- 1Department of Respiratory Medicine, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
- 2Department of Respiratory Medicine, Jiashan County Yaozhuang Town Health Centre, Jiaxing, Zhejiang, China
- 3Department of Respiratory Medicine, Bengbu Medical College, Bengbu, Anhui, China
- 4The Key Laboratory, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
- 5Clinical Laboratory, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
Objective: To investigate the clinical features, diagnosis, and treatment of Chlamydia psittaci (C. psittaci) pneumonia.
Methods: We retrospectively analyzed the clinical data of six patients with C. psittaci pneumonia who were admitted to the Division of Pulmonary and Critical Care Medicine of the Second Hospital of Jiaxing from December 2021 to September 2022.
Results: All patients reported a fever and other accompanying symptoms, including cough (5/6), chest tightness (1/6), fatigue (2/6), and headache (1/6). Laboratory results showed that all patients had high levels of C-reactive protein (CRP≥70 mg/L), procalcitonin (PCT; 2 patients with PCT levels ≥0.5 ng/L), and erythrocyte sedimentation rate (ESR). Lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) levels were elevated in 3/6 and of 2/6 patients, respectively. Chest computed tomography (CT) of most patients showed patchy, high-density shadows with partial consolidation, accompanied by air bronchogram signs and pleural effusion. Six patients were diagnosed with C. psittaci pneumonia using metagenomic next-generation sequencing (mNGS). They showed favorable outcomes following immediate adjustment of the regimen to doxycycline-based therapy and hydration, nutrition, and other follow-up treatments. In the imaging findings obtained at one-two month, the lesions were completely cleared, suggesting a favorable prognosis.
Conclusion: Patients with C. psittaci pneumonia commonly present sepsis and rapidly progressing disease. Early diagnosis is critical for C. psittaci pneumonia using mNGS, which can lead to favorable prognoses via immediate adjustment therapies.
1 Introduction
C. psittaci pneumonia, whose causative agent is the Gram-negative and obligate intracellular bacterium C. psittaci (Kong et al., 2021), is commonly transmitted via close contact with infected birds (such as parrots and poultry) or inhalation of aerosols from nasal secretions and dust from feces or feathers (Chen et al., 2022). C. psittaci pneumonia cases constitute approximately 1% of all community-acquired pneumonia (CAP) cases (Zhang A. et al., 2022). Owing to the non-specificity of its clinical manifestations, early diagnosis is difficult, and a rapid progression of the disease is observed in patients (Zhao et al., 2022). Additionally, antibiotics can promote the development of a severe and life-threatening disease. With the development and wide applicability of metagenomic next-generation sequencing (mNGS), the pathogenesis underlying C. psittaci pneumonia has been gradually elucidated (Teng et al., 2021). To better understand the clinical characteristics of patients with C. psittaci pneumonia, this study analyzed the clinical data of six patients who were admitted to the Second Hospital of Jiaxing in the last ten months.
2 Subjects and methods
2.1 Subjects
We identified six patients with pneumonia who were hospitalized at the Second Hospital of Jiaxing from December 2021 to September 2022 and showed positive mNGS results for C. psittaci. Demographic characteristics, including age, sex, occupational history, and history of bird exposure, were collected. Clinical manifestations, signs (maximum temperature and clinical symptoms), and laboratory and imaging findings were also recorded. This study was approved by the ethical committee of the Second Hospital of Jiaxing (approval number JXEY-2022SW067) and was in accordance with the ethical standards formulated in the Declaration of Helsinki. All patients whose clinical data were collected provided written informed consent and signed informed consent forms.
2.2 Diagnostic criteria
The diagnosis was confirmed via bronchoalveolar lavage fluid (BALF) samples collected from all patients based on the presence of C. psittaci sequences determined by mNGS.
