- 1Department of Microbiology, Chrisland University, Abeokuta, Ogun State, Nigeria
- 2Department of Pharmaceutical Microbiology, University of Ibadan, Ibadan, Oyo State, Nigeria
- 3Department of Paediatrics, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria
- 4Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria
- 5Department of Community Medicine, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria
- 6Department of Medical Microbiology & Parasitology, University College Hospital, Ibadan, Oyo State, Nigeria
Introduction: Diarrhoea can be debilitating in young children. Few aetiological investigations in Africans living with human immunodeficiency virus (HIV) have been performed since antiretrovirals became widely available.
Methods: Stool specimens from children with diarrhoea living with HIV, and HIV-uninfected controls, recruited at two hospitals in Ibadan, Nigeria, were screened for parasites and occult blood, and cultured for bacteria. Following biochemical identification of at least five colonies per specimen, diarrhoeagenic Escherichia coli and Salmonella were confirmed by PCR. Data were line-listed and comparisons were made using Fisher’s Exact test.
Results: Only 10 children living with HIV could be enrolled during the 25-month study period and 55 HIV-uninfected children with diarrhoea were included for comparison. The most common pathogens overall were enteroaggregative E. coli (18/65, 27.7%), enteroinvasive E. coli (10/65, 15.4%), Cryptosporidium parvum (8/65, 12.3%) and Cyclospora cayetanensis (7/65, 10.8%). At least one pathogen was detected from seven of ten children living with HIV and 27 (49.1%) HIV-uninfected children. Parasite detection was associated with HIV positive status (p=0.03) with C. parvum specifically recovered more commonly from children living with HIV (p=0.01). Bacterial-parasite pathogen combinations were detected in specimens from four of ten children living with HIV but only 3(5.5%) HIV-uninfected children (p=0.009). Stools from five of ten children living with HIV and 7(12.7%) HIV-negative children (p = 0.014) contained occult blood.
Discussion: Even though children living with HIV present infrequently to Ibadan health facilities with diarrhoea, their greater propensity for mixed and potentially invasive infections justifies prioritizing laboratory diagnosis of their stools.
Introduction
Diarrhoea is the passage of three or more loose stools per day and can lead to appetite loss, dehydration and subsequent weight loss (Jensen et al., 2014). The incidence of diarrhoea is particularly high in low-income settings where sanitary conditions are largely inadequate, of which there are many in Africa (Botero et al., 2003). Unfortunately, many of these areas also have a high proportion of people living with human immunodeficiency virus (HIV) who, when immunocompromised, are susceptible to a plethora of opportunistic infections that can result in diarrhoea (Nwosu et al., 2014). Deaths from diarrhoea are declining but children under five in Africa may have up to an annual average of twelve episodes which places them at risk of death, malnutrition and significantly affects their well-being (Okeke, 2009; Farthing et al., 2013; Guarino et al., 2014; Zollner-Schwetz and Krause, 2015; Bruzzese et al., 2018).
Infectious diarrhoea can be caused by many viral, bacterial and parasitic agents. These include but are not limited to Salmonella and diarrhoeagenic Escherichia coli, including enteroaggregative E. coli (EAEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enterotoxigenic E. coli (ETEC), diffusively adherent E. coli (DAEC) and Shiga-toxin producing E. coli (STEC) (Hien et al., 2008; de Mello Santos et al., 2020). Intestinal parasites such as Cryptosporidium parvum and Cystoisospora belli (Ogunlesi et al. 2006; Sunnotel et al., 2006) as well as viruses including norovirus, adenoviruses and rotaviruses have also been implicated as agents of diarrhoea (World Gastroenterology Organization, 2012).
Years ago, highly active antiretroviral therapy (HAART) was not readily available for and accessible to many people living with HIV leading to high mortality rates, but the trend has changed since access to HAART has improved (Ogunbosi et al., 2011). Moreover, Prevention of Mother-to-Child Transmission (PMTCT) programmes have achieved some success averting several potential paediatric HIV infections. In Nigeria, Olakunde et al. (2019) reported 89.5% increase in PMTCT coverage from 2009 to 2016. All young children are vulnerable to diarrhoea but it is possible that children living with HIV, who may be more significantly impacted by diarrhoea sequelae, are infected with a wider repertoire of pathogens and/or experience more severe infections (Tiruneh et al., 2022). Among indicators of diarrhoeal disease severity are the presence of occult blood in the stools, which may also be connected to colorectal cancer and other forms of carcinomas (Antoniou et al., 2015) but is sparsely investigated in the context of diarrhoeal infections. Diarrhoea is the chief predictor of HIV among children but very few African studies have focused on microbial aetiology of this syndrome in children living with HIV (Okeke and Nataro, 2001; Samie et al., 2007; Ogunbosi et al., 2011). Studies focused on the repertoire of diarrhoea aetiologies among people with HIV in Africa are scarce overall with information largely unavailable in Nigeria, and no information on aetiologies since HAART access improved. This study investigates the facultative aerobic bacterial and parasitic (but not viral) aetiologies of diarrhoea, and stool occult blood, in children living with HIV and compares them to aetiology in children without HIV.
Materials and methods
Study area and patient enrolment
The study was conducted in two healthcare facilities, the University College Hospital (UCH) and Adeoyo Maternity Teaching Hospital (AMTH), both in Ibadan, Nigeria. The research was approved by the UI/UCH Ethics Committee with assigned number UI/EC/18/0335 and Oyo State Ethical Review Committee with assigned number AD13/479/1378. Patients’ parents or guardians provided informed consent prior to enrollment.
Children living with HIV and HIV-uninfected controls, aged 0-15 years, were recruited between January 2019 and February 2021. Children Living with HIV were recruited at HIV clinics or wards while HIV-negative controls were recruited at children emergency wards or children outpatient clinics. Recruitments were guided by the national guidelines for screening children for HIV and managing them (Federal Ministry of Health, Abuja, Nigeria, 2016). Briefly, children less than 18 months are screened for HIV using HIV DNA polymerase chain reaction (PCR). Children with positive HIV DNA PCR results are subsequently placed on HAART. Children with negative results get antibody test at 18 months after weaning. For children ≥ 18 months, antibody tests are carried out using Determine™ rapid kit (Abbott Diagnostics, Japan). If reactive, a confirmatory test is done using Stat Pak® (ChemBio Diagnostic Systems, USA). Uni-Gold™ (Trinity Biotech, Ireland) is used as tie breaker in the event of discordant results.
