
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 23 May 2022
Sec. Microbiome in Health and Disease
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.881899
This article is part of the Research Topic The Pivotal Role of Oral Microbiota Dysbiosis and Microbiota-host Interactions in Diseases - Volume II View all 11 articles
 Tong Tong Wu1
Tong Tong Wu1 Jin Xiao2*
Jin Xiao2* Samantha Manning1
Samantha Manning1 Prakaimuk Saraithong3
Prakaimuk Saraithong3 Komkham Pattanaporn4
Komkham Pattanaporn4 Bruce J. Paster5
Bruce J. Paster5 Tsute Chen5
Tsute Chen5 Shruti Vasani2
Shruti Vasani2 Christie Gilbert6
Christie Gilbert6 Yan Zeng2
Yan Zeng2 Yihong Li7*
Yihong Li7*Early childhood caries (ECC) is not only the most common chronic childhood disease but also disproportionately affects underserved populations. Of those, children living in Thailand have been found to have high rates of ECC and severe ECC. Frequently, the cause of ECC is blamed on a handful of cariogenic organisms, such as Streptococcus mutans and Streptococcus sobrinus. However, ECC is a multifactorial disease that results from an ecological shift in the oral cavity from a neutral pH (~7.5) to an acidic pH (<5.5) environment influenced by the host individual’s biological, socio-behavioral, and lifestyle factors. Currently, there is a lack of understanding of how risk factors at various levels influence the oral health of children at risk. We applied a statistical machine learning approach for multimodal data integration (parallel and hierarchical) to identify caries-related multiplatform factors in a large cohort of mother-child dyads living in Chiang Mai, Thailand (N=177). Whole saliva (1 mL) was collected from each individual for DNA extraction and 16S rRNA sequencing. A set of maternal and early childhood factors were included in the data analysis. Significantly, vaginal delivery, preterm birth, and frequent sugary snacking were found to increase the risk for ECC. The salivary microbial diversity was significantly different in children with ECC or without ECC. Results of linear discriminant analysis effect size (LEfSe) analysis of the microbial community demonstrated that S. mutans, Prevotella histicola, and Leptotrichia hongkongensis were significantly enriched in ECC children. Whereas Fusobacterium periodonticum was less abundant among caries-free children, suggesting its potential to be a candidate biomarker for good oral health. Based on the multimodal data integration and statistical machine learning models, the study revealed that the mode of delivery and snack consumption outrank salivary microbiome in predicting ECC in Thai children. The biological and behavioral factors may play significant roles in the microbial pathobiology of ECC and warrant further investigation.
Early childhood caries (ECC) is the single most common chronic childhood disease, known to disproportionately afflict more than 73% of underprivileged preschool children worldwide (Dye et al., 2007; Dye et al., 2012).
More than 50% of 3-year-old Thai children experienced ECC (Bureau of Dental Health, 2018). Chronically persistent ECC can progress to severe-ECC (S-ECC) impacting the primary dentition and adding a significant financial burden to the involved families. Several studies have depicted a higher prevalence of ECC in Thailand, with as high as 44.5% non-cavitated initial lesions and 24.5% cavitated advanced lesions being reported among 15–19-month-old children in Central Thailand (Ledder et al., 2018). In addition, a prospective study demonstrated ECC progressed rapidly among 9-18-month-old children in Southern Thailand (reported incidence of 2% at nine months, 22.8% at 12 months, and 68.1% at 18 months of age) (Thitasomakul et al., 2006). Nationwide, the ECC prevalence among 3-year-olds and 5-year-olds was 52.9% and 75.6%, respectively; 98% of them had untreated caries (Bureau of Dental Health, 2018). This highlights the urgent need to raise awareness among Thai parents/caregivers and healthcare providers to implement more effective preventive strategies for ECC control.
While ECC is an infectious disease initiated by cariogenic pathogens, it is now understood as a multifactorial and ecology-based disease (Simon-Soro and Mira, 2015), with the interplay between host, environment, and oral microbiota affecting the onset and severity of the disease (Pitts et al., 2017; Bowen et al., 2018). Factors such as environmental (exposure to water fluoridation and fluoride toothpaste, oral hygiene), biological (dental plaque accumulation, cariogenic microbial composition), lifestyle (breastfeeding pattern, frequent sugar consumption, obesity), and socio-cultural (low socio-economic status, marital status, maternal oral health perception, utilization of dental care) have been linked to ECC (Chanpum et al., 2020). A longitudinal cohort study among 3-year-old children in Northern Thailand reported that suboptimal water fluoridation, low socioeconomic status, frequent sugar consumption, and dental plaque accumulation contributed to high ECC prevalence (44.1%) (Peltzer and Mongkolchati, 2015). Additionally, studies have reported inconclusive results for the association between ECC and prolonged breastfeeding habits. However, one cross-sectional study indicated that ECC’s higher prevalence and severity among breastfed Thai children was significantly associated with factors such as age, children’s oral health status, and breastfeeding pattern/duration (Chanpum et al., 2020). Furthermore, our previous study among 182 3-year-old and 166 5-year-old Thailand preschool children revealed that mode of birth delivery was significantly correlated with Streptococcus mutans colonization and caries outcomes in young Thai children (Pattanaporn et al., 2013; Saraithong et al., 2015). Taken together, findings from these studies confirm that ECC is a public health concern in Thailand owing to the rising disease burden. With a thorough understanding of the underlying risk factors and effective prevention strategies, it is hoped that this knowledge will help reduce the ECC disease burden among Thai children as well as children worldwide.
Due to the multifactorial etiology of ECC, a valid prediction model that utilizes sensitive microbial markers from oral microbiota (including but not limited to S. mutans) at early life, together with medical-socio-behavior and environmental factors, would offer a substantial opportunity to predict ECC and help generate a personalized preventive regimen. A few recent studies have demonstrated machine learning predictive modeling using 16S rRNA sequencing of oral samples, but they lack consideration for multifactorial nature of tooth decay (Teng et al., 2015; Grier et al., 2020). Our team has recently developed a multivariate ECC prediction model that uses a machine learning approach incorporating microorganism composition and demographic-environmental factors among a small group of US mother-child dyads (Wu et al., 2021). Here, we further developed stepwise, parallel integrative, statistical machine learning (ML) models to identify caries-related multiplatform factors [environment, biomedical (child and maternal), socio-behavior-feeding, and oral microorganisms) in a large Thai mother-child dyad.
This cross-sectionally-designed study is a subset of a parent study detailed previously (Pattanaporn et al., 2013). The current study enrolled 177 3-year-old children and their biological mothers during the children’s immunization visit at the Health Promoting Hospital in Chiang Mai, Thailand, from July to December of 2009. Children who had significant congenital anomalies, chronic illness, or had taken any antibiotics within six weeks prior to examinations were excluded. A structured questionnaire was used to obtain data on family socio-demographics (age, sex, primary care provider, and maternal/family background), maternal pregnancy and past medical history (mode of delivery, gestational age, birth weight), as well as child’s feeding practice (breastfeeding, bottle feeding, and mother pre-chewing food), dietary habits (consumption of fruit juice, snacks, gum, lollipop candy, dried fruit, soft drink, child sleeping with bottle), and oral health practices. Information about delivery methods, gestational age, and child’s birth weight were further verified from accessing the hospital medical records. Chi-squared statistics were used to examine the differences between categorical variables. The Ethical Committee approved the protocol for this study of the Faculty of Dentistry, Chiang Mai University, Thailand (No. 12/2008). Written informed consent was obtained from all mothers or responsible caregivers at the time of children’s hospital visits.
Two calibrated dentists performed a comprehensive dental examination for all children and their mothers using the WHO criteria of decayed, missing, and filled teeth (DMFT for the mothers and dmft for the children) (Organization. WH, 1997). The presence of ECC was also recorded as a detectable white-spot lesion or cavity following American Academy of Pediatric Dentistry criteria (American Academy of Pediatric Dentistry, 2022). Approximately 1 ml whole saliva was collected from the mothers and their children after chewing a piece of paraffin wax for 1 min under the close supervision of a medical professional. The saliva samples were stored in centrifuge tubes and immediately transferred to a -20°C freezer in the microbiology laboratory at the Chiang Mai University Faculty of Dentistry until further microbiome analysis.
The microbial DNA extractions were performed at the microbiology laboratory at New York University College of Dentistry using a standard procedure (Saraithong et al., 2015). Briefly, 500µl of each saliva sample was used for whole-genome DNA extraction using Epicentre MasterPure™ DNA Purification kit. The 16S rRNA sequencing was conducted at the Forsyth Institute, Cambridge, MA. A total of 129 genus-specific probes and 638 species-level probes were used for bacterial identification. QIIME 1.9.1 (Caporaso et al., 2010) was used to quantify the composition and diversity of each community based on its open-reference OTU picking facility. Sequencing data that passed quality controls were included in this study to develop a caries prediction model and were assigned to operational taxonomic units (OTUs). OTUs having zero counts across all the samples or only appearing in one sample were removed from further analysis.