2.3 Laboratory and imaging studies
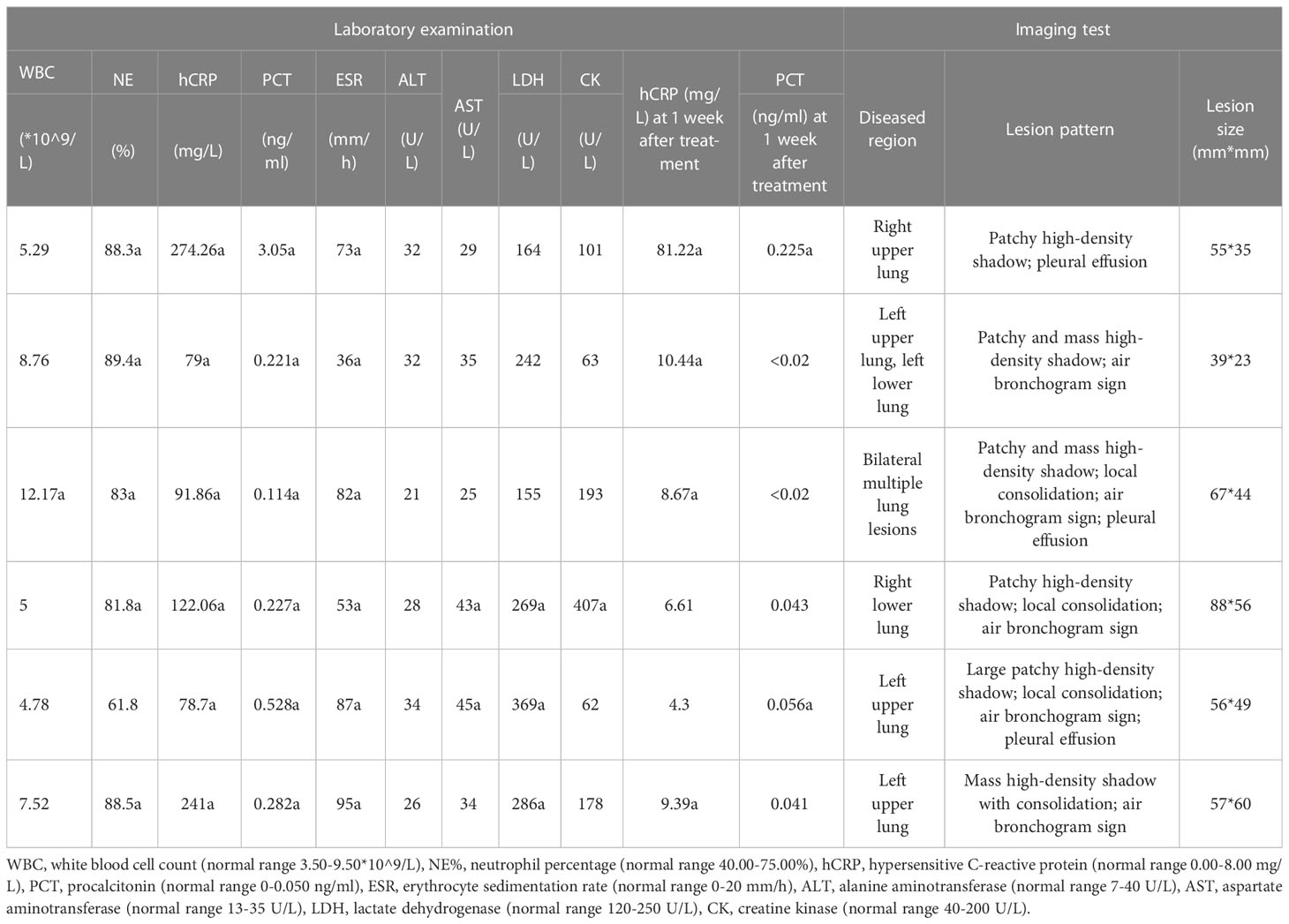
White blood cell (WBC) count, neutrophil percentage, hypersensitive C-reactive protein (hCRP) and procalcitonin (PCT) levels, erythrocyte sedimentation rate (ESR) along with the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase (CK) were recorded on the day of admission.
All patients underwent lung computed tomography (CT) prior to admission. The distribution, number, and type of lesions were described separately. The CT images were analyzed and characterized based on the following imaging features: ground-glass opacity, consolidation shadow, air bronchogram sign, reversed halo sign, centrilobular nodules, vacuole sign, lymphadenectasis, and pleural effusion.
BALF samples were obtained for smear and culture analysis. Samples were also subjected to sequencing by mNGS technology, which was performed via BGI2000 (More BioMedical, Hangzhou).
