
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 15 February 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1139449
This article is part of the Research Topic Diversity and Molecular Diagnostics of Fungi and Oomycetes in Plants View all 18 articles
Pseudohydnum is characterized by gelatinous basidiomata with hydnoid hymenophores and longitudinally septate basidia. In this study, samples of the genus from North China were examined morphologically and phylogenetically using a dataset of the internal transcribed spacer of the ribosomal RNA gene and the nuclear large subunit rDNA. This study describes three new species, namely Pseudohydnum abietinum, Pseudohydnum candidissimum, and Pseudohydnum sinobisporum. Pseudohydnum abietinum is characterized by pileate and pale clay pink basidiomata when fresh, with a rudimentary stipe base, four-celled basidia, and broadly ellipsoid to ovoid or subglobose basidiospores (6−7.5 × 5−6.3 μm). P. candidissimum is characterized by very white basidiomata when fresh, frequently four-celled basidia, and broadly ellipsoid to subglobose basidiospores (7.2−8.5 × 6−7 μm). P. sinobisporum is characterized by ivory basidiomata when fresh, two-celled basidia, ovoid to broadly ellipsoid, or subglobose basidiospores (7.5−9.5 × 5.8−7.2 μm). The main characteristics, type localities, and hosts of Pseudohydnum species are listed.
Pseudohydnum P. Karst., typified by P. gelatinosum (Scop.) P. Karst. (Karsten, 1868), has high nutritional and medicinal values (Wang, 2012; Wu et al., 2019). The genus belongs to Auriculariales and is characterized by gelatinous basidiomata with conical spines, a monomitic hyphal system with clamp connections on generative hyphae, longitudinally septate basidia, and ovoid to ellipsoid or globose basidiospores (Niveiro and Popoff, 2011; Chen et al., 2020). Unlike the transversely septate (auricularioid) basidia, the genus has longitudinally cruciate-septate (tremellioid) basidia and thus was treated in Tremellales (Karsten, 1868; Breitenbach and Kränzlin, 1986; Courtecuisse and Lowy, 1990; Niveiro and Popoff, 2011). However, Ingold (1982; 1985) noted that Pseudohydnum and Exidia have a relatively close relationship based on spore germination. Morphologically, Bandoni (1984) redefined the concept of Auriculariales, and the family Aporpiaceae was used to accommodate taxa with myxarioid basidia, including Pseudohydnum. Weiss and Oberwinkler (2001) verified that Pseudohydnum has a close relationship with Auriculariales based on phylogenetic analyses; however, the position of Pseudohydnum in Auriculariales was ambiguous.
Eight species have been recognized in Pseudohydnum. The type species P. gelatinosum was found in Europe (Scopoli, 1772), and two varieties, P. gelatinosum var. bisporum Lowy & Courtec. and P. gelatinosum var. paucidentatum Lowy, were discovered in North America (Lowy, 1959, 1971; Courtecuisse and Lowy, 1990). Three species were described from Oceania: P. orbiculare J.A. Cooper, P. tasmanicum Y.C. Dai & G.M. Gates, and P. totarae (Lloyd) J.A. Cooper (Zhou et al., 2022). Four species were described from Asia: P. translucens Lloyd, P. brunneiceps Y.L. Chen et al., P. himalayanum Y.C. Dai et al., and P. sinogelatinosum Y.C. Dai et al., of which the last three species were described from China (Chen et al., 2020; Zhou et al., 2022). In addition, two forms, P. gelatinosum f. album (Bres.) Kobayasi and P. gelatinosum f. fuscum (Bres.) Kobayasi, have been described from Japan (Lloyd, 1925; Kobayasi, 1954).
During an investigation of jelly fungi in North China, several samples belonging to Pseudohydnum were collected, and three unknown species were found. To confirm the affinity of the taxa, phylogenetic analysis was performed based on the internal transcribed spacer (ITS) and large subunit nuclear ribosomal RNA gene (LSU) sequences.
The specimens were collected from the provinces of Jinlin, Heilongjiang, and Gansu in North China. They were deposited in the herbaria of Beijing Forestry University (BJFC) and the Mycology Department of Jinlin Agriculture University (HMJAU). Samples were photographed when fresh in the field, and their habitats were recorded. Microscopic structures were discussed by Chen et al. (2020), Fan et al. (2021), and Zhou et al. (2022). Special color terms were set by Anonymous (1969) and Petersen (1996). A Nikon Digital Sight DS-L3 or Leica ICC50 HD camera (magnification ×1,000) was used to examine hand-cut sections of basidiomata, which were first treated with 5% KOH for a few minutes and then with 1% phloxine B (C20H4Br4Cl2K2O5). At least 30 basidiospores of each species were examined. The values were expressed as a mean with 5% of the measurements excluded from each end of the range, given in parentheses. Stalks were excluded for basidia measurement, and the hilar appendages were excluded for basidiospore measurement.
The following abbreviations are used in the descriptions: IKI, Melzer’s reagent; IKI−, neither amyloid nor dextrinoid; CB, cotton blue; CB−, acyanophilous in cotton blue; L, the arithmetic average of spore lengths; W, the arithmetic average of spore widths; Q, L/W ratio; and n (a/b), number of spores (a) measured from a given number (b) of specimens.
The CTAB rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd., Beijing) was used to obtain DNA from dried specimens and PCR was performed according to the manufacturer’s instructions with some modifications (Chen and Dai, 2021). Two DNA gene fragments, ITS and LSU, were amplified using the primer pairs ITS5/ITS4 (White et al., 1990) and LR0R/LR7, respectively (Hopple and Vilgalys, 1994).
