- National Centre of Meat Quality and Safety Control, Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, College of Food Science and Technology, Nanjing Agricultural University, Nanjing, China
Listeria monocytogenes, as a model organism, is a causative agent of enteric pathogen that causes systemic infection. However, the interaction of L. monocytogenes and small intestinal epithelium has not been fully elucidated yet. In this study, mice and intestinal organoids were chosen as the models to investigate the influence of L. monocytogenes infection on the intestinal secretory cells and its differentiation-related pathways. Results confirmed the phenomenon of intestinal damage that L. monocytogenes infection could lead to villi damage in mice, which was accompanied by the increase of TNF-α production in jejunum as well as lipopolysaccharide (LPS) secretion in serum. Moreover, it was demonstrated that L. monocytogenes infection increased the number of goblet and Paneth cells in mice and intestinal organoids and upregulated the expression of Muc2 and Lyz. Furthermore, L. monocytogenes decreased the relative expression of Notch pathway-related genes (Jag1, Dll4, Notch1, and Hes1) while upregulating the relative expression of Math1 gene in mice and intestinal organoids. This indicated that L. monocytogenes infection caused the inhibition of Notch pathway, which may be the reason for the increased number of goblet and Paneth cells in the intestine. Collectively, these results are expected to provide more information on the mechanism of L. monocytogenes infection in the intestine.
1 Introduction
Listeria monocytogenes is a major foodborne pathogen that causes listeriosis and has a high mortality rate ranging from 20 to 30% (de Noordhout et al., 2014). Its severity is attributed to the fact that it can cross the intestinal, placental, and blood-brain barriers in immunocompromised individuals, inducing gastroenteritis, abortions, and meningitis (Doganay, 2003).
As the first defense barrier, the intestine plays a vital role in L. monocytogenes defense (Gahan and Hill, 2014; Becattini and Pamer, 2018). Under normal conditions, mucosal epithelial cells, mucus-secreting cells, and immune cells form a protective barrier against invading pathogens (Ruch and Engel, 2017). Related studies mainly focused on the protective effects of immune cells during L. monocytogenes infection (Cho et al., 2017; Wilharm et al., 2021). However, the intestinal epithelial cells compose numerous cell types that help maintain intestinal homeostasis and defend against enteric pathogen invasion. Specifically, goblet cells can produce mucin glycoproteins and form mucus, and Paneth cells, at the bottom of intestinal crypts, can secret antimicrobial peptides (AMPs). It had been found that L. monocytogenes could damage intestinal homeostasis and affect the differentiation of intestinal epithelial cells. A study on intestinal infection by L. monocytogenes demonstrated a significant effect on the differentiation of epithelial cells by increasing the number of goblet cells in mice (Pian et al., 2020). In addition, it was reported that the counts of Paneth cells in organoids decreased after L. monocytogenes infection for 1 h but increased after infection for 18 h through the regulation of Wnt signaling pathway (Huang et al., 2021). Therefore, these studies indicated that the differentiation of epithelial cells, especially the secretory cells, could be affected by bacteria invasion in vivo or in vitro.
In literature, the proliferation and differentiation of intestinal epithelial cells were confirmed to be regulated by a variety of signaling pathways, such as Wnt, EGF, BMP, and Notch signaling pathways (Date and Sato, 2015). Among them, the Notch signaling pathway played a crucial role in regulating the differentiation of intestinal secretory cells, such as goblet and Paneth cells. Recently, some studies have demonstrated that mice exposed to Cadmium or Salmonella infection led to a loss of goblet cells through the activation of Notch-signaling pathway (Wu et al., 2018; Xie et al., 2020). Conversely, blockade of the Notch pathway using γ-secretase inhibitors led to the conversion of all intestinal epithelial cells into goblet cells (Wong et al., 2004; van der Flier and Clevers, 2009). These studies indicated that the Notch signaling pathway regulated the differentiation of secretory cells under physiological and pathological conditions. However, researches on the influence of intestinal infection caused by L. monocytogenes on the Notch pathway are insufficient.
Animals and cells models are often used to investigate the infection mechanism of the enteric pathogens in the intestine. However, most traditional intestinal cell models have immortalized 2-D cell lines, such as Caco-2 cells containing a single cell type, which cannot reproduce some characteristics of natural infection (Wilson et al., 2015). Recently, intestinal organoids were served as a more effective model to study pathogen-host interactions (Hill and Spence, 2017). In contrast to traditional cell lines, intestinal organoids occupied 3-dimensional space. They formed complex microenvironments that facilitated differentiation and persistence of epithelial subtypes and the formation of villus-like structures, comprised of Paneth cells, Goblet cells, enterocytes, enteroendocrine cells, and stem cells (Watson et al., 2014; Finkbeiner et al., 2015). In addition, it was reported that intestinal organoids were used to visualize the invasiveness of Salmonella and the morphologic changes of the organoids (Zhang et al., 2014). And intestinal organoids also were used to model the infection of L. monocytogenes (Huang et al., 2021), and pathogenic Escherichia coli strains (Vandussen et al., 2014; Rajan et al., 2018), which provided important insights into the pathogenesis of the intestine.
Therefore, in this study, mice and intestinal organoids were used to establish an invasion model of L. monocytogenes to explore its influence on the differentiation of secretory cells and differentiation-related Notch pathway, which will provide more information on the mechanism of L. monocytogenes infection in the intestine.
2 Materials and Methods
2.1 Bacterial Strain Culture
The L. monocytogenes 10403s strain used in this study was supplied by Prof. Weihuan Fang (Zhejiang University). L. monocytogenes 10403s was grown in brain heart infusion (BHI) broth supplemented with 5 μg/ml erythromycin for 16 h at 37°C with shaking (180 rpm).
