- 1Department of Otolaryngology Head and Neck Surgery, Peking University Third Hospital, Beijing, China
- 2Department of Otolaryngology Head and Neck Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 3Department of Otolaryngology Head and Neck Surgery, The First Affiliated Hospital of Wenzhou Medical University, Zhejiang, Wen Zhou, China
Background: Disease control is a primary treatment goal for patients with chronic rhinosinusitis (CRS). This study aims to summarize the evaluation parameters of disease control and then identify predictors of poorly controlled CRS.
Methods: A systematic review of the literature was performed on PubMed, Google Scholar, Scopus, and Cochrane databases to identify studies relating to disease control in CRS.
Results: The concept of disease control in patients with CRS involved the longitudinal assessment of the disease state and was also an important goal of treatment. As a metric of the disease state, the disease control reflected the ability to keep disease manifestations within certain limits, the efficacy after treatment, and the impact on quality of life. Validated measurements, such as EPOS2012 criteria, EPOS2020 criteria, Sinus Control Test, and patient/physician-reported global level of CRS control, have been utilized in clinical practice. These existing disease control instruments incorporated various disease manifestations and categorized patients into two (well-controlled and poor-controlled), three (uncontrolled, partly controlled, and controlled), or five (not at all, a little, somewhat, very, and completely) control categories. Eosinophilia, high computerized tomography score, bilateral sinonasal disease, asthma, allergic rhinitis, female gender, aspirin intolerance, revision surgery, low serum amyloid A, and specific T cell subtype would predict poorly controlled CRS.
Conclusion: The concept of disease control and its application were gradually developed in patients with CRS. The existing disease control instruments demonstrated a lack of uniformity regarding the controlled criteria and included parameters.
Introduction
Chronic rhinosinusitis (CRS) is a chronic inflammatory sinonasal disease that afflicts 10.9% of Europeans (Hastan et al., 2011), 11.9% of Americans (Hirsch et al., 2017), and 8% of Chinese (Shi et al., 2015). Despite maximal medical therapy, up to 60% of patients with CRS have persistent symptoms (Baguley et al., 2014), which are called difficult-to-treat rhinosinusitis or refractory CRS (Fokkens et al., 2012). Furthermore, the revision rate after endoscopic sinus surgery is about 15–20% after five to ten years of follow-up (Alanin and Hopkins, 2020). For patients with chronic rhinosinusitis with nasal polyps (CRSwNP), polyp recurrence after endoscopic sinus surgery is as high as 70% of patients after 18 months of follow-up (DeConde et al., 2017). It seems that patients with CRS need a long-term treatment strategy and there is a growing consensus that the primary treatment goal of CRS is to maintain clinical control (Van der Veen et al., 2017). Identifying the patients with poor disease control states is necessary to guide therapy alterations.
An increasing number of studies are exploring the different aspects of disease control in patients with CRS (Snidvongs et al., 2014). It is important to note that the evaluation parameters of disease control in patients with CRS differ across studies. The 2012 European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS 2012) defined disease control as a disease state free from bothersome symptoms and having a healthy mucosa without the need for systemic medication (Fokkens et al., 2012). It proposed an assessment of CRS disease control based on a combination of symptoms, endoscopy, and systemic medication used (Fokkens et al., 2012). The EPOS2012 criteria have categorized patients into uncontrolled, partly controlled, and controlled CRS. Since the establishment of EPOS2012 criteria for disease control in CRS, several studies have attempted to develop criteria like Physician Global Assessment (PGA), Sinus Control Test (SCT), and patient report of change in global nasal function (Snidvongs et al., 2014; Banglawala et al., 2016; Gray et al., 2017a; Campbell et al., 2018; Sedaghat et al., 2018; McCann et al., 2021; Phillips et al., 2021). In addition, patient/physician-reported global level of CRS control was also developed. These tools classified the patients with CRS into five (Not at all, a little, somewhat, very, and completely) control levels (Gray et al., 2017a; Campbell et al., 2018; Sedaghat et al., 2018; McCann et al., 2021; Phillips et al., 2021). Moreover, several recent studies have explored the predictors of poor disease control, which help to guide for escalation of the CRS treatment regimen (Van der Veen et al., 2017; Tao et al., 2018; Wang et al., 2019; Lu et al., 2021; Penttilä et al., 2021; Jiang et al., 2022). However, these studies utilized varied components of CRS disease control assessments. A systematic review of the current literature on disease control assessment would lay a foundation for developing a gold standard to assess disease control in CRS. Therefore, this systematic review aimed to summarize characteristics of the evaluation measurements of disease control in patients with CRS and then identify predictors of poorly controlled CRS.
Materials and methods
Literature search strategy
An evidence-based systematic review was performed utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A comprehensive search of PubMed, Scopus, Cochrane databases, and Google Scholar was conducted to identify studies relating to disease control in CRS on July 14, 2022. A combination of terms was used in this review: chronic rhinosinusitis, chronic sinusitis, control, and disease control.