The PCR procedure for ITS was as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles at 94 °C for 40 s, 54 °C for 45 s, and 72 °C for 1 min; and a final extension at 72 °C for 10 min. The PCR procedure for LSU was as follows: initial denaturation at 94 °C for 1 min, followed by 35 cycles at 94 °C for 30 s, 50 °C for 1 min, and 72 °C for 1.5 min; and a final extension at 72 °C for 10 min. DNA sequencing was performed at the Beijing Genomics Institute. All newly generated sequences were submitted to GenBank and are listed in Table 1.
Sequences generated for this study were aligned, with additional sequences downloaded from GenBank. Both ITS and LSU sequences were aligned using MAFFT v.7 (https://mafft.cbrc.jp/alignment/server/), adjusting the direction of nucleotide sequences according to the first sequence (accurate enough for most cases), and selecting the G-INS-i iterative refinement method (Katoh et al., 2019). Alignments were manually adjusted to maximize alignment and minimize gaps with BioEdit v.7.0.9 (Hall, 1999). A dataset composed of concatenated ITS + LSU sequences was used to determine the phylogenetic position of new species. The aligned sequences were deposited in TreeBase (https://www.treebase.org/treebase-web/home.html; submission ID 29962). Protomerulius subreflexus (Lloyd) O. Miettinen & Ryvarden and P. substuppeus (Berk. & Cooke) Ryvarden were selected as outgroups following Chen et al. (2020).
Maximum likelihood (ML) analysis was performed using the CIPRES Science Gateway (Miller et al., 2009) based on the dataset using the RA × ML-HPC BlackBox tool, with setting RA × ML halt bootstrapping automatically and 0.25 for maximum hours and obtaining the best tree using ML search. Other parameters in ML analysis used default settings, and statistical support values were obtained using nonparametric bootstrapping with 1,000 replicates.