Alpha diversity analysis was performed using the phyloseq package (McMurdie and Holmes, 2013). The results were plotted across samples and reviewed as box plots for each group or experimental factor. Further, the statistical significance of grouping based on experimental factor was also estimated using a t-test. Beta-diversity similarity or distance between sample was measured using non-phylogenetic Bray-Curtis distance. Ordination-based methods Principal Coordinate Analysis (PCoA) was used to visualize these matrices in the 2D plot where each point represents the entire microbiome of a single sample. The statistical significance of the clustering pattern in ordination plots for beta diversity was evaluated using anyone among Permutational MANOVA (PERMANOVA). A P value less than 0.05 was considered statistically significant.
The Core microbiome analysis was adopted from the core function in the R package microbiome. The result of this analysis was represented in the form of a heatmap of core taxa or features where Y-axis represents the prevalence level of core features across the detection threshold (Relative abundance) range on the X-axis. A heat tree map depicting the OTU classifications and differential abundance comparison between salivary microbiome of children with ECC and caries-free children was employed. Taxa that have a significant abundance difference between ECC children vs. caries-free children was measured by the Wilcoxon Rank Sum test. Linear discriminant analysis (LDA) coupled with effect size (LEfSe) analysis (Segata et al., 2011) and non-parametric factorial Kruskal-Wallis sum-rank test were used to identify differentially abundant bacterial taxa regarding ECC status. Bacterial features were considered to be significant based on the logarithmic LDA score threshold >2.0 and false discovery rate <0.1.
The primary outcome was the caries status (Y/N) of children. The independent variables were grouped into three platforms based on the distal to proximal relationship to ECC: 1) Maternal socio-demographic-behavior-environmental factors (seven variables); 2) Children’s socio-demographic-behavior-environmental factors (20 variables); 3) Children’s oral microbial factors (386 variables). The characteristics of the two groups (ECC and Caries-free children) were compared using t-test for continuous data and Chi-square or Fisher’s exact tests for categorical data.
OTUs with fewer than 10 reads were removed from the dataset to ensure the sufficient depths. The centered log-ratio (CLR) transformation was applied to the relative abundance of taxa, where for each subject, the sample vector undergoes a transformation based on the logarithm of the ratio between the individual elements and the geometric mean of the vector. CLR removes the value-range restriction of percentages (relative abundance is a percentage) but keeps the sum constraint of compositional data.
First, we took the parallel integration approach to integrate data from different platforms. Data collected on the three platforms were put together and fitted in penalized logistic regression model using least absolute shrinkage and selection operator (LASSO) with the response variable being the binary indicator of whether the subject has caries or not (1 = caries present, 0 = no caries present). The LASSO tuning parameter was chosen using k-fold (k = 10) cross-validation. The solution path was created to show the order of the variables entering the model.
Then, the hierarchical integration approach, a two-step procedure, was used to integrate data collected on the three platforms listed above. First, a LASSO penalized logistic regression model was fit for each of the four platforms, with the same response variable of having caries or not. Next, the LASSO tuning parameter was chosen using k-fold (k = 10) cross-validation for each model. The set of variables selected for each of the four platforms were then collected together and used as candidate variables to fit the final LASSO-penalized logistic regression model. Again, 10-fold cross-validation was used to determine the optimal number of variables in the final model. Finally, the solution paths for the four models and the final model were created.
The demographic characteristics of the children with or without ECC are shown in Table 1. No significant differences were found between the ECC and caries-free children regarding maternal age and antibiotic use. There was an increase of ECC in mothers who prechewed food for their children, although the difference was marginally significant (p=0.051). In terms of children’s factors, significantly more ECC children than caries-free children were born vaginally [63.27% vs. 43.04%, p=0.007; Odd’s ratio = 2.28, 95% CI (1.19, 4.37)] or preterm (45.95% vs. 30.38%, p=0.035). More than 83% ECC children were colonized with high levels of S. mutans compared to 20% in caries-free children (p<0.001). More caries-free children were found to use fluoridated toothpaste (p=0.009); were bottle fed (p=0.040); and less frequently consumed soft drinks (p=0.003), snacks (p=0.003), lollipop (p=0.017), and other candies (p=0.015).
The microbiome data include 79,001 OTUs [excluding OTUs with fewer than two sequences and sequences that fail to align with PyNAST (Caporaso et al., 2010)] for 354 samples. The sequence reads of all samples in the study are deposited in the NCBI Sequence Read Archive (SRA) as a study under the accession number of PRJNA824062. For the rarefaction curves, see Figure S1. Microbial community variation measured by alpha diversity was seen among children from different age groups, the mode of delivery, mother chew food practice, and children’s ECC status (Figure 1). For instance, the salivary microbiome of 2-year-old children and born vaginally had a higher alpha diversity (Observed and Fischer index) than 3-year-old children and born with C-section (Figures 1A, B) . The alpha diversity was significantly higher in children who frequently consumed snacks (Figure 1D).
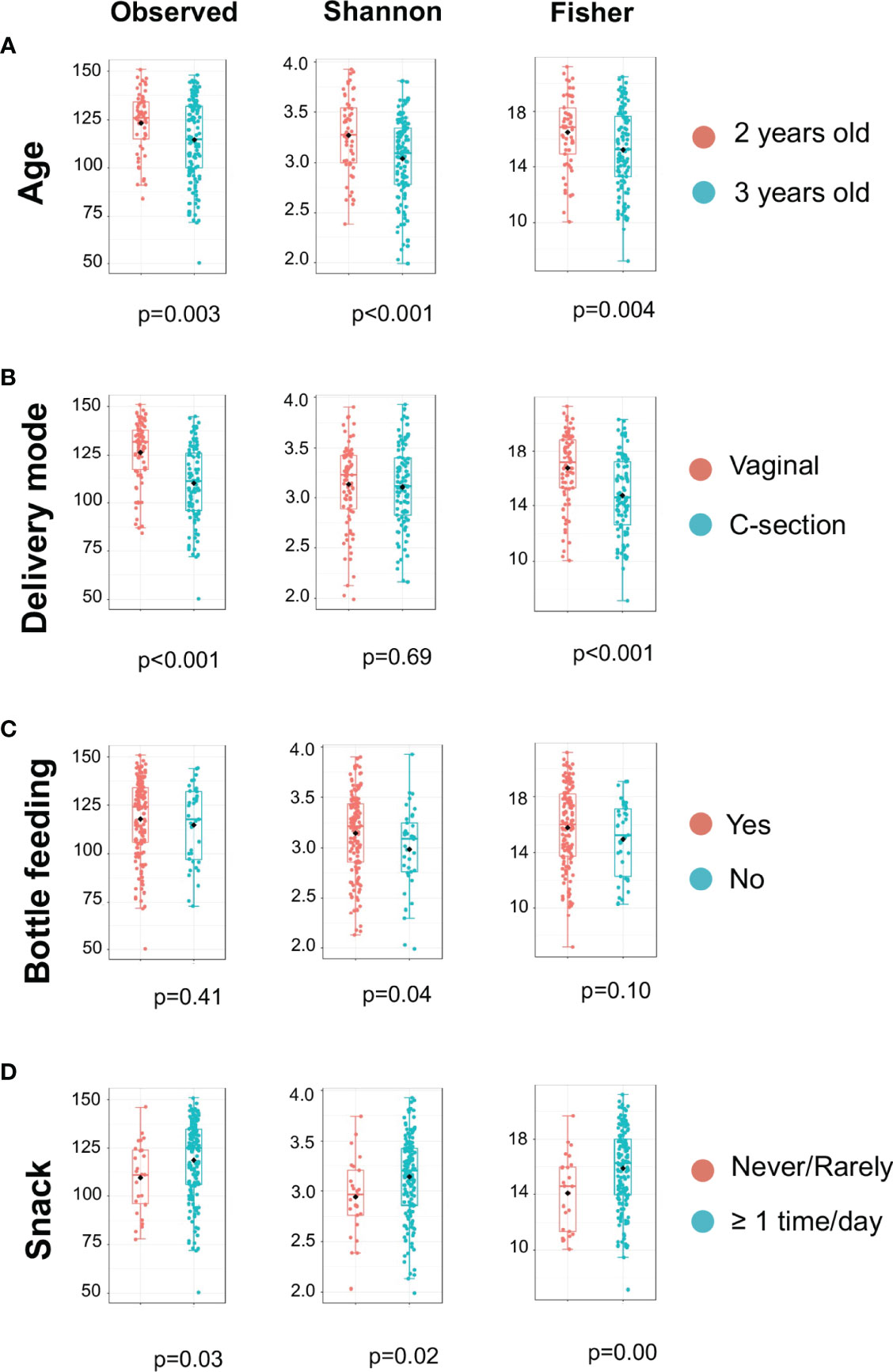
Figure 1 Alpha Diversity of salivary microbiome among children. Microbial variation measured by alpha diversity index among children from different age group (A), mode of delivery (B), Bottle feeding (C), and Consumption of snacks (D). T-test was used for the statistical comparisons.
Principle coordinate analysis (PCOA) plot was generated using OTU metrics based on beta diversity (Bray-Curtis index). The study demonstrated the beta diversity of the salivary microbiome of children differs depending on sex (p<0.01), age (p=0.02), mode of delivery (p<0.001), whether mother chews food for the child (p=0.02), and children’s caries severity measured by dmft (p<0.01) (Figure 2). However, the overall beta diversity was not significantly different among ECC and caries-free children (when ECC is defined using AAPD criteria) (Figure 2F).