2.2 Animals and Intestinal Organoids
2.2.1 Animals
Twenty-four C57BL/6 mice (4 weeks old, specific-pathogen-free (SPF) female) were purchased from the Animal Research Centre of Yang Zhou University. All animals were randomly divided into two groups and orally administrated sterile PBS (control group, CK, n=6) and L. monocytogenes 10403s (109 CFU/ml, LM, n=18). The mice were sacrificed on day 4, and tissue samples were collected for further analysis. All animal studies were approved by the Nanjing Agriculture University Committee on Animal Resources Committee and the National Institutes of Health guidelines for the performance of animal experiments.
2.2.2 Intestinal Organoids
2.2.2.1 Isolation and Culture of Intestinal Organoids
Intestinal organoids were isolated from the small intestine of 4-week-old SPF C57/BL6 mice. The intestine samples were cleaned with phosphate-buffer saline (PBS) and cut into small pieces. After that, Gentle Cell Dissociation Reagent (Stem Cell, Canada) was added, and the mixture was digested at 20°C for 15 min. After incubation, crypts were filtered through a 70-μm sterile cell strainer and centrifuged at 300 g for 5 min at 4°C. The cells were resuspended by Matrigel (Corning, USA) and IntestiCult™ OGM Mouse Basal Medium (Stem cell, Canada) and then plated in 24-well plates. The plates were polymerized at 37°C for 20 min before the addition of culture medium. The medium was changed every 2-3 days.
2.2.2.2 L. Monocytogenes Infection of Organoid Cells
The methods of organoids infection were referenced from Huang et al. (Huang et al., 2021) with some modifications. Firstly, L. monocytogenes culture was centrifuged at 5000 rpm for 5 min and washed with PBS, before being resuspended in culture medium to 108 CFU/ml. After removing the Matrigel with cold PBS, organoids were pipetted up and down and were resuspended in culture medium for 1 h. Subsequently, the organoids were reseeded with Matrigel and cultured with medium containing gentamicin (100 μg/ml, Gbico) for 18 h.
2.3 The Location of L. monocytogenes in Organoids
After centrifugation at 5000rpm for 5min, the collected bacteria were washed once with 1 ml 0.1M NaHCO3, re-suspended in a solution containing 0.2 mg/mL fluoresceine isothiocyanate (FITC) dissolved in 0.1M NaHCO3 and incubated in the dark at 37°C for 1h. The FITC labeled bacteria were washed twice with PBS and the concentration was set to obtain 108 CFU/mL. Then the labeled bacteria were used to infect crypts before the following steps, and observed in organoids by using a Leica DMi8 Laser Scanning confocal microscope.
2.4 Morphology of Intestine Tissue
To observe the pathological change of the intestine, the intestinal tissue was fixed in 4% paraformaldehyde for 24 h, dehydrated in ethanol (for 1h in 70%, 80%, 90%, and 100%, respectively), xylene for 40 s, and embedded in paraffin wax. The paraffin blocks were cut to a thickness of 5 microns and stained with hematoxylin-eosin (H&E) staining.
2.5 ELISA
The production of TNF-α was analyzed with Mouse TNF-α ELISA kits (NeoBioscience, China), and Lipopolysaccharide (LPS) was measured with LPS ELISA kits (NeoBioscience, China) according to the manufacturer’s protocols.
2.5 Real-Time Quantitative PCR
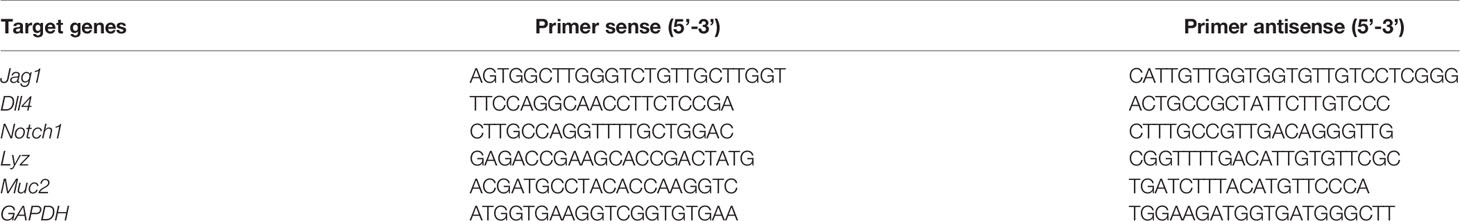
Total RNA of tissue and organoid samples was extracted using TRIzol (Ambion, USA), after which reverse transcription PCR was performed. Using the primers (Table 1), 1 μL of template cDNA was reacted with a master mix in a final volume of 10 μL. The thermal cycling procedure was 30 s at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 60°C using an Applied Biosystems 7500 real-time PCR system.
2.6 Immunofluorescence Assay
Intestinal tissue slides were deparaffinized with xylene and rehydrated with an alcohol gradient. To enhance immunoreactivity, the slides were incubated in 10 mM sodium citrate for 15 min at 95°C. Then the slides were cooled to room temperature, washed in PBS for 5 min (five times in total), blocked for 2 h with 5% Bovine Serum Albumin (BSA), and incubated for 2 h with Ulex europaeus agglutinin-1 (UEA-1). Finally, 4′,6-diamidino-2-phenylindole(DAPI)was used to counterstain nuclei. For lysozyme (Lyz) staining, cells were stained with anti-rabbit lysozyme antibody (1:200, Abcam) overnight at 4°C. The samples were incubated with goat anti-rabbit to Alexa Fluor 594 (1:250, Abcam) for 90 min, followed by DAPI for 5 min at room temperature. For in vitro imaging, infected organoids were embedded in Matrigel on glass chamber slides. The 0.5% Triton X-100 was used for 20 min to permeabilize the cells. Thereafter, the slides were washed with PBS three times and incubated for 1 h in 5% BSA. Subsequently, UEA-1 and Lyz were used to visualize goblet cells and Paneth cells in organoids, respectively, and the staining was observed with a Leica DMi8 Laser Scanning confocal microscope (Leica, Germany).
