- 1Institute of Microbiology, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2Faculty of Agronomy and Life Sciences, Zhaotong University, Zhaotong, Yunnan, China
Two new corticioid fungi in the family Phanerochaetaceae, Phanerochaete shenghuaii and Rhizochaete variegata, are described and illustrated from Southwest China based on morphological characteristics and molecular data. Phanerochaete shenghuaii is characterized by annual, effused, inseparable basidiocarps from substrate, ivory white to cream hymenial surface when juvenile, buff to yellowish brown with age, buff in KOH, a monomitic hyphal system, smooth cystidia, and ellipsoid basidiospores measuring 4.8–6 × 2.5–3.8 µm. Rhizochaete variegata is characterized by annual, effused, easily separable basidiocarps from substrate, buff-yellow to clay-pink fresh hymenial surface becoming cream to buff upon drying, violet in KOH, a monomitic hyphal system, encrusted cystidia, and ellipsoid basidiospores measuring 3–4 × 2.2–3 µm. The phylogenetic analyses based on ITS + nLSU rDNA sequences confirm the placement of the two new species, respectively, in the Phanerochaete clade and the Rhizochaete clade of Phanerochaetaceae. Phylogenetically related and morphologically similar species to these two new species are discussed.
Introduction
A phlebioid clade is a large group of Polyporales, comprising three families (Phanerochaetaceae Jülich, Irpicaceae Spirin & Zmitr., and Meruliaceae Rea), which accommodates massive corticioid fungi (Wu et al., 2010; Dai, 2011; Justo et al., 2017; He et al., 2019). Most members of the phlebioid clade are saprotrophs on dead wood, causing white rot, which plays an essential role in the maintenance of forest ecosystems (Justo et al., 2017; Ryvarden and Melo, 2017). However, compared with the antrodia and core polyporoid fungi in Polyporales, the phlebioid clade, especially corticioid fungi, has not been intensively studied, with some corticioid genera being known as paraphyletic or polyphyletic, and their members are scattered in different lineages, not fully consistent with the morphological features (Ortiz-Santana et al., 2013; Justo et al., 2017; Cui et al., 2019).
Phanerochaete P. Karst., established based on P. velutina (DC.) P. Karst., is the largest corticioid genus with more than 100 described species in Phanerochaetaceae (Burdsall, 1985; Kirk et al., 2008; Wu et al., 2010; Ghobad-Nejhad et al., 2015). The genus has a worldwide distribution and is characterized by white-rot, resupinate, and membranaceous basidiocarps; smooth or tuberculate hymenial surface; a monomitic hyphal system; generative hyphae mostly simple septate; the presence of smooth or encrusted cystidia; and thin-walled, non-amyloid, and acyanophilous basidiospores (Wu, 2000; Wu et al., 2010; Floudas and Hibbett, 2015; Ghobad-Nejhad et al., 2015). The diversity and taxonomy of Phanerochaete s.l. in China have been studied for 30 years (Wu, 1990; Wu, 1995; Wu, 1998; Wu, 2000; Wu, 2004; Wu, 2007; Xiong and Dai, 2009; Wu et al., 2010; Ghobad-Nejhad et al., 2015; Liu and He, 2016; Chen et al., 2018; Wu et al., 2018a; Wu et al., 2018b). Early studies focused on fungi of Taiwan Province and were mostly based solely on morphology. Recent studies have confirmed that the genus is highly polyphyletic and its species are distributed throughout the phlebioid clade, comprising a number of Phanerochaete species assembled in a highly supported clade, referred to as the core Phanerochaete clade, containing the type P. velutina (Wu et al., 2010; Floudas and Hibbett, 2015; Justo et al., 2017; Chen et al., 2021).
Rhizochaete is a small genus introduced by Greslebin et al. (2004), based on R. brunnea Gresl. et al., as a segregate of Phanerochaete, differing mainly by the reaction of basidiocarps and rhizomorphs (hyphal cords) with KOH: basidiocarps of Rhizochaete become red or violet in KOH, while they keep unchanged in Phanerochaete. Rhizochaete is characterized by resupinate, loosely adnate basidiocarps, with smooth to tuberculate hymenophore, usually turning red to violet in KOH, a monomitic hyphal system with simple septa or clamp connections, cylindrical to ellipsoid basidiospores, usually non-amyloid and acyanophilous (Nakasone et al., 2017; Gu and Zhao, 2021). Since Rhizochaete was erected, the number of newly named species is increasing continuously. Based on studying the parenthesome structure of some corticioid fungi, Bianchinotti et al. (2005) reported that three Rhizochaete species had perforate septal dolipore caps or parenthesomes. Nakasone et al. (2017) described a new species of Rhizochaete from Belize and transferred three additional species to the genus based on morphological and molecular data. Gu and Zhao (2021) reported two new species based on a combination of morphological features and molecular evidence. So far, approximately 17 species have been accepted in Rhizochaete worldwide (Greslebin et al., 2004; Chikowski et al., 2016; Nakasone et al., 2017; Gu and Zhao, 2021). Recently, a family-level classification of Polyporales or phlebioid fungi has shown that the genus Rhizochaete nested within Phanerochaetaceae, grouped with Hapalopilus P. Karst., Phaeophlebiopsis Floudas & Hibbett, and Phlebiopsis Jülich (Greslebin et al., 2004; Wu et al., 2010; Ghobad-Nejhad et al., 2015; Chen et al., 2021; Zhao et al., 2021).
During investigations on the diversity of wood-rotting fungi from China, four unknown corticioid specimens were collected from Southwest China, and their morphology corresponded to the concepts of Phanerochaete and Rhizochaete. To confirm their affinity, phylogenetic analyses based on the internal transcribed spacer (ITS) and nLSU rDNA sequences were carried out. Both morphological characteristics and molecular evidence demonstrated that these four corticioid specimens represent two new species of Phanerochaetaceae. So, we describe them in the present paper.
