
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 16 August 2022
Sec. Microbiome in Health and Disease
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.969526
This article is part of the Research TopicRising stars in Microbiome in Health and Disease: 2022View all 10 articles
The gut dysbiosis has emerged as a prominent player in the pathogenesis and development of colorectal cancer (CRC), which in turn intensifies dysregulated gut microbiota composition and inflammation. Since most drugs are given orally, this dysbiosis directly and indirectly impinges the absorption and metabolism of drugs in the gastrointestinal tract, and subsequently affects the clinical outcome of patients with CRC. Herbal medicine, including the natural bioactive products, have been used traditionally for centuries and can be considered as novel medicinal sources for anticancer drug discovery. Due to their various structures and pharmacological effects, natural products have been found to improve microbiota composition, repair intestinal barrier and reduce inflammation in human and animal models of CRC. This review summarizes the chemo-preventive effects of extracts and/or compounds derived from natural herbs as the promising antineoplastic agents against CRC, and will provide innovative strategies to counteract dysregulated microbiota and improve the lives of CRC patients.
The incidence of colorectal cancer (CRC) has boosted greatly in the last decades and has become the third leading cause of cancer death, which now accounts for approximately 10% of cancer-related mortality in the world (Bray et al., 2018). The high incidence of CRC has been attributed to the increasingly aging population, unfavorable dietary habits, low physical exercise and excessive obesity (Lund et al., 2011). With the in-depth application of high-throughput sequencing technologies, such as 16S rRNA, metagenomics and metatranscriptomics, in human gut microbiota, emerging evidence has implicated the microbiota in the pathogenesis and prognosis of CRC (Watanabe et al., 2020). Some bacterial species including Fusobacterium spp., Enterococcus spp., Escherichia coli, and Bacteroides spp. are most commonly associated with the onset and progression of CRC (Lennard et al., 2016). Changes in microbiota composition (dysbiosis) impair the gut barrier function of epithelial tight junctions and the mucus layer. Consequently, it increases the exposure of the epithelium to bacteria and their toxic metabolites, which may have carcinogenic potential to interfere with cell cycle regulation or directly damage DNA. Bacterial translocation also induces chronic inflammation and triggers a cascade of suppressive immune responses associated with the production of procarcinogens or chemicals such as reactive oxygen species (ROS), bacterial genotoxins (colibactin), and hydrogen sulfide (H2S) (Gagnière et al., 2016). In turn, the excessive oxidative stress aggravates colitis and neoplastic processes. Thus, targeting and improving gut microbial dysbiosis could be plausible therapeutic strategies for the prevention and treatment of CRC.
Herbal medicines have been used to prevent and treat diseases for thousands of years which are being developed into decoction and liquid extract for clinical application. When herbal medicines enter the digestive system, they will inevitably come into contact with gut microbes, which could limit excessive inflammatory response and maintain intestinal homeostasis (Chen et al., 2017). Some prodrugs derived from herbal medicines are produced under the metabolism of gut microbes, and subsequently display their antitumor effects to reduce tumor mass and prevent tumorigenesis through several mechanism (Meng et al., 2013; Chen et al., 2016). In addition, bioactive ingredients in herbal medicines may stimulate microorganisms to secrete certain endogenous substances which can enhance barrier stabilization and immune surveillance (Vivarelli et al., 2019). Despite these advances, the underlying molecular mechanism of herbal medicines and their bioactive compounds on microbe-mediated CRC remains extremely deficient. In this review, we highlight the importance of herbal medicine intervention on the intestinal microbiota as an instrument for dysbacteriosis, and consequently, for the prevention of colorectal cancer, suggesting anti-inflammatory, antioxidant and anticarcinogenic properties.
The gut microbiota constitutes a natural defensive barrier to infection. Growing evidence has demonstrated the role of gut microbes in promoting inflammatory responses, creating a suitable microenvironment for the development of skewed interactions between the gut microbiota and cancer initiation (Perillo et al., 2020). Thus, the gut microbiota has been proposed as a novel therapeutic target in light of recent promising data in which it seems to modulate the response to cancer immunotherapy. Moreover, the microbiota involves in numerous protective, structural and metabolic roles in the intestinal epithelium to maintain gut homeostasis. The human intestinal mucosal surface area is more than 200 m2. There are about 103 different microorganisms, which are 10-fold more than the total number of human cells (Sekirov et al., 2010). More than 3×107 genes in the gut microbiota are considered as the second genome of humans, and approximately 10% of the metabolite cycles occur in the human intestinal micro-ecological environment (Gill et al., 2006; Wu et al., 2013). CRC is frequently associated with dramatic alterations in the microbial composition of the tumor and adjacent mucosa (Figure 1). Clinical trials proved that the abundance of Fusobacterium nucleatum started at stage 0 and increased as the CRC progressed, while Atopobium parvulum and Actinomyces odontolyticus were significantly increased in patients with multiple polypoid adenomas and/or stage 0 but no longer increased in more advanced stages, Peptostreptococcus anaerobius, Peptostreptococcus stomatis, and Parvimonas micra increased at stage I~IV (Sobhani et al., 2011). In addition, the number of beneficial bacteria such as Bifidobacterium, Helicobacter oxysporum, and Haemophilus was reduced from the polyps to CRC stage 0 (Mizutani et al., 2020). Although the causal relationship between gut dysbiosis and CRC remains unclear, gut dysbiosis exacerbates the development of colorectal cancer mainly via intestinal inflammation, immunotolerance, and oxidative stress (Figure 2).
