
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 25 January 2023
Sec. Virus and Host
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1082390
This article is part of the Research Topic Precision medicine in viral hepatitis: Progress and prospects towards elimination View all 9 articles
 Mengyao Yu1,2†
Mengyao Yu1,2† Lei Huang3†
Lei Huang3† Shichang Zhang1,2†
Shichang Zhang1,2† Longfeng Jiang4
Longfeng Jiang4 Yuexinzi Jin1,2
Yuexinzi Jin1,2 Min Gu1,2
Min Gu1,2 Jun Liao5*
Jun Liao5* Jiexin Zhang1,2*
Jiexin Zhang1,2*Introduction: Chronic viral hepatitis (CH) is a stage prior to cirrhosis and primary cancer. Standard protocols for CH assessment during the long follow-up period are of great importance for precise treatment and living quality improvement. In this study, we aimed to analyze multiple serum indexes in chronic hepatitis B (CHB)-infected patients and to discuss their combined values in clinical applications.
Methods: Total 503 lines of laboratory data from 2012 to 2021 were extracted from103 CHB patients who were followed-up in our hospital. They were divided into the remission group and the progression group according to their complete clinical information and laboratory data. A series of models of serum indexes were analyzed to illustrate the fluctuation trend of @ach index in a time-dependent manner.
Results: The models revealed that abundant serum alpha-fetoprotein (AFP) in the remission group was characteristically associated with hepatocyte destruction markers aspartate aminotransferase (AST) and alanine aminotransferase and favored a much longer progression-free period (P 0.0001). A model-derived equation consisting of serum AFP and AST values showed a good performance (83% reliability) to distinguish the two groups.
Discussion: This study clearly demonstrates the intrinsic quantitative relationship between serum AFP and liver aminotransferases involving antivirus treatment response. The model-based equation compensates for serum hepatitis B virus DNA detection during outpatient follow-up and it may serve as a useful laboratory tool for CHB progression assessment.
Viral hepatitis is a severe public health problem affecting hundreds of millions of people worldwide. Hepatotropic viruses mainly include hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV) and hepatitis E virus, among which HBV, HCV and HDV are the major causes of progressive hepatic fibrosis, liver failure, and hepatocellular carcinoma (HCC) (Buti et al., 2022). China has an over-weighted HBV infection burden, with an estimated 93 million carriers of hepatitis B surface antigen (HBsAg), of which 20~30 million have progressed to chronic viral hepatitis (CVH) (Cui and Jia, 2013; Wang et al., 2014; Liu et al., 2019). Approximately 10~15% of chronic hepatitis type B (CHB) patients are coinfected with HCV and 5% with HDV, which increases morbidity and mortality rates (Seto et al., 2018).
CHB is a chronic necroinflammatory disease of the liver caused by persistent HBV infection (Fung et al., 2022). The different stages of CHB can vary depending on the dynamic interactions between the virus and the liver microenvironment which consists of hepatic parenchymal cells, nonparenchymal cells and local immune cells. Some patients may experience lifelong quiescence, but others will develop more severe complications characterized by fluctuations in HBV DNA and alanine aminotransferase (ALT) levels (Gish et al., 2015). HBV provokes a persistent and chronic immune response to destroy hepatocytes followed by necrosis and fibrosis in situ. With the persistent high viremia as well as the expansion of inflammation and destruction back and forth, ignored or inappropriately treated CHB will progress to cirrhosis or HCC within years (Gines et al., 2021). Therefore, decision making on initiating therapy refers to the virological assessment and the primary goal is to eliminate or significantly suppress HBV replication which is often represented by serum HBV DNA level (Lok et al., 2016; Naggie and Lok, 2021). The first-line therapies worldwide are pegylated interferon alfa, entecavir (ETV), tenofovir disoproxil fumarate, and tenofovir alafenamide (Martin et al., 2022). Nucleotide/nucleoside lamivudine (LAM) was once a commonly used therapy in China because of its established long-term safety and low-cost despite of its comparatively higher risk of the emergence of therapy-resistant mutations (Sarin et al., 2016). In order to achieve a functional cure (HBsAg loss), leading professional associations, such as the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, and the Asian Pacific Association for the Study of the Liver, agree on the requirement for indefinite long-term or life-long therapy in the vast majority of patient (Block et al., 2021).
The Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh (CTP) score are two systems that are comprehensively used to evaluate the severity of liver cirrhosis and have achieved fruitful clinical results so far. However, there is still a lack of systematic data or evidence related to the establishment of CHB models, especially those that can be used to assess the real-time status of the remaining healthy hepatocytes during outpatient follow-up.
Alpha-fetoprotein (AFP) is derived from embryonic endoderm tissue cells and is a constitutional component of fetal circulation. The serum AFP level gradually decreases after birth to less than 10 ng/mL in adulthood, in which it is synthesized and secreted by liver stem/progenitor cells (LPCs) (Jiang et al., 2022). Overexpressed AFP is well known as an HCC biomarker. Nevertheless, some studies have focused on its indication in liver regeneration (Bloomer et al., 1977; Taketa, 1990; Chen et al., 2017; Jiao et al., 2021). Wang et al. demonstrated that a high serum AFP level (log10AFP≥ 2.04) can predict 180-day survival and better outcomes [decreased total bilirubin (TBIL) and international normalized ratio (INR)] in patients with hepatitis B-related acute-on-chronic liver failure (Wang et al., 2018). Their further study revealed that AFP is a candidate marker to be representative of active liver regeneration and it is also an independent prognostic factor in these patients (Wang et al., 2020).
