
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Cell. Infect. Microbiol., 09 August 2022
Sec. Parasite and Host
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.955974
This article is part of the Research TopicAdvances in Diagnosis and Therapeutic Intervention for Foodborne Parasitic Diseases, Volume IIIView all 5 articles
 Xinyu Wang1†
Xinyu Wang1† Aizhe Li1†
Aizhe Li1† Ruizhe Wang1†
Ruizhe Wang1† Tianji Hou1
Tianji Hou1 Huixin Chen1
Huixin Chen1 Jing Wang1
Jing Wang1 Mingyuan Liu1,2
Mingyuan Liu1,2 Chen Li1*
Chen Li1* Jing Ding1*
Jing Ding1*Trichinellosis is a major food-borne parasitosis caused by ingesting raw or semi-raw meat products from pigs infected with Trichinella spiralis (T. spiralis). Although China is the largest consumer of pork in the world, the current diagnostic method of T. spiralis is exclusively performed in a laboratory setting, due to its complexity and laborious procedure. Here, in order to solve the detection problems in the pig breeding industry, a rapid, sensitive, and on-site serological diagnosis method was developed. The novel lateral flow immunoassay strip (ICS) is based on europium(III) chelate microparticle (ECM) to detect T. spiralis-specific IgG antibody in the serum and whole blood samples from pigs. The structure of the blood-filtering pad and the conjugate pad was added to the ICS, allowing for whole blood samples to be detected and enabling on-site deployment. By comparing the detection results of the serum samples and the whole blood samples, the detection limit of this method was evaluated. Thereafter, this method was used to investigate Trichinella infection in Chongqing, Sichuan, Inner Mongolia, Guangxi, and Liaoning provinces of China, and the results were almost consistent with the standard method of artificial digestion. Taking advantage of its user-friendly procedure, short detection time (3 min), and sensitivity, the ECM-ICS could be employed for monitoring the epidemic of Trichinella infection and ensuring meat safety.
Trichinellosis is a neglected food-borne zoonotic parasitic disease caused by nematodes of the genus Trichinella, which infects more than 150 species of animals, including humans (Dupouy-Camet, 2000; Pozio, 2007; Murrell, 2016). According to the International Commission on Trichinellosis (ICT), a total of 65,813 people in the world were diagnosed with trichinellosis as a result of eating raw or semi-raw meat from 1986 to 2009 (Murrell and Pozio, 2011). Furthermore, in 2015, nearly 40,000 cases of Trichinella infections were reported in China (Yang et al., 2020). In recent years, outbreaks of trichinellosis have occurred several times in remote areas of central and western China where people have a habit of eating uncooked pork (Bai et al., 2017). Hence, there is an urgent need to carry out epidemiological monitoring of trichinellosis and to strengthen the prevention and control of Trichinella infections.
According to the recommendation of the ICT and the World Organization for Animal Health (OIE), serological methods are an acceptable method of detecting Trichinella infection in animals (Gamble et al., 2004; Bruschi et al., 2019). The most widely used serological method is enzyme-linked immunosorbent assay (ELISA) based on excretory–secretory (ES) antigens (Gamble et al., 1988; Kapel and Gamble, 2000; Gomez-Morales et al., 2009). Depending on their accuracy and sensitivity, ELISAs serve as a reliable method to detect Trichinella in a diagnostic lab. However, ELISA-based detection is plagued by lengthy, laborious, and user-unfriendly procedures.
The immunoassay strip (ICS) is a sensitive, convenient, and rapid option for detecting pathogen infection. More recently, ICS has played a key role in the field of detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, further promoting the advantage of ICS in epidemic disease outbreaks (Chen et al., 2021; Zhang et al., 2021). Traditional ICS methods employ the use of gold nanoparticles, quantum dots, or fluorescent microspheres (Liang et al., 2017; Willen et al., 2018; Lee et al., 2019). These different ICS methods have previously been developed for the diagnosis of Trichinella infection, albeit using serum samples from pigs and in a laboratory setting (Zhang et al., 2006; Fu et al., 2013; Xu et al., 2021). Europium(III) chelate microparticle (ECM) is a fluorescent microsphere-based ICS and has the advantages of sensitivity and specificity. Additionally, ECM benefits from long fluorescence bursts and is less susceptible to stray light and long Stokes displacement ICS (Hu et al., 2017). No previous literature has been published on investigating the potential of ECM-based ICS for on-site detection of Trichinella infection and its promise as a powerful tool for epidemic monitoring.
In our previous work, we developed an ECM-based ICS capable of detecting T. spiralis-specific IgG antibody in pig serums; the sensitivity, specificity, and cross-reaction were evaluated (Wang et al., 2021). Aiming for clinical whole blood samples, we simplified the procedure and improved the effectiveness of the ECM-based ICS. A blood-filtering pad and a conjugate pad were incorporated into the ICS, which retained high sensitivity and accuracy. More importantly, the novel ICS can be used for whole blood samples detected and is potentially suitable for a wider range of applications.
Bovine serum albumin (BSA) and Tween-20 were purchased from Solarbio (Beijing, China). 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (NHS) were purchased from Tokyo Chemical Industry (TCI Shanghai, China). COOH-modified europium nanoparticles (EuNPs) were purchased from Thermo Fisher (USA). Mouse anti-pig monoclonal IgG antibodies and rabbit anti-goat IgG antibodies were purchased from Beijing Biolab Technology (Beijing, China). Goat anti-rabbit IgG was purchased from Cell Signal Technology (USA). The NC membrane (CN140) was purchased from Sartorius (USA), and Ultra-15 3 kDa, Millipore 135, and Millipore 90 centrifugal filters were purchased from Millipore (USA). The sample pads; the conjugate pads SB06, SB08, VL78, VL98, RB45, and RB65; the blood filter membranes; the absorbent pads; and the plastic backing were obtained from Jinbiao Biotech (Shanghai, China). The BSA protein assay kit was obtained from Beyotime Biotechnology (Shanghai, China).
The XYZ3060 dispenser was obtained from BioDot (USA). The TRF fluorescence quantitative analyzer was obtained from Weice Biotech (Nanjing, China). A UV lamp was obtained from Shenzhen Feike Technology (Shenzhen, China).