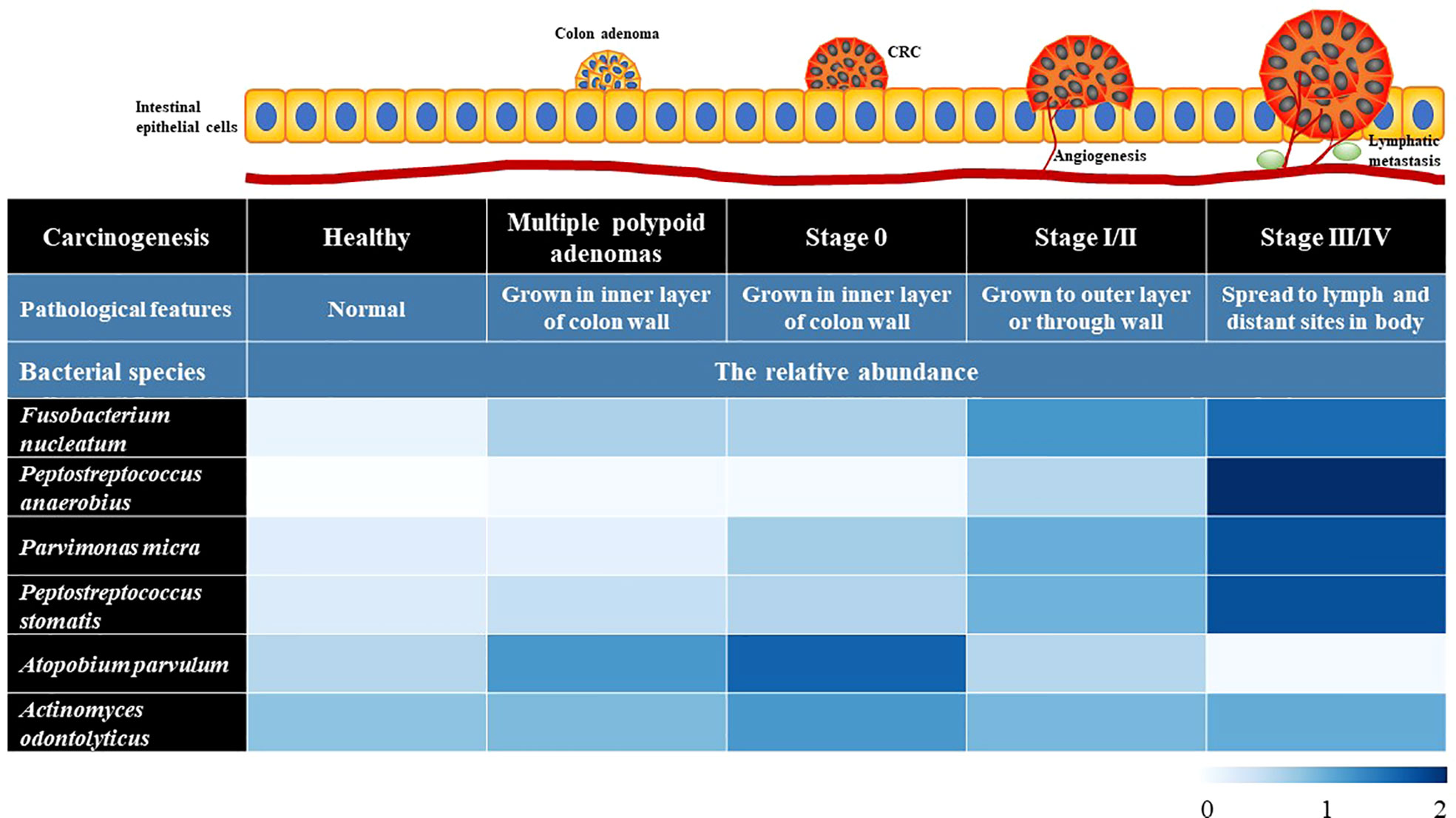
Figure 1 The profiles of gut microbiota in the intestinal tissues of healthy people and the CRC patients at different stages. The data in heatmap were obtained from the reference (Sobhani et al., 2011). The microbiome signature potentially can be used as auxiliary diagnostic biomarkers.
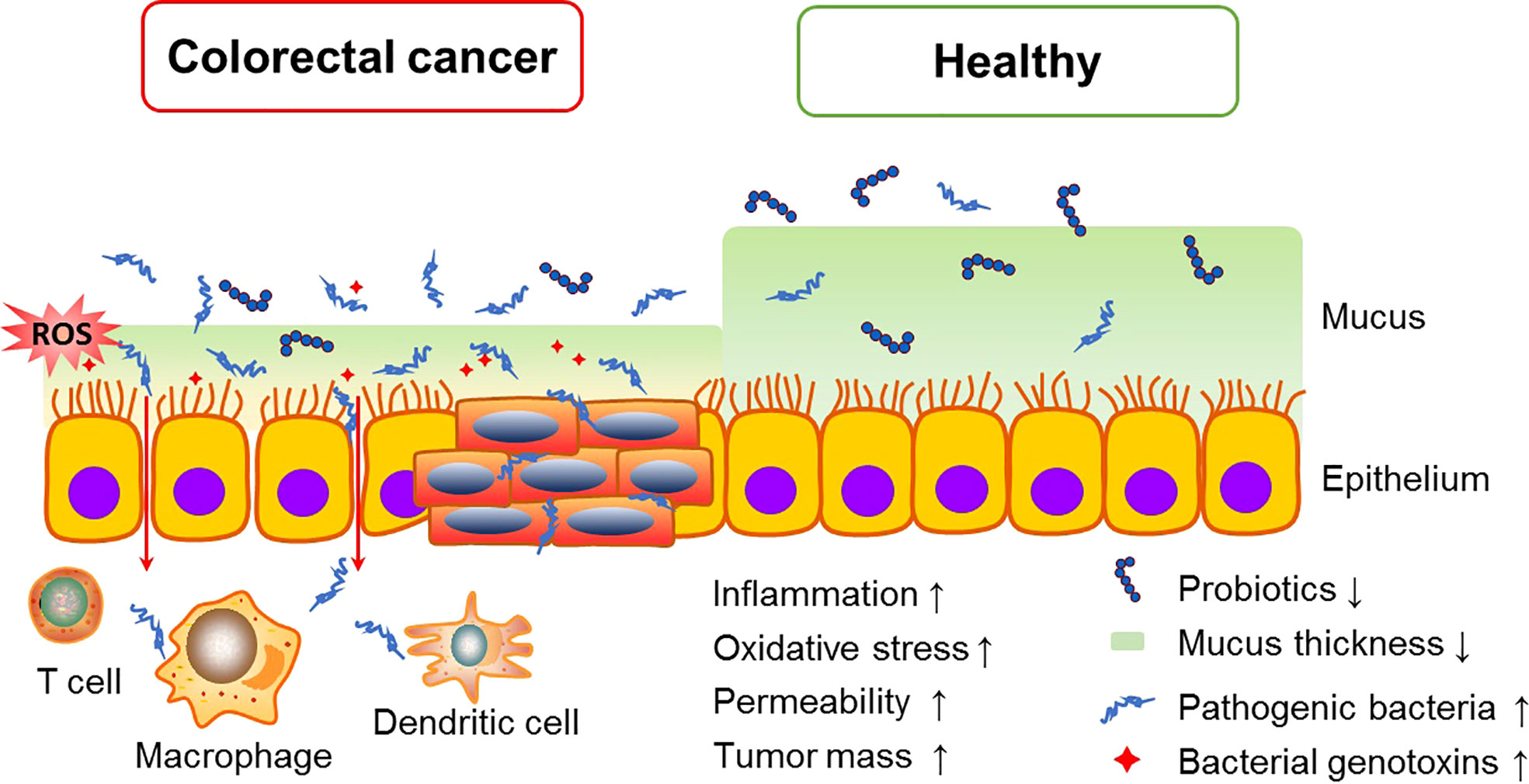
Figure 2 Intestinal dysbiosis accelerate CRC tumorigenesis. Overgrowth of pathogenic bacteria produce toxic metabolites, which can interfere with the cell cycle regulation and directly damage DNA, and also induce chronic inflammation and oxidative stress, consequently promoting CRC growth and spread.