Eligibility criteria for participation in this study include verified HIV status known to and confirmed by the attending medical personnel, passage of loose stools three or more times within a 24-hour period over the past three days and obtained informed consent from their parents or guardians.
Stool microscopy
Microscopic examination of the stool samples was done using the method of Winn et al. (2006). Drops of normal saline and iodine were separately placed on a clean glass slide and with an applicator stick, an aliquot of stool sample was picked and placed on the drops of normal saline and iodine to make a smear. A cover slip was placed over each wet preparation which was mounted for microscopic observation using the x10 and x40 objectives.
Modified Ziehl-Neelsen method (Fayer et al., 2000; Tahvildar-Biderouni and Salehi, 2014) was also employed to examine the samples for coccidian parasites Cryptosporidium parvum, Cylospora cayetanensis and Cystoisospora belli. Stool smear was made on clean glass slide and allowed to dry. The dried smear was fixed in methanol for three minutes and subsequently stained with strong carbol fuchsin for 20 minutes and then rinsed in tap water. The smear was counterstained with methylene blue for 60 seconds and rinsed with tap water, then dried. Examination of the slides was done using x40 and x100 objectives.
Testing for presence of occult blood
Detection of occult blood in the stool samples was done using a rapid kit (Cromatest®, Linear Chemicals, Spain). This was done by transferring an aliquot of the stool sample into the specimen collection tube containing the extraction buffer and mixed together by shaking. Subsequently, three drops of the extracted stool sample were transferred to the test cassette and observed for positivity indicated by a red line showing on the region marked “T” (for test) as well as the region marked “C” (control) on the device. Specimens testing negative lacked the red line on the test region but showed the red line on the control region (Yamamoto and Nakama, 2000).
Bacteriology
The stool samples were cultured on MacConkey agar and Eosin Methylene Blue (EMB) agar for the isolation of Escherichia coli and other enteric pathogens. For recovery of Salmonella and Shigella, the stool samples were first enriched overnight in selenite F broth before being sub-cultured onto xylose lysine deoxycholate (XLD) agar. Incubation was done at 370C for 18-24 hours (Medina et al., 2010). For the detection of Yersinia species, stool samples were initially enriched in phosphate buffered saline (PBS) for a 21-day period at 4°C before being sub-cultured onto Yersinia selective agar (YSA) and allowed to incubate at room temperature for 24-48 hours. Isolates were purified via sub-culturing and cryopreserved in 50:50 Luria Broth-glycerol mixture.
Isolate identification was done by conventional biochemical tests and confirmation using the Vitek2 Identification system (Biomerieux Inc., Hazelwood, MO, USA) that allowed suspensions of the isolates in cassettes to undergo a 64-well reagent test for identification. Salmonella spp and diarrhoeagenic E. coli were confirmed/differentiated by PCR.
DNA extraction and PCR
The DNA of isolates extracted using the Promega® wizard genomic extraction kit was used to template PCR employing primers for the detection of virulence genes. The genes sought delineating different pathotypes of diarrhoeagenic E. coli and Salmonella spp. listed in Table 1. Strains were deemed to belong to the respective pathotype if they contained at least one of the gene targets sought for that pathogenic type.
Data analysis
Data obtained in this study were analyzed using Chi square with p set at 0.05. In the many instances where expected values were <5, the Fisher’s Exact test was used.
Results and discussion
The United Nations International Children’s Emergency Fund (UNICEF, 2006) report observed that 90% of new cases of HIV among children are acquired through transmission from mother to child. Since then, very few studies have examined the aetiology of diarrhoea in children living with HIV. This study aimed to identify diarrhoeal pathogens in children living with HIV. We enrolled fewer individuals than anticipated when the study was designed five years ago because the target population is on the decline due to improved access to antiretroviral therapy. Olakunde et al. (2019) reported a significant increase in coverage of prevention of mother-to-child transmission (PMTCT) in Nigeria, accounting for the low recruitment of diarrhoeic children living with HIV that limits our analyses. However, the small data we have will support their management and remains relevant as the goal to end paediatric AIDS within the current decade is as yet unmet (Bagcchi, 2022). This study screened for bacterial and parasitic aetiologies of diarrhoea in children living with HIV and HIV-negative controls. Viral agents were unfortunately not sought owing to non-availability of required resources, a second limitation of this study.
Enteric pathogen detection in diarrhoea stools and co-infections
In this study, only ten children living with HIV who had diarrhoea could be recruited during the study’s 25-month duration and 55 HIV-uninfected children with diarrhoea were recruited as controls. EAEC, EIEC, C. parvum and C. cayetanensis were the most frequently encountered pathogens in the study subjects, while STEC, Shigella and Yersinia were not detected at all. The full and repertoire of bacterial and parasitic pathogens detected/isolated from them are presented on Table 2.