In this study, we retrospectively collected the clinical and laboratory data of patients who had suffered from CHB for years to investigate the relationship of serum AFP and other serum indexes in model establishment and to evaluate their significance in CHB progression or regression.
We screened treatment-naïve CHB patients in our hospital and the enrolled CHB patients must had: (1) viremia (serum HBV DNA copy number ≥ 1 × 103 IU/mL) before antiviral drug (ETV or LAM) administration between 2012 to 2019; (2) virological response (serum HBV DNA copy number < 20 IU/mL) after antiviral therapy; (3) continuous administration of the same antiviral drug during outpatient follow-up; and (4) at least one time point of serum AFP, ALT, aspartate aminotransferase (AST), TBIL, direct bilirubin (DBIL), total protein (TP), albumin (ALB), and coagulation detection during viremia and at least one time point after virological response.
The exclusion criteria were as follows: (1) only one time point or no serum AFP, ALT, and AST values; (2) irregular antiviral drug administration; (3) lost to follow-up (including death); and those with (4) acute hepatitis; (5) alcoholic cirrhosis; (6) prior decompensation; (7) metabolic fatty liver; (8) hepatic cancer; (9) autoimmune hepatitis; (10) non-hepatitis B virus infection or HBV-combined hepatitis virus infection; and (11) other diseases, including cardiovascular disease, renal insufficiency, hypersplenism, and metabolic disorders.
Total 103 enrolled CHB patients were classified into two groups: the progression group and the remission group. The progression group contained 52 patients including 18 with CTP grade A/B. After virological response, they had acute hepatitis flare with recurrent viremia (increase of HBV DNA by > 1 × log10 IU/mL, n=17) or a re-elevated serum ALT level greater than 2 times the upper limit of normal (> 70 U/L for men and > 50 U/L for women, n=11), or they had persistent liver inflammation and dysfunction with higher levels of multiple serum indexes [e.g., ALT, AST, TBIL, DBIL, and international normalized ratio (INR), n=24] (Vittal and Ghany, 2019). The remission group contained 51 patients including 16 with CTP grade A/B. They showed persistently undetectable serum HBV DNA levels and normal serum indexes levels after virological response.
All follow-up laboratory test results were documented backwards and forwards from the day of initial viremia and were sorted by the detection date of serum indexes. Data collection stopped just before documented therapy switch or disease progressed in the progression group. Total 503 lines of data were acquired from 2012 to 2021 and were respectively analyzed by month intervals between the dates of follow-up test and initial viremia (Supplementary Figures 1, 2). This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2021-SR-570).
Scatter plots (ggplot2 package), quantile-quantile plots (car package), predicted plots of the generalized-additive-model (GAM) (mgcv package), predicted GAMs for serum indexes of log transformation with 90% bootstrap pointwise confidence intervals, and 2D partitions plot of the Quadratic-Discriminant-Analysis (QDA) (klaR package) were all established by R language (Version 4.1.2, R Core Team, Austria) with the original data.
By adjusting R2 and mean square error (MSE), we performed a stepwise selection and chose a model with best performance in total six nonlinear models (polynomial regression, step function, regression spline, smoothing regression, local regression, and GAM).
Interval validation was performed by bootstrap sampling. The GAM was fit on the bootstrap dataset for each of the 1000 bootstrap samples. The outcomes on the full dataset were predicted and the R2 and MSE from these predictions were calculated. Then, the mean R2 and MSE across bootstrap samples as well as standard errors were calculated to obtain the final confidence intervals.
Total six classification models were used to respectively predict the qualitative response variable, which were logistic regression, linear discriminant analysis, support vector classifier, support vector machine with linear kernel, support vector machine with radial kernel, and QDA. A model with the lowest test error was chosen for subsequent analysis.
Best subset selection was used to identify the best model for daily clinical practice. All combinations of the predictors were performed and were compared to fit model. Leave-One-Out-Cross-Validation (LOOCV) was used to calculate the test classification error of each model. A model with the smallest error was selected as the optimal model for subsequent analysis.
Data were analyzed using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA, https://www.graphpad.com/scientific-software/prism/). Descriptive analyses were conducted using the chi-square test for categorical variables and Student’s t-test for continuous variables. Group differences were examined using unpaired t tests for normally distributed variables or using Mann–Whitney U tests for nonnormally distributed data. The significance level was set at P<0.05.