Materials and methods
Morphological studies
The studied specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Macro-morphological descriptions are based on field notes and measurements of herbarium specimens. Micro-morphological data and drawings are obtained from the dried specimens and observed under a light microscope following Chen et al. (2021) and Wu et al. (2022b). Color terms followed Petersen (1996). Sections were studied at a magnification up to ×1,000 using a Nikon Eclipse 80i microscope with phase contrast illumination (Nikon, Tokyo, Japan). Drawings were made with the aid of a drawing tube. Microscopic features, measurements, and drawings were made from slide preparations stained with Cotton Blue and Melzer’s reagent. Basidiospores were measured from sections cut from the hymenophore. To present the variation of basidiospores size, 5% of measurements were excluded from each end of the range and are given in parentheses. The following abbreviations are used: IKI = Melzer’s reagent; IKI− = neither amyloid nor dextrinoid; KOH = 5% potassium hydroxide; CB = Cotton Blue; CB− = acyanophilous; L = arithmetic average of all basidiospores length; W = arithmetic average of all basidiospores width; Q = variation in the L/W ratios between the specimens studied, (n = x/y) = the number of basidiospores (x) measured from a given number of specimens (y).
DNA extraction and sequencing
A cetyltrimethylammonium bromide (CTAB) rapid plant genome extraction kit (Aidlab Biotechnologies, Co., Ltd., Beijing, China) was used to extract DNA (Wu et al., 2020). The following primer pairs were used to amplify the DNA: ITS5 (5′‐GGA AGT AAA AGT CGT AAC AAG G‐3′) and ITS4 (5′‐TCC TCC GCT TAT TGATAT GC‐3′) for the ITS regions (White et al., 1990); LR0R (5′‐ACC CGC TGA ACT TAA GC‐3′) and LR7 (5′‐TAC TAC CAC CAA GAT CT‐3′) for nuclear large subunit rDNA (nLSU) (Vilgalys and Hester, 1990). The PCR products were purified with a Gel Extraction and PCR Purification Combo Kit (Spin-column) at Beijing Genomics Institute (BGI), China. The purified products were then sequenced on an ABI-3730-XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA) using the same primers as in the original PCR amplifications. All newly generated sequences were submitted to GenBank and are listed in Table 1.
Phylogenetic analyses
New sequences, deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) (Table 1), were aligned with additional sequences retrieved from GenBank (Table 1) using BioEdit 7.0.5.3 (Hall, 1999) and ClustalX 1.83 (Thompson et al., 1997), followed by manual adjustment. Sequence alignment was deposited at TreeBase (http://purl.org/phylo/treebase/; submission ID 29897). Sequences of Bjerkandera adusta (Willd.) P. Karst. and B. centroamericana Kout et al. were used as outgroups (Chen et al., 2021). Maximum likelihood (ML) and Bayesian inference (BI) methods were used for the phylogenetic analysis. The GTR + I + G model was estimated as the best-fit evolutionary model by PhyloSuite 1.2.2 (Zhang et al., 2020) using the Akaike information criterion. The ML analysis was carried out with RAxML 8.2.12 (Stamatakis, 2006; Silvestro and Michalak, 2012), and the BI tree reconstruction was carried out with MrBayes 3.2.5 (Ronquist et al., 2012). Four Markov chains were run for two runs from random starting trees for 10 million generations, and trees were sampled every 1,000 generations. The burn-in was set to discard 25% of the trees. A majority rule consensus tree of all the remaining trees was calculated. Branches that received bootstrap support for ML and Bayesian posterior probabilities (BPP) greater than or equal to 75% (ML) and 0.95 (BPP) were considered as significantly supported.
Results
Phylogeny
The ITS + nLSU dataset included 155 fungal collections representing 101 taxa of the family Phanerochaetaceae. PhyloSuite suggested GTR + I + G to be the best-fit models of nucleotide evolution for BI. Bayesian analysis resulted in a concordant topology with an average standard deviation of split frequencies = 0.006701. The ML and BI analyses resulted in nearly identical topologies, and thus, only the ML tree is presented with the ML and BPP when they were greater than or equal to 50% and 0.90, respectively.
The phylogram inferred from ITS + nLSU sequences within the family Phanerochaetaceae highlighted two undescribed species nested in Phanerochaete and Rhizochaete, respectively. Phanerochaete shenghuaii formed an independent lineage with a robust support (ML = 99, BPP = 1.0) and stably nested within the core Phanerochaete clade. Rhizochaete variegata clustered in Rhizochaete clade with high support (ML = 99, BPP = 1.0) and grouped with Rhizochaete radicata (Henn.) Gresl. et al. and R. grandinosa C.L. Zhao & Z.R. Gu.
Taxonomy
Phanerochaete shenghuaii Q.Y. Zhang, Y.C. Dai & Jing Si, sp. nov., Figures 1, 2

Figure 1 Basidiocarps of Phanerochaete shenghuaii (holotype, Dai 24610). (A) In situ. (B) Detailed view of the margin. (C) Reaction with KOH. Scale bars: (A) = 1 cm, (B) = 2 mm, (C) = 0.5 cm.