Inflammations caused by gut microbes are the main mechanisms to induce tumorigenesis (Konstantinov et al., 2013). The disorder of gut microbiota induces the hyperpermeability of the intestinal wall, and then helps the pathogenic bacteria and its endotoxin to break out into the bloodstream, resulting in chronic inflammatory response and immuno-suppression (Avril and DePaolo, 2021). Chronic inflammation reshapes the tumor microenvironment, and promote tumorigenesis and even metastasis via activating numerous exogenous and endogenous signaling pathways (Yu and Fang, 2015). Pathogen recognition receptors (PPRs) are a series of innate immune receptors mainly including toll-like receptors (TLRs) and Nod-like receptors (NLRs). Once the intestinal epithelial barrier is breached, PPRs rapidly sense nucleic acids and antigen components from bacteria and subsequently induce the secretion of type I interferon and antimicrobial peptides to defense against intestinal pathogens. However, inappropriately vigorous innate immune responses can also activate cell survival signaling mainly via NF-κB, STAT3 and MAPK pathways, which elevate the transcription of pro-inflammatory cytokines. Then secondary enteric inflammatory challenges prolong systemic inflammation and expedite proliferation and metastasis of tumor cells (Fukata and Abreu, 2009). Indeed, the patients with inflammatory bowel disease (IBD) presented an increased risk of developing CRC (Feagins et al., 2009; Rogler, 2014). In those patients, the number of probiotics such as Lactobacillus and Bifidobacteria is reduced, while Parvimonas micra, Phascolarctobacterium, Streptococcus bovis and S. gallolyticus are increased (Uronis et al., 2009; Richard et al., 2018). Oral administration of antibiotics, probiotic preparation or antioxidants significantly decrease the number of mucosal nodules and suppress colon tumorigenesis in the azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced CRC mouse model (Hattori et al., 2019; Luo et al., 2019), suggesting that altering mucosa-associated bacterial microbiota and chronic inflammation in the IBD patients may be beneficial for CRC prevention.
Gut microbiota is also involved in the production of various enzymes and metabolites. During gastrointestinal tumorigenesis, the physiological capacities of several bacteria are changed, resulting in the odd levels of bacterial enzymes and their metabolites. Bacterial enzymes including β-glucuronidase, nitroso-reductase, nitrate reductase, β-glucosidase, azo-reductase and 7α-dehydrooxygenase from gut microbiota disorders can induce the alteration of intestinal metabolites (such as secondary bile acids and H2S), thereby producing various carcinogens and promoting the occurrence of colorectal cancer (Azcárate-Peril et al., 2011). Primary bile acids excreted into the gut are converted into secondary bile acids which can increase reactive oxygen species through microbial derived-metabolism, such as hydrolase, leading to DNA damage and genomic instability, and finally induce the growth of tumors (Saracut et al., 2015). Clostridium converts primary bile acid into deoxycholic acid, increasing free radicals and ROS to induce chronic inflammation and colorectal cancer. H2S is a metabolite produced by sulfate-reducing bacteria in the gut tract which can cause DNA damage, free radical release, colonic mucosal inflammation and hyperplasia, suppress cytochrome oxidase and DNA methylation, and ultimately contribute to tumor initiation (Wang et al., 2021).
ROS is also blamed as being a driving force behind CRC initiation. Enterococcus faecalis releases extracellular superoxide, and after transformed by hydrogen peroxide, these free radicals as powerful mutagens can cause DNA breakage, and local genomic instability in CRC patients with colorectal cancer (Kabwe et al., 2021; Rivas-Domínguez et al., 2021). Similarly, Helicobacter pylori promotes the development of gastrointestinal inflammation and carcinogenesis via elevating ROS and reactive nitrogen species (RNS) to upregulate oncogenic pathways such as HIF-1α, NF-κB and PI3K/AKT (Liu et al., 2019; Lu et al., 2020). Therefore, targeting those pathogenic bacteria or counteract their deleterious effects (reducing ROS generation) have been considered as potential strategies for preventing CRC.
Herbal medicines including herbal formulas, extracts, and compounds have been studied for many years in the treatment of gut-related diseases via regulating gut micro-ecosystem. It can apparently alter the composition and metabolism of gut microbiota, and dramatically affect the number and function of intestinal epithelial cells to achieve the rebalancing of gut microecology (Figure 3) (Li et al., 2021).
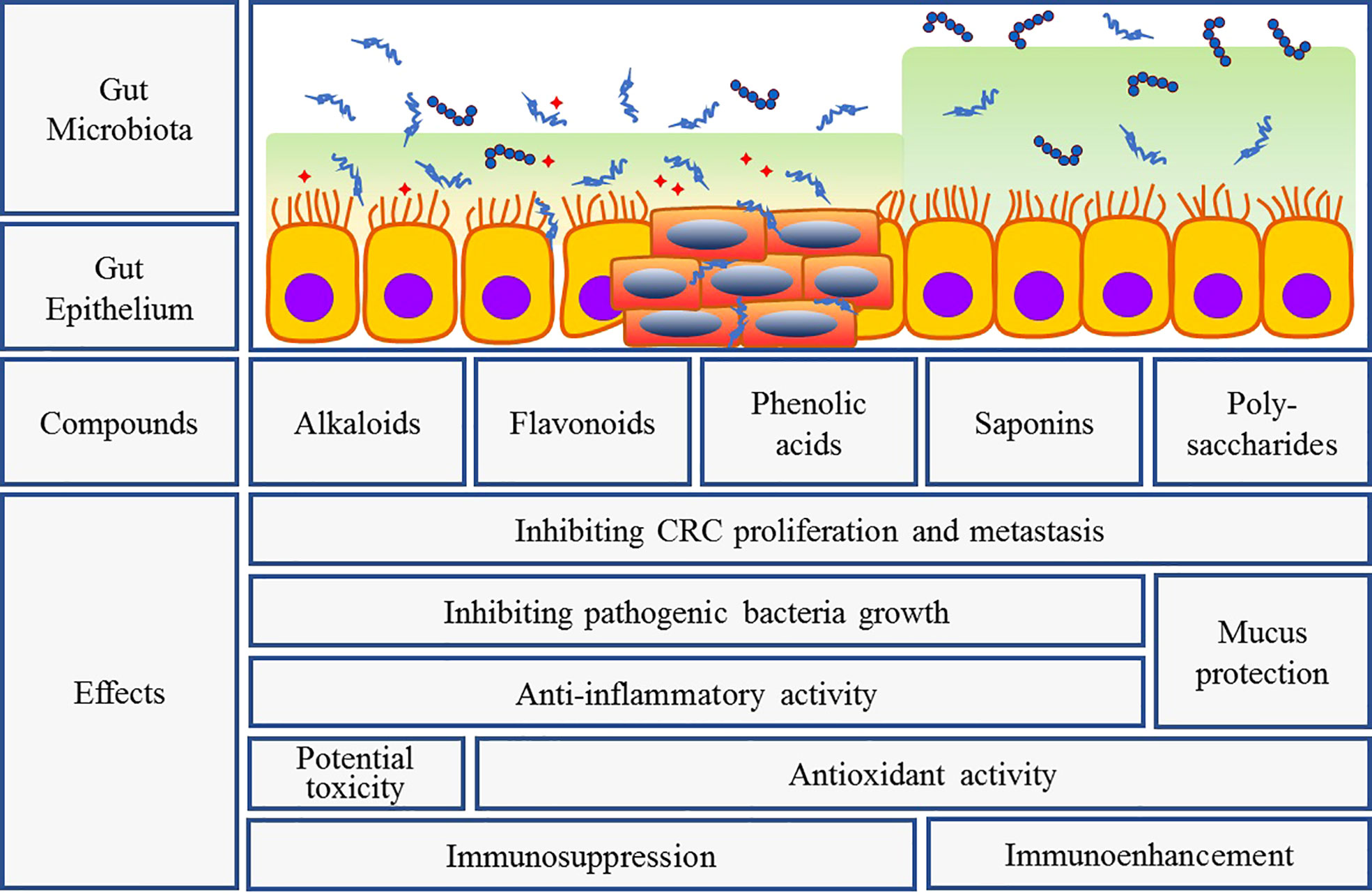
Figure 3 Intervention of herbal bioactive components on gut bacteria and CRC. Interaction between herbal medicine and gut microbiota can fight tumor growth and prevent tumorigenesis through several mechanisms: (1) inhibiting pathogenic bacteria overgrowth and promoting probiotics growth; (2) anti-inflammatory and antioxidant activities as well as intestinal mucosal protection and immune regulation; (3) direct anti-tumor activity. But different components exert their respective features.