A total of 103 middle-aged CHB patients were enrolled, of which 75.7% were male. According to the medical records after virological response, all patients were divided into the progression group and the remission group (see details in “Patient grouping” in Materials and methods). The progression group contained 234 lines of data from 52 patients and the remission group contained 269 lines of data from 51 patients. During initial viremia, a flare of hepatocyte destruction was indicated in the remission group with statistically higher serum ALT (P<0.0001) and AST (P=0.0002) levels (Table 1). In the meantime, the average serum AFP level was significantly higher in the remission group (median: 30.1 ng/mL) than in the progression group (median: 3.1 ng/mL) (P<0.0001; Table 1). Then, a GAM was established to observe the serum AFP fluctuation trend in each group based on the follow-up time. We found that the optimal fitting of time width by GAM analysis was 30 months (Figure 1). The remission group had a quick increase followed by a rapid decrease to the baseline, whereas the progression group barely exhibited an elevation (Figure 1). Moreover, the magnitudes of the two AFP curves were completely different.
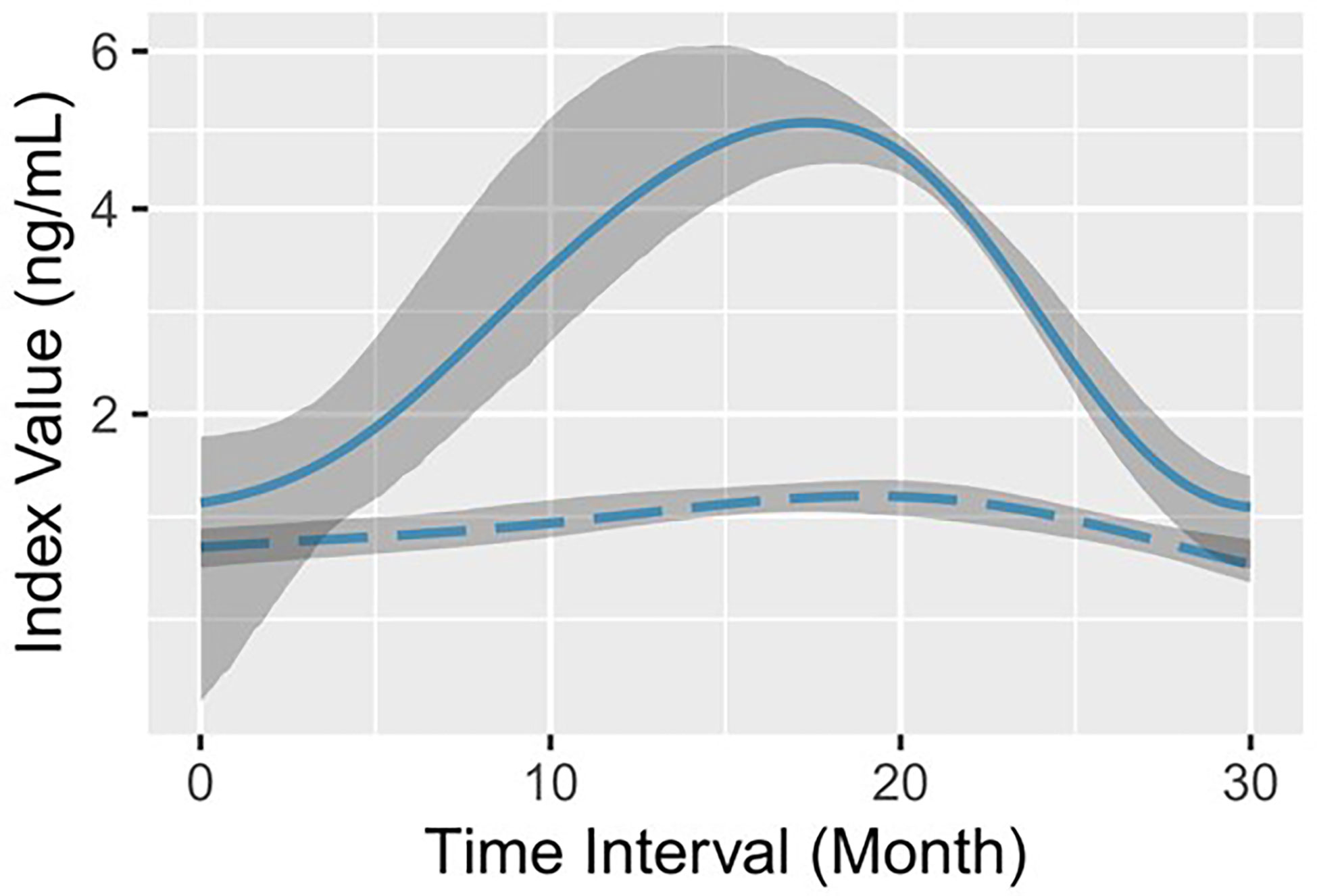
Figure 1 Log-predicted GAMs with 90% bootstrap pointwise confidence intervals for serum AFP. The solid line (representing remission) and the long-dashed line (representing progression) indicate the log-fitted GAM of serum AFP in the remission group and the progression group, respectively. The shadow area corresponds to the 90% bootstrap resampling pointwise confidence interval.
