- Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, Changsha, China
Gut microbiota and its metabolites play an important role in maintaining host homeostasis. Pulmonary arterial hypertension (PAH) is a malignant clinical syndrome with a frightening mortality. Pulmonary vascular remodeling is an important feature of PAH, and its pathogenesis is not well established. With the progress of studies on intestinal microbes in different disease, cumulative evidence indicates that gut microbiota plays a major role in PAH pathophysiology. In this review, we will systematically summarize translational and preclinical data on the correlation between gut dysbiosis and PAH and investigate the role of gut dysbiosis in the causation of PAH. Then, we point out the potential significance of gut dysbiosis in the diagnosis and treatment of PAH as well as several problems that remain to be resolved in the field of gut dysbiosis and PAH. All of this knowledge of gut microbiome might pave the way for the extension of novel pathophysiological mechanisms, diagnosis, and targeted therapies for PAH.
Highlights
1. It is an indisputable fact that intestinal dysbiosis exists in patients with PAH and rodent models.
2. Gut dysbiosis plays an important role in the pathophysiology of PAH by mediating systemic inflammation or immunity via bacteria-related metabolites.
3. Abnormalities in intestinal microflora composition and metabolites contribute to the diagnosis of PAH, and ameliorating intestinal dysbiosis and subsequent disorders are beneficial to the control of PAH, which provides a potentially therapeutic target for PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a malignant pulmonary vascular disease characterized by pulmonary vascular remodeling, resulting in right heart failure (RHF) and imposing an enormous economic burden on the society (Thenappan et al., 2018; Humbert et al., 2019). Although targeted drugs significantly improved patients’ quality of life and survival by dilating pulmonary arterials, PAH cannot be completely cured. Gut microbiota can synthesize and secrete metabolites, which is critical for maintaining general homeostasis, and, accordingly, gut dysbiosis is associated with the initiation and development of a variety of diseases, such as obesity (Gordon et al., 2006; Henao-Mejia et al., 2012), diabetes (Cani et al., 2008; Khan et al., 2014), atherosclerosis (Brown and Hazen, 2018), hypertension (Marques et al., 2018; Sharma et al., 2019), and heart failure (HF) (Tang et al., 2019). Benefiting from the development of metagenomics, metabonomics, and microbiology, the research studies of gut microbiota and PAH provide a new perspective for elucidating the pathogenesis of PAH. In this review, we discuss the current literature and perspective on the role of gut microbiota in PAH.
Alteration of Gut Microbiota and Metabolites in PAH: Pre-Clinical and Translational Evidence
PAH is a complex systemic disease involving multiple organs such as the lung, gut, and brain (Oliveira et al., 2020). Profound understanding of the cellular (Archer et al., 2008; Caruso et al., 2017; Zhang et al., 2017), genetic (Drake et al., 2013; Long et al., 2015; Evans et al., 2016), and epigenetic (Rexhaj et al., 2011; Maresca, 2015; Momparler et al., 2017) changes implicated in pulmonary vascular remodeling in patients with PAH has been proved over the past decades (Thenappan et al., 2018; Humbert et al., 2019). Although the precise molecular mechanism of gut microbiota in PAH has not been completely identified, cumulative preclinical and clinical evidence highlights the participation of gut microbiota and its metabolites in the pathogenesis of PAH.
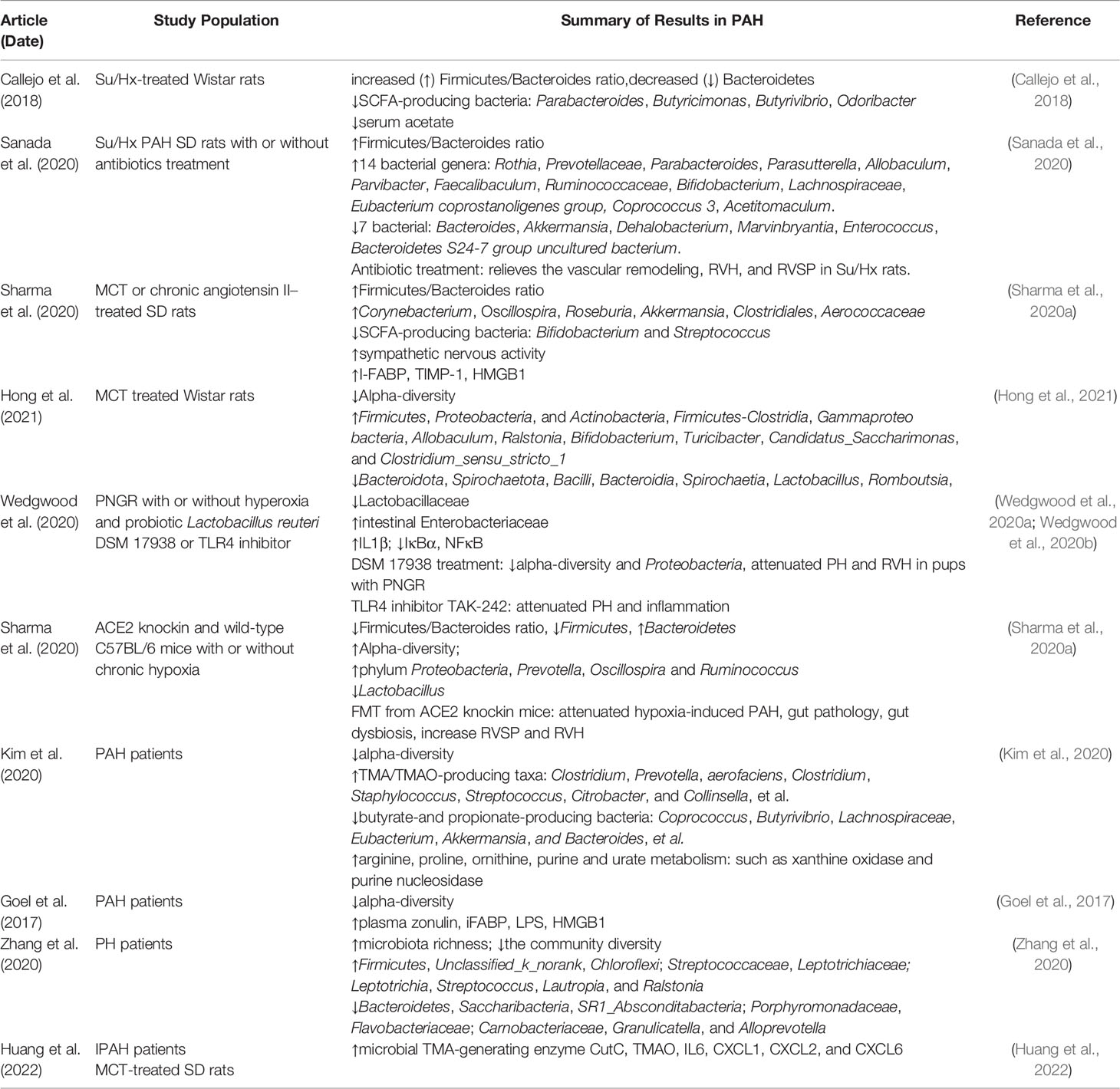
Gut Dysbiosis in PAH: Pre-Clinical Evidence
Numerous studies have identified that abnormal alterations in gut microbial communities are present both in patients and various animal models with PAH (Table 1). The imbalance of the ratio of Firmicutes to Bacteroidetes (F/B), an important characteristic of gut dysbiosis, has been reported in a variety of diseases, such as hypertension (Yang et al., 2015; Marques et al., 2017), HF (Mayerhofer et al., 2020), and obesity (Murphy et al., 2013; Chang et al., 2015). Callejo et al. analyzed the microbial composition of feces in Su5416/Hypoxia (Su/Hx)–treated Wistar rats by 16S rRNA gene sequencing and bioinformatics analysis and observed that the F/B ratio increased by three times compared with control rats, which was mainly related to the decrease of Bacteroidetes (Callejo et al., 2018). At the same time, the researchers demonstrated that both acetate-producing bacteria and the level of serum acetate were decreased in PAH rats (Callejo et al., 2018). Similarly, the fecal F/B ratio of Su/Hx-treated SD rats was also significantly higher than that of control and simple hypoxia group. Compared with the control group, the abundance of 14 bacterial genera (e.g., Rosia and Prevotellaceae) and seven bacterial genera (e.g., Bacteroides and Akkermansia) in Su/Hx rats increased and decreased, respectively (Sanada et al., 2020). In addition to Su/Hx rat model, Sharma et al. noted that the F/B ratio and pathogenic microorganisms in the fecal of Monocrotaline (MCT)–treated SD rats were profoundly higher than those in the healthy control group, whereas beneficial symbiotic bacteria were richer in healthy control group (Sharma et al., 2020a). Recently, Hong and colleagues found that MCT significantly reduced the microbial diversity and altered the abundance of intestinal flora in Wistar rats, for example, the abundance of Firmicutes, Proteobacteria, Actinobacteria, Firmicutes-Clostridia, and Gammaproteo bacteria was increased in the PAH group, whereas the abundance of Bacteroidota, Spirochaetota, Bacilli, Bacteroidia, and Spirochaetia was lower in PAH group than in the control group (Hong et al., 2021). Interestingly, Wedgwood et al. also demonstrated intestinal dysbiosis in the cecum and distal small intestine in postnatal growth restriction (PNGR)–induced PAH rats, including the difference of α/β diversity, the increase in Enterobacteriaceae, and decrease in Lactobacillaceae (Wedgwood et al., 2020a; Wedgwood et al., 2020b). In addition, a distinct characteristic of microbial community was also presented in C57BL/6 mice subjected to hypoxia, namely, increased α-diversity and decreased F/B ratio, where the genera Prevotella, Oscillospira, and Ruminococcus were increased and Lactobacillus was decreased, which was associated with intestinal pathology (Sharma et al., 2020b). Notably, although a few results are controversial, different PAH animal models have similar changes in gut microbiota, namely, an increase in the abundance of pathogenic microorganisms and a decrease in probiotics, suggesting the specific alteration of gut microbiota profile inextricably linked with PAH.
