
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Cell. Infect. Microbiol., 28 September 2022
Sec. Clinical Microbiology
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.971933
This article is part of the Research TopicLong COVID-19: Ultimate reasoning as a need for the search of proximate solutionsView all 7 articles
During the COVID-19 pandemic, there have been an increasing number of COVID-19 patients with cavitary or cystic lung lesions, re-positive or long-term positive nucleic acid tests, but the mechanism is still unclear. Lung cavities may appear at long time interval from initial onset of coronavirus infection, generally during the absorption phase of the disease. The main histopathological characteristic is diffuse alveolar damage and may have more severe symptoms after initial recovery from COVID-19 and an increased mortality rate. There are many possible etiologies of pulmonary cavities in COVID-19 patients and we hypothesize that occult SARS-CoV-2, in the form of biofilm, is harbored in the airway lacuna with other pathogenic microorganisms, which may be the cause of pulmonary cavities and repeated and long-term positive nucleic acid tests.
Novel coronavirus disease 2019 (COVID-19) is a kind of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The typical imaging findings of COVID-19 are bilateral grand glass opacities in the lungs (Adams et al., 2020; Chen et al., 2020a; Guan et al., 2020). Cavitary or cystic lung lesions are seldom found and can be ignored from time to time, however, they may lead to serious consequences like pneumothorax (Zoumot et al., 2021; Kalenchic et al., 2022), thus require more attention. Cavitary or cystic lung lesions in COVID-19 patients may be the result of direct SARS-CoV-2 infection, co-infection with bacterial, fungal or mycobacterial pathogens, secondary to co-existing interstitial lung lesions, cystic bronchiectasis, or barotrauma related to mechanical ventilation, malignancy or metastasis (Aggarwal et al., 2021). COVID-19 patient complicated with pulmonary cavities has a wide age span, including infant, child, adult and elderly patient (Chen et al., 2021; Egoryan et al., 2021; Ozgur and Dogan, 2021; Zoumot et al., 2021; He et al., 2022), can appear in both severe and mild COVID-19 (Selvaraj and Dapaah-Afriyie, 2020; Chen et al., 2020a; Afrazi et al., 2021; Aggarwal et al., 2021; Chen et al., 2021; Jafari et al., 2021; Zoumot et al., 2021; Kalenchic et al., 2022). Some scholars consider lung cavity formation as a late complication during COVID-19 recovery (Zoumot et al., 2021; Egoryan et al., 2021). However, whether pulmonary cavity is related to the presence of SARS-CoV-2 and the mechanism of occurrence still remain unclear. Besides, the number of patients with long-term positive nucleic acid tests or re-positive results during COVID-19 convalescence period has soared (He et al., 2020; Lu et al., 2020a; Wang et al., 2020b; Qiao et al., 2020; Lu et al., 2020a; Liang et al., 2021; Wu et al., 2021; Zhu et al., 2021; He et al., 2022; Maccio et al., 2022). The source of SARS-CoV-2 and the mechanism of re-positive have not been clarified either. Herein, we propose a relevant hypothesis based on existing reports.
As an aggregation form of microbes, biofilms are closely associated with nosocomial infections and over 80% bacterial infection is contained to biofilms (Liu et al., 2012; Gomez-Carretero et al., 2017; Sun et al., 2017; Arciola et al., 2018; Strom et al., 2020). According to research, the resistance of biofilm is approximately 100 to 1000 times that of its corresponding planktonic mode, thus obtaining a favorable adaption to adverse external environment. Biofilms can act as a barrier, shielding pathogenic microorganisms from drugs and patients’ immune response (Samrot et al., 2021; Ciofu et al., 2022). Multispecies biofilms exist both externally and within the host in nature (Von Borowski and Trentin, 2021). Fahrenfeld et al. detected the N1 and N2 genes of SARS-CoV-2 in sewage biofilms, which can be considered as a vitro model (Fahrenfeld et al., 2022). Actually, biofilms with viruses have been reported to exist in multiple microbiotas. Multispecies biofilms which encompass Gram-negative bacteria and filamentous fungi and enteric viruses have been spotted, as well as Candida albicans biofilms comprised with the coxsackievirus type B5 (CVB5) or herpes simplex virus 1 (HSV-1). Gomes’s team confirmed the presence of SARS-CoV-2 in oral biofilm samples (Gomes et al., 2021; Von Borowski and Trentin, 2021). In this paper, we hypothesize the possible mechanism of the long-term retention of SARS-CoV-2 in the form of biofilm which leads to lung cavities, re-positive and long-term positive nucleic acid tests (Figure 1).