The preparation of muscle larvae (ML) ES antigens follows the procedures described previously (Bai et al., 2016; Wang et al., 2020). A total of eight specific pathogen-free (SPF) SD rats were orally infected with 4,500 T. spiralis (T1 ISS534) ML each. Subsequently, the rats were euthanized at 35 dpi and muscle samples were used to recover T. spiralis ML by following the artificial digestion method. Then, the ML was washed several times with 0.9% saline solution until clean and resuspended with serum-free RPMI-1640 medium including antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin); the culture condition was 37°C for 18 h in 5% CO2. Finally, the culture supernatant was concentrated by Ultra-15 3 kDa centrifugal filters, and the concentration of the protein was measured by BCA kits.
The preparation of preadult worm (PAW) ES antigens was performed according to the procedures described in Sun et al. (Sun et al., 2015). An additional eight SPF SD rats were infected with 10,000 T. spiralis ML each and euthanized at 6 h post-infection. The tissue of the small intestine was harvested from the abdominal cavity and was put in 0.9% saline solution (containing 200 U/ml of penicillin and 200 μg/ml of streptomycin) at 37°C for 2 h after being split longitudinally. The cultivation and preparation of PAW-ES antigens were performed the same as the ML-ES antigens.
Four Landrace pigs aged 10 weeks were inoculated with 400, 600, 600, and 800 doses of T. spiralis ML, and a total of 60 serum samples were collected at 0, 7, 9, 11,13, 15, 17, 19, 21, 25, 30, 35, 45, 60, and 90 dpi. Five Landrace pigs aged 10 weeks were inoculated with 0, 200, 600, 1,000, and 10,000 T. spiralis ML, and a total of 20 serum samples and 20 whole blood samples were collected at 0, 21, 25, 30, and 35 dpi. The whole blood samples collected from pigs infected with 10,000 T. spiralis at 60 dpi were served as a standard positive control.
A total of 1,500 whole blood samples were collected from slaughterhouses in Chongqing, Sichuan, Inner Mongolia, Guangxi, and Liaoning provinces of China.
The preparation of the fluorescent probe was followed by the previous protocol of Yan et al. (Yan et al., 2021). Firstly, 20 μl of ECM was centrifuged at 18,000×g for 10 min, and subsequently, the liquid supernatant was discarded. Then, 20 µl of 1 mg/ml EDC, 20 µl of 1 mg/ml NHS, and 200 µl of MES (50 mmol/L, pH 6.0) were mixed with the ECM and incubated for 1 h. Then, the mixture was centrifuged at 18,000×g for 10 min to remove the phosphate and glycerol. Once removed, 20 μg of PAW-ES antigens and ML-ES antigens at the ratio of 1:1 in 200 µl of PBS buffer (50 mmol/L, pH 8.0) were added to it. The mixture was incubated for 3 h, followed by a centrifuging step at 18,000×g for 10 min; the supernatant was discarded. Then, the mixture was blocked by 200 µl of 5% BSA overnight at 4°C. Finally, the ECM-ES fluorescent probes were dissolved in a preserved buffer (100 mmol/L of PBS containing 1.5% BSA and 1.5% ProClin). The method of ECM conjugated with goat anti-rabbit IgG was the same as the ECM-ES.
The ICS consisted of an absorbent pad, nitrocellulose membrane (NC membrane), conjugate pad, blood filter pad, and baseboard. Afterward, the ECM-ES with ECM-goat anti-rabbit IgG was mixed at 3:1. The fluorescent probes were sprayed on a conjugate pad using the XYZ3060 dispenser. Meanwhile, mouse anti-pig monoclonal IgG (1 mg/ml) and rabbit anti-goat IgG (1 mg/ml) were sprayed on the position of the testing line (T-line) and the control line (C-line) of the NC membrane by the XYZ3060 dispenser, respectively. Following a 2-h drying step at 37°C, the five parts were assembled forming the ICS. Finally, the ICS was cut at a width of 3.8 mm and placed in a white cassette.
The addition of a conjugate pad is a key step toward the commercialization of the novel ICS. In order to achieve a perfect release of fluorescent particles by the ICS, the relevant reaction steps of the conjugate pad were optimized. Firstly, the materials of the conjugate pads SB06, SB08, VL78, VL98, RB45, and RB65 made of glass fiber and polyester fiber were assessed for suitability for use in the novel ICS. Secondly, the fluorescent particles conjugated with ES antigens at a ratio of 10:1, 20:1, 30:1, and 40:1 were evaluated on the novel ICS. Thirdly, the fluorescent particles sprayed on the conjugate pad at the amounts of 0.4, 0.8, and 1.2 µg were evaluated on the ICS. In order to achieve desirable incubation times, multiple NC membranes, CN140, Millipore 135, and Millipore 90 were tested on the novel ICS. The serum obtained from the pigs infected with 10,000 T. spiralis ML at 60 dpi was used to optimize the reaction conditions in this part. For all conditions, visual results were observed by a 365-nm UV lamp 3 min after the samples were added to the ICS.
After collecting the whole blood samples using anticoagulation tubes, these samples were detected by ICS at the dilution of 1:50, 1:100, 1:200, 1:400, 1:800, 1:1,600, 1:3,200, 1:6,400, 1:12,800, and 1:25,600. The whole blood samples from pigs infected with 10,000 T. spiralis ML at 60 dpi and uninfected pigs were chosen as the standard positive and negative controls. Visual results were observed by a 365-nm UV lamp, and the fluorescent signal was analyzed by a fluorescent reader 3 min after the samples were added.
To evaluate the sensitivity of ICS after employing the conjugate pad, the serum samples from pigs infected with 400, 600, 600, and 800 T. spiralis ML at 0–90 dpi were detected by ICS. In these three infectious doses, the pork samples contain approximately 0.5–1.5 larvae per gram (LPG). Previous studies indicated that the detection limit of the gold standard method (artificial digestion) is 1–3 LPG (Gamble, 1998). If the detection effect is better than the digestion method, it can be assumed that the sensitivity of the method is higher than that of the current gold standard.