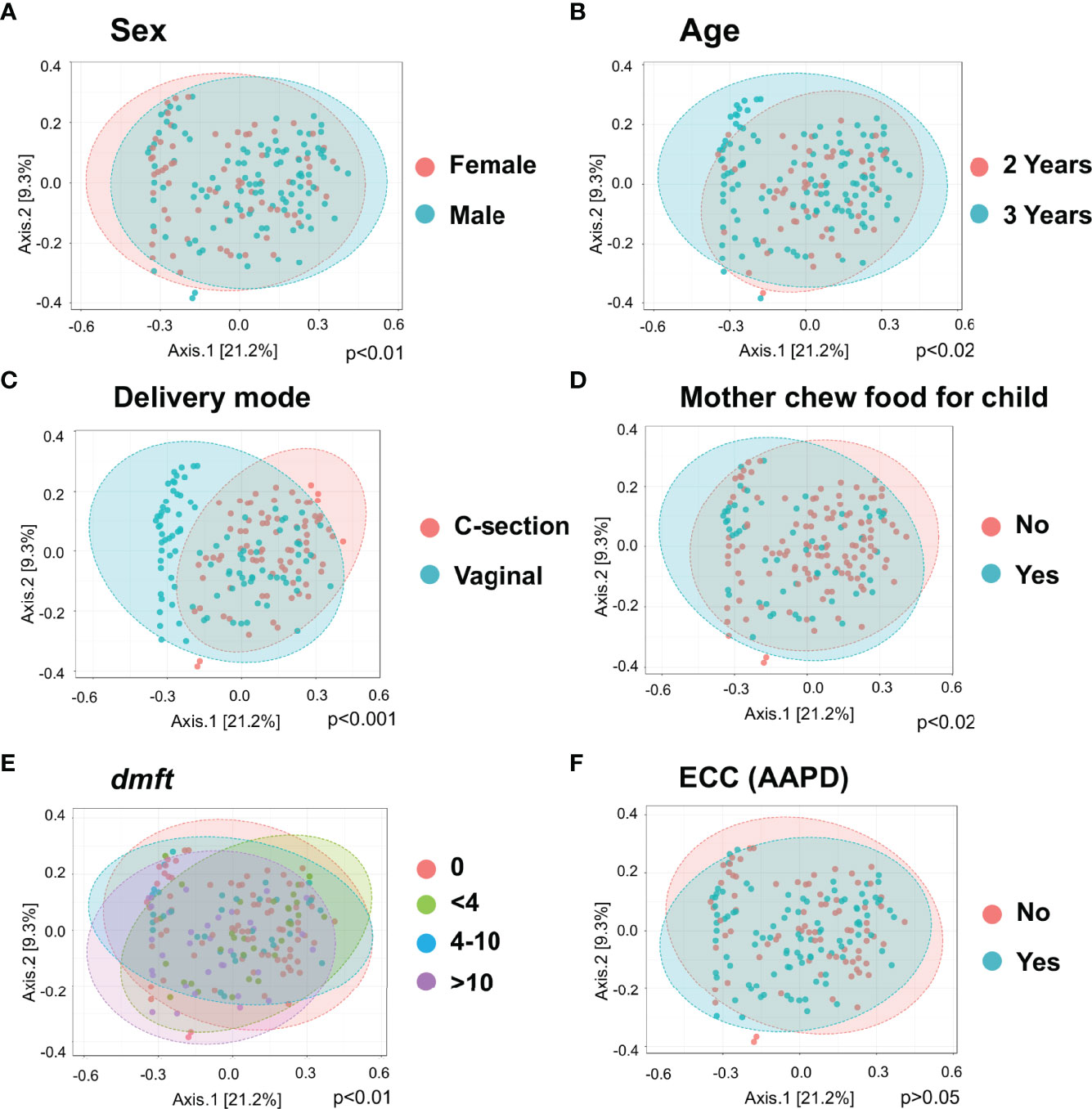
Figure 2 Diversity of salivary microbiome among children. Principle coordinate analysis (PCOA) plot is generated using OTU metrics based on beta diversity (Bray-Curtis index) for different sex groups (A), Age (B), Mode of delivery (C), Mother chew food for child (D), Child caries severity dmft (E), and ECC status (F). Permutational MANOVA (PERMANOVA) was used for these statistical comparisons between or among the categorical groups.
Taxa at the genus and species level with more than 20% prevalence and more than 0.01% relative abundance are depicted. Interestingly, most of the genera and species were the same among the ECC and the caries-free children. However, several core taxa relative abundance and prevalence differed between the two groups. For instance, Rothia aeria, Prevotella melaninogenica, Streptococcus sanguinis, and Corynebacterium durum were more prevalent and enriched in caries-free children, while Leptotrichia shahii and Corynebacterium matruchotii were more prevalent and enriched in ECC children (Figure 3).
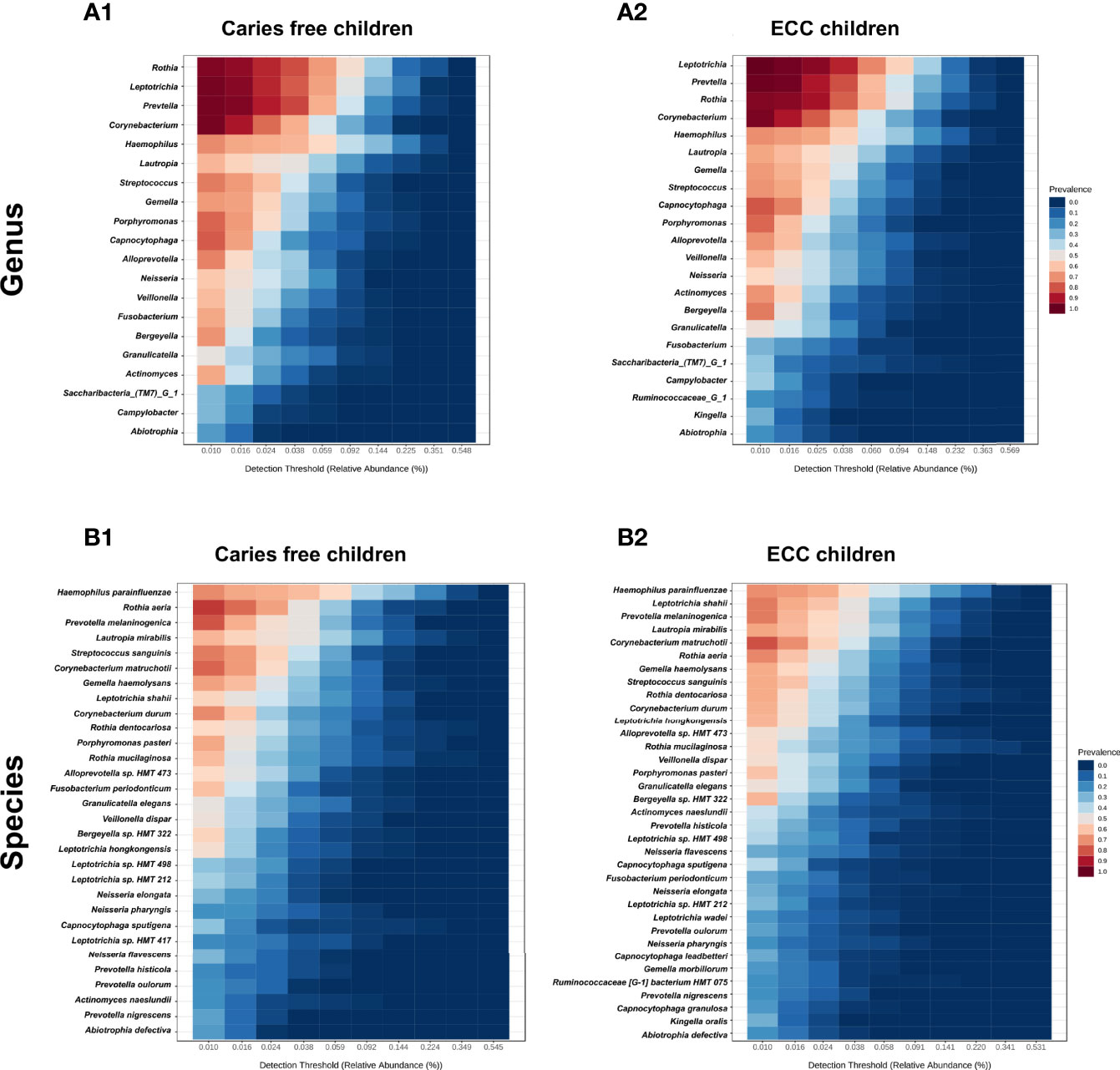
Figure 3 Core salivary microbiome of ECC and carries-free children. Taxa at the genus and species level with more than 20% prevalence and more than 0.01% relative abundance are depicted. in caries free children (A1, B1) and ECC children (A2, B2).