2.7 Western Blot
Tissue and cell samples were lysed in RIPA buffer containing a protease inhibitor cocktail. Protein concentration in the lysed sample was detected using a bicinchoninic acid (BCA) assay kit (Thermo Scientific, USA). After that, samples containing 5× load buffer were heated for 5 min at 95°C. Equal amounts of protein were separated by 4-20% SDS-PAGE, and transferred to PVDF membranes (BIO-RAD, USA). Then, the membranes were blocked with 5% non-fat milk in TBS with 0.1% Tween-20 for 1 h and incubated with rabbit anti-GAPDH (Abcam, 1:10000), rabbit anti-lysozyme (Abcam,1:1000) overnight, respectively. After the washing, goat anti-rabbit secondary antibodies (Bioss, 1:1500) were used to incubate the membranes. Finally, the optical protein bands were developed using efficient chemiluminescence (ECL) kit, and light emission was captured using the Versa DOC 4000 imaging system.
2.8 Statistical Analysis
All statistical analyses were performed using GraphPad Prism 7. A t-test was employed to determine the significant difference between the two groups. The significance levels were shown as *P < 0.05, **P < 0.01 and ***P < 0.001. Data were combined from at least three independent experiments unless otherwise stated and expressed as means ± SD.
3 Results
3.1 The Intestinal Pathological Changes in Mice After L. monocytogenes Infection
Compared with the control group, L. monocytogenes infection led to the disorder of intestinal villi in the jejunum (Figure 1A). Moreover, after L. monocytogenes infection, the concentration of LPS in the serum of mice increased significantly (Figure 1B). Figure 1C showed that the protein expression level of TNF-α in the jejunum was significantly higher than that in the control group. These results confirmed that L. monocytogenes infection could lead to intestinal pathological changes in mice.
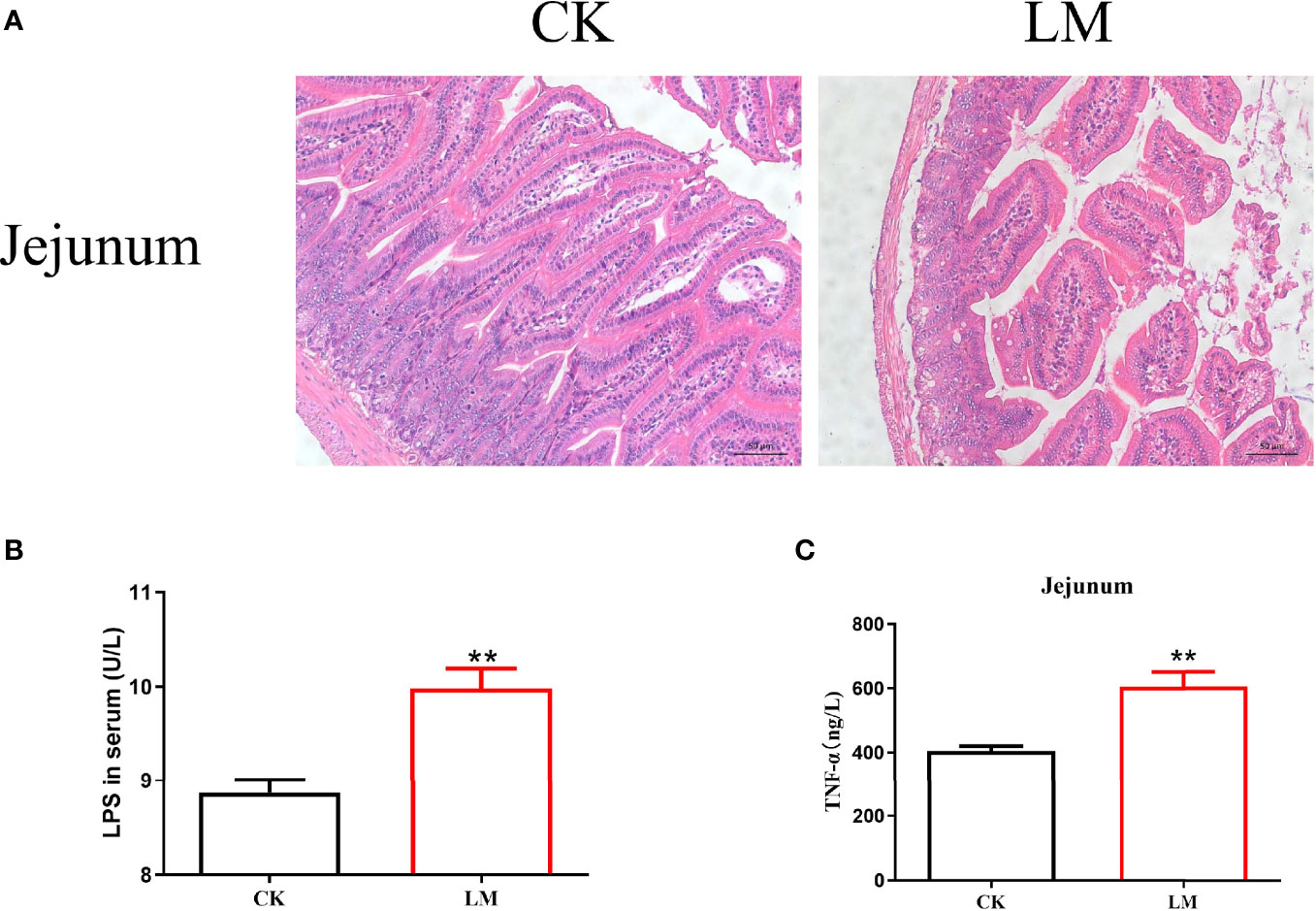
Figure 1 The intestinal pathological changes in mice after L. monocytogenes infection. CK, control, orally challenge with PBS; LM, L. monocytogenes-infected group. (A) Histopathological changes in jejunum tissues were examined by hematoxylin eosin (HE) staining. (B) The concentration of LPS was measured in serum. (C) The concentration of TNF-α was measured in jejunum. Data is presented as mean ± SD. **P < 0.01. Data combined from at least three independent experiments unless otherwise stated.