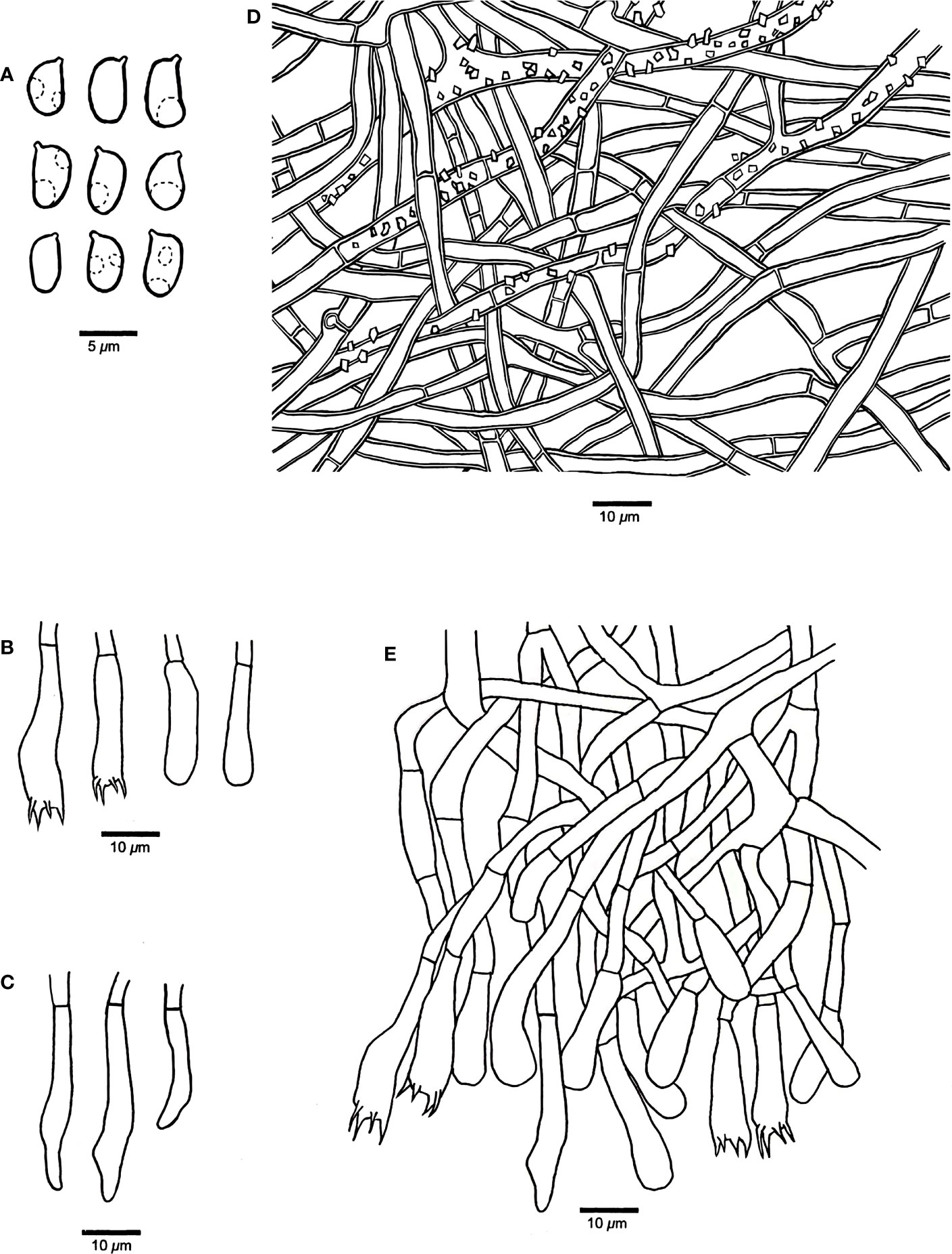
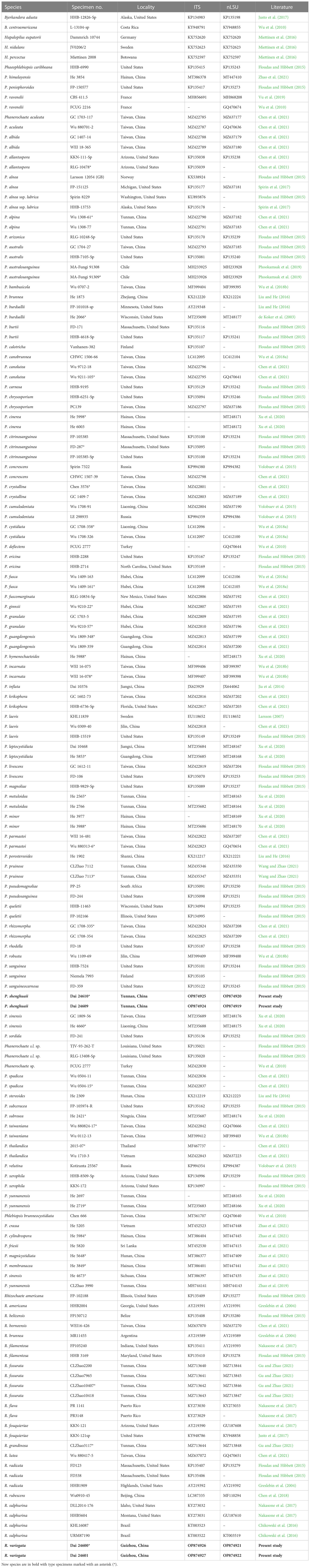
Figure 2 Microscopic structures of Phanerochaete shenghuaii (drawn from the holotype, Dai 24610). (A) Basidiospores. (B) Basidia and basidioles. (C) Cystidia. (D) A vertical section of the subiculum. (E) A vertical section of the hymenium.
MycoBank: 847200
Type — China, Yunnan Province, Zhaotong, Daguan County, Huanglianhe Scenic Spot, on fallen liana branch, 16 July 2022, Dai 24610 (holotype, BJFC038931).
Etymology — Shenghuaii (Lat.): In honor of Professor Sheng-Hua Wu, the Chinese mycologist.
Basidiocarps — Annual, effused, adnate, inseparable from substrate, membranaceous to subceraceous, up to 2.5 cm long, 1.5 cm wide, and 0.2 mm thick in section. Hymenial surface ivory white to cream when juvenile, buff to yellowish brown with age, buff in KOH, smooth, uncracked; margin concolorous with hymenial surface, thinning out, usually rhizomorphic.