To identify discriminate taxa between the ECC and caries-free children, heat trees depicted the OTU classifications and differential abundance comparison at the genus level (Figure 4A) and at the species level (Figure 4B) between the salivary microbiome of children with and without ECC. In the heat trees, size and color of nodes and edges are correlated with the abundance ratio of organisms in ECC children vs. caries-free children. Taxa colored in red are enriched in ECC Children (e.g., S. mutans, P. histicola, L. hongkongesis, and L. shahii, etc.). Taxa colored in blue are enriched in caries-free children (e.g., F. periodonticum, Actinomyces gerensceriae, Oribacterium sinus, and Veillonella rogosae, etc.). Taxa with labels had a significant differential abundance between ECC children and caries-free children measured by the Wilcoxon Rank Sum test, p<0.05. The significant differential abundances were further verified in the linear discriminant analysis effect size (LEfSe) analysis and random forest at the species level (Figure 5A). The ECC group had an increased abundance in S. mutans, P. histicola, and L. hongkongesis compared to an increased abundance in F. periodonticum, Leptotrichia sp., V. rogosae, O. sinus, and P. nigrescens in caries-free groups (Figure 5B).
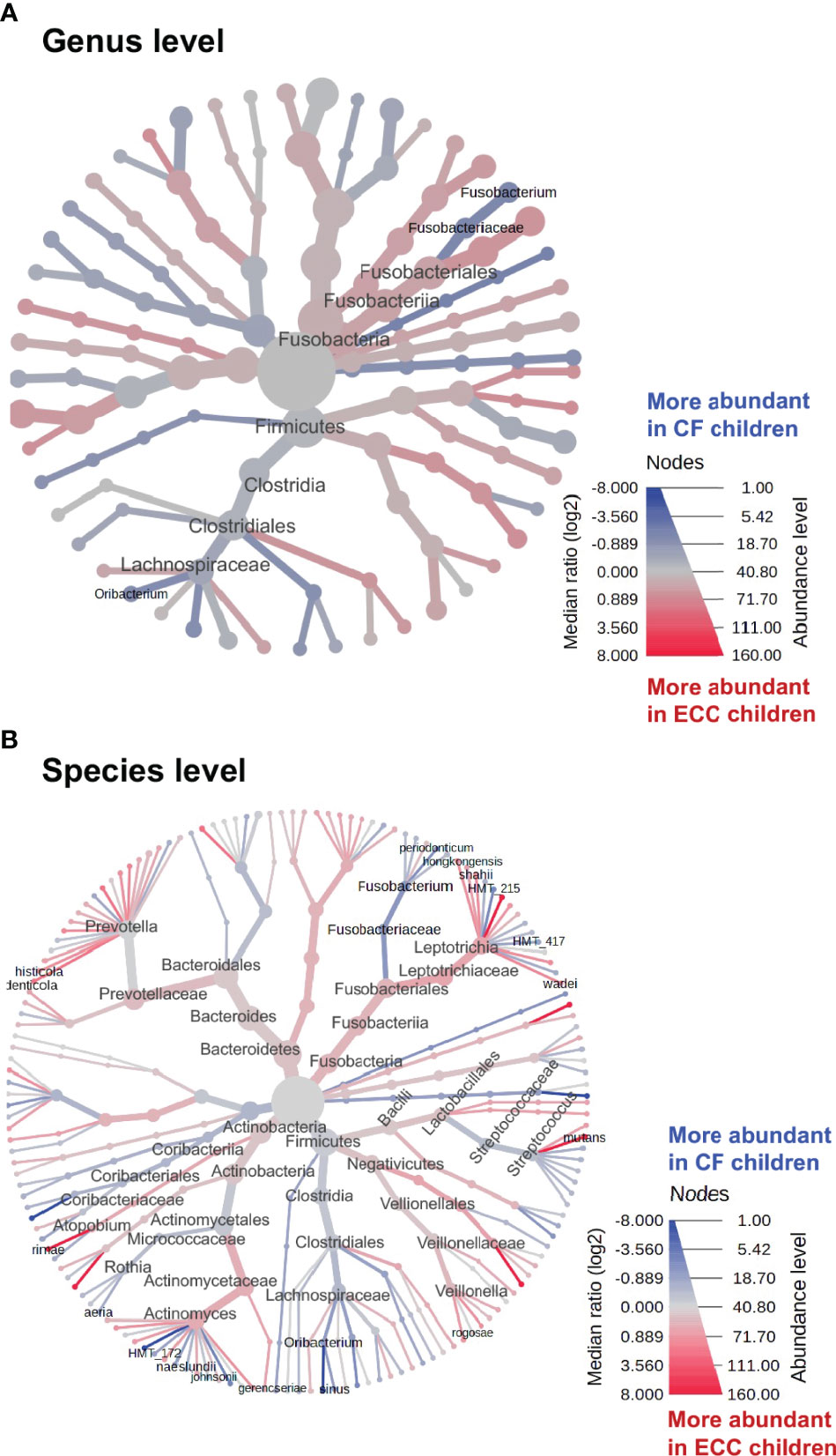
Figure 4 Heat trees of salivary microbiome abundance among ECC and caries-free children. The heat trees depict the OTU classifications and differential abundance comparison at the genus level (A) and at the species level (B) between salivary microbiome of children with ECC and without ECC. In the heat trees, size and color of nodes and edges are correlated with the abundance ratio of organisms in ECC children vs. caries-free children. Taxa colored in red are enriched in ECC Children, whereas taxa colored in blue are enriched in caries-free children. Taxa with labels indicate a significant abundance difference between ECC children vs. caries-free children measured by the Wilcoxon Rank Sum test (p<0.05).
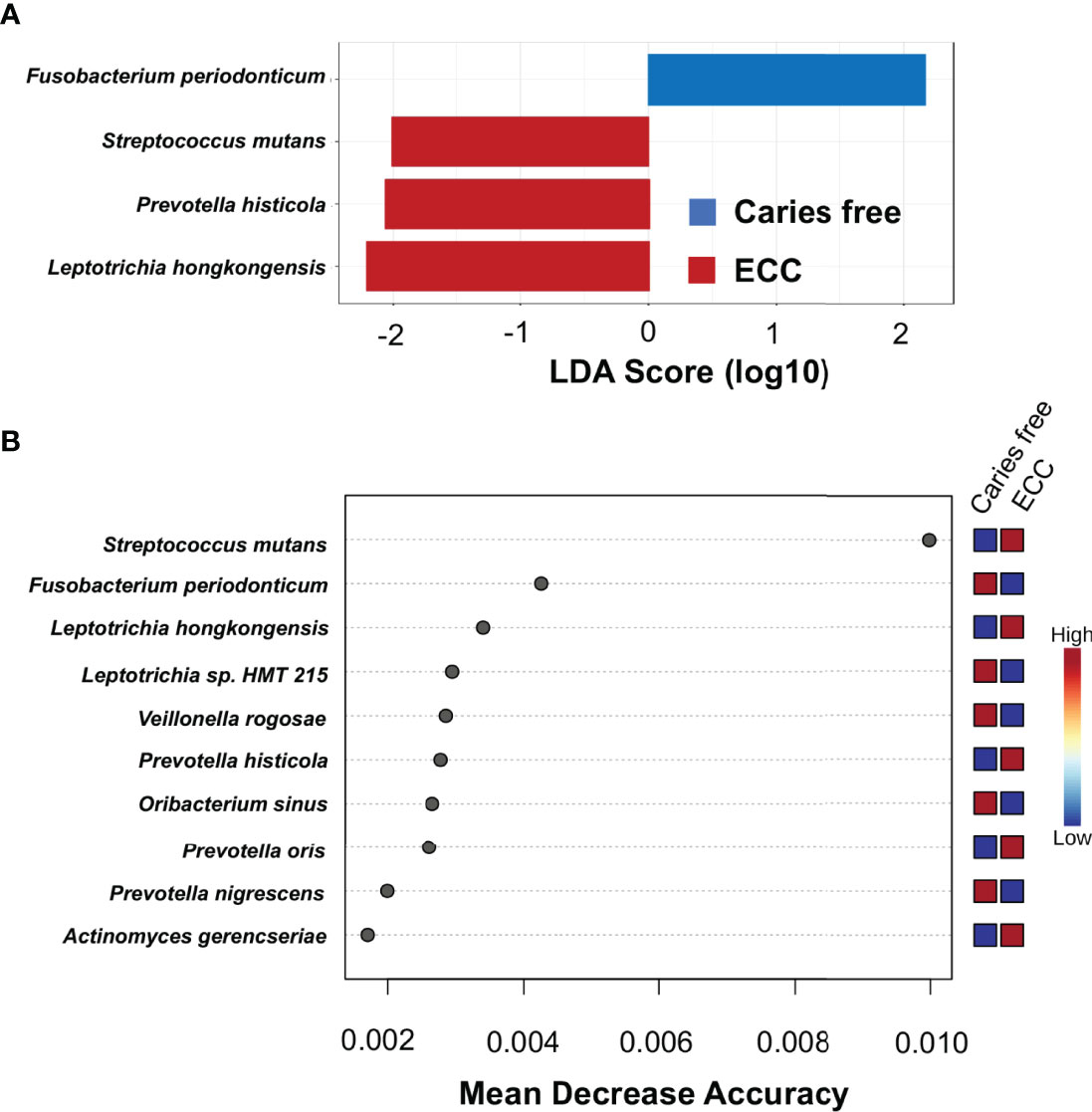
Figure 5 Taxa at genus level differently enriched in ECC and caries-free children. (A) Linear discriminant analysis (LDA) effect size (LEfSe) method was performed to compare taxa between ECC and caries-free children. The bar plot lists the significantly differential taxa based on effect size (LDA score log10 >2.0 and FDR <0.1). (B) Random forest identified important features at the species level that were differently enriched among ECC and caries-free children. Red indicates a higher abundance in ECC, whereas blue indicates a higher abundance in caries-free children.