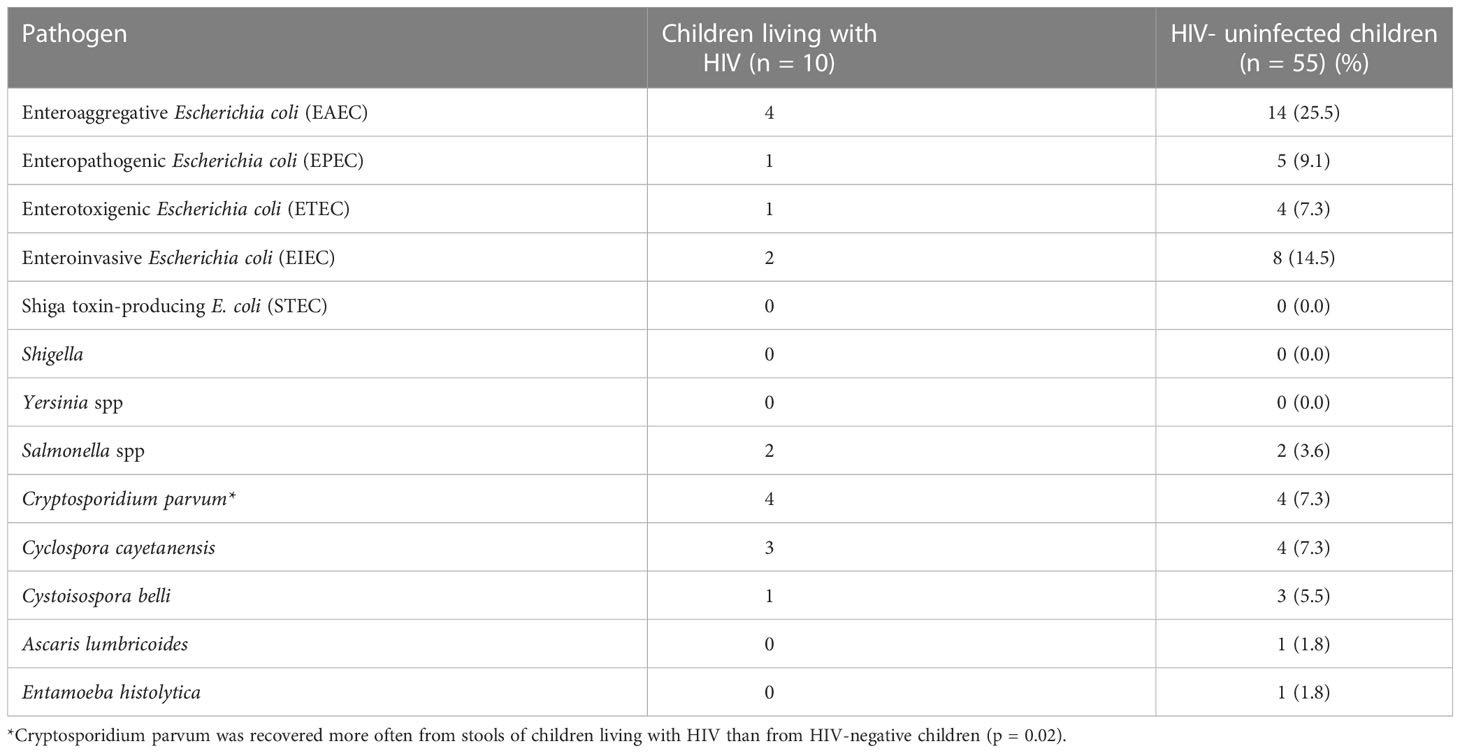
Table 2 Detection of pathogens in the stools of children living with HIV and HIV-uninfected children with diarrhea.
Other aetiologic studies performed in Ibadan and other locations in Nigeria have found diarrhoeagenic E. coli, Cryptosporidium parvum and Cystoisospora belli commonly associated with diarrhoea (Adesiji et al., 2007; Abdullahi et al., 2010; Saka et al., 2019; Karshima and Karshima, 2021; Akinlabi et al., 2022). These pathogens were all detected in stools from both children living with HIV and the of HIV- uninfected children who served as controls in study (Table 2) with comparable recovery rates seen in both patient groups for almost all pathogens. An important exception is C. parvum, which was recovered from four of ten children living with HIV but only 4 (7.3%) HIV- uninfected children (p = 0.02, Table 2). C. parvum, is a major protozoan parasite commonly detected in immunocompromised individuals, particularly people living with HIV, including those in Nigeria (Botero-Garces et al., 2021; Karshima and Karshima, 2021). A meta-analysis of 131 studies by Wang et al. (2018) revealed low prevalence of Cryptosporidium (and Cystoisospora) among People Living with HIV in high income studies and a much higher prevalence, with diarrhoea association in sub-Saharan Africa. Although we found lower frequencies, Cyclospora cayetanensis is also commonly reported from people living with HIV (Stark et al., 2009; Feasey et al., 2011) and Cystoisospora belli is increasingly being recognized as a cause of diarrhoea in this sub-population (Casmo et al., 2018). As shown in Figure 1 at least one parasite was detected in 5/10 of children living with HIV but only 9/55 (16.4%) of children without HIV infection (p = 0.03) showing that parasite detection is associated with HIV.
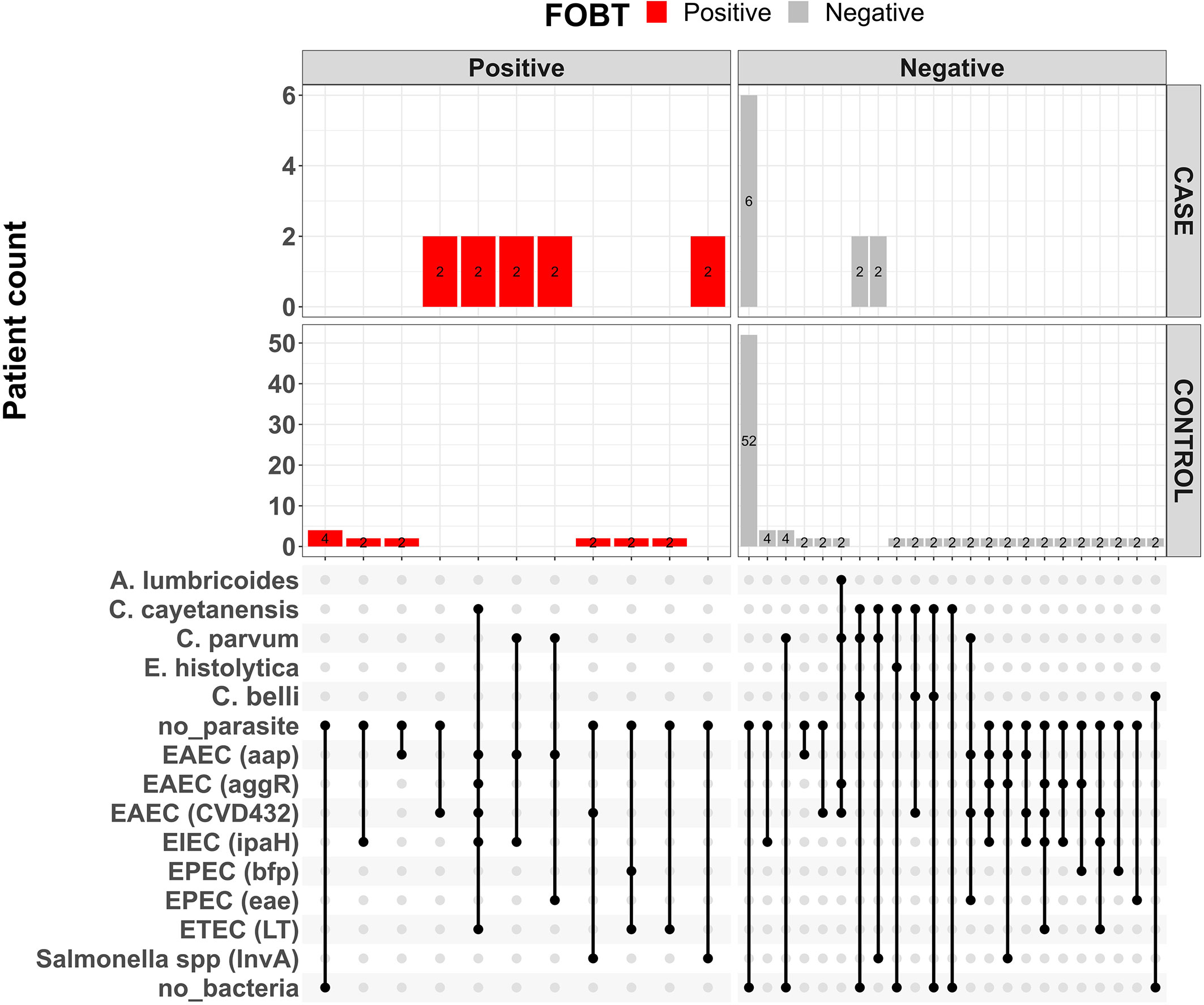
Figure 1 Upsett plot showing pathogen combinations for co-infections and faecal occult blood status among children living with HIV (cases) and HIV-uninfected children (controls).
