
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 25 March 2022
Sec. Clinical Microbiology
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.818167
This article is part of the Research Topic Congruities between Cancer and Infectious Diseases: Lessons to be learned from these distinct yet analogous fields View all 6 articles
The co-reactivation of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) in patients undergoing hematopoietic stem cell transplantation (HSCT) has been found. Research has shown that the reactivation of CMV or EBV is closely related to poor HSCT outcomes. In this study, we describe the clinical characteristics of HSCT patients with co-reactivation of CMV and EBV. We retrospectively reviewed the medical records of 327 patients who underwent HSCT at the Peking University People’s Hospital Institute of Hematology. Co-reactivation of CMV and EBV was observed in a total of 75 patients (22.9%) who also had a higher incidence of hemorrhagic cystitis (P=0.000). HSCT patients with CMV and co-reactivation of CMV and EBV had a significantly lower 1-year overall survival (OS; P=0.050). Further, COX regression analysis showed that viral infection was a risk factor for 1-year OS (HR, 12.625 for co-reactivation vs. no reactivation, p=0.021, and HR 13.580 for CMV reactivation vs. no reactivation, P=0.013). In conclusion, the patients with CMV reactivation had poorer outcome after HSCT regardless of EBV reactivation.
Despite advanced treatment and prevention measures, double-stranded DNA (dsDNA) viruses can still increase the mortality of patients treated with allogeneic hematopoietic stem cell transplantation (HSCT). The reactivation of multiple herpes viruses, especially cytomegalovirus (CMV) reactivation, commonly occurs following HSCT (Hill et al., 2017; Stern et al., 2021). Epstein-Barr virus (EBV) reactivation is also prevalent and can cause serious complications, such as post-transplant lymphoproliferative disorder (PTLD) (Diop et al., 2021; Enok Bonong et al., 2021). The reported incidences of virus reactivation after HSCT fluctuate widely from 0.1% to 63% for EBV (Styczynski et al., 2016b) and from 30% to 70% for CMV (Styczynski et al., 2016a; Ljungman et al., 2019) with ambiguous effects on transplant outcomes (Auger et al., 2014; Teira et al., 2016).
CMV and EBV are the most closely related clinical viruses with a clear therapeutic association (Einsele et al., 2020; Yoshimori et al., 2021). There seems to be a bidirectional relationship between the two viruses, and a high incidence/low clearance rate of CMV infection and a high incidence and delayed immune reconstitution of EBV-PTLD are key to all of these findings (Green et al., 2013; Chiereghin et al., 2019). Therefore, we speculate that the co-activation of CMV and EBV after HSCT might lead to worse clinical outcomes in transplant patients compared to those with single virus reactivation. However, few studies have investigated the effects of CMV and EBV co-reactivation following HSCT. Our study analyzed the clinical characteristics of HSCT patients who developed co-reactivation of CMV and EBV, investigated the influence of CMV and EBV co-reactivation on HSCT outcomes, and analyzed the underlying risk factors.
In total, 327 patients who underwent allo-HSCT for the first time between January 2018 and January 2019 at the Peking University People’s Hospital Institute of Hematology were included in our study. We performed a retrospective review of their medical records. The Ethics Committee of the Peking University People’s Hospital approved our study. Moreover, informed consent was waived owing to the retrospective nature of this study.
The transplantation process is described as follows. The adjustment therapy was modified rabbit anti-thymocyte globulin (2.5 mg/kg/day; Sang Stat, Lyon, France) plus busulfan/cyclophosphamide (BU/CY; busulfan, 9.6 mg/kg i.v., given as 12 doses on days −8 to−-6) and cyclophosphamide, 1.8 g/m2/day i.v. on days −5 to −4) for the unrelated donor (URD) HSCT and haplo-HSCT groups (Wang et al., 2011) and modified BU/CY for the identical sibling donor (ISD) patients. The ISD and haplo-HSCT patients were given mobilized granulocyte colony-stimulating factor (G-CSF), which primed peripheral blood stem cells (PBSCs). The URD HSCT patients were given unmanipulated PBSCs (3.0 × 108 cells per kg) and fresh G-CSF-mobilized (5 μg kg−1 daily for 5–6 days). To prevent graft-versus-host disease (GVHD), all patients were given mycophenolate mofetil, cyclosporine, and short-term methotrexate (Wang, Liu, Xu, Liu, Chen, Chen, Han, Shi and Huang 2011).