3.2 The Influence of L. monocytogenes on Goblet and Paneth Cells in Mice
Intestinal secretory cells, such as goblet and Paneth cells, could protect the mucosal barrier and defend against L. monocytogenes invasion. The immunofluorescence assay results showed that the oral administration of L. monocytogenes could increase the number of goblet cells stained with UEA-1 (Figure 2A) and increase the relative expression level of Muc2 gene significantly in the jejunum of mice (Figure 2D). Additionally, the number of Paneth cells stained with Lyz was significantly increased through immunofluorescence analysis (Figure 2B). The protein and mRNA expression levels of Lyz were also upregulated to 3.08-fold and 1.79-fold after L. monocytogenes infection, respectively (Figures 2C, E). These results indicated that L. monocytogenes caused the abnormal increase of goblet and Paneth cells in the jejunum of mice.
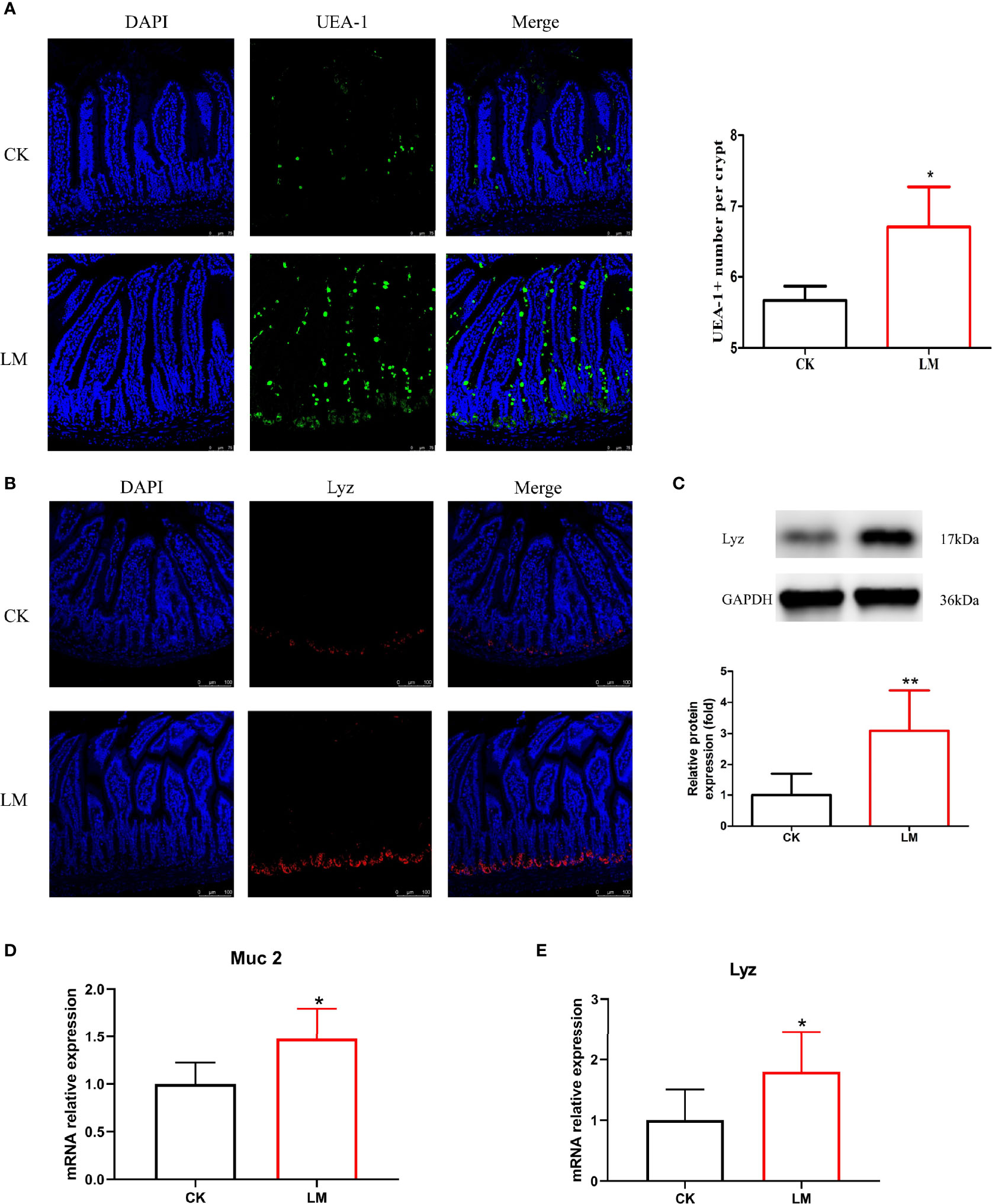
Figure 2 The influence of L. monocytogenes on the differentiation of intestinal secretory cells in mice. CK, control, orally challenge with PBS; LM, L. monocytogenes-infected group. (A) Confocal microscopy analysis of mucus stained with UEA-1 in jejunum sections. (B) Confocal microscopy analysis of lysozyme in jejunum sections. (C) Western blot of lysozyme in jejunum. (D) mRNA levels of Muc2 from homogenized jejunum samples. (E) mRNA levels of Lyz from homogenized jejunum samples. Data is presented as mean ± SD. *P < 0.05, **P < 0.01. Data combined from at least three independent experiments unless otherwise stated.