We calculated the time interval between the initial viremia and the subsequent rescue therapy for acute hepatitis flare within 30 months for each patient. The maximum interval was 900 days. The median progression-free period was 900 days in the remission group and 88.5 days in the progression group (HR: 0.5214, 95% CI: 0.3418 to 0.7954, P<0.0001; Figure 2A). Next, we divided each group of patients into general CHB and CTP grade A/B to respectively analyze their progression-free period. As shown in Figure 2B, the general CHB patients in the remission group had significantly longer progression-free period (846 days) than those in the progression group (86 days; HR: 0.5038, 95% CI: 0.2977 to 0.8525, P=0.001). A similar difference was also confirmed between the two CTP grade A/B groups (900 days vs. 149 days; HR: 0.5484, 95% CI: 0.2689 to 1.118, P=0.0261; Figure 2C).
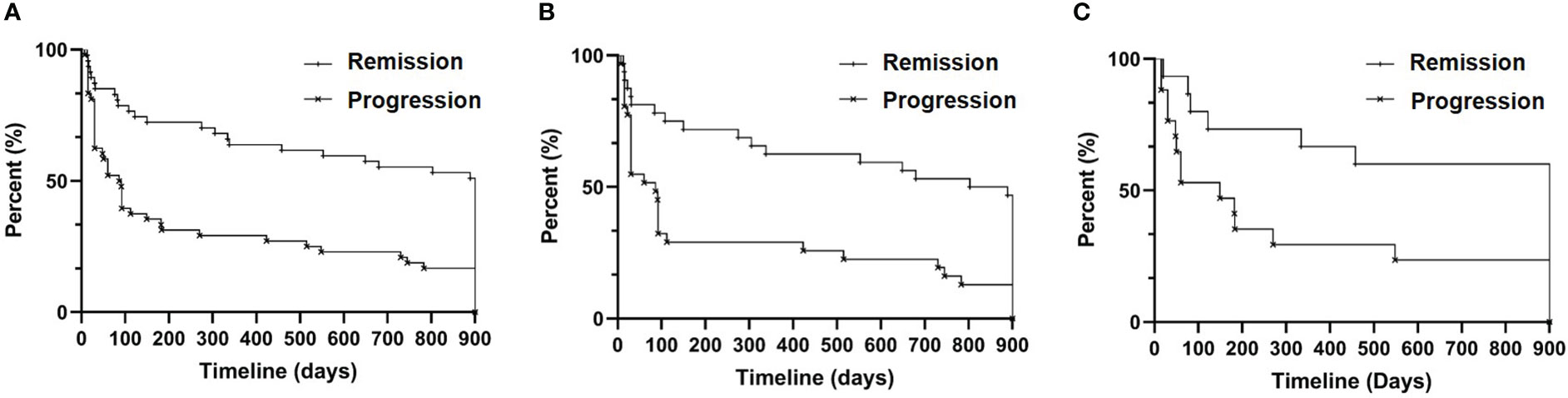
Figure 2 Progression-free survival analysis. (A) All patients (n=95, eight patients without documented drug dosage were excluded); (B) CHB patients (n=63, six patients without documented drug dosage were excluded); and (C) CTP grade A/B patients (n=32, two patients without documented drug names were excluded).
Next, we combined the hepatocyte markers ALT and AST with AFP to explore whether they interact by GAM analysis. Interestingly, they presented an exquisite coherent relationship in the remission group (Figure 3A). The results showed that 1) the wave crest of ALT emerged ahead of that of AST; 2) in response to increased serum ALT and AST concentrations, AFP secretion was upregulated; 3) serum ALT and AST levels were then alleviated; and 4) the serum AFP concentration gradually decreased as liver function was restored. Overall, the dynamic changes in serum ALT, AST and AFP exhibited a nested bell-shaped diagram during the 30-month. By contrast, there was no obvious pattern in the progression group (Figures 3B, C). Spearman analysis confirmed the strong correlation between serum AFP and AST (r=0.4787, P<0.0001) or ALT (r=0.4425, P<0.0001) in the remission group (Supplementary Figure 3).
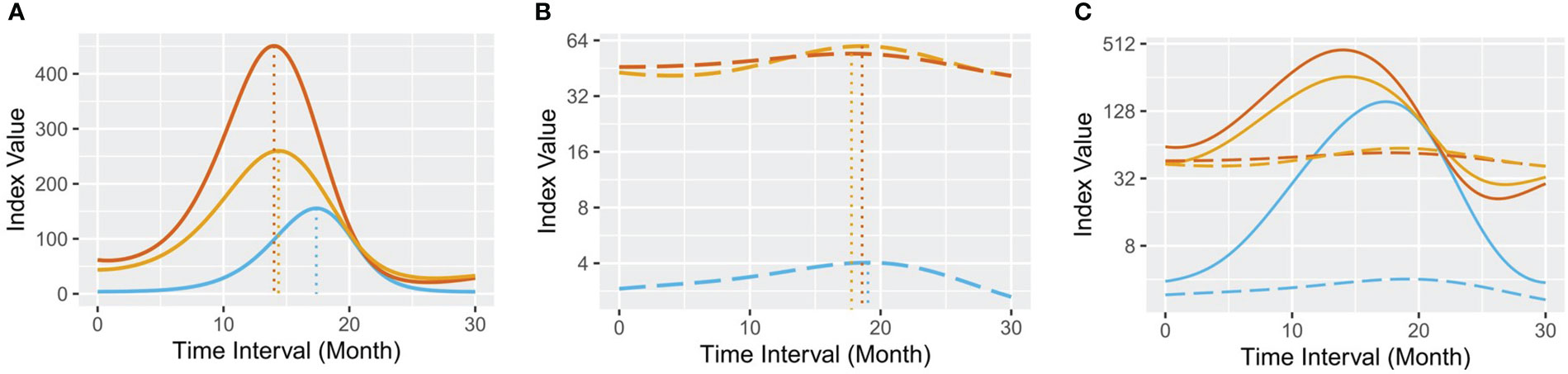
Figure 3 Predicted GAM plots for serum AFP, ALT and AST. (A) the remission group; (B) the progression group; and (C) all samples. In each plot, the blue line represents the GAM of serum AFP, the red line represents the GAM of serum ALT, and the orange line represents the GAM of serum AST. The full lines represent the remission group, and the long-dashed lines represent the progression group.