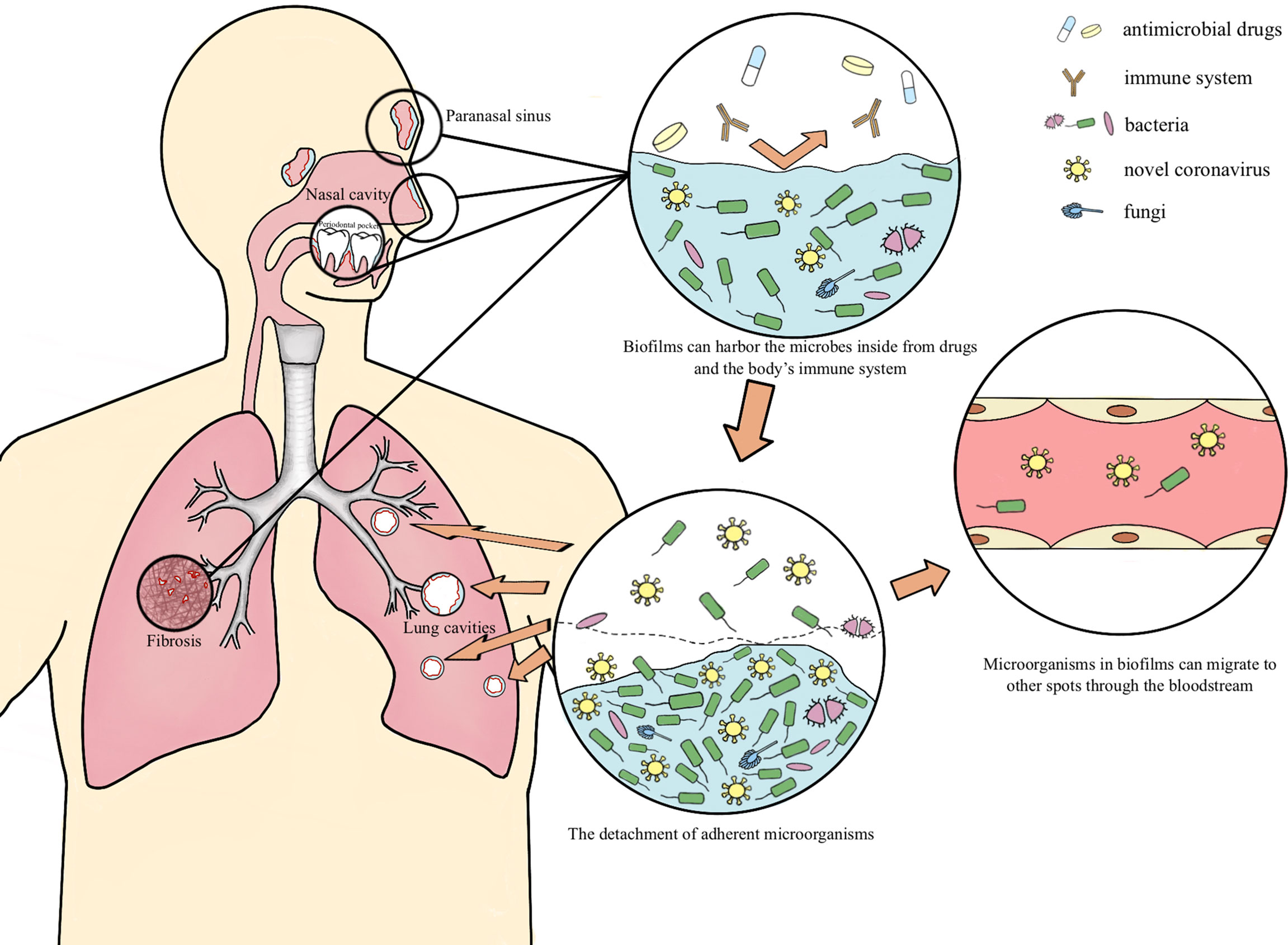
Figure 1 The possible mechanism of the long-term retention of SARS-CoV-2 Occult SARS-CoV-2 may coexist with other pathogenic microorganisms in respiratory lacuna (paranasal sinuses, periodontal clearance, bronchioloalveolar necrotic tissue and pulmonary fibrosis tissue, etc.) in the form of biofilm, and transfers into the lung then multiply when the body is in an immunocompromised situation, as well as migrates to other organs at the distance of the entrance through the bloodstream, accounting for the cavitation, re-positive and long-term positive nucleic acid tests during the COVID-19 rehabilitation.
Occult SARS-CoV-2 may coexist with other pathogenic microorganisms in respiratory lacuna (nasal sinuses, periodontal clearance, bronchioloalveolar necrotic tissue and pulmonary fibrosis tissue, etc.) in the form of biofilm, and transfers into the lung then multiply when the body is in an immunocompromised situation, accounting for the cavitation, re-positive and long-term positive nucleic acid tests during the COVID-19 rehabilitation (Figure 1).
Abundant cases of SARS-CoV-2 co-infection with other microorganisms have been reported, which were associated with venerable age, immunosuppression, cardiovascular disease, diabetes, intensive care unit attending, mechanical ventilation treatment, long-term antibiotic use, glucocorticoid therapy, prolonged hospitalization, exacerbation of symptoms, and poor prognosis (Bao et al., 2020; Blasco et al., 2020; Contou et al., 2020; Wang et al., 2020a; Alhumaid et al., 2021; He et al., 2021; Yasmin et al., 2021; Gomes et al., 2022; Hedberg et al., 2022; Ortega-Pena et al., 2022; Rouze et al., 2022; SeyedAlinaghi et al., 2022; Shetty et al., 2022). M. pneumoniae, P. aeruginosa, H. influenzae, and K. pneumoniae are bacterial co-pathogens usually detected (Lansbury et al., 2020). Streptococcus, Klebsiella and Staphylococcus are generally involved in pulmonary cavitation (Aggarwal et al., 2021). Diffuse alveolar damage is proved to be the primary histopathological manifestation of lung cavities, and pulmonary fibrosis can also be noticed when cavities appear in the late phase of COVID-19 (Aggarwal et al., 2021). Lung cavities can be present in patients with both severe and mild COVID-19 (Chen et al., 2020a; Selvaraj and Dapaah-Afriyie, 2020; Afrazi et al., 2021; Aggarwal et al., 2021; Chen et al., 2021; Jafari et al., 2021; Zoumot et al., 2021). During the convalescent period of COVID-19, SARS-CoV-2 may colonize in alveolar necrotic tissue and fibrotic lung tissue by hiding inside biofilms, resulting in sustained injury and necrosis, and eventually the formation of lung cavities. SARS-Cov-2 may be hidden in biofilms for a long time, and possible latent sites include nasal sinuses, oral cavity, bronchoalveolar necrotic tissue, fibrotic lung tissue, etc.