Bayesian inference (BI) analysis based on the dataset was performed using MrBayes v.3.2.6 (Ronquist et al., 2012). The best substitution model for the dataset was selected by ModelFinder (Kalyaanamoorthy et al., 2017) using a Bayesian information criterion, and the model was used for Bayesian analysis. Four Markov chains were run from random starting trees for 0.8 million generations. Trees were sampled every 1,000th generation. The first 25% of sampled trees were discarded as burn-in, whereas other trees were used to construct a 50% majority consensus tree and for calculating Bayesian posterior probabilities (BPPs).
The concatenated ITS + LSU dataset included 30 ITS and 22 LSU sequences from 30 samples representing 14 taxa. The best model for the concatenated ITS + LSU dataset estimated and applied for BI analysis was “SYM + I + G”, datatype = DNA, nucmodel = 4by4, lset nst = 6, rates = invgamma; state frequencies had a Dirichlet prior (1,1,1,1), and the distribution was approximated using four categories. BI analysis yielded a similar topology to ML analysis, with an average standard deviation of split frequencies of 0.007485. The ML tree was provided (Figure 1). Branches that received bootstrap support for ML (ML-BS) and BI (BPP) ≥70% (ML-BS), and 0.85 (BPP) were considered significantly supported, respectively.
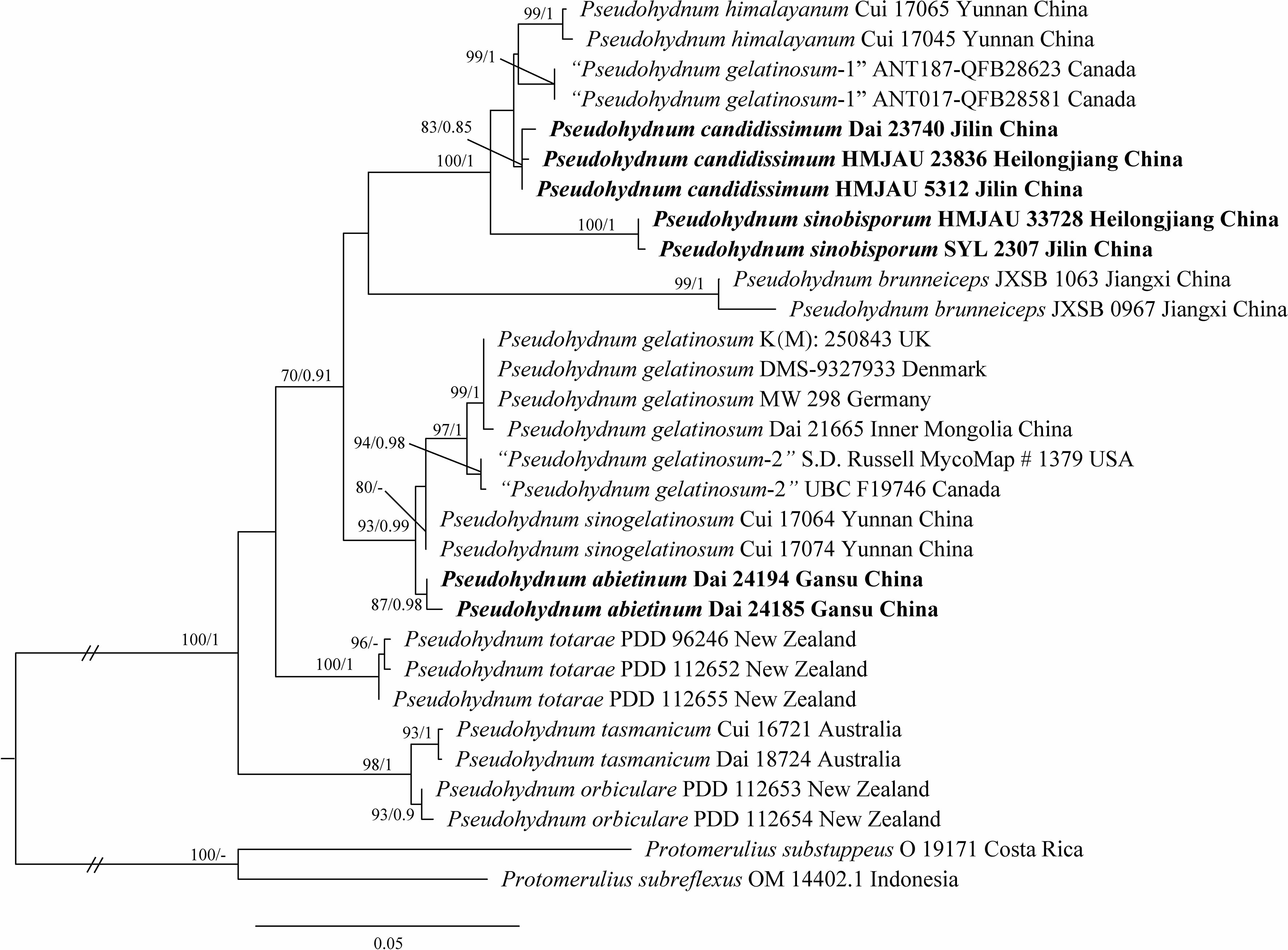
Figure 1 Phylogeny of Pseudohydnum species generated by maximum likelihood (ML) based on ITS + LSU sequences. Branches are labeled with ML bootstrap ≥70% and Bayesian posterior probabilities ≥0.85.
Phylogenetic analysis placed all Pseudohydnum samples in a fully supported clade (100/1, Figure 1). Five specimens from Northeast China formed two lineages, namely P. candidissmum and P. sinobisporum, clustered with P. himalayanum as strong support (100/1, Figure 1). The two specimens from Northwest China were named P. abietinum, sister to P. sinogelatinosum and P. gelatinosum. The samples from North America were treated as “P. gelatinosum-1” and “P. gelatinosum-2.”
Pseudohydnum abietinum H.M. Zhou & Jing Si, sp. nov. Figure 2
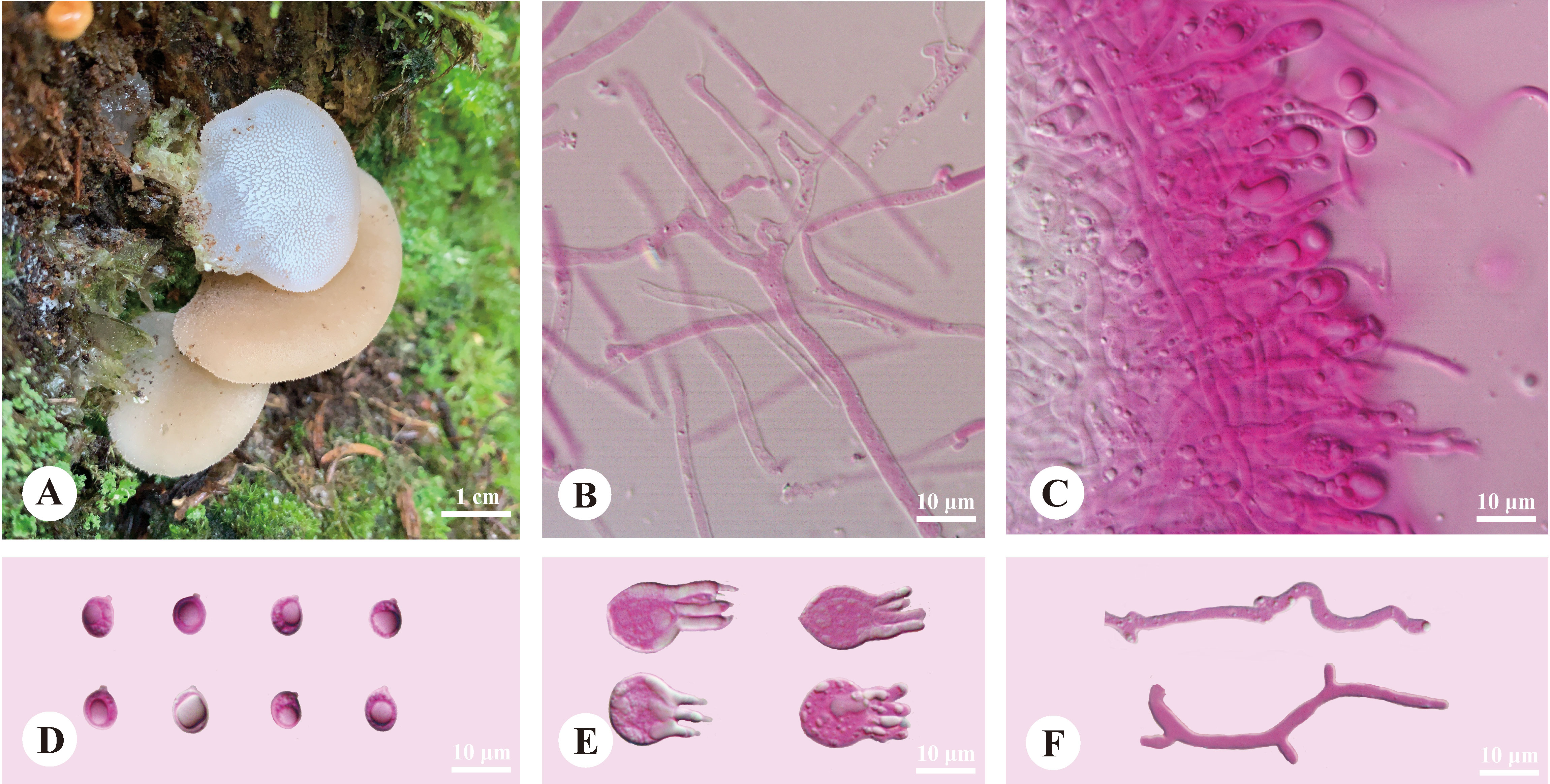
Figure 2 Basidiomata and microscopic structures of Pseudohydnum abietinum (holotype, Dai 24185). (A) Basidiomata; (B) Tramal hyphae; (C) A section of hymenium; (D) Basidiospores; (E) Basidia; (F) Hyphidia.