All children had more than 100 OTU reads per subject. Ninety OTUs with fewer than 10 reads were removed from the dataset to ensure sufficient depths. The final dataset included 177 children with 416 variables in total, in which seven were maternal socio-demographic-behavior-environmental factors (platform 1), 20 were children’s socio-demographic-behavior-environmental factors (platform 2), and 386 were children’s oral microbial factors (platform 3). In the two-step model building approach, three separate LASSO penalized logistic regression models were fitted for the three platforms in step 1. Two maternal socio-demographic-behavior-environmental factors from the platform 1 (Figure 6A), 11 children’s socio-demographic-behavior-environmental factors from the platform 2 (Figure 6B), and 11 children’s oral microbial factors from the platform 3 (Figure 6C) were selected based on 10-fold cross-validation.
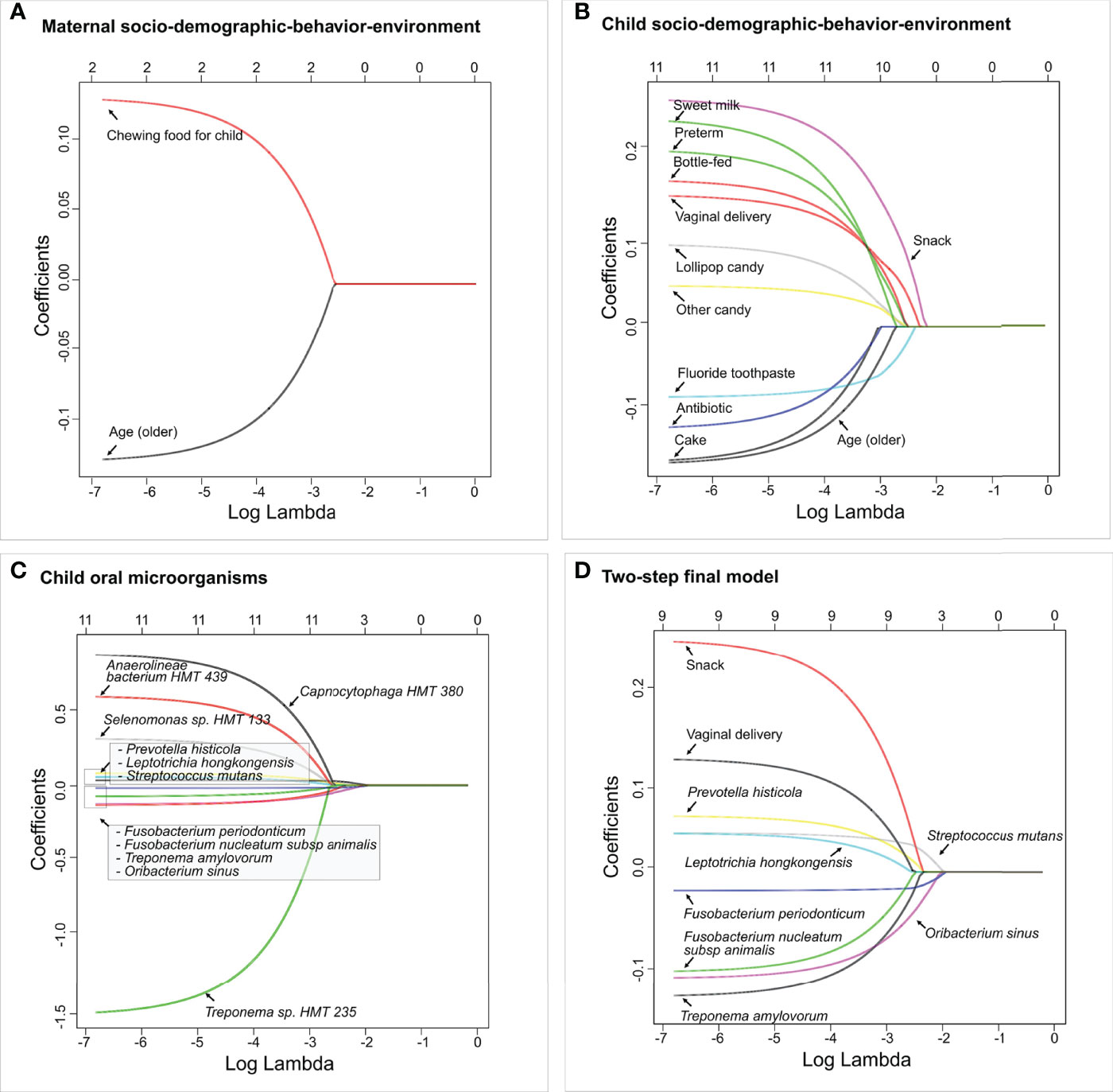
Figure 6 Identified factors associated with child’s caries risk using factors via two-step model. LASSO penalized logistic regression modeling was used for caries predictor selection based on 413 variables, including children’s saliva samples. Specifically, variables from three separate platforms were identified shown in (A) Maternal socio-demographic-behavior-environmental factors, (B) Children’s socio-demographic-behavior-environmental factors, and (C) Children’s salivary microorganisms. The final two-step model using variables identified from (A–C) is shown in (D). The LASSO solution path above shows how the model is built sequentially by adding one variable at a time to the active set. The 2-step predictive model is the following (area under the curve: 0.85).
In step 2, the 24 variables from the previous step were used as the candidate variables and the final model selects nine. Nine variables entered the final logistic regression model, they were, in order, F. periodonticum, S. mutans, O. sinus, P. histicola, snack, Treponema amylovorum, F. nucleatum subsp. animalis, vaginal delivery, and L. hongkongensis (Figure 6D). In particular, vaginal delivery, snack, L. hongkongensis, P. histicola, and S. mutans had a positive effect on ECC with an increased risk of having caries. F. nucleatum subsp. animalis, F. periodonticum, O. sinus, and T. amylovorum showed decreased risk for having ECC in our cohort. As a comparison, the one-step model with the same nine variables resulted in the same regression coefficients (Figure S2). The area under the curve (AUC) value for the two-step predictive model was 0.85.
Caries occurrence in preschool-age children depends on several host and environmental factors, which can ultimately disturb the oral microbiome equilibrium. Studies of caries risk assessment and prediction at an individual level are consequential for a clinical decision-making at a patient-care level and design appropriate evidence-based caries preventive interventions at a community level (Chanpum et al., 2020). The highlight of this study is the interdisciplinary approach incorporating data platforms from multiple sources, which include 1) the carefully acquired mother-child dyads dataset containing information of socio-demographic, medical, and delivery information; caries examination, oral health behavior, and dietary practice; 2) the salivary microbiome 16S rRNA gene sequencing dataset; 3) the use of linear discriminant analysis coupled with effect size (LEfSe) analysis (Segata et al., 2011) to determine differentially abundant bacterial taxa associated with ECC or caries-free children; and 4) the application of a novel machine learning approach to develop a multi-platform caries prediction model with quantifiable coefficients.
The initial objective of this study sought to examine the caries-associated risk factors among the 177 mother-child dyads. The significant risk factors for ECC included preterm birth, high levels of S. mutans, increased consumption of soft drinks, snacks, and candies, and used less fluoride toothpaste. Vaginal-born children are 2.27 times more likely to experience ECC compared to their counterparts. These findings are consistent with those evidenced in earlier studies (Pattanaporn et al., 2013; Twetman et al., 2020). What is not yet clear is how those plausible caries-associated risk factors impact on oral microbial composition in 2- to 3-year-old children. In addition to microbes, our predictive model was successful at identifying behavioral and environmental risk factors for developing caries; the conceptual inference is drawn in Figure 7. Interestingly, our model revealed that vaginal delivery predicted the onset of caries in these Thai children. While there is limited knowledge on how the delivery route impacts the oral ecology of overall oral health, several studies have demonstrated a relationship between the delivery route and oral microbiome development (Xiao et al., 2020). For example, a birth cohort study among Irish infants revealed a higher oral microbial community diversity in children born by cesarean. However, the impact of birth mode on the oral microbiome was only observed up to the first week of age. This influence diminished after the first week of life. Another study examined the oral microbiome of very low birth weight infants and found a higher relative abundance of Ureaplasma and Pantoea in the vaginal-born infants, but a higher colonization prevalence of Corynebacterium, Methylobacterium, and Variovorax in cesarean-born infants (Li et al., 2020). Other studies indicated that the bacterial profile colonized in infants born vaginally resembles mothers’ vaginal bacterial communities, whereas the microbial community of infants born by cesarean section resembles those present on mothers’ skin (Dominguez-Bello et al., 2010; Lif Holgerson et al., 2011; Drell et al., 2017). Worth noting that research on infants’ gut microbiome development and delivery mode revealed similar findings to the oral microbiome. These findings included an increased similarity to their mother’s gut microbiome when born vaginally (Bäckhed et al., 2015), delayed colonization of prominent commensals in cesarean born infants (Dogra et al., 2015), and lower diversity in cesarean born infants when compared to vaginally-born infants (Hesla et al., 2014). Due to the importance of understanding the impact of the maternal oral microbiome on children’s caries outcome, our future study will further analyze the relatedness of the maternal and children’s salivary bacteriome and their impact on ECC. Importantly, we also found a lower diversity in the cesarean-born children in our study (Figure 1A). Further, 3-year-old children had a lower alpha diversity than the children at 2 years of age in our study (Figure 1B). This could be reflective of the stabilization of the oral microbiome that occurs after 2 years of age; at this point, children become stably colonized by resident bacteria of the oral cavity and have fewer bacteria that are from environmental exposures (Dashper et al., 2019).