Rhoades et al. ‘s study found that the nasal microbiome of COVID-19 patients had an increase in bacterial pathogens, such as Pseudomonas aeruginosa, Acinetobacter, Roche, etc., and have positive correlation with SARS-CoV-2 RNA load (Rhoades et al., 2021). The presence of biofilms in the sinuses of experimental animals infected with Pseudomonas aeruginosa was identified by scanning electron microscopy (Vlastarakos et al., 2007). A systematic review of COVID-19 and Mucor co-infection shows that the most common co-infection spots are the nasal cavity, sinuses, and orbit, and can further co-infect with Aspergillus (SeyedAlinaghi et al., 2022). In this review, 45 (33.6%) out of 134 co-infected patients died, and related co-infection risk factors include diabetes, glucocorticoids therapy, immunomodulatory drugs use, immunocompromised status, hypertension, hematological malignancies, etc. Sufficient evidences have been documented to prove the existence of biofilm in the sinus mucosa of patients with chronic sinusitis (Bendouah et al., 2006; Palmer, 2006; Hunsaker and Leid, 2008; Bezerra et al., 2009; Kłodzińska et al., 2016; Michalik et al., 2018). There are more opportunistic bacteria and less symbiotic microorganisms in the nasal sinus of chronic sinusitis patients (Taylor et al., 2021). Biofilms can also be observed in healthy sinus mucosa (Vlastarakos et al., 2007; Mladina et al., 2010). We speculate that biofilms in nasal cavity and sinuses might harbor SARS-CoV-2, especially in patients with a history of chronic inflammation at these sites (Figure 1).
The periodontal pocket provides a unique subgingival environment and is an ideal location for pathogenic microorganisms to gather and produce biofilm. The dental root wall permits the formation of subgingival biofilms (Colombo and Tanner, 2019; Badran et al., 2020). SARS-CoV-2 has a certain resistance to the external environment, permitting its attachment to dental biofilms before being eliminated, which depends on the organic polymers such as polysaccharides, glycoproteins and lipids inside biofilms (Rabin et al., 2015; Scheller et al., 2020; Loveday et al., 2021; Samrot et al., 2021). Gomes’s team has confirmed the presence of SARS-CoV-2 in oral biofilm samples (Gomes et al., 2021). In this study, a total of 70 participants who had positive results for SARS-CoV-2 RNA from nasal/oropharyngeal swab samples using real-time quantitative polymerase chain reaction analysis were included. The result revealed that SARS-CoV-2 RNA were positive in 13 biofilm samples. Their another study showed that SARS-CoV-2 RNA was detected in oral biofilms in 26 out of 52 COVID-19 patients from the ICU, and it’s turn out that 96.2% positive samples were from subgingival specimens (Gomes et al., 2022). Furthermore, there are also other kinds of viral components within dental plaque (Das et al., 2012; Edlund et al., 2015), such as bacteriophage, herpes simplex virus (HSV) and Epstein-Barr virus (EBV) etc. Viruses in oral biofilms can also migrate to other spots through the bloodstream (Badran et al., 2020). SARS-CoV-2 may obtain strong resistance of viral drugs and human immune system and remain latent in the body for a long time by co-existing with other pathogenic microorganisms in subgingival biofilms. At the stage of biofilm detachment, due to anaerobic environment, nutrition deficiency and temperature altering, the secretion of dispersin B begin to increase (Samrot et al., 2021). Dispersin B is an enzyme which exists in the microbial extracellular matrix with the function of degrading polysaccharides in extracellular polymeric substances, permitting the detachment of adherent microorganisms (Samrot et al., 2021). We conjecture that SARS-CoV-2 can disengage from the biofilm along with bacteria, enter the oral cavity, mix with saliva, thus can be detected, and can also pass through the respiratory tract, reach the lung then cause cavitary lung lesions, and even spread to other parts through bloodstream, especially in immunocompromised patients (Figure 1).
A great number of research studies have indicated that lung microbial communities include bacteria and other non-bacterial organisms, comprising fungi and viruses. SARS-CoV-2 may diffuse in the lung as aerosols, then colonize though pulmonary mucus (Nguyen et al., 2015; Chen et al., 2022). Gherlan et al. reported a case that the COVID-19 patient got the entire right lung removed, the direct microscopic detection confirmed A. baumannii, Mucorales, Candida species (Gherlan et al., 2022). Maccio et al. noticed accompanying bacterial pneumonia (23/35,66%) and diffuse alveolar damage (24/35,69%) in the autopsies of 35 COVID-19 patients, and lung aspergillosis was an associative double infection (Maccio et al., 2022). Edler et al. also found that some COVID-19 patients have bacterial superinfected bronchopneumonia (Edler et al., 2020). Chen et al. found Veillonella and Capnocytophaga in the bronchoalveolar lavage fluid (BALF) of COVID-19 patients (Chen et al., 2020b). Hedberg et al. discovered the presence of bacterial co-infection through the culture of samples from lower respiratory tract (Hedberg et al., 2022). In addition, some reports spot that COVID-19 can co-exist with tuberculosis or latent tuberculosis infection (Ata et al., 2020; Luke et al., 2022). Wang et al. collected BALF and sputum samples from COVID-19 patients and cultured Aspergillus (Wang et al., 2020a). Song et al.’ retrospective analysis indicates the presence of fungal and SARS-CoV-2 co-infections, particularly in immunocompromised and severe COVID-19 patients (Song et al., 2020). COVID-19 patients co-infected with Candida, Mucor and Cryptococcus have been reported as well (Zhu et al., 2020). Shen et al. found that there are similarities between the microorganisms of patients with community-acquired pneumonia (CAP) and COVID-19 patients, both contain an increasing number of symbiotic bacteria or are occupied by pathogens in oral and upper respiratory tract (Shen et al., 2020). Ren et al. detected Pseudomonas, Streptococcus and Acinetobacter Baumannii in the BALF of 5 hospitalized COVID-19 patients (Ren et al., 2020). Divisi et al. also determined the existence of Pseudomonas aeruginosa in COVID-19 patients’ pleural empyema (Divisi et al., 2021). SARS-CoV-2 can lead to a relatively anoxic environment in the lung, which is conducive to the presence and growth of oral anaerobe and facultative anaerobe (Bao et al., 2020). Hazardous factors, for instance, ventilation treatment, cough, increased inhalation and inferior oral hygiene offer opportunity for oral microbiome to migrate into lower respiratory tract (Bao et al., 2020).