3.3 The Influence of L. monocytogenes on the Notch Signaling Pathway in Mice
The differentiation of intestinal secretory cells was regulated by the Notch signaling pathway, where Dll4 and Jag1 are two Notch ligands that regulate the expression of the Notch target gene, Hes1. The relative expression of the genes showed that the mRNA relative expression of Jag1, Dll4, Notch1, and Hes1 were down-regulated to 0.57-fold, 0.66-fold, 0.92-fold, and 0.64-fold, respectively (Figures 3A-D). Additionally, the relative expression of Math1 gene in the jejunum, which governs the differentiation of goblet and Paneth cells, was significantly increased after L. monocytogenes infection (Figure 3E). These results indicated that L. monocytogenes infection could inhibit the Notch signaling pathway, which may be the reason for the increase in the number of goblet and Paneth cells in mice.
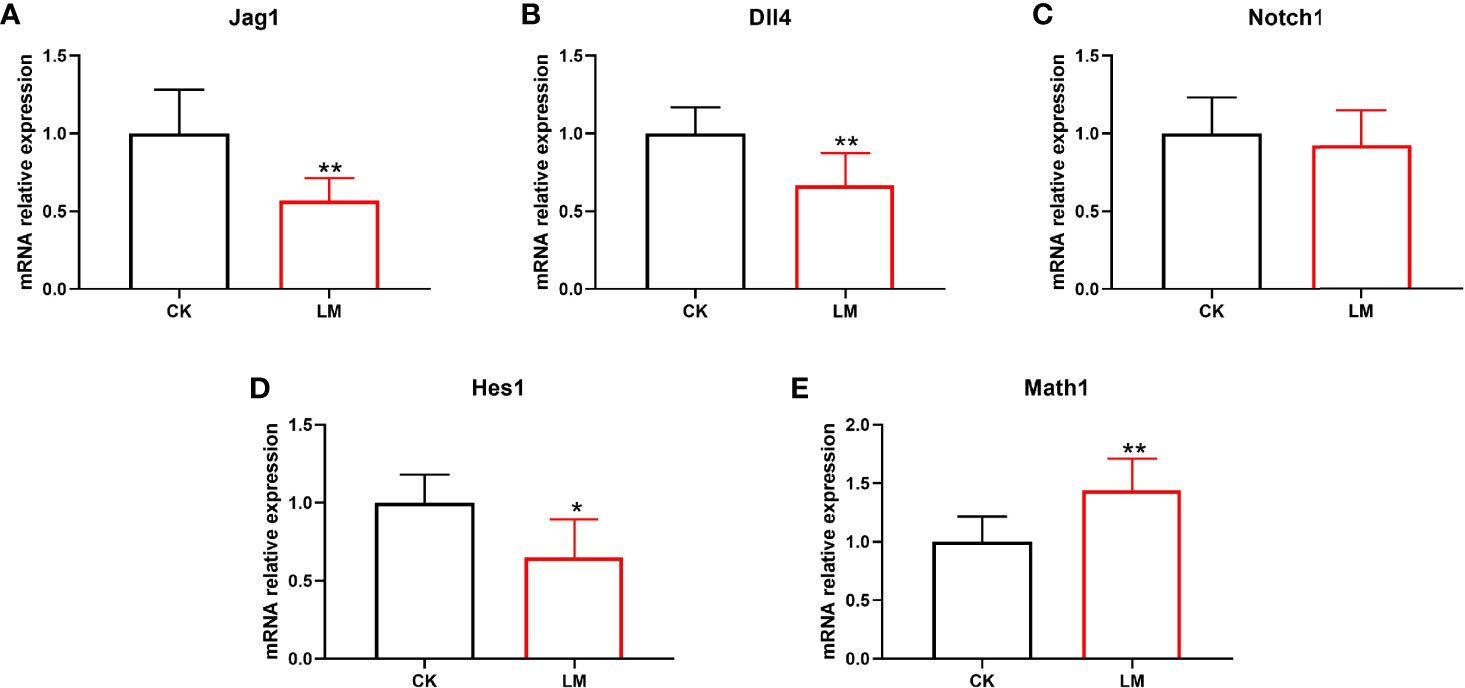
Figure 3 The influence of L. monocytogenes on the Notch pathway in mice. CK, control, orally challenged with PBS; LM, L. monocytogenes-infected group. (A-E) The expression of Jag1, Dll4, Notch1, Hes1 and Math1 genes were determined by quantitative RT-PCR and normalized by the expression of GAPDH. Data is presented as mean ± SD. *P < 0.05, **P < 0.01. Data combined from at least three independent experiments unless otherwise stated.