Hyphal structure — Hyphal system monomitic; generative hyphae mostly simple septate, occasionally with clamp connections in subiculum, IKI−, CB−; tissue unchanged in KOH.
Subiculum — Subicular hyphae hyaline, slightly thick-walled, frequently simple septate, occasionally with clamp connections, frequently branched, usually strongly encrusted with crystal granules, interwoven, 3–5 μm in diameter.
Hymenophore — Subhymenial hyphae hyaline, thin-walled, smooth, simple septate, frequently branched, interwoven, 2.5–5 μm in diameter; cystidia smooth, immersed or projecting from hymenium, narrowly fusiform or clavate with pointed tips, hyaline, thin-walled, smooth, with a simple septum at the base, 18–35 × 3–5 µm; basidia clavate, with a basal simple septum and four sterigmata, 22–30 × 4–5 µm; basidioles similar to basidia in shape, but slightly smaller.
Basidiospores — Ellipsoid with a distinct apiculus, hyaline, thin-walled, smooth, occasionally with one or two guttules, IKI−, CB−, (4.5–)4.8–6(–6.4) × 2.5–3.8(–4) µm, L = 5.26 µm, W = 3.01 µm, Q = 1.71–1.79 (n = 60/2).
Additional specimen (paratype) examined — China, Yunnan Province, Zhaotong, Daguan County, Huanglianhe Scenic Spot, on fallen angiosperm branch, 16 July 2022, Dai 24609 (BJFC038930).
Rhizochaete variegata Q.Y. Zhang, Y.C. Dai & Jing Si, sp. nov., Figures 3, 4

Figure 3 Basidiocarps of Rhizochaete variegata (holotype, Dai 24600). (A) In situ. (B) Detailed view of the margin. (C) Reaction with KOH. Scale bars: (A) = 1 cm, (B) = 1 mm, (C) = 0.5 cm.
Figure 4 Microscopic structures of Rhizochaete variegata (drawn from the holotype, Dai 24600). (A) Basidiospores. (B) Basidia and basidioles. (C) Cystidia. (D) A vertical section of the subiculum. (E) A vertical section of the hymenium.
MycoBank: 847201
Type — China, Guizhou Province, Zunyi, Suiyang County, Kuankuoshui Nature Reserve, on fallen angiosperm trunk, 07 July 2022, Dai 24600 (holotype, BJFC038928).
Etymology — Variegata (Lat.): referring to the species having variable cystidia.
Basidiocarps — Annual, effused, loosely adnate, easily separable from substrate, membranaceous, soft, fragile, up to 9 cm long, 3.5 cm wide, and 1 mm thick in section. Hymenial surface buff-yellow to clay-pink when fresh, cream to buff upon drying, violet in KOH, smooth or locally tuberculate, occasionally cracked; margin darker or concolorous with hymenial surface, thinning out, usually rhizomorphic.
Hyphal structure — Hyphal system monomitic; generative hyphae simple septate, IKI−, CB−; tissue unchanged in KOH.
Subiculum — Subicular hyphae hyaline, slightly thick-walled, simple septate, rarely branched, bearing abundant crystal granules, strongly interwoven, 3.5–6 μm in diameter.
Hymenophore — Subhymenial hyphae hyaline, slightly thick-walled, smooth, simple septate, more or less regularly arranged, 3–5 μm in diameter. Hymenium contains a dense palisade of cystidia and basidia, IKI−, CB−; cystidia numerous, immersed or projecting from hymenium, clavate, subfusiform or subulate with an obtuse apex, hyaline, slightly thick-walled, some with thin-walled apex, with a simple septum at the base, some apically or centrally encrusted, 28–52 × 5–8 µm; basidia narrowly clavate, with a basal simple septum and four sterigmata, 30–45 × 4–5 µm; basidioles similar to basidia in shape, but slightly smaller.
Basidiospores — Ellipsoid with a distinct apiculus, hyaline, thin-walled, smooth, occasionally with one or two small guttules, IKI−, CB−, 3–4(–4.2) × (2–)2.2–3(–3.2) µm, L = 3.61 µm, W = 2.72 µm, Q = 1.27–1.38 (n = 60/2).
Additional specimen (paratype) examined — China, Guizhou Province, Zunyi, Suiyang County, Kuankuoshui Nature Reserve, on fallen angiosperm trunk, 07 July 2022, Dai 24601 (BJFC038929).
Discussion
Southwest China has a complex topography and geography, luxuriant vegetation, and virgin forests and has highly variable weather including tropical, subtropical, and alpine climates, thus providing a favorable region for the growth and reproduction of higher fungi (Yuan and Dai, 2008; Dai et al., 2021; Wu et al., 2022a). The extremely high fungal diversity in this area has attracted much attention from mycologists both at home and abroad (Feng and Yang, 2018). It is worth noting that the two new corticioid species P. shenghuaii and R. variegata were collected from Northeast Yunnan and Northwest Guizhou, respectively, and the type locality of the two new species is in a typical subtropical climate.
Phanerochaete shenghuaii is characterized by white to cream basidiocarps with rhizomorphic margin, encrusted subicular hyphae, and smooth cystidia. Morphologically, three species, Phanerochaete rhizomorpha C.C. Chen et al., P. leptocystidiata Y.L. Xu & S.H. He, and P. sinensis Y.L. Xu et al., are similar to P. shenghuaii by sharing similar basidiocarps, rhizomorphic margin, and smooth cystidia. However, P. rhizomorpha is described from Taiwan Province, China, and differs from P. shenghuaii by its subcapitate to cylindrical cystidia with obtuse apices and smaller basidiospores (3.9–5.3 × 2.1–3 μm vs. 4.8–6 × 2.5–3.8 µm, Chen et al., 2021). Phanerochaete leptocystidiata is widely distributed in South China and differs from P. shenghuaii by its basidiocarps easily separable from substrate and longer cystidia (30–70 μm in length vs. 18–35 µm in length, Xu et al., 2020). Phanerochaete sinensis is distinguished from P. shenghuaii in having longer cystidia (35–50 µm in length vs. 18–35 µm in length) and smaller basidiospores (4–5 × 2–2.5 μm vs. 4.8–6 × 2.5–3.8 µm, Xu et al., 2020).