Characteristics of cases that SARS-CoV-2 co-infects with other pathogens include venerable age, glucocorticoid therapy, immunocompromised state, long-term antibiotic use history, diabetes, cardiovascular comorbidities, intensive care unit attending, ventilation treatment, prolonged hospitalization time, and exacerbation of symptoms (Bao et al., 2020; Blasco et al., 2020; Contou et al., 2020; Wang et al., 2020a; Alhumaid et al., 2021; He et al., 2021; Yasmin et al., 2021; Gomes et al., 2022; Hedberg et al., 2022; Ortega-Pena et al., 2022; Rouze et al., 2022; SeyedAlinaghi et al., 2022; Shetty et al., 2022). Bacterial pathogens generally isolated cover M. pneumoniae, P. aeruginosa, H. influenzae, K. pneumoniae and S. pneumoniae (Lansbury et al., 2020). Streptococcus, Klebsiella and Staphylococcus are generally involved in pulmonary cavitation (Aggarwal et al., 2021). Langford et al. found bacterial co-infection and secondary bacterial infection account for 3.5% and 14.3% of COVID-19 patients respectively. Bacterial infection was found in 6.9% of COVID-19 patients overall, which was more frequent in seriously ill patients (Langford et al., 2020). A multi-center study showed that about 9.5% of the COVID-19 patients included in this study had clinically diagnosed bacterial co-infection, with an increased risk of bacterial co-infection in patients with advanced age or cardiovascular comorbidities (He et al., 2021). The total proportion of mortality in SARS-CoV-2 and fungal co-infection patients was 0.17, and there is an obvious distinction between the proportion of mixed hospitalized population and ICU patients (0.06 vs 0.36) (Peng et al., 2021). Contou et al. reported that 28% of severe COVID-19 patients had bacterial co-infection in their course of ICU hospitalization, related bacteria include Enterobacteriaceae, Staphylococcus aureus, Streptococcus pneumoniae and Haemophilus influenzae (Contou et al., 2020). The total mortality of COVID-19 patients with other pathogens co-infection increased significantly (Contou et al., 2020; Mirzaei et al., 2020; Alhumaid et al., 2021; He et al., 2021; Peng et al., 2021; Maccio et al., 2022; Rouze et al., 2022; SeyedAlinaghi et al., 2022). Many other studies have documented antibiotic resistance in COVID-19 patients with bacterial infections (He et al., 2021). The formation of biofilms can significantly enhance the resistance of microorganisms to antibiotics and the host immune attack, resulting in persistent and chronic infections, which is connected with high morbidity and mortality of diseases (Samrot et al., 2021). Some researchers recommend that patients be regularly screened for bacterial and fungal co-infections following a definite diagnose of SARS-CoV-2 infection (Zhu et al., 2020; White et al., 2021).
There are increasing cases of COVID-19 patients with cavitary lung lesions, re-positive or long-term positive nucleic acid tests (Chen et al., 2020a; He et al., 2020; Qiao et al., 2020; Selvaraj and Dapaah-Afriyie, 2020; Lu et al., 2020a; Lu et al., 2020a; Wang et al., 2020b; Aggarwal et al., 2021; Afrazi et al., 2021; Chen et al., 2021; Egoryan et al., 2021; Jafari et al., 2021; Liang et al., 2021; Ozgur and Dogan, 2021; Wu et al., 2021; Zhu et al., 2021; Zoumot et al., 2021; He et al., 2022; Maccio et al., 2022). Lung cavities appear at a long-time interval from initial novel coronavirus infection, generally during the absorption phase of the disease, may have severer symptoms after initial recovery and also an increasing mortality rate (Aggarwal et al., 2021; Chen et al., 2021; Egoryan et al., 2021; He et al., 2022; Zoumot et al., 2021). Maccio et al. performed autopsies on 35 COVID-19 patients and found that patients could still show diffuse alveolar damage 2 months after the initial diagnosis of COVID-19 (Maccio et al., 2022). Diffuse alveolar damage has a significant correlation with the persistence of SARS-CoV-2 RNA in the lung (Maccio et al., 2022). The main histopathological manifestations of lung cavities were also diffuse alveolar damage (Aggarwal et al., 2021). SARS-CoV-2 can multiply in alveolar epithelial cells and bronchiole mucosa, resulting in diffuse alveolar damage, alveolar cell hyperplasia, fibroblast hyperplasia and pulmonary fibrosis (Chen et al., 2021; Huang and Tang, 2021). These evidences suggest that SARS-CoV-2 may stay persistent and latent in bronchoalveolar necrotic tissue or fibrotic lung tissue. We hypothesize that occult SARS-CoV-2 may hide in the biofilm to evade the attack of antiviral drugs and the host immune system attack, and remain latent in the body, then, lead to cavitary lung lesions and re-positive nucleic acid tests through replication for a period of time (Figure 1). Persistent and repeated positive nucleic acid tests suggest that SARS-CoV-2 may retain for a long time and cause chronic lung damage, possibly associated with more serious pulmonary complications. The limitations about this hypothesis are that: the chance of contamination of upper respiratory population do exist during the bronchoalveolar lavage, although much measures have been adapted to minimize the possibility, such as the standard sterile procedure, the use of sterile reagent and the disinfectant immersion of the bronchoscope before use, we still cannot exclude the contamination completely (Pang et al., 1989). Besides, diffuse alveolar damage is not only the underlying pathophysiology of cystic or cavitary lung lesions, but the underlying pathophysiology of acute respiratory distress syndrome (ARDS) which represents a typical condition for severe COVID-19 patients. This kind of patients usually need mechanical ventilation which itself can induce barotrauma and ventilator associated lung injury (VILI). Thus, being a potential factor in the formation of cystic or cavitary pulmonary lesion (Cardinal-Fernandez et al., 2017). But the existence of some mild COVID-19 cases with cystic or cavitary lung lesions relatively increases the possibility of SARS-CoV-2 directed lung lesions for the low chance of ARDS in mild cases (Chen et al., 2021; He et al., 2022).