Study selection
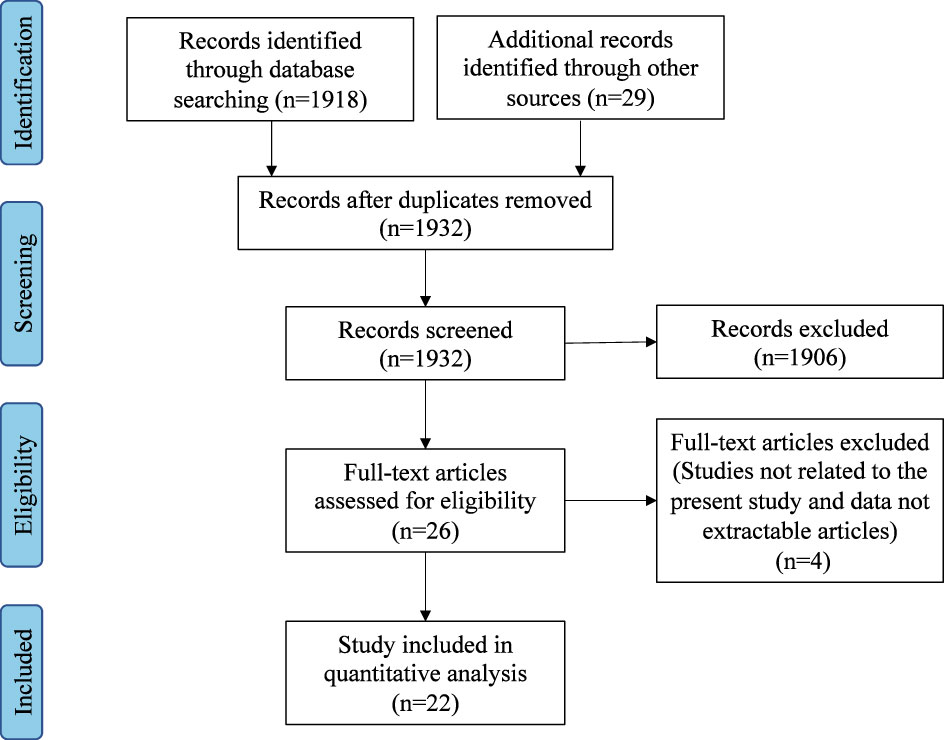
Titles and abstracts of all the relevant studies were reviewed by two independent authors (DW and JZ). Included studies addressed the concept, criteria, characteristics, and predictors of disease control in CRS. All included studies were downloaded, and both authors reviewed the full text. Figure 1 outlines the search strategy and inclusion process used to find relevant studies.
Data extraction and analysis
Data included the year of publication, study design, concept, clinical application, disease control criteria, intervention, result, and conclusion. After analysis of each article, summary tables were developed. The quality of each article was assessed by the Oxford Center for Evidence-Based Medicine Levels of Evidence categorization (Burns et al., 2011).
Results
Include studies
The initial database search identified 1947 articles. After the removal of duplicates and abstract screening, 1906 articles were excluded. A total of 26 articles underwent a full-text assessment for eligibility, and 22 articles met the final inclusion criteria for systematic review.
Different disease control measures in patients with CRS
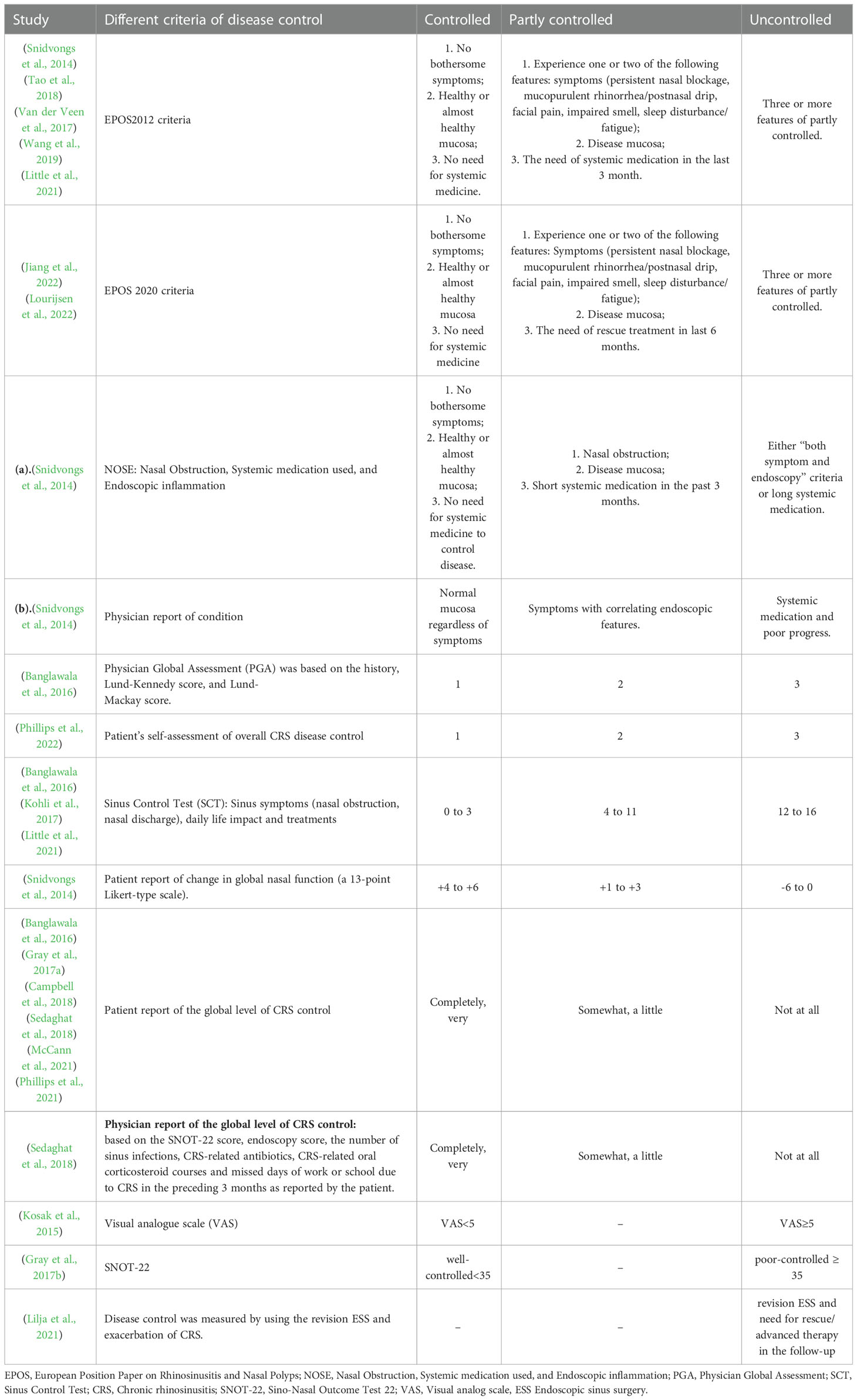
A total of thirteen disease control measures in patients with CRS in seventeen studies were summarized, including EPOS2012 criteria, EPOS2020 criteria, Nasal Obstruction Systemic medication used Endoscopic inflammation (NOSE), physician report of condition, PGA, patient’s self-assessment of overall CRS disease control, SCT, patient report of change in global nasal function, patient/physician-reported global level of CRS control with five control levels, Visual analog scale (VAS), Sino-Nasal Outcome Test 22 (SNOT-22) scores, and controlled CRS measured by the revision ESS and exacerbation of CRS during the follow-up (Table 1).