CMV and EBV reactivation was tested twice per week using plasma samples with real-time quantitative polymerase chain reaction (PCR). All patients were given ganciclovir between days −9 and −2 (Huang et al., 2009). Pre-emptive therapy with either intravenous foscarnet (90 mg/kg/day) or intravenous ganciclovir (5 mg/kg, twice daily) was started when the patients had confirmed CMV viremia reactivation, and therapy was continued until the CMV DNA was not detected on two consecutive tests. Antiviral drugs, such as foscarnet, were given to patients with EBV reactivation. Moreover, rituximab was administered when EBV viremia persisted or deteriorated to EBV disease. The salvage therapy was EBV-specific CTL.
Our study defined myeloid engraftment as the first day of three continuous days with an absolute neutrophil count ≥0.5×109/L, and platelet engraftment was recognized as the first day of seven consecutive days with a platelet count ≥20×109/L without blood transfusion. Viral pneumonia mainly includes lung infections caused by influenza A, influenza B, paramyxoviruses, CMV, EBV, respiratory syncytial virus, adenovirus, and rhinoviruses. CMV and EBV reactivation were defined as the first of two continuous viral DNA tests in which the viral DNA reached or surpassed 1,000 copies/mL and 500 copies/mL, respectively. Co-reactivation of CMV and EBV was defined as a test of the EBV load and CMV load over 1 year after HSCT. The duration of viremia was defined as the number of days between the first day of viremia and the first day when the virus load was no longer detected Time to relapse was defined as the days between the date of HSCT and the date of disease relapse. Non-relapse mortality (NRM) was defined death as from all causes other than those directly related to the blood disease itself, occurring at any time after transplantation. Overall survival (OS) was defined as the days from HSCT to death from any cause. Leukemia-free survival (LFS) was defined as the days from HSCT to disease progression after HSCT.
Categorical variables between the groups were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using the Mann-Whitney U test. Multivariate Cox congress models were applied to test the hazards assumption and nonlinearity of the time-by-covariate interaction. The competing risk model was suggested to calculate the cumulative incidence of virus reactivation. IBM SPSS 25.0 statistical software (IBM SPSS Statistics, USA) and SAS (version 9.4; SAS institute Inc) were used in this research.
In total, 327 patients participated in this study. Patient characteristics are shown in Tables 1 and 2. Of these patients, 193 (59.0%) were men. The median age was 33 (25, 44) years. The majority of patients had acute myeloid leukemia (n=154, 47.1%) and acute lymphoblastic leukemia (n=102, 31.2%). Two hundred and forty-one (73.7%) patients received HSCT from haploidentical donors and 12 (3.7%) underwent HSCT originating from unrelated donors. Myeloid and platelet engraftments were found in 324 (99.1%) patients at a median of 13 (12–16) days and in 212 (64.8%) patients at a median of 15 (12– 20) days after HSCT, respectively. The morbidity of grade 1–2 acute GVHD and total acute GVHD was 32.4% (n=106) and 50.6% (n=119), respectively. The 1-year OS and LFS rates were 88.3% and 75.7%, respectively. The NRM and relapse rates causing mortality were 7.0% and 3.0%, respectively.
CMV viremia was found in 70.0% of the patients (n=229), among which, 47 patients were infected by CMV twice or more during 1 year after HSCT. The median time of the first CMV viremia reactivation after HSCT was 40 (33, 47) days, and the median duration was 18 (13, 25) days. The median CMV DNA copy number in patients was 2.27×103 (1.42, 4.30×103). Eighty-six patients (26.3%) had EBV reactivation. EBV viremia was reactivated at a median of 49 (41, 63) days after HSCT and lasted for a median of 13 (5, 20) days. EBV DNA copies reached 5.10×103 (1.66, 2.62×103). According to the aforementioned definition, 75 (22.9%) patients were classified as having co-reactivation of CMV and EBV. In total, 154 (47.1%) patients had CMV reactivation only and 87 (26.6%) patients had no reactivation of either virus (Tables 1, 2).