2.4 Treatment and outcomes
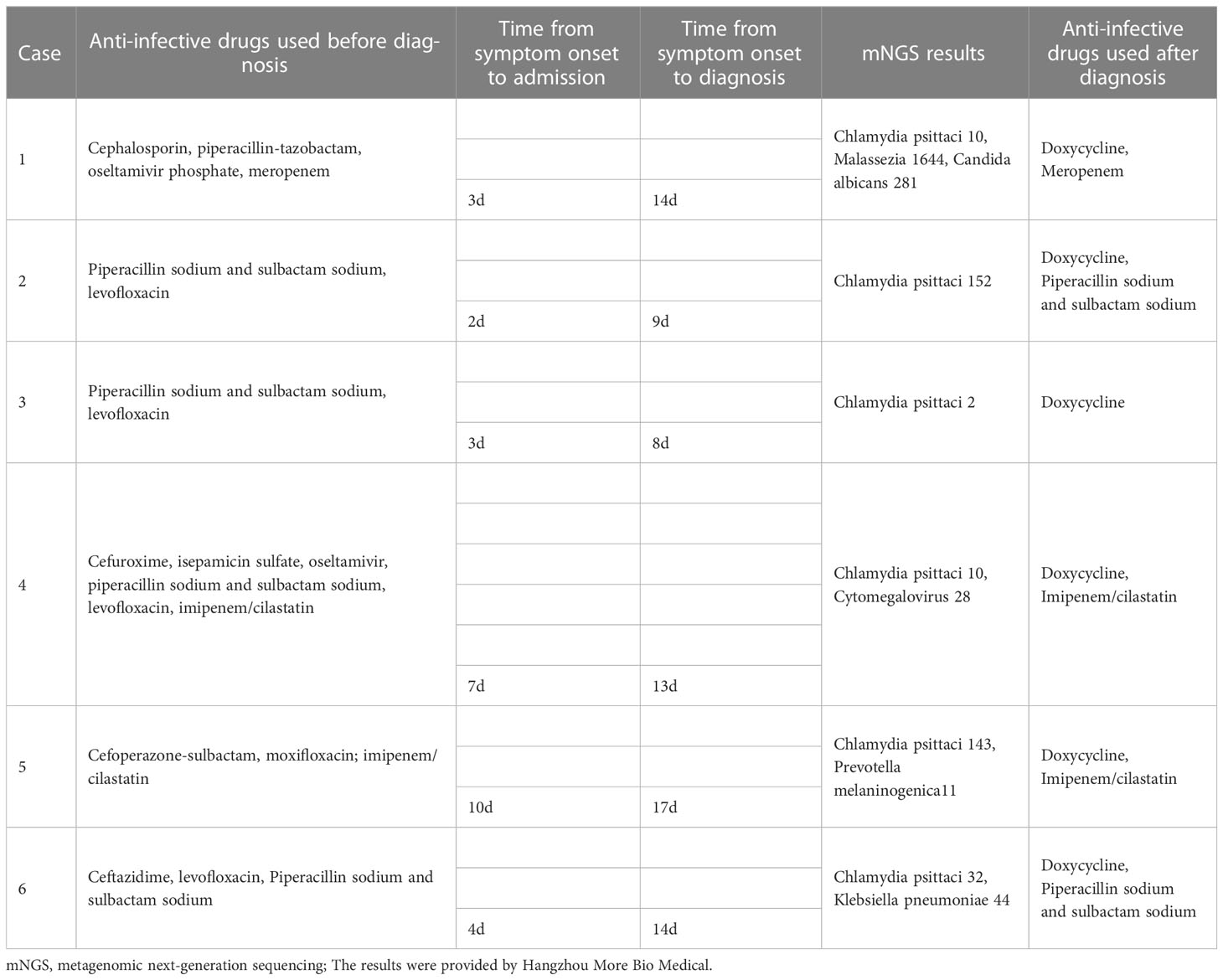
The types of antibiotics were recorded using a BGISEQ-2000 sequencing platform before and after the diagnosis of C. psittaci pneumonia. Body temperatures were recorded after administration. The interval from symptom onset to admission and that from symptom onset to diagnosis was recorded.
3 Results
3.1 General data of patients
The six patients enrolled in this study comprised four males and two females, with a median age of 65.5 years (range, 51–69 years). No patient reported a history of respiratory disease. Four patients had a history of combined hypertension, one had a history of hepatitis A, one had a history of gout, and two patients were immunocompromised (one with carcinoma in situ of the colon and one with thymoma). Four patients demonstrated a clear history of exposure to birds (Table 1).
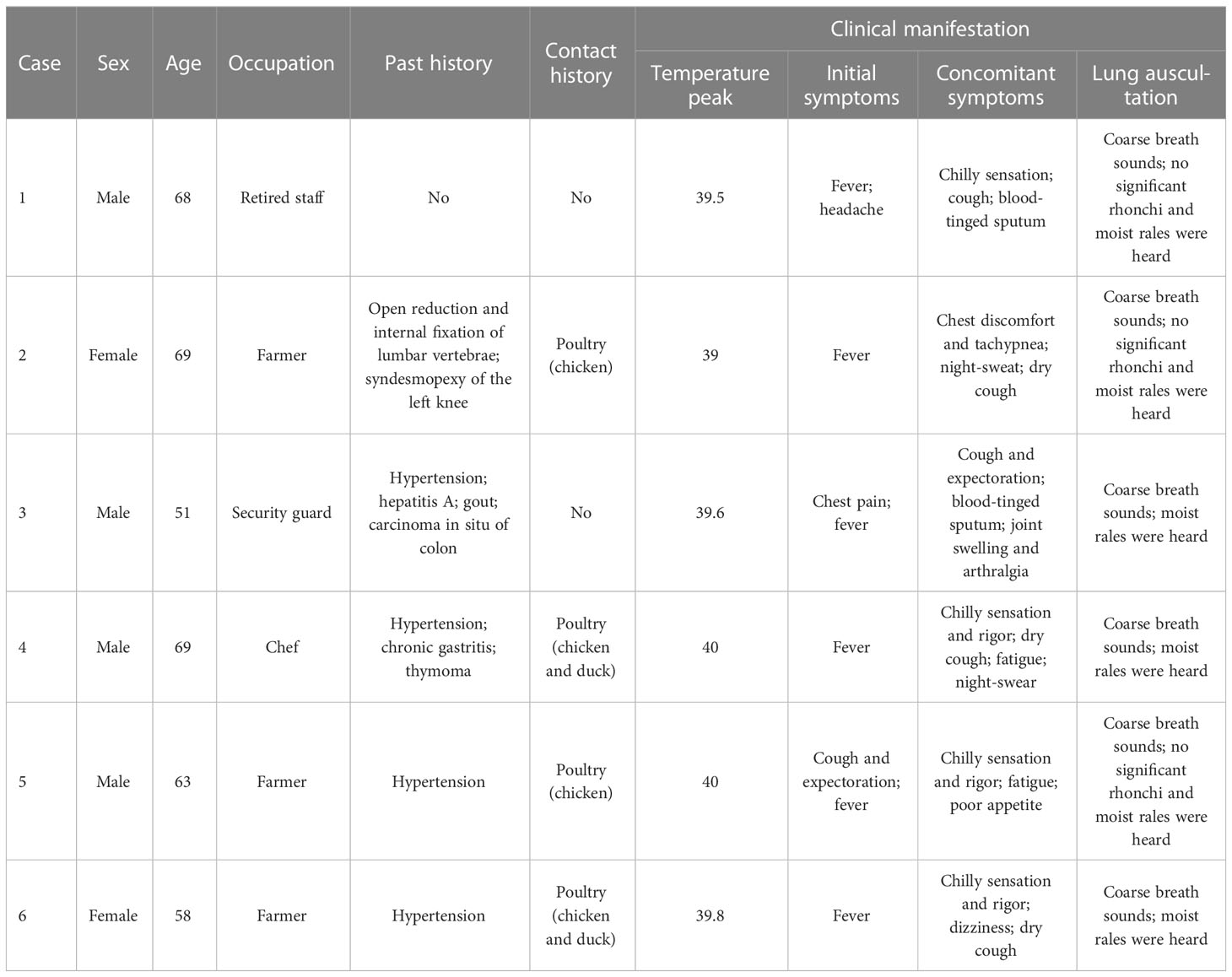
Table 1 Baseline characteristics and clinical manifestations of patients with Chlamydia psittaci pneumonia.
3.2 Clinical manifestations
All six patients developed fever, with a maximum body temperature of 39.0-40.0 °C reported in four patients and ≥40.0 °C observed in two patients. Five patients reported the development of cough, including dry cough (3/6) and blood-tinged sputum (2/6). Accompanying symptoms included headache (1/6), chilly sensation (4/6), rigor (3/6), night sweats (2/6), chest discomfort and tachypnea (1/6), chest pain (1/6), fatigue (2/6), poor appetite (1/6), and joint swelling and arthralgia (1/6). All patients reported the absence of nasal obstruction, pharyngodynia, muscular soreness, abdominal pain, diarrhea, nausea, or vomiting. Moist rales were heard in three of the six patients, without signs of combined cyanosis, barrel chest, wheezing phlegm, wheezing rale, or pleural friction rubs (Table 1).