In addition, the diversity of flora of seed plants and the distinctly diverse climates in Yunnan Province both contribute to the suitable substrates and environments for Phanerochaete species. Recently, a large number of Phanerochaete species have been found in Yunnan Province (Xiong and Dai, 2009; Wu et al., 2010; Xu et al., 2020; Chen et al., 2021; Wang and Zhao, 2021). Among them, Phanerochaete yunnanensis Y.L. Xu & S.H. He is similar to P. shenghuaii by growing on dead liana and fallen angiosperm branches but differs by grandinioid basidiocarps and the absence of cystidia. Phanerochaete pruinose C.L. Zhao and D.Q. Wang is similar to P. shenghuaii by sharing white and smooth hymenophore, but differs by lacking cystidia and having thinner basidiospores (1.5–2.7 μm in width vs. 2.5–3.8 µm in width, Wang and Zhao, 2021). It is still noteworthy that P. rhizomorpha C.L. Zhao and D.Q. Wang described from Yunnan Province is an invalid name, attributed to the priority of P. rhizomorpha C.C. Chen et al. (Chen et al., 2021; Wang and Zhao, 2021). In addition, the two taxa represent two independent species according to their distinctive DNA sequences and morphology.
Our phylogenetic analysis demonstrates that Rhizochaete is monophyletic with a low support and clusters as a sister clade to Hapalopilus, Phaeophlebiopsis, and Phlebiopsis. Two specimens of R. variegata form a lineage with a strong support (ML = 99, BPP = 1.0, Figure 5). Rhizochaete variegata is closely related to R. grandinosa and R. radicata (ML = 100, BPP = 1, Figure 5), and these three species share curry-yellow hymenial surface, violet in KOH, thick-walled and encrusted subicular hyphae, and similar-sized basidiospores. However, R. variegata has abundant variable and slightly thick-walled cystidia with a thin-walled apex, which can be readily distinguished from R. grandinosa and R. radicata (Greslebin et al., 2004; Gu and Zhao, 2021). Furthermore, there are differences of more than eight base pairs between their sequences, which amounts to 2% nucleotides in the ITS regions. Morphologically, Rhizochaete sulphurosa (Bres.) Chikowski et al. may be confused with R. variegata by sharing yellow basidiocarps, hymenial surface violet in KOH, and thin or slightly thick-walled (<1 µm) cystidia. Nevertheless, R. sulphurosa differs from R. variegata by its longer basidiospores (4.5–5.5 µm in length vs. 3–4 µm in length, Chikowski et al., 2016).
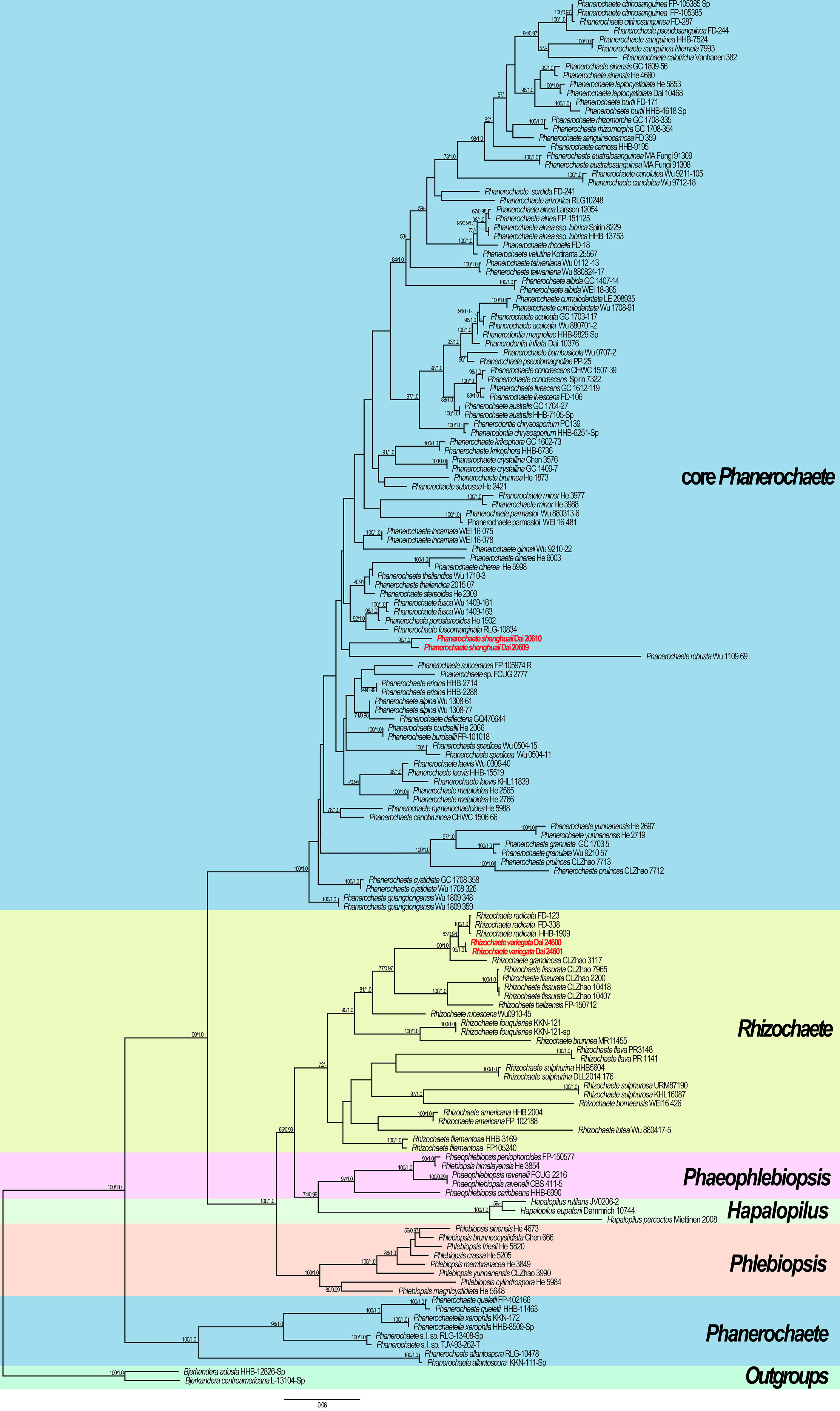
Figure 5 Maximum likelihood (ML) tree illustrating the phylogeny of the two new species in Phanerochaetaceae based on ITS + nLSU sequences. Branches are labeled with ML bootstrap >50% and Bayesian posterior probabilities (BPP) >0.90, respectively. New species are highlighted by red text.