3.4 The Influence of L. monocytogenes on Intestinal Secretory Cell Differentiation and Notch Signaling Pathway in Organoids
Intestinal organoids, as an in vitro model, were used to verify the influence of L. monocytogenes on intestinal secretory cell differentiation. Figure 4A showed that the FITC-labeled L. monocytogenes were observed in organoids, which indicated that L. monocytogenes could invade in organoids after 18h co-culture. In addition, results showed that L. monocytogenes infection increased the number of UEA-1+ cells (Figure 4B) and markedly increased Muc2 expression (Figure 4E). Furthermore, the number of Lyz+ cells were increased (Figure 4C), and the mRNA and protein expression of Lyz were increased significantly after L. monocytogenes infection (Figures 4D, F), which indicated that L. monocytogenes infection increased the Paneth cells of organoids. Also, L. monocytogenes significantly decreased the relative expression of Jag1, Dll4, Notch1, and Hes1 genes and upregulated the relative expression of Math1 gene in organoids (Figures 5A-E). Overall, the results of organoids further confirmed those of mice findings, which also indicated that the inhibition of Notch signaling pathway during L. monocytogenes infection may induce the expansion of goblet and Paneth cell population.
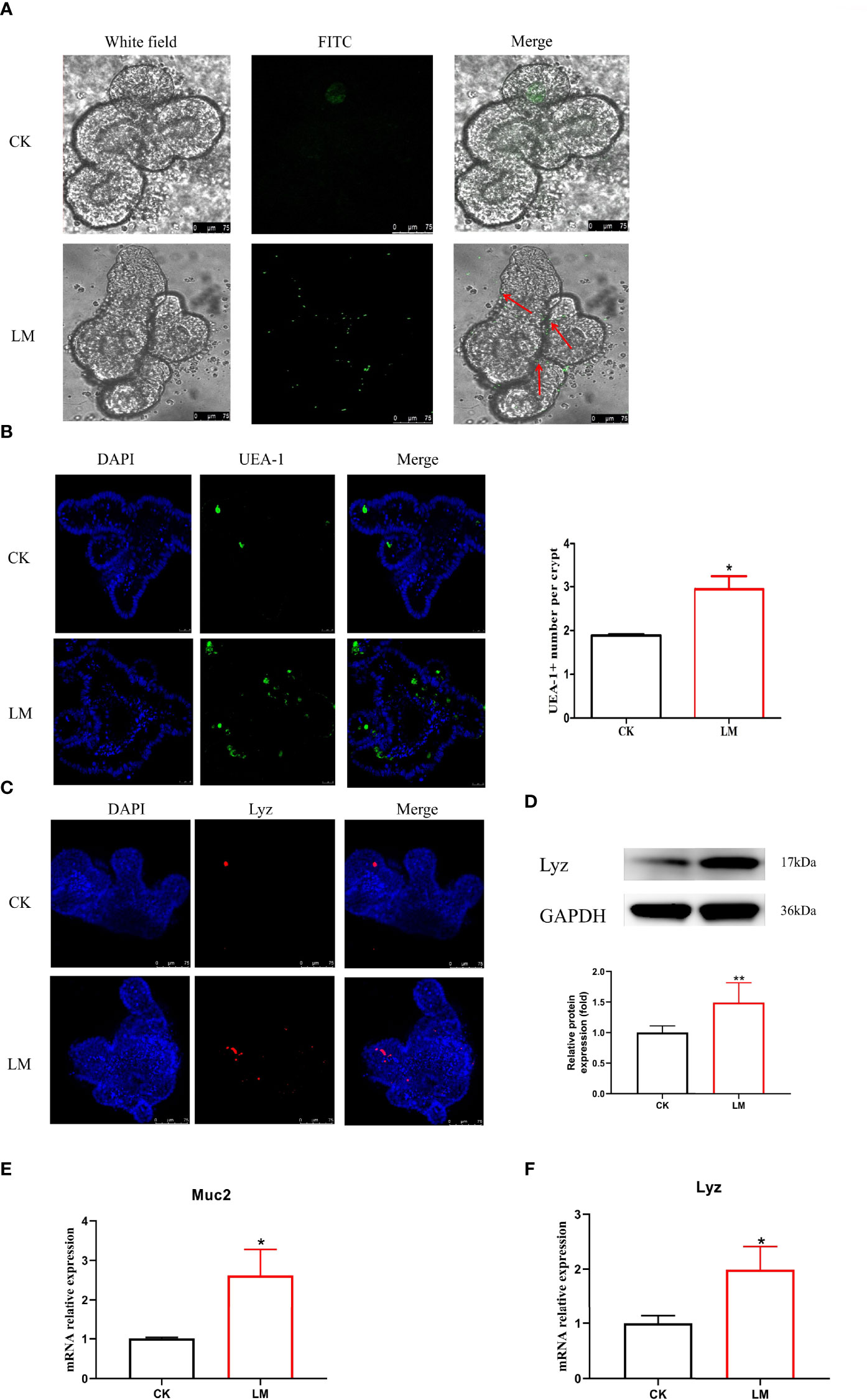
Figure 4 The influence of L. monocytogenes on the differentiation of intestinal secretory cells. CK, control; LM, L. monocytogenes-infected group. (A) The location of L. monocytogenes in organoids. (B) Confocal microscopy analysis of UEA-1+ cells in organoids. (C) Confocal microscopy analysis of Lyz+ cells in organoids. (D) Western blot of lysozyme in organoids. (E) mRNA levels of Muc2 from organoids samples. (F) mRNA levels of Lyz from organoids samples. Data is presented as mean ± SD. *P < 0.05, **P < 0.01. Data combined from at least three independent experiments unless otherwise stated.
