- 1West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, China
- 2Division of Infectious Diseases, State Key Laboratory of Biotherapy and Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, China
- 3Dental Department, 363 Hospital, Chengdu, China
- 4Health Management Center, West China Hospital of Sichuan University, Chengdu, China
- 5Helicobacter pylori Research Laboratory, School of Biomedical Sciences, Marshall Centre for Infectious Disease Research and Training, University of Western Australia, Nedlands, WA, Australia
- 6School of Biomedical Engineering, Marshall Laboratory of Biomedical Engineering, Shenzhen University Health Science Center, Shenzhen, China
Background: Association of gastric atrophy or cancer with levels of serum pepsinogens, gastrin-17 and anti-Helicobacter pylori IgG antibody have been extensively studied. However, the association of serum pepsinogen and gastrin-17 with H. pylori infection has not been studied in a large population.
Aim: To investigate the impact of H. pylori infection on serum levels of pepsinogens and gastrin-17.
Methods: A total of 354, 972 subjects who underwent health check-ups were included. Serum levels of pepsinogens and gastrin-17 were measured using the enzyme-linked immunosorbent assay. H. pylori infection was detected using 14C-urea breath test (UBT). Multivariable logistic regression analysis was used to investigate the association of serum pepsinogen and gastrin-17 with H. pylori infection.
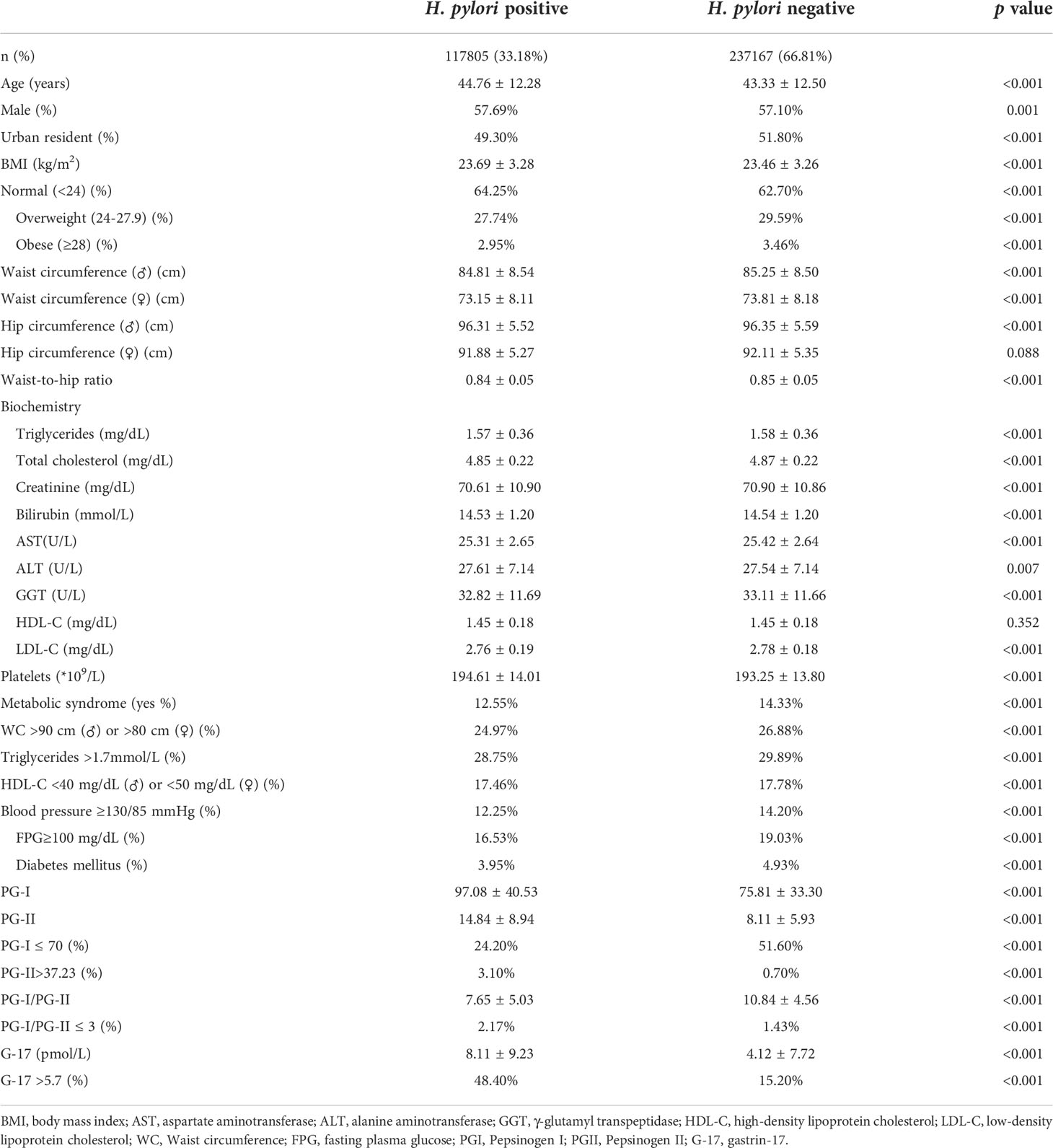
Results: H. pylori prevalence was 33.18% in this study. The mean levels of pepsinogens and gastrin-17 were higher, while the mean pepsinogen-I/II ratio were lower among H. pylori-positive than -negative subjects. In H. pylori-positive subjects, pepsinogen and gastrin-17 levels correlated positively, whereas the pepsinogen-I/II ratio correlated negatively with UBT values (e.g., the mean serum level of pepsinogen-I in subjects with UBT values in the range of 100-499dpm, 500-1499dpm, and ≥1500dpm was 94.77 ± 38.99, 102.77 ± 43.59, and 111.53 ± 47.47 ng/mL, respectively). Compared with H. pylori-negative subjects, the adjusted odds ratio (aOR) of having pepsinogen-I ≤ 70 ng/mL in the three H. pylori-positive but with different UBT value groups was 0.31 (p<0.001), 0.16 (p<0.001), and 0.08 (p<0.001), respectively; while the aOR of having G-17>5.70 pmol/L was 4.56 (p<0.001), 7.43 (p<0.001), and 7.12 (p<0.001). This suggested that H. pylori-positive subjects with higher UBT values were less likely to have pepsinogen-I ≤70 ng/mL (a serum marker for gastric atrophy), but more likely to have gastrin-17 >5.70 pmol/L (a marker for peptic ulcer).
Conclusions: H. pylori-positive subjects with higher UBT values are unlikely to have gastric atrophy, but may have greater risk of severe gastritis or peptic ulcers. Our study suggests that H. pylori-positive patients with high UBT values may benefit the most from H. pylori eradication.
Introduction
Helicobacter pylori is one of the most common human bacterial pathogens, infecting approximately half of the world’s population (Hooi et al., 2017). Chronic H. pylori infection, usually acquired in childhood, causes active gastritis in all infected subjects (Chey et al., 2017; Malfertheiner et al., 2017). Without antibiotic treatment, H. pylori gastritis can progress to gastric atrophy, intestinal metaplasia, dysplasia, and eventually gastric cancer (Sugano et al., 2015; Malfertheiner et al., 2017; Liu et al., 2018). With more than 1 million new cases diagnosed in 2020 and approximately 0.77 million deaths, gastric cancer is the fifth most frequent cancer and the fourth leading cause of cancer death worldwide (Sung et al., 2021).