Next, we used QDA as the best-fit classification model to distinguish the two group patients. As a result, a total of fifteen classification models were generated (Table 2), and one model subset [log(AFP) + log(AST)] had the smallest classification error whose area under curve was 0.815 with sensitivity of 0.9283, specificity of 0.7018, and accuracy of 0.8235 (Supplementary Figure 4). Then, we generated an accessible equation (Equation 1) that can classify CHB patients into the remission group (rm) or the progression group (pr) based on serum AFP and AST values. According to the larger output value (Equation 2 into Equation 1 vs. Equation 3 into Equation 1), a result with approximately 83% reliability was expected.
We reviewed studies published in the last twenty years on clinical laboratory indexes that can reflect hepatocyte death or dysfunction. It seems that their optimistic effects on reducing mortality or complication incidence in CVH patients did not meet requirements. Although AFP is of great importance in damage repair during CVH, there is still a lack of documentation on how to use it other than as a tumor exclusion indicator to evaluate the capability of hepatocyte regeneration (Notarpaolo et al., 2017).
Both the CTP and MELD scores are used to determine ongoing cirrhosis (Su et al., 2019; Jepsen et al., 2020). The CTP score incorporates three common laboratory indicators (prothrombin time, serum albumin, and bilirubin) as well as clinical indicators (ascites, hepatic encephalopathy and nutritional status) (Su et al., 2019). Bias may arise due to subjective reasoning among clinicians and the therapeutic impact on exogenous serum albumin (Kamath et al., 2001). The MELD score is based on three variables: serum creatinine (CREA), TBIL and the INR. While CREA indicates muscle cell metabolism and is a sensitive index for renal function evaluations (Mindikoglu and Pappas, 2018), bilirubin is the main metabolite of iron porphyrin compounds in senescent red blood cells and reflects the excretion function of the liver (Sullivan and Rockey, 2017). Impeded hepatic cell function also reduces prothrombin production, leading to an increase in the INR (Garg et al., 2012). Other non-disease factors, an insufficient blood volume and excessive diuretic usage contribute to the cumulative error in the final scores. Therefore, all of these indexes are indirect evidence of hepatocyte death, and both of the scoring systems ignore the transition process of the remaining cells (such as regenerating cells) during the lifelong course of CVH.
Based on a comprehensive analysis of the medical records and test results of 103 patients, we found that the remission group had abundant serum AFP levels (Figure 1) and distinguishable longer progression-free period (Figure 2). For a long time, AFP was the most widely used serum marker for the diagnosis of HCC, and its increase indicates a high probability of HCC (the diagnostic criterion is > 20 ng/mL) (Trevisani et al., 2001). Nevertheless, the serum AFP concentration here showed a bell-shaped curve rather than a curve indicating a consistently high level. Our data also indicated that serum AFP has a strong concomitant response to hepatocyte death, characterized by a lagging fluctuation after serum ALT/AST changes in the remission group (Figure 3A). This is fundamentally different from that observed in HCC.
LPCs are necessary for liver regeneration. When under attack (such as a virus, drug, or alcohol), LPCs re-express AFP for tissue self-repair and proliferation (Peterson et al., 2011; Kakisaka et al., 2015). Frank’s team measured serum AFP levels on admission (median: 8.1 ng/mL) and on day 3 (median: 17.6 ng/mL) in 162 patients suffering from acute liver failure, and the result showed that AFP upregulation within day 3 may indicate a better prognosis (Schiodt et al., 2006). Schmidt et al. obtained serial serum AFP values at peak in 239 patients with severe acetaminophen-induced liver injury and found that the serum AFP levels in the survivors were significantly higher (from 4.2 ± 3.1 ng/mL to 68.6 ± 77.9 ng/mL) than those in the deceased starting on the day of peak ALT (Schmidt and Dalhoff, 2005). They proposed a combined analysis of AFP and the INR to provide additional prognostic information. From our point of view, a self-limited fluctuation of serum AFP concentration (possibly caused by viral replication and hepatocyte destruction) indicates a longer progression-free period in CHB patients.