3.3 Laboratory indices
Five out of six patients showed an elevated WBC count and elevated neutrophil percentage. The levels of CRP, PCT, and ESR were increased in all patients and were recorded in the ranges of 78.70-274.26 mg/L, 0.114-3.050 ng/ml, and 36-95 mm/h, respectively, during initial consultations. ALT levels in all six patients were within the normal range; an elevated AST level was present in 2/6 patients. Elevated LDH levels were recorded in three cases in the range of 269-369 U/L at initial consultations. An elevated CK level was present in 1/6 patients; the other patients showed levels within the normal range. After one week of empirical anti-infective therapy, a substantial decrease in CRP and PCT levels was observed in all cases. CRP levels and PCT levels were reduced to those within the normal range in 2/6 patients and 4/6 patients, respectively (Table 2).
3.4 Imaging findings
All patients underwent chest CT examinations upon admission. All lung lesions were extensive, with a maximum diameter of 39-88<nbsp/>mm and median diameter of 56.5<nbsp/>mm. Five out of six patients showed the lesions involving unilateral lung, and 1/6 patients had lesions with bilateral lung involvement. The lesions showed patchy high-density exudation shadows with local consolidation, accompanied by air bronchogram signs (5/6) and pleural effusion (3/6) (Table 2 and Figure 1).