Herbal medicines are rich in chemical constituents which contains not only bioactive ingredients such as glycosides, flavonoids, alkaloids, quinones and steroids, but also nutrients such as protein and vitamins, leading to the variety of their pharmacodynamic effects. The mechanism of herbal medicine in improving gut microbiota imbalance includes two aspects: inhibiting pathogenic bacterial overgrowth and promoting probiotics growth. At present, CRC-associated probiotics are mainly divided into three categories: Lactobacillus, Bifidobacteriums, and Gram-positive cocci. On the one hand, probiotics indirectly inhibit the growth and invasion of pathogenic bacteria through strengthening the barrier function of intestinal epithelial cells and producing beneficial metabolites (Kaur et al., 2021). On the other hand, probiotics have physiologically positive effects on the host (animals or human) through regulating the immune function of the host mucosa and system, or improving the balance of gut nutrition and microbiota composition. What’s more, probiotics also play a role in inhibiting allergies, controlling serum cholesterol level, and regulating immune function such as metabolic transformation and metabolic detoxification for preventing the gastrointestinal carcinogenesis (Paveljšek et al., 2021).
Many herbal medicines, such as Ganoderma Lucidum, American Ginseng, Red Ginseng, Gynostemma Pentaphyllum, and Curcumin, significantly inhibit the growth of pathogenic bacteria such as Clostridium, Escherichia, Staphylicus, Verrucomicrobia but increase the number of probiotic Bifidobacteria and lactobacilli, resulting in the increased diversity of the gut microbiota in DSS-induced CRC mouse model (Guo et al., 2015; McFadden et al., 2015; Yu et al., 2015; Chen et al., 2016; Wang et al., 2016; Gong et al., 2019; Lv et al., 2019 ;Jiang et al., 2020; Shen et al., 2020; Sui et al., 2020; Sun et al., 2020 ;Zhang et al., 2020; Hao et al., 2021; Wang et al., 2021; Zhang et al., 2021; Zhu et al., 2021). These anti-CRC profiles of herbal medicine on gut microbiota are summarized as shown in Table 1.
It has been known that chronic intestinal inflammation is closely related to gut microenvironment, and gut inflammation mostly leads to colorectal cancer (Konstantinov et al., 2013; Avril and DePaolo, 2021). Under physiological conditions, both gut bacteria and viruses can’t transmit through the mucosa. However, when inflammatory and neoplastic intestinal disorders exist, the permeability of intestinal barrier will increase, causing higher translocation of bacteria and viruses into the bloodstream. The active ingredients of herbal medicines can alleviate the stimulating effect of gut microbiota on tumors by improving the tumor microenvironment, such as inflammation and immunosuppression, thus inhibiting the development of tumors and even the metastasis and recurrence after operation.
Coptidis Rhizoma (also called as Huanglian), is derived from the rhizome of Coptis chinensis Franch., Coptis deltoidea C.F. Cheng et Hisao or Coptis teeta Wall. Berberine is a main activealkaloid isolated and identified from this herb. Evidences support the folkloric medicinal properties of Coptidis Rhizoma, in particular berberine, as a promising anticancer candidate in CRC via inducing AMPK activation and autophagic cell death (Huang et al., 2017; La et al., 2017). Berberine mitigates intestinal inflammation and oxidant stress through blocking the IL-6/STAT3, Nrf2 and PPAR pathways on colitis-associated tumorigenesis in mice (Li et al., 2017; Zhu et al., 2019). Moreover, it can not only significantly increase the abundance of Brucella, Bacteroides, Clostridium butyricum and Helicobacter in gut tract, but also protects intestinal epithelial cells against CRC-induced intestinal barrier dysfunction (Zhu et al., 2019).
Genistein is the predominant isoflavone found in Leguminous plants (such as Sophora japonica L. and Glycine max) and acts as the strong tyrosine kinase inhibitor, topoisomerase inhibitor and PPARγ agonist. It exerts phytoestrogenic, antioxidant and anti-inflammatory effects on gut microenvironment via regulating COX-2-related signaling pathway in CRC mice (He et al., 2016; Song et al., 2018). Crocin, a natural carotenoid from saffron (Croci stigma) and gardenia (Gardeniae fructus), can inhibit the expression of pro-inflammatory cytokines and inducible inflammatory enzymes in AOM/DSS-induced colorectal inflammation model, and significantly reduce inflammation and mucosal ulcer (Kawabata et al., 2012). The flavanol-rich foods as well as red wine polyphenolics inhibited ROS generation and NF-κB activation in colon cells by inducing miR-126 and miR-146a (Noratto et al., 2011; Angel-Morales et al., 2012).
FCT, a synbiotic combination of probiotic Lactobacillus gasseri 505 (LG) and Cudrania tricuspidata leaf extract (CT), reduced the risk of colitis-associated colon cancer via regulating inflammation, carcinogenesis, and gut microbiota composition. Compared with CT and LG, FCT significantly down-regulated pro-inflammatory mediators (TNF-α, IFN-γ, IL-1β, IL-6, iNOS and COX-2), and up-regulated anti-inflammatory cytokines (IL-4 and IL-10). In addition, FTC enhanced gut barrier function via up-regulating mucus layer markers (MUC-2 and TFF3) and tight junction (occludin and ZO-1), decreasing Staphylococcus and increasing Lactobacillus, Bifidobacterium, and Akkermansia, resulting in the increased production of short-chain fatty acids (SCFAs) (Oh et al., 2020).
Most notably, clinical responses to immune checkpoint inhibitors are closely associated with the abnormal gut microbiome composition, especially the relative abundance of Akkermansia muciniphila (Routy et al., 2018). Although several herbal medicines had been demonstrated to prevent the CRC growth, reduce side effects of chemotherapy and enhance the efficacy of PD-1 inhibitors via modulating the gut microbiota composition and CD4+ T cell proportion in tumor beds (Lv et al., 2019; Zhang et al., 2021; Huang et al., 2022; Messaoudene et al., 2022), the key orchestrators responsible for the primary resistance to PD-1 blockers remain unclear.