The non-invasive serological biopsy including 5 stomach-specific circulating biomarkers (serum pepsinogen I, PG-I; PG-II; the PG-I/PG-II ratio; gastrin-17, G-17; and anti-H. pylori IgG antibody) have been widely used in clinical practice for the assessment of gastric atrophy and gastric cancer risk (Bodger et al., 2001; Miki and Urita, 2007; Miki, 2011; Tu et al., 2017; Cai et al., 2019). PG-I is secreted mainly by chief cells and neck mucous cells in the gastric corpus and fundus glands, while PG-II is secreted not only by the fundus glands but also by the pyloric glands of the gastric antrum and the Brunner’s glands of the proximal duodenum. The serum PG-I decreases with the atrophy of the gastric fundus, while the PG-II level remains relatively constant. Thus, low serum PG-I level (≤70 ng/ml) and/or low PG-I/PG-II ratio (≤3.0) are usually used to indicate the chronic atrophic gastritis in the fundus, a high risk factor for the development of gastric cancer (Miki et al., 1993; Kitahara et al., 1999; Tu et al., 2017). Of note, the PGs have been used in clinical practice as biomarkers for chronic atrophic gastritis even before the discovery of H. pylori (Miki and Urita, 2007; Miki, 2011).
Serum G-17 is the amidated and biologically active gastrin secreted by the G cells in the gastric antrum. It plays an important role in gastric physiology by stimulating acid secretion (mainly in response to low acidity in the stomach) and maintaining gastric mucosal homeostasis (Watson et al., 2006). It has been suggested that an elevated intragastric pH induced by chronic atrophic gastritis in the corpus may cause hypersecretion of G-17 from the antrum (Fourmy et al., 2011). G-17 is also a pro-proliferative and anti-apoptotic hormone which has been proposed to play an important role in gastric carcinogenesis (Watson et al., 2006; Takaishi et al., 2009). It has been reported that the serum G-17 level in patients with gastric cancer is higher than those without (Sun et al., 2014; Cai et al., 2019; Shen et al., 2021). Thus, elevated serum G-17 level is commonly used to identify individuals at high risk of developing gastric cancer (Zagari et al., 2017; Cai et al., 2019).
There are numerous studies that have assessed the different combination of PG-I, PG-II, PG-I/PG-II ratio, G-17 and anti-H. pylori IgG antibody as independent risk factors in screening patients with high risk of gastric cancer (Miki et al., 1993; Miki and Urita, 2007; Miki, 2011; Tu et al., 2017; Zagari et al., 2017; Cai et al., 2019; Shen et al., 2021). H. pylori infection is the most important risk factor for gastric cancer, as nearly 90% of gastric cancer cases can be ascribed to this gastric bacterium (Dang and Graham, 2017; Malfertheiner et al., 2017). However, studies specifically investigating the association of serum PGs and G-17 with H. pylori infection are scarce. Here, with the inclusion of 354, 972 subjects who underwent a health checkup in West China Hospital, we investigated the effects of H. pylori infection (assessed by 14C urea breath test, UBT) on serum PGs and G-17.
Materials and methods
Subjects
A total of 455,733 subjects who underwent medical examination at the Physical Examination Centre of West China Hospital, Sichuan University between January 2014 and December 2017 were investigated. Subjects who underwent UBT and aged 18 years or older were included. Subjects with incomplete data and aged less than 18 years were excluded. If the included participants had more than one time of health check-up records, only the first-time medical record was used for this cross-sectional study. A total of 354, 972 subjects were included in the final analysis. The study was performed in accordance with the ethical guidelines in the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. The study was approved by the Ethics Committee of West China Hospital of Sichuan University. All participants provided informed consent.
Data collection
The data was automatically extracted using a Hospital Information System (HIS). Using calibrated instruments with standard protocols, a range of physical measurements including height, weight, hip and waist circumferences and blood pressure etc. were recorded by trained technicians. The body mass index (BMI) of the subjects was calculated by dividing the subject’s weight (kg) by the square of their height (m2). A BMI <24 kg/m2 was defined as normal weight, whereas a BMI in the range of 24-27.9 kg/m2 was considered as overweight, and a BMI ≥28 kg/m2 was defined as obese (Zhou, 2002). For each subject, overnight (12 h) fasting blood samples were taken for routine biochemical analyses including complete blood count, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), triglycerides (TG), total cholesterol, creatinine, bilirubin, and platelets.
Metabolic syndrome was defined as having central obesity (waist circumference ≥ 90 cm in men or ≥80 cm in women) plus any two of the following four factors: 1) raised triglyceride, defined by triglyceride levels ≥1.7 mmol/L (150 mg/dL) or use of lipid-lowering agents; 2) reduced HDL-C, defined by HDL-C < 1.03 mmol/L (40 mg/dL) in men or <1.29 mmol/L (50 mg/dL) in women or use of lipid-lowering agents; 3) raised blood pressure, determined by blood pressure ≥130/85 mm Hg or use of blood pressure-lowering agents; 4) raised FPG, defined by FPG level ≥ 5.6 mmol/L (100 mg/dL) or previously diagnosed type 2 diabetes or use of blood glucose-lowering agents (Alberti et al., 2006; Alberti et al., 2009).
The presence of H. pylori infection was detected using the 14C UBT (Shenzhen Zhonghe Headway Bio-Sci & Tech Co. Ltd., Shenzhen, Guangdong, China). Subjects with the measured disintegrations per minute (dpm) value ≥ 100 were diagnosed as having H. pylori infection (Bielański et al., 1996). Positive H. pylori infected subjects were further divided into three groups based on UBT values in the range of 100-499 dpm, 500-1499 dpm, and ≥1500 dpm, respectively.
Serum G-17, PGI, and PGII were measured using the enzyme-linked immunosorbent assay according to the manufacturer’s instructions. Abnormal serum PGs and G-17 were defined as PGI ≤ 70 ng/mL, PGII>37.23 ng/mL, PGI/PGII ≤ 3 and G-17>5.70 pmol/L (Miki et al., 1993; Miki and Urita, 2007; Miki, 2011; Bang et al., 2019; Cai et al., 2019).
Statistical analysis
Data were reported as the mean ± standard deviation for normal and median (interquartile range) for non-normal continuous variables, while frequency was used for discrete variables. In the univariate comparisons, we used the Student’s t-test and ANOVA with Bonferroni adjustments for continuous samples and chi-square test or Fisher’s exact test for the qualitative ones. Non-parametric alternatives (Mann–Whitney U and Kruskal-Wallis tests) were used for non-normal distributions.
Pearson’s correlation test was performed to determinate the correlation of serum levels of PGs, PGI/PGII ratio, G-17 with UBT values, age, sex, and BMI. Logistic regression models were used to estimate adjusted odds ratios (aORs) of specific disease prevalence associated with outcome. Covariates were selected for analysis according to their biologically plausible potential to act as confounders or predictors for each outcome. The potential predictors were as follows: age, gender, location (rural or urban), year of health check-up year, BMI, H. pylori infection. The collinearity between factors included in the multivariable analyses was checked by using variance inflation factor (VIF) and tolerance (1/VIF) values. Variables with very high VIF values indicating possible redundancy entered into different multivariable models.
The method used for missing data was complete-case analysis since statistical packages excluded individuals with any missing value. All CIs, significance tests, and resulting p values were 2-sided, values were supposed to be statistically significant when p <0.05. Statistical analysis was performed using STATA (version 14) and PRISM (version 8).