Five studies reported EPOS2012 criteria (Snidvongs et al., 2014; Van der Veen et al., 2017; Tao et al., 2018; Wang et al., 2019; Little et al., 2021), and two studies reported EPOS2020 criteria (Jiang et al., 2022; Lourijsen et al., 2022). Both criteria were based on symptoms, endoscopy, and systemic medication used. Based on validation studies (Snidvongs et al., 2014; Van der Veen et al., 2017; Calus et al., 2019), the EPOS2020 criteria have some improvements over EPOS2012. EPOS2020 criteria recommend using a VAS scale for all symptoms (Fokkens et al., 2020). EPOS2012 focused on systemic medication needed in the last three months, while EPOS2020 focused on rescue treatment in the last six months. In EPOS2012 criteria, uncontrolled CRS needs long-term systematic medication (Fokkens et al., 2012) while patients with uncontrolled CRS still had symptoms after rescue treatment in EPOS2020. Snidvongs et al. (2014) proposed a simpler NOSE system just using nasal obstruction, the systemic medication used, and endoscopic inflammation. Meanwhile, they put forward physician reports of conditions based on the nasal mucosa, symptoms, systemic medication, and progress. The above four measures would divide the patients with CRS into three levels of control, including controlled, partly controlled, and uncontrolled conditions according to the combination of different subjective and objective parameters.
Another two measures including PGA and patient’s self-assessment of overall CRS disease control would directly classify patients with CRS into controlled, partly controlled, and uncontrolled conditions. The treating physicians completed the PGA questionnaire using a 3-point scale. The control rating was based on the history, endoscopic sinus examination, and computed tomography findings. The patients with CRS rated their overall CRS disease control as controlled, partly controlled, and uncontrolled condition. The SCT was utilized in three studies to evaluate disease control in patients with CRS based on the sinus symptoms (nasal obstruction, nasal discharge), daily life impact, and treatments (Banglawala et al., 2016; Kohli et al., 2017; Little et al., 2021). Snidvongs et al. (2014) used a 13-point Likert-type scale to report the change in global nasal function.
Patient self-assessment based on perception or patient report of the global level of CRS control was utilized in six studies where patients were classified into five control levels (Banglawala et al., 2016; Gray et al., 2017a; Campbell et al., 2018; Sedaghat et al., 2018; McCann et al., 2021; Phillips et al., 2021). Similarly, Sedaghat et al. (2018) used physician reports of the global level of CRS control based on the SNOT-22 score, endoscopy score, the number of sinus infections, CRS-related antibiotics, CRS-related oral corticosteroid courses, and missed days of work or school due to CRS in the preceding three months as reported by the patient. Moreover, the patients were divided into five control levels. Kosak, et al (Kosak et al., 2015). used VAS to assess disease control in patients with CRS. A VAS score of less than 5 indicated a well-controlled condition, and a VAS score of more than 5 indicated an uncontrolled condition. Similarly, Gray et al. (2017b) used scores of SNOT-22 to distinguish between patients with well or poor-controlled CRS symptoms. Lilja et al. (2021) identified patients with a revision of ESS and the need for rescue/advanced therapy in the follow-up as uncontrolled CRS.
Characteristics of different disease control measures
We summarized the characteristics of different disease control measures in thirteen studies (Table 2). Snidvongs et al. (2014) proposed NOSE criteria which were simpler than EPOS 2012 criteria, and found that both EPOS 2012 and NOSE significantly correlated with physicians and patient reports. Furthermore, Van der Veen et al. (2017) found that the levels of control measured by EPOS2012 corresponded to mean total SNOT-22 and short form (36) health survey (SF-36) scores (p < 0.05). Lu et al. (2021) found that increased serum amyloid A (SAA) level was associated with better disease control as measured by EPOS2012 criteria in patients with CRSwNP after ESS.