Gut microbiota modifies the chemical composition of herbal medicine through their own enzymatic system. Intestinal cells also influence the metabolism and absorption of herbal medicine through transporter proteins and metabolic enzymes. After underwent by gut microbiota biotransformation (including hydrolysis, oxidation and reduction reaction), the chemical composition, pharmacological activity and toxicity of the herbal medicines will be changed, and it will form new active metabolites. Therefore, the biotransformation induced by gut microbiota has a central impact on exerting the efficiency of herbal medicine (Shen et al., 2013).
Most glycosides, including saponins, flavonoids and anthraquinones, will be hydrolyzed by gut microbiota to remove glycosyl groups and form aglycones, which reduces polarity, increases lipo-solubility and facilitates absorption into the blood. Both licorice and ginseng contain saponins. Glycyrrhetinic acid could be detected in normal rats after oral administration of glycyrrhizin, but not in sterile rats, indicating that gut microbiota could be converted into glycyrrhetinic acid and then absorbed by organisms (Takeda et al., 1996). Gut microbiota can promote the absorption and metabolic transformation of ginsenoside Rb1 and ginsenoside Rd, which can promote the biosynthesis of RNA and protein, regulate body metabolism and enhance immune function (Kim et al., 2014). Compound K, the main metabolite of ginseng saponin, induces apoptosis of colorectal cancer cells by inhibiting histone deacetylase activity (Kang et al., 2013). Most flavonoids, such as baicalin and isoquercitrin, are α-glucosylated by gut microbiota, but those enzymatical modification enhances their intestinal absorption and pharmacological action (Wang et al., 2015; Lee et al., 2017; Terao, 2017). More notably, degraded by Clostridium orbiscindens, diet flavonoids were cracked and converted into desaminotyrosine, which can up-regulate the signal pathway of type I interferon and enhance the host antiviral immune response (Steed et al., 2017).
Alkaloids are a class of nitrogen-containing organic compounds, which have strong pharmacological activities in the central nervous system, cardiovascular system, immune function, anti-bacterial, anti-inflammatory and anti-cancer. As like as flavonoids, many alkaloids, such as berberine, aconitine and scopolamine, are usually characterized by small molecules, or by ether bonds and coordination bond which are prone to hydrolysis and dehydration under the action of gut microbiota. Under the action of gut bacteria, aconitine, a poisonous alkaloid mainly obtained from Aconitum carmichaeli Debxwhich shows chondroprotective activity can produce new monoester, diester and lipid alkaloids through deacetylation, demethylation, dehydroxylation and esterification, which greatly reduces the toxicity of aconitine and alleviates intestinal irritation (Tong et al., 2014). α-Chaconine, a potato glycoside alkaloid, induces apoptosis of HT-29 colon cancer cells by activating caspase-3 and inhibiting ERK 1/2 phosphorylation, and inhibits Enterobacter aerogenes, Escherichia coli and Staphylococcus aureus (Yang et al., 2006).
Phenylpropanoids generally have lactone structure, including phenylpropionic acid, coumarin and lignans. After the catalysis of gut microbiota, lactone structure can be broken or demethylated. The metabolic transformation of silymarin in Eubacterium limosum produced demethylsilybin A, demethylsilybin B, demethylisosilybin A and demethylisosilybin B which had stronger inhibitory effects on Alzheimer’s amyloid protein-β42 (Zhang et al., 2014). Proteasome degraded flaxseed lignans in human intestinal tract bacteria. Hydrolysis and deglycosylation removed two sugars to form isopine resin diol diester (SECO). Pine resin diol diester was produced by digestive Streptococcus Petostreptococcus productus, Eubacterium limosum, Clodium methoxybenzo-vorans and Eggelentala tarda under the action of digestive peptococcus, Eubacterium limosum, Clodium methoxybenzo-vorans and Eggetala tarda. They were demethylated and dehydroxylated to form enterediol and enterolactone (Eeckhaut et al., 2008; Woting et al., 2010; Mabrok et al., 2012). A study of the metabolic mechanism of lignans in flaxseed, demonstrated that Ruminal Prevotella was the main microbial group for lignans metabolism (Schogor et al., 2014).
Herbal medicines contain a variety of bioactive compounds and have unique advantages on maintenance of intestinal homeostasis and regulation of host immune. It precisely regulates the microbiota composition to indirectly prevent the CRC occurrence and development. On the other hand, the active ingredients in herbal medicine can directly inhibit the growth of colon cancer cells. Gut microorganisms produce many metabolic enzymes during their growth and reproduction, such as hydrolase, lyase, transferase and redox enzymes, which improve the bioavailability of the effective components of herbal medicine by biotransformation. Many active ingredients of Chinese herbal medicines can be transformed by gut microorganisms to produce metabolites with strong pharmacological effects, which can be easily absorbed by the body and exert anticancer activity. Thus, herbal medicine has the promise of preventing or delaying CRC progression via maintenance of intestinal homeostasis.
However, it should be pointed out that, in terms of current research, there is no evidence that herbal medicine can cure tumors only by improving gut microbiota, or more by exerting direct effects on cancer cells. Secondly, it is difficult to determine the sequence of the effects of herbal medicine and gut microbiota, just like the problem of “eggs and chickens” which one appears first. Although some specific bacteria that cause a precancerous phenotype in vivo have been identified, whether gut dysbiosis is the culprit behind CRC rather than a result of inflammation is still in dispute. Thus, deep signal regulation pathways and key targets of gut microbiota as a potent herbal medicine intervention in colorectal cancer need to be further explored.
H-ZY, WX, and M-CW searched the articles and drafted the manuscript. J-QH and H-HZ checked the contents. H-ZY and C-HY revised the manuscript. C-HY was responsible for the project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
This work is supported by National Natural Science Foundation of China (No. 82104526) and Zhejiang province of medical science (No. 2022KY917).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AMPK, adenosine 5’-monophosphate-activated protein kinase; APC, adenomatosis polyposis coli; AOM, azoxymethane; CAC, colitis associated cancer; COX2, cyclo-oxygenase-2; CRC, colorectal cancer; DSS, dextran sodium sulfate; FOXO3, forkhead box O3; IL-17, interleukin 17; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-B; PI3K, phosphoinositide 3-kinase; TGF-β1, transforming growth factor-β1; ROS, reactive oxygen species; SCFAs, short chain fatty acids; STAT3, signal transducer and activator of transcription 3.