Although more taxa of Phanerochaetaceae have been described (Greslebin et al., 2004; Bianchinotti et al., 2005; Chen et al., 2021), the taxonomy of corticioid fungi in Polyporales is woefully understudied. Many closely related genera are difficult to differentiate based on apparently plesiomorphic morphology, such as Phanerochaete, Rhizochaete, Phaeophlebiopsis, and Hapalopilus (Bianchinotti et al., 2005; Chen et al., 2021). Rhizochaete is separated from Phanerochaete mainly by their basidiocarp reaction with KOH (Greslebin et al., 2004; Chen et al., 2021). Indeed, this character is delimited in most species of the two genera. However, there are still some species of Phanerochaete exhibiting red in KOH, such as P. affinis (Burt) Parmasto and P. aurantiobadia Ghob.-Nejh. et al. (Punugu et al., 1980; Ghobad-Nejhad et al., 2015). Therefore, more samples from worldwide and multigene phylogeny analysis are urgently needed for understanding the diversity of corticioid species of Polyporales.
Southwest China is a hotspot for fungal diversity, and numerous taxa of wood-inhabiting fungi have been described from this area based on morphological and molecular phylogenetic analyses (Dai, 2010; Zhao et al., 2015; Zhou et al., 2016; Dai et al., 2021; Guan and Zhao, 2021; Wang et al., 2021; Wu et al., 2021; Wu et al., 2022b). Notably, the species diversity of corticioid fungi in this area is still not well-known, and therefore, the present paper confirms that more unknown species exist in this area.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OP874919, OP874920, OP874921, OP874922, OP874924, OP874925, OP874926, OP874927.
Author contributions
Q-YZ, Z-BL, and JS designed the research and contributed to data analysis and interpretation. Q-YZ prepared the samples and drafted the manuscript. Z-BL conducted molecular experiments and analyzed the data. H-GL and JS discussed the results and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by the National Natural Science Foundation of China (Nos. 32270016 and 32070016).
Acknowledgments
The authors would like to express their deep appreciation to Prof. Yu-Cheng Dai (Beijing Forestry University, China) for allowing them to study his specimens.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bianchinotti, M. V., Rajchenberg, M., Greslebin, A. G. (2005). Parenthesome structure of some corticioid fungi. Mycol. Res. 109, 923–926. doi: 10.1017/S0953756205003333
Burdsall, H. H. (1985). A contribution to the taxonomy of the genus Phanerochaete. Mycol. Mem. 10, 1–165.
Chen, C. C., Chen, C. Y., Wu, S. H. (2021). Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (Phanerochaetaceae, irpicaceae, meruliaceae) of polyporales. Fungal Divers. 111, 337–442. doi: 10.1007/s13225-021-00490-w
Chen, C. C., Wu, S. H., Chen, C. Y. (2018). Hydnophanerochaete and Odontoefibula, two new genera of phanerochaetoid fungi (Polyporales, basidiomycota) from East Asia. MycoKeys 39, 75–96. doi: 10.3897/mycokeys.39.28010
Chikowski, R. S., Larsson, K. H., Gibertoni, T. B. (2016). Three new combinations in Rhizochaete (Agaricomycetes, fungi) and a new record to the Brazilian Amazonia. Nova Hedwig. 102, 185–196. doi: 10.1127/nova_hedwigia/2015/0298
Cui, B. K., Li, H. J., Ji, X., Zhou, J. L., Song, J., Si, J., et al. (2019). Species diversity, taxonomy and phylogeny of polyporaceae (Basidiomycota) in China. Fungal Divers. 97, 137–392. doi: 10.1007/s13225-019-00427-4
Dai, Y. C. (2010). Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 45, 131–343. doi: 10.1007/s13225-010-0066-9
Dai, Y. C. (2011). A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 52, 69–79. doi: 10.1007/S10267-010-0068-1
Dai, Y. C., Yang, Z. L., Cui, B. K., Wu, G., Yuan, H. S., Zhou, L. W., et al. (2021). Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 40, 770–805. doi: 10.13346/j.mycosystema.210036
de Koker, T., Nakasone, K. K., Haarhof, J., Burdsall, H. H., Janse, B. J. H. (2003). Phylogenetic relationships of the genus Phanerochaete inferred from the internal transcribed spacer region. Mycol. Res. 107, 1032–1040. doi: 10.1017/S095375620300827X
Feng, B., Yang, Z. (2018). Studies on diversity of higher fungi in yunnan, southwestern China: A review. Plant Divers. 40, 165–171. doi: 10.1016/j.pld.2018.07.001
Floudas, D., Hibbett, D. S. (2015). Revisiting the taxonomy of Phanerochaete (Polyporales, basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol. 119, 679–719. doi: 10.1016/j.funbio.2015.04.003
Ghobad-Nejhad, M., Liu, S. L., Langer, E., Dai, Y. C. (2015). Molecular and morphological evidence reveal a new non-cystidiate species belonging to the core Phanerochaete (Polyporales). Mycol. Prog. 14, 1–7. doi: 10.1007/s11557-015-1072-9
Greslebin, A., Nakasone, K. K., Rajchenberg, M. (2004). Rhizochaete, a new genus of phanerochaetoid fungi. Mycologia 96, 260–271. doi: 10.1080/15572536.2005.11832976