Results
The prevalence of H. pylori infection was different among different age groups
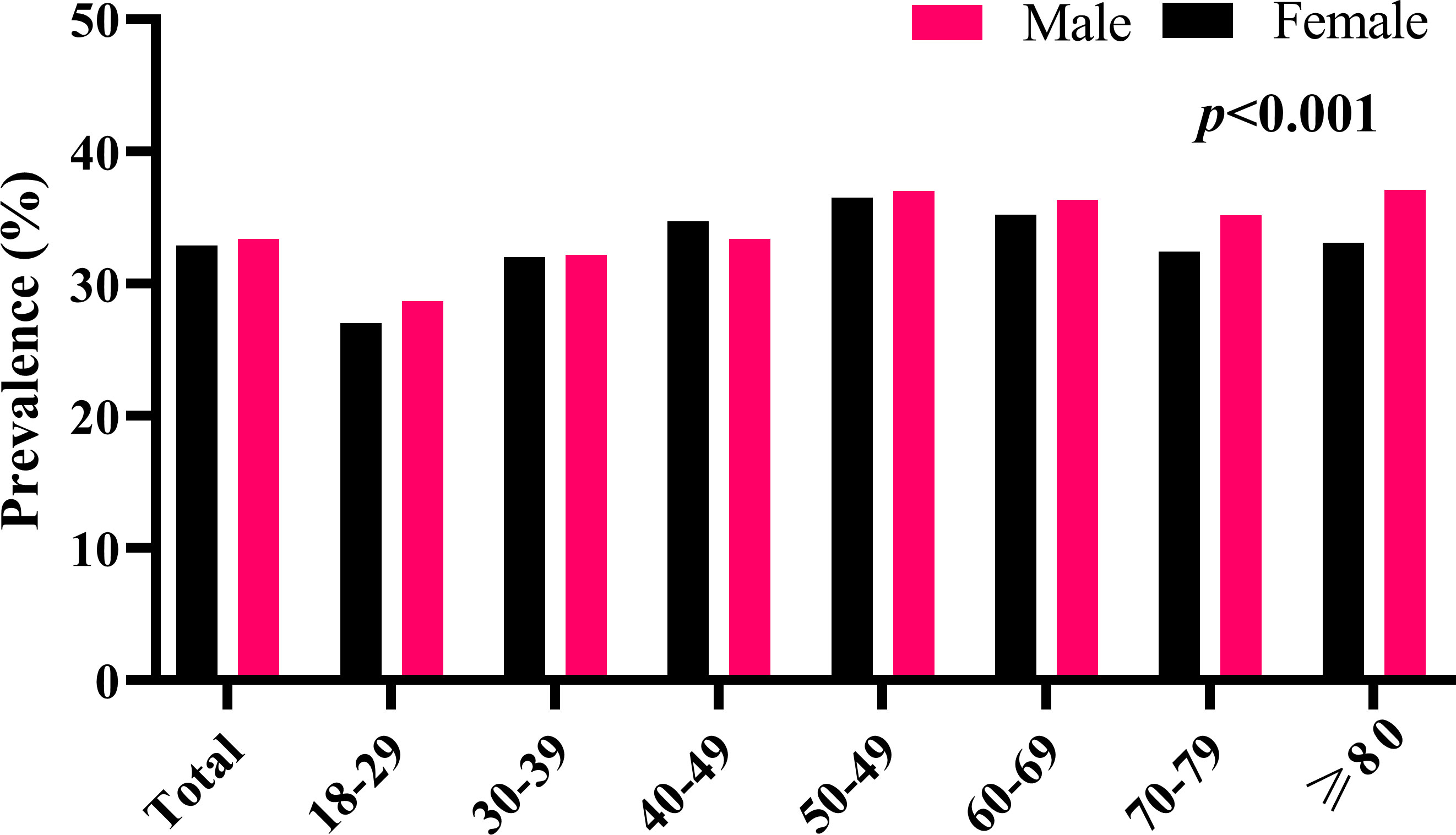
A total of 354, 972 subjects (203,384 males, and 151,588 females) with a mean age of 43.80 ± 12.44 years were included in the final analysis. The overall prevalence of H. pylori infection was 33.18% (117,805/354,972) (Table 1). The prevalence of H. pylori infection in males and females was 33.42% (67,964/203,384) and 32.88% (49,841/151,588), respectively. Interestingly, among the 8 different age groups (18-29, 30-39, 40-49, 50-59, 60-69, 70-79, and ≥80 years), the prevalence of H. pylori infection in subjects aged between 18-29 years was the lowest in both males and females (27.86% and 28.69%), which gradually increased to 36.79% and 36.97% in subjects aged between 50-59 years (Figure 1).
Of note, the mean serum levels of PGs, and G-17 increased, whereas the PGI/PGII ratio decreased gradually with age. Overall, the male subjects had higher serum levels of PGs and PGI/PGII ratio, whereas lower serum level of G-17 than that of their female counterparts (Figure 2). Based on Pearson’s correlation test, there was a positive correlation between age and PGI (r=0.196, p<0.001), PGII (r= 0.204, p<0.001), and G-17 (r=0.078, p<0.001); whereas a negative correlation between age and PGI/PGII ratio (r=-0.114, p<0.001). There was a positive correlation between male gender and PGI (r=0.183, p<0.001), PGII (r= 0.038, p<0.001), and PGI/PGII ratio (r=0.053, p<0.001), whereas a negative correlation between male gender and G-17 (r=-0.035, p<0.001). Interestingly, the Pearson’s correlation test revealed a positive correlation between BMI and serum levels of PGI (r=0.016, p=0.015), and PGII (r=0.018, p=0.005).
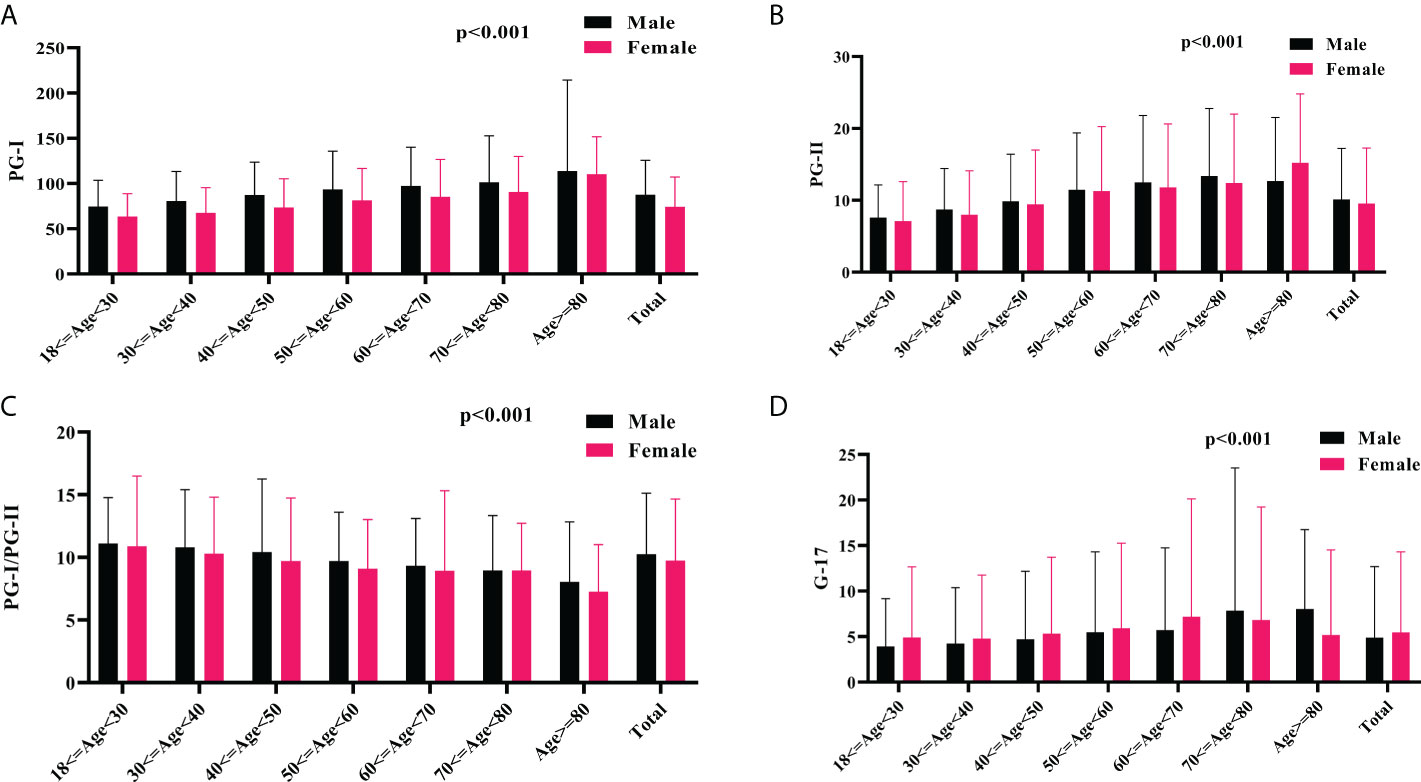
Figure 2 The association of serum PGs and G-17 with age and gender. The mean serum levels of PGI (A), PGII (B), PGI/PGII ratio (C), and G-17 (D) among different gender and age groups. PG, pepsinogen; G-17, gastrin-17.