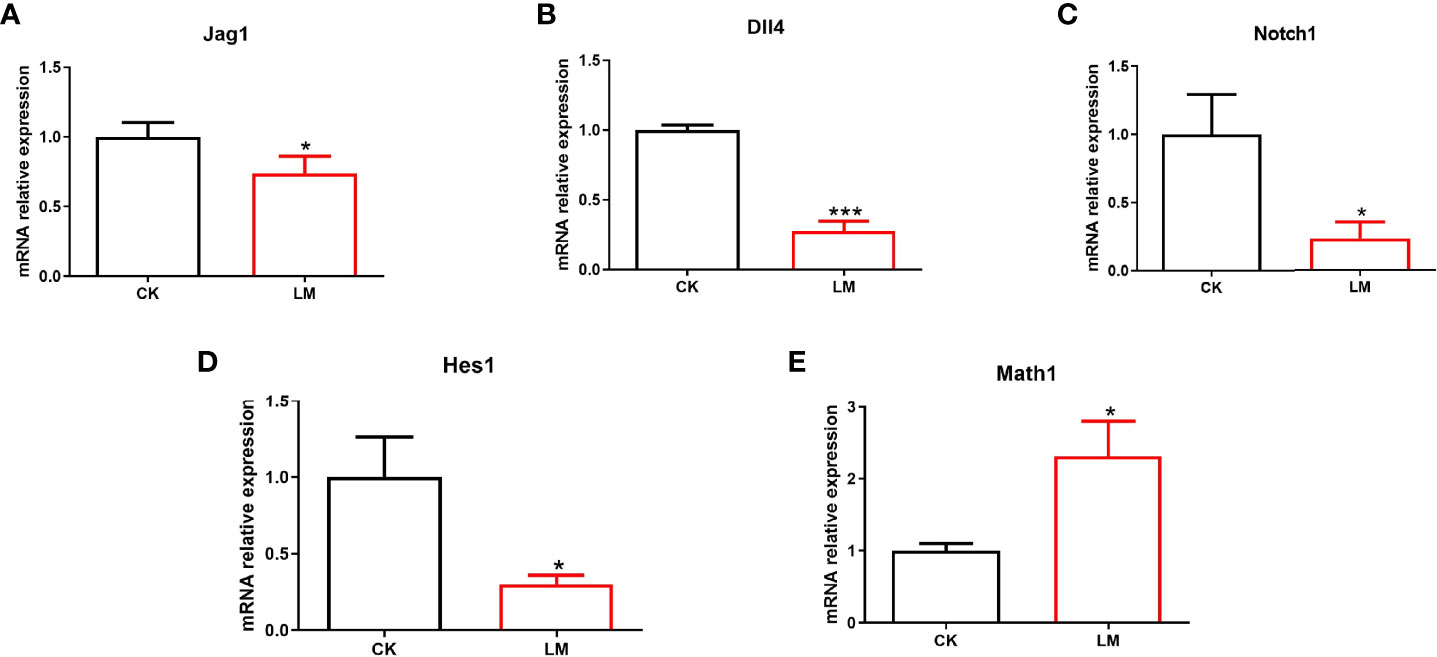
Figure 5 The influence of L. monocytogenes on the Notch pathway in organoids. CK, control; LM, L. monocytogenes-infected group. (A-E) The expression of Jag1, Dll4, Notch1, Hes1 and Math1 genes were determined by quantitative RT-PCR and normalized by the expression of GAPDH. Data is presented as mean ± SD. *P < 0.05, ***P < 0.001. Data combined from at least three independent experiments unless otherwise stated.
4 Discussion
L. monocytogenes infection is a severe foodborne disease worldwide, reported in more than 30 countries, including the USA, Canada, Germany, Portugal, and Austria (Desai et al., 2019; Jamshidi and Zeinali, 2019). During L. monocytogenes infection, the intestine is a vital defense line. This study showed that L. monocytogenes infection could lead to villi damage with H&E staining, which was closely related to the increase of pro-inflammatory cytokines TNF-α secretion. This corroborated the study of Alkhuriji et al. that L. monocytogenes infection in mice could cause epithelial cells exfoliation and degeneration of the lamina propria and induce the production of TNF-α in the intestine (Alkhuriji et al., 2020). In addition, L. monocytogenes increased the level of LPS in the serum, which indicated that the intestinal permeability was increased (Mokkala, K. et al., 2017).
As specialized intestinal epithelial cells, goblet and Paneth cells play an essential role in mucosal homeostasis and bacterial defense. During infection, goblet cell secretion, which contains mucopolysaccharides, critical in forming the first line of defense by protecting the intestinal barrier against pathogenic invasion, was described extensively (Pelaseyed et al., 2014). This study demonstrated that the number of goblet cells, in vivo and in vitro, and the relative expression of Muc2 gene were significantly increased, which was consistent with the previous study (Pian et al., 2020). Notably, Paneth cells, located at the bottom of the intestinal crypt, could produce various antibacterial peptides to kill pathogens, including regenerating 3γ (reg3γ), lysozyme, and defensin (Bevins and Salzman, 2011). This study showed that the count of Lyz+ cells was increased in mice, which was consistent with the protein and mRNA expression levels in mice and organoids. Moreover, it was reported that L. monocytogenes increased the number of Paneth cells during co-culture with organoids for 18 h in vitro (Huang et al., 2021). Although the increasing number of goblet and Paneth cells shielded against epithelial damage, excessive secretory cells may disrupt the intestinal homeostasis by consuming the stem cells (Putman et al., 2011), which could rejuvenate tissue homeostasis and repair injured tissues (Umar, 2002). Therefore, how to preserve the number and function of goblet and Paneth cells during the treatment of bacterial infection is imperative.