To effectively detect the whole blood samples, a blood filter pad was incorporated into the ICS, which could filter out the red blood cells. To evaluate the limit of detection of the ICS for whole blood samples and serum samples, two kinds of samples collected from pigs infected with 0, 200, 600, 1,000, and 10,000 T. spiralis ML at 0, 21, 25, 30, and 35 dpi were analyzed by ICS. Following a 3-min incubation period, visual results were observed by a 365-nm UV lamp, and the fluorescent signal was analyzed by a fluorescent reader.
The ultimate aim of this research was to apply this method to the clinical detection of Trichinella. A total of 1,500 whole blood samples were collected from slaughterhouses in the villages of Chongqing, Sichuan, Inner Mongolia, Guangxi, and Liaoning provinces in China where Trichinella infection has been widely reported. The novel ICS detection method’s accuracy and its potential clinical application were verified using artificial digestion.
The fluorescence intensity of the T-line of ICS was analyzed by a fluorescent reader, and the results were expressed as the mean ± SD using GraphPad Prism8.
The principle of the ICS can be considered a classical indirect detection method; the ECM-ES fluorescent probes served as capture probes and the ECM-goat anti-rabbit IgG fluorescent probes served as indicative probes. Firstly, T. spiralis-specific IgG antibodies were captured by fluorescent ECM-ES probes, and these, in turn, react with mouse anti-pig IgG antibodies on the T-line. Meanwhile, the fluorescent ECM-goat anti-rabbit IgG probes were immobilized on the C-line by rabbit anti-goat IgG antibodies. Therefore, when ICS detected a positive sample, both the T-line and the C-line had a fluorescent signal. When ICS detected a negative sample, only the C-line had a fluorescent signal. Due to the addition of a blood filter membrane into the ICS, both serum samples and whole blood samples could be detected (Figure 1).
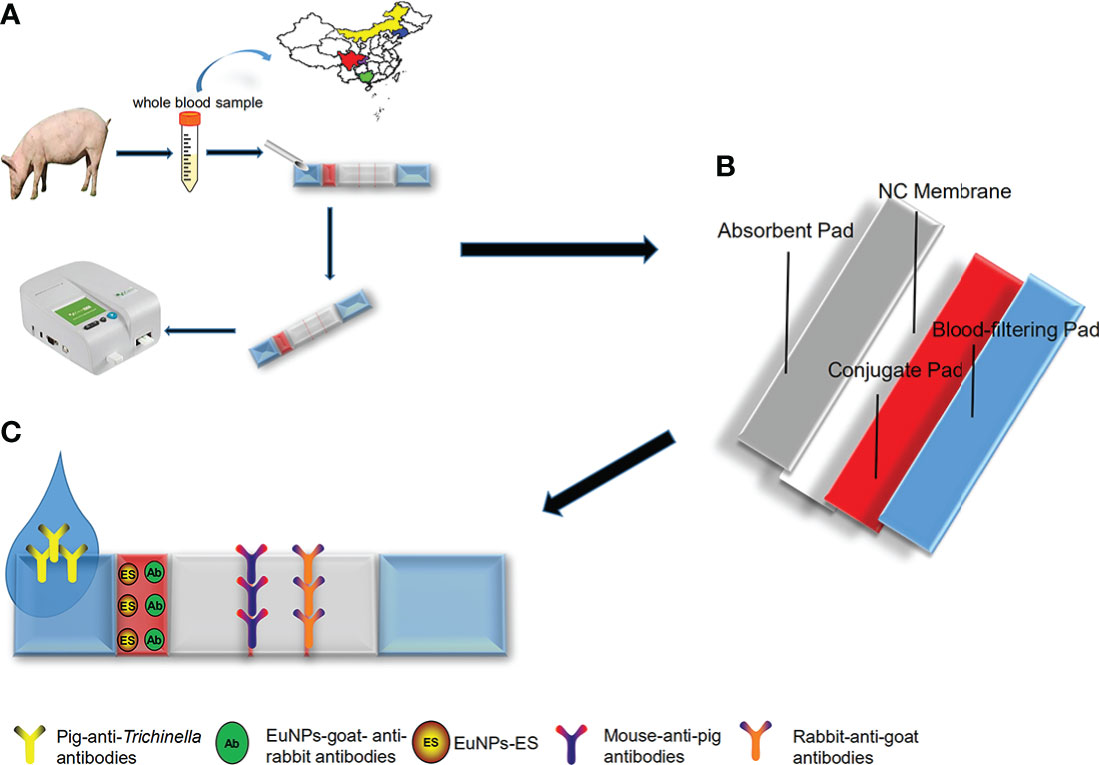
Figure 1 The schematic of europium(III) chelate microparticle-immunoassay strip (ECM-ICS). (A) Detecting the whole blood sample by ECM-ICS. (B) Schematic depiction of the construction of the ECM-ICS. (C) Immunochromatographic process of the ECM-ICS.
Optimizing the reaction conditions of the conjugate and NC membrane pads in the ICS conformed to the principle that fluorescent particles were realized adequately and the T-line broke out the stronger fluorescent signal. The use of the 0.8-µg fluorescent particles at the ratio of 10:1 conjugated with ES antigens, which was sprayed on the conjugate pad, yielded the best results. Moreover, it was determined that the VL78 conjugate pad and the CN140 NC produced the desired result (Figure 2).