Gut Dysbiosis in PAH: Translational Clinical Evidence
In addition to PAH animal models, a growing body evidence confirmed the presence of gut microbial dysbiosis in patients with PAH (Goel et al., 2017; Kim et al., 2020; Zhang et al., 2020). Goel et al. demonstrated that the abundance, diversity, and evenness of gut microbiota in patients with PAH were profoundly decreased, whereas Gram-positive and facultative-anaerobic genera were increased. Seungbum et al. accomplished a landmark study in the field of gut microbiota and PAH via shotgun metagenomics, which firstly observed a unique profile of gut microbial communities in patients with PAH (Kim et al., 2020). Fecal analysis of 18 patients with type 1 PAH and 13 reference subjects revealed that PAH-associated flora involving in the synthesis of arginine, proline, and ornithine, as well as colonies related to trimethylamine/trimethylamine N-oxide (TMA/TMAO) and purine metabolism were increased in PAH cohorts, whereas the abundance of butyric- and propionate-producing bacteria such as Coprococcus and Butyrivibrio were richer in health controls. This study noted that the distinct gut microbiome was effective in predicting PAH with 83% accuracy. Furthermore, virome analysis confirmed Enterococcal enrichment and relative depletion of Lactococcal phages in patients with PAH (Kim et al., 2020). In addition, Zhang and colleagues studied the microbiota profile of oropharyngeal rather than fecal samples in patients with pulmonary hypertension (PH), and the results indicated that the relative abundance and diversity of microbiota in patients with PH were profoundly different from healthy subjects, including that Streptococcus, Lautropia, and Ralstonia were enriched in PH. Although Saemophilus, Rothia, Granulicatella, Capnocytophage, and Sccharibacteria were relatively abundant in healthy subjects, this difference may be a potential predictor for distinguishing PH from reference subjects (Zhang et al., 2020). Although limited by sample size and geographic area, this study was partly consistent with preclinical studies and reflected the characteristics of gut microbiota composition alteration in PAH. In addition, future randomized controlled studies with larger sample sizes await further confirm these findings.
Gut Microbial Metabolites in PAH
Intestinal microflora can synthesize and secrete specific metabolites, which play a vital role in regulating various host physiological function. Given that, the alteration in serum levels of these metabolites may reflect the taxonomic and functional changes of gut microbiota and diversity in PAH. For example, Callejo and colleagues measured serum concentrations of short-chain fatty acids (SCFAs) in Su/Hx PAH rats by nuclear magnetic resonance spectroscopy and found a significant decrease in acetate, which was consistent with the decrease in acetate-producing bacteria (Callejo et al., 2018). Serum endotoxin, another important metabolite of gut microbiota (Kazemian et al., 2020), was involved in PAH pathophysiology by inducing inflammation and subsequently pulmonary vascular remodeling (Thenappan et al., 2011). Common bile duct ligation in rats resulted in elevated serum endotoxin levels and pulmonary vascular disease (Thenappan et al., 2011; Ranchoux et al., 2017). More directly, increased serum endotoxin concentration in the portal vein was also observed in MCT-PAH rats (Ranchoux et al., 2017). These studies suggest that changes of the gut microbial metabolites arising from gut dysbiosis may contribute to the initiation, maintenance, or aggravation of pulmonary vascular adverse remodeling in PAH.
The Causal Relationship Between Gut Microbiota and PAH
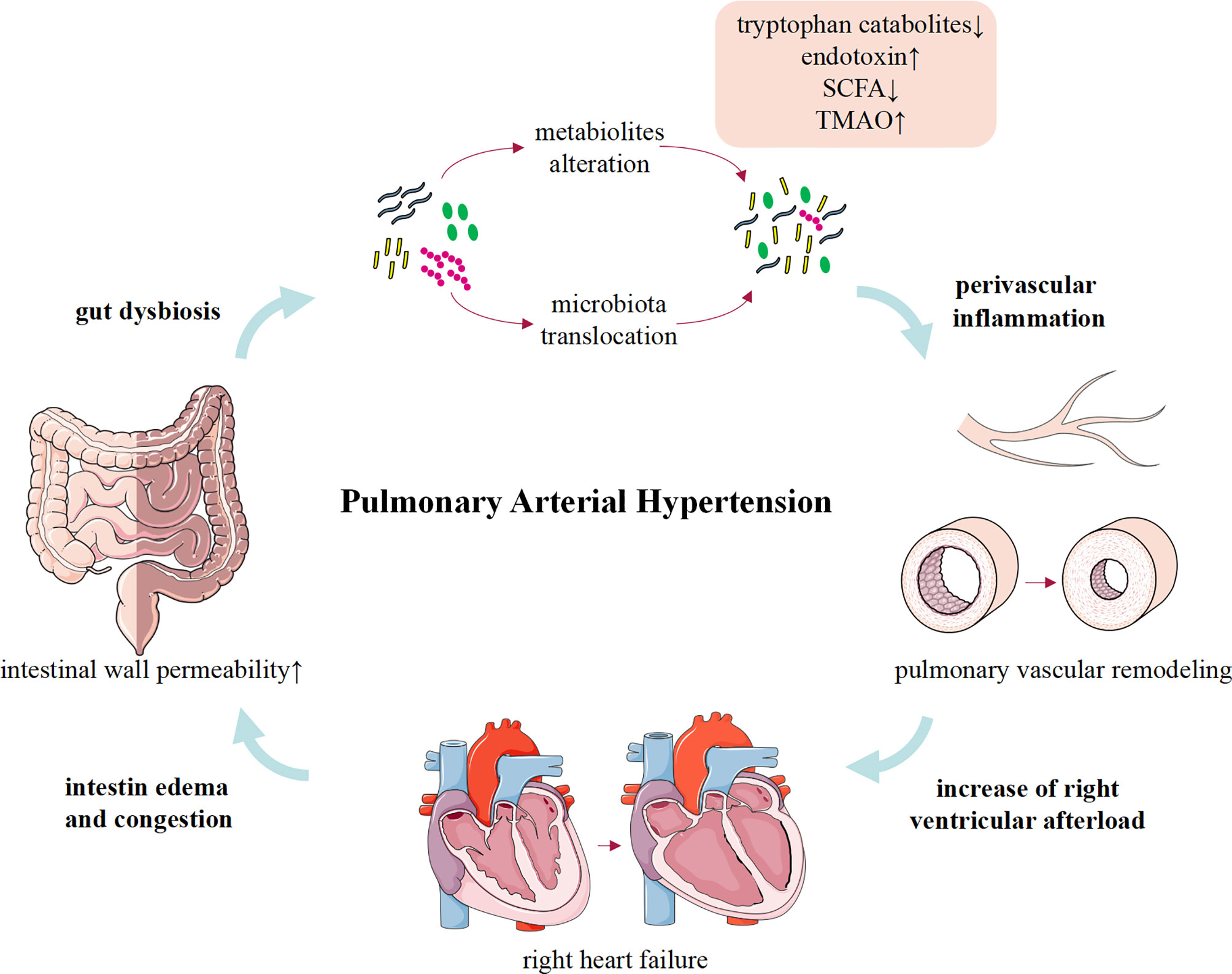
Although limited to rare human studies, convincing animal data linking gut dysbiosis to PAH have been available (Figure 1). Gut microbiota can modulate the host’s immune condition, and alterations of gut microbiota composition and the activation of inflammation support that gut dysbiosis involves in the inflammation process of PAH (Rabinovitch et al., 2014; Marsh et al., 2018; Sweatt et al., 2019). Meanwhile, PAH is a complex clinical syndrome and gut dysbiosis may be a concomitant symptom of RHF or other pathogenic factors. Therefore, more comprehensive research studies are urgently required to determine whether the gut dysbiosis is a cause or merely a consequence of PAH.
Gut Dysbiosis and Inflammation in PAH
Despite lacking in direct evidence to elucidate that gut microbiota contributes to the development of PAH, gut dysbiosis causes pathophysiological changes similar to PAH. Intriguingly, an important feature of pulmonary arterial lesions in patients with PAH and experimental models is varying degrees of perivascular inflammatory infiltration. Although the mechanisms that trigger and/or aggravate the inflammatory response are not fully understood, inflammation precedes vascular lesion supports that immune dysregulation is responsible for vascular remodeling in PAH (Tamosiuniene et al., 2011). Increasing evidence suggested that a healthy gut microbial community contributes to maintaining the host immune homeostasis (Kim et al., 2016; Clavel et al., 2017), and gut dysbiosis is associated with a variety of inflammation-related diseases, including atherosclerosis (Jonsson and Bäckhed, 2017; Yoshida et al., 2018; Wu et al., 2021), obesity (Yoshimoto et al., 2013; Canfora et al., 2019; Arnoriaga-Rodríguez et al., 2020), multiple sclerosis (Kadowaki et al., 2019; Kadowaki and Quintana, 2020; Pröbstel et al., 2020), chronic transplantation rejection (Wu et al., 2020), autoimmune diseases (Manfredo Vieira et al., 2018; Fine et al., 2020; McPherson et al., 2021; Pandey et al., 2021), and cancers (Zhu et al., 2020; McPherson et al., 2021). Considering that inflammation is a crucial factor in the initiation and progress of PAH (Almodovar et al., 2011; Stacher et al., 2012; Graham et al., 2013; Simonneau et al., 2013; Saito et al., 2017; Qian et al., 2019). Recently, accumulating animal and clinical evidence reveals that gut dysbiosis may play an important role in inducing or aggravating the inflammatory response in PAH via the following aspects. First, gut dysbiosis disrupts gut barrier function and migrates gut microbiota into the host circulation triggering the immunoreaction (Manfredo Vieira et al., 2018; Fine et al., 2020). Second, gut microbiota participates in the regulation of host immune response by governing the metabolism of endotoxin, SCFAs, tryptophan, and TMA/TMAO (Thenappan et al., 2019).