Both episodic hepatitis flares and intermittent ALT elevations may occur and repeat during anti-HBV therapy, which more frequently leads to the development of cirrhosis, hepatic decompensation, failure, or even death (Liaw et al., 1988; Chang and Liaw, 2014). Based on this, all recruited patients in this study were divided into two groups. We simultaneously analyzed two more serum indexes, AST and AFP, in CHB patients with adequate liver reserve and highlighted the specific fluctuation pattern of each index in a time-dependent manner before and after effective antiviral therapy. We found that three serum indexes presented an exquisite a nested bell-shaped diagram in the remission group (Figure 3A). Jeng et al., reported a phenomenon, called “host-dominating flare” or “beneficial flare”, of greater AFP and ALT fluctuation on HBV DNA fall and rapid HBsAg decline (Jeng et al., 2016; Liaw, 2021). Unlike indexes used in CTP or MELD score system, serum AST and ALT elevation directly reflects aggravated hepatocyte destruction. The hepatocyte regeneration marker AFP whose serum concentration is more critical regarding prognosis was also integrated in our models. The model-derived equation (Equ 1) quantifies the relationship between serum AFP and AST and it is convenient for laboratory application. The output of the equation indicates the real-time status of hepatocytolysis and regeneration and help to assess CHB progression in a cost-effectiveness way along with serum HBV DNA measurement during outpatient follow-up. Studies suggested that host-dominating flare is not associated antiviral therapy per se (Chang and Liaw, 2022). Therefore, in-depth research of patients in the remission group may identify the trigger and involved immune responses. It has been proven that fibrosis and early cirrhosis are reversible (Bedossa, 2015). It is also important to recognize patients in the progression group by Equ 1 and to apply other intervention protocols in a timely manner to improve progression-free period.
Hepatitis B e antigen (HBeAg)-negative CHB patients were not included in this study. They are characterized by impaired HBeAg expression, lower serum HBV DNA levels, more frequent hepatitis flares, and progressive liver damage (Marcellin et al., 2004). Antiviral treatment strategy is primarily urged according to the recommended cut-offs of indications (serum HBV DNA and ALT) for HBeAg-negative CHB, which is quite different from that for HBeAg-positive CHB (Sarin et al., 2016). Since ETV was reported to better improve virological response and liver inflammation than LAM in treatment-naïve HBeAg-negative CHB patients after a 48-week course of treatment (Lai et al., 2006), it is interesting to explore the change patterns of serum indexes in these patients during nucleos(t)ide analogues therapy.
Serum index detection is more acceptable than other invasive examinations for CHB patients during the long course of treatment (usually a lifetime). By establishing connections among the virus, patients and clinicians, this equation provides alternative access to follow-up management from which CHB patients will benefit the most.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital with Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
JZ concepted and designed the research. LH, YJ, and MG collected, analyzed, or interpreted the data. JL performed statistical analysis. MY wrote the manuscript. SZ and LJ provided critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by the “The Six Top Talent Project” of Jiangsu Province (no. WSW-004) and the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (ZDXKB2016005).
We thank Prof. Yali Weng from the Department of Infectious Diseases in our hospital for sharing clinic knowledge and intellectual content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1082390/full#supplementary-material
Bedossa, P. (2015). Reversibility of hepatitis b virus cirrhosis after therapy: Who and why? Liver. Int. 35 Suppl 1, 78–81. doi: 10.1111/liv.12710
Block, T. M., Chang, K. M., Guo, J. T. (2021). Prospects for the global elimination of hepatitis b. Annu. Rev. Virol. 8, 437–458. doi: 10.1146/annurev-virology-091919-062728
Bloomer, J. R., Waldmann, T. A., McIntire, K. R., Klatskin, G. (1977). Serum alpha-fetoprotein in patients with massive hepatic necrosis. Gastroenterology. 72 (3), 479–492.