Sugary snacks are known to serve as the substrate for oral microbial metabolism, leading to acidification of the oral environment, favoring aciduric and cariogenic microorganisms, and causing tooth hard tissue demineralization and, ultimately, caries (Marsh, 2018). More than two-thirds (68%) of US 2-year-olds and roughly three-quarters (74%) of 3-year-olds consumed some type of dessert or candy in a day (Fox et al., 2010). Our findings that frequent snacking is a risk factor for caries is supported by previous studies that have emphasized the importance of limiting cariogenic or sugary snack consumption as part of a good oral health routine to prevent caries onset (Hong et al., 2014; Moimaz et al., 2014).
To build the caries-predictive model, the study first identified commonly recognized caries risk microbial factors, such as children’s age, model of delivery, preterm birth, frequency of snack consumption, and presence of S. mutans and other potential caries-associated bacteria. Additionally, we detected several other microbial species, either caries-associated or protective that traditionally have received less attention.
For the penalized regression models, both the variables selected into the model and the order they enter the model were important. A variable entering a model sooner (i.e., at a higher lambda value, the tuning parameter) indicates that it is deemed significant under harsher penalties. In our final logistic regression model, the coefficients of vaginal delivery, snack, presence of S. mutans, L. hongkongensis, and P. histicola, were positive, indicating the change in log-odds of an individual having caries due to a one-unit increase in those variables. More specifically, the coefficient for vaginal delivery in the 2-step model was 0.1174. The odds ratio of having caries for a child who was delivered by vaginal, compared with a child who was c-sectionally delivered, could be 12% higher. Likewise, having snacks increases the odds of having caries by 27%.
Meanwhile, the coefficients of presence of F. periodonticum, F. nucleatum subsp. animalis, O. sinus, and T. amylovorum were negative, indicating a decrease in the log-odds of an individual having caries, which means that for a negative coefficient, the subject is less likely to have caries as those variable increase. The results suggest that the enriched levels of those four bacterial species in the saliva present a reduced risk for ECC.
This study identified the plausible caries-promoting microbial species, which were enriched in ECC samples, were S. mutans, P. histicola, and L. hongkongensis.
ECC children are known to have a higher relative abundance of salivary and plaque S. mutans, well-known for its acidogenicity, aciduricity, and capability of synthesizing extracellular matrix using carbohydrates (Banas, 2004) (Tanner et al., 2011; Gross et al., 2012; Yang et al., 2012; Ma et al., 2015; Johansson et al., 2016; Richards et al., 2017; Xiao et al., 2018). Not surprisingly, this species was firstly found as caries promoting species in our model.
Prevotella histicola was found in two independent studies (Teng et al., 2015; Wu et al., 2021) that used a machine learning approach to identify discriminative species in caries-active and caries-free children. Despite previously reported association between P. histicola and increased caries risk in children (Hurley et al., 2019), it remains unclear about the potential cariogenicity of P. histocola and its interaction with other oral microorganisms in leading to a caries-prone condition in the oral cavity.
Leptotrichia species normally reside in the oral cavity, gastrointestinal system, and urogenital system. They are typically not considered pathogenic, but may cause opportunistic infections in an immune-suppressed host (Eribe and Olsen, 2017). Xu et al. characterized microbial composition in supragingival plaque samples from children younger than 30 months old and observed that L. hongkongensis was identified in caries-affected subjects (Xu et al., 2014). L. hongkongensis was also more predominant in adults with active caries (Johansson et al., 2016). Interestingly, L. hongkongensis co-occurred with other Leptotrichia and Fusobacterium species that contributed to black stains of dental caries in primary dentition. Despite the observed associations of Leptotrichia species with caries, there remains a gap in understanding their role in the progression of caries.
This study further identified several potentially protective microbial species enriched in caries-free children: F. periodonticum, F. nucleatum subsp. animalis, Treponema amylovorum, O. sinus. Interestingly, three of these species (F. periodonticum, F. nucleatum, T. amylovorum) are commonly known periodontal pathogens.
F. periodonticum, although without clear evidence on its role in the etiopathogenesis of periodontal disease, is considered to be an opportunistic pathogen residing in deep periodontal pockets and gingival sulcus (Park et al., 2010). Jiang and colleagues observed that F. periodonticum was significantly predominant in the saliva of caries-free children compared to caries-affected, which could be suggestive of a caries protective role (Jiang et al., 2016). These findings were also consistent with a similar case-control cohort study examining bacterial profiles of adults with caries (Belstrøm et al., 2014). A more recent study analyzed the variation of the tongue microbiota and its association with dental caries among 6-7-year-old and 11-12-year-old children. The study results demonstrated a more frequent presentation of F. periodonticum in children without history of dental caries (Zhang et al., 2021). Thus, salivary counts of F. periodonticum can be used as a biomarker and may help caries risk screening (Belstrøm et al., 2014; Jiang et al., 2016).
F. nucleatum is a commonly recognized periodontal pathogen and regarded as a bridging colonizer that adheres to the early colonizers of dental plaque, followed by adhesion from late plaque colonizers (Aruni et al., 2015). Its FadA adhesin has been identified as a virulence factor for both F. nucleatum and F. periodonticum, which is absent in other fusobacterium species and are considered to assist them in binding to host cells (Han, 2015). Studies have reportedly detected Fusobacterium species including F. nucleatum in abundance in the healthy oral microbiota among children (Teng et al., 2015). Tanner et al. reported F. nucleatum as more frequently detected in caries-free children (Tanner et al., 2011). Heinrich-Weltzien et al., indicated that F. nucleatum was found more frequently in non-discolored plaque samples (Tanner et al., 2011).
T. amylovorum species are highly motile, fastidious, saccharolytic gram-negative spirochetes majorly associated with subgingival plaque in periodontal disease (Wyss et al., 1997; Siqueira and Rôças, 2004). Although the majority of the scientific evidence links T. amylovorum with periodontal and endodontic diseases, our study results indicated that the presence and higher abundance of T. amylovorum in Thai children are associated with a lower risk for ECC, which is a novel finding.
O. sinus was initially isolated from purulent discharge of a 6-year-old-child with bilateral maxillary sinusitis (Carlier et al., 2004). A longitudinal cohort study examined the oral microbiome development during the first four years of life and subsequent development of ECC (Dashper et al., 2019). Although O. sinus had a higher (≥90%) prevalence when children are between 1-4 years of age, making its way to the ‘core oral microbiome’ of young children, the relative abundance of O. sinus was not suggested to be significantly associated with caries status (Dashper et al., 2019).
V. rogosae has been identified as a species associated with caries-free condition from other discriminate methods. Veillonella species, including V. rogosae, are early plaque colonizers with metabolic requirements that are dependent upon organic acids, including lactic acid, produced by Streptococcus. Thus, together these species are considered to co-aggregate and contribute to biofilm formation and maturation in early stages (Mashima and Nakazawa, 2014). Studies have reported that age, geographic location, diet, and oral health behaviors influence the proportion of oral Veillonella species. One such study (Djais et al., 2019) examined the proportion of oral Veillonella species in the saliva samples of Japanese children 4- to 14-years-old as a measure of their oral health status. Interestingly, the detection of V. rogosae declined with deteriorating oral hygiene status (49.1% in good hygiene vs. 44.4% in moderate hygiene vs. 34.1% in poor oral hygiene). A study in Thai children with different oral hygiene status (good, moderate, and poor) revealed similar findings. V. rogosae prevalence was significantly lower in the poor oral hygiene group than in the good oral hygiene groups, whereas, Veillonella parvula and Veillonella tobetsuensis were significantly more prevalent in the poor oral hygiene group (Mashima et al., 2016; Theodorea et al., 2017). These findings, together with ours, suggest that the abundance of V. rogosae may be a predictor of good oral hygiene status and potentially a caries protective factor among children.
We took two data integration approaches in this paper. The first parallel integration approach treats factors on different platforms equally and assumes no specific correlation structures among factors. The second hierarchical integration approach is indeed an integration with pre-selection on each platform first. It can be considered to take into account the correlation structure within each platform. The two approaches usually produce different outcomes since the pools of candidate predictor variables are different but are expected to have a great overlap. It is a coincidence that the two models in this paper are exactly the same, which also indicates good reproducibility and reliability.
The following limitations need to be considered when interpreting the study results: 1) The study was conducted in one Thailand city. Thus, generalization to other populations is unreliable due to the difference in racial, ethnical, cultural, and dietary background; 2) With the dataset being cross-sectional, the models are built upon the existing caries status, not through the longitudinal onset of caries. Future validations of our models are warranted using longitudinal dataset. There is an additional need for a more mechanistic understanding of how bacteria, such as V. rogosae and L. hongkongensis, are involved in protecting the oral cavity from caries progression.