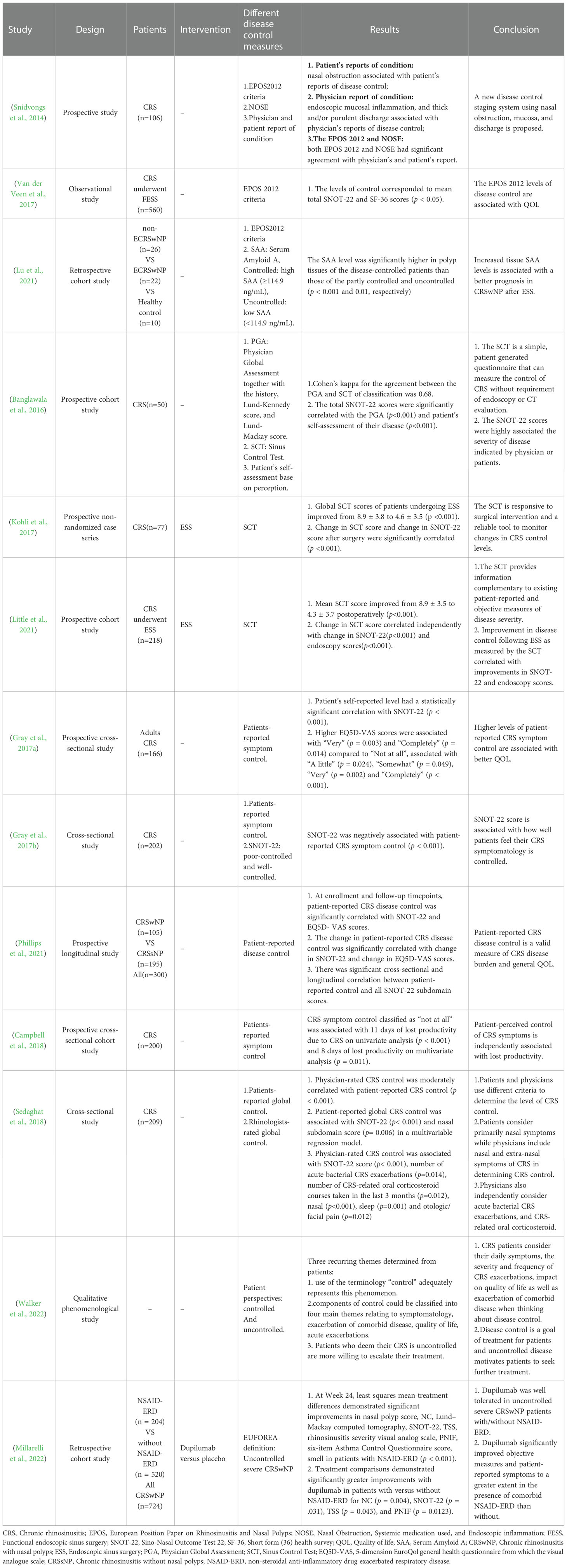
Table 2 Characteristics among different disease control measures and their association with disease burden and clinical parameters.
Banglawala, et al (Banglawala et al., 2016). proposed PGA and SCT to measure the control of CRS and found that SCT correlated with PGA. In addition, SNOT-22 scores were significantly associated with disease control indicated by physicians or patients. Furthermore, both Kohli et al. and Little et al (Kohli et al., 2017; Little et al., 2021). found that SCT scores decreased postoperatively, and change in SCT score was significantly correlated with change in SNOT-22 score and endoscopy score. It was demonstrated in two studies that higher levels of patient-reported disease control were associated with better quality of life as measured by SNOT-22 or 5-dimension EuroQol general health questionnaire from the visual analogue scale (EQ-5D VAS) (Gray et al., 2017b; Phillips et al., 2022). Besides, Campbell et al. (2018) found that a low level of patient-reported symptom control was independently related to lost productivity.
Sedaghat, et al (Sedaghat et al., 2018). found that patients and physicians utilized different criteria to determine the level of CRS control. Specifically, nasal and extra-nasal symptoms, acute bacterial CRS exacerbations, and CRS-related oral corticosteroids were associated with physician-rated CRS control, while primarily nasal symptoms were associated with patient-reported global CRS control. Both the CRS control rated by patients and rhinologists had a significant association with SNOT-22 (Sedaghat et al., 2018). Furthermore, Walker et al. (2022) reported that patients would consider daily symptoms, CRS exacerbations, quality of life, and exacerbations of comorbid disease when talking about disease control and uncontrolled disease motivated patients to seek further treatment. Millarelli et al. (2022) defined uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP) when the patients with CRSwNP presented with persistent or recurring CRSwNP despite long-term intranasal corticosteroid (INCS) and having received at least one course of systemic corticosteroids in the preceding two years and/or previous sinonasal surgery. They also defined severe CRSwNP based on the bilateral CRSwNP with a nasal polyp score (NPS) of ≥4, and persistent symptoms despite long-term INCS with the need for add-on treatment. In this study, the high efficiency of dupilumab in patients with uncontrolled severe CRSwNP patients was demonstrated.
Predictors of poor disease control in patients with chronic rhinosinusitis
We further summarized the predictors of poor disease control in patients with CRS (Table 3). Five studies identified poor disease control in patients with CRS based on EPOS2012, and risk factors included tissue or blood eosinophilia, high CT score, bilateral disease, asthma, and allergic rhinitis. Furthermore, tissue eosinophil ratio >0.206, blood eosinophil ratio >0.025, Lund-Mackay score≥ 15, CT ethmoid score≥maxillary score, female gender, aspirin intolerance, and revision Functional endoscopic sinus surgery (FESS), low serum amyloid A (SAA) were independently associated with the poor disease control (Van der Veen et al., 2017; Tao et al., 2018; Lu et al., 2021). Additionally, Wang et al. (2019) found that concordant blood and tissue eosinophilia can predict poor control better than isolated blood or tissue eosinophilia. Tao et al. (2018) found that prediction models based on tissue or blood eosinophil ratio together with CT score had significantly different uncontrolled levels.
EPOS2020 criteria were used in two studies (Penttilä et al., 2021; Jiang et al., 2022). Jiang et al. (2022) found that the presence of asthma, allergy rhinitis (AR), tissue eosinophil ratio (TER), and peripheral blood eosinophil count (PEBC) affected disease control postoperatively, and the sum nomogram showed the highest accuracy (AUC=0.760). Similarly, Penttilä et al. (2021) reported that the sum model of baseline eosinophilia, asthma/non-steroidal anti-inflammatory drug exacerbated respiratory disease (NERD), and oral corticosteroids (OCS) could well predict uncontrolled CRSwNP.