Patients were divided into four groups according to CMV and EBV reactivation based on the following definitions: (1) co-reactivation group, (2) CMV reactivation group, (3) EBV reactivation group, and (4) no reactivation group. The characteristics of the four groups are presented in Table 1. Neutrophil engraftment was comparable among the four groups (100% vs. 100% vs. 100% vs. 97.0% for the co-reactivation, CMV reactivation, EBV reactivation, and no reactivation groups, respectively, P=0.375). However, neutrophil engraftment seemed to be delayed in patients with no virus reactivation, in contrast to that in the other groups (13 vs. 13 vs. 13 vs.14 days for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.010). According to platelet engraftment, the ratio of patients (61.3% vs. 63.6% vs. 54.5% vs. 71.3% for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.460) and days of engraftment (15 vs. 15 vs. 20 vs.13 days for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.080) were comparable among the four groups. The morbidity of acute GVHD was very higher in the reactivation group compared to that in the no reactivation group (56.3% vs. 61.5% vs. 55.6% vs. 37.0% for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.005). Patients in the reactivation group were more likely than those in the other groups to progress to viral pneumonia (4.0% vs. 4.5% vs. 0% vs. 1.1% for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.484), and a similar trend was observed for viral enteritis (2.7% vs. 1.3% vs. 0% vs. 0% for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.473). Hemorrhagic cystitis was more common in the reactivation group than in the other groups (37.5% vs. 35.2% vs. 14.1% vs. 14.1% for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.000, Table 2).
Co-reactivation and CMV reactivation were found in 22.9% (n=75) and 47.1% (n=154) of patients, respectively. In the co-reactivation group, the time of first CMV viremia reactivation after HSCT preceded that in the CMV reactivation group (40 [31, 47] vs. 40 [33, 49] days, respectively, P=0.370). The duration of CMV viremia in the co-reactivation group was longer than that in the CMV reactivation group (21 [15,26] vs.18 [11,24], days, respectively, P=0.007). The highest CMV viral load in the CMV reactivation group was higher than that in the CMV reactivation group (1.71 [1.14,3.68] vs.1.69 [0.77,3.50] ×103 copies/mL, respectively, P=0.315). EBV reactivation was observed in 3.3% of the patients (n=11). The differences in the time of first EBV viremia reactivation after HSCT and the duration of EBV viremia were not statistically different between the two groups. Moreover, the highest EBV viral load was also not statistically different between the two groups.
The 1-year OS was lower in the reactivation groups than in the no reactivation group (86.7% vs. 85.1% vs. 90.0% vs. 95.4% for co-reactivation, CMV reactivation, EBV reactivation, and no reactivation, respectively, P=0.050). There were no significant differences in 1-year LFS among these groups. Viral reactivation was a risk factor for 1-year OS (Figure 1). Further Cox regression analysis showed that viral infection was a risk factor affecting the 1-year OS (Table 3; HR, 12.625 for co-reactivation vs. no reactivation, P=0.021, and HR, 13.580 for CMV reactivation vs. no reactivation, P=0.013). However, viral reactivation was not a risk factor for LFS (Figure 2; p=0.348). The GVHD was a risk factor affecting the 1-year LFS (HR, 2.099 for GVHD vs. no GVHD, P=0.016). The 1-year OS and 1-year LFS hazards are summarized in Table 3.
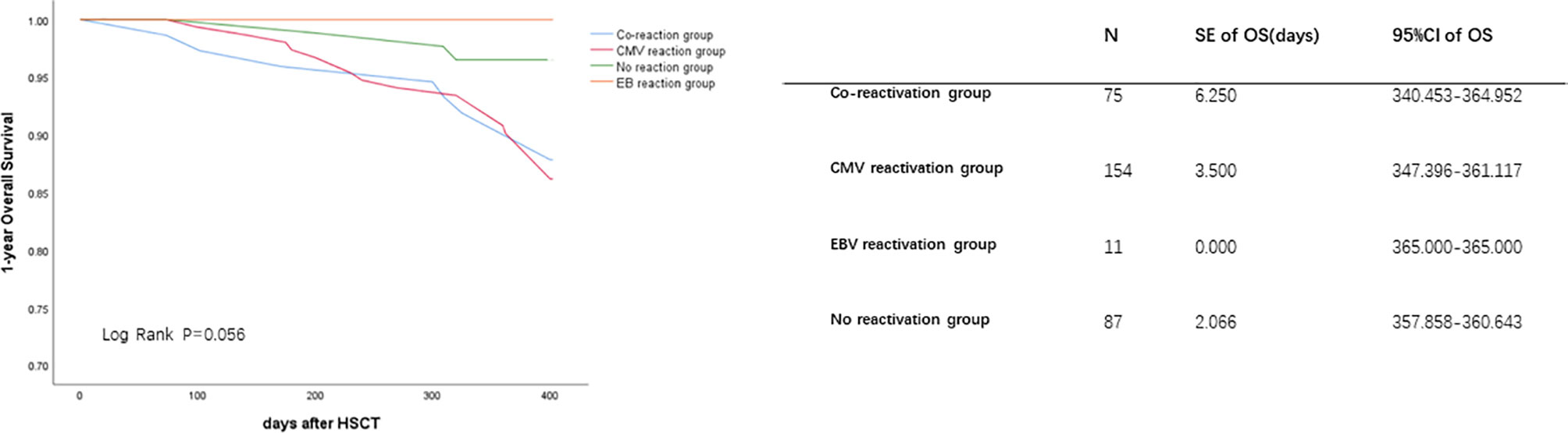
Figure 1 Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) co-reactivation was identified as one of the risk factors for 1-year OS. The OS were performed by K-M method. P value was determined by Log-rank test. OS, overall survival; SE, standard error; CI, confidence interval.