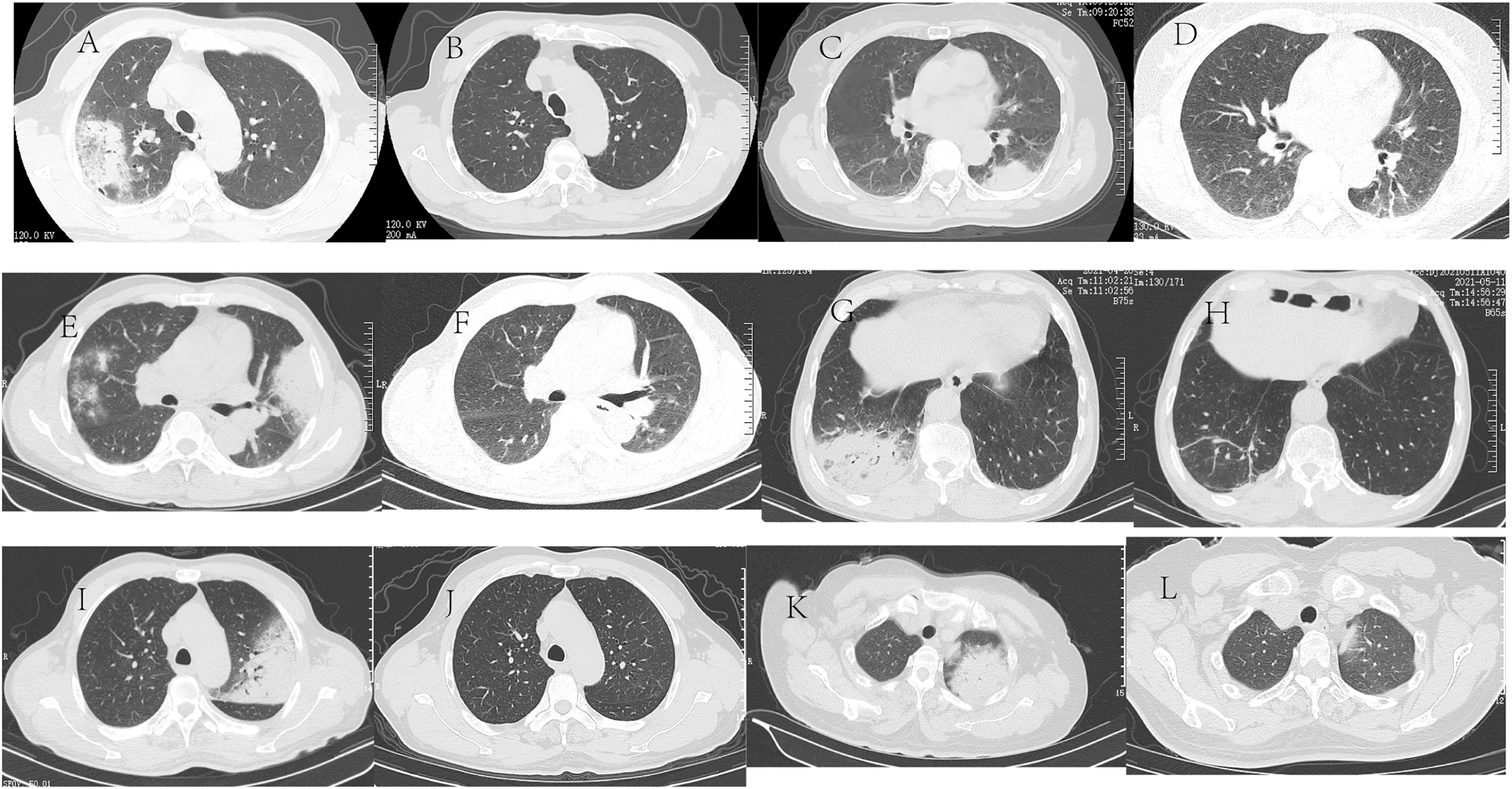
Figure 1 Chest CT of six patients with C psittaci pneumonia Chest CT of six patients with C psittaci pneumonia. Case 1: A 68-year-old male was admitted because of a fever and headache for three days. (A) Chest CT shows a patchy high-density shadow subpleural region in the right upper lung, with a small amount of pleural effusion in the right pleural cavity. (B) Two months after discharge from the hospital, chest CT showed that the inflammation in the upper lobe of the right lung almost disappeared. Case 2: A 69-year-old female was admitted because of a fever for two days. (C) Chest CT shows patchy and mass high-density shadow in the upper and lower lobes of the left lung, with a sign of an air bronchogram. (D) Two months after discharge from the hospital, chest CT showed that the lesions in the upper and lower lobes of the left lung almost disappeared. Case 3, A 51-year-old male was admitted because of left-sided chest pain with a fever for three days. (E) Chest CT shows bilateral multiple patchy and mass high-density shadows, accompanied by local consolidation and air bronchogram sign with a small amount of bilateral pleural effusion. (F) One month after discharge from the hospital, chest CT showed that the inflammation in the bilateral lungs disappeared. Case 4: A 69-year-old male was admitted because of a fever for seven days. (G) Chest CT shows a patchy high-density shadow in the lower lobe of the right lung, with local consolidation and air bronchogram sign. (H) One month after discharge from the hospital, chest CT showed that the inflammation in the lower lobe of the right lung disappeared. Case 5: A 63-year-old male was admitted because of a cough and fever for ten days. (I) Chest CT shows a large patchy high-density shadow in the left upper lung, with local consolidation; A few consolidation shadows in the left lower lung and a small amount of fluid in the left pleural cavity. (J) Two months after discharge from the hospital, chest CT showed that the inflammation in the upper lobe of the left lung almost disappeared. Case 6<nbsp/>A 58-year-old female was admitted because of a fever for four days. (K) Chest CT shows a mass high-density shadow in the left upper lung, with consolidation and air bronchogram sign. (L) One month after discharge from the hospital, chest CT showed that the inflammation in the upper lobe of the left lung disappeared.