Patient report of five levels of CRS control was used in three studies (Gray et al., 2017a; McCann et al., 2021; Phillips et al., 2021). It is reported that hyposmia was not associated with poor control because hyposmia seldom occurs without nasal obstruction/drainage (McCann et al., 2021). Furthermore, only nasal obstruction and nasal discharge scores were significantly associated with CRS symptom control. Phillips et al. (2021) reported that SNOT-22 scores>25 or EQ-5D VAS scores <77 had the predictive significance of poorly controlled CRS, while another study by Gray, et al (Gray et al., 2017b). showed that SNOT-22 scores of 35 accurately distinguished poor or well-controlled CRS. A recent study by Phillips et al. (2022) utilized three levels of patient-rated overall CRS control and found that VAS symptom scores >3.5 related to the uncontrolled status on the corresponding binary descriptive EPOS symptom scale for all five symptoms, but the rhinorrhea/postnasal drip descriptive symptom scale translates disparately worse to VAS scores. A study by Kosak et al. (2015) defined VAS≥5 as uncontrolled CRS and showed that T17 CD4-CD8-CCR6+ cells predicted uncontrolled CRSwNP while Tc CD8+ cells predicted uncontrolled chronic rhinosinusitis without nasal polyps (CRSsNP).
Discussion
There is a growing awareness among physicians that poorly controlled CRS remains a chronic disease, and regular assessment of the disease control level of CRS is crucial to maintain long-term effective treatment of CRS (Guo et al., 2021). Currently, the concept of disease control in patients with CRS has been widely accepted (Fokkens et al., 2020; Orlandi et al., 2021). The definition, evaluation methods of disease control, and their application in CRS management are still active areas of study. Thus, a comprehensive understanding of disease control in patients with CRS would facilitate individualized treatment and also help to unify the application and interpretation of the existing evaluation methods.
We summarized the clinical application of disease control in patients with CRS. Disease control in CRS was first defined as a disease state where disease manifestations were limited to a certain extent (Snidvongs et al., 2014; Kohli et al., 2017; Van der Veen et al., 2017; Tao et al., 2018), reflecting the disease burden or disease severity, efficacy after ESS or medical therapy, the prediction of treatment response or prognosis, and the impact on quality of life. It can be inferred that patients with CRS would be classified into different levels of CRS control. Furthermore, the definition of disease control in CRS among the studies involved the longitudinal assessment of the disease state based on symptoms, sinonasal mucosa, and systematic or local medication, which helps to identify uncontrolled or well-controlled CRS at different points in time (Gray et al., 2017b; Sedaghat et al., 2018). The terminology “disease control” (also known as controlled or well-controlled CRS) also represented an important goal of treatment for patients with CRS (McCann et al., 2021; Phillips et al., 2021; Walker et al., 2022), especially in patients with difficult-to-treat CRS. There is no consensus on the criteria of CRS disease control, and critical components of CRS disease control should be explored in future studies.
We next summarized the characteristics of different disease control measures in patients with CRS. EPOS2012 utilized binary symptoms, endoscopy, and systemic medication used to assess disease control, in which patients with CRS were divided into three control levels including controlled, partly controlled, and uncontrolled (Fokkens et al., 2020). Previous studies validated the reliability of EPOS2012 criteria and showed that EPOS2012 related to physicians and patient reports of overall CRS disease control, total SNOT-22, and SF-36 scores (Snidvongs et al., 2014; Van der Veen et al., 2017). However, a study by Van der Veen et al. showed that only 4 of 21 patients who thought themselves had controlled CRS met the criteria of being controlled (Van der Veen et al., 2017), which indicated that EPOS2012 criteria might overrate the proportion of uncontrolled CRS. On this basis, EPOS2020 criteria recommend using a VAS scale for all symptoms, and a VAS score of more than 5 defines their clinical significance (Fokkens et al., 2020). A recent study by Sedaghat et al. also demonstrated that EPOS2020guidelines regularly assess worse CRS control than those assessed by patients (Sedaghat et al., 2022). The binary symptoms criteria and inclusion of nasal endoscopy may contribute to the discordance of EPOS2020 with patient-reported CRS control (Sedaghat et al., 2022).
Apart from the CRS control criteria proposed by EPOS2012 and EPOS2020, alternative tools for the assessment of CRS control have been proposed. A simpler NOSE system just using nasal obstruction, the systemic medication used, and the presence of endoscopic inflammation was proved to be significantly associated with physicians and patient reports of CRS disease control (Snidvongs et al., 2014). Besides, the PGA was based on the history, endoscopic sinus examination, and computed tomography findings and the physicians rated CRS disease control as controlled, partly controlled, and uncontrolled (Banglawala et al., 2016). Based on the sinus symptoms (nasal obstruction, nasal discharge), daily life impact, and treatments, the SCT was proposed, which was proved to have a significant agreement with PGA (Banglawala et al., 2016). Furthermore, SCT scores in postoperative patients significantly decreased, and the change in SCT score was significantly associated with the change in SNOT-22 score and endoscopy score (Kohli et al., 2017; Little et al., 2021). It can be speculated that the SCT provided information about the degrees of CRS control level and was responsive to change after endoscopic sinus surgery, indicating a complementary role of SCT to the existing assessment of disease control in CRS.
Patient/physician-report CRS disease control was widely applied in studies (Snidvongs et al., 2014; Banglawala et al., 2016; Gray et al., 2017b; Campbell et al., 2018; Sedaghat et al., 2018; McCann et al., 2021; Phillips et al., 2022). However, physician-rated CRS control was moderately correlated with patient-reported CRS control (Sedaghat et al., 2018). The difference between patient-rated and physician-assessed disease control levels may suggest that they considered different factors when assessing disease control. Physicians always take symptoms, acute exacerbations, and oral corticosteroids into account, while patients pay more attention to primarily nasal symptoms (Sedaghat et al., 2018). From a clinical point of view, care should be taken to balance the focus of the patient and the physician. The poorly controlled CRS perceived by the patients motivates them to seek further treatment, and physician-assessed disease control levels would directly inform management decisions. Above all, both objective and subjective parameters should be taken into consideration when conducting the evaluation of disease control in patients with CRS.