Subjects with H. pylori infection have higher serum levels of PGs and G-17, but lower PGI/PGII ratio than those without
The mean PGI and PGII levels in H. pylori-positive subjects were 97.08 ± 40.53 and 14.84 ± 8.94 ng/mL, which were much higher than the 75.81 ± 33.30 and 8.11 ± 5.93 ng/mL in H. pylori-negative subjects (P<0.001) (Table 1). The percentage of individuals having PGI ≤ 70 ng/mL was much lower in H. pylori-positive subjects than that of the H. pylori-negative subjects (24.20% vs 51.60%, p<0.001), whereas the percentage of individuals having PGII≤37.23 ng/mL among H. pylori-positive subjects was higher than that of the H. pylori-negative subjects (3.10% vs 0.70%, p<0.001). The mean PGI/PGII ratio in the H. pylori-positive subjects was 7.65 ± 5.03, which was much lower than the mean ratio of 10.84 ± 4.56 in H. pylori-negative subjects (p<0.001). The percentage of subjects with PGI/PGII ≤ 3 in H. pylori-positive and H. pylori-negative subjects was 2.17% and 1.43%, respectively (Table 1).
The mean serum G-17 level in the H. pylori-positive subjects was 8.11 ± 9.23 pmol/L, which was much higher than the mean value of 4.12 ± 7.72 pmol/L in the H. pylori-negative subjects (p<0.001) (Table 1). The percentage of individuals having G-17 >5.7 among H. pylori-positive subjects was higher than that of the H. pylori-negative subjects (48.40% vs 15.20%, p<0.001).
H. pylori-positive subjects with higher UBT values have higher serum levels of PGs but lower PGI/II ratio
Among the H. pylori-positive subjects with different UBT values, we analysed whether the serum levels of PGs and G-17, and the PGI/II ratio were different. We demonstrated that the mean PGI levels in H. pylori-positive subjects with UBT values in the range of 100-499 dpm, 500-1499 dpm, and ≥1500 dpm were 94.77 ± 38.99, 102.77 ± 43.59, and 111.53 ± 47.47 ng/mL, respectively (Figure 3A). The mean PGII level among the three different UBT value groups was 14.20 ± 8.85, 16.48 ± 8.94, and 18.19 ± 9.00 ng/mL, respectively (Figure 3B). The PGI/PGII ratio in the three different UBT value groups was 7.88 ± 5.54, 7.04 ± 3.26, and 6.69 ± 2.33, respectively (Figure 3C). The serum G-17 levels in the three groups were 7.82 ± 9.45, 8.89 ± 8.65, and 8.50 ± 7.05 pmol/L, respectively (Figure 3D).
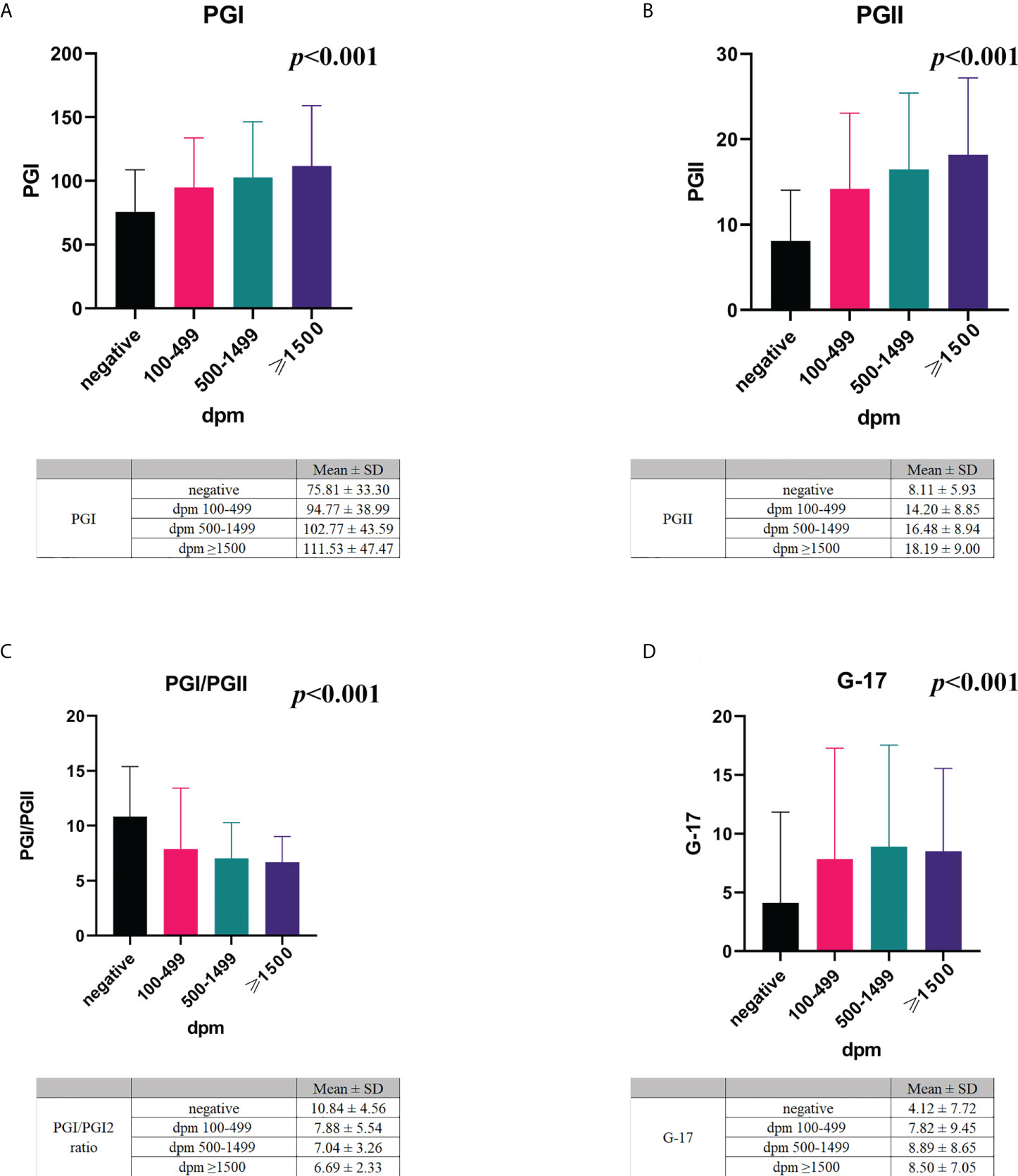
Figure 3 The association of serum PGs and G-17 with H. pylori infection. The mean serum levels of PGI (A), PGII (B), PGI/PGII ratio (C), and G-17 (D) in H. pylori-negative and -positive subjects with different UBT values. PG, pepsinogen; G-17, gastrin-17; dpm, disintegrations per minute.