MycoBank: 847486
Type—China. Gansu Province, Gannan, Zhuoni County, Taohe National Nature Reserve, Boyu Valley, on a stump of Abies, elev. 2,900 m, August 19, 2022, Dai 24185 (holotype, BJFC).
Etymology—Abietinum (Lat.): referring to the species growing on Abies.
Diagnosis—Differed from other Pseudohydnum species in having pileate basidiomata, with a rudimentary stipe base, pale clay pink pileal surface when fresh, hymenophore with spines 2−3 per mm at the base, broadly ellipsoid to ovoid or subglobose basidiospores measuring 6−7.5 × 5−6.3 μm, and occurring in Gansu Province, Northwest China.
Basidiomata—Annual, pileate with a rudimentary stipe base, gelatinous when fresh, brittle when dry, usually solitary. Pilei were dimidiated to flabelliform, projecting up to 1.5 cm, 1.4 cm wide, and 1.9 mm thick when dry. Pileal surfaces were pale clay pink when fresh, and hazel when dry. Spines were white and conical when fresh, cream when dry, 2−3 per mm at the base, and up to 1.5-mm long when dry. The context was translucent when fresh.
Hyphal structure—Monomitic; generative hyphae with clamp connections. Contextual hyphae were hyaline, thin- to slightly thick-walled, frequently branched, interwoven, and 2−6 μm in diam. Tramal hyphae were hyaline, thin-walled, frequently branched, interwoven, and 1.5−2 μm in diam. Hyphidia were occasionally branched. Basidia were four-celled, barrel-shaped, globose to subglobose, with guttules, and 9.5−12 × 7.5−12 μm; sterigmata were up to 12-μm long and 1.5−2 μm in diam. Probasidia were fusiform to lageniform and 10−13 × 8−10.5 μm. Basidiospores were broadly ellipsoid to ovoid or subglobose, hyaline, thin-walled, with a big guttule, IKI−, CB−, 6−7.5(−8) × 5−6.3(−6.8) μm, L = 6.84 μm, W = 5.59 μm, and Q = 1.20−1.24 (60/2).
Additional specimen examined (paratype)—China. Gansu Province, Gannan, Zhuoni County, Taohe National Nature Reserve, Boyu Valley, on rotten wood of Abies, elev. 2,900 m, August 19, 2022, Dai 24194 (BJFC).
Pseudohydnum candidissimum H.M. Zhou, T. Bau & Jing Si, sp. nov. Figure 3
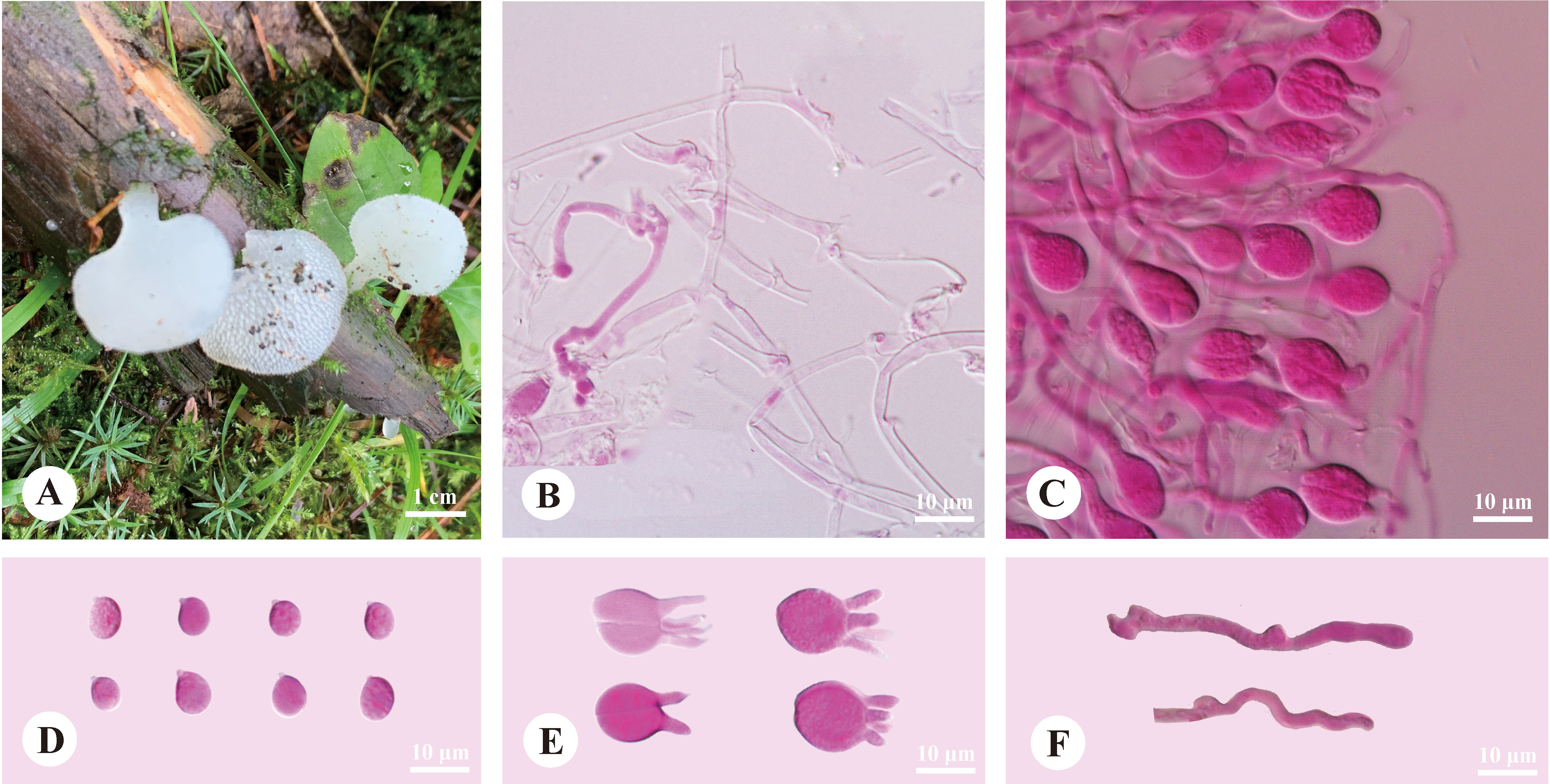