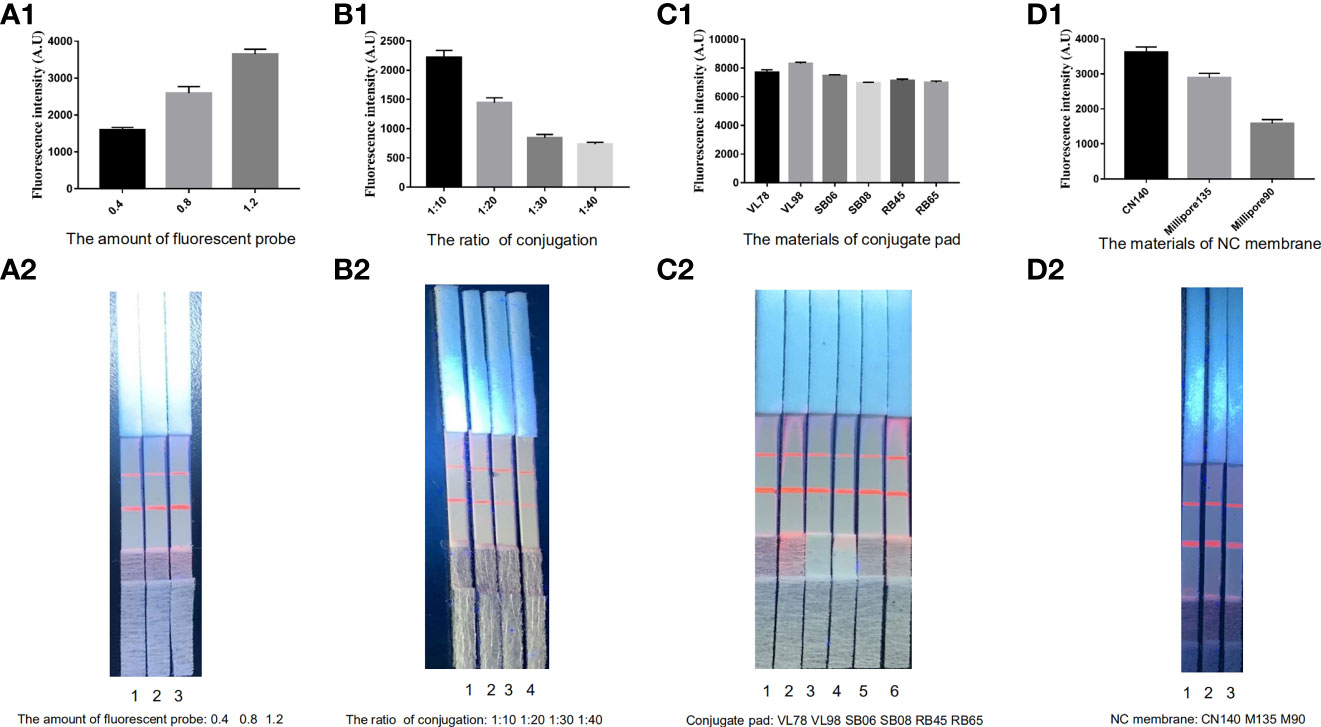
Figure 2 Optimization of the ECM-ICS. (A) Fluorescent particles sprayed on conjugate pads 1–3: 0.4, 0.8, and 1.2 µg. (B) The rate of ES antigens conjugated with fluorescent particles 1–4: 1:10, 1:20, 1:30, and 1:40. (C) The materials of conjugate pads 1–6: VL78, VL98, SB06, SB08, RB45, and RB65. (D) The materials of NC membranes 1–3: CN140, Millipore 135, and Millipore 90. ECM-ICS was analyzed by the TRF reader (A1, B1, C1, D1). Visual results of the ICS under ultraviolet light (A2, B2, C2, D2).
The fluorescence intensity of ICS was the strongest when whole blood samples were diluted 1,600 times. Moreover, the visual ranking was consistent with the fluorescence analysis results. The novel ICS was able to produce a fluorescence signal for positive samples, even when diluted 25,600 times. Considering the fact that whole blood samples cannot be diluted too much in practical applications, we recommend diluting the sample 1,600 times (Figure 3).
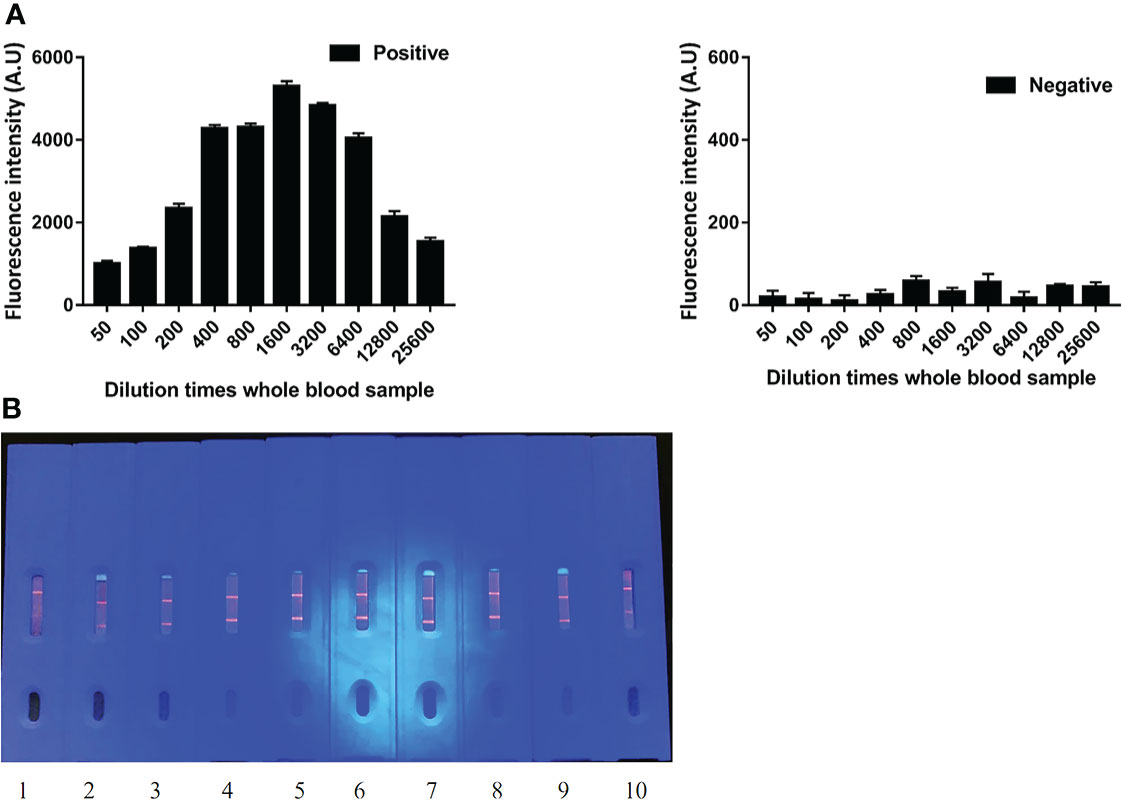
Figure 3 The dilution limit of whole blood samples by ECM-ICS. (A) ECM-ICS was analyzed by the TRF reader. (B) Visual results of ICS under ultraviolet light. 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 represent the whole blood samples diluted 50, 100, 200, 400, 800, 1,600, 3,200, 6,400, 12,800, and 25,600 times.