EAEC was the most frequently detected pathogen in diarrhoeal stools among both children living with HIV and HIV-uninfected children. Reports from within Nigeria (Onanuga et al., 2014; Saka et al., 2019; Akinlabi et al., 2022) and elsewhere (Kotloff et al., 2013; Shah et al., 2016) show that, in the relatively infrequent instances where it is sought, EAEC is commonly the most frequently detected diarrhoeagenic E. coli pathotype in children with diarrhoea although it can also be recovered from children without diarrhoea (Franç et al., 2013; Platts-Mills et al., 2015). Pathogen and host factors may be important determinants of virulence and children without diarrhoea who carry EAEC may be at risk of nutrient malabsorption and other less visible sequelae (Nataro et al., 1992; Nataro et al., 1998; Platts-Mills et al., 2015).
Certain enteric bacterial pathogens, notably Shigella and ETEC have been prioritized for vaccine development. ETEC strains were recovered from children with and without HIV infection in this study, whereas, Shigella, though commonly reported as a pathogen of significance in Africa, was not found in this study (Breurec et al., 2018). While the study size was small, EIEC, which carry the Shigella invasive plasmid were recovered from children with and without HIV. Cabal et al. (2016) reported EIEC association with diarrhoea in patients from Spain. Meanwhile, recent studies in Nigeria have not recovered Shigella or reported relatively low recovery (Joseph et al., 2017; Akinwumi et al., 2021), adding to evidence supporting earlier speculation that this pathogen may be less important in this setting and therefore the importance of understanding pathogen epidemiologies in a range of settings (Lindsay et al., 2015; Okeke et al., 2016).
Overall, the genes delineating EAEC (aap. aggR and aatA) were the most commonly detected by PCR, further confirming the preponderance of EAEC among diarrhoeic children in our setting as previously reported by Saka et al. (2019) and Akinlabi et al. (2022) and elsewhere (Weintraub, 2007). In contrast to EAEC, EPEC and Salmonella spp. were less commonly recovered with other bacterial pathogens. Of the six (6) EPEC strains recovered, including the one isolate from a child living with HIV, three (3) were typical, that is had the EPEC adherence factor plasmid-borne bfpA gene, and three (3) were atypical, that is lacked the gene
Multiple pathogens were detected among five of the ten children living with HIV (Table 3), representing half of the children in the group whereas multiple detection of pathogens was observed in 14 (25.5%) of the 55 HIV-uninfected children. Ledwaba et al. (2018) reported bacteria-bacteria co-infections among diarrhoiec children of rural communities of Vhembe district in South Africa, an observation also made in the current study. In a study done in Uruguay (Varela et al., 2015), mixed infections were observed only in 2 (3.4%) of the diarrhoea patients. Unsurprisingly in this study, there is preponderance of EAEC in the mixed infections, as it was the most frequently encountered pathogen overall. However, certain combinations were encountered more frequently than would be predicted by chance alone. For example, as shown in Table 2, EAEC and EIEC were seen in combination in three of the ten HIV-uninfected children, whereas probabilities predict that 0-1 children would be expected to carry both pathogens by chance alone based on overall detection rate. Similarly, the combinations of EAEC and Salmonella spp. as well as EAEC, EIEC and ETEC were each detected in two HIV-uninfected children with expected carriage rates of ¾1 child respectively. The detection of multiple pathogens (including combinations of bacterial and parasitic agents, that is bacterial-parasite co-infections) in some of the subjects in this study underscores the multifactorial nature of diarrhoea as was previously reported by Kotloff et al. (2013) in the Global Enteric Multicentre Study (GEMS). Zhang et al. (2016) also reported enteric pathogens co-infections among diarrhoeic children in China. Chattaway et al. (2013) reported common co-infections of EAEC with other pathogens albeit being more significantly recovered from diarrhoeic cases than healthy controls.
Figure 1 shows a plot of the pathogen combinations observed in this study. The repertoire of pathogens among the children living with HIV and HIV-uninfected children appear similar. However, the proportion of children living with HIV who had either parasites or bacteria detected/recovered from their stools was higher than HIV-uninfected children. The detection of similar repertoire of diarrhoeagenic E. coli pathogens from HIV-infected and HIV-uninfected children has previously been reported by Samie et al. (2007). Even though viruses are part of the leading causes of childhood diarrhea (Nguyen et al., 2007), they were not sought in this study and therefore it is possible that more infections might have been mixed than we are able to report.
Occult blood in stool
Gross or occult (hidden) in faeces can have different origins, ranging from injury to the epithelium and mucosal linings of the gastrointestinal tract by invasive pathogens (Bardhan et al., 2000) to colorectal cancer (CRC) (Borges et al., 2018). Blood in the stool delineates acute watery diarrhoea from dysentery, which is important since the two syndromes are managed differently and understanding which pathogens produce either or both syndromes is important for understanding the pathogenesis of diarrhoeal pathogens in hosts of differing susceptibilities. When blood in stool is not macroscopically visible, that is occult, it has to be detected through tests for blood components in stool, which are generally reliable although they may also pick up heme components that have been ingested with food (Rose et al., 1990; Ransohoff and Lang, 1997; Craven, 2001). For infectious diarrhoea, occult blood is a marker of severe disease caused by invasive pathogens such as Shigella, Salmonella and Entamoeba histolytica sensu stricto. Gassama-Sow et al. (2004) had reported high levels of severe diarrhoea among people living with HIV.