We also reviewed the predictors of poor disease control in patients with CRS. Tissue or blood eosinophilia, high CT score, bilateral disease, asthma, allergic rhinitis, female gender, aspirin intolerance, and revision FESS were all independent predictors of poor controlled CRS (Van der Veen et al., 2017; Tao et al., 2018; Wang et al., 2019; Penttilä et al., 2021; Jiang et al., 2022), and concordant blood and tissue eosinophilia predicted better than isolated blood or tissue eosinophilia (Wang et al., 2019). Meanwhile, several sum models based on the above factors were demonstrated to have higher prediction accuracy (Tao et al., 2018; Penttilä et al., 2021; Jiang et al., 2022). Furthermore, low SAA was independently associated with poor disease control. Unexpectedly, as a common symptom caused by inflammatory injury (Smith et al., 2021) or obstruction (Rombaux et al., 2016), hyposmia often occurs together with nasal obstruction/rhinorrhea (McCann et al., 2021). Moreover, without nasal obstruction/drainage, hyposmia had no significant association with patients’ perceptions of CRS control level (Snidvongs et al., 2014; Banglawala et al., 2016; McCann et al., 2021).. However, an impaired sense of smell was associated with the patient’s agreement with EPOS2020 guidelines on having “uncontrolled” CRS (Sedaghat et al., 2022). Regarding the T cell subtype, T17 CD4-CD8-CCR6+ cells were associated with poorly controlled CRSwNP, while Tc CD8+ cells predicted poorly controlled CRSsNP (Kosak et al., 2015). Besides, high SNOT-22 scores, low EQ-5D scores, and high VAS symptom scores were also significantly associated with poorly controlled CRS, which reflected high disease burden and poor QOL (Gray et al., 2017a; Phillips et al., 2021; Phillips et al., 2022). These findings on prediction factors would facilitate preventive interventions and upgrade treatment to achieve disease control.
Some meaningful factors have not been studied in CRS disease control but may inform predictions of poorly controlled CRS. Vitamin D is recognized for its anti-inflammation and antiproliferation effects in previous studies (Stokes and Rimmer, 2016). Compared to healthy subjects, patients with CRS presented a lower level of serum vitamin D (Li et al., 2021). Meanwhile, patients with CRSwNP had a significantly lower level of Vitamin D than patients with CRSsNP (Stokes and Rimmer, 2016; Bavi et al., 2019; Li et al., 2021). It can be inferred that serum vitamin D levels were highly associated with CRS phenotypes. More studies are needed to explore the association of low levels of Vitamin D with poor disease control. It is proved that various fungal related components are able induce type 2 inflammation among patients with sinonasal diseases (Tyler et al., 2021; Challapalli et al., 2022). Eosinophilic CRS is characterized by a type 2 immune response (Tyler et al., 2021) and is frequently associated with a poor disease control status. Further studies should clarify the role of the fungus in the uncontrolled patients with CRS.
Conclusions
As a refractory disease, the concept of disease control and its application were gradually developed, and a longitudinal assessment of disease control in patients with CRS is crucial to achieving a satisfactory outcome. There is currently no consensus on the notion and critical components of CRS disease control. The existing disease control instruments demonstrated a lack of uniformity regarding the controlled criteria and included parameters. Our review of the literature suggests that eosinophilia, high CT score, bilateral disease, asthma, allergic rhinitis, female gender, aspirin intolerance, revision FESS, low SAA, and T cell subtype would predict poorly controlled CRS. For higher reliability and accuracy, more studies are needed to build a consensus on the certain definition and criteria of disease control in patients with CRS, which is based on the combination of subjective parameters and objective indicators.
Author contributions
JZ and DW drafted the manuscript. FY and TH analyzed the data. LZ reviewed and revised this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Natural Science Foundation of China (82000954), Beijing Science and Technology Nova Program (Z201100006820086), Beijing Hospitals Authority Youth Program (QML20190617), and Beijing Hospitals Authority Clinical Medicine Development of Special Funding (XMLX202136).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alanin, M. C., Hopkins, C. (2020). Effect of functional endoscopic sinus surgery on outcomes in chronic rhinosinusitis. Curr. Allergy Asthma Rep. 20 (7), 27. doi: 10.1007/s11882-020-00932-6
Baguley, C., Brownlow, A., Yeung, K., Pratt, E., Sacks, R., Harvey, R. (2014). The fate of chronic rhinosinusitis sufferers after maximal medical therapy: the fate of CRS sufferers after MMT. Int. Forum Allergy Rhinol. 4 (7), 525–532. doi: 10.1002/alr.21315
Banglawala, S. M., Schlosser, R. J., Morella, K., Chandra, R., Khetani, J., Poetker, D. M., et al. (2016). Qualitative development of the sinus control test: a survey evaluating sinus symptom control: qualitative development of the SCT. Int. Forum Allergy Rhinol. 6 (5), 491–499. doi: 10.1002/alr.21690