The fluorescent particles were fully released and the T-line produced a strong fluorescent signal; in the pigs infected with 400, 600, 600, and 800 ML, seroconversion was first detectable by ICS at 30, 25, 25, and 21 dpi, respectively (Figure 4). Compared with other results (Gondek et al., 2018), the novel ICS could detect early infection even the pigs were infected with low dose of Trichinella, which indicated ICS has high sensitivity. Furthermore, the fluorescence intensity of the T-line increased with days post-infection (Figure 4).
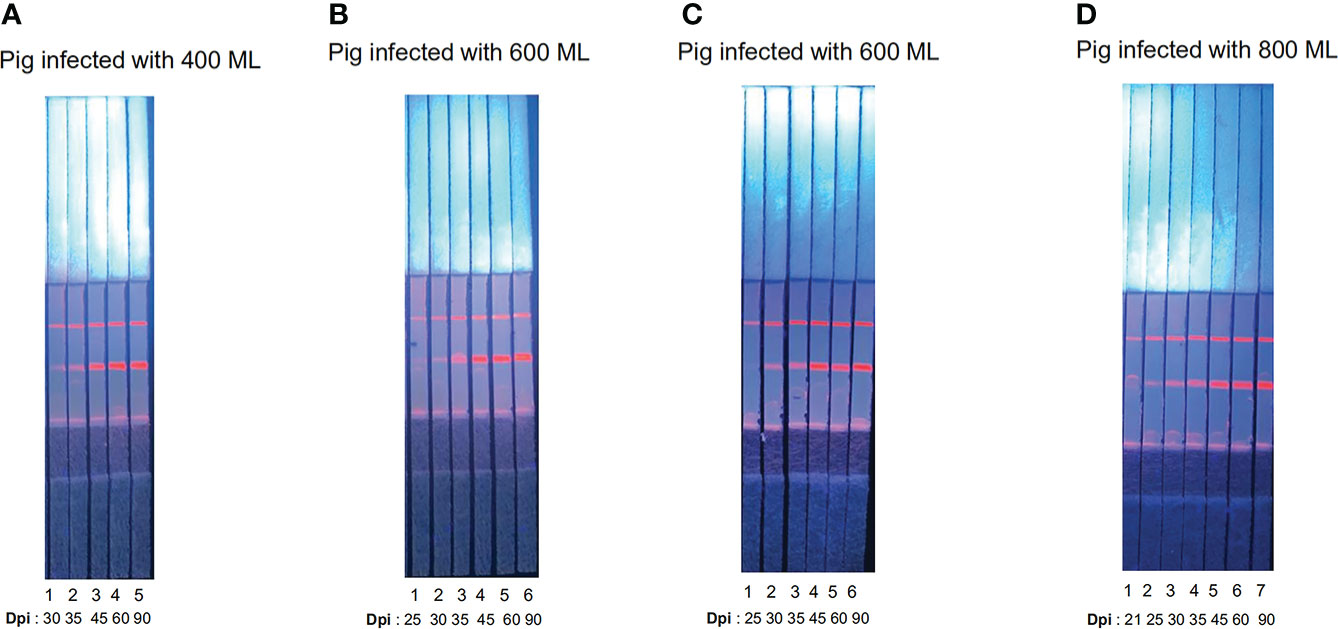
Figure 4 Serum samples detected by ECM-ICS. (A) Samples from pigs infected with 400 Trichinella. (B) Samples from pigs infected with 600 Trichinella. (C) Samples from pigs infected with 600 Trichinella. (D) Samples from pigs infected with 800 Trichinella. (A1–5) 30, 35, 45, 60, and 90 dpi; (B1–6) 25, 30, 35, 45, 60, and 90 dpi; (C1–6) 25, 30, 35, 45, 60, and 90 dpi; (D1–7) 21, 25, 30, 35, 45, 60, and 90 dpi.
As shown in Figure 3C, in pigs infected with 200, 600, 1,000, and 10,000 ML, seroconversion was first detected by ICS at 25, 25, 21, and 21 dpi, respectively. Although the signal of whole blood is slightly weaker than that of serum, the results of both samples were consistent when visually rated. More interesting, regardless of the sample type, the fluorescence intensity of the T-line increased with the dose of infection, which indicated that the level of antibody produced by the host is positively correlated with the dose of infection (Figure 5).