Some COVID-19 patients had an elevated level of inflammatory cytokines and biomarkers associated with bacterial co-infection (Mirzaei et al., 2020). Joseph et al. proposed that bioluminescent imaging technique may be considered as a promising method to confirm the relevant biofilm load of COVID-19 patients, providing new strategy for clinical diagnosis (Joseph and Steier, 2022). The hypothesis that SARS-CoV-2 may co-exist in biofilms with other microorganisms depends on available evidences and clinical observation. If this hypothesis is confirmed, future researches are supposed to focus on finding suitable biomarkers or applying new techniques to help identify SARS-CoV-2 hiding in biofilms and using antibiofilm agents (such as bioactive compounds) in combination concerning COVID-19 treatment.
DH and CF wrote the initial manuscript. DH drew the figure. MN and XH revised the manuscript. YC and SS coordinated, supervised, and critically reviewed the manuscript for important intellectual content. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
National Natural Science Foundation of China (No: 82272980, 82060426); Yunnan Health Training Project of High-Level Talents (H-2018025).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, H. J. A., Kwee, T. C., Yakar, D., Hope, M. D., Kwee, R. M. (2020). Chest CT imaging signature of coronavirus disease 2019 infection: In pursuit of the scientific evidence. Chest 158, 1885–1895. doi: 10.1016/j.chest.2020.06.025
Afrazi, A., Garcia-Rodriguez, S., Maloney, J. D., Morgan, C. T. (2021). Cavitary lung lesions and pneumothorax in a healthy patient with active coronavirus-19 (COVID-19) viral pneumonia. Interact. Cardiovasc. Thorac. Surg. 32, 150–152. doi: 10.1093/icvts/ivaa238
Aggarwal, A., Tandon, A., Bhatt, S., Aggarwal, A., Dagar, S., Bansal, H. (2021). COVID19 pneumonia with cavitation and cystic lung changes: multi-detector computed tomography spectrum of a gamut of etiologies. BJR. Open 3, 20210007. doi: 10.1259/bjro.20210007
Alhumaid, S., Al Mutair, A., Al Alawi, Z., Alshawi, A. M., Alomran, S. A., Almuhanna, M. S., et al. (2021). Coinfections with bacteria, fungi, and respiratory viruses in patients with SARS-CoV-2: A systematic review and meta-analysis. Pathogens 10 (7), 809. doi: 10.3390/pathogens10070809
Arciola, C. R., Campoccia, D., Montanaro, L. (2018). Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 16, 397–409. doi: 10.1038/s41579-018-0019-y
Ata, F., Yousaf, Q., Veliyankodan Parambil, J., Parengal, J., Mohamedali, M. G., Yousaf, Z. (2020). A 28-Year-Old man from India with SARS-Cov-2 and pulmonary tuberculosis Co-infection with central nervous system involvement. Am. J. Case Rep. 21, e926034. doi: 10.12659/AJCR.926034
Badran, Z., Gaudin, A., Struillou, X., Amador, G., Soueidan, A. (2020). Periodontal pockets: A potential reservoir for SARS-CoV-2? Med. Hypotheses 143, 109907. doi: 10.1016/j.mehy.2020.109907
Bao, L., Zhang, C., Dong, J., Zhao, L., Li, Y., Sun, J. (2020). Oral microbiome and SARS-CoV-2: Beware of lung Co-infection. Front. Microbiol. 11, 1840. doi: 10.3389/fmicb.2020.01840
Bendouah, Z., Barbeau, J., Hamad, W. A., Desrosiers, M. (2006). Biofilm formation by staphylococcus aureus and pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol. Head Neck Surg. 134, 991–996. doi: 10.1016/j.otohns.2006.03.001
Bezerra, T. F., Pádua, F. G., Ogawa, A. I., Gebrim, E. M., Saldiva, P. H., Voegels, R. L. (2009). Biofilm in chronic sinusitis with nasal polyps: pilot study. Braz. J. Otorhinolaryngol. 75, 788–793. doi: 10.1590/S1808-86942009000600003
Blasco, M. L., Buesa, J., Colomina, J., Forner, M. J., Galindo, M. J., Navarro, J., et al. (2020). Co-Detection of respiratory pathogens in patients hospitalized with coronavirus viral disease-2019 pneumonia. J. Med. Virol. 92, 1799–1801. doi: 10.1002/jmv.25922
Cardinal-Fernandez, P., Lorente, J. A., Ballen-Barragan, A., Matute-Bello, G. (2017). Acute respiratory distress syndrome and diffuse alveolar damage. new insights on a complex relationship. Ann. Am. Thorac. Soc. 14, 844–850. doi: 10.1513/AnnalsATS.201609-728PS
Chen, Y., Chen, W., Zhou, J., Sun, C., Lei, Y. (2021). Large Pulmonary cavity in COVID-19 cured patient case report. Ann. Palliat. Med. 10, 5786–5791. doi: 10.21037/apm-20-452
Chen, Y., Huang, Y., Ding, X., Yang, Z., He, L., Ning, M., et al. (2022). A multi-omics study of familial lung cancer: Microbiome and host gene expression patterns. Front. Immunol. 13, 827953. doi: 10.3389/fimmu.2022.827953
Chen, L., Liu, W., Zhang, Q., Xu, K., Ye, G., Wu, W., et al. (2020b). RNA Based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 wuhan outbreak. Emerg. Microbes Infect. 9, 313–319. doi: 10.1080/22221751.2020.1725399
Chen, J., Peng, S., Zhang, B., Liu, Z., Liu, L., Zhang, W. (2020a). An uncommon manifestation of COVID-19 pneumonia on CT scan with small cavities in the lungs: A case report. Med. (Baltimore). 99, e21240. doi: 10.1097/MD.0000000000021240
Ciofu, O., Moser, C., Jensen, P. O., Hoiby, N. (2022). Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol 20 (10), 621–35. doi: 10.1038/s41579-022-00682-4
Colombo, A. P. V., Tanner, A. C. R. (2019). The role of bacterial biofilms in dental caries and periodontal and peri-implant diseases: A historical perspective. J. Dent. Res. 98, 373–385. doi: 10.1177/0022034519830686
Contou, D., Claudinon, A., Pajot, O., Micaelo, M., Longuet Flandre, P., Dubert, M., et al. (2020). Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 10, 119. doi: 10.1186/s13613-020-00736-x