Buti, M., Craxi, A., Foster, G. R., Maticic, M., Negro, F., Zeuzem, S., et al. (2022). Viral hepatitis elimination: Towards a hepatitis-free world. J. Hepatol. 77 (5), 1444–1447. doi: 10.1016/j.jhep.2022.06.034
Chang, M. L., Liaw, Y. F. (2014). Hepatitis b flares in chronic hepatitis b: Pathogenesis, natural course, and management. J. Hepatol. 61, 1407–1417. doi: 10.1016/j.jhep.2014.08.033
Chang, M. L., Liaw, Y. F. (2022). Hepatitis b flare in hepatitis b e antigen-negative patients: A complicated cascade of innate and adaptive immune responses. Int. J. Mol. Sci. 23, 1552. doi: 10.3390/ijms23031552
Chen, S., Chen, H., Gao, S., Qiu, S., Zhou, H., Yu, M., et al. (2017). Differential expression of plasma microRNA-125b in hepatitis b virus-related liver diseases and diagnostic potential for hepatitis b virus-induced hepatocellular carcinoma. Hepatol. Res. 47 (4), 312–320. doi: 10.1111/hepr.12739
Cui, Y., Jia, J. (2013). Update on epidemiology of hepatitis b and c in China. J. Gastroenterol. Hepatol. 28 Suppl 1, 7–10. doi: 10.1111/jgh.12220
Fung, S., Choi, H. S. J., Gehring, A., Janssen, H. L. A. (2022). Getting to HBV cure: The promising paths forward. Hepatology. 76, 233–250. doi: 10.1002/hep.32314
Garg, H., Kumar, A., Garg, V., Sharma, P., Sharma, B. C., Sarin, S. K. (2012). Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig. Liver. Dis. 44 (2), 166–171. doi: 10.1016/j.dld.2011.08.029
Gines, P., Krag, A., Abraldes, J. G., Sola, E., Fabrellas, N., Kamath, P. S. (2021). Liver cirrhosis. Lancet. 398 (10308), 1359–1376. doi: 10.1016/S0140-6736(21)01374-X
Gish, R. G., Given, B. D., Lai, C. L., Locarnini, S. A., Lau, J. Y., Lewis, D., et al. (2015). Chronic hepatitis b: virology, natural history, current management and a glimpse at future opportunities. Antivir. Res. 121, 47–58. doi: 10.1016/j.antiviral.2015.06.008
Jeng, W. J., Chen, Y. C., Chang, M. L., Liaw, Y. F. (2016). α-fetoprotein level-dependent early hepatitis b surface antigen decline during entecavir therapy in chronic hepatitis b with hepatitis flare. J. Antimicrob. Chemother. 71, 1601–1608. doi: 10.1093/jac/dkw019
Jepsen, P., Watson, H., Macdonald, ,. S., Vilstrup, H., Jalan, R. (2020). MELD remains the best predictor of mortality in outpatients with cirrhosis and severe ascites. Aliment. Pharmacol. Ther. 52 (3), 492–499. doi: 10.1111/apt.15882
Jiang, L., Wang, X., Ma, F., Wang, X., Shi, M., Yan, Q., et al. (2022). PITX2C increases the stemness features of hepatocellular carcinoma cells by up-regulating key developmental factors in liver progenitor. J. Exp. Clin. Cancer. Res. 41 (1), 211. doi: 10.1186/s13046-022-02424-z
Jiao, Y., Lu, W., Xu, P., Shi, H., Chen, D., Chen, Y., et al. (2021). Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure. Hepatol. Int. 15 (4), 957–969. doi: 10.1007/s12072-021-10217-3
Kakisaka, K., Kataoka, K., Onodera, M., Suzuki, A., Endo, K., Tatemichi, Y., et al. (2015). Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatol. Res. 45 (10), E12–E20. doi: 10.1111/hepr.12448
Kamath, P. S., Wiesner, R. H., Malinchoc, M., Kremers, W., Therneau, T. M., Kosberg, C. L., et al. (2001). A model to predict survival in patients with end-stage liver disease. Hepatology. 33 (2), 464–470. doi: 10.1053/jhep.2001.22172
Lai, C. L., Shouval, D., Lok, A. S., Chang, T. T., Cheinquer, H., Goodman, Z., et al. (2006). Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis b. N. Engl. J. Med. 354, 1011–1020. doi: 10.1056/NEJMoa051287
Lanini, S., Ustianowski, A., Pisapia, R., Zumla, A., Ippolito, G. (2019). Viral hepatitis: Etiology, epidemiology, transmission, diagnostics, treatment, and prevention. Infect. Dis. Clin. North. Am. 33 (4), 1045–1062. doi: 10.1016/j.idc.2019.08.004
Liaw, Y. F. (2021). Hepatitis b flare after cessation of nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis b: To retreat or not to retreat. Hepatology. 73, 843–852. doi: 10.1002/hep.31525
Liaw, Y. F., Tai, D. I., Chu, C. M., Chen, T. J. (1988). The development of cirrhosis in patients with chronic type b hepatitis: A prospective study. Hepatology. 8, 493–496. doi: 10.1002/hep.1840080310
Liu, J., Liang, W., Jing, W., Liu, M. (2019). Countdown to 2030: Eliminating hepatitis b disease, China. Bull. World. Health Organ. 97 (3), 230–238. doi: 10.2471/BLT.18.219469
Lok, A. S. F., McMahon, B. J., Brown, J. R. S., Wong, J. B., Ahmed, A. T., Farah, W., et al. (2016). Antiviral therapy for chronic hepatitis b viral infection in adults: A systematic review and meta-analysis. Hepatology. 63, 284–306. doi: 10.1002/hep.28280