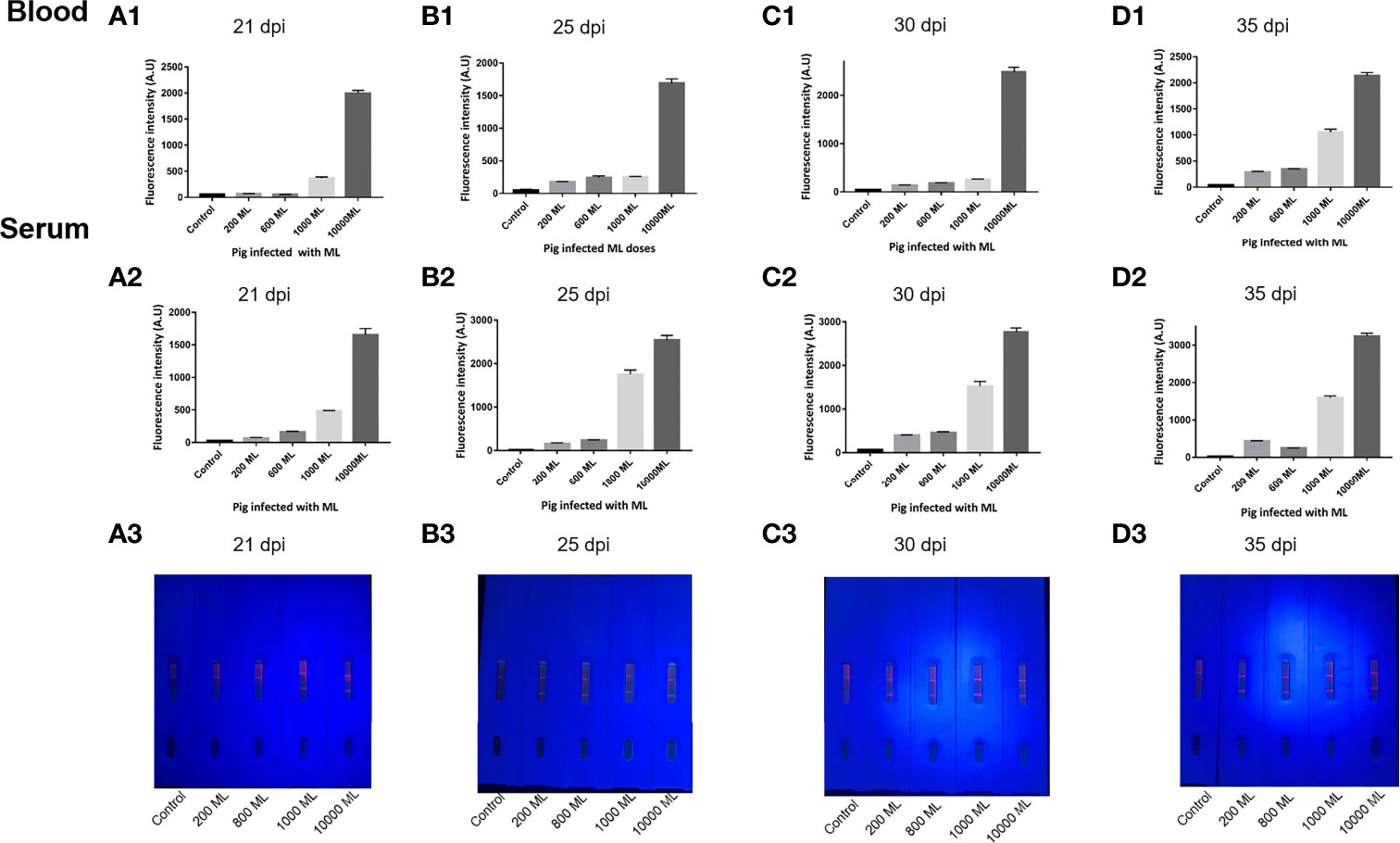
Figure 5 Comparison of ECM-ICS for detecting whole blood and serum samples. (A) Samples from pigs infected with Trichinella at 21 dpi. (B) Samples from pigs infected with Trichinella at 25 dpi. (C) Samples from pigs infected with Trichinella at 30 dpi. (D) Samples from pigs infected with Trichinella at 35 dpi. (A1, B1, C1, D1) ECM-ICS for detecting whole blood samples analyzed by the TRF reader; (A2, B2, C2, D2) ECM-ICS for detecting serum samples analyzed by the TRF reader;(A3, B3, C3, D3) the whole blood samples detected by ECM-ICS and the visual results under ultraviolet light.
The performance of the ICS for detecting whole blood was shown, and the process of detection could be completed within 3 min without specialized equipment or the need for specially trained personnel (Video 1). Based on this method, five suspected trichinosis endemic areas were screened using the novel test strip. Results showed that five whole blood samples were detected as positive by ICS and three pork samples were detected as positive by artificial digestion (Table 1).
Developing an on-site detection method for Trichinella infections is of critical importance, which will contribute to epidemiological investigations and monitoring of potential epidemic outbreaks. Based on our previous research, this study is a further research for the exploration of ICS based on ECM detection in clinical application. Here, we demonstrated that the seroconversion time detected by means of the developed novel ICS for use with whole blood samples was consistent with serum samples. Moreover, we used this method to complete an epidemiological survey of five provinces in China.
Optimization of the conjugate pad material employed for the novel ICS played an important role in allowing the use of the ICS to be more convenient and user-friendly. Firstly, our result shows that attention should be given to the application of fluorescent particles to the conjugate pad. The spraying of fluorescent particles required exact precision because excessive fluorescent particles will not react with the antibody and cause high fluorescence background on the NC membrane. In contrast, insufficient fluorescent particles will result in an insufficient reaction between the antigen and the antibody. Furthermore, the selection of the NC membrane is another key factor in developing the ICS method. If the detection reaction rate is defined as R, it is reflected by the affinity of the antibody for the antigen K and the concentration of the antigen (Ag) and the antibody (Ab) on the NC membrane (see equation). Different NC membranes have different pore sizes, which determine the crawling speed of the antibody on the membrane, thereby affecting the reaction time of the antigen-antibody. Therefore, on the premise of ensuring the specificity of the ICS, increasing the incubation time of the antigen with the antibody on the NC membrane could improve the sensitivity of the ICS.
The use of the blood-filtering pad makes ICS drastically decrease the test time, which allows the ICS to detect the whole blood samples. Despite the addition of this extra structure, the limit of detection of the novel ICS was not affected. Moreover, compared with the previous study (Gondek et al., 2018), the novel ICS could detect early anti-Trichinella antibodies in pigs, and this may have been due to the method employing the use of the PAW-ES antigens. We were able to confirm previous findings, which reported that the use of early antigens could shorten the diagnostic window of Trichinella infections (Liu et al., 2016; Wang et al., 2017a; Wang et al., 2017b). Due to the ML-ES being easily acquired and widely used in the detection of Trichinella infection, we determined to combine the two antigens at a ratio of 1:1 in this coupling system.
Investigating Trichinella infection in China is of great significance. The difference in the results between the ICS and artificial digestion may be due to the host interacting with the worms, causing a response that results in the expulsion of the parasites in low doses, or due to the habit of feeding insecticides in rural areas. In summary, this study provides a useful tool for performing on-site detection of Trichinella infection, having the advantages of being sensitive, rapid, and convenient. The use of a conjugate pad and a blood filter membrane facilitated the use of the novel ICS in clinical applications. Our novel detection method is superior compared to more mainstream serological detection methods for the detection of early Trichinella infection.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study was reviewed and approved by Administration of Affairs Concerning Experimental Animals in China, The protocol was approved by the Institutional Animal Care and Use Committee of Jilin University (20170318).