Angel-Morales, G., Noratto, G., Mertens-Talcott, S. (2012). Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: Potential role of microRNA-126. Food Funct. 3 (7), 745–752. doi: 10.1039/c2fo10271d
Avril, M., DePaolo, R. W. (2021). "Driver-passenger" bacteria and their metabolites in the pathogenesis of colorectal cancer. Gut Microbes 13 (1), 1941710. doi: 10.1080/19490976.2021.1941710
Azcárate-Peril, M. A., Sikes, M., Bruno-Bárcena, J. M. (2011). The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer. Am. J. Physiol. Gastrointest Liver Physiol. 301 (3), G401–G424. doi: 10.1152/ajpgi.00110.2011
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi: 10.3322/caac.21492
Chen, L., Brar, M. S., Leung, F. C., Hsiao, W. L. (2016). Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 7 (21), 31226–31242. doi: 10.18632/oncotarget.8886
Chen, F., Jiang, J., Tian, D. D., Wen, Q., Li, Y. H., Zhang, J. Q., et al. (2017). Targeting obesity for the prevention of chronic cardiovascular disease through gut microbiota-herb interactions: An opportunity for traditional herbs. Curr. Pharm. Des. 23 (8), 1142–1152. doi: 10.2174/1381612822666161014115724
Chen, F., Wen, Q., Jiang, J., Li, H. L., Tan, Y. F., Li, Y. H., et al. (2016). Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs. J. Ethnopharmacol. 179, 253–264. doi: 10.1016/j.jep.2015.12.031
Eeckhaut, E., Struijs, K., Possemiers, S., Vincken, J. P., Keukeleire, D. D., Verstraete, W. (2008). Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J. Agric. Food Chem. 56 (12), 4806–4812. doi: 10.1021/jf800101s
Feagins, L. A., Souza, R. F., Spechler, S. J. (2009). Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 6 (5), 297–305. doi: 10.1038/nrgastro.2009.44
Fukata, M., Abreu, M. T. (2009). Pathogen recognition receptors, cancer and inflammation in the gut. Curr. Opin. Pharmacol. 9 (6), 680–687. doi: 10.1016/j.coph.2009.09.006
Gagnière, J., Raisch, J., Veziant, J., Barnich, N., Bonnet, R., Buc, E., et al. (2016). Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 22 (2), 501–518. doi: 10.3748/wjg.v22.i2.501
Gill, S. R., Pop, M., Deboy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. S., et al. (2006). Metagenomic analysis of the human distal gut microbiome. Science 312 (5778), 1355–1359. doi: 10.1126/science.1124234
Gong, Y., Dong, R., Gao, X., Li, J., Jiang, L., Zheng, J., et al. (2019). Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol. Res. 148, 104460. doi: 10.1016/j.phrs.2019.104460
Guo, M., Ding, S., Zhao, C., Gu, X., He, X., Huang, K., et al. (2015). Red ginseng and semen coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J. Ethnopharmacol. 162, 7–13. doi: 10.1016/j.jep.2014.12.029
Hao, W., Wu, J., Yuan, N., Gong, L., Huang, J., Ma, Q., et al. (2021). Xiaoyaosan improves antibiotic-induced depressive-like and anxiety-like behavior in mice through modulating the gut microbiota and regulating the NLRP3 inflammasome in the colon. Front. Pharmacol. 12. doi: 10.3389/fphar.2021.619103
Hattori, N., Niwa, T., Ishida, T., Kobayashi, K., Imai, T., Mori, A., et al. (2019). Antibiotics suppress colon tumorigenesis through inhibition of aberrant DNA methylation in an azoxymethane and dextran sulfate sodium colitis model. Cancer Sci. 110 (1), 147–156. doi: 10.1111/cas.13880
He, X., Bai, Y., Zhao, Z., Wang, X., Fang, J., Huang, L., et al. (2016). Local and traditional uses, phytochemistry, and pharmacology of sophora japonica l.: A review. J. Ethnopharmacol. 187, 160–182. doi: 10.1016/j.jep.2016.04.014
Huang, J., Liu, D., Wang, Y., Liu, L., Li, J., Yuan, J., et al. (2022). Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71 (4), 734–745. doi: 10.1136/gutjnl-2020-321031
Huang, T., Xiao, Y., Yi, L., Li, L., Wang, M., Tian, C., et al. (2017). Coptisine from rhizoma coptidis suppresses HCT-116 cells-related tumor growth in vitro and in vivo. Sci. Rep. 7, 38524. doi: 10.1038/srep38524
Jiang, F., Liu, M., Wang, H., Shi, G., Chen, B., Chen, T., et al. (2020). Wu Mei wan attenuates CAC by regulating gut microbiota and the NF-kB/IL6-STAT3 signaling pathway. BioMed. Pharmacother. 125, 109982. doi: 10.1016/j.biopha.2020.109982
Kabwe, M., Meehan-Andrews, T., Ku, H., Petrovski, S., Batinovic, S., Chan, H. T., et al. (2021). Lytic bacteriophage EFA1 modulates HCT116 colon cancer cell growth and upregulates ROS production in an enterococcus faecalis Co-culture system. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.650849
Kang, K. A., Piao, M. J., Kim, K. C., Zheng, J., Yao, C. W., Cha, J. W., et al. (2013). Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. Int. J. Oncol. 43 (6), 1907–1914. doi: 10.3892/ijo.2013.2129
Kaur, H., Gupta, T., Kapila, S., Kapila, R. (2021). Protective effects of potential probiotic lactobacillus rhamnosus (MTCC-5897) fermented whey on reinforcement of intestinal epithelial barrier function in a colitis-induced murine model. Food Funct. 12 (13), 6102–6116. doi: 10.1039/d0fo02641g
Kawabata, K., Tung, N. H., Shoyama, Y., Sugie, S., Mori, T., Tanaka, T. (2012). Dietary crocin inhibits colitis and colitis-associated colorectal carcinogenesis in Male ICR mice. Evid. Based. Complement Alternat. Med. 2012, 820415. doi: 10.1155/2012/820415
Kim, K. A., Yoo, H. H., Gu, W., Yu, D. H., Jin, M. J., Choi, H. L., et al. (2014). Effect of a soluble prebiotic fiber, NUTRIOSE, on the absorption of ginsenoside Rd in rats orally administered ginseng. J. Ginseng Res. 38 (3), 203–207. doi: 10.1016/j.jgr.2014.03.003
Konstantinov, S. R., Kuipers, E. J., Peppelenbosch, M. P. (2013). Functional genomic analyses of the gut microbiota for CRC screening. Nat. Rev. Gastroenterol. Hepatol. 10 (12), 741–745. doi: 10.1038/nrgastro.2013.178
La, X., Zhang, L., Li, Z., Yang, P., Wang, Y. (2017). Berberine-induced autophagic cell death by elevating GRP78 levels in cancer cells. Oncotarget 8 (13), 20909–20924. doi: 10.18632/oncotarget.14959
Lee, Y. S., Woo, J. B., Ryu, S. I., Moon, S. K., Han, N. S., Lee, S. B. (2017). Glucosylation of flavonol and flavanones by bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability. Food Chem. 229, 75–83. doi: 10.1016/j.foodchem.2017.02.057