Guan, Q. X., Zhao, C. L. (2021). Two new corticioid species, Hyphoderma sinense and H. floccosum (Hyphodermataceae, polyporales), from southern China. Mycosystema 40, 447–461. doi: 10.13346/j.mycosystema.200382
Gu, Z. R., Zhao, C. L. (2021). The hidden wood-decaying fungal diversity: Rhizochaete from east Asia. Diversity 13, 503. doi: 10.3390/d13100503
Hall, T. A. (1999). Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. doi: 10.1021/bk-1999-0734.ch008
He, M. Q., Zhao, R. L., Hyde, K. D., Begerow, D., Kemler, M., Yurkov, A., et al. (2019). Notes, outline and divergence times of basidiomycota. Fungal Divers. 99, 105–367. doi: 10.1007/s13225-019-00435-4
Jia, B. S., Zhou, L. W., Cui, B. K., Rivoire, B., Dai, Y. C. (2014). Taxonomy and phylogeny of Ceriporia (Polyporales, basidiomycota) with an emphasis of Chinese collections. Mycol. Prog. 13, 81–93. doi: 10.1007/s11557-013-0895-5
Justo, A., Miettinen, O., Floudas, D., Ortiz-Santana, B., Sjokvist, E., Lindner, D., et al. (2017). A revised family-level classification of the polyporales (Basidiomycota). Fungal Biol. 121, 798–824. doi: 10.1016/j.funbio.2017.05.010
Kirk, P. M., Cannon, P. F., Minter, D. W., Stalpers, J. A. (2008). Dictionary of the fungi. 10th edn (Wallingford: CABI).
Larsson, K. H. (2007). Re-thinking the classification of corticioid fungi. Mycol. Res. 111, 1040–1063. doi: 10.1016/j.mycres.2007.08.001
Liu, S. L., He, S. H. (2016). Phanerochaete porostereoides, a new species in the core clade with brown generative hyphae from China. Mycosphere 7, 648–655. doi: 10.5943/mycosphere/7/5/10
Miettinen, O., Spirin, V., Vlasák, J., Rivoire, B., Stenroos, S., Hibbett, D. (2016). Polypores and genus concepts in phanerochaetaceae (Polyporales, basidiomycota). MycoKeys 17, 1–46. doi: 10.3897/mycokeys.17.10153
Nakasone, K. K., Draeger, K. R., Ortiz-Santana, B. (2017). A contribution to the taxonomy of Rhizochaete (Polyporales, basidiomycota). Cryptogam. Mycol. 38, 81–99. doi: 10.7872/crym/v38.iss1.2017.81
Ortiz-Santana, B., Lindner, D. L., Miettinen, O., Justo, A., Hibbett, D. S. (2013). A phylogenetic overview of the antrodia clade (Basidiomycota, polyporales). Mycologia 105, 1391–1411. doi: 10.3852/13-051
Petersen, J. H. (1996). “The Danish mycological society’s colour-chart,” in Greven: Foreningen til svampekundskabens fremme, Denmark, 1–6.
Phookamsak, R., Hyde, K. D., Jeewon, R., Bhat, D. J., Jones, E. B. G., Maharachchikumbura, S. S. N., et al. (2019). Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 95, 1–273. doi: 10.1007/s13225-019-00421-w
Punugu, A., Dunn, M. T., Welden, A. L. (1980). The peniophoroid fungi of the West indies. Mycotaxon 10, 428–454.
Ronquist, F., Teslenko, M., van der Mark, P., Avres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice, across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Silvestro, D., Michalak, I. (2012). RaxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 12, 335–337. doi: 10.1007/s13127-011-0056-0
Spirin, V., Volobuev, S., Okun, M., Miettinen, O., Larsson, K. H. (2017). What is the type species of Phanerochaete (Polyporales, basidiomycota)? Mycol. Prog. 16, 171–183. doi: 10.1007/s11557-016-1267-8
Stamatakis, A. (2006). RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinf. (Oxford England) 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., Higgins, D. G. (1997). The Clustal_X windows interface: fexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. doi: 10.1093/nar/25.24.4876
Vilgalys, R., Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Volobuev, S., Okun, M., Ordynets, A., Spirin, V. (2015). The Phanerochaete sordida group (Polyporales, basidiomycota) in temperate Eurasia, with a note on Phanerochaete pallida. Mycol. Prog. 14, 1–13. doi: 10.1007/s11557-015-1097-0
Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., et al. (2019). Large-Scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 92, 1–20. doi: 10.1016/j.simyco.2018.05.001
Wang, K., Chen, S. L., Dai, Y. C., Jia, Z. F., Li, T. H., Liu, T. Z., et al. (2021). Overview of china's nomenclature novelties of fungi in the new century, (2000–2020). Mycosystema 40, 822–833. doi: 10.13346/j.mycosystema.210064
Wang, D. Q., Zhao, C. L. (2021). Morphological and phylogenetic evidence for recognition of two new species of Phanerochaete from East Asia. J. Fungi 7, 1063. doi: 10.3390/jof7121063
White, T. J., Bruns, T. D., Lee, S., Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: A guide to methods and applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, T. J. (New York Academic Press: US), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, S. H. (1990). The corticiaceae (Basidiomycetes) subfamilies phlebioideae, phanerochaetoideae and hyphodermoideae in Taiwan. Acta Bot. Fenn. 142, 1–123.