The intestinal mucosal barrier maintains homeostasis by preventing pathogenic microorganisms or toxins from entering other tissues, organs, or blood (Citi, 2018). A healthy gut microbial community is beneficial to maintain normal intestinal barrier, whereas gut dysbiosis impaired the integrity of barrier function (Castillo et al., 2019). Intestinal pathological examination in MCT-PAH rats showed significantly increased intestinal fibrosis and muscular thickness, decreased goblet cells, and shortened villus length. Meanwhile, plasma intestinal fatty acid binding protein (I-FABP) in MCT-PAH rats increased by 2.3 times compared with healthy rats (Sharma et al., 2020a). This intriguing study firstly identified that MCT-PAH is associated with impairment of intestinal barrier function and increased intestinal permeability. All these changes significantly affect the host–microbiota interaction, which may trigger a host immune response and destroy the homeostasis of the gut-lung axis. Furthermore, these pathological changes may also provide a suitable growth environment for pathogenic microbiota and produce a variety of pro-inflammatory substances, consequently affecting the development of PAH. However, these data need to be validated in more patients with PAH and animal models in the future.
With the alteration of gut microbial composition and the increase of intestinal permeability, endotoxins translocate into the host circulation through the intestinal wall and cause metabolic endotoxemia (Bowser et al., 2020). Previous studies demonstrated that serum endotoxin and sCD14 levels were significantly increased in patients with PAH and MCT-treated rats (Ranchoux et al., 2017), which promoted immune response and pulmonary vascular remodeling via activation of the Toll-like Receptor 4 (TLR4)/nuclear factor kB (NF-kB) inflammatory signaling pathway and confirmed intestinal bacterial translocation and macrophage activation in PAH (Perros et al., 2011; Bauer et al., 2014; Ranchoux et al., 2017). Accordingly, depleted macrophages or targeted therapy improved bacterial translocation and pulmonary vascular remodeling (Thenappan et al., 2011; Ranchoux et al., 2017). These studies suggest that gut dysbiosis, impaired barrier function, and elevated circulating endotoxin levels may be important pathways that trigger the inflammatory response of PAH.
Gut microbiota produce a variety of metabolites, and their role in regulating host metabolic balance has been confirmed in past descades (Tilg et al., 2020). SCFAs (such as acetate, propionate, and butyrate), tryptophan metabolites, TMA/TMAO, and bile acids involved in the pathogenesis of PAH have been extensively studied. SCFAs, the end products of microbial fermentation, exert anti-inflammatory immunomodulatory effects by activating G protein–coupled receptor or inhibiting histone deacetylase (Kaisar et al., 2017; Ratajczak et al., 2019; Zhan et al., 2019). Previous studies demonstrated that SCFA-producing bacteria and SCFAs were significantly reduced in patients with PAH and rodent models (Callejo et al., 2018; Kim et al., 2020). Although direct data remain scarce, these results suggest that gut dysbiosis leads to the reduction of SCFAs and exacerbates the inflammatory response and PAH.
Indole, a tryptophan catabolite synthesized by intestinal bacterial possessing tryptophanase, was also reported to play important roles in inflammation and immune tolerance via inhibiting TNF-αmediated NF-κB activation, expression of IL-8, and increasing expression of the anti-inflammatory IL-10 (Bansal et al., 2010). In addition, indole also plays a beneficial effect on increasing the expression of epithelial junction complex molecules and on improving intestinal epithelial cell and barrier function (Bansal et al., 2010; Shimada et al., 2013). It is intriguing that indole is produced by various commensal Gram-positive and Gram-negative bacteria (Lee and Lee, 2010; Lee et al., 2015), and the amount of several indole-producing bacteria, such as Escherichia coli (Lee et al., 2007), Lactobacillus strain (Natividad et al., 2018; Sharma et al., 2020b), Prevotella (Sanada et al., 2020; Sharma et al., 2020b), and Bacteroidetes (Callejo et al., 2018; Sharma et al., 2020a), is extremely abnormal in patients with PAH and rodent models. In view of this, we conclude that the adverse effects of abnormal inflammation and immune response caused by gut dysbiosis in PAH are partially mediated by indoles. Recent study using metagenomic and linear discriminant analysis effect size analysis observed increase in Streptococcus, Coprococcus, and bacterial tryptophan biosynthesis in patients with PAH (Kim et al., 2020). Previous studies have shown that tryptophan hydroxylase 1 inhibitor can ameliorate pulmonary vascular pathology (MacLean, 2007). These findings suggest gut microbiome affect PAH by regulating the metabolism of tryptophan and inflammation.
TMA is synthesized by gut microbes using choline, carnitine, and other food ingredients and is converted to TMAO in the liver, which contributes to vascular endothelial dysfunction via inducing inflammation and oxidative stress (Li T et al., 2017). Previous studies have confirmed that TMAO was actively participated in the development of various vascular disease (Gregory et al., 2015; Senthong et al., 2016; Li XS et al., 2017), and the level is affected by the composition and diversity of gut microbiota. TMA/TMAO-producing bacteria, such as Clostridium, Desulfovibrio, Enterobacter, Escherichia, Klebsiella, Pseudomonas, Rothia, Prevotella, Clostridium, Staphylococcus, Streptococcus, Citrobacter, and Collinella, were significantly increased in patients with PAH, which were negatively correlated with TMA/TMAO production (Kim et al., 2020). Intriguingly, circulating TMAO was elevated in severe patients with PAH and MCT-treated rats compared to healthy control and hypoxia-induced mouse models (Huang et al., 2022), and the reason for this difference remains to be further elucidated (Aldred, 2022). In addition, the study also confirmed that TMAO promotes the proliferation and migration of PASMCs via upregulating inflammatory factors secretion from macrophages, and reducing the production of TMAO with 3,3-dimethyl-1-butanol (DMB) partially alleviated pulmonary vascular remodeling by reducing inflammatory factors (Huang et al., 2022). This indicates that the inflammatory pathway involved in TMAO may directly contribute to the pathogenesis of PAH.
Gut Dysbiosis and RHF in PAH
RHF is a serious complication in patients with end-stage PAH and suggests a poor prognosis. Available data indicated that intestinal morphology and permeability were altered in patients with HF (Sandek et al., 2007; Arutyunov et al., 2008). Patients with HF may present with intestinal wall thickening, intestinal wall edema, and impaired barrier function (Sandek et al., 2008). While decreased cardiac output and activation of RAAS in HF results in adaptive redistribution of blood between different organs to satisfy perfusion of vital organs, which further restricts intestinal blood flow. Interestingly, Sandek et al. and Krack et al. demonstrated that restricted intestinal blood flow in patients with HF results in abnormal growth of intestinal bacteria and impaired intestinal barrier function (Krack et al., 2005; Sandek et al., 2008). In addition, previous studies have identified the specific characteristics of intestinal microbiome profile in patients with HF, namely, decreased intestinal microbial diversity (mainly driven by Blautia and Collinsella) and downregulation of important microflora, as well as the overgrowth of intestinal pathogenic bacteria such as Campylobacter, Shigella, Salmonella, Yersinia enterocolitica, and Candida species (Pasini et al., 2016; Luedde et al., 2017). Significantly, microbial metabolites such as endotoxin, SCFAs, and TMAO are affected by gut dysbiosis, which were consistent with changes in intestinal microbes and were also observed in PAH animal models and patients (Kim et al., 2020). On the one hand, bacterial translocation and gut dysbiosis lead to persistent low-grade chronic inflammation in patients with HF (Dick and Epelman, 2016; Tang et al., 2017). On the other hand, the toxic effects of microbial metabolites, such as TMAO, may directly lead to cardiac mitochondrial dysfunction, cardiac hypertrophy, and fibrosis (Organ et al., 2016; Makrecka-Kuka et al., 2017; Li et al., 2019). Therefore, gut dysbiosis and HF are mutually progressive “vicious circle”. Together, these changes in intestinal pathology, microbiota, and barrier function facilitate the translocation of bacteria and/or metabolites and subsequently systemic inflammation, which increase susceptibility to HF. In future studies, it is necessary to further clarify the relationship between gut dysbiosis and HF in PAH by matching the pathophysiological status of enrolled subjects.
Pathogenic Factor and Gut Dysbiosis in PAH
Apart from idiopathic PAH (IPAH), PAH is always associated with other conditions, including infectious disorders [such as human immunodeficiency virus (HIV) (Vujkovic-Cvijin and Somsouk, 2019) and schistosomiasis infection (Jenkins et al., 2018; Floudas et al., 2019; Hu et al., 2020)], portal hypertension (Yokoyama et al., 2020), and connective tissue diseases [systemic lupus erythematosus (SLE) (Luo et al., 2018), multiple sclerosis (Chen et al., 2016), and rheumatoid arthritis (RA) (Lerner and Matthias, 2015; Manasson et al., 2018)], and these primary diseases are usually accompanied with gut dysbiosis. The potential association between the primary disease and gut dysbiosis in PAH needs to be investigated. Rexhaj and colleagues analyzed the gut microbiota of fecal in rats with systemic hypertension and MCT-PAH and found that Bifidobacterium and Streptococcus were significantly increased in hypertension group, whereas the number of Spirillum, Rosa, and akermansia was increased in MCT-PAH group (Sharma et al., 2020a). It suggested that the composition of gut microbiota in diverse diseases may present unique characteristics. Distinguish disease related unique gut microbiota alteration might be beneficial in identifying types of PAH.