Figure 3 Basidiomata and microscopic structures of Pseudohydnum candidissimum (holotype, Dai 23740). (A) Basidiomata; (B) Tramal hyphae; (C) A section of hymenium; (D) Basidiospores; (E) Basidia; (F) Hyphidia.
Mycobank: 847487
Type—China. Jilin Province, Yanbian, Antu County, Changbaishan Nature Reserve, on a fallen trunk of Larix, July 24, 2022, Dai 23740 (holotype, BJFC).
Etymology—Candidissimum (Lat.): referring to the species having very white basidiomata when fresh.
Diagnosis—Differed from other Pseudohydnum species in having very white basidiomata when fresh, simple hyphidia, broadly ellipsoid to subglobose, measuring 7.2−8.5 × 6−7 μm, and occurring in Northeast China.
Basidiomata—Annual, gelatinous when fresh, brittle when dry, usually solitary, with a lateral stipe. Pilei flabelliform to dimidiate, projecting up to 1.5 cm, 1.2 cm wide, and 0.6-mm thick when dry. The pileal surface was white when fresh and pale mouse-gray when dry. Spines were white and conical when fresh, buff-yellow when dry, 2−3 per mm at the base, and up to 0.5-mm long. The context was translucent when fresh. Stipe concolorous with pileal surface, translucent when fresh, up to 5-mm long and 3 mm in diam. when dry.
Hyphal structure—Monomitic; generative hyphae with clamp connections. Contextual hyphae were hyaline, thin- to slightly thick-walled, frequently branched, interwoven, 1.5−3 μm in diam. Tramal hyphae were hyaline, thin-walled, frequently branched, interwoven, 1.5−2 μm in diam. Hyphidia simple. Basidia were frequently four-celled, occasionally two-celled, barrel-shaped, ellipsoid to subglobose, 11−14 × 10.5−13 μm; sterigmata up to 10-μm long and 2−3.5 μm in diam. Probasidia were fusiform to lageniform, 11−14 × 6.5−10 μm. Basidiospores were broadly ellipsoid to subglobose, hyaline, thin-walled, IKI−, CB−, (7−)7.2−8.5(−9.2) × 6−7(−7.5) μm, L = 7.97 μm, W = 6.56 μm, and Q = 1.19−1.24 (90/3).
Additional specimens examined (paratypes)—China. Heilongjiang Province, Yichun, Fenglin National Nature Reserve, July 25, 2010, HMJAU 23836; Jilin Province, Yanbian, Antu County, Erdaobai River, on rotten wood, September 13, 2007, HMJAU 5312.