Wu, S. H. (1995). A study of the genus Phanerochaete (Aphyllophorales) with brown subicular hyphae. Mycotaxon 54, 163–172.
Wu, S. H. (1998). Nine new species of Phanerochaete from Taiwan. Mycol. Res. 102, 1126–1132. doi: 10.1017/S0953756298006091
Wu, S. H. (2000). Six new species of Phanerochaete from Taiwan. Bot. Bull. Acad. Sin. (Taipei). 41, 165–174.
Wu, S. H., Chen, C. C., Wei, C. L. (2018a). Three new species of Phanerochaete (Polyporales, basidiomycota). MycoKeys 41, 91–106. doi: 10.3897/mycokeys.41.29070
Wu, S. H., Chen, Y. P., Wei, C. L., Floudas, D., Dai, Y. C. (2018b). Two new species of Phanerochaete (Basidiomycota) and redescription of P. robusta. Mycol. Prog. 17, 425–435. doi: 10.1007/s11557-017-1368-z
Wu, F., Li, S. J., Dong, C. H., Dai, Y. C., Papp, V. (2020). The genus Pachyma (syn. wolfiporia) reinstated and species clarification of the cultivated medicinal mushroom “Fuling” in China. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.590788
Wu, F., Man, X. W., Tohtirjap, A., Dai, Y. C. (2022a). A comparison of polypore funga and species composition in forest ecosystems of China, north America, and Europe. For. Ecosyst. 9, 100051. doi: 10.1016/j.fecs.2022.100051
Wu, S. H., Nilsson, H. R., Chen, C. T., Yu, S. Y., Hallenberg, N. (2010). The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phleboid clade of the polyporales (Basidiomycota). Fungal Divers. 42, 107–118. doi: 10.1007/s13225-010-0031-7
Wu, F., Tohtirjap, A., Fan, L. F., Zhou, L. W., Alvarenga, R. L. M., Gibertoni, T. B., et al. (2021). Global diversity and updated phylogeny of Auricularia (Auriculariales, basidiomycota). J. Fungi 7, 933. doi: 10.3390/jof7110933
Wu, F., Zhou, L. W., Vlasák, J., Dai, Y. C. (2022b). Global diversity and systematics of hymenochaetaceae with poroid hymenophore. Fungal Divers. 113, 1–192. doi: 10.1007/s13225-021-00496-4
Xiong, H., Dai, Y. C. (2009). Notes on lignicolous corticioid fungi in China 3. Phanerochaete basidiomycota, polyporales in China. Mycosystem 28, 29–35. doi: 10.13346/j.mycosystema.2009.01.006
Xu, Y. L., Cao, Y. F., Nakasone, K. K., Chen, C. C., He, S. H. (2020). Taxonomy and phylogeny of Phanerochaete sensu stricto (Polyporales, basidiomycota) with emphasis on Chinese collections and descriptions of nine new species. Mycosphere 11, 1527–1552. doi: 10.5943/mycosphere/11/1/12
Yuan, H. S., Dai, Y. C. (2008). Polypores from northern and central yunnan province, southwestern China. Sydowia 60, 147–159.
Zhang, D., Gao, F., Jakovlić, I., Zou, H., Zhang, J., Li, W. X., et al. (2020). PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 20, 348–355. doi: 10.1111/1755-0998.13096
Zhao, C. L., Cui, B. K., Song, J., Dai, Y. C. (2015). Fragiliporiaceae, a new family of polyporales (Basidiomycota). Fungal Divers. 70, 115–126. doi: 10.1007/s13225-014-0299-0
Zhao, Y. N., He, S. H., Nakasone, K. K., Wasantha Kumara, K. L., Chen, C. C., Liu, S. L., et al. (2021). Global phylogeny and taxonomy of the wood-decaying fungal genus Phlebiopsis (Polyporales, basidiomycota). Front. Microbiol. 12. doi: 10.3389/fmicb.2021.622460
Zhao, C. L., Liu, X. F., Ma, X. (2019). Phlebiopsis yunnanensis sp. nov. (Polyporales, basidiomycota) evidenced by morphological characters and phylogenetic analysis. Nova Hedwig. 108, 265–279. doi: 10.1127/nova_hedwigia/2018/0508
Zhou, L. W., Vlasák, J., Decock, C., Assefa, A., Stenlid, J., Abate, D., et al. (2016). Global diversity and taxonomy of the Inonotus linteus complex (Hymenochaetales, basidiomycota): Sanghuangporus gen. nov., Tropicoporus excentrodendri and T. guanacastensis gen. et spp. nov., and 17 new combinations. Fungal Divers. 77, 335–347. doi: 10.1007/s13225-015-0335-8
Keywords: new taxa, phlebioid clade, phylogeny, taxonomy, wood-decaying fungi
Citation: Zhang Q-Y, Liu Z-B, Liu H-G and Si J (2023) Two new corticioid species of Phanerochaetaceae (Polyporales, Basidiomycota) from Southwest China. Front. Cell. Infect. Microbiol. 13:1105918. doi: 10.3389/fcimb.2023.1105918
Received: 23 November 2022; Accepted: 18 January 2023;
Published: 03 February 2023.
Edited by:
Jia-Jia Chen, Jiangsu Vocational College of Agriculture and Forestry, ChinaReviewed by:
James A. Fraser, The University of Queensland, AustraliaHaixia Ma, Chinese Academy of Tropical Agricultural Sciences, China
Copyright © 2023 Zhang, Liu, Liu and Si. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Si, amluZ3NpMTc4OEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Qiu-Yue Zhang
Qiu-Yue Zhang Zhan-Bo Liu
Zhan-Bo Liu Hong-Gao Liu
Hong-Gao Liu Jing Si
Jing Si