Schistosomiasis-associated PAH (Sch-PAH) is a serious complication of chronic hepatosplenic schistosomiasis, which is related to inflammation and transforming growth factor (TGF) signaling pathway, and histopathologic features are similar to that of IPAH (Lapa et al., 2009; Knafl et al., 2020). Recent studies reported that Schistosoma mansoni, the primary etiology of Sch-PAH, could regulate the composition and diversity of intestinal microbiota and immunity (Jenkins et al., 2018; Floudas et al., 2019). Analysis of fecal microbiota of Schistosoma mansoni–infected mice revealed that the abundance of Alistipes, Bacteroides, Parabacteroides, and Helicobacter was increased, whereas the abundance of Lactobacillus was decreased. In addition to dysbacteria, Schistosoma mansoni also altered the metabolic signature of infected mice, including increased glycolysis and decreased microbial-related metabolites (e.g., SCFA), which well known to play important roles in PAH (Wang et al., 2004).
HIV infection increased the risk of a variety of infectious and non-infectious pulmonary conditions (Almodovar et al., 2011) and is a well-established risk factor for PH (Opravil and Sereni, 2008; Hai-long et al., 2013). Aberrant inflammation and immune activation impaired gastrointestinal barrier function, and then, intestinal bacteria and bacterial products were transferred to the systemic circulation and further promote inflammation and disease progression, which plays an important role in morbidity and mortality in patients with HIV (Marchetti et al., 2013; Deeks et al., 2013). Numerous studies have confirmed that a series of changes in the composition of the gut microbiome in HIV-infected patients (Ellis et al., 2011; Marchetti et al., 2013). Gori and colleagues demonstrated that the fecal microbiota of HIV-infected patients had higher levels of opportunistic pathogens (e.g., P. aeruginosa and C. albicans) and lower levels of protective bacteria (e.g., lactobacilli and bifidobacteria) compared with healthy individuals (Gori et al., 2008), similar to previous preclinical and clinical studies on PAH. In addition, HIV infection–related hypercoagulable, endothelial damage, and dysfunction also contribute to the development of PAH. However, the pathogenesis of intestinal flora in HIV-associated PH remains to be elucidated constantly and further investigated.
SLE is complex autoimmune diseases. Intestinal microbiota dysbiosis has been observed in patients with SLE and lupus models. Luo and colleagues reported that the composition and diversity of intestinal microorganisms in lupus-prone mice (NZB/W F1) and patients were considerably altered and correlated with disease severity (Luo et al., 2018). In patients with SLE, some Gram-negative bacteria, such as Proteobacteria, were significantly increased. Whereas different from reduced F/B ratio in patients with SLE in remission in a previous study (Hevia et al., 2014), no significantly difference was observed in the F/B ratio between patients with and without SLE in this study. In addition, increased bacterial diversity was also confirmed in lupus-prone MRL/LPR and SWRxNZB F1 (SNF1) mouse models, characterized by a decrease in Lactobacillaceae and an increase in Rikenellaceae family or Lachnospiraceae, which was associated with lupus-like symptoms (Zhang et al., 2014; Johnson et al., 2015).
In short, intestinal dysbiosis is manifested in various primary diseases, and correction of intestinal dysbiosis is beneficial to alleviate these diseases. However, intestinal microbiota is easily affected by various factors, such as health status, diet, and drugs. Therefore, it is difficult to determine the contribution of these primary diseases to intestinal dysbiosis, and the role of dysbiosis in PAH progress needs to be further explored.
Future Prospects of Intestinal Microbiota in PAH
Gut microbiota and its metabolites are closely related to host homeostasis (Fan and Pedersen, 2021). Therefore, identifying specific alteration of gut microbial composition and function may provide potential diagnostic and therapeutic approaches. Recent studies noted that the composition of intestinal flora in PAH rats is significantly different from that of healthy control subjects and systemic hypertension rats (Callejo et al., 2018; Sharma et al., 2020a). In Seungbum’s study, patients with PAH were predicted with 85% accuracy according to the composition of gut microbiota. This evidence suggested that integrating existing studies and dynamically monitoring the composition of gut microbiota in large-scale PAH population is expected to screen out valuable biomarkers for clinical diagnosis based on the changes of gut microbiota. However, its repeatability must be confirmed in future research to improve the stability and feasibility of intestinal flora as a diagnostic marker of PAH.
The accumulating evidence demonstrated that modification of the gut microbiota can suppress the progression of several chronic diseases (Olofsson and Bäckhed, 2022; Schupack et al., 2022), including metabolic diseases (e.g., diabetes, obesity), cardiovascular diseases (e.g., atherosclerosis and hypertension) and tumors. Considering the contribution to PAH, intestinal flora has emerged as a potential therapeutic target for PAH (Sanada et al., 2020). Numerous factors such as age, dietary patterns, drugs, genetics, and environmental microbes have profound effects on host microbiota, and naturally regulating the composition of gut microbiome through diet, antibiotics, probiotics, or fecal microbiota transplantation (FMT) may be an effective option for PAH.
The adverse effects of high-fat diet on intestinal microbiome and metabolites have been demonstrated in a host of diseases, and high fat aggravates pulmonary vascular abnormalities and right ventricular hypertrophy (RVH) in apolipoprotein E–null mice (Lawrie et al., 2011; Wallström et al., 2012). Specific dietary patterns, such as foods rich in dietary fiber and antioxidant food components, play important roles in maintaining gut homeostasis and cardiovascular protection by improving lipid profiles, reducing inflammation, and modulating gut microbiota and its metabolites (e.g., SCFAs) (Gill et al., 2018; Hills et al., 2019; Martin-Gallausiaux et al., 2020; Zhou et al., 2021). For example, the Mediterranean diet was associated with improved health status and predicted future cardiovascular disease risk independently of traditional risk factors (Li et al., 2020). In addition, nutritional alterations such as iron deficiency are prevalent in PAH and may trigger or exacerbate disease progression (Callejo et al., 2020). Available evidence indicates that dietary components such as polyphenols alleviate disease progression in animal models of PAH. However, the effects of dietary nutrition intervention and timing choices targeting intestinal microbiota on PAH remain to be further confirmed (Aldred, 2022).
Antibiotics are prevalent anti-infection drugs in clinical practice that can collaterally alter the gut microbial profiles (Zimmermann et al., 2021). Sanada et al. observed that antibiotic intervention could alter the composition of intestinal flora and alleviated pulmonary vascular remodeling and RVH index in Su/Hx-rats (Sanada et al., 2020). Notably, antibiotics are strongly related to microbial dysbiosis (Blaser, 2016), such as fungal enrichment, and strict clinical indications limit the use of antibiotics in PAH to a certain extent.
Evidence has confirmed that probiotics, living microorganisms, ameliorate certain disease (e.g., irritable bowel syndrome and inflammatory bowel disease) by replenishing depleted host microbes, which provide reference for the treatment of PAH with probiotics (Ford et al., 2014; Olofsson and Bäckhed, 2022). Wedgwood and colleagues demonstrated that intervention with Lactobacillus reuteri DSM 17938 in PNGR rats reduced α-diversity of gut microbiota and prevented PNGR-associated PH and RVH (Wedgwood et al., 2020a). Sharma et al. first confirmed that overexpressed ACE2 prevents intestinal flora and intestinal pathology related to PH, and transplantation of feces of mice with overexpressed ACE2 was beneficial to correct these pathological changes and relieve the increased right ventricular systolic pressure and RVH caused by hypoxia in wild-type mice (Sharma et al., 2020b). The aforementioned studies suggest that probiotics and/or FMT may be a promising complementary therapy for PAH, and the complementarity between donor and recipient of fecal bacteria may further improve the therapeutic efficacy. Recently, Daphne et al. achieved a Food and Drug Administration–approved phase I safety and feasibility trial of microbiota transplantation for PAH, which is the first step to explore FMT as a treatment for patients with PAH. If FMT is both safe and feasible in PAH, then future clinical studies could further examine its efficacy (Moutsoglou, 2022). In addition, gut dysbiosis results in corresponding changes in bacterial metabolites, and previous studies have demonstrated that supplementing exogenous SCFAs (Maniar et al., 2018; Karoor et al., 2021) or reducing TMAO (Huang et al., 2022) can relieve pulmonary vascular disease, suggesting that correction of abnormal metabolites may be another potential therapeutic strategy for PAH.
Conclusion
PAH is a clinical syndrome involving multiple systems, and accumulating evidence demonstrated that intestinal microbiota plays an important role in PAH. Targeting intestinal microbiota, such as probiotics and FMT, has profoundly therapeutic potential in PAH and may be an essential complement to targeted vasodilation therapies. It is remarkably, however, that the improvement of intestinal microflora in pulmonary vascular remodeling is based primarily on animal experiments, and there is still a long way to go for intestinal microbiota as a therapeutic target in clinical practice. First, the causal relationship between gut dysbiosis and changes in metabolites and PAH awaits further determined. Second, well-designed large-scale clinical studies are necessary to verify the role of intestinal microbiota in PAH. In conclusion, the exploration of intestinal flora and its function provides a new perspective for examining the pathogenesis and treatment strategies of PAH.