The Pearson’s correlation test revealed a positive correlation between UBT values and serum levels of PGI (r=0.232, p<0.001), PGII (r=0.352, p<0.001), and G-17 (r=0.178, p<0.001), whereas a negative correlation between PGI/PGII ratio and the UBT values (r=-0.241, p<0.001).
H. pylori-positive subjects with higher UBT values are less likely to have PGI ≤ 70 ng/mL, but more likely to have G-17>5.70 pmol/L
Multivariable analysis was carried out to investigate whether H. pylori-positive subjects with different UBT values had different effects on the abnormalities of serum PGs and G-17. After adjustment for sociodemographic characteristics including age, sex, region (urban or rural), year of health check-up, and BMI, we demonstrated that compared with H. pylori-negative subjects, the aOR of having PGI ≤ 70 ng/mL in H. pylori-positive subjects with UBT values in the range of 100-499 dpm, 500-1499 dpm, and ≥1500 dpm was 0.31 (95% CI 0.29-0.33, p<0.001), 0.16 (95% CI 0.14-0.18, p<0.001), and 0.08 (95% CI 0.04-0.15, p<0.001), respectively; and the aOR of having G-17>5.70 pmol/L was 4.56 (95% CI 4.24-4.91, p<0.001), 7.43 (95% CI 6.67-8.28, p<0.001), and 7.12 (95% CI 4.66-10.88, p<0.001) (Table 2). This suggested that H. pylori-positive subjects with higher UBT values were less likely to have PGI ≤ 70 ng/mL, but more likely to have G-17>5.70 pmol/L.
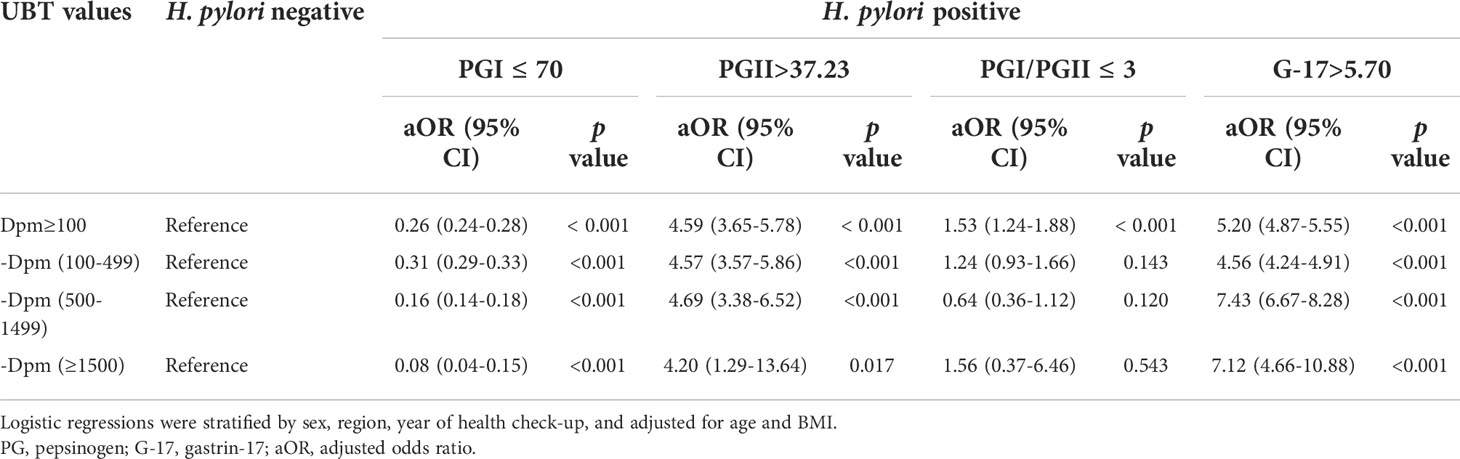
Table 2 Adjusted ORs for abnormalities of serum PGs and G-17 in H. pylori-positive subjects with different UBT values.
The abnormalities of serum PGs and G-17 are associated with age and gender
As age and gender are associated with risk of many cancers, multivariable logistic regression analysis was performed to investigate whether different age and gender had different effects on the abnormalities of serum PGs and G-17. We demonstrated that H. pylori-positive subjects aged less than 40 years had a lower chance to have PGI ≤ 70 ng/mL than those H. pylori-positive subjects aged 40 years and older (aOR 0.19 vs aOR 0.29, p<0.001), but a higher chance to have G-17>5.70 pmol/L (aOR 6.27 vs aOR 4.87, p<0.001). Similarly, our results demonstrated that female H. pylori-positive subjects were less likely to have PGI ≤ 70 ng/mL than their male counterparts (aOR 0.18 vs aOR 0.38, p<0.001), but more likely to have G-17>5.70 pmol/L (aOR 5.90 vs aOR 4.62, p<0.001) (Table 3).
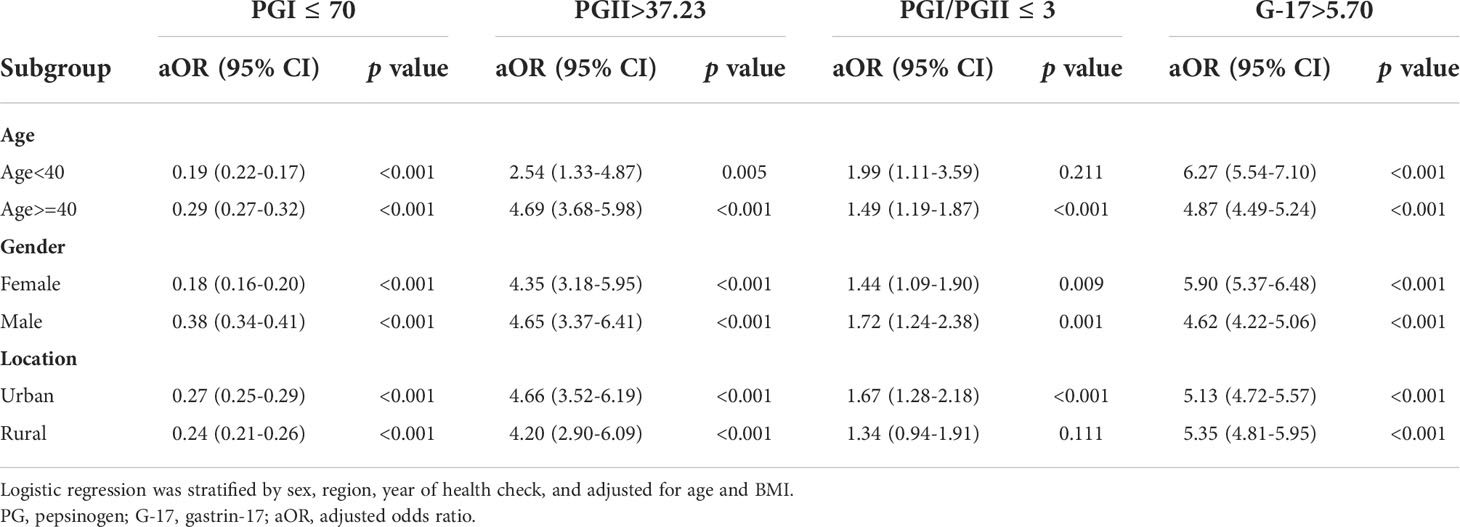
Table 3 Subgroup analysis of adjusted ORs for abnormalities of serum PGs and G-17 in H. pylori-positive subjects.