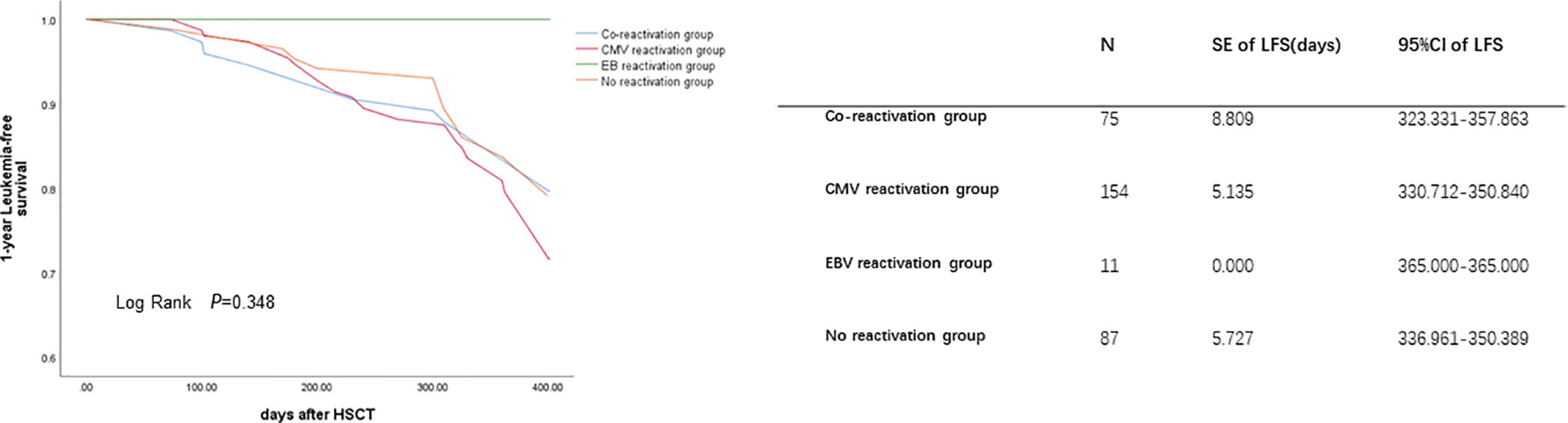
Figure 2 Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) co-reactivation was identified as one of the risk factors for 1-year LFS. The LFS were performed by K-M method. P value was determined by Log-rank test. LFS, leukemia free survival; SE, standard error; CI, confidence interval.
Patients with co-reactivation of CMV and EBV were compared with all other patients to identify risk factors associated with co-reactivation. The competing risk model showed that age(>=40years vs. <40years)was one risk factor for co-reactivation of CMV and EBV (Table 4; HR:95%CI 1.360(1.008-1.833). Another risk factor was HLA match (HR:95%CI (4.442(1.710-11.535) for unrelated donor vs. Identical, P=0.002; HR:95%CI 5.178(3.333-8.047) for haploidentical vs. Identical, P=0.000). GVHD and disease status were excluded as a risk factor for CMV and EBV co-reactivation in the competing risk model(P>0.05).