Author Contributions
PW and TZ drafted and contributed equally to this manuscript. ZT, SC, and ZF revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldred, M. A. (2022). Food For Thought: The Emerging Role Ofintestinal Microbiota in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 66 (4), 361–62. doi: 10.1165/rcmb.2022-0005ED
Almodovar, S., Hsue, P. Y., Morelli, J., Huang, L., Flores, S. C. (2011). Pathogenesis of HIV-Associated Pulmonary Hypertension: Potential Role of HIV-1 Nef. Proc. Am. Thorac. Soc. 8 (3). doi: 10.1513/pats.201006-046WR
Archer, S. L., Gomberg-Maitland, M., Maitland, M. L., Rich, S., Garcia, J. G. N., Weir, E. K. (2008). Mitochondrial Metabolism, Redox Signaling, and Fusion: A Mitochondria-ROS-HIF-1a-Kv1.5 O2 -Sensing Pathway at the Intersection of Pulmonary Hypertension and Cancer. Am. J. Physiol. Heart CirculatoryPhysiol 294 (2), H570–H578. doi: 10.1152/ajpheart.01324.2007
Arnoriaga-Rodríguez, M., Mayneris-Perxachs, J., Burokas, A., Contreras-Rodríguez, O., Blasco, G., Coll, C., et al. (2020). Obesity Impairs Short-Term and Working Memory Through Gut Microbial Metabolism of Aromatic Amino Acids. Cell Metab., 32 (4), 548–560.e7. doi: 10.1016/j.cmet.2020.09.002
Arutyunov, G. P., Kostyukevich, O. I., Serov, R. A., Rylova, N. V., Bylova, N. A. (2008). Collagen Accumulation and Dysfunctional Mucosal Barrier of the Small Intestine in Patients With Chronic Heart Failure. Int. J. Cardiol., 125 (2), 240–245. doi: 10.1016/j.ijcard.2007.11.103
Bansal, T., Alaniz, R. C., Wood, T. K., Jayaraman, A. (2010). The Bacterial Signal Indolen Increases Epithelial-Cell Tight-Junction Resistance and Attenuates Indicators of Inflammation. Proc. Natl. Acad. Sci., 107 (1), 228–233. doi: 10.1073/pnas.0906112107
Bauer, E. M., Chanthaphavong, R. S., Sodhi, C. P., Hackam, D. J., Billiar, T. R., Bauer, P. M. (2014). Genetic Deletion of Toll-Like Receptor 4 on Platelets Attenuates Experimental Pulmonary Hypertension. Circ. Res. 114 (10), 1596–1600. doi: 10.1161/CIRCRESAHA.114.303662
Blaser, M. J. (2016). Antibiotic Use and Its Consequences for the Normal Microbiome. Science 352 (6285), 544–545. doi: 10.1126/science.aad9358
Bowser, S. M., McMillan, R. P., Boutagy, N. E., Tarpey, M. D., Smithson, A. T., Osterberg, K. L., et al. (2020). Serum Endotoxin, Gut Permeability and Skeletal Muscle Metabolic Adaptations Following a Short Term High Fat Diet Inhumans. Metabol. Clin. Exp. 103, 154041–154041. doi: 10.1016/j.metabol.2019.154041
Brown, J. M., Hazen, S. L. (2018). Microbial Modulation of Cardiovascular Disease. Nat. Rev. Microbiol. 16 (3), 171–181. doi: 10.1038/nrmicro.2017.149
Callejo, M., Barberá, J. A., Duarte, J., Perez-Vizcaino, F. (2020). Impact of Nutrition on Pulmonary Arterial Hypertension. Nutrients 12 (1), 169. doi: 10.3390/nu12010169
Callejo, M., Mondejar-Parreño, G., Barreira, B., Izquierdo-Garcia, J. L., Morales-Cano, D., Esquivel-Ruiz, S., et al. (2018). Pulmonary Arterial Hypertension Affects Therat Gut Microbiome. Sci. Rep. 8 (1). doi: 10.1038/s41598-018-27682-w
Canfora, E. E., Meex, R. C. R., Venema, K., Blaak, E. E. (2019). Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15 (5), 261–273. doi: 10.1038/s41574-019-0156-z
Cani, P. D., Bibiloni, R., Knauf, C., Waget, A., Neyrinck, A. M., Delzenne, N. M., et al. (2008). Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes, 1470–1481, 57 (6). doi: 10.2337/db07-1403
Caruso, P., Dunmore, B. J., Schlosser, K., Schoors, S., Dos Santos, C., Perez-Iratxeta, C., et al. (2017). Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension Via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 136 (25), 2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034
Castillo, D. J., Rifkin, R. F., Cowan, D. A., Potgieter, M. (2019). The Healthy Human Blood Microbiome: Factor Fiction? Front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00148
Chang, C., Lin, C., Lu, C., Martel, J., Ko, Y., Ojcius, D. M., et al. (2015). Ganoderm Lucidum Reduces Obesity in Mice by Modulating the Composition of the Gut Microbiota. Nat. Commun. 6 (1). doi: 10.1038/ncomms8489
Chen, J., Chia, N., Kalari, K. R., Yao, J. Z., Novotna, M., PazSoldan, M. M., et al. (2016). Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci. Rep. 6 (1). doi: 10.1038/srep28484
Citi, S. (2018). Intestinal Barriers Protect Against Disease. Sci. (American Assoc. Adv. Sci.) 359 (6380), 1097–1098. doi: 10.1126/science.aat0835
Clavel, T., Gomes-Neto, J. C., Lagkouvardos, I., Ramer-Tait, A. E. (2017). Deciphering Interactions Between the Gut Microbiota and the Immune System Via Microbial Cultivation and Minimal Microbiomes. Immunol. Rev. 279 (1), 8–22. doi: 10.1111/imr.12578
Deeks, S. G., Tracy, R., Douek, D. C. (2013). Systemic Effects of Inflammation on Health During ChronicHiv Infection. Immunity 39 (4), 633–645. doi: 10.1016/j.immuni.2013.10.001
Dick, S. A., Epelman, S. (2016). Chronic Heart Failure and Inflammation. Circ. Res. 119 (1), 159–176. doi: 10.1161/CIRCRESAHA.116.308030
Drake, K. M., Dunmore, B. J., McNelly, L. N., Morrell, N. W., Aldred, M. A. (2013). Correction of Nonsense Bmpr2 Andsmad9 Mutations by Ataluren I Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 49 (3), 403– 409. doi: 10.1165/rcmb.2013-0100OC
Ellis, C. L., Ma, Z., Mann, S. K., Li, C., Wu, J., Knight, T. H., et al. (2011). Molecular Characterization of Stool Microbiota in HIV-Infected Subjects by Panbacterial and Order-Level 16s Ribosomal DNA (Rdna) Quantification and Correlations With Immune Activation. JAIDS J. Acquir. Immune Defic. Syndr. 57 (5), 363– 409. doi: 10.1097/QAI.0b013e31821a603c
Evans, J. D. W. M., Girerd, B. P., Montani, D. M., Wang, X. P., Galiè, N. M., Austin, E. D. M., et al. (2016). Bmpr2 Mutations and Survival in Pulmonary Arterial Hypertension: An Individual Participant Data Meta-Analysis. Lancet Respir. Med. 4 (2), 129–137. doi: 10.1016/S2213-2600(15)00544-5
Fan, Y., Pedersen, O. (2021). Gut Microbiota in Human Metabolic Healthand Disease. Nat. Rev. Microbiol. 19 (1), 55–71. doi: 10.1038/s41579-020-0433-9
Fine, R. L., Manfredo Vieira, S., Gilmore, M. S., Kriegel, M. A. (2020). Mechanisms and Consequences of Gut Commensal Translocation in Chronic Diseases. Gut Microbes 11 (2), 217–230. doi: 10.1080/19490976.2019.1629236
Floudas, A., Aviello, G., Schwartz, C., Jeffery, I. B., O'Toole, P. W., Fallon, P. G. (2019). ). Schistosoma Mansoni Worm Infection Regulates the Intestinal Microbiota and Susceptibility to Colitis. Infect. Immun. 87 (8), e00275–e00219. doi: 10.1128/IAI.00275-19
Ford, A. C., Quigley, E. M., Lacy, B. E., Lembo, A. J., Saito, Y. A., Schiller, L. (2014). Efficacy of Prebiotics, Probiotics, and Synbiotics in Chronic Idiopathic Constipation: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 1091562 (10), 1547–1561. doi: 10.1038/ajg.2014.202
Gill, P. A., van Zelm MC, M. (2018). Review Article:Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Aliment. Pharmacol. Ther. 48 (1), 15–34. doi: 10.1111/apt.14689
Goel, R., Kim, S., Rigatto, K., Shapiro, B., Ray, J., Qi, Y., et al. (2017). Abstract 20620: Increased Gut Dysbiosis and Leakiness Inpatients With Pulmonary Arterial Hypertension A20620–A20620 (suppl_1). doi: 10.1161/circ.136.suppl_1.20620
Gordon, J. I., Ley, R. E., Klein, S., Turnbaug, P. J. (2006). Microbial Ecology Human GutMicrobes Associated With Obesity. Nat. (London) 444 (7122), 1022–1023. doi: 10.1038/4441022a
Gori, A., Tincati, C., Rizzardini, G., Torti, C., Quirino, T. (2008). Early Impairment of Gut Function and Gut Flora Supporting a Role for Alteration of Gastrointestinal Mucosa in Human Immunodeficiency Virus PathogenesisJ. Clin. Microbiol 46, 2, 757–758. doi: 10.1128/JCM.01729-07
Graham, B. B., Chabon, J., Kumar, R., Kolosionek, E., Gebreab, L., Debella, E., et al. (2013). Protective Role of IL-6 in Vascular Remodeling Inschistosoma Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 49 (6), 951–959. doi: 10.1165/rcmb.2012-0532OC