Discussion
This study included a large health checkup population (354, 972 subjects) to analyze the impact of H. pylori infection on serum PGI and PGII levels, PGI/PGII ratio, and G-17 levels. In H. pylori positive subjects, the serum PGI, PGII, and G-17 levels were much higher, while the PGI/PGII ratio was much lower than that of the H. pylori-negative subjects. We further demonstrated a positive correlation between the levels of PGI and PGII and the UBT values, while a negative correlation between the PGI/PGII ratio and the UBT values. Multivariable analysis demonstrated that H. pylori-positive subjects with higher UBT values were more likely to have G-17>5.70 pmol/L, but less likely to have PGI ≤ 70 ng/mL. Compared with older and male H. pylori-positive subjects, younger and female H. pylori-positive subjects are less likely to have PGI ≤ 70 ng/mL.
In the present study, we demonstrated that the prevalence of H. pylori infection was 33.18% in this study, which was much lower than the previously reported H. pylori prevalence rate of approximately 50% in China (Hooi et al., 2017; Wang et al., 2019; Xiong et al., 2020). The relatively low prevalence of H. pylori infection in this study and also in another recent study (27.5%) (Shu et al., 2019) might be partly explained by the fact that the subjects were recruited in the capital city of the province where the economic and educational levels are higher than the general population (Nagy et al., 2016; Leja et al., 2019). It has been reported that H. pylori infection prevalence in urban populations was much lower the rural populations, as the improved living standards associated with urbanization play an important role in reducing the horizontal transmission of H. pylori (Nagy et al., 2016). It is expected that the prevalence of H. pylori infection will continue to decrease with sustainable urbanization in China.
H. pylori infection is considered to be the most important etiological factor for gastric cancer (Malfertheiner et al., 2017; El-Serag et al., 2018; Liu et al., 2018). It is now well-accepted that H. pylori infection is the crucial and initiating factor in the Correa Cascade model of gastric cancer: H. pylori infection→acute gastritis→chronic gastritis→gastric atrophy→intestinal metaplasia→dysplasia→gastric cancer (Malfertheiner et al., 2017). Previous studies have shown significant associations between H. pylori infection and elevated levels of PGI, PGII, and G-17, and reduced PG-I/PG-II ratio in H. pylori-related non-atrophic chronic gastritis (Asaka et al., 1992; Bodger et al., 2001; Ohkusa et al., 2004; Miki and Urita, 2007; Miki, 2011; Gong et al., 2014). It has been reported that H. pylori-mediated gastrin induction is type IV secretion system (T4SS)-dependent (Rieder et al., 2005; Gunawardhana et al., 2017). H. pylori infection induces the expression of heparin-binding EGF-like growth factor (HB-EGF) via T4SS. Subsequently, the increased HB-EGF binds to the EGF receptor in G cells to sequentially activate C-Raf, Mek-1, and Erk2 in the mitogen-activated protein kinase (MAPK) pathway, resulting in an increased gastrin expression (Gunawardhana et al., 2017). H. pylori lipopolysaccharide (LPS) has been reported to be responsible for stimulating pepsinogen secretion in a dose-dependent manner (Young et al., 1992; Young et al., 2002). Both the composition and structure of the LPS molecule play a modulatory role in pepsinogen release. LPS from other gastrointestinal bacterial species including Helicobacter nemestrinae and Campylobacter jejuni have no effect on pepsinogen secretion (Young et al., 2002).
In this study, we demonstrated a progressive increase in serum PG levels with increasing UBT values among this health check-up population, which is consistent with a previous study (Huang et al., 2016). It has been reported that the UBT values correlate positively with the intragastric H. pylori bacterial load (Perri et al., 1998; Zagari et al., 2005). Thus, it can be inferred that the higher the serum PG levels, the denser of H. pylori infection in the stomach. As the H. pylori-associated gastritis progresses, the gastric mucosa may become inhospitable for the colonization of H. pylori, leading to a reduced UBT value or even a negative H. pylori serology test (Ohata et al., 2004; Tu et al., 2017). The serum PG I level decreases with the reduced H. pylori bacterial load and the loss of fundic gland mucosa (Bodger et al., 2001; Ohata et al., 2004; Miki and Urita, 2007; Tu et al., 2017). It has been reported that in H. pylori-infected patients, serum PG-I level positively correlates with the severity of antral inflammation, while a stepwise decrease of serum PG-I level was observed with progression from an antral predominant gastritis, through pangastritis, to a corpus predominant gastritis (Bodger et al., 2001). The lowest serum PG-I level and the PG-I/PG-II ratio were observed in patients with severe corpus atrophy and gastric cancer (Bodger et al., 2001). Hence, the combination of UBT and PG-I might be used in clinical practice to predict the functional and histological status of the gastric mucosa. H. pylori-infected patients having high levels of serum PG-I and UBT value may have antral predominant gastritis and high risk of peptic ulcers but low risk of gastric atrophy and cancer. In contrast, H. pylori-infected subjects having decreased levels of serum PG-I and UBT values (or even negative UBT) are very likely to have high risk of gastric atrophy and cancer.
Older age and male sex are always associated with a higher risk of gastric cancer (Rawla and Barsouk, 2019; Etemadi, et. al., 2020; Machlowska et al., 2020; Lou et al., 2020; Sung et al., 2021; Wong et al., 2021). The incidence rate of gastric cancer increases progressively with age, especially after 40 years old. In a recently developed gastric cancer risk prediction rule consisting of seven variables (age, sex, PG I/II ratio, G-17, anti-H. pylori infection IgG status, pickled food, and fried food), points assigned to age 60-69, and older than 69 were 6 and 9, respectively, which were much higher than the points assigned to other variables (Cai et al., 2019). For males, the age-standardized incidence rate of gastric cancer is approximately twice the rate for females (Rawla and Barsouk, 2019; Etemadi, et. al., 2020; Machlowska et al., 2020; Sung et al., 2021). The lower gastric cancer rate in women might be partly explained by the protective effect of estrogen (Chandanos and Lagergren, 2008; Rawla and Barsouk, 2019). Taking the age and gender into account, it would be expected that the effect of H. pylori infection on subjects with different age and gender is different. Indeed, in the present study, we demonstrated that compared with older and male H. pylori-positive subjects, younger and female H. pylori-positive subjects are less likely to have PGI ≤ 70 ng/mL (Table 3).