Multimodal data integration using statistical machine learning models revealed that the mode of delivery and sugary snack consumption outranks salivary microbiome in predicting dental caries in a large cohort of mother-child dyads living in Thailand. Future machine learning approaches that include microbial and environmental risk factors are needed to comprehensively assess the dynamic changes of children’s caries risk factors in a longitudinal cohort.
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: NCBI; PRJNA824062.
The Ethical Committee approved the protocol for this study of the Faculty of Dentistry, Chiang Mai University, Thailand (No. 12/2008). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
TTW, JX, and YL contributed to the conception, design, data acquisition, analysis, and interpretation, drafting and critically revising the manuscript. PS and KP contributed to the clinical data collection. PS was responsible for microbial DNA extraction. BP and CG were responsible for DNA sequencing. SM, SV, and YZ contributed to data acquisition, analysis, and critically revising the manuscript. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Dr. JX research was supported by the National Institute of Dental and Craniofacial Research grant K23DE027412.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.881899/full#supplementary-material
American Academy of Pediatric Dentistry. (2020). Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. The Reference Manual of Pediatric Dentistry. (Chicago, Ill.: American Academy of Pediatric Dentistry), 79–81. Available at: https://www.aapd.org/media/policies_guidelines/p_eccclassifications.pdf.
Aruni, A. W., Dou, Y., Mishra, A., Fletcher, H. M. (2015). The Biofilm Community-Rebels With a Cause. Curr. Oral. Health Rep. 2 (1), 48–56. doi: 10.1007/s40496-014-0044-5
Bäckhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., et al. (2015). Dynamics and Stabilization of the Human Gut Microbiome During the First Year of Life. Cell Host Microbe 17 (5), 690–703. doi: 10.1016/j.chom.2015.04.004
Banas, J. A. (2004). Virulence Properties of Streptococcus Mutans. Front. Biosci. 9, 1267–1277. doi: 10.2741/1305
Belstrøm, D., Fiehn, N. E., Nielsen, C. H., Holmstrup, P., Kirkby, N., Klepac-Ceraj, V., et al. (2014). Altered Bacterial Profiles in Saliva From Adults With Caries Lesions: A Case-Cohort Study. Caries Res. 48 (5), 368–375. doi: 10.1159/000357502
Bowen, W. H., Burne, R. A., Wu, H., Koo, H. (2018). Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 26 (3), 229–242. doi: 10.1016/j.tim.2017.09.008
Bureau of Dental Health (2018). The 8th Thailand National Oral Health Survey Report 2017 (Nonthaburi: Ministry of Public Health, Thailand), 1–330.
Caporaso, J. G., Bittinger, K., Bushman, F. D., DeSantis, T. Z., Andersen, G. L., Knight, R. (2010). PyNAST: A Flexible Tool for Aligning Sequences to a Template Alignment. Bioinformatics 26 (2), 266–267. doi: 10.1093/bioinformatics/btp636
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 7 (5), 335–336. doi: 10.1038/nmeth.f.303
Carlier, J. P., K'Ouas, G., Bonne, I., Lozniewski, A., Mory, F. (2004). Oribacterium Sinus Gen. Nov., Sp. Nov., Within the Family 'Lachnospiraceae' (Phylum Firmicutes). Int. J. Syst. Evol. Microbiol. 54 (Pt 5), 1611–1615. doi: 10.1099/ijs.0.63060-0
Chanpum, P., Duangthip, D., Trairatvorakul, C., Songsiripradubboon, S. (2020). Early Childhood Caries and Its Associated Factors Among 9- to 18-Month Old Exclusively Breastfed Children in Thailand: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 17 (9), 3194–3204. doi: 10.3390/ijerph17093194
Dashper, S. G., Mitchell, H. L., Lê Cao, K. A, Carpenter, L., Gussy, M. G., Calache, H., et al. (2019). Temporal Development of the Oral Microbiome and Prediction of Early Childhood Caries. Sci. Rep. 9 (1), 19732. doi: 10.1038/s41598-019-56233-0
Djais, A. A., Theodorea, C. F., Mashima, I., Otomo, M., Saitoh, M., Nakazawa, F. (2019). Identification and Phylogenetic Analysis of Oral Veillonella Species Isolated From the Saliva of Japanese Children. F1000Res 8, 616. doi: 10.12688/f1000research.18506.5
Dogra, S., Sakwinska, O., Soh, S. E., Ngom-Bru, C., Brück, W. M., Berger, B., et al. (2015). Dynamics of Infant Gut Microbiota Are Influenced by Delivery Mode and Gestational Duration and Are Associated With Subsequent Adiposity. mBio 6 (1), e02419-14. doi: 10.1128/mBio.02419-14
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., et al. (2010). Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota Across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. U. S. A. 107 (26), 11971–11975. doi: 10.1073/pnas.1002601107
Drell, T., Stsepetova, J., Simm, J., Rull, K., Aleksejeva, A., Antson, A., et al. (2017). The Influence of Different Maternal Microbial Communities on the Development of Infant Gut and Oral Microbiota. Sci. Rep. 7 (1), 9940. doi: 10.1038/s41598-017-09278-y
Dye, B. A., Li, X., Thorton-Evans, G. (2012). Oral Health Disparities as Determined by Selected Healthy People 2020 Oral Health Objectives for the United States, 2009-2010. NCHS Data Brief 104, 1–8.
Dye, B. A., Tan, S., Smith, V., Lewis, B. G., Barker, L. K., Thornton-Evans, G., et al. (2007). Trends in Oral Health Status: United States, 1988-1994 and 1999-2004. Vital Health Stat Ser. 11 Data Natl. Health Survey 248), 1–92.
Eribe, E. R. K., Olsen, I. (2017). Leptotrichia Species in Human Infections II. J. Oral. Microbiol. 9 (1), 1368848. doi: 10.1080/20002297.2017.1368848
Fox, M. K., Condon, E., Briefel, R. R., Reidy, K. C., Deming, D. M. (2010). Food Consumption Patterns of Young Preschoolers: Are They Starting Off on the Right Path? J. Am. Diet Assoc. 110 (12 Suppl), S52–S59. doi: 10.1016/j.jada.2010.09.002
Grier, A., Myers, J. A., O'Connor, T. G., Quivey, R. G., Gill, S. R., Kopycka-Kedzierawski, D. T. (2020). Oral Microbiota Composition Predicts Early Childhood Caries Onset. J. Dent. Res. 100 (6), 599–607. doi: 10.1177/0022034520979926
Gross, E. L., Beall, C. J., Kutsch, S. R., Firestone, N. D., Leys, E. J., Griffen, A. L. (2012). Beyond Streptococcus Mutans: Dental Caries Onset Linked to Multiple Species by 16S rRNA Community Analysis. PLoS One 7 (10), e47722. doi: 10.1371/journal.pone.0047722
Han, Y. W. (2015). Fusobacterium Nucleatum: A Commensal-Turned Pathogen. Curr. Opin. Microbiol. 23, 141–147. doi: 10.1016/j.mib.2014.11.013
Hesla, H. M., Stenius, F., Jäderlund, L., Nelson, R., Engstrand, L., Alm, J., et al. (2014). Impact of Lifestyle on the Gut Microbiota of Healthy Infants and Their Mothers—the ALADDIN Birth Cohort. FEMS Microbiol. Ecol. 90 (3), 791–801. doi: 10.1111/1574-6941.12434
Hong, C. H., Bagramian, R. A., Hashim Nainar, S. M., Straffon, L. H., Shen, L., Hsu, C. Y. (2014). High Caries Prevalence and Risk Factors Among Young Preschool Children in an Urban Community With Water Fluoridation. Int. J. Paediatric Dentis. 24 (1), 32–42. doi: 10.1111/ipd.12023
Hurley, E., Barrett, M. P. J., Kinirons, M., Whelton, H., Ryan, C. A., Stanton, C., et al. (2019). Comparison of the Salivary and Dentinal Microbiome of Children With Severe-Early Childhood Caries to the Salivary Microbiome of Caries-Free Children. BMC Oral. Health 19 (1), 13. doi: 10.1186/s12903-018-0693-1
Jiang, S., Gao, X., Jin, L., Lo, E. C. (2016). Salivary Microbiome Diversity in Caries-Free and Caries-Affected Children. Int. J. Mol. Sci. 17 (12), 1978–1991. doi: 10.3390/ijms17121978
Johansson, I., Witkowska, E., Kaveh, B., Lif Holgerson, P., Tanner, A. C. (2016). The Microbiome in Populations With a Low and High Prevalence of Caries. J. Dent. Res. 95 (1), 80–86. doi: 10.1177/0022034515609554
Ledder, R. G., Kampoo, K., Teanpaisan, R., McBain, A. J. (2018). Oral Microbiota in Severe Early Childhood Caries in Thai Children and Their Families: A Pilot Study. Front. Microbiol. 9, 2420. doi: 10.3389/fmicb.2018.02420
Lif Holgerson, P., Harnevik, L., Hernell, O., Tanner, A. C., Johansson, I. (2011). Mode of Birth Delivery Affects Oral Microbiota in Infants. J. Dent. Res. 90 (10), 1183–1188. doi: 10.1177/0022034511418973
Li, H., Zhang, Y., Xiao, B., Xiao, S., Wu, J., Huang, W. (2020). Impacts of Delivery Mode on Very Low Birth Weight Infants' Oral Microbiome. Pediatr. Neonatol. 61 (2), 201–209. doi: 10.1016/j.pedneo.2019.10.004
Ma, C., Chen, F., Zhang, Y., Sun, X., Tong, P., Si, Y., et al. (2015). Comparison of Oral Microbial Profiles Between Children With Severe Early Childhood Caries and Caries-Free Children Using the Human Oral Microbe Identification Microarray. PLoS One 10 (3), e0122075. doi: 10.1371/journal.pone.0122075
Marsh, P. D. (2018). In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dental Res. 29 (1), 60–65. doi: 10.1177/0022034517735295
Mashima, I., Nakazawa, F. (2014). The Influence of Oral Veillonella Species on Biofilms Formed by Streptococcus Species. Anaerobe 28, 54–61. doi: 10.1016/j.anaerobe.2014.05.003
Mashima, I., Theodorea, C. F., Thaweboon, B., Thaweboon, S., Nakazawa, F. (2016). Identification of Veillonella Species in the Tongue Biofilm by Using a Novel One-Step Polymerase Chain Reaction Method. PloS One 11 (6), e0157516. doi: 10.1371/journal.pone.0157516
McMurdie, P. J., Holmes, S. (2013). Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One 8 (4), e61217. doi: 10.1371/journal.pone.0061217
Moimaz, S. A., Garbin, A. J., Lima, A. M., Lolli, L. F., Saliba, O., Garbin, C. A. (2014). Risk Factors in the Mother-Child Relationship That Predispose to the Development of Early Childhood Caries. Eur. Arch. Paediatric Dentistry Off. J. Eur. Acad. Paediatric Dentistry. 15 (4), 245–250. doi: 10.1007/s40368-014-0108-1
Organization. WH (1997). Oral Health Surveys - Basic Methods. 4th ed (Geneva: World Health Organization).