Gregory, J. C., Buffa, J. A., Org, E., Wang, Z., Levison, B. S., Zhu, W., et al. (2015). Transmission of Atherosclerosis Susceptibility With Gut Microbial Transplantation. J. Biol. Chem. 290 (9), 5647–5660. doi: 10.1074/jbc.M114.618249
Hai-long, D., Yi-bing, L. U., Xue-feng, G., Zhi-cheng, X., Ming, Z., Wei-hua, Z. (2013). Bosentan and Antiretroviral Therapy in an HIV/HBV/HCV Coinfected Chinese Patient With Hiv-Related Pulmonary Arterial Hypertension. Chin.Med. J. 126 (6), 1197–1198. doi: 10.3760/cma.j.issn.0366-6999.20123277
Henao-Mejia, J., Elinav, E., Jin, C., Hao, L., Mehal, W. Z., Strowig, T., et al. (2012). Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 482 (7384), 179–185. doi: 10.1038/nature10809
Hevia, A., Milani, C., López, P., Cuervo, A., Arboleya, S., Duranti, S., et al. (2014). Intestinal Dysbiosis Associated With Systemic Lupus Erythematosus. mBio5 5), e01548–e01514. doi: 10.1128/mBio.01548-14
Hills, R. J., Pontefract, B. A., Mishcon, H. R., Black, C. A., Sutton, S. C., Theberge, C. R. (2019). Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 11 (7), 1613. doi: 10.3390/nu11071613
Hong, W., Mo, Q., Wang, L., Peng, F., Zhou, Y., Zou, W., et al. (2021). Changes in the Gut Microbiome and Metabolome in a Rat Model of Pulmonary Arterial Hypertension. Bioengineered 12 (1), 5173–5183. doi: 10.1080/21655979.2021.1952365
Huang, Y., Lin, F., Tang, R., Bao, C., Zhou, Q., Ye, K., et al. (2022). Gut Microbial Metabolite Trimethylamine N-Oxide Aggravates Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 66 (4), 452–460. doi: 10.1165/rcmb.2021-0414OC
Hu, Y., Chen, J., Xu, Y., Zhou, H., Huang, P., Ma, Y., et al. (2020). Alterations of Gut Microbiome and Metabolite Profiling in Mice Infected by Schistosoma Japonicum. Front. Immunol. 11. doi: 10.3389/fimmu.2020.569727
Humbert, M., Guignabert, C., Bonnet, S., Dorfmüller, P., Klinger, J. R., Nicolls, M. R., et al. (2019). Pathology and Pathobiology of Pulmonary Hypertension: State of the Art and Research Perspectives. Eur. Respir. J. 53 (1), 1801887. doi: 10.1183/13993003.01887-2018
Jenkins, T. P., Peachey, L. E., Ajami, N. J., MacDonald, A. S., Hsieh, M. H., Brindley, P. J., et al. (2018). Schistosoma Mansoni Infection Is Associated With Quantitative and Qualitative Modifications of the Mammalian Intestinal Microbiota. Sci.Rep 8 (1), 12072. doi: 10.1038/s41598-018-30412-x
Johnson, B. M., Gaudreau, M. C., Al-Gadban, M. M., Gudi, R. Vasu C.(2015).Impact of Dietary Deviation on Disease Progressionand Gut Microbiome Composition in Lupus-Prone Snf1 Mice. Clin. Exp. Immunol. 181 (2), 323–337. doi: 10.1111/cei.12609
Jonsson, A. L., Bäckhed, F. (2017). Role of Gut Microbiota in Atherosclerosis. Nat. Rev. Cardiol. 14 (2), 79–87. doi: 10.1038/nrcardio.2016.183
Kadowaki, A., Quintana, F. J. (2020). The Gut–CNS Axis in Multiple Sclerosis. Trends Neurosci., 622–634, 43 (8). doi: 10.1016/j.tins.2020.06.002
Kadowaki, A., Saga, R., Lin, Y., Sato, W., Yamamura, T. (2019). Gut Microbiota-Dependent Ccr9+Cd4+ T Cells Are Altered in Secondary Progressive Multiple Sclerosis. Brain 142 (4), 916–931. doi: 10.1093/brain/awz012
Kaisar, M. M. M., Pelgrom, L. R., van der Ham, A. J., Yazdanbakhsh, M., Everts, B. (2017). Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells Via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109a Signaling. Front. Immunol. 8. doi: 10.3389/fimmu.2017.0142
Karoor, V., Strassheim, D., Sullivan, T., Verin, A., Umapathy, N. S., Dempsey, E. C., et al. (2021). The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 22 (18). doi: 10.3390/ijms22189916
Kazemian, N., Mahmoudi, M., Halperin, F., Wu, J. C., Pakpour, S. (2020). Gut Microbiota and Cardiovascular Disease:Opportunities and Challenges. Microbiome 8 (1). doi: 10.1186/s40168-020-00821-0
Khan, M. T., Nieuwdorp, M., Bäckhed, F., Sahlgrenska, A., Wallenberg, L., et al. (2014). Microbial Modulation of Insuli Sensitivity. Cell Metab., 753–760, 20 (5). doi: 10.1016/j.cmet.2014.07.006
Kim, M., Qie, Y., Park, J., Kim, C. H. (2016). Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 20 (2), 202–214. doi: 10.1016/j.chom.2016.07.001
Kim, S., Rigatto, K., Gazzana, M. B., Knorst, M. M., Richards, E. M., Pepine, C. J., et al. (2020). Altered Gut Microbiome Profile in Patients With Pulmonary Arterialhypertension. Hypertension 75 (4), 1063–1071. doi: 10.1161/HYPERTENSIONAHA.119.14294
Knafl, D., Gerges, C., King, C. H., Humbert, M., Bustinduy, A. L. (2020). Schistosomiasis-Associated Pulmonary Arterial Hypertension: A Systematic Review. Eur. Respir. Rev. 29 (155), 190089. doi: 10.1183/16000617.0089-2019
Krack, A., Sharma, R., Figulla, H. R., Anker, S. D. (2005). The Importance of the Gastrointestinal System in the Pathogenesis of Heart Failure. Eur. Heart J. 26 (22), 2368–2374. doi: 10.1093/eurheartj/ehi389
Lapa, M., Dias, B., Jardim, C., Fernandes, C. J. C., Dourado, P. M. M., Figueiredo, M., et al. (2009). Cardiopulmonary Manifestations of Hepatosplenic Schistosomiasis. Circulation 119 (11), 1518–1523. doi: 10.1161/CIRCULATIONAHA.108.803221
Lawrie, A., Hameed, A. G., Chamberlain, J., Arnold, N., Kennerley, A., Hopkinson, K., et al. (2011). Paigen Diet–Fed Apolipoprotein E Knockout Mice Develop Severe Pulmonary Hypertension in an Interleukin-1–Dependent Manner. Am. J. Pathol. 179 (4), 1693–1705. doi: 10.1016/j.ajpath.2011.06.037
Lee, J., Jayaraman, A., Wood, T. K. (2007). Indole Is an Inter-Species Biofilm Signal Mediated by Sdia. BMC Microbiol. 7, 42. doi: 10.1186/1471-2180-7-42
Lee, J. H., Lee, J. (2010). Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 34 (4), 426–444. doi: 10.1111/j.1574-6976.2009.00204.x
Lee, J., Wood, T. K., Lee, J. (2015). Roles of Indole as an Interspecies an Interkingdom Signaling Molecule. Trends Microbiol. 23 (11), 707–718. doi: 10.1016/j.tim.2015.08.001
Lerner, A., Matthias, T. (2015). Rheumatoidarthritis-Celiac Disease Relationship: Joints Get That Gut Feeling. Autoimmun. Rev. 14 (11), 1038–1047. doi: 10.1016/j.autrev.2015.07.007
Li, T., Chen, Y., Gua, C., Li, X. (2017). Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats Through Vascular Inflammation and Oxidative Stress. Front. Physiol. 8. doi: 10.3389/fphys.2017.00350
Li, J., Guasch-Ferré, M., Chung, W., Ruiz-Canela, M., Toledo, E., Corella, D., et al. (2020). The Mediterranean Diet, Plasma Metabolome, and Cardiovascular Disease Risk Cardiovascular Disease Risk. Eur. Heart J. 41 (28), 2645–2656. doi: 10.1093/(eurheartj/ehaa209
Li, X. S., Obeid, S., Klingenberg, R., Gencer, B., Mach, F., Räber, L., et al. (2017). Gut Microbiota-Dependent Trimethylamine N-Oxide in Acute Coronary Syndromes: A Prognostic Marker for Incident Cardiovascular Events Beyond Traditional Risk Factors. Eur. Heart J. 38 (11), , 814–, 824. doi: 10.1093/eurheartj/ehw582
Li, Z., Wu, Z., Yan, J., Liu, H., Liu, Q., Deng, Y., et al. (2019). Gut Microbe-Derived Metabolite Trimethylamine N-Oxide Induces Cardiac Hypertrophy and Fibrosis. Lab. Invest. 99 (3), 346–357. doi: 10.1038/s41374-018-0091-y
Long, L., Ormiston, M. L., Yang, X., Southwood, M., Gräf, S., Machado, R. D., et al. (2015). Selective Enhancement of Endothelial Bmpr-Ii With Bmp9 Reverses Pulmonary Arterial Hypertension. Nat. Med. 21 (7), 777–785. doi: 10.1038/nm.3877
Luedde, M., Winkler, T., Heinsen, F., Rühlemann, M. C., Spehlmann, M. E., Bajrovic, A., et al. (2017). Heart Failure Is Associated With Depletion of Core Intestinal Microbiota. ESC Heart Fail 4 (3), 282–290. doi: 10.1002/ehf2.12155
Luo, X. M., Edwards, M. R., Mu, Q., Yu, Y., Vieson, M. D., Reilly, C. M., et al. (2018). Gut Microbiota in Human Systemic Lupus Erythematosus and a Mouse Model of Lupus. Appl. Environ. Microbiol. 84 (4). doi: 10.1128/AEM.02288-17
MacLean, M. R. (2007). Pulmonary Hypertension and the Seroton in Hypothesis: Where Are We Now? Int. J. Clin. Pract. 61, 27–31. doi: 10.1111/j.1742-1241.2007.01497.x
Makrecka-Kuka, M., Volska, K., Antone, U., Vilskersts, R., Grinberga, S., Bandere, D., et al. (2017). Trimethylamine N-Oxide Impairs Pyruvate and Fatty Acid Oxidation in Cardiac Mitochondria. Toxicol. Lett. 267, 32–38. doi: 10.1016/j.toxlet.2016.12.017