Das, S., Krithiga, G. S., Gopalakrishnan, S. (2012). Detection of human herpes viruses in patients with chronic and aggressive periodontitis and relationship between viruses and clinical parameters. J. Oral. Maxillofac. Pathol. 16, 203–209. doi: 10.4103/0973-029X.98502
Divisi, D., Zaccagna, G., Angeletti, C., Cicerone, E., De Vico, A., Moretti, R., et al. (2021). Pleural empyema associated with alveolar-pleural fistulas in severe acute respiratory syndrome coronavirus 2. Clin. Case Rep. 9 (6), e04262. doi: 10.1002/ccr3.4262
Edler, C., Schroder, A. S., Aepfelbacher, M., Fitzek, A., Heinemann, A., Heinrich, F., et al. (2020). Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Legal. Med. 134, 1275–1284. doi: 10.1007/s00414-020-02317-w
Edlund, A., Santiago-Rodriguez, T. M., Boehm, T. K., Pride, D. T. (2015). Bacteriophage and their potential roles in the human oral cavity. J. Oral. Microbiol. 7, 27423. doi: 10.3402/jom.v7.27423
Egoryan, G., Hyser, E., Mushtaq, A. H., Yanez-Bello, M. A., Trelles-Garcia, D. P., Friedman, H. J., et al. (2021). Development of cavitary lung disease as a long-term complication of coronavirus disease 2019 in a young previously healthy patient: a case report. J. Med. Case Rep. 15, 377. doi: 10.1186/s13256-021-02961-9
Fahrenfeld, N. L., Morales Medina, W. R., D'elia, S., Modica, M., Ruiz, A., Mclane, M. (2022). Comparison of residential dormitory COVID-19 monitoring via weekly saliva testing and sewage monitoring. Sci. Total. Environ. 814, 151947. doi: 10.1016/j.scitotenv.2021.151947
Gherlan, G. S., Hoara, M. C., Smadu, S. G., Popescu, C. P., Ionescu, P., Florescu, S. A. (2022). Histopathologically confirmed pulmonary mucormycosis as a complication of COVID-19: a case report from Romania and insight into pathology. Maedica. (Bucur). 17, 215–225. doi: 10.26574/maedica.2022.17.1.215
Gomes, S. C., Da Fonseca, J. G., Miller, L. M., Manenti, L., Angst, P. D. M., Lamers, M. L., et al. (2022). SARS-CoV-2 RNA in dental biofilms: Supragingival and subgingival findings from inpatients in a COVID-19 intensive care unit. J. Periodontol 3, 10.1002/JPER.21-0623. doi: 10.1002/JPER.21-0623
Gomes, S. C., Fachin, S., Da Fonseca, J. G., Angst, P. D. M., Lamers, M. L., Da Silva, I. S. B., et al. (2021). Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J. Clin. Periodontol. 48, 880–885. doi: 10.1111/jcpe.13471
Gomez-Carretero, S., Nybom, R., Richter-Dahlfors, A. (2017). Electroenhanced antimicrobial coating based on conjugated polymers with covalently coupled silver nanoparticles prevents staphylococcus aureus biofilm formation. Adv. Healthc. Mater. 6 (20). doi: 10.1002/adhm.201700435
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. doi: 10.1056/NEJMoa2002032
Hedberg, P., Johansson, N., Ternhag, A., Abdel-Halim, L., Hedlund, J., Naucler, P. (2022). Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect. Dis. 22, 108. doi: 10.1186/s12879-022-07089-9
He, S., Liu, W., Jiang, M., Huang, P., Xiang, Z., Deng, D., et al. (2021). Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: A multi-center study. PloS One 16, e0249668. doi: 10.1371/journal.pone.0249668
He, D., Sun, C., Shang, Z., Yang, Y., Chen, Y. (2022). Multiple small pneumatoceles as a complication of a SARS-CoV-2 infection in a child from Myanmar. J. Travel. Med 29 (3), taac026. doi: 10.1093/jtm/taac026
He, S., Zhou, K., Hu, M., Liu, C., Xie, L., Sun, S., et al. (2020). Clinical characteristics of "re-positive" discharged COVID-19 pneumonia patients in wuhan, China. Sci. Rep. 10, 17365. doi: 10.1038/s41598-020-74284-6
Huang, W. J., Tang, X. X. (2021). Virus infection induced pulmonary fibrosis. J. Transl. Med. 19, 496. doi: 10.1186/s12967-021-03159-9
Hunsaker, D. H., Leid, J. G. (2008). The relationship of biofilms to chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 16, 237–241. doi: 10.1097/MOO.0b013e3282fdc6d5
Jafari, R., Cegolon, L., Masghsoudi, H., Zhao, S., Fathi, S., Khedmat, L., et al. (2021). Simultaneous giant cavity pulmonary lesion and pneumothorax following COVID-19 pneumonia. Radiol. Case Rep. 16, 2534–2536. doi: 10.1016/j.radcr.2021.06.026
Joseph, B., Steier, L. (2022). Bioluminescence and ventilator-associated pneumonia caused by oral biofilm in ICU during COVID-19 -is there a possible relationship? Med. Hypotheses 159, 110760. doi: 10.1016/j.mehy.2021.110760
Kalenchic, T. I., Kabak, S. L., Primak, S. V., Melnichenko, Y. M., Kudelich, O. A. (2022). Bilateral parapneumonic pleural effusion with pneumothorax in a patient with covid 19 pneumonia: case report. Radiol. Case Rep. 17, 869–874. doi: 10.1016/j.radcr.2021.12.039
Kłodzińska, S. N., Priemel, P. A., Rades, T., Mørck Nielsen, H. (2016). Inhalable antimicrobials for treatment of bacterial biofilm-associated sinusitis in cystic fibrosis patients: Challenges and drug delivery approaches. Int. J. Mol. Sci. 17 (10), 1688. doi: 10.3390/ijms17101688
Langford, B. J., So, M., Raybardhan, S., Leung, V., Westwood, D., Macfadden, D. R., et al. (2020). Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin. Microbiol. Infect. 26, 1622–1629. doi: 10.1016/j.cmi.2020.07.016
Lansbury, L., Lim, B., Baskaran, V., Lim, W. S. (2020). Co-Infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 81, 266–275. doi: 10.1016/j.jinf.2020.05.046
Liang, L., Guo, Q., Zhang, H., Lin, S., Zheng, H., Li, B., et al. (2021). Low infectious risk of re-positive COVID-19 patients: a single-center study. Int. J. Infect. Dis. 111, 5–9. doi: 10.1016/j.ijid.2021.08.019
Liu, L., Tan, X., Jia, A. (2012). [Relationship between bacterial quorum sensing and biofilm formation–a review]. Wei. Sheng. Wu. Xue. Bao. 52, 271–278.