In our study, we proved that CMV and EBV co-reactivation in HSCT patients were associated with OS and LFS. Our findings are thus clinically relevant. CMV reactivation is known to be significantly associated with EBV reactivation (Fan et al., 2016), but CMV and EBV co-reactivation is relatively less widespread compared to that of the other double-stranded DNA viruses. In our study, CMV and EBV co-reactivation was found in 22.9% of patients after HSCT (n=75). This is consistent with a previous study that reported CMV-EBV coinfection, in which the rates of co-infection post-transplantation varied between 3.0% and 32.7% (Anderson-Smits et al., 2020). In the study reporting the lowest rate (3.0%,12/404), the total study population included patients who were not included in the groups at a high risk of EBV-related complications, in high-stakes patients, and in those who were not high stakes but had improved moderate-to-poor steroid-refractory GVHD (Garcia-Cadenas et al., 2015). Although the proportion of patients with CMV-EBV co-reactivation was reported with EBV-PTLD (post-transplant lymphoproliferative disorders), it is unclear whether this occurs for all cases of co-reactivation (i.e., CMV-EBV co-reactivation without PTLD), which might have helped to decrease the rate of co-reactivation. In the report citing the highest rate of CMV-EBV co-reactivation (32.7%; 33/101), all cases of co-reactivation involved both EBV and CMV (defined by viral load) (Zallio et al., 2013), and a similar definition of co-reactivation was used to that in our study. Another study reported CMV-EBV coinfection rates of 22.7% (10/44) (Fan et al., 2016) with a similar incidence of co-reactivation under the same definition.
Our study proved that CMV and EBV co-reactivation were related to a decreased 1-year OS. However, the causes of death (NRM or relapse) did not significantly differ among the groups (P>0.05). This result is partly in accordance with that of a previous study. Co-reactivation of CMV and EBV was found to be related to a decreased 1-year OS, which was mainly because of an increase in NRM (Zhou et al., 2020). With co-reactivation, the 1-year NRM was higher, in contrast to that found by two other groups, the difference was not remarkably significant (P=0.053), and no deaths occurred due to relapse (Zhou et al., 2020). Another report showed that a CMV and EBV co-reactivation group had a significantly higher 6-month non-relapse mortality than the other groups with CMV or EBV reactivation alone (Song et al., 2014). According to our findings, CMV reactivation alone after HSCT was also associated with 1-year OS; however, EBV reactivation alone after HSCT was not. One meta-analysis showed that CMV reactivation is associated with an increased risk of overall mortality and NRM in allo-HSCT recipients (Gimenez et al., 2019).
According to our results, the duration of CMV viremia was found in the co-reactivation group as compared to that in the other reactivation groups, reflecting the effect of EBV reactivation on CMV reactivation. CMV induces NKG2C + CD57 + KIR+ natural killer (NK) cell expansion 3–6 months after HSCT (Zuo and Zhao, 2021). NK cells are the earliest reconstituting immune cells, achieving normal numbers within weeks after patients undergo HSCT and helping in the graft-versus-tumor function along with T cells (Farag et al., 2002). Meanwhile, the cytokine-producing and cytotoxic functions of NK cells were found to be lower until 3–6 months after HSCT (Foley et al., 2011) and reached common reactivity levels at the first year and were maintained during later times (Haas et al., 2011). Immunoreactivation of one virus mediated by another virus has been previously reported with both HSCT and solid organ transplantation. This could also demonstrate poor immune reconstitution as previously shown with poor CD3+ and CD4+25+ cell counts (on day 30) (Zhou et al., 2020). CMV and EBV co-reactivation induces an increase in CD56dim/NKG2A+/CD57+NK cell numbers, which remain elevated up to 6 months after reactivation and leads to a decrease in the absolute quantity of immature CD56bright/CD16− NK cells in the blood (Lunemann et al., 2013; Hendricks et al., 2014). One study showed that CMV is an especially active inducer of some members of the herpesvirus family and implied that the interplay between CMV and EBV occurs unidirectionally in vivo (Aalto et al., 1998). However, this study was not based on HSCT patients.
Our study identified HLA match (unrelated donor and haploidentical vs. Identical) as an independent risk factor for CMV and EBV co-reactivation. Compared with HLA identical sibling transplantation, patients undergoing HLA-haploidentical stem cell transplantation(haploSCT) usually receive more intensive immunosuppressors to guarantee engraftment and later prevent graft-versus-host disease (GVHD) (Luo et al., 2021). Previous studies have shown that risk factors for CMV reactivation after HSCT include a donor or recipient seropositive for CMV, mismatched or unrelated donors, pre-allo-HSCT viremia, and use of alemtuzumab (Sousa et al., 2014). Another findings show patients undergoing hematopoietic stem cell transplantation or solid organ transplantation can experience post-transplant lymphoproliferative disorders due to dysfunction or suppression of host’s immune system, or uncontrolled proliferation of EBV-infected cells (Fujimoto and Suzuki, 2020). Some study uncovered a significant correlation of recovered Vδ2 with EBV reactivation following haploSCT (Liu et al., 2018). HSCT is a process comprising total immune reconstruction, and the interplay of various viruses in this process makes the condition of HSCT patients more complicated and diverse.