Marcellin, P., Lau, G. K., Bonino, F., Farci, P., Hadziyannis, S., Jin, R., et al. (2004). Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis b. N. Engl. J. Med. 351, 1206–1217. doi: 10.1056/NEJMoa040431
Martin, P., Nguyen, M. H., Dieterich, D. T., Lau, D. T., Janssen, H. L. A., Peters, M. G., et al. (2022). Treatment algorithm for managing chronic hepatitis b virus infection in the united states: 2021 update. Clin. Gastroenterol. Hepatol. 20, 1766–1775. doi: 10.1016/j.cgh.2021.07.036
Mindikoglu, A. L., Pappas, S. C. (2018). New developments in hepatorenal syndrome. Clin. Gastroenterol. Hepatol. 16 (2), 162–177 e161. doi: 10.1016/j.cgh.2017.05.041
Naggie, S., Lok, A. S. (2021). New therapeutics for hepatitis b: The road to cure. Annu. Rev. Med. 72, 93–105. doi: 10.1146/annurev-med-080119-103356
Notarpaolo, A., Layese, R., Magistri, P., Gambato, M., Colledan, M., Magini, G., et al. (2017). Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J. Hepatol. 66 (3), 552–559. doi: 10.1016/j.jhep.2016.10.038
Peterson, M. L., Ma, C., Spear, B. T. (2011). Zhx2 and Zbtb20: novel regulators of postnatal alpha-fetoprotein repression and their potential role in gene reactivation during liver cancer. Semin. Cancer. Biol. 21 (1), 21–27. doi: 10.1016/j.semcancer.2011.01.001
Sarin, S. K., Kumar, M., Lau, G. K., Abbas, Z., Chan, H. L. Y., Chen, C. J., et al. (2016). Asian-Pacific clinical practice guidelines on the management of hepatitis b: a 2015 update. Hepatol. Int. 10, 1–98. doi: 10.1007/s12072-015-9675-4
Schiodt, F. V., Ostapowicz, G., Murray, N., Satyanarana, R., Zaman, A., Munoz, S., et al. (2006). Alpha-fetoprotein and prognosis in acute liver failure. Liver. Transpl. 12 (12), 1776–1781. doi: 10.1002/lt.20886
Schmidt, L. E., Dalhoff, K. (2005). Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 41 (1), 26–31. doi: 10.1002/hep.20511
Seto, W. K., Lo, Y. R., Pawlotsky, J. M., Yuen, M. F. (2018). Chronic hepatitis b virus infection. Lancet. 392 (10161), 2313–2324. doi: 10.1016/s0140-6736(18)31865-8
Sullivan, J. I., Rockey, D. C. (2017). Diagnosis and evaluation of hyperbilirubinemia. Curr. Opin. Gastroenterol. 33 (3), 164–170. doi: 10.1097/MOG.0000000000000354
Su, T. S., Yang, H. M., Zhou, Y., Huang, Y., Liang, P., Cheng, T., et al. (2019). Albumin-bilirubin (ALBI) versus child-Turcotte-Pugh (CTP) in prognosis of HCC after stereotactic body radiation therapy. Radiat. Oncol. 14 (1), 50. doi: 10.1186/s13014-019-1251-y
Taketa, K. (1990). Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 12 (6), 1420–1432. doi: 10.1002/hep.1840120625
Trevisani, F., D'Intino, P. E., Morselli-Labate, A. M., Mazzella, G., Accogli, E., Caraceni, P., et al. (2001). Serum α-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J. Hepatol. 34 (4), 570–575. doi: 10.1016/s0168-8278(00)00053-2
Vittal, A., Ghany, M. G. (2019). WHO guidelines for prevention, care and treatment of individuals infected with HBV: A US perspective. Clin. Liver. Dis. 23 (3), 417–432. doi: 10.1016/j.cld.2019.04.008
Wang, F. S., Fan, J. G., Zhang, Z., Gao, B., Wang, H. Y. (2014). The global burden of liver disease: the major impact of China. Hepatology. 60 (6), 2099–2108. doi: 10.1002/hep.27406
Wang, X., Shen, C., Yang, J., Yang, X., Qin, S., Zeng, H., et al. (2018). Alpha-fetoprotein as a predictive marker for patients with hepatitis b-related acute-on-Chronic liver failure. Can. J. Gastroenterol. Hepatol. 2018, 1232785. doi: 10.1155/2018/1232785
Wang, X., Sun, M., Yang, X., Gao, L., Weng, M., Yang, D., et al. (2020). Value of liver regeneration in predicting short-term prognosis for patients with hepatitis b-related acute-on-Chronic liver failure. Biomed. Res. Int. 2020, 5062873. doi: 10.1155/2020/5062873
World Health Organization (2017) Global hepatitis report 2017. Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (Accessed March 3,2019).
Keywords: chronic hepatitis B, alpha-fetoprotein, aspartate aminotransferase, acute hepatitis flare, outpatient follow-up
Citation: Yu M, Huang L, Zhang S, Jiang L, Jin Y, Gu M, Liao J and Zhang J (2023) Follow-up value of serum AFP and aminotransferases in chronic hepatitis B progression. Front. Cell. Infect. Microbiol. 13:1082390. doi: 10.3389/fcimb.2023.1082390
Received: 28 October 2022; Accepted: 10 January 2023;
Published: 25 January 2023.
Edited by:
Alexander V. Ivanov, Engelhardt Institute of Molecular Biology (RAS), RussiaReviewed by:
Qing-Lei Zeng, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2023 Yu, Huang, Zhang, Jiang, Jin, Gu, Liao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiexin Zhang, amlleGluemhhbmdAbmptdS5lZHUuY24=; Jun Liao, bGlhb2p1bkBjcHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.