Bavi, F., Movahed, R., Salehi, M., Hossaini, S., Bakhshaee, M. (2019). Chronic rhinosinusitis with polyposis and serum vitamin d levels. Acta Otorhinolaryngol. Ital. 39 (5), 336–340. doi: 10.14639/0392-100X-2439
Burns, P. B., Rohrich, R. J., Chung, K. C. (2011). The levels of evidence and their role inevidence-based medicine. Plast. Reconstr. Surg. 128 (1), 305–310. doi: 10.1097/PRS.0b013e318219c171
Calus, L., Van Bruaene, N., Bosteels, C., Dejonckheere, S., Van Zele, T., Holtappels, G., et al. (2019). Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin. Transl. Allergy 9 (1), 30. doi: 10.1186/s13601-019-0269-4
Campbell, A. P., Hoehle, L. P., Phillips, K. M., Caradonna, D. S., Gray, S. T., Sedaghat, A. R. (2018). Symptom control in chronic rhinosinusitis is an independent predictor of productivity loss. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 135 (4), 237–241. doi: 10.1016/j.anorl.2017.05.005
Challapalli, S. D., McKee, S., Luong, A. U. (2022). The role of fungus in the pathogenesis of chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 30 (1), 58–62. doi: 10.1097/MOO.0000000000000775
DeConde, A. S., Mace, J. C., Levy, J. M., Rudmik, L., Alt, J. A., Smith, T. L. (2017). Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis: polyp recurrence after ESS. Laryngoscope 127 (3), 550–555. doi: 10.1002/lary.26391
Fokkens, W. J., Lund, V. J., Hopkins, C., Hellings, P. W., Kern, R., Reitsma, S., et al. (2020). European Position paper onrhinosinusitis and nasal polyps 2020. Rhinology 58 (Suppl S29), 1–464. doi: 10.4193/Rhin20.600
Fokkens, W. J., Lund, V. J., Mullol, J., Bachert, C., Alobid, I., Baroody, F., et al. (2012). EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. a summary for otorhinolaryngologists. Rhinology 50 (1), 1–12. doi: 10.4193/Rhino12.000
Gray, S. T., Hoehle, L. P., Phillips, K. M., Caradonna, D. S., Sedaghat, A. R. (2017a). Patient-reported control of chronic rhinosinusitis symptoms is positively associated with general health-related quality of life. Clin. Otolaryngol. 42 (6), 1161–1166. doi: 10.1111/coa.12841
Gray, S. T., Phillips, K. M., Hoehle, L. P., Caradonna, D. S., Sedaghat, A. R. (2017b). The 22-item sino-nasal outcome test accurately reflects patient-reported control of chronic rhinosinusitis symptomatology: SNOT-22 and CRS symptom control. Int. Forum Allergy Rhinol. 7 (10), 945–951. doi: 10.1002/alr.21992
Guo, C. L., Liao, B., Liu, J. X., Pan, L., Liu, Z. (2021). Predicting difficult-to-treat chronic rhinosinusitis by noninvasive biological markers. Rhinology 59 (1), 81–90. doi: 10.4193/Rhin20.103
Hastan, D., Fokkens, W. J., Bachert, C., Newson, R. B., Bislimovska, J., Bockelbrink, A., et al. (2011). Chronic rhinosinusitis in Europe - an underestimated disease. a GA2LEN study: chronic rhinosinusitis in Europe. Allergy 66 (9), 1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x
Hirsch, A. G., Stewart, W. F., Sundaresan, A. S., Young, A. J., Kennedy, T. L., Scott Greene, J., et al. (2017). Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy 72 (2), 274–281. doi: 10.1111/all.13042
Jiang, L., Wang, K., Lin, T., Jiang, Y., Gao, W., Li, C., et al. (2022). A novel risk score for disease control prediction of chronic rhinosinusitis. Clin. Otolaryngol. 47 (5), 568–576. doi: 10.1111/coa.13949
Kohli, P., Soler, Z. M., Storck, K. A., Shahangian, A., Banglawala, S. M., Schlosser, R. J. (2017). Responsiveness and reliability of the sinus control test in chronic rhinosinusitis. Rhinology 55 (1), 39–44. doi: 10.4193/Rhin16.208
Kosak, T. S., Silar, M., Kern, I., Boltezar, I. H., Korosec, P. (2015). Uncontrolled chronic rhinosinusitis with and without polyps is predicted by T cell subtype. Clin. Transl. Allergy 5 (S4), 1–1. doi: 10.1186/2045-7022-5-S4-O9
Li, B., Wang, M., Zhou, L., Wen, Q., Zou, J. (2021). Association between serum vitamin d and chronic rhinosinusitis: a meta-analysis. Braz. J. Otorhinolaryngol. 87 (2), 178–187. doi: 10.1016/j.bjorl.2019.08.007
Lilja, M. J., Koskinen, A., Virkkula, P., Vento, S. I., Myller, J., Hammarén-Malmi, S., et al. (2021). Factors affecting the control of chronic rhinosinusitis with nasal polyps: a comparison in patients with or without NERD. Allergy Rhinol. Provid. RI 12, 21526567211003844. doi: 10.1177/21526567211003844
Little, R. E., Schlosser, R. J., Smith, T. L., Storck, K. A., Alt, J. A., Beswick, D. M., et al. (2021). Disease control after surgery for chronic rhinosinusitis: prospective, multi-institutional validation of the sinus control test. Int. Forum Allergy Rhinol. 11 (2), 106–114. doi: 10.1002/alr.22659
Lourijsen, E. S., Reitsma, S., Vleming, M., Hannink, G., Adriaensen, G. F., Cornet, M. E., et al. (2022). Endoscopic sinus surgery with medical therapy versus medical therapy for chronic rhinosinusitis with nasal polyps: a multicentre, randomised, controlled trial. Lancet Respir. Med. 10 (4), 337–346. doi: 10.1016/S2213-2600(21)00457-4
Lu, H., Liu, H., Wang, K., Shi, J., Sun, Y. (2021). Association between serum amyloid a expression and disease control after endoscopic sinus ssurgery in patients with chronic rhinosinusitis with nasal polyps. Ear Nose Throat J., 014556132110513. doi: 10.1177/01455613211051311