Loveday, E. K., Hain, K. S., Kochetkova, I., Hedges, J. F., Robison, A., Snyder, D. T., et al. (2021). Effect of inactivation methods on SARS-CoV-2 virion protein and structure. Viruses 13 (4), 562. doi: 10.3390/v13040562
Luke, E., Swafford, K., Shirazi, G., Venketaraman, V. (2022). TB and COVID-19: An exploration of the characteristics and resulting complications of Co-infection. Front. Biosci. (Schol. Ed). 14, 6. doi: 10.31083/j.fbs1401006
Lu, J., Peng, J., Xiong, Q., Liu, Z., Lin, H., Tan, X., et al. (2020a). Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. EBioMedicine 59, 102960. doi: 10.1016/j.ebiom.2020.102960
Maccio, U., Zinkernagel, A. S., Schuepbach, R., Probst-Mueller, E., Frontzek, K., Brugger, S. D., et al. (2022). Long-term persisting SARS-CoV-2 RNA and pathological findings: Lessons learnt from a series of 35 COVID-19 autopsies. Front. Med. (Lausanne). 9, 778489. doi: 10.3389/fmed.2022.778489
Michalik, M., Samet, A., Marszałek, A., Krawczyk, B., Kotłowski, R., Nowicki, A., et al. (2018). Intra-operative biopsy in chronic sinusitis detects pathogenic escherichia coli that carry fimG/H, fyuA and agn43 genes coding biofilm formation. PloS One 13, e0192899. doi: 10.1371/journal.pone.0192899
Mirzaei, R., Goodarzi, P., Asadi, M., Soltani, A., Aljanabi, H. A. A., Jeda, A. S., et al. (2020). Bacterial co-infections with SARS-CoV-2. IUBMB Life 72, 2097–2111. doi: 10.1002/iub.2356
Mladina, R., Skitarelić, N., Musić, S., Ristić, M. (2010). A biofilm exists on healthy mucosa of the paranasal sinuses: a prospectively performed, blinded, scanning electron microscope study. Clin. Otolaryngol. 35, 104–110. doi: 10.1111/j.1749-4486.2010.02097.x
Nguyen, L. D., Viscogliosi, E., Delhaes, L. (2015). The lung mycobiome: an emerging field of the human respiratory microbiome. Front. Microbiol. 6, 89. doi: 10.3389/fmicb.2015.00089
Ortega-Pena, S., Rodriguez-Martinez, S., Cancino-Diaz, M. E., Cancino-Diaz, J. C. (2022). Staphylococcus epidermidis controls opportunistic pathogens in the nose, could it help to regulate SARS-CoV-2 (COVID-19) infection? Life (Basel). 12 (3), 341. doi: 10.3390/life12030341
Ozgur, C., Dogan, C. (2021). Multiple pneumatoceles and diffuse ground-glass opacities in a 20-month-old boy with COVID-19 pneumonia. Respirol. Case Rep. 9, e0842. doi: 10.1002/rcr2.842
Palmer, J. (2006). Bacterial biofilms in chronic rhinosinusitis. Ann. Otol. Rhinol. Laryngol. Suppl. 196, 35–39. doi: 10.1177/00034894061150S906
Pang, J. A., Cheng, A. F., Chan, H. S., French, G. L. (1989). Special precautions reduce oropharyngeal contamination in bronchoalveolar lavage for bacteriologic studies. Lung 167, 261–267. doi: 10.1007/BF02714955
Peng, J., Wang, Q., Mei, H., Zheng, H., Liang, G., She, X., et al. (2021). Fungal co-infection in COVID-19 patients: evidence from a systematic review and meta-analysis. Aging (Albany. NY). 13, 7745–7757. doi: 10.18632/aging.202742
Qiao, X. M., Xu, X. F., Zi, H., Liu, G. X., Li, B. H., Du, X., et al. (2020). Re-positive cases of nucleic acid tests in discharged patients with COVID-19: A follow-up study. Front. Med. (Lausanne). 7, 349. doi: 10.3389/fmed.2020.00349
Rabin, N., Zheng, Y., Opoku-Temeng, C., Du, Y., Bonsu, E., Sintim, H. O. (2015). Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 7, 493–512. doi: 10.4155/fmc.15.6
Ren, L. L., Wang, Y. M., Wu, Z. Q., Xiang, Z. C., Guo, L., Xu, T., et al. (2020). Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. (Engl). 133, 1015–1024. doi: 10.1097/CM9.0000000000000722
Rhoades, N. S., Pinski, A. N., Monsibais, A. N., Jankeel, A., Doratt, B. M., Cinco, I. R., et al. (2021). Acute SARS-CoV-2 infection is associated with an increased abundance of bacterial pathogens, including pseudomonas aeruginosa in the nose. Cell Rep. 36, 109637. doi: 10.1016/j.celrep.2021.109637
Rouze, A., Lemaitre, E., Martin-Loeches, I., Povoa, P., Diaz, E., Nyga, R., et al. (2022). Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: a European multicenter comparative cohort study. Crit. Care 26, 11. doi: 10.1186/s13054-021-03874-1
Samrot, A. V., Abubakar Mohamed, A., Faradjeva, E., Si Jie, L., Hooi Sze, C., Arif, A., et al. (2021). Mechanisms and impact of biofilms and targeting of biofilms using bioactive compounds-a review. Medicina (Kaunas) 57 (8), 839. doi: 10.3390/medicina57080839