In this study, fecal occult blood detection was not associated with number and nature of pathogen recovered but showed significant association with HIV positive status (p = 0.01) as shown in Table 4. Bacterial pathogens were recovered from 6 of the children living with HIV, 5 of who had occult blood detected in their stool samples whereas of the 20 HIV-negative children from whom bacterial pathogens were isolated, only 5 had their stool samples positive for occult blood (p = 0.02). Similarly, 3 of the 5 parasite-positive children living with HIV also had their stool samples positive for occult blood compared to none (0) of the 9 parasite-positive children without HIV infection in the control group (p = 0.003). Barring earlier stated confounders such as possibility of colorectal cancer and dietary influences among recruited subjects, our data suggest that invasive pathogens may exert the worst toll on children living with HIV.
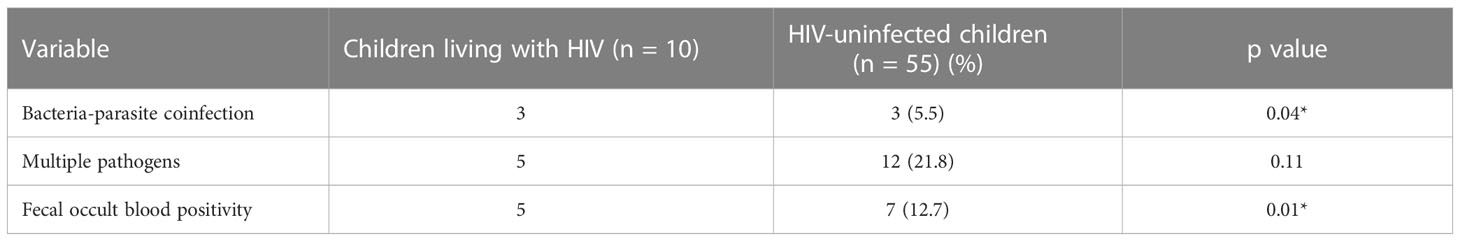
Table 4 Markers of severity: Multiple pathogens and occult blood in stool specimens from children with diarrhoea.
A limitation of this study, in addition to the unavoidably small sample size, is that it did not investigate other pathogens such as Campylobacter species and viruses that have been implicated in diarrhoea aetiology (Junaid et al., 2011; Imade and Eghafona, 2015). Even though the study focused on a limited number of bacterial and parasitic etiologies of diarrhoea, multiple pathogens in form of bacteria-bacteria, bacteria-parasite and parasite-parasite co-infections were observed in the specimens. Meanwhile, the overall breadth of pathogens detected in specimens from children living with HIV compares with that of the HIV-uninfected children (Table 4). However, bacteria-parasite co-infection in specimens from children living with HIV when compared to those from children without HIV infection (p = 0.004).
Conclusion
A broad range of diarrhoeal pathogens are detectable in the stools of children living with HIV that present with diarrhoea in Ibadan and the pathogen repertoire is similar to what is seen with HIV-uninfected children with diarrhoea. Children living with HIV who have diarrhoea often present with blood in the stool suggesting that their infections could be invasive and therefore more severe. Mixed infections are common, particularly among children living with HIV so that general Water, Sanitation and Hygiene (WASH)-based interventions are most likely to have significant protective effects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by University of Ibadan/University College Hospital Ethics Committee and Oyo State Ethical Review Committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
OB: conceptualization, data collection, formal analysis, investigation, methodology, project administration, and visualization, writing first draft. MO: investigation, data collection. BO. conceptualization, investigation, methodology, supervision and resources. OA: conceptualization, investigation, methodology, supervision and resources KA: conceptualization, investigation, methodology, supervision and resources TI: conceptualization, investigation, methodology, supervision and resources VO investigation, methodology, supervision VN: investigation AG-A investigation, data collection EA: investigation, data collection IO: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, resources and visualization. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by an African Research Leader’s Award to IO jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. IO is a Calestous Juma Fellow supported by the Bill and Melinda Gates Foundation.
Acknowledgments
We thank A Oladipo Aboderin and Olabisi C Akinlabi for helpful discussions and Ayorinde Afolayan for assisting with data visualization. We also thank the staff and management of the children clinics of University College Hospital and Adeoyo Maternity Teaching Hospital who helped with recruitment of subjects in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullahi, M., Olonitola, S. O., Inabo, I. H. (2010). Isolation of bacteria associated with diarrhoea among children attending some hospitals in Kano metropolis, Kano State, Nigeria. Bayero J. Pure Applied Sci. 3 (1), 10–15.