McCann, A. C., Trope, M., Walker, V. L., Kavoosi, T. A., Speth, M. M., Gengler, I., et al. (2021). Olfactory dysfunction is not a determinant of patient-reported chronic rhinosinusitis disease control. Laryngoscope 131 (7), E2116–E2120. doi: 10.1002/lary.29280
Millarelli, S., Loperfido, A., Mammarella, F., Giorgione, C., Celebrini, A., Del Ninno, M., et al. (2022). A practical clinical protocol for monitoring patients with severe uncontrolled chronic rhinosinusitis with nasal polyposis treated with biologics. Allergy Rhinol. Provid. RI 13, 21526567221074336. doi: 10.1177/21526567221074335
Orlandi, R. R., Kingdom, T. T., Smith, T. L., Bleier, B., DeConde, A., Luong, A. U., et al. (2021). International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int. Forum Allergy Rhinol. 11 (3), 213–739. doi: 10.1002/alr.22741
Penttilä, E., Sillanpää, S., Vento, S. I., Myller, J., Koskinen, A., Hammarén-Malmi, S., et al. (2021). Eosinophilia, asthma, NERD and the use of oral corticosteroids predict uncontrolled chronic rhinosinusitis with nasal polyps after surgery [published online ahead of print, 2021 sep 5. Asian Pac. J. Allergy Immunol. doi: 10.12932/AP-310321-1102
Phillips, K. M., Houssein, F. A., Singerman, K., Boeckermann, L. M., Sedaghat, A. R. (2021). Patient-reported chronic rhinosinusitis disease control is a valid measure of disease burden. Rhinology 59 (6), 545–551. doi: 10.4193/Rhin21.282
Phillips, K. M., Singerman, K. W., Sedaghat, A. R. (2022). Individual symptom visual analogue scale severity scores for determining EPOS guideline-based chronic rhinosinusitis disease control. Rhinology 60 (3), 229–235. doi: 10.4193/Rhin21.446
Rombaux, P., Huart, C., Levie, P., Cingi, C., Hummel, T. (2016). Olfaction in chronic rhinosinusitis. Curr. Allergy Asthma Rep. 16 (5), 41. doi: 10.1007/s11882-016-0617-6
Sedaghat, A. R., Hoehle, L. P., Gray, S. T. (2018). Chronic rhinosinusitis control from the patient and physician perspectives: understanding CRS disease control. Laryngoscope Investig. Otolaryngol. 3 (6), 419–433. doi: 10.1002/lio2.208
Sedaghat, A. R., Singerman, K. W., Phillips, K. M. (2022). Discordance of chronic rhinosinusitis disease control between EPOS guidelines and patient perspectives identifies utility of patient-rated control assessment. Rhinology 60 (6), 444–452. doi: 10.4193/Rhin22.160
Shi, J. B., Fu, Q. L., Zhang, H., Cheng, L., Wang, Y. J., Zhu, D. D., et al. (2015). Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven C hinese cities. Allergy 70 (5), 533–539. doi: 10.1111/all.12577
Smith, T. L., Schlosser, R. J., Soler, Z. M., Mace, J. C., Mattos, J. L., Ramakrishnan, V. R., et al. (2021). Olfactory cleft mucus inflammatory proteins in CRS: a case-control study. Int. Forum Allergy Rhinol. 11 (9), 1321–1335. doi: 10.1002/alr.22770
Snidvongs, K., Heller, G. Z., Sacks, R., Harvey, R. J. (2014). Validity of european position paper on rhinosinusitis disease control assessment and modifications in chronic rhinosinusitis. Otolaryngol. Neck Surg. 150 (3), 479–486. doi: 10.1177/0194599813517080
Stokes, P. J., Rimmer, J. (2016). The relationship between serum vitamin d and chronic rhinosinusitis: a systematic review. Am. J. Rhinol. Allergy 30 (1), 23–28. doi: 10.2500/ajra.2016.30.4267
Tao, X., Chen, F., Sun, Y., Wu, S., Hong, H., Shi, J., et al. (2018). Prediction models for postoperative uncontrolled chronic rhinosinusitis in daily practice: prediction models for CRS in daily practice. Laryngoscope 128 (12), 2673–2680. doi: 10.1002/lary.27267
Tyler, M. A., Lam, K., Marino, M. J., Yao, W. C., Schmale, I., Citardi, M. J., et al. (2021). Revisiting the controversy: the role of fungi in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 11 (11), 1577–1587. doi: 10.1002/alr.22826
Van der Veen, J., Seys, S. F., Timmermans, M., Levie, P., Jorissen, M., Fokkens, W. J., et al. (2017). Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy 72 (2), 282–290. doi: 10.1111/all.12983
Walker, V., Trope, M., Tichavakunda, A. A., Speth, M. M., Sedaghat, A. R., Phillips, K. M. (2022). Disease control in chronic rhinosinusitis: a qualitative study of patient perspectives. Rhinology 60 (4), 282–292. doi: 10.4193/Rhin21.448
Keywords: sinusitis, treatment outcome, patient outcome assessment, risk factors, prognosis
Citation: Zhou J, Yuan F, Huang T, Zhu L and Wu D (2023) Current understanding of disease control and its application in patients with chronic rhinosinusitis. Front. Cell. Infect. Microbiol. 13:1104444. doi: 10.3389/fcimb.2023.1104444
Received: 21 November 2022; Accepted: 22 May 2023;
Published: 05 June 2023.
Edited by:
Noureddin Nakhostin Ansari, Tehran University of Medical Sciences, IranReviewed by:
Manish Sharma, Emory University, United StatesJim Bartley, The University of Auckland, New Zealand
Copyright © 2023 Zhou, Yuan, Huang, Zhu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawei Wu, ZGF2aWR3dW9ybEAxNjMuY29t; Li Zhu, cHJsaXpodUBxcS5jb20=
 Jiahui Zhou1
Jiahui Zhou1 Fan Yuan
Fan Yuan Tianhao Huang
Tianhao Huang Dawei Wu
Dawei Wu