Our study has several limitations. Firstly, the diagnosis of H. pylori infection was based on UBT rather than the gold standard, biopsy/culture. However, biopsy/culture requires endoscopy, which is invasive and usually not included in a routine health check-up package. Although UBT is not the gold standard, the advantages of easy to operate and non-invasive procedure, and also the both high sensitivity and specificity (approximately 95%) make it the best and most frequently used test for the diagnosis of H. pylori infection (Malfertheiner et al., 2017; El-Serag et al., 2018). Secondly, H. pylori IgG assay was not performed to the health check-up population in this study. Due to the retrospective nature of this study, we were unable to conduct the H. pylori IgG assay to compare the results of UBT and H. pylori IgG assay in analyzing the effect of H. pylori infection on serum pepsinogens and G-17. Thirdly, the information of medication usage such as the proton pump inhibitors and antibiotics was not recorded during the health checkup examination, which may impact the diagnosis of H. pylori infection by UBT. Fourthly, this study was a single hospital-based cross-sectional study, so the generated findings may not be generalized to the entire Chinese population. Nevertheless, our study included the largest health checkup population so far (354, 972 subjects) to analyze the association between H. pylori infection and serum pepsinogen and gastrin levels.
In summary, we demonstrate that H. pylori infection is closely associated with elevated levels of PGs and G-17, but with a reduced PG-I/PG-II ratio among the health checkup population. The serum levels of PGs are positively correlated, while the PGI/PGII ratio is negatively correlated with the intragastric H. pylori bacterial load as reflected by the UBT values. H. pylori-positive subjects with higher UBT values are less likely to have PGI ≤ 70 ng/mL (a serum marker for gastric atrophy), but more likely to have G-17>5.70 pmol/L (stimulating gastric acid secretion and peptic ulcers), suggesting that H. pylori-positive patients with high UBT values are unlikely to have gastric atrophy, but may have a greater risk of severe gastritis and peptic ulcers. Thus, H. pylori-positive patients with high UBT values, and high levels of serum PGs and G-17 may benefit the most from H. pylori eradication.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-pZ, C-hL, B-wL, and HL wrote the manuscript. MB, BJM, Y-jW, and HT edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (82072248) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZY2016201).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120, 1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644
Alberti, K. G., Zimmet, P., Shaw, J. (2006). Metabolic syndrome–a new world-wide definition. a consensus statement from the international diabetes federation. Diabetes Med. 23, 469–480. doi: 10.1111/j.1464-5491.2006.01858.x
Asaka, M., Kimura, T., Kudo, M., Takeda, H., Mitani, S., Miyazaki, T., et al. (1992). Relationship of helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 102, 760–766. doi: 10.1016/0016-5085(92)90156-S
Bang, C. S., Lee, J. J., Baik, G. H. (2019). Prediction of chronic atrophic gastritis and gastric neoplasms by serum pepsinogen assay: A systematic review and meta-analysis of diagnostic test accuracy. J. Clin. Med. 8, 657. doi: 10.3390/jcm8050657
Bielański, W., Konturek, S. J., Dobrzańska, M. J., Pytko-Polończyk, J., Sito, E., Marshall, B. J. (1996). Microdose 14C-urea breath test in detection of helicobacter pylori. J. Physiol. Pharmacol. 47, 91–100.
Bodger, K., Wyatt, J. I., Heatley, R. V. (2001). Variation in serum pepsinogens with severity and topography of helicobacter pylori-associated chronic gastritis in dyspeptic patients referred for endoscopy. Helicobacter 6, 216–224. doi: 10.1046/j.1523-5378.2001.00031.x
Cai, Q., Zhu, C., Yuan, Y., Feng, Q., Feng, Y., Hao, Y., et al. (2019). Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: A nationwide multicentre study. Gut 68, 1576–1587. doi: 10.1136/gutjnl-2018-317556
Chandanos, E., Lagergren, J. (2008). Oestrogen and the enigmatic male predominance of gastric cancer. Eur. J. Cancer 44, 2397–2403. doi: 10.1016/j.ejca.2008.07.031
Chey, W. D., Leontiadis, G. I., Howden, C. W., Moss, S. F. (2017). ACG clinical guideline: Treatment of helicobacter pylori infection. Am. J. Gastroenterol. 112, 212–239. doi: 10.1038/ajg.2016.563
Dang, B. N., Graham, D. Y. (2017). Helicobacter pylori infection and antibiotic resistance: A WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 14, 383–384. doi: 10.1038/nrgastro.2017.57
El-Serag, H. B., Kao, J. Y., Kanwal, F., Gilger, M., Lovecchio, F., Moss, S. F., et al. (2018). Houston Consensus conference on testing for helicobacter pylori infection in the united states. Clin. Gastroenterol. Hepatol. 16, 992–1002.e6. doi: 10.1016/j.cgh.2018.03.013
Etemadi, A., Safiri, S., Sepanlou, S. G., Ikuta, K., Bisignano, C., Shakeri, R., et al (2020). The global, regional, and national burden of stomach cancer in 195 countries 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol 5, 42–54. doi: 10.1016/S2468-1253(19)30328-0
Fourmy, D., Gigoux, V., Reubi, J. C. (2011). Gastrin in gastrointestinal diseases. Gastroenterology 141, 814–818.e1-3. doi: 10.1053/j.gastro.2011.07.006
Gong, Y., Wei, W., Yuan, Y. (2014). Association between abnormal gastric function risk and helicobacter pylori infection assessed by ELISA and 14C-urea breath test. Diagn. Microbiol. Infect. Dis. 80, 316–320. doi: 10.1016/j.diagmicrobio.2014.09.009
Gunawardhana, N., Jang, S., Choi, Y. H., Hong, Y. A., Jeon, Y. E., Kim, A., et al. (2017). Helicobacter pylori-induced HB-EGF upregulates gastrin expression via the EGF receptor, c-raf, Mek1, and Erk2 in the MAPK pathway. Front. Cell Infect. Microbiol. 7, 541. doi: 10.3389/fcimb.2017.00541
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., Tanyingoh, D., et al. (2017). Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429. doi: 10.1053/j.gastro.2017.04.022
Huang, R. G., Xiao, H. L., Zhou, B., Song, X. H., Zhang, J., Wang, C. M., et al. (2016). Serum pepsinogen levels are correlated with age, sex and the level of helicobacter pylori infection in healthy individuals. Am. J. Med. Sci. 352, 481–486. doi: 10.1016/j.amjms.2016.08.005
Kitahara, F., Kobayashi, K., Sato, T., Kojima, Y., Araki, T., Fujino, M. A. (1999). Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut 44, 693–697. doi: 10.1136/gut.44.5.693
Leja, M., Grinberga-Derica, I., Bilgilier, C., Steininger, C. (2019). Review: Epidemiology of helicobacter pylori infection. Helicobacter 24 Suppl 1, e12635. doi: 10.1111/hel.12635
Liu, W. Z., Xie, Y., Lu, H., Cheng, H., Zeng, Z. R., Zhou, L. Y., et al. (2018). Fifth Chinese national consensus report on the management of helicobacter pylori infection. Helicobacter 23, e12475. doi: 10.1111/hel.12475