Adesiji, Y. O., Lawal, R. O., Taiwo, S. S., Fayemiwo, S. A., Adeyeba, O. A. (2007). Cryptosporidiosis in HIV infected patients with diarrhoea in osun state southwestern Nigeria. Eur. J. Gen. Med. 4 (3), 119–122. doi: 10.29333/ejgm/82505
Akinlabi, O. C., Nwoko, E. Q., Dada, A. ,. R., Ekpo, S., Omotuyi, A., Adepoju, A., et al. (2022). Epidemiology and risk factors for diarrhoeagenic escherichia coli carriage among children in northern ibadan (Nigeria). doi: 10.1101/2022.09.26.22280249
Akinwumi, F. O., Igbeneghu, O. A., Oyelami, O. A., Lamikanra, A. (2021). A study of bacterial pathogens associated with diarrhoea in children under 2 years in ile-ife, Nigeria. Afr. J. Microbiol. Res. 15 (2), 82–88. doi: 10.5897/AJMR2020.9462
Antoniou, T., Jambere, N., Saskin, R., Kopp, A., Glazier, R. H. (2015). A population-based study of the extent of colorectal cancer screening in men with HIV. BMC Health Serv. Res. 15, 51. doi: 10.1186/s12913-015-0711-9
Aranda, K. R., Fabbricotti, S. H., Fagundes-Neto, U., Scaletsky, I. C. (2007). Single multiplex assay to identify simultaneously enteropathogenic, enteroaggregative, enterotoxigenic, enteroinvasive and shiga toxin-producing Escherichia coli strains in Brazilian children. FEMS Microbiol. Lett. 267, 145–150. doi: 10.1111/j.1574-6968.2006.00580.x
Aranda, K. R., Fagundes/Neto, U., Scaletsky, I. C. (2004). Evaluation of multiplex PCRs for diagnosis of infection with diarrhoeagenic Escherichia coli and Shigella spp. J. Clin. Microbiol. 42 (12), 5849–5853. doi: 10.1128/JCM.42.12.5849/5853.2004
Bagcchi, S. (2022). Global alliance to tackle HIV in children. Lancet Infect. Dis. 22 (10), 1426. doi: 10.1016/S1473-3099(22)00605-3
Bardhan, P., Beltinger, J., Beltinger, R., Hossain, A., Mahalanabis, D., Gyr, K. (2000). Screening of patients with acute infectious diarrhoea: Evaluation of clinical features, faecal microscopy, and faecal occult blood testing. Scandinavian J. Gastroenterology. 35, 54–60. doi: 10.1080/003655200750024533
Borges, L. V., Mattar, R., da Silva, J. M. K., da Silva, A. L. W., Carrilho, F. J., Hashimoto, C. L. (2018). Fecal occult blood: a comparison of chemical and immunochemical tests. Arq Gastroenterol. 55 (2), 128–132. doi: 10.1590/s0004-2803.201800000-22
Botero, J. H., Castano, A., Montoya, M. N., Ocampo, N. E., Hurtado, M. I., Lopera, M. M. (2003). A preliminarystudy of the prevalence of intestinal parasites in immunocompromised patients with andwithout gastrointestinal manifestations. Rev. Inst Med. Trop. Sao Paulo 4, 197–200. doi: 10.1590/S0036-46652003000400004
Botero-Garces, J., Villegas-Arbelaez, E., Giraldo, S., Uran-Velasquez, J., Arias-Agudelo, L., Alzate-Angel, J. C., et al. (2021). Prevalence of intestinal parasites in a cohort of HIV-infectedpatients from antioquia, Colombia. Biomédica 41 (Supl.2), 153–164. doi: 10.7705/biomedica.5992
Breurec, S., Rafai, C., Onambele, M., Frank, T., Farra, A., Legrand, A., et al. (2018). Serotype distribution and antimicrobial resistance of Shigellaspecies in Bangui, central African republic, from 2002 to 2013. Am. J. Trop. Med. Hygiene 99 (2), 283–286. doi: 10.4269/ajtmh.17-0917
Bruzzese, E., Giannattasio, A., Guarino, A. (2018). Antibiotic treatment of acute gastroenteritis in children. F1000Research 7, 193. doi: 10.12688/f1000research.12328.1
Cabal, A., Garcia-Castillo, M. C., Canton, R., Gortazar, C., Dominguez, L., Alvarez, J., et al. (2016). Prevalence of Escherichia coli virulence genes in patients with diarrhea and a subpopulation of healthy volunteers in Madrid, Spain. Front. Microbiol. 7, 1–8. doi: 10.3389/fmicb.2016.00641
Casmo, V., Lebbad, M., Maungate, S., Lindh, J. (2018). Occurrence of Cryptosporidium spp. and Cystoisospora belli among adult patients with diarrhoea in Maputo, Mozambique. Heliyon 4 (9), 1–13. doi: 10.1016/j.heliyon.2018.e00769
Cerna, J. F., Nataro, J. P., Estrada-Garcia, T. (2003). Multiplex PCR for detection of Three Plasmid-Borne Genes of Enteroaggregative Escherichia coli. J. Clin. Microbiol. 41 (5), 2138–2140.
Chattaway, M. A., Harris, R., Jenkins, C., Tam, C., Coia, J. E., Gray, J., et al. (2013). Investigating the link between the presence of enteroaggregative Escherichia coli and infectious intestinal disease in the united kingdo To 1996 and 2008 to 2009. Euro Surveill 18 (37), pii=20582. doi: 10.2807/1560-7917.ES2013.18.37.20582
Craven, O. (2001). Screening for colorectal cancer using the fecal occult blood test: a critical literature review. Eur. J. Oncol. Nurs. 5, 234–243. doi: 10.1054/ejon.2001.0137
de Mello Santos, A. C., Santos, F. F., Silva, R. M., Gomes, T. A. T. (2020). Diversity of hybrid-and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell Infect. Microbiol. 10, 339. doi: 10.3389/fcimb.2020.00339
Farthing, M., Salam, M. A., Lindberg, G., Dite, P., Khalif, I., Salazar-Lindo, E., et al. (2013). Acute diarrhoea in adults and children: a global perspective. J. Clin. Gastroenterol. 47, 12–20. doi: 10.1097/MCG.0b013e31826df662
Fayer, R., Morgan, U., Upton, S. J. (2000). Epidemiology of cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30, 1305–1322. doi: 10.1016/S0020-7519(00)00135-1
Feasey, N. A., Healey, P., Gordon, M. A. (2011). Review article: the aaetiology, investigation and management of diarrhoea in the HIV-positive patient. Alimentary Pharmacol. Ther. 34, 587–603. doi: 10.1111/j.1365-2036.2011.04781.x
Federal Ministry of Health, Abuja, Nigeria (2016) National guidelines on HIV prevention, treatment and care. Available at: https://www.prepwatch.org/wp-content/uploads/2017/08/nigeria_national_guidelines_2016.pdf.