3.5 Pathogen examination
BALF samples from six patients with suspected C. psittaci pneumonia were obtained by bronchoscopy and sent to Hangzhou More BioMedical for mNGS to detect C. psittaci. C. psittaci with cytomegalovirus was detected in one case (Table 3).
3.6 Treatment and outcomes
All patients received empirical anti-infective therapy prior to a definitive diagnosis, with two-five antibiotics in succession. Two out of six patients were treated with concomitant oseltamivir antiviral therapy because viral pneumonia could not be excluded. After one week of anti-infective therapy, one patient continued to report fever, and five patients reported a reduction of body temperatures back to those within the normal range. However, all six patients did not show substantial improvement in non-specific symptoms, such as cough and expectoration. The time from symptom onset to diagnosis was 8-17 days among six patients, with a median duration of 13.5 days. After diagnosis, as the immediate adjustment of the regimen to doxycycline-based anti-infective therapy, all patients had favorable prognoses and were discharged. All patients underwent chest CT within 1-2 months after treatment, which suggested that the inflammatory lesions had almost disappeared (Table 3 and Figure 1).
4 Discussion
C. psittaci pneumonia is caused by infection with the pathogen C. psittaci, an obligate intracellular gram-negative bacterium that can survive in vitro (Guedel et al., 1990). Humans can be infected via inhalation of aerosols contaminated with urine, feces, or other excreta from infected birds. Human-to-human transmission is possible but rare. Other potential transmission routes, such as contact with flocks of city pigeons, have been reported but are uncommon (Bourne et al., 2003). In this study, four patients had a clear history of bird exposure, which supports the hypothesis of earlier studies that over 50% of patients with C. psittaci pneumonia have a history of bird exposure. The findings highlight the importance of a detailed consultation to identify the disease as early as possible, thereby providing effective treatment.
In this study, two of the cases were immunocompromised and the remaining four were immunocompetent, without substantial specificity in the signs and symptoms of all patients, suggesting no apparent association between the presence or absence of an immunocompromised state and the severity of clinical manifestations. Our data provide further evidence in support of previous reports that C. psittaci is not a common infection in immunocompromised populations, and hence, does not pose a significant threat to these individuals (Fang et al., 2021).
The incubation period for C. psittaci pneumonia is approximately 5-14 days. Its clinical symptoms are diverse and vary in severity, ranging from mild influenza-like symptoms to systemic symptoms dominated by atypical pneumonia (Fang et al., 2021). In this study, all patients presented with hyperpyrexia (temperature ≥39.0°C), and other symptoms such as cough and expectoration were non-specific. No influenza-like symptoms, such as nasal obstruction and discharge, or myalgia were observed, and lung auscultation lacked specificity. One patient presented with joint swelling, arthralgia, and a history of gout. According to previous reports, C. psittaci infections act as a trigger for the development of acute gout in approximately 4% of patients (Su et al., 2021), and in this case, C. psittaci infection was considered to cause gout.
In this study, the admission hCRP and ESR levels were substantially elevated in all patients, and the admission PCT level was slightly elevated in general. After one week of empirical anti-infective therapy, hCRP, ESR, and PCT levels were significantly reduced in all patients, but no significant improvement was observed in clinical symptoms and imaging manifestations. Instead, an exacerbation of the disease was observed. Thereafter, two possibilities were considered: i. hCRP, ESR, and PCT had little diagnostic value for C. psittaci, and ii. There was a possibility of co-infection with other pathogens.
Three patients were admitted with a short onset, mostly 2-3 days; however, all patients showed extensive pulmonary lesions. Middle-aged or elderly patients with underlying diseases were at risk of severe pneumonia because of rapid disease progression. The progression of C. psittaci pneumonia may be associated with old age, underlying diseases (especially cardiopulmonary disease), smoking, nutrition, and timely diagnosis and treatment (Fang et al., 2021). Imaging findings illustrated that pulmonary lesions in six cases mainly involved the unilateral lung. All chest CT scans showed patchy high-density exudation shadows with local consolidation and air bronchogram signs, mostly accompanied by pleural effusion, which is in accordance with previous findings. After treatment, the disappearance of pulmonary lesions was observed in the absence of residual fibrous cord-like exudation, suggesting a good outcome.