There are several limitations to this study. First, the retrospective nature of the study has inherent risks of bias; nevertheless, the patient characteristics and the HSCT complications did not differ significantly from those reported in prospective research. Second, we did not study other herpesviruses, which might have had a significant effect on these findings. However, we studied the two most clinically significant viruses that have defined treatment options. Despite these limitations, we believe that this study provides clinical hematologists with scientific evidence of CMV and EBV co-reactivation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Peking University People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
SL and MS: design of the study; SL and NZ: data acquisition, analysis, and interpretation; MJ: drafted the article and critically revised the manuscript; MS: gave final approval for the version to be submitted. All authors contributed to the article and approved the submitted version.
This study was approved by the National Natural Science Foundation of China to MS (Grant No. 81870196).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.818167/full#supplementary-material
Aalto, S. M., Linnavuori, K., Peltola, H., Vuori, E., Weissbrich, B., Schubert, J., et al. (1998). Immunoreactivation of Epstein-Barr Virus Due to Cytomegalovirus Primary Infection. J. Med. Virol. 56, 186–191. doi: 10.1002/(SICI)1096-9071(199811)56:3<186::AID-JMV2>3.0.CO;2-3
Anderson-Smits, C., Baker, E. R., Hirji, I. (2020). Coinfection Rates and Clinical Outcome Data for Cytomegalovirus and Epstein-Barr Virus in Post-Transplant Patients: A Systematic Review of the Literature. Transpl. Infect. Dis. 22, e13396. doi: 10.1111/tid.13396
Auger, S., Orsini, M., Ceballos, P., Fegueux, N., Kanouni, T., Caumes, B., et al. (2014). Controlled Epstein-Barr Virus Reactivation After Allogeneic Transplantation is Associated With Improved Survival. Eur. J. Haematol. 92, 421–428. doi: 10.1111/ejh.12260
Chiereghin, A., Piccirilli, G., Belotti, T., Prete, A., Bertuzzi, C., Gibertoni, D., et al. (2019). Clinical Utility of Measuring Epstein-Barr Virus-Specific Cell-Mediated Immunity After HSCT in Addition to Virological Monitoring: Results From a Prospective Study. Med. Microbiol. Immunol. 208, 825–834. doi: 10.1007/s00430-019-00629-2
Diop, N. S., Enok Bonong, P. R., Buteau, C., Duval, M., Lacroix, J., Laporte, L., et al. (2021). Association Between Antiviral Prophylaxis and Cytomegalovirus and Epstein-Barr Virus DNAemia in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplant. Vaccines (Basel) 7, 9. doi: 10.3390/vaccines9060610
Einsele, H., Ljungman, P., Boeckh, M. (2020). How I Treat CMV Reactivation After Allogeneic Hematopoietic Stem Cell Transplantation. Blood 135, 1619–1629. doi: 10.1182/blood.2019000956
Enok Bonong, P. R., Buteau, C., Duval, M., Lacroix, J., Laporte, L., Tucci, M., et al. (2021). Risk Factors for Post-Transplant Epstein-Barr Virus Events in Pediatric Recipients of Hematopoietic Stem Cell Transplants. Pediatr. Transplant. 25, e14052. doi: 10.1111/petr.14052
Fan, J., Jing, M., Yang, M., Xu, L., Liang, H., Huang, Y., et al. (2016). Herpesvirus Infections in Hematopoietic Stem Cell Transplant Recipients Seropositive for Human Cytomegalovirus Before Transplantation. Int. J. Infect. Dis. 46, 89–93. doi: 10.1016/j.ijid.2016.03.025
Farag, S. S., Fehniger, T. A., Ruggeri, L., Velardi, A., Caligiuri, M. A. (2002). Natural Killer Cell Receptors: New Biology and Insights Into the Graft-Versus-Leukemia Effect. Blood 100, 1935–1947. doi: 10.1182/blood-2002-02-0350
