., et al. (2022). Lockdown measures during the COVID-19 pandemic strongly impacted the circulation of respiratory pathogens in southern China. Sci. Rep. 12 (1), 16926. doi: 10.1038/s41598-022-21430-x
Weinberger Opek, M., Yeshayahu, Y., Glatman-Freedman, A., Kaufman, Z., Sorek, N., Brosh-Nissimov, T. (2021). Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, ashdod, Israel 2021. Euro surveillance: Bull. Europeen sur les maladies transmissibles = Eur. communicable Dis. Bull. 26 (29). doi: 10.2807/1560-7917.Es.2021.26.29.2100706
Ye, Q., Liu, H. (2022). Impact of non-pharmaceutical interventions during the COVID-19 pandemic on common childhood respiratory viruses - an epidemiological study based on hospital data. Microbes Infection 24 (1), 104911. doi: 10.1016/j.micinf.2021.104911
Yeoh, D. K., Foley, D. A., Minney-Smith, C. A., Martin, A. C., Mace, A. O., Sikazwe, C. T., et al. (2021). Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 72 (12), 2199–2202. doi: 10.1093/cid/ciaa1475
Zhao, Z., Li, X., Liu, F., Jin, R., Ma, C., Huang, B., et al. (2021). Stringent nonpharmaceutical interventions are crucial for curbing COVID-19 transmission in the course of vaccination: A case study of south and southeast Asian countries. Healthcare 9, (10). doi: 10.3390/healthcare9101292
Keywords: COVID-19, SARS-CoV-2, nonpharmaceutical interventions, epidemiology, pediatrics, respiratory virus
Citation: Zhu L, Luo T, Yuan Y, Yang S, Niu C, Gong T, Wang X, Xie X, Luo J, Liu E, Fu Z and Tian D (2023) Epidemiological characteristics of respiratory viruses in hospitalized children during the COVID-19 pandemic in southwestern China. Front. Cell. Infect. Microbiol. 13:1142199. doi: 10.3389/fcimb.2023.1142199
Received: 13 January 2023; Accepted: 16 March 2023;
Published: 04 April 2023.
Edited by:
Mariana Baz, WHO Collaborating Centre for Reference and Research on Influenza (VIDRL), AustraliaReviewed by:
Sandra Isabel, The Hospital for Sick Children, CanadaCopyright © 2023 Zhu, Luo, Yuan, Yang, Niu, Gong, Wang, Xie, Luo, Liu, Fu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daiyin Tian, dF9keUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.