Lennard, K. S., Goosen, R. W., Blackburn, J. M. (2016). Bacterially-associated transcriptional remodelling in a distinct genomic subtype of colorectal cancer provides a plausible molecular basis for disease development. PloS One 11 (11), e0166282. doi: 10.1371/journal.pone.0166282
Liu, I. L., Tsai, C. H., Hsu, C. H., Hu, J. M., Chen, Y. C., Tian, Y. F., et al. (2019). Helicobacter pylori infection and the risk of colorectal cancer: A nationwide population-based cohort study. QJM 112 (10), 787–792. doi: 10.1093/qjmed/hcz157
Li, X., Wu, D., Niu, J., Sun, Y., Wang, Q., Yang, B., et al. (2021). Intestinal flora: A pivotal role in investigation of traditional Chinese medicine. Am. J. Chin. Med. 49 (2), 237–268. doi: 10.1142/S0192415X21500130
Li, D., Zhang, Y., Liu, K., Zhao, Y., Xu, B., Xu, L., et al. (2017). Berberine inhibits colitis-associated tumorigenesis via suppressing inflammatory responses and the consequent EGFR signaling-involved tumor cell growth. Lab. Invest. 97 (11), 1343–1353. doi: 10.1038/labinvest.2017.71
Lund, E. K., Belshaw, N. J., Elliott, G. O., Johnson, I. T. (2011). Recent advances in understanding the role of diet and obesity in the development of colorectal cancer. Proc. Nutr. Soc. 70 (2), 194–204. doi: 10.1017/S0029665111000073
Luo, S., Wen, R., Wang, Q., Zhao, Z., Nong, F., Fu, Y., et al. (2019). Rhubarb peony decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J. Ethnopharmacol. 231, 39–49. doi: 10.1016/j.jep.2018.08.033
Lu, Y., Rong, J., Lai, Y., Tao, L., Yuan, X., Shu, X. (2020). The degree of helicobacter pylori infection affects the state of macrophage polarization through crosstalk between ROS and HIF-1α. Oxid. Med. Cell Longev. 2020, 5281795. doi: 10.1155/2020/5281795
Lv, J., Jia, Y., Li, J., Kuai, W., Li, Y., Guo, F., et al. (2019). Gegen qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 10 (6), 415. doi: 10.1038/s41419-019-1638-6
Mabrok, H. B., Klopfleisch, R., Ghanem, K. Z., Clavel, T., Blaut, M., Loh, G. (2012). Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis 33 (1), 203–208. doi: 10.1093/carcin/bgr256
McFadden, R. M., Larmonier, C. B., Shehab, K. W., Midura-Kiela, M., Ramalingam, R., Harrison, C. A., et al. (2015). The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis. 21 (11), 2483–2494. doi: 10.1097/MIB.0000000000000522
Meng, Q. X., Roubin, R. H., Hanrahan, J. R. (2013). Ethnopharmacological and bioactivity guided investigation of five TCM anticancer herbs. J. Ethnopharmacol. 148 (1), 229–238. doi: 10.1016/j.jep.2013.04.014
Messaoudene, M., Pidgeon, R., Richard, C., Ponce, M., Diop, K., Benlaifaoui, M., et al. (2022). A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov 12 (4), 1070–1087. doi: 10.1158/2159-8290.CD-21-0808
Mizutani, S., Yamada, T., Yachida, S. (2020). Significance of the gut microbiome in multistep colorectal carcinogenesis. Cancer Sci. 111 (3), 766–773. doi: 10.1111/cas.14298
Noratto, G. D., Kim, Y., Talcott, S. T., Mertens-Talcott, S. U. (2011). Flavonol-rich fractions of yaupon holly leaves (Ilex vomitoria, aquifoliaceae) induce microRNA-146a and have anti-inflammatory and chemopreventive effects in intestinal myofibroblast CCD-18Co cells. Fitoterapia 82 (4), 557–569. doi: 10.1016/j.fitote.2011.01.013
Oh, N. S., Lee, J. Y., Kim, Y. T., Kim, S. H., Lee, J. H. (2020). Cancer-protective effect of a synbiotic combination between lactobacillus gasseri 505 and a cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 12 (1), 1785803. doi: 10.1080/19490976.2020.1785803
Paveljšek, D., Ivičak-Kocjan, K., Treven, P., Benčina, M., Jerala, R., Rogelj, I. (2021). Distinctive probiotic features share common TLR2-dependent signalling in intestinal epithelial cells. Cell Microbiol. 23 (1), e13264. doi: 10.1111/cmi.13264
Perillo, F., Amoroso, C., Strati, F., Giuffrè, M. R., Díaz-Basabe, A., Lattanzi, G., et al. (2020). Gut microbiota manipulation as a tool for colorectal cancer management: Recent advances in its use for therapeutic purposes. Int. J. Sci. 21 (15), 5389. doi: 10.3390/ijms21155389
Richard, M. L., Liguori, G., Lamas, B., Brandi, G., da Costa, G., Hoffmann, T. W., et al. (2018). Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 9 (2), 131–142. doi: 10.1080/19490976.2017.1379637
Rivas-Domínguez, A., Pastor, N., Martínez-López, L., Colón-Pérez, J., Bermúdez, B., Orta, M. L. (2021). The role of DNA damage response in dysbiosis-induced colorectal cancer. Cells 10 (8), 1934. doi: 10.3390/cells10081934
Rogler, G. (2014). Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 345 (2), 235–241. doi: 10.1016/j.canlet.2013.07.032
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillère, R., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359 (6371), 91–97. doi: 10.1126/science.aan3706
Saracut, C., Molnar, C., Russu, C., Todoran, N., Vlase, L., Turdean, S., et al. (2015). Secondary bile acids effects in colon pathology. Exp. Mice Study Acta Cir. Bras. 30 (9), 624–631. doi: 10.1590/S0102-865020150090000007
Schogor, A. L., Huws, S. A., Santos, G. T., Scollan, N. D., Hauck, B. D., Winters, A. L., et al. (2014). Ruminal prevotella spp. may play an important role in the conversion of plant lignans into human health beneficial antioxidants. PloS One 9 (4), e87949. doi: 10.1371/journal.pone.0087949
Sekirov, I., Russell, S. L., Antunes, L. C., Finlay, B. B. (2010). Gut microbiota in health and disease. Physiol. Rev. 90 (3), 859–904. doi: 10.1152/physrev.00045.2009
Shen, H., Leung, W. I., Ruan, J. Q., Li, S. L., Lei, J. P., Wang, Y. T., et al. (2013). Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin. Med. 8 (1), 22. doi: 10.1186/1749-8546-8-22
Shen, J., Li, P., Liu, S., Liu, Q., Li, Y., Zhang, Z., et al. (2020). The chemopreventive effects of huangqin-tea against AOM-induced preneoplastic colonic aberrant crypt foci in rats and omics analysis. Food Funct. 11 (11), 9634–9650. doi: 10.1039/d0fo01731k
Sobhani, I., Tap, J., Roudot-Thoraval, F., Roperch, J. P., Letulle, S., Langella, P., et al. (2011). Microbial dysbiosis in colorectal cancer (CRC) patients. PloS One 6 (1), e16393. doi: 10.1371/journal.pone.0016393