Foley, B., Cooley, S., Verneris, M. R., Curtsinger, J., Luo, X., Waller, E. K., et al. (2011). NK Cell Education After Allogeneic Transplantation: Dissociation Between Recovery of Cytokine-Producing and Cytotoxic Functions. Blood 118, 2784–2792. doi: 10.1182/blood-2011-04-347070
Fujimoto, A., Suzuki, R. (2020). Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disorders After Hematopoietic Stem Cell Transplantation: Pathogenesis, Risk Factors and Clinical Outcomes. Cancers (Basel) 12. doi: 10.3390/cancers12020328
Garcia-Cadenas, I., Castillo, N., Martino, R., Barba, P., Esquirol, A., Novelli, S., et al. (2015). Impact of Epstein Barr Virus-Related Complications After High-Risk Allo-SCT in the Era of Pre-Emptive Rituximab. Bone Marrow Transplant. 50, 579–584. doi: 10.1038/bmt.2014.298
Gimenez, E., Torres, I., Albert, E., Pinana, J. L., Hernandez-Boluda, J. C., Solano, C., et al. (2019). Cytomegalovirus (CMV) Infection and Risk of Mortality in Allogeneic Hematopoietic Stem Cell Transplantation (Allo-HSCT): A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Am. J. Transplant. 19, 2479–2494. doi: 10.1111/ajt.15515
Green, M. L., Leisenring, W. M., Xie, H., Walter, R. B., Mielcarek, M., Sandmaier, B. M., et al. (2013). CMV Reactivation After Allogeneic HCT and Relapse Risk: Evidence for Early Protection in Acute Myeloid Leukemia. Blood 122, 1316–1324. doi: 10.1182/blood-2013-02-487074
Haas, P., Loiseau, P., Tamouza, R., Cayuela, J. M., Moins-Teisserenc, H., Busson, M., et al. (2011). NK-Cell Education is Shaped by Donor HLA Genotype After Unrelated Allogeneic Hematopoietic Stem Cell Transplantation. Blood 117, 1021–1029. doi: 10.1182/blood-2010-02-269381
Hendricks, D. W., Balfour, H. H., Jr., Dunmire, S. K., Schmeling, D. O., Hogquist, K. A., Lanier, L. L. (2014). Cutting Edge: NKG2C(hi)CD57+ NK Cells Respond Specifically to Acute Infection With Cytomegalovirus and Not Epstein-Barr Virus. J. Immunol. 192, 4492–4496. doi: 10.4049/jimmunol.1303211
Hill, J. A., Mayer, B. T., Xie, H., Leisenring, W. M., Huang, M. L., Stevens-Ayers, T., et al. (2017). The Cumulative Burden of Double-Stranded DNA Virus Detection After Allogeneic HCT is Associated With Increased Mortality. Blood 129, 2316–2325. doi: 10.1182/blood-2016-10-748426
Huang, X. J., Liu, D. H., Liu, K. Y., Xu, L. P., Chen, H., Han, W., et al. (2009). Treatment of Acute Leukemia With Unmanipulated HLA-Mismatched/Haploidentical Blood and Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 15, 257–265. doi: 10.1016/j.bbmt.2008.11.025
Liu, J., Bian, Z., Wang, X., Xu, L. P., Fu, Q., Wang, C., et al. (2018). Inverse Correlation of Vdelta2(+) T-Cell Recovery With EBV Reactivation After Haematopoietic Stem Cell Transplantation. Br. J. Haematol. 180, 276–285. doi: 10.1111/bjh.15037
Ljungman, P., de la Camara, R., Robin, C., Crocchiolo, R., Einsele, H., Hill, J. A., et al. (2019). Guidelines for the Management of Cytomegalovirus Infection in Patients With Haematological Malignancies and After Stem Cell Transplantation From the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e260–e272. doi: 10.1016/S1473-3099(19)30107-0
Lunemann, A., Vanoaica, L. D., Azzi, T., Nadal, D., Munz, C. (2013). A Distinct Subpopulation of Human NK Cells Restricts B Cell Transformation by EBV. J. Immunol. 191, 4989–4995. doi: 10.4049/jimmunol.1301046
Luo, X. H., Zhu, Y., Chen, Y. T., Shui, L. P., Liu, L. (2021). CMV Infection and CMV-Specific Immune Reconstitution Following Haploidentical Stem Cell Transplantation: An Update. Front. Immunol. 12, 732826. doi: 10.3389/fimmu.2021.732826
Song, T., Chen, G., Zhang, X., Xu, Y., Chen, J., Wang, Y., et al. (2014). Clinical Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation Patients With Co-Activation of Cytomegalovirus and Epstein-Barr Virus. Zhonghua Yi Xue Za Zhi 94, 3135–3139. doi: 10.3760/cma.j.issn.0376-2491.2014.40.005