Pseudohydnum sinobisporum T. Bau, H.M. Zhou & Jing Si, sp. nov. Figure 4
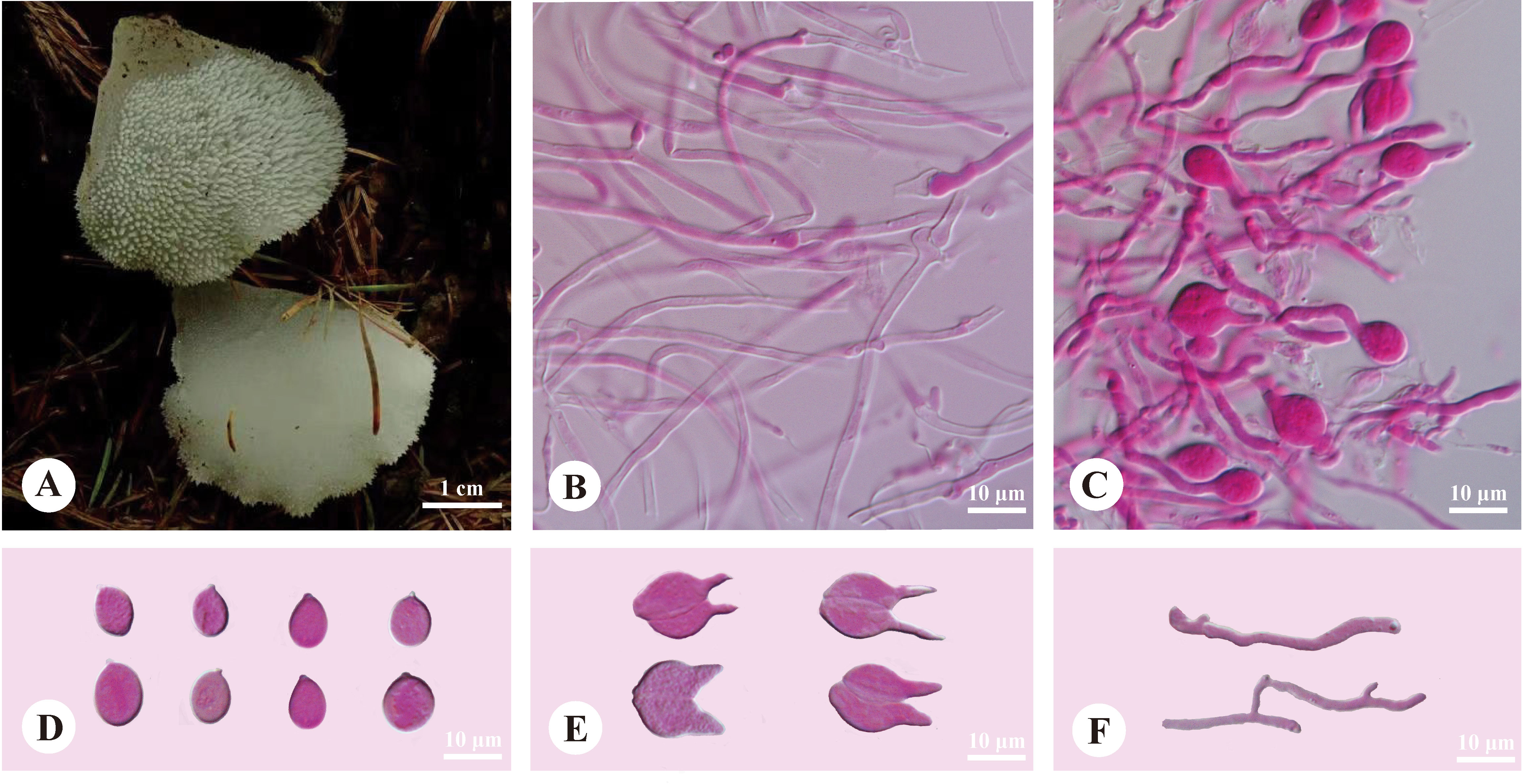
Figure 4 Basidiomata and microscopic structures of Pseudohydnum sinobisporum (holotype, SYL 2307). (A) Basidiomata; (B) Tramal hyphae; (C) A section of hymenium; (D) Basidiospores; (E) Basidia; (F) Hyphidia.
MycoBank: 847488
Type—China. Jilin Province, Yanbian, Tianfozhishan National Nature Reserve, on a stump of Quercus mongolica, August 23, 2020, SYL 2307 (holotype, HMJAU).
Etymology—Sinobisporum (Lat.): referring to the species having two spores on each basidium and being found in China.
Diagnosis—Differed from other Pseudohydnum species in having ivory basidiomata, two spores on each basidium, branched hyphidia, ovoid to broadly ellipsoid or subglobose, measuring 7.5−9.5 × 5.8−7.2 μm, and occurring in Northeast China.
Basidiomata—Annual, gelatinous when fresh, brittle when dry, solitary, with a lateral stipe. Pilei was shell-shaped, projecting up to 1.2 cm, 1 cm wide, and 1.2 mm thick when dry. Pileal surfaces were ivory when fresh and hazel when dry. Spines were white and conical when fresh, cream when dry, 2−3 per mm at the base, and up to 1 mm long when dry. The context was translucent when fresh. Stipe concolorous with pileal surface, shrinking to the base, translucent when fresh, up to 5.5-mm long and 5 mm in diam. when dry.
Hyphal structure—Monomitic; generative hyphae with clamp connections. Contextual hyphae were hyaline, thin- to slightly thick-walled, frequently branched, interwoven, and 1.5−3 μm in diam. Tramal hyphae were hyaline, thin-walled, occasionally branched, interwoven, and 1−2 μm in diam. Hyphidia were occasionally branched. Basidia were two-celled, barrel-shaped, ellipsoid to subglobose, 11−11.5 × 9−12 μm; sterigmata were up to 11-μm long and 2−3 μm in diam. Probasidia were fusiform to lageniform, 11−15 × 8−11.5 μm. Basidiospores were ovoid to broadly ellipsoid or subglobose, hyaline, thin-walled, IKI−, CB−, (7.2−)7.5−9.5(−10) × (5.5−)5.8−7.2(−7.5) μm, L = 8.29 μm, W = 6.36 μm, Q = 1.30−1.31 (60/2).
Additional specimen examined (paratype)—China. Heilongjiang Province, Tahe County, on the ground in the forest of Larix, August 19, 2015, HMJAU 33728.
Morphological examination and phylogenetic analysis identified eight species of Pseudohydnum (Chen et al., 2020; Zhou et al., 2022). In this study, three new species of Pseudohydnum were identified in North China: P. abietinum, P. candidissimum, and P. sinobisporum.