With regard to the pathogenic examination, sputum cultures were negative in all patients. PCR is the most specific and rapid method to genotype positive strains and is of paramount importance to identify the source of infection; however, sensitivity is observed only during the acute phase of infection and is rarely applied in clinical practice (Nieuwenhuizen et al., 2018; Lei et al., 2020; Zhang et al., 2021). C. psittaci infections are often overlooked, resulting in a lack of timely diagnoses. Owing to the deficiencies of traditional methods, mNGS is an increasingly popular alternative for clinical diagnosis (Nieuwenhuizen et al., 2018; Lei et al., 2020; Zhang et al., 2021). Unknown infection sources can be detected by mNGS, and the relative abundance of pathogens can be quantified. It has the potential to assist doctors in the diagnosis and treatment of mixed infections. In this study, C. psittaci was detected in the BALF samples collected from all patients, with unique sequence/reads <<nbsp/>15 in three cases, as tested by the mNGS report. Two reasons were considered, which are outlined as follows: C. psittaci is an intracellular pathogen, and multiple antibiotic treatments had been administered before BALF samples were sent for examination. Cytomegalovirus was also detected by mNGS in one patient with a history of underlying thymoma. It causes latent infection, and the retained virus can be activated in immunocompromised patients (Zhang Z. et al., 2022).
The mNGS results alone are insufficient to underpin the final diagnosis, which can be confirmed by a combination of medical history, clinical symptoms, laboratory examination, and imaging findings. Clinical symptoms, laboratory findings, and imaging findings of the patients lacked specificity. Routine culture and serological tests yielded no positive results. After one course of empirical anti-infective therapy, imaging findings revealed unsatisfactory disappearance of lesions, and BALF was then subjected to mNGS to identify the pathogen. However, the late intervention of mNGS led to a delayed diagnosis, with a time from symptom onset to diagnosis of approximately two weeks. In recent years, retaining respiratory specimens for mNGS testing has been highly recommended for patients with respiratory infections if the pathogen is not clearly defined by routine laboratory tests within three days, and empirical anti-infective therapy is ineffective (Li et al., 2022).
With the identification of noninfectious lesions, a variety of antibiotics were administered prior to diagnosis. However, these were ineffective due to the low sensitivity of C. psittaci to these antibiotics and large lesions. All patients were administered antibiotics that were not sufficiently effective. In line with the results of recommended antimicrobial therapies, all patients took a favorable turn following the adjustment to a doxycycline-based anti-infective regimen administered for 10-14 days after diagnosis (Shen et al., 2021). Doxycycline or minocycline is preferred for the treatment of C. psittaci pneumonia, followed by azithromycin, clarithromycin, erythromycin, and chloramphenicol, and the antibiotics are administered for a minimum of ten days (Wen et al., 2021).
In conclusion, notwithstanding the complexity, variety, and non-specificity of clinical manifestations in the context of C. psittaci pneumonia, certain general clinical characteristics observed in patients are listed as follows: a history of bird exposure; hyperpyrexia with a temperature ≥39.0°C, accompanied by cough; normal WBC count, with elevated neutrophil percentage and mildly elevated hCRP and PCT levels; imaging findings of lesions mostly showing patchy high-density exudation shadows with local consolidation; difficulties in routine pathogen examination and culture, with the confirmation of diagnosis by mNGS; sensitivity to tetracyclines (doxycycline); complete disappearance of the lesion; and good outcome.
Ethics statement
Written informed consent was obtained for the publication of this case report.
Author contributions
JD dealt with the case and drafted the manuscript. XuL, JM and XiL assisted collected case data and literature and carried out all the documentary and article work out. WM gave some constructive suggestions for this paper during the revision and production period. HW and JJ contributed to conception, design, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Health Bureau of Zhejiang Province (grant no. 2022PY026), and Science and Technology Bureau of Jiaxing (grant no. 2022AD30028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bourne, D., Beck, N., Summerton, C. B. (2003). Chlamydia psittaci pneumonia presenting as acute generalised peritonism. Emerg. Med. J. 20, 386–387. doi: 10.1136/emj.20.4.386
Chen, M., Zhang, M., Shi, M., Hu, X. (2022). Diagnosis and analysis of clinical characteristics of chlamydia psittaci pneumonia. Vector Borne Zoonotic Dis. 22, 499–504. doi: 10.1089/vbz.2022.0013
Fang, C., Xu, L., Lu, J., Xie, Y., Tan, H., Lin, J. (2021). Analysis of clinical features of 16 cases with chlamydia psittaci pneumonia. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 33, 1366–1369.