Sousa, H., Boutolleau, D., Ribeiro, J., Teixeira, A. L., Pinho Vaz, C., Campilho, F., et al. (2014). Cytomegalovirus Infection in Patients Who Underwent Allogeneic Hematopoietic Stem Cell Transplantation in Portugal: A Five-Year Retrospective Review. Biol. Blood Marrow Transplant. 20, 1958–1967. doi: 10.1016/j.bbmt.2014.08.010
Stern, A., Su, Y., Dumke, H., Fang, J., Tamari, R., Jakubowski, A., et al. (2021). Cytomegalovirus Viral Load Kinetics Predict Cytomegalovirus End-Organ Disease and Mortality After Hematopoietic Cell Transplant. J. Infect. Dis. 224, 620–631. doi: 10.1093/infdis/jiab212
Styczynski, J., Czyzewski, K., Wysocki, M., Gryniewicz-Kwiatkowska, O., Kolodziejczyk-Gietka, A., Salamonowicz, M., et al. (2016a). Increased Risk of Infections and Infection-Related Mortality in Children Undergoing Haematopoietic Stem Cell Transplantation Compared to Conventional Anticancer Therapy: A Multicentre Nationwide Study. Clin. Microbiol. Infect. 22, 179 e171–179 e110. doi: 10.1016/j.cmi.2015.10.017
Styczynski, J., van der Velden, W., Fox, C. P., Engelhard, D., de la Camara, R., Cordonnier, C., et al. (2016b). Management of Epstein-Barr Virus Infections and Post-Transplant Lymphoproliferative Disorders in Patients After Allogeneic Hematopoietic Stem Cell Transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) Guidelines. Haematologica 101, 803–811. doi: 10.3324/haematol.2016.144428
Teira, P., Battiwalla, M., Ramanathan, M., Barrett, A. J., Ahn, K. W., Chen, M., et al. (2016). Early Cytomegalovirus Reactivation Remains Associated With Increased Transplant-Related Mortality in the Current Era: A CIBMTR Analysis. Blood 127, 2427–2438. doi: 10.1182/blood-2015-11-679639
Wang, Y., Liu, D. H., Xu, L. P., Liu, K. Y., Chen, H., Chen, Y. H., et al. (2011). Superior Graft-Versus-Leukemia Effect Associated With Transplantation of Haploidentical Compared With HLA-Identical Sibling Donor Grafts for High-Risk Acute Leukemia: An Historic Comparison. Biol. Blood Marrow Transplant. 17, 821–830. doi: 10.1016/j.bbmt.2010.08.023
Yoshimori, M., Shibayama, H., Imadome, K. I., Kawano, F., Ohashi, A., Nishio, M., et al. (2021). Antineoplastic and Anti-Inflammatory Effects of Bortezomib on Systemic Chronic Active EBV Infection. Blood Adv. 5, 1805–1815. doi: 10.1182/bloodadvances.2020002417
Zallio, F., Primon, V., Tamiazzo, S., Pini, M., Baraldi, A., Corsetti, M. T., et al. (2013). Epstein-Barr Virus Reactivation in Allogeneic Stem Cell Transplantation Is Highly Related to Cytomegalovirus Reactivation. Clin. Transpl. 27, E491–E497. doi: 10.1111/ctr.12172
Zhou, J. R., Shi, D. Y., Wei, R., Wang, Y., Yan, C. H., Zhang, X. H., et al. (2020). Co-Reactivation of Cytomegalovirus and Epstein-Barr Virus Was Associated With Poor Prognosis After Allogeneic Stem Cell Transplantation. Front. Immunol. 11, 620891. doi: 10.3389/fimmu.2020.620891
Keywords: co-reactivation, CMV, EBV, HSCT, outcomes
Citation: Li S-s, Zhang N, Jia M and Su M (2022) Association Between Cytomegalovirus and Epstein-Barr Virus Co-Reactivation and Hematopoietic Stem Cell Transplantation. Front. Cell. Infect. Microbiol. 12:818167. doi: 10.3389/fcimb.2022.818167
Received: 19 November 2021; Accepted: 28 January 2022;
Published: 25 March 2022.
Edited by:
Shyamala Thirunavukkarasu, Washington University in St. Louis, United StatesReviewed by:
Depei Wu, The First Affiliated Hospital of Soochow University, ChinaCopyright © 2022 Li, Zhang, Jia and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Su, c3VtaW5nQGJqbXUuZWR1LmNu
†These authors have contributed equally to work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.