Phylogenetically, P. abietinum formed a sister group with P. gelatinosum, P. sinogelatinosum, and “P. gelatinosum-2” (Figure 1). However, P. gelatinosum had smaller basidiospores than P. abietinum (5−6 × 4.5−5.5 μm vs. 6−7.5 × 5−6.3 μm, Breitenbach and Kränzlin, 1986), and P. sinogelatinosum had wider basidiospores than P. abietinum (6−7.2 μm vs. 5−6.3 μm, Zhou et al., 2022) (Table 2). Samples of “P. gelatinosum-2” were not evaluated in this study.
Pseudohydnum candidissimum and P. sinobisporum were related to P. himalayanum and “P. gelatinosum-1” (Figure 1); however, P. himalayanum had denser spines at the base (5−6 per mm vs. 2−3 per mm, Zhou et al., 2022) and was clay-pink to cinnamon basidiomata when fresh (Table 2). Samples of “P. gelatinosum-1” were not evaluated in this study.
Morphologically, P. himalayanum and P. abietinum had similar basidiomata and were easily confused; however, P. himalayanum had wider basidiospores than P. abietinum (6−7.2 µm vs. 5−6.3 µm, Zhou et al., 2022). Pseudohydnum tasmanicum and P. abietinum shared a rudimentary stipe; however, P. tasmanicum had wider basidiospores than P. abietinum (6−7.2 µm vs. 5−6.3 µm, Zhou et al., 2022).
Similar to P. candidissimum, P. gelatinosum and P. gelatinosum var. paucidentatum had white basidiomata (Figure 3; Table 2); however, P. gelatinosum had smaller basidiospores than P. candidissimum (5−6 × 4.5−5.5 μm vs. 7.2−8.5 × 6−7 μm, Breitenbach and Kränzlin, 1986). Compared to P. candidissimum, P. gelatinosum var. paucidentatum had widely scattered spines, the color of its basidiomata remained unchanged upon drying (Lowy, 1959, 1971), and it is distributed in tropical America.
Pseudohydnum brunneiceps, P. gelatinosum var. bisporum, and P. sinobisporum had two-celled basidia (Figure 4; Table 2). However, P. brunneiceps had brownish basidiomata and occurs in subtropical China; P. gelatinosum var. bisporum had short elliptical to subglobose basidiospores and is distributed in French Guiana and South America (Courtecuisse and Lowy, 1990), whereas the newly discovered P. sinobisporum had ivory basidiomata, which were ovoid to broadly ellipsoid or subglobose, and is distributed in boreal to temperate China.
Pseudohydnum candidissimum and P. sinobisporum had overlapping distributions in Northeast China; however, P. candidissimum had very white basidiomata and mostly four-celled basidia (Figure 3; Table 2), and P. sinobisporum had ivory basidiomata and two-celled basidia (Figure 4; Table 2).
Jelly fungi are a special group of wood-inhabiting basidiomycetes and most species belong to the taxa form phragmobasidia (Wells, 1994). Most belong to Auriculariales and Tremellales, and some are edible mushrooms (Dai et al., 2010; Luo et al., 2022; Yao et al., 2022; Zhang et al., 2022). However, the diversity of the Chinese jelly fungi is not well-known, and recently, new species were described from China based on both morphology and phylogeny (Wu et al., 2020, 2021; Fan et al., 2021; Zhou et al., 2022). Advanced techniques, including molecular phylogeny and omics, will aid in discovering new species of jelly fungi in the future.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OP965345-OP965351 and https://www.ncbi.nlm.nih.gov/genbank/, OP965365-OP965371.
Design of the research: H-MZ, TB, and JS. Performance of the research: H-MZ and JS. Data analysis and interpretation: H-MZ and JS. Collection of materials: H-MZ and TB. Writing and revising the manuscript: H-MZ, JS, and TB. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The research was supported by the National Natural Science Foundation of China (Nos. 32070016 and 32270016).
The authors would like to express their deep thanks to Prof. Yu-Cheng Dai (Beijing Forestry University, China) who allowed us to study his specimens.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anonymous (1969). Flora of British fungi. color identification chart (Edinburgh, UK: Her Majesty’s Stationery Office).
Bandoni, R. J. (1984). The tremellales and auriculariales: An alternative classification. Trans. Mycol. Soc Japan. 25, 489–530.
Breitenbach, J., Kränzlin, F. (1986). Fungi of Switzerland volume 2: Non-gilled fungi, heterobasidiomycetes, aphyllophorales, gasteromycetes (Switzerland: Verlag Mykologia Lucerne).
Chen, J. J., Dai, Y. C. (2021). Two new species of Physisporinus (Polyporales, basidiomycota) from yunnan, southwest China. Mycol. Prog. 20, 1–10. doi: 10.1007/s11557-020-01647-8
Chen, Y. L., Su, M. S., Zhang, L. P., Zou, Q., Wu, F., Zeng, N. K., et al. (2020). Pseudohydnum brunneiceps (Auriculariales, basidiomycota), a new species from central China. Phytotaxa 441, 87–94. doi: 10.11646/phytotaxa.441.1.8
Courtecuisse, R., Lowy, B. (1990). Elements for a mycological inventory of the vicinity of ‘Saut pararé’ (Arataye river) and ‘Nouragues inselberg’ (French Guiana) III. heterobasidiomycetideae. studies on the flora of the guianas, no. 52. Mycotaxon 39, 329–344.