Lou, L., Wang, L., Zhang, Y., Chen, G., Lin, L., Jin, X., et al. (2020). Sex difference in incidence of gastric cancer: An international comparative study based on the global burden of disease study 2017. BMJ Open 10, e033323. doi: 10.1136/bmjopen-2019-033323
Machlowska, J., Baj, J., Sitarz, M., Maciejewski, R., Sitarz, R. (2020). Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 21, 4012. doi: 10.3390/ijms21114012
Malfertheiner, P., Megraud, F., O'morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of helicobacter pylori infection-the maastricht V/Florence consensus report. Gut 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Miki, K. (2011). Gastric cancer screening by combined assay for serum anti-helicobacter pylori IgG antibody and serum pepsinogen levels - "ABC method". Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 87, 405–414. doi: 10.2183/pjab.87.405
Miki, K., Ichinose, M., Ishikawa, K. B., Yahagi, N., Matsushima, M., Kakei, N., et al. (1993). Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn. J. Cancer Res. 84, 1086–1090. doi: 10.1111/j.1349-7006.1993.tb02805.x
Miki, K., Urita, Y. (2007). Using serum pepsinogens wisely in a clinical practice. J. Dig. Dis. 8, 8–14. doi: 10.1111/j.1443-9573.2007.00278.x
Nagy, P., Johansson, S., Molloy-Bland, M. (2016). Systematic review of time trends in the prevalence of helicobacter pylori infection in China and the USA. Gut. Pathog. 8, 8. doi: 10.1186/s13099-016-0091-7
Ohata, H., Kitauchi, S., Yoshimura, N., Mugitani, K., Iwane, M., Nakamura, H., et al. (2004). Progression of chronic atrophic gastritis associated with helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 109, 138–143. doi: 10.1002/ijc.11680
Ohkusa, T., Miwa, H., Nomura, T., Asaoka, D., Kurosawa, A., Sakamoto, N., et al. (2004). Improvement in serum pepsinogens and gastrin in long-term monitoring after eradication of helicobacter pylori: comparison with h. pylori-negative patients. Aliment Pharmacol. Ther. 20 Suppl 1, 25–32. doi: 10.1111/j.1365-2036.2004.01970.x
Perri, F., Clemente, R., Pastore, M., Quitadamo, M., Festa, V., Bisceglia, M., et al. (1998). The 13C-urea breath test as a predictor of intragastric bacterial load and severity of helicobacter pylori gastritis. Scand. J. Clin. Lab. Invest. 58, 19–27. doi: 10.1080/00365519850186797
Rawla, P., Barsouk, A. (2019). Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 14, 26–38. doi: 10.5114/pg.2018.80001
Rieder, G., Merchant, J. L., Haas, R. (2005). Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128, 1229–1242. doi: 10.1053/j.gastro.2005.02.064
Shen, H., Xiong, K., Wu, X., Cheng, S., Lou, Q., Jin, H., et al. (2021). The diagnostic value of serum gastrin-17 and pepsinogen for gastric cancer screening in Eastern China. Gastroenterol. Res. Pract. 2021, 6894248. doi: 10.1155/2021/6894248
Shu, L., Zheng, P. F., Zhang, X. Y., Feng, Y. L. (2019). Dietary patterns and helicobacter pylori infection in a group of Chinese adults ages between 45 and 59 years old: An observational study. Med. (Baltimore) 98, e14113. doi: 10.1097/MD.0000000000014113
Sugano, K., Tack, J., Kuipers, E. J., Graham, D. Y., El-Omar, E. M., Miura, S., et al. (2015). Kyoto Global consensus report on helicobacter pylori gastritis. Gut 64, 1353–1367. doi: 10.1136/gutjnl-2015-309252
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Sun, L., Tu, H., Liu, J., Gong, Y., Xu, Q., Jing, J., et al. (2014). A comprehensive evaluation of fasting serum gastrin-17 as a predictor of diseased stomach in Chinese population. Scand. J. Gastroenterol. 49, 1164–1172. doi: 10.3109/00365521.2014.950693
Takaishi, S., Tu, S., Dubeykovskaya, Z. A., Whary, M. T., Muthupalani, S., Rickman, B. H., et al. (2009). Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am. J. Pathol. 175, 365–375. doi: 10.2353/ajpath.2009.081165
Tu, H., Sun, L., Dong, X., Gong, Y., Xu, Q., Jing, J., et al. (2017). A serological biopsy using five stomach-specific circulating biomarkers for gastric cancer risk assessment: A multi-phase study. Am. J. Gastroenterol. 112, 704–715. doi: 10.1038/ajg.2017.55
Wang, W., Jiang, W., Zhu, S., Sun, X., Li, P., Liu, K., et al. (2019). Assessment of prevalence and risk factors of helicobacter pylori infection in an oilfield community in hebei, China. BMC Gastroenterol. 19, 186. doi: 10.1186/s12876-019-1108-8
Watson, S. A., Grabowska, A. M., El-Zaatari, M., Takhar, A. (2006). Gastrin - active participant or bystander in gastric carcinogenesis? Nat. Rev. Cancer 6, 936–946. doi: 10.1038/nrc2014
Wong, M. C. S., Huang, J., Chan, P. S. F., Choi, P., Lao, X. Q., Chan, S. M., et al. (2021). Global incidence and mortality of gastric cancer 1980-2018. JAMA Netw. Open 4, e2118457. doi: 10.1001/jamanetworkopen.2021.18457
Xiong, X., Chen, J., He, M., Wu, T., Yang, H. (2020). Helicobacter pylori infection and the prevalence of hypertension in Chinese adults: The dongfeng-tongji cohort. J. Clin. Hypertens. (Greenwich) 22, 1389–1395. doi: 10.1111/jch.13928
Young, G. O., Brown, S., Stemmet, N., Lastovica, A. J., Marks, I. N., Modlin, I. M., et al. (2002). The pepsinogen releasing effect of helicobacter pylori lipopolysaccharide. Helicobacter 7, 30–38. doi: 10.1046/j.1523-5378.2002.00053.x
Young, G. O., Stemmet, N., Lastovica, A., van der Merwe, E. L., Louw, J. A., Modlin, I. M., et al. (1992). Helicobacter pylori lipopolysaccharide stimulates gastric mucosal pepsinogen secretion. Aliment Pharmacol. Ther. 6, 169–177. doi: 10.1111/j.1365-2036.1992.tb00260.x
Zagari, R. M., Pozzato, P., Martuzzi, C., Fuccio, L., Martinelli, G., Roda, E., et al. (2005). 13C-urea breath test to assess helicobacter pylori bacterial load. Helicobacter 10, 615–619. doi: 10.1111/j.1523-5378.2005.00358.x
Zagari, R. M., Rabitti, S., Greenwood, D. C., Eusebi, L. H., Vestito, A., Bazzoli, F. (2017). Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol. Ther. 46, 657–667. doi: 10.1111/apt.14248
Keywords: Helicobacter pylori, pepsinogen, gastrin-17, urea breath test, gastric cancer
Citation: Zhou J-p, Liu C-h, Liu B-w, Wang Y-j, Benghezal M, Marshall BJ, Tang H and Li H (2022) Association of serum pepsinogens and gastrin-17 with Helicobacter pylori infection assessed by urea breath test. Front. Cell. Infect. Microbiol. 12:980399. doi: 10.3389/fcimb.2022.980399
Received: 28 June 2022; Accepted: 01 August 2022;
Published: 16 August 2022.
Edited by:
Feng Wang, Affiliated Hospital of Nantong University, ChinaReviewed by:
Sujit Chaudhuri, AMRI Hospitals, IndiaQinbo Cai, The First Affiliated Hospital of Sun Yat-sen University, China
Nazefah Abdul Hamid, Universiti Sains Islam Malaysia, Malaysia
Copyright © 2022 Zhou, Liu, Liu, Wang, Benghezal, Marshall, Tang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Li, YW5ob25nOTk5OTlAMTYzLmNvbQ==; Hong Tang, aHRhbmc2MTk4QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Jun-peng Zhou
Jun-peng Zhou Chang-hai Liu
Chang-hai Liu Bo-wen Liu3†
Bo-wen Liu3† Mohammed Benghezal
Mohammed Benghezal Barry James Marshall
Barry James Marshall Hong Tang
Hong Tang Hong Li
Hong Li