Manasson, J., Shen, N., Garcia Ferrer, H. R., Ubeda, C., Iraheta, I., Heguy, A., et al. (2018). Gut Microbiota Perturbations in Reactive Arthritisand Postinfectious Spondyloarthritis. Arthritis Rheumatol 70 (2), 242–254. doi: 10.1002/art.40359
Manfredo Vieira, S., Hiltensperger, M., Kumar, V., Zegarra-Ruiz, D., Dehner, C., Khan, N., et al. (2018). Translocation of a Gut Pathobiont Drives Autoimmunity in Mice and Humans. Sci. (American Assoc. Adv. Sci.) 359 (6380), 1156–1161. doi: 10.1126/science.aar7201
Maniar, K., Singh, V., Moideen, A., Bhattacharyya, R., Chakrabarti, A., Banerjee, D. (2018). Inhalational Supplementation of Metformin Butyrate: A Strategy for Prevention and Cure of Various Pulmonary Disorders. Biomed. Pharmacother. 107, 495–506. doi: 10.1016/j.biopha.2018.08.021
Marchetti, G., Tincati, C., Silvestri, G. (2013). Microbial Translocation in the Pathogenesis of HIV Infection and AIDS. Clini. Microbiol. Rev. 26 (1), 2–1. doi: 10.1128/CMR.00050-12
Maresca, A. (2015). Dna Methyltransferase 1 Mutations and Mitochondrial Pathology: Is Mtdna Methylated? Front. Genet. 6. doi: 10.3389/fgene.2015.00090
Marques, F. Z., Mackay, C. R., Kaye, D. M. (2018). Beyond Gut Feelings: How the Gu Microbiota Regulates Blood Pressure. Nat. Rev. Cardiol. 15 (1), 20–32. doi: 10.1038/nrcardio.2017.120
Marques, F. Z., Nelson, E., Chu, P., Horlock, D., Fiedler, A., Ziemann, M., et al. (2017). High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 135 (10), 964–977. doi: 10.1161/CIRCULATIONAHA.116.024545
Marsh, L. M., Jandl, K., Grünig, G., Foris, V., Bashir, M., Ghanim, B., et al. (2018). The Inflammatory Cell Landscape in the Lungsof Patients With Idiopathic Pulmonary Arterial Hypertension. Eur. Respir. J., 51 (1)1701214. doi: 10.1183/13993003.01214-2017
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P., Lapaque, N. (2020). Scfa: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 80 (1), 37–49. doi: 10.1017/S0029665120006916
Mayerhofer, C. C. K., Kummen, M., Holm, K., Broch, K., Awoyemi, A., Vestad, B., et al. (2020). Low Fibre Intake Is Associated With Gut Microbiota Alterations in Chronic Heart Failure. ESC Heart Fail 7 (2), 456–466. doi: 10.1002/ehf2.12596
McPherson, A. C., Pandey, S. P., Bender, M. J., Meisel, M. (2021). Systemic Immunoregulatory Consequences of Gut Commensal Translocation. Trends Immunol. 42 (2), 137–150. doi: 10.1016/j.it.2020.12.005
Momparler, R. L., Côté, S., Momparler, L. F., Idaghdour, Y. (2017). Inhibition of DNA and Histone Methylation by 5Aza-2′-Deoxycytidine (Decitabine) and 3-Deazaneplanocin-A on Antineoplastic Action and Gene Expression in Myeloid Leukemic Cells. Front. Oncol. 7. doi: 10.3389/fonc.2017.00019
Moutsoglou, M. (2022). 2021American Thoracic Society BEAR Cage Winning Proposal: Microbiome Transplantin Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 205 (1), 13–16. doi: 10.1164/rccm.202108-1833ED
Murphy, E. F., Cotter, P. D., Hogan, A., O'Sullivan, O., Joyce, A., Fouhy, F., et al. (2013). Divergent Metabolic Outcomes Arising From Targeted Manipulation of the Gu Microbiota in Diet-Induced Obesity. Gut 62 (2), 220–226. doi: 10.1136/gutjnl-2011-300705
Natividad, J. M., Agus, A., Planchais, J., Lamas, B., Jarry, A. C., Martin, R., et al. (2018). Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab., 737–749. e4, 28(5). doi: 10.1016/j.cmet.2018.07.001
Oliveira, A. C., Richards, E. M., Raizada, M. K. (2020). Pulmonary Hypertension: Pathophysiology Beyond the Lung. Pharmacol. Res. 151, 104518. doi: 10.1016/j.phrs.2019.104518
Olofsson, L. E., Bäckhed, F. (2022). The Metabolic Role and Therapeutic Potential of the Microbiome. Endocrine Rev. Endocr. Rev., bnac004. doi: 10.1210/endrev/bnac004
Opravil, M., Sereni, D. (2008). Natural History of HIV-Associated Pulmonary Arterial Hypertension: Trends in the HAART Era. AIDS (London) 22, S35–S40. doi: 10.1097/01.aids.0000327514.60879.47
Organ, C. L., Otsuka, H., Bhushan, S., Wang, Z., Bradley, J., Trivedi, R., et al. (2016). Choline Diet and Its Gut Microbe–Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload–Induced Heart Failure. Circ.: Heart Fail 9 (1). doi: 10.1161/CIRCHEARTFAILURE.115.002314
Pandey, S. P., Bender, M. J., McPherson, A. C. (2021). Loss of Tet2 in T Cells Drives Translocated Pathobiont Derived Aryl Hydrocarbon Receptor Agonist-Induced Tc1 Cell Autoimmune Hepatitis. Available at: https://ssrn.com/abstract=3942586http://dx.doi.org/10.2139/ssrn.3942586.
Pasini, E. M., Aquilani, R. M., Testa, C. M., Baiardi, P. P., Angioletti, S. M., Boschi, F. P., et al. (2016). Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC. Heart Failure 4 (3), 220–227. doi: 10.1016/j.jchf.2015.10.009
Perros, F., Lambrecht, B. N., Hammad, H. (2011). Tlr4 Signalling in Pulmonary Stromal Cells Is Critical for Inflammation and Immunity in the Airways. Respir. Res. 12 (1), 1–8. doi: 10.1186/1465-9921-12-125
Pröbstel, A. K., Zhou, X., Baumann, R., Wischnewski, S., Kutza, M., Rojas, O. L., et al. (2020). Gut Microbiota – Specific Iga+ B Cells Traffic to the CNS in Active Multiple Sclerosis. Sci. Immunol. 5 (53). doi: 10.1126/sciimmunol.abc7191
Qian, J., Li, M., Zhao, J., Wang, Q., Tian, Z., Zeng, X. (2019). Inflammation in SLE-PAH Good News or Not. Ann. Rheum Dis., 78(12), e135. doi: 10.1136/annrheumdis-2018-214605
Rabinovitch, M., Guignabert, C., Humbert, M., Nicolls, M. R. (2014). Inflammation and Immunity in the Pathogenesis of Pulmonary Arterial Hypertension. Circ. Res. 115 (1), 165–175. doi: 10.1161/CIRCRESAHA.113.301141
Ranchoux, B., Bigorgne, A., Hautefort, A., Girerd, B., Sitbon, O., Montani, D., et al. (2017). Gut-Lung Connection in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 56 (3), 402–405. doi: 10.1165/rcmb.2015-0404LE
Ratajczak, W., Rył, A., Mizerski, A., Walczakiewicz, K., Sipak, O., Laszczyńska, M. (2019). Immunomodulatory Potential of Gut Microbiome-Derived Short-Chai Fatty Acids (Scfas). Acta Biochim. Pol. 66 (1), 1–1. doi: 10.18388/abp.2018_2648
Rexhaj, E., Bloch, J., Jayet, P., Rimoldi, S. F., Dessen, P., Mathieu, C., et al. (2011). Fetal Programming of Pulmonary Vascular Dysfunction in Mice: Role Ofepigenetic Mechanisms. Am. J. Physiol. Heart Circulatory Physiol. 301 (1), H247–H252. doi: 10.1152/ajpheart.01309.2010
Saito, T., Miyagawa, K., Chen, S., Tamosiuniene, R., Wang, L., Sharpe, O., et al. (2017). Upregulation of Human Endogenous Retrovirus-K Is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension. Circulation 136 (20), 1920–1935. doi: 10.1161/CIRCULATIONAHA.117.027589
Sanada, T. J., Hosomi, K., Shoji, H., Park, J., Naito, A., Ikubo, Y., et al. (2020). Gut Microbiota Modification Suppresses the Development of PulmonaryArterial Hypertension in an SU5416/hypoxia Rat Model. Pulm. Circ. 10 (3), 204589402092914. doi: 10.1177/2045894020929147
Sandek, A., Bauditz, J., Swidsinski, A., Buhner, S., Weber-Eibel, J., von Haehling, S., et al. (2007). Altered Intestinal Function in Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 50 (16), 1561–1569. doi: 10.1016/j.jacc.2007.07.016
Sandek, A., Rauchhaus, M., Anker, S. D., von Haehling, S. (2008). The Emerging Role of the Gut in Chronic Heartfailure. Curr. Opin. Clin. Nutr. Metab. Care 11, 5, 632–639. doi: 10.1097/MCO.0b013e32830a4c6e
Schupack, D. A., Mars, R. A. T., Voelker, D. H., Abeykoon, J. P., Kashyap, P. C. (2022). The Promise of the Gut Microbiome as Partof Individualized Treatment Strategies. Nat. Rev. Gastroenterol. Hepatol. 19 (1), 7–25. doi: 10.1038/s41575-021-00499-1
Senthong, V., Wang, Z., Fan, Y., Wu, Y., Hazen, S. L., Tang, W. H. (2016). Trimethylamine N-Oxide and Mortality Riskin Patients With Peripheral Artery Disease. J. Am. Heart Assoc. 5 (10), e004237. doi: 10.1161/JAHA.116.004237
Sharma, R. K., Oliveira, A. C., Yang, T., Karas, M. M., Li, J., Lobaton, G. O., et al. (2020b). Gut Pathology and Its Rescue by ACE2 (Angiotensinconverting Enzyme 2) in Hypoxia-Induced Pulmonary Hypertension. Hypertension 761), 206–216. doi: 10.1161/HYPERTENSIONAHA.120.14931