Song, S., Cheng, D., Wei, S., Wang, X., Niu, Y., Qi, W., et al. (2018). Preventive effect of genistein on AOM/DSS-induced colonic neoplasm by modulating the PI3K/AKT/FOXO3 signaling pathway in mice fed a high-fat diet. J. Funct. Foods 46, 237–242. doi: 10.1016/j.jff.2018.05.006
Steed, A. L., Christophi, G. P., Kaiko, G. E., Sun, L., Goodwin, V. M., Jain, U., et al. (2017). The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 357 (6350), 498–502. doi: 10.1126/science.aam5336
Sui, H., Zhang, L., Gu, K., Chai, N., Ji, Q., Zhou, L., et al. (2020). YYFZBJS ameliorates colorectal cancer progression in ApcMin/+ mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun. Signal. 18 (1), 113. doi: 10.1186/s12964-020-00596-9
Sun, L., Yan, Y., Chen, D., Yang, Y. (2020). Quxie capsule modulating gut microbiome and its association with T cell regulation in patients with metastatic colorectal cancer: Result from a randomized controlled clinical trial. Integr. Cancer Ther. 19, 1534735420969820. doi: 10.1177/1534735420969820
Takeda, S., Ishthara, K., Wakui, Y., Amagaya, S., Maruno, M., Akao, T., et al. (1996). Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; relevance to the intestinal bacterial hydrolysis. J. Pharm. Pharmacol. 48 (9), 902–905. doi: 10.1111/j.2042-7158.1996.tb05998.x
Terao, J. (2017). Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 139, 15–23. doi: 10.1016/j.bcp.2017.03.021
Tong, P., Xu, S., Cao, G., Jin, W., Guo, Y., Cheng, Y., et al. (2014). Chondroprotective activity of a detoxicated traditional Chinese medicine (Fuzi) of aconitum carmichaeli debx against severe-stage osteoarthritis model induced by mono-iodoacetate. J. Ethnopharmacol. 151 (1), 740–744. doi: 10.1016/j.jep.2013.11.048
Uronis, J. M., Mühlbauer, M., Herfarth, H. H., Rubinas, T. C., Jones, G. S., Jobin, C. (2009). Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PloS One 4 (6), e6026. doi: 10.1371/journal.pone.0006026
Vivarelli, S., Salemi, R., Candido, S., Falzone, L., Santagati, M., Stefani, S., et al. (2019). Gut microbiota and cancer: From pathogenesis to therapy. Cancers (Basel) 11 (1), 38. doi: 10.3390/cancers11010038
Wang, Y., Nguyen, L. H., Mehta, R. S., Song, M., Huttenhower, C., Chan, A. T. (2021). Association between the sulfur microbial diet and risk of colorectal cancer. JAMA Netw. Open 4 (11), e2134308. doi: 10.1001/jamanetworkopen.2021.34308
Wang, C. Z., Yu, C., Wen, X. D., Chen, L., Zhang, C. F., Calway, T., et al. (2016). American Ginseng attenuates colitis-associated colon carcinogenesis in mice: Impact on gut microbiota and metabolomics. Cancer Prev. Res. (Phila) 9 (10), 803–811. doi: 10.1158/1940-6207.CAPR-15-0372
Wang, C. Z., Zhang, C. F., Chen, L., Anderson, S., Lu, F., Yuan, C. S. (2015). Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int. J. Oncol. 47 (5), 1749–1758. doi: 10.3892/ijo.2015.3173
Wang, M., Zhou, B., Cong, W., Zhang, M., Li, Z., Li, Y., et al. (2021). Amelioration of AOM/DSS-induced murine colitis-associated cancer by evodiamine intervention is primarily associated with gut microbiota-Metabolism-Inflammatory signaling axis. Front. Pharmacol. 12. doi: 10.3389/fphar.2021.797605
Watanabe, D., Murakami, H., Ohno, H., Tanisawa, K., Konishi, K., Tsunematsu, Y., et al. (2020). Association between dietary intake and the prevalence of tumourigenic bacteria in the gut microbiota of middle-aged Japanese adults. Sci. Rep. 10 (1), 15221.doi: 10.1038/s41598-020-72245-7
Woting, A., Clavel, T., Loh, G., Blaut, M. (2010). Bacterial transformation of dietary lignans in gnotobiotic rats. FEMS Microbiol. Ecol. 72 (3), 507–514. doi: 10.1111/j.1574-6941.2010.00863.x
Wu, N., Yang, X., Zhang, R., Li, J., Xiao, X., Hu, Y., et al. (2013). Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Eco. 66 (2), 462–470. doi: 10.1007/s00248-013-0245-9
Yang, S. A., Paek, S. H., Kozukue, N., Lee, K. R., Kim, J. A. (2006). Alpha-chaconine, a potato glycoalkaloid, induces apoptosis of HT-29 human colon cancer cells through caspase-3 activation and inhibition of ERK 1/2 phosphorylation. Food Chem. Toxicol. 44 (6), 839–846. doi: 10.1016/j.fct.2005.11.007
Yu, Y. N., Fang, J. Y. (2015). Gut microbiota and colorectal cancer. Gastrointest. Tumors 2 (1), 26–32. doi: 10.1159/000380892
Yu, Y. N., Yu, T. C., Zhao, H. J., Sun, T. T., Chen, H. M., Chen, H. Y., et al. (2015). Berberine may rescue fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget 6 (31), 32013–32026. doi: 10.18632/oncotarget.5166
Zhang, S. L., Mao, Y. Q., Zhang, Z. Y., Li, Z. M., Kong, C. Y., Chen, H. L., et al. (2021). Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 11 (9), 4155–4170. doi: 10.7150/thno.54476
Zhang, Z., Shao, S., Zhang, Y., Jia, R., Hu, X., Liu, H., et al. (2020). Xiaoyaosan slows cancer progression and ameliorates gut dysbiosis in mice with chronic restraint stress and colorectal cancer xenografts. BioMed. Pharmacother. 132, 110916. doi: 10.1016/j.biopha.2020.110916
Zhang, Y., Yang, D. H., Zhang, Y. T., Chen, X. M., Li, L. L., Cai, S. Q. (2014). Biotransformation on the flavonolignan constituents of silybi fructus by an intestinal bacterial strain eubacterium limosum ZL-II. Fitoterapia 92, 61–71. doi: 10.1016/j.fitote.2013.10.001
Zhang, M. M., Yin, D. K., Rui, X. L., Shao, F. P., Li, J. C., Xu, L., et al. (2021). Protective effect of pai-Nong-San against AOM/DSS-induced CAC in mice through inhibiting the wnt signaling pathway. Chin. J. Nat. Med. 19 (12), 912–920. doi: 10.1016/S1875-5364(22)60143-2
Zhu, L., Gu, P., Shen, H. (2019). Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 68, 242–251. doi: 10.1016/j.intimp.2018.12.036
Keywords: intestinal homeostasis, natural products, chronic inflammation, probiotic, immunoenhancement, tumor microenvironment
Citation: Ying H-Z, Xie W, Wang M-C, He J-Q, Zhang H-H and Yu C-H (2022) Gut microbiota: An emerging therapeutic approach of herbal medicine for prevention of colorectal cancer. Front. Cell. Infect. Microbiol. 12:969526. doi: 10.3389/fcimb.2022.969526
Received: 15 June 2022; Accepted: 01 August 2022;
Published: 16 August 2022.
Edited by:
Ping Li, Zhejiang Gongshang University, ChinaCopyright © 2022 Ying, Xie, Wang, He, Zhang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-Huan Yu, eXVjaGVuaHVhbjIwMDJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.