Franç, F. L. S., Wells, T. J., Browning, D. F., Nogueira, R. T., Sarges, F. S., Pereira, A. C., et al. (2013). Genotypic and phenotypic characterisation of enteroaggregative Escherichia coli from children in Rio de Janeiro, Brazil. PloS One 8 (7), 1–10. doi: 10.1371/journal.pone.0069971
Gassama-Sow, A., Sow, P. S., Guėye, M., Guėye-N’diaye, A., Perret, J. L., M’boup, S., et al. (2004). Characterization of pathogenic Escherichia coli in human immunodeficiency virus-related diarrhoea in Senegal. J. Infect. Dis. 189, 75–78. doi: 10.1086/380489
Guarino, A., Ashkenazi, S., Gendrel, D., Vecchio, A. L., Shamir, R., Szajewska, H. (2014). European Society forpediatric gastroenterology, hepatology, and nutrition/European society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J. Pediatr. Gastroenterol. Nutr. 59, 132–152. doi: 10.1097/MPG.0000000000000375
Hien, B. T., Scheutz, F., Cam, P. D., Serichantalergs, O., Huong, T. T., Thu, T. M., et al. (2008). Diarrhoeagenic Escherichia coli and Shigella strains isolated from children in a hospital case-control study in Hanoi, Vietnam. J. Clin. Microbiol. 46, 996–1004. doi: 10.1128/JCM.01219-07
Imade, P. E., Eghafona, N. O. (2015). Viral agents of diarrhoea in young children in two primary health centers in edo state, Nigeria. Int. J. Microbiol. 2015, 1–5. doi: 10.1155/2015/685821
Jensen, H. B., Olsen, K. E., Struve, C., Krogfelt, K. A., Petersen, A. M. (2014). Epidemiology and clinical manifestations of enteroaggregative Escherichia coli. Clin. Microbiol. Rev. 27 (3), 614–630. doi: 10.1128/CMR.00112-13
Joseph, A. A., Odimayo, M. S., Oluwayemi, I. O., Fadeyi, A., Dada, S. A. (2017). An overview of the aetiologic agents of diarrhoea diseases in children: How far have we gone in management and control? Med. J. Zambia 55 (4), 266–275.
Junaid, S. A., Umeh, C., Olabode, A. O., Banda, J. M. (2011). Incidence of rotavirus infection in children with gastroenteritis attending jos university teaching hospital, Nigeria. Virol. J. 8, 233. doi: 10.1186/1743-422X-8-233
Karshima, S. N., Karshima, M. N. (2021). Epidemiology of Cryptosporidium infections among people living with HIV/AIDS in Nigeria: Results of systematic review and meta-analysis. Acta Parasitol. 66 (1), 60–74. doi: 10.1007/s11686-020-00253-8
Kotloff, K. L., Nataro, J. P., Blackwelder, N., Nasrin, D., Farag, T. (2013). Burden and aaetiology of diarrhoea disease in infants and young children in developing countries (the global enteric multicenter study, GEMs): a prospective, case-control study. Lancet 382 (9888), 209–222. doi: 10.1016/S0140-6736(13)60844-2
Ledwaba, S. E., Kabue, J. P., Barnard, T. G., Traore, A. N., Potgieter, N. (2018). Enteric pathogen co-infections in the paediatric population from rural communities in the vhembe district, south Africa. South Afr. J. Child Health 12 (4), 170–174. doi: 10.7196/SAJCH.2018.v12i4.1550
Lindsay, B., Saha, D., Sanogo, D., Das, S. K., Omore, R., Farag, T. H., et al. (2015). Association between shigella infection and diarrhea varies based on location and age of children. Am. J. Trop. Med. Hyg 93, 918–924. doi: 10.4269/ajtmh.14-0319
Medina, A. M., Rivera, F. P., Romero, L. M., Kolevic, L. A., Castillo, M. E. (2010). Diarrheagenic Escherichia coli in human immunodeficiency virus (HIV) pediatric patients in Lima. Am. J. Trop. Med. Hygiene 83 (1), 158–163. doi: 10.4269/ajtmh.2010.09-0596
Nataro, J. P., Deng, Y., Maneval, D. R., German, A. L., Martin, W. C., Levine, M. M. (1992). Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60, 2297–2304. doi: 10.1128/iai.60.6.2297-2304.1992
Nataro, J. P., Kaper, J. B. (1998). Diarrhoeagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201. doi: 10.1128/CMR.11.1.142
Nguyen, T. A., Yagyu, F., Okame, M. (2007). Diversity of viruses associated with acute gastroenteritis in children hospitalized with diarrhea in ho chi minh city, Vietnam. J. Med. Virol. 79 (5), 582–590. doi: 10.1002/jmv.20857
Nwosu, F. C., Avershina, E., Wilson, R., Rudi, K. (2014). Gut microbiota in HIV infection: Implication for disease progression and management. Gastroenterol Research & Practice 1–6. doi: 10.1155/2014/803185
Ogunbosi, B. O., Oladokun, R. E., Brown., J. B., Osinusi, K. I. (2011). Prevalence and clinical pattern of paediatric HIV infection at the university college hospital, ibadan, Nigeria: a prospective cross-sectional study. Ital. J. Paediatrics 37, 29–34. doi: 10.1186/1824-7288-37-29
Ogunlesi, T., Okeniyi, J., Oseni, S., Oyelami, O., Njokanma, F., Dedeke, O. (2006). Parasitic aetiology of childhood diarrhoea. Indian J. Paediatrics 73, 1–5. doi: 10.1007/BF02763049
Okeke, I. N. (2009). Diarrhoeagenic Escherichia coli in sub-Saharan Africa: status, uncertainties and necessities. J. Infect. Dev. Ctries 23 (11), 817–842. doi: 10.3855/jidc.586
Okeke, I. N., Aboderin, A. O., Opintan, J. A. (2016). Enteroinvasive escherichia coli may account for uncultured shigella. Am. J. Trop. Med. Hygiene 94 (2), 480–481. doi: 10.4269/ajtmh.15-0777a
Okeke, I. N., Nataro, J. P. (2001). Enteroaggregative escherichia coli. Lancet Infect. Dis. 1, 304–313. doi: 10.1016/S1473-3099(01)00144-X
Olakunde, B. O., Adeyinka, D. A., Olawepo, J., Pharr, J. R., Ozigbu, C. E., Wakdok, S., et al. (2019). Towards the elimination of mother-to-child transmission of HIV in Nigeria: a health system perspective of the achievements and challenges. Int. Health 11 (4), 240–249. doi: 10.1093/inthealth/ihz018
Onanuga, A., Igbeneghu, O., Lamikanra, A. (2014). A study of the prevalence of diarrhoeagenic Escherichia coli in children from Gwagwalada, Federal Capital Territory, Nigeria. Pan African Med. J. 17, 146. doi: 10.11604/pamj.2014.17.146.3369
Platts-Mills, J. A., Babji, S., Bodhidatta, L., Gratz, J., Haque, R., Havt, A., et al. (2015). Pathogen-specific burdens of community diarrhoea in developing countries (MAL-ED): a multisite birth cohort study. Lancet Global Health 3 (9), 1–22.