Sharma, R. K., Oliveira, A. C., Yang, T., Kim, S., Zubcevic, J., Aquino, V., et al. (2020a). Pulmonary Arterial Hypertension-Associated Changes in Gut Pathology and Microbiota. ERJ Open Res., 00253– 02019, 6 (3). doi: 10.1183/23120541.00253-2019
Sharma, R. K., Yang, T., Oliveira, A. C., Lobaton, G. O., Aquino, V., Kim, S., et al. (2019). Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin Ii-Induced Hypertension. Circ.Res. 124 (5), 727–736. doi: 10.1161/CIRCRESAHA.118.313882
Shimada, Y., Kinoshita, M., Harada, K., Mizutani, M., Masahata, K., Kayama, H., et al. (2013). Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PloS One 8 (11), e80604– e80604. doi: 10.1371/journal.pone.0080604
Simonneau, G., Gatzoulis, M. A., Adatia, I., Celermajer, D., Denton, C., Ghofrani, A., et al. (2013). Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol. 62 (25), D34–D41. doi: 10.1016/j.jacc.2013.10.029
Stacher, E., Graham, B. B., Hunt, J. M., Gandjeva, A., Groshong, S. D., McLaughlin, V. V., et al. (2012). Modern Age Pathology of Pulmonary Arterial Hypertension. Am. J.Respir. Crit. Care Med. 186 (3), 261–272. doi: 10.1164/rccm.201201-0164OC
Sweatt, A. J., Hedlin, H. K., Balasubramanian, V., Hsi, A., Blum, L. K., Robinson, W. H., et al. (2019). Discovery of Distinct Immune Phenotypes Using Machine Learningin Pulmonary Arterial Hypertension. Circ. Res. 124 (6), 904–919. doi: 10.1161/CIRCRESAHA.118.313911
Tamosiuniene, R., Tian, W., Dhillon, G., Wang, L., Sung, Y. K., Gera, L., et al. (2011). Regulatory T Cells Limit Vascular Endothelial Injury and Prevent Pulmonary Hypertension. Circ. Res. 109 (8), 867–879. doi: 10.1161/CIRCRESAHA.110.236927
Tang, W. H. W., Kitai, T., Hazen, S. L. (2017). Gut Microbiota in CardiovascularHealth and Disease. Circ. Res. 120 (7), 1183–1196. doi: 10.1161/CIRCRESAHA.117.309715
Tang, W. H. W., Li, D. Y., Hazen, S. L. (2019). Dietary Metabolism, the Gut Microbiome, and Heart Failure. Nat. Rev. Cardiol. 1 6 (3), 137–154. doi: 10.1038/s41569-018-0108-7
Thenappan, T., Goel, A., Marsboom, G., Fang, Y., Toth, P. T., Zhang, H. J., et al. (2011). A Central Role for CD68(+) Macrophages in Hepatopulmonary Syndrome. Am. J. Respir. Crit. Care Med. 183 (8), 1080–1091. doi: 10.1164/rccm.201008-1303OC
Thenappan, T., Khoruts, A., Chen, Y., Weir, E. K. (2019). Can Intestinal Microbiota an Circulating Microbial Products Contribute to Pulmonary Arterial Hypertension? Am. J. Physiol. Heart Circulatory Physiol. 317 (5), H1093– H1101. doi: 10.1152/ajpheart.00416.2019
Thenappan, T., Ormiston, M. L., Ryan, J. J., Archer, S. L. (2018). Pulmonary Arterial Hypertension: Pathogenesis and Clinical Management. BMJ (Online), j5492–j5492, 360. doi: 10.1136/bmj.j5492
Tilg, H., Zmora, N., Adolph, T. E., Elinav, E. (2020). The Intestinal Microbiota Fuelling Metabolic Inflammation. Nat. Rev. Immunol. 20 (1), 40–54. doi: 10.1038/s41577-019-0198-4
Vujkovic-Cvijin, I., Somsouk, M. (2019). HIV and the GutMicrobiota: Composition, Consequences, and Avenues for Amelioration. Curr. HIV/AIDS Rep. 16 (3), 204–213. doi: 10.1007/s11904-019-00441-w
Wallström, P., Sonestedt, E., Hlebowicz, J., Ericson, U., Drake, I., Persson, M., et al. (2012). Dietary Fiber and Saturated Fat Intake Associations With Cardiovascular Disease Differ by Sex in the Malmö Diet and Cancercohort: A Prospective Study. PloS One 7 (2), e31637–e31637. doi: 10.1371/journal.pone.0031637
Wang, Y., Holmes, E., Nicholson, J. K., Cloarec, O., Chollet, J., Tanner, M., et al. (2004). Metabonomic Investigations in Mice Infected With Schistosoma Mansoni:An Approach for Biomarker Identification. Proc. Natl. Acad. Sci. - PNAS 101 (34), 12676–12681. doi: 10.1073/pnas.0404878101
Wedgwood, S., Gerard, K., Halloran, K., Hanhauser, A., Monacelli, S., Warford, C., et al. (2020b). Intestinal Dysbiosis and the Developing Lung: The Role of Toll-Like Receptor 4 in the Gut-Lung Axis. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00357
Wedgwood, S., Warford, C., Agvatisiri, S. R., Thai, P. N., Chiamvimonvat, N., Kalanetra, K. M., et al. (2020a). The Developing Gut-Lung Axis: Postnatal Growth Restriction, Intestinal Dysbiosis, and Pulmonary Hypertension in a Rodent Model. Pediatr. Res. 87 (3), 472–479. doi: 10.1038/s41390-019-0578-2
Wu, W., Ivanova, E. A., Orekhov, A. N. (2021). Gut Microbiome: A Possible Commo Therapeutic Target for Treatment of Atherosclerosis and Cancer. Semin. Cancer Biol. 70, 85–97. doi: 10.1016/j.semcancer.2020.06.017
Wu, H., Singer, J., Kwan, T. K., Loh, Y. W., Wang, C., Tan, J., et al. (2020). Gut Microbial Metabolites Induce Donor-Specific Tolerance of Kidney Allografts Through Induction of T Regulatory Cells by Short-Chain Fatty Acids. J. Am. Soc Nephrol. 31 (7), 1445–1461. doi: 10.1681/ASN.2019080852
Yang, T., Santisteban, M. M., Rodriguez, V., Li, E., Ahmari, N., Carvajal, J. M., et al. (2015). Gut Dysbiosis Is Linked to Hypertension. Hypertension. 65 (6), 1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315
Yokoyama, K., Tsuchiya, N., Yamauchi, R., Miyayama, T., Uchida, Y., Shibata, K., et al. (2020). Exploratory Research on the Relationship Betweenhuman Gut Microbiota and Portal Hypertension. Internal Med. 59 (17), 2089–2094. doi: 10.2169/internalmedicine.4628-20
Yoshida, N., Emoto, T., Yamashita, T., Watanabe, H., Hayashi, T., Tabata, T., et al. (2018). Bacteroides Vulgatus Andbacteroides Dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 138 (22), 2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714
Yoshimoto, S., Loo, T. M., Atarashi, K., Kanda, H., Sato, S., Oyadomari, S., et al. (2013). Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer Through Senescence Secretome. Nature 499 (7456), 97–101. doi: 10.1038/nature12347
Zhang, H., Liao, X., Sparks, J. B., Luo, X. M. (2014). Dynamics of Gut Microbiota in Autoimmune Lupus. Appl. Environ. Microbiol 80 (24), 7551–7560. doi: 10.1128/AEM.02676-14
Zhan, K., Gong, X., Chen, Y., Jiang, M., Yang, T., Zhao, G. (2019). Short-Chain Fatty Acids Regulate Theimmune Responses Via G Protein-Coupled Receptor 41 in Bovine Rumen Epithelial Cells. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02042
Zhang, H., Wang, D., Li, M., Plecitá-Hlavatá, L., D Alessandro, A., Tauber, J., et al. (2017). Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PT (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 136 (25), 2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069
Zhang, C., Zhang, T., Lu, W., Duan, X., Luo, X., Liu, S., et al. (2020). Altered Air Way Microbiota Compositionin Patients With Pulmonary Hypertension. Hypertension 76 (5), 1589–1599. doi: 10.1161/HYPERTENSIONAHA.120.15025
Zhou, D., Luo, M., Shang, A., Mao, Q., Li, B., Gan, R., et al. (2021). Antioxidant Food Components for the Prevention and Treatment of Cardiovascular Diseases: Effects, Mechanisms, and Clinical Studies. Oxid. Med. Cell. Longev, 1–17. doi: 10.1155/2021/6627355
Zhu, Z., Huang, J., Li, X., Xing, J., Chen, Q., Liu, R., et al. (2020). Gut Microbiota Regulate Tumor Metastasis Via circRNA/miRNA Networks. Gut Microbes 12 (1), 1788891. doi: 10.1080/19490976.2020.1788891
Keywords: gut dysbiosis, pulmonary arterial hypertension, vascular remodeling, inflammation, treatment
Citation: Wu P, Zhu T, Tan Z, Chen S and Fang Z (2022) Role of Gut Microbiota in Pulmonary Arterial Hypertension. Front. Cell. Infect. Microbiol. 12:812303. doi: 10.3389/fcimb.2022.812303
Received: 14 November 2021; Accepted: 30 March 2022;
Published: 06 May 2022.
Edited by:
Xi Ma, China Agricultural University, ChinaReviewed by:
Surya Prakash Pandey, University of Pittsburgh, United StatesAshraf Yusuf Rangrez, University of Kiel, Germany
Copyright © 2022 Wu, Zhu, Tan, Chen and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenfei Fang, ZmFuZ3poZW5mZWlAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Panyun Wu
Panyun Wu Tengteng Zhu
Tengteng Zhu Zhen Tan
Zhen Tan Shenglan Chen
Shenglan Chen Zhenfei Fang
Zhenfei Fang