Dai, Y. C., Zhou, L. W., Yang, Z. L., Wen, H. A., Bao, T., Li, T. H. (2010). A revised checklist of edible fungi in China. Mycosystema 29, 1–21. doi: 10.13346/j.mycosystema.2010.01.022
Fan, L. F., Alvarenga, R. L. M., Gibertoni, T. B., Wu, F., Dai, Y. C. (2021). Four new species in the Tremella fibulifera complex (Tremellales, basidiomycota). MycoKeys 82, 33–56. doi: 10.3897/mycokeys.82.63241
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. doi: 10.1021/bk-1999-0734.ch008
Hopple, J. S., Vilgalys, R. (1994). Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86, 96–107. doi: 10.1080/00275514.1994.12026378
Ingold, C. T. (1982). Basidiospore germination and conidium formation in Exidia glandulosa and Tremella mesenterica. Trans. Bri. Mycol. Soc 79, 370–373. doi: 10.1016/S0007-1536(82)80135-6
Ingold, C. T. (1985). Observations on spores and their germination in certain heterobasidiomycetes. Trans. Bri. Mycol. Soc 85, 417–423. doi: 10.1016/S0007-1536(85)80035-8
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Karsten, P. A. (1868). Auriculariei, clavariei et tremellini in paroecia tammela crescentes. not. sällskapets fauna flora fenn. förhandlingar, Vol. 9. 365–374.
Katoh, K., Rozewicki, J., Yamada, K. D. (2019). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. doi: 10.1093/bib/bbx108
Lowy, B. (1959). New or noteworthy tremellales from Bolivia. Mycologia 51, 840–850. doi: 10.1080/00275514.1959.12024864
Luo, X. F., Wu, M. W., Li, X. L., Wu, M. S., Chen, B. Z., Jiang, Y. J. (2022). Preventive effects of powder of Auricularia heimuer fruiting bodies on nutritional obesity of high-fat model mice. Mycosystema 41, 1099–1111. doi: 10.13346/j.mycosystema.210451
Miller, M. A., Holder, M. T., Vos, R., Midford, P. E., Liebowitz, T., Chan, L., et al. (2009)The CIPRES portals. In: CIPRES. Available at: http://www.phylo.org/sub_sections/portal (Accessed September 4, 2022).
Niveiro, N., Popoff, O. F. (2011). Pseudohydnum gelatinosum (Tremellales, basidiomycota) en las yungas argentinas. Bol. Soc Argent. Bot. 46, 223–226.
Petersen, J. H. (1996). The Danish mycological society’s colour-chart (Greve, Denmark: Foreningen til Svampekundskabens Fremme).
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Scopoli, J. A. (1772). Flora carniolica (Vindobona: Impensis ioannis Pauli Krauss, Bibliopolae Vindobonensis).
Wang, Z. J. (2012). Optimizing the extraction process of total sugar from Pseudohydnum gelatinosum (Scop.:Fr.) karst and studying antioxidant activities of the extract. J. Henan Normal Univers. (Natural Sci. Edition) 40, 111–115. doi: 10.1098/rstb.2016.0146
Weiss, M., Oberwinkler, F. (2001). Phylogenetic relationships in auriculariales and related groups-hypotheses derived from nuclear ribosomal DNA sequences. Mycol. Res. 105, 403–415. doi: 10.1017/S095375620100363X
Wells, K. (1994). Jelly fungi, then and now! Mycologia 86, 18–48. doi: 10.1080/00275514.1994.12026372
White, T. J., Bruns, T. D., Lee, S., Taylor, J. W. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: A guide to methods and applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, T. J. (New York: Academic Press), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, F., Tohtirjap, A., Fan, L. F., Zhou, L. W., Alvarenga, R. L. M., Gibertoni, T. B., et al. (2021). Global diversity and updated phylogeny of Auricularia (Auriculariales, basidiomycota). J. Fungi 7, 933. doi: 10.3390/jof7110933
Wu, F., Zhao, Q., Yang, Z. L., Ye, S. Y., Rivoire, B., Dai, Y. C. (2020). Exidia yadongensis, a new edible species from East Asia. Mycosystema 39, 1203–1214. doi: 10.13346/j.mycosystema.200205
Wu, F., Zhou, L. W., Yang, Z. L., Bau, T., Li, T. H., Dai, Y. C. (2019). Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 98, 1–76. doi: 10.1007/s13225-019-00432-7
Yao, C. X., Tian, G. T., Wang, H., Yao, Y., Sun, Y. M. (2022). A new Auricularia heimuer cultivar ‘Gaoyuanyuner 3’. Mycosystema 41, 318–320. doi: 10.13346/j.mycosystema.210230
Zhang, Q. H., Liu, J. L., Li, J. H., Chen, L. D., Kong, X. Q., Sun, S. J. (2022). A new Tremella fuciformis cultivar ‘Xiuyin 1’. Mycosystema 41, 163–165. doi: 10.13346/j.mycosystema.210293
Keywords: taxonomy, phylogeny, hydnoid fungi, gelatinous fungi, temperate forests, species diversity
Citation: Zhou H-M, Bau T and Si J (2023) Morphological and phylogenetic evidence reveal three new Pseudohydnum (Auriculariales, Basidiomycota) species from North China. Front. Cell. Infect. Microbiol. 13:1139449. doi: 10.3389/fcimb.2023.1139449
Received: 07 January 2023; Accepted: 01 February 2023;
Published: 15 February 2023.
Edited by:
Yusufjon Gafforov, Academy of Science of the Republic of Uzbekistan, UzbekistanReviewed by:
Viktor Papp, Szent István University, HungaryCopyright © 2023 Zhou, Bau and Si. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Si, amluZ3NpMTc4OEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.