Park, S. N., Park, J. Y., Kook, J. K. (2010). Development of Species-Specific Polymerase Chain Reaction Primers for Detection of Fusobacterium Periodonticum. Microbiol. Immunol. 54 (12), 750–753. doi: 10.1111/j.1348-0421.2010.00279.x
Pattanaporn, K., Saraithong, P., Khongkhunthian, S., Aleksejuniene, J., Laohapensang, P., Chhun, N., et al. (2013). Mode of Delivery, Mutans Streptococci Colonization, and Early Childhood Caries in Three- to Five-Year-Old Thai Children. Commun. Dent. Oral. Epidemiol. 41 (3), 212–223. doi: 10.1111/cdoe.12013
Peltzer, K., Mongkolchati, A. (2015). Severe Early Childhood Caries and Social Determinants in Three-Year-Old Children From Northern Thailand: A Birth Cohort Study. BMC Oral. Health 15, 108. doi: 10.1186/s12903-015-0093-8
Pitts, N. B., Zero, D. T., Marsh, P. D., Ekstrand, K., Weintraub, J. A., Ramos-Gomez, F., et al. (2017). Dental Caries. Nat. Rev. Dis. Primers. 3, 17030. doi: 10.1038/nrdp.2017.30
Richards, V. P., Alvarez, A. J., Luce, A. R., Bedenbaugh, M., Mitchell, M. L., Burne, R. A., et al. (2017). Microbiomes of Site-Specific Dental Plaques From Children With Different Caries Status. Infect. Immun. 85 (8), e00106–17. doi: 10.1128/IAI.00106-17
Saraithong, P., Pattanaporn, K., Chen, Z., Khongkhunthian, S., Laohapensang, P., Chhun, N., et al. (2015). Streptococcus Mutans and Streptococcus Sobrinus Colonization and Caries Experience in 3- and 5-Year-Old Thai Children. Clin. Oral. Investigat. 19 (8), 1955–1964. doi: 10.1007/s00784-015-1437-0
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic Biomarker Discovery and Explanation. Genome Biol. 12 (6), R60. doi: 10.1186/gb-2011-12-6-r60
Simon-Soro, A., Mira, A. (2015). Solving the Etiology of Dental Caries. Trends Microbiol. 23 (2), 76–82. doi: 10.1016/j.tim.2014.10.010
Siqueira, J. F., Jr., Rôças, I. N. (2004). Treponema Species Associated With Abscesses of Endodontic Origin. Oral. Microbiol. Immunol. 19 (5), 336–339. doi: 10.1111/j.1399-302x.2004.00156.x
Tanner, A. C., Kent, R. L., Jr., Holgerson, P. L., Hughes, C. V., Loo, C. Y., Kanasi, E., et al. (2011). Microbiota of Severe Early Childhood Caries Before and After Therapy. J. Dent. Res. 90 (11), 1298–1305. doi: 10.1177/0022034511421201
Tanner, A. C., Mathney, J. M., Kent, R. L., Chalmers, N. I., Hughes, C. V., Loo, C. Y., et al. (2011). Cultivable Anaerobic Microbiota of Severe Early Childhood Caries. J. Clin. Microbiol. 49 (4), 1464–1474. doi: 10.1128/JCM.02427-10
Teng, F., Yang, F., Huang, S., Bo, C., Xu, Z. Z., Amir, A., et al. (2015). Prediction of Early Childhood Caries via Spatial-Temporal Variations of Oral Microbiota. Cell Host Microbe 18 (3), 296–306. doi: 10.1016/j.chom.2015.08.005
Theodorea, C. F., Mashima, I., Thaweboon, B., Thaweboon, S., Nakazawa, F. (2017). Molecular Detection of Oral Veillonella Species in the Saliva of Children With Different Oral Hygiene Statuses. Int. J. Curr. Microbiol. Appl. Sci. 6 (7), 449–461. doi: 10.20546/ijcmas.2017.607.054
Thitasomakul, S., Thearmontree, A., Piwat, S., Chankanka, O., Pithpornchaiyakul, W., Teanpaisan, R., et al. (2006). A Longitudinal Study of Early Childhood Caries in 9- to 18-Month-Old Thai Infants. Commun. Dent. Oral. Epidemiol. 34 (6), 429–436. doi: 10.1111/j.1600-0528.2006.00292.x
Twetman, S., Boustedt, K., Roswall, J., Dahlgren, J. (2020). Systematic Review Suggests a Relationship Between Moderate to Late Preterm Birth and Early Childhood Caries. Acta Paediatr. 109 (12), 2472–2478. doi: 10.1111/apa.15424
Wu, T. T., Xiao, J., Sohn, M. B., Fiscella, K. A., Gilbert, C., Grier, A., et al. (2021). Machine Learning Approach Identified Multi-Platform Factors for Caries Prediction in Child-Mother Dyads. Front. Cell Infect. Microbiol. 11, 727630. doi: 10.3389/fcimb.2021.727630
Wyss, C., Choi, B. K., Schüpbach, P., Guggenheim, B., Göbel, U. B. (1997). Treponema Amylovorum Sp. Nov., A Saccharolytic Spirochete of Medium Size Isolated From an Advanced Human Periodontal Lesion. Int. J. Syst. Bacteriol. 47 (3), 842–845. doi: 10.1099/00207713-47-3-842
Xiao, J., Fiscella, K. A., Gill, S. R. (2020). Oral Microbiome: Possible Harbinger for Children's Health. Int. J. Oral. Sci. 12 (1), 12. doi: 10.1038/s41368-020-0082-x
Xiao, J., Grier, A., Faustoferri, R. C., Alzoubi, S., Gill, A. L., Feng, C., et al. (2018). Association Between Oral Candida and Bacteriome in Children With Severe ECC. J. Dent. Res. 97 (13), 1468–1476. doi: 10.1177/0022034518790941
Xu, H., Hao, W., Zhou, Q., Wang, W., Xia, Z., Liu, C., et al. (2014). Plaque Bacterial Microbiome Diversity in Children Younger Than 30 Months With or Without Caries Prior to Eruption of Second Primary Molars. PLoS One 9 (2), e89269. doi: 10.1371/journal.pone.0089269
Yang, F., Zeng, X., Ning, K., Liu, K. L., Lo, C. C., Wang, W., et al. (2012). Saliva Microbiomes Distinguish Caries-Active From Healthy Human Populations. ISME J. 6 (1), 1–10. doi: 10.1038/ismej.2011.71
Keywords: oral microbiome, saliva, multimodal analysis, early childhood caries, machine learning, diet
Citation: Wu TT, Xiao J, Manning S, Saraithong P, Pattanaporn K, Paster BJ, Chen T, Vasani S, Gilbert C, Zeng Y and Li Y (2022) Multimodal Data Integration Reveals Mode of Delivery and Snack Consumption Outrank Salivary Microbiome in Association With Caries Outcome in Thai Children. Front. Cell. Infect. Microbiol. 12:881899. doi: 10.3389/fcimb.2022.881899
Received: 23 February 2022; Accepted: 20 April 2022;
Published: 23 May 2022.
Edited by:
Yuan Liu, University of Pennsylvania, United StatesCopyright © 2022 Wu, Xiao, Manning, Saraithong, Pattanaporn, Paster, Chen, Vasani, Gilbert, Zeng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yihong Li, eWlob25nLmxpQGNvcm5lbGwuZWR1; Jin Xiao, amluX3hpYW9AdXJtYy5yb2NoZXN0ZXIuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.