Guedel, S., Hilpert, F., Fouet, P., Toledano, D. (1990). Chlamydia psittaci pneumonia. diagnostic and therapeutic problems. Presse Med. 19, 1282–1283. doi: 10.3201/eid0204.960406
Kong, C. Y., Zhu, J., JJ, L., Xu, Z. H. (2021). Clinical characteristics of chlamydia psittaci pneumonia. Chin. Med. J. (Engl) 134, 353–355. doi: 10.1097/CM9.0000000000001313
Lei, G., Wei, L., Meng, R., Jing, L., Guo, Y., Jia, Y., et al. (2020). The application of metagenomic next-generation sequencing in diagnosing chlamydia psittaci pneumonia: A report of five cases. BMC Pulm Med. 20 (1), 65. doi: 10.1186/s12890-020-1098-x
Li, H., Hao, B., Wang, Y., Yu, D., Chen, Z., Du, D., et al. (2022). Metagenomic next-generation sequencing for the diagnosis of chlamydia psittaci pneumonia. Clin. Respir. J. 16, 513–521. doi: 10.1111/crj.13519
Nieuwenhuizen, A. A., Dijkstra, F., Notermans, D. W., Hoek, W. (2018). Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect. Dis. 18 (1), 442. doi: 10.1186/s12879-018-3317-0
Shen, L., Tian, X. J., Liang, R. Z., Cheng, Y., Kong, X. L., He, F., et al. (2021). Clinical and imaging features of chlamydia psittaci pneumonia: An analysis of 48 cases in China. Zhonghua Jie He He Hu Xi Za Zhi 44, 886–891. doi: 10.3760/cma.j.cn112147-20210127-00082
Su, S., Su, X., Zhou, L., Lin, P., Chen, J., Chen, C., et al. (2021). Severe chlamydia psittaci pneumonia: Clinical characteristics and risk factors. Ann. Palliat Med. 10, 8051–8060. doi: 10.21037/apm-21-1502
Teng, X. Q., Gong, W. C., TT, Q., GH, L., Qu, Q., Lu, Q., et al. (2021). Clinical analysis of metagenomic next-generation sequencing confirmed chlamydia psittaci pneumonia: A case series and literature review. Infect. Drug Resist. 14, 1481–1492. doi: 10.2147/IDR.S305790
Wen, W., Gu, L., Zhao, L. W., Chen, M. Y., Yang, W. Q., Liu, W., et al. (2021). [Diagnosis and treatment of chlamydia psittaci pneumonia: Experiences of 8 cases]. Zhonghua Jie He He Hu Xi Za Zhi 44, 531–536. doi: 10.3760/cma.j.cn112147-20210205-00097
Zhang, Q., Li, S., Zhou, W., Zheng, L., Ren, Y., Dong, L., et al. (2021). Application of metagenomic next-generation sequencing (mNGS) combined with rapid on-site cytological evaluation (ROSCE) for the diagnosis of chlamydia psittaci pneumonia. Int. J. Clin. Exp. Pathol. 14, 389–398.
Zhang, Z., Wang, P., Ma, C., Wang, J., Li, W., Quan, C., et al. (2022). Host inflammatory response is the major factor in the progression of chlamydia psittaci pneumonia. Front. Immunol. 13, 929213. doi: 10.3389/fimmu.2022.929213
Zhang, A., Xia, X., Yuan, X., Lv, Y., Liu, Y., Niu, H., et al. (2022). Clinical characteristics of 14 cases of severe chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing: A case series. Med. (Baltimore) 101, e29238. doi: 10.1097/MD.0000000000029238
Keywords: psittacosis, chlamydia, pneumonia, mNGS, doxycycline
Citation: Dai J, Lian X, Mo J, Li X, Mo W, Wang H and Jiang J (2023) Case report: A clinical case study of six patients with Chlamydia psittaci pneumonia. Front. Cell. Infect. Microbiol. 13:1084882. doi: 10.3389/fcimb.2023.1084882
Received: 31 October 2022; Accepted: 06 February 2023;
Published: 24 February 2023.
Edited by:
Joseph D. Lykins, Virginia Commonwealth University Health System, United StatesReviewed by:
Michael F. Minnick, University of Montana, United StatesTong Zhou, Second Affiliated Hospital of Soochow University, China
Copyright © 2023 Dai, Lian, Mo, Li, Mo, Wang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiqin Wang, d19oYWlxaW5AMTYzLmNvbQ==; Jianping Jiang, anh4ejA2MTNAMTYzLmNvbQ==
 Jinmeng Dai1,2
Jinmeng Dai1,2 Juanfen Mo
Juanfen Mo Xiaosi Li
Xiaosi Li Haiqin Wang
Haiqin Wang Jianping Jiang
Jianping Jiang