XW, AL, RW, and JD participated in the data collection and drafted the manuscript. CL, ML, and JW participated in designing this study and revising the manuscript. TH, HC, and XW participated in collecting the samples. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (NSFC31872467, 31902290) and the Program for JLU Science and Technology Innovative Research Team (2017TD-32).
Author ML was employed by Changchun Institute of Biological Products Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/fcimb.2022.955974/full#supplementary-material
Bai, X., Hu, X., Liu, X., Tang, B., Liu, M. (2017). Current research of trichinellosis in China. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01472
Bai, X., Wang, X. L., Tang, B., Shi, H. N., Boireau, P., Rosenthal, B., et al. (2016). The roles of supernatant of macrophage treated by excretory-secretory products from muscle larvae of Trichinella spiralis on the differentiation of C2C12 myoblasts. Vet. Parasitol. 231, 83–91. doi: 10.1016/j.vetpar.2016.07.033
Bien, J., Cabaj, W., Moskwa, B. (2013). Recognition of antigens of three different stages of the Trichinella spiralis by antibodies from pigs infected with T. Spiralis. Exp. Parasitol. 134 (2), 129–137. doi: 10.1016/j.exppara.2013.02.007
Bruschi, F., Gomez-Morales, M. A., Hill, D. E. (2019). International commission on trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and humans. Food Waterborne. Parasitol. 14, e00032. doi: 10.1016/j.fawpar.2018.e00032
Chen, R., Ren, C., Liu, M., Ge, X., Qu, M., Zhou, X., et al. (2021). Early detection of sars-Cov-2 seroconversion in humans with aggregation-induced near-infrared emission nanoparticle-labeled lateral flow immunoassay. ACS Nano. 15 (5), 8996–9004. doi: 10.1021/acsnano.1c01932
Dupouy-Camet, J. (2000). Trichinellosis: A worldwide zoonosis. Vet. Parasitol. 93 (3-4), 191–200. doi: 10.1016/s0304-4017(00)00341-1
Fu, B. Q., Li, W. H., Gai, W. Y., Yao, J. X., Qu, Z. G., Xie, Z. Z., et al. (2013). Detection of anti-Trichinella antibodies in serum of experimentally-infected swine by immunochromatographic strip. Vet. Parasitol. 194 (2-4), 125–127. doi: 10.1016/j.vetpar.2013.01.036
Gamble, H. R. (1996). Detection of trichinellosis in pigs by artificial digestion and enzyme immunoassay. J. Food Prot. 59 (3), 295–298. doi: 10.4315/0362-028x-59.3.295
Gamble, H. R. (1998). Sensitivity of artificial digestion and enzyme immunoassay methods of inspection for Trichinae in pigs. J. Food Prot. 61 (3), 339–343. doi: 10.4315/0362-028x-61.3.339
Gamble, H. R., Anderson, W. R., Graham, C. E., Murrell, K. D. (1983). Diagnosis of swine trichinosis by enzyme-linked immunosorbent assay (Elisa) using an excretory–secretory antigen. Vet. Parasitol. 13 (4), 349–361. doi: 10.1016/0304-4017(83)90051-1
Gamble, H. R., Pozio, E., Bruschi, F., Nockler, K., Kapel, C. M., Gajadhar, A. A. (2004). International commission on trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and man. Parasite 11 (1), 3–13. doi: 10.1051/parasite/20041113
Gamble, H. R., Rapic, D., Marinculic, A., Murrell, K. D. (1988). Evaluation of excretory-secretory antigens for the serodiagnosis of swine trichinellosis. Vet. Parasitol. 30 (2), 131–137. doi: 10.1016/0304-4017(88)90160-4
Gomez-Morales, M. A., Ludovisi, A., Pezzotti, P., Amati, M., Cherchi, S., Lalle, M., et al. (2009). International ring trial to detect anti-Trichinella igg by Elisa on pig sera. Vet. Parasitol. 166 (3-4), 241–248. doi: 10.1016/j.vetpar.2009.09.005
Gondek, M., Bien, J., Nowakowski, Z. (2018). Use of Elisa and Western blot for serological detection of antibodies to ES antigens of Trichinella spiralis muscle larvae in sera of swine experimentally infected with Trichinella spiralis. Vet. Immunol. Immunopathol. 203, 13–20. doi: 10.1016/j.vetimm.2018.07.010
Hu, L. M., Luo, K., Xia, J., Xu, G. M., Wu, C. H., Han, J. J., et al. (2017). Advantages of time-resolved fluorescent nanobeads compared with fluorescent submicrospheres, quantum dots, and colloidal gold as label in lateral flow assays for detection of ractopamine. Bios. Bioelect. 91, 95–103. doi: 10.1016/j.bios.2016.12.030
Kapel, C. M., Gamble, H. R. (2000). Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. In. Experiment. Infect. Pigs. Int. J. Parasitol. 30 (2), 215–221. doi: 10.1016/s0020-7519(99)00202-7
Korinkova, K., Kovarcik, K., Pavlickova, Z., Svoboda, M., Koudela, B. (2008). Serological detection of Trichinella spiralis in swine by Elisa (Enzyme-linked immunosorbent assay) using an excretory-secretory (E/S) antigen. Parasitol. Res. 102 (6), 1317–1320. doi: 10.1007/s00436-008-0911-x
Lee, C., Noh, J., O'Neal, S. E., Gonzalez, A. E., Garcia, H. H., Cysticercosis Working Group in P, et al. (2019). Feasibility of a point-of-Care test based on quantum dots with a mobile phone reader for detection of antibody responses. PLoS Negl. Trop. Dis. 13 (10), e0007746. doi: 10.1371/journal.pntd.0007746
Liang, R. L., Deng, Q. T., Chen, Z. H., Xu, X. P., Zhou, J. W., Liang, J. Y., et al. (2017). Europium (III) chelate microparticle-based lateral flow immunoassay strips for rapid and quantitative detection of antibody to hepatitis b core antigen. Sci. Rep. 7 (1), 14093. doi: 10.1038/s41598-017-14427-4
Liu, R. D., Qi, X., Sun, G. G., Jiang, P., Zhang, X., Wang, L. A., et al. (2016). Proteomic analysis of Trichinella spiralis adult worm excretory-secretory proteins recognized by early infection sera. Vet. Parasitol. 231, 43–46. doi: 10.1016/j.vetpar.2016.10.008
Murrell, K. D. (2016). The dynamics of Trichinella spiralis epidemiology: Out to pasture? Vet. Parasitol. 231, 92–96. doi: 10.1016/j.vetpar.2016.03.020
Murrell, K. D., Pozio, E. (2011). Worldwide occurrence and impact of human trichinellosis 1986-2009. Emerg. Infect. Dis. 17 (12), 2194–2202. doi: 10.3201/eid1712.110896
Nockler, K., Serrano, F. J., Boireau, P., Kapel, C. M., Pozio, E. (2005). Experimental studies in pigs on trichinella detection in different diagnostic matrices. Vet. Parasitol. 132 (1-2), 85–90. doi: 10.1016/j.vetpar.2005.05.033
Pozio, E. (2007). World distribution of trichinella spp. infections in animals and humans. Vet. Parasitol. 149 (1-2), 3–21. doi: 10.1016/j.vetpar.2007.07.002
Smith, H. J. (1987). Evaluation of the Elisa for the serological diagnosis of trichinosis in Canadian swine. Can. J. Vet. Res. 51 (2), 194–197.