Scheller, C., Krebs, F., Minkner, R., Astner, I., Gil-Moles, M., Watzig, H. (2020). Physicochemical properties of SARS-CoV-2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control. Electrophoresis 41, 1137–1151. doi: 10.1002/elps.202000121
Selvaraj, V., Dapaah-Afriyie, K. (2020). Lung cavitation due to COVID-19 pneumonia. BMJ Case Rep. 13 (7), e237245. doi: 10.1136/bcr-2020-237245
SeyedAlinaghi, S., Karimi, A., Barzegary, A., Pashaei, Z., Afsahi, A. M., Alilou, S., et al. (2022). Mucormycosis infection in patients with COVID-19: A systematic review. Health Sci. Rep. 5, e529. doi: 10.1002/hsr2.529
Shen, Z., Xiao, Y., Kang, L., Ma, W., Shi, L., Zhang, L., et al. (2020). Genomic diversity of severe acute respiratory syndrome-coronavirus 2 in patients with coronavirus disease 2019. Clin. Infect. Dis. 71, 713–720. doi: 10.1093/cid/ciaa203
Shetty, S., Shilpa, C., Kavya, S., Sundararaman, A., Hegde, K., Madhan, S. (2022). Invasive aspergillosis of nose and paranasal sinus in COVID-19 convalescents: Mold goes viral? Indian J. Otolaryngol. Head Neck Surg. 14, 1–6. doi: 10.1007/s12070-022-03073-6
Song, G., Liang, G., Liu, W. (2020). Fungal Co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia 185, 599–606. doi: 10.1007/s11046-020-00462-9
Strom, M., Crowley, T., Shigdar, S. (2020). Novel detection of nasty bugs, prevention is better than cure. Int. J. Mol. Sci. 22 (1), 149. doi: 10.3390/ijms22010149
Sun, Y., Sun, F., Feng, W., Qiu, X., Liu, Y., Yang, B., et al. (2017). Hyperoside inhibits biofilm formation of pseudomonas aeruginosa. Exp. Ther. Med. 14, 1647–1652. doi: 10.3892/etm.2017.4641
Taylor, A., Fuzi, J., Sideris, A., Banks, C., Havas, T. E. (2021). Non-steroid, non-antibiotic anti-biofilm therapy for the treatment of chronic rhinosinusitis: a systematic review. J. Laryngology. Otology. 135, 196–205. doi: 10.1017/S0022215121000542
Vlastarakos, P. V., Nikolopoulos, T. P., Maragoudakis, P., Tzagaroulakis, A., Ferekidis, E. (2007). Biofilms in ear, nose, and throat infections: how important are they? Laryngoscope 117, 668–673. doi: 10.1097/MLG.0b013e318030e422
Von Borowski, R. G., Trentin, D. S. (2021). Biofilms and coronavirus reservoirs: a perspective review. Appl. Environ. Microbiol. 87, e0085921. doi: 10.1128/AEM.00859-21
Wang, Q. X., Huang, K. C., Qi, L., Zeng, X. H., Zheng, S. L. (2020b). No infectious risk of COVID-19 patients with long-term fecal 2019-nCoV nucleic acid positive. Eur. Rev. Med. Pharmacol. Sci. 24, 5772–5777. doi: 10.26355/eurrev_202005_21370
Wang, J., Yang, Q., Zhang, P., Sheng, J., Zhou, J., Qu, T. (2020a). Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in zhejiang, China: a retrospective case series. Crit. Care 24, 299. doi: 10.1186/s13054-020-03046-7
White, P. L., Dhillon, R., Cordey, A., Hughes, H., Faggian, F., Soni, S., et al. (2021). A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin. Infect. Dis. 73, e1634–e1644. doi: 10.1093/cid/ciaa1298
Wu, X., Wang, Z., He, Z., Li, Y., Wu, Y., Wang, H., et al. (2021). A follow-up study shows that recovered patients with re-positive PCR test in wuhan may not be infectious. BMC Med. 19, 77. doi: 10.1186/s12916-021-01954-1
Yasmin, F., Najeeb, H., Naeem, A., Dapke, K., Phadke, R., Asghar, M. S., et al. (2021). COVID-19 associated mucormycosis: A systematic review from diagnostic challenges to management. Diseases 9 (4), 65. doi: 10.3390/diseases9040065
Zhu, X., Ge, Y., Wu, T., Zhao, K., Chen, Y., Wu, B., et al. (2020). Co-Infection with respiratory pathogens among COVID-2019 cases. Virus Res. 285, 198005. doi: 10.1016/j.virusres.2020.198005
Zhu, X., Wang, M., Yang, F., Sun, Z., Yang, X., Yan, Y. (2021). Clinical characteristics and outcomes of COVID-19 long-term nucleic acid positive patients. Technol. Health Care 29, 849–858. doi: 10.3233/THC-212921
Keywords: SARS-CoV-2, biofilm, COVID-19, lung cavity, re-positive, co-infection
Citation: He D, Fu C, Ning M, Hu X, Li S and Chen Y (2022) Biofilms possibly harbor occult SARS-CoV-2 may explain lung cavity, re-positive and long-term positive results. Front. Cell. Infect. Microbiol. 12:971933. doi: 10.3389/fcimb.2022.971933
Received: 17 June 2022; Accepted: 14 September 2022;
Published: 28 September 2022.
Edited by:
Leo Pruimboom, Pontifical University of Salamanca, SpainReviewed by:
Shreya Singh, Dr. B.R. Ambedkar Institute of Medical Sciences, IndiaCopyright © 2022 He, Fu, Ning, Hu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Chen, MzY0MTAwMDhAcXEuY29t; Shanshan Li, ZmVuZ2xpeWFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.