Ransohoff, D. F., Lang, C. A. (1997). Screening for colorectal cancer with the fecal occult blood test: a background paper. American college of physicians. Ann. Intern. Med. 126, 811–822. doi: 10.7326/0003-4819-126-10-199705150-00014
Rose, I. S., Young, G. P., St. John, D. J. B., Deacon, M. C., Blake, D., Henderson, R. W. (1990). Effect of ingestion of hemoproteins on fecal excretion of hemes and porphyrins. Clin. Chem. 35 (12), 2290–2296.
Saka, H. K., Dabo, N. T., Muhammad, B., Garcia-Soto, S., Ruiz, M. U., Alvarez, J. (2019). Diarrhoeagenic Escherichia coli pathotypes from children younger than 5 years in Kano state. Front. Public Health 7 (348), 1–8. doi: 10.3389/fpubh.2019.00348
Samie, A., Obi, C. L., Dillingham, R., Pinkerton, R. C., Guerrant, R. L. (2007). Enteroaggregative Escherichia coli in venda, south Africa: distribution of virulence-related genes by multiplex polymerase chain reaction in stool samples of human immunodeficiencyvirus (HIV)-positive and HIV-negative individuals and primary school children. Am. J. Trop. Med. Hygiene 77, 142–150. doi: 10.4269/ajtmh.2007.77.142
Shah, S., Kongre, V., Kumar V. and Bharadwaj, R. (2016). A study of parasitic and bacterial pathogens associated with diarrhoea in HIV-positive patients. Cureus 8 (9), 1–13. doi: 10.7759/cureus.807
Stark, D., Barratt, J. L., van Hal, S. (2009). Clinical significance of enteric protozoa in the immunosuppressed human population. Clin. Microbiol. Rev. 22, 634–650. doi: 10.1128/CMR.00017-09
Sunnotel, O., Lowery, C. J., Moore, J. E., Dooley, J. S. G., Xiao, L., Millar, B. C. (2006). Cryptosporidium. Letter Appl. Microbiol. 43, 7–16. doi: 10.1111/j.1472-765X.2006.01936.x
Tahvildar-Biderouni, F., Salehi, N. (2014). Detection of cryptosporidium infection by modified ziehl-neelsen and PCR methods in children with diarrhoeal samples in pediatric hospitals in Tehran. Gastroenterol. Hepatol. Bed to Bench 7 (2), 125–130. https://pubmed.ncbi.nlm.nih.gov/24834304
Tiruneh, C. M., Emiru, T. D., Tibebu, N. S., Abate, M. W., Nigat, A. B., Bantie, B., et al. (2022). Underweight and its associated factors among pediatrics attending HIV care in south gondar zone public health facities, Northwest ethiopi. BMC Pediatr. 22 (575), 1–6. doi: 10.1186/s12887-022-03630-6
Varela, G., Batthyany, L., Bianco, M. N., Perez, W., Pardo, L., Algorta, G., et al. (2015). Enteropathogens associated with acute diarrhea in children from households with high socioeconomic level in Uruguay. Int. J. Microbiol. 2015, 1–8. doi: 10.1155/2015/592953
Wang, Z., Liu, Q., Liu, H.-H., Li, S., Zhang, L., Zhao, Y.-K., et al. (2018). Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasites Vectors. 11 (28), 1–19. doi: 10.1186/s13071-017-2558-x
Weintraub, A. (2007). Enteroaggregative Escherichia coli: epidemiology, virulence and detection. J. Med. Microbiol. 56, 4–8. doi: 10.1099/jmm.0.46930-0
Winn, W., Jr, Allen, S., Janda, W., Koneman, E., Procop, G., Schreckenberger, P., et al. (2006). Koneman’s colour atlas and textbook of diagnostic microbiology. 6th Edition. Eds. Winn, W., Jr, Allen, S., Janda, W., Koneman, E., Procop, G., Schreckenberger, P., et al. (Lippincott Williams & Wilkins, Philadelphia).
World Gastroenterology Organization (2012). Acute diarrhea in adults and children: a global perspective. (Milwaukee, WI, USA: World Gastroenterology Publication) 53202–3823.
World Health Organization (2017) Diarrhoea disease: WHO fact sheet updated. Available at: http://www.who.int/hiv/data/en/.
Yamamoto, M., Nakama, H. (2000). Cost-effectiveness analysis of immunochemical occult blood screening for colorectal cancer among three fecal sampling methods. Hepatogastroenterology 47 (32), 396–399.
Zhang, S.-X., Zhou, Y.-M., Xu, W., Tian, L.-G., Chen, J.-X., Chen, S.-H., et al. (2016). Impact of co-infections with enteric pathogens on children suffering from acute diarrhea in southwest China. Infect. Dis. Poverty 5 (64), 1–13. doi: 10.1186/s40249-016-0157-2
Keywords: diarrhoea, human immunodeficiency virus, diarrhoeagenic Escherichia coli, enteroaggregative Escherichia coli, enteric pathogens, faecal occult blood, Nigeria
Citation: Bejide OS, Odebode MA, Ogunbosi BO, Adekanmbi O, Akande KO, Ilori T, Ogunleye VO, Nwachukwu VU, Grey-Areben A, Akande ET and Okeke IN (2023) Diarrhoeal pathogens in the stools of children living with HIV in Ibadan, Nigeria. Front. Cell. Infect. Microbiol. 13:1108923. doi: 10.3389/fcimb.2023.1108923
Received: 26 November 2022; Accepted: 20 February 2023;
Published: 13 March 2023.
Edited by:
Theresa Jean Ochoa, Universidad Peruana Cayetano Heredia, PeruReviewed by:
Waldir P. Elias, Butantan Institute, BrazilMegha Sharma, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2023 Bejide, Odebode, Ogunbosi, Adekanmbi, Akande, Ilori, Ogunleye, Nwachukwu, Grey-Areben, Akande and Okeke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iruka N. Okeke, aXJ1a2Eubi5va2VrZUBnbWFpbC5jb20=
 Oyeniyi S. Bejide
Oyeniyi S. Bejide Mariam A. Odebode2
Mariam A. Odebode2 Babatunde O. Ogunbosi
Babatunde O. Ogunbosi Elizabeth T. Akande
Elizabeth T. Akande Iruka N. Okeke
Iruka N. Okeke