Smith, H. J., Snowdon, K. E. (1989). Comparative assessment of a double antibody enzyme immunoassay test kit and a triple antibody enzyme immunoassay for the diagnosis of Trichinella spiralis and Trichinella spiralis nativa infections in swine. Can. J. Vet. Res. 53 (4), 497–499.
Sun, G. G., Wang, Z. Q., Liu, C. Y., Jiang, P., Liu, R. D., Wen, H., et al. (2015). Early serodiagnosis of trichinellosis by Elisa using excretory-secretory antigens of Trichinella spiralis adult worms. Parasit. Vectors 8, 484. doi: 10.1186/s13071-015-1094-9
van der Leek, M. L., Dame, J. B., Adams, C. L., Gillis, K. D., Littell, R. C. (1992). Evaluation of an enzyme-linked immunosorbent assay for diagnosis of trichinellosis in swine. Am. J. Vet. Res. 53 (6), 877–882.
Wang, N., Bai, X., Ding, J., Lin, J., Zhu, H., Luo, X., et al. (2020). Trichinella infectivity and antibody response in experimentally infected pigs. Vet. Parasitol. 297, 109111. doi: 10.1016/j.vetpar.2020.109111
Wang, Z. Q., Liu, R. D., Sun, G. G., Song, Y. Y., Jiang, P., Zhang, X., et al. (2017a). Proteomic analysis of Trichinella spiralis adult worm excretory-secretory proteins recognized by sera of patients with early trichinellosis. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00986
Wang, Z. Q., Shi, Y. L., Liu, R. D., Jiang, P., Guan, Y. Y., Chen, Y. D., et al. (2017b). New insights on serodiagnosis of trichinellosis during window period: Early diagnostic antigens from Trichinella spiralis intestinal worms. Infect. Dis. Pov. 6 (1), 41. doi: 10.1186/s40249-017-0252-z
Wang, X., Tang, B., Zhao, Y., Ding, J., Wang, N., Liu, Y., et al. (2021). Development of a rapid and sensitive immunochromatographic strip based on eunps-Es fluorescent probe for the detection of early Trichinella spiralis-specific igg antibody in pigs. Vet. Res. 52 (1), 85. doi: 10.1186/s13567-021-00951-9
Willen, L., Mertens, P., Volf, P. (2018). Evaluation of the Rsp03b sero-strip, a newly proposed rapid test for canine exposure to phlebotomus perniciosus, vector of Leishmania infantum. PLoS Negl. Trop. Dis. 12 (8), e0006607. doi: 10.1371/journal.pntd.0006607
Xu, N., Liu, Y., Li, Y., Tang, B., Liang, X., Yang, Y., et al. (2021). Rapid quantum dot nanobead-mab probe-based immunochromatographic assay for antibody monitoring of Trichinella spiralis infection. Int. J. Nanomed. 16, 2477–2486. doi: 10.2147/IJN.S304845
Yang, X. D., Xu, C. Y., Wang, S. Y., Gao, H. Y., Liang, J. B. (2020). Epidemiology, diagnosis, treatment and control measures of trichinellosis in China: an overview. Zhongguo. Xue. Xi. Chong. Bing. Fang. Zhi. Za. Zhi. 32 (5), 448–452. doi: 10.16250/j.32.1374.2020251
Yan, J., Peng, B., Chen, H., Jin, Z., Cao, D., Song, Q., et al. (2021). On-site differential diagnostic detection of hp-prrsv and c-prrsv using eunps-mab fluorescent probe-based immunoassay. Anal. Bioanal. Chem. 413 (23), 5799–5810. doi: 10.1007/s00216-021-03558-3
Zhang, G. P., Guo, J. Q., Wang, X. N., Yang, J. X., Yang, Y. Y., Li, Q. M., et al. (2006). Development and evaluation of an immunochromatographic strip for trichinellosis detection. Vet. Parasitol. 137 (3-4), 286–293. doi: 10.1016/j.vetpar.2006.01.026
Keywords: Trichinella spiralis, excretory–secretory, europium(III) chelate microparticle, immunochromatographic strip, on-site detecting
Citation: Wang X, Li A, Wang R, Hou T, Chen H, Wang J, Liu M, Li C and Ding J (2022) Lateral flow immunoassay strips based on europium(III) chelate microparticle for the rapid and sensitive detection of Trichinella spiralis infection in whole blood samples of pigs. Front. Cell. Infect. Microbiol. 12:955974. doi: 10.3389/fcimb.2022.955974
Received: 29 May 2022; Accepted: 15 July 2022;
Published: 09 August 2022.
Edited by:
Xiao-Xuan Zhang, Qingdao Agricultural University, ChinaReviewed by:
Nian-Zhang Zhang, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (CASS), ChinaCopyright © 2022 Wang, Li, Wang, Hou, Chen, Wang, Liu, Li and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Li, bGMyMDE4QGpsdS5lZHUuY24=; Jing Ding, ZGoxOUBqbHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.