The Notch signaling pathway is a development switch for intestinal secretory and absorptive cells. However, its suppression leads to the inhibition of enterocytes differentiation and a dramatic expansion in goblet and Paneth cell numbers (van Es et al., 2005). The Notch signaling was activated when one of the Delta or Jagged Notch transmembrane receptors interacted with one of the five Notch ligands triggering proteolytic cleavage of the receptor (Scoville et al., 2008). The cleavage released the free Notch 1 intracellular domain (NICD) that translocated into the nucleus to upregulate target genes, mainly of Hes class, such as Hes1 in the intestine, which suppressed the Math1 gene (Yang et al., 2001). It was reported that constitutive overexpression of the Notch1 receptor reduced the differentiation of enteroendocrine and Paneth cells, thus decreasing their numbers (Robine et al., 2005). Also, deficiency in Hes1 mice led to an abundance of goblet, enteroendocrine, and Paneth cells, but a reduced number of enterocytes (Suzuki et al., 2005). Conversely, the intestine from Math1 deficient mice exhibited an intestinal epithelium formed only by enterocytes (Yang et al., 2001).
Furthermore, under pathological conditions, Wu et al. found that Salmonella infection induced the loss of goblet cells and reduced the mRNA expression of Muc2 by increasing the expression of Dll1, Dll4, and Hes1 genes, indicating the activation of Notch signaling pathway (Wu et al., 2018). However, to the best of our knowledge, the influence of L. monocytogenes infection on the Notch signaling pathway has not been documented in mice and organoids. Based on the increase in goblet and Paneth cells, our findings speculated whether L. monocytogenes promoted the differentiation of intestinal secretory cells by inhibiting the Notch signaling pathway. Consistent with this hypothesis, the relative expression of related genes of Notch pathway (Jag1, Dll4, Nocth1, and Hes1) were detected and decreased, while the mRNA relative expression of Math1 was upregulated in mice. Also, the intestinal organoids, an effective intestinal cell model, were used to establish an infection model in vitro. The mRNA relative expression of Jag1, Dll4, Nocth1, and Hes1 were down-regulated significantly, while the relative expression of Math1 gene was significantly increased, which was consistent with a previous study (Huang et al., 2021). As such, the decreased expression of the Notch signaling pathway genes demonstrated L. monocytogenes inhibition on the Notch pathway. Combined with these results, the Notch pathway inhibition may be the reason for the increased number of goblet and Paneth cells after L. monocytogenes infection in mice and organoids.
In summary, this study indicated that L. monocytogenes infection damaged the intestinal barrier and upregulated LPS level in serum, and increased the expression of TNF-α in the jejunum. Furthermore, L. monocytogenes infection inhibited the Notch pathway, which may lead to the increasing number of goblet and Paneth cells in the intestine of mice. Therefore, this study provides insight to study the mechanism of L. monocytogenes damage to the intestinal mucosal barrier, which is helpful to control L. monocytogenes pathogenic infection.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Nanjing Agriculture University Committee on Animal Resources Committee and the National Institutes of Health guidelines for the performance of animal experiments.
Author Contributions
CZ: Data curation, Formal analysis, Writing - original draft. YYZ: Writing - original draft. AB: Writing - original draft. JH: Writing - original draft. YFZ: Writing - original draft. KY: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing - original draft, Writing - review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (32172267) and Program for Student Innovation through Research and Training (202110307047).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alkhuriji, A. F., Majrashi, N. A., Alomar, S., El-Khadragy, M. F., Awad, M. A., Khatab, A. R., et al. (2020). The Beneficial Effect of Eco-Friendly Green Nanoparticles Using Garcinia Mangostana Peel Extract Against Pathogenicity of Listeria Monocytogenes in Female BALB/C Mice. Animals 10 (4), 573. doi: 10.3390/ani10040573
Becattini, S., Pamer, E. G. (2018). Multifaceted Defense Against Listeria Monocytogenes in the Gastro-Intestinal Lumen. Pathogens 7 (1), 1. doi: 10.3390/pathogens7010001
Bevins, C. L., Salzman, N. H. (2011). Paneth Cells, Antimicrobial Peptides and Maintenance of Intestinal Homeostasis. Nat. Rev. Microbiol. 9 (5), 356–368. doi: 10.1038/nrmicro2546
Cho, S. S. L., Han, J., James, S. J., Png, C. W., Weerasooriya, M., Alonso, S., et al. (2017). Dual-Specificity Phosphatase 12 Targets P38 Map Kinase to Regulate Macrophage Response to Intracellular Bacterial Infection. Front. Immunol. 8. doi: 10.3389/fimmu.2017.01259
Date, S., Sato, T. (2015). Mini-Gut Organoids: Reconstitution of Stem Cell Niche. Annu. Rev. Cell Dev. Biol. 31, 269–289. doi: 10.1146/annurev-cellbio-100814-125218
de Noordhout, C. M., Devleesschauwer, B., Angulo, F. J., Verbeke, G., Haagsma, J., Kirk, M., et al. (2014). The Global Burden of Listeriosis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 14 (11), 1073–1082. doi: 10.1016/S1473-3099(14)70870-9
Desai, A. N., Anyoha, A., Madoff, L. C., Lassmann, B. (2019). Changing Epidemiology of Listeria Monocytogenes Outbreaks, Sporadic Cases, and Recalls Globally: A Review of Promed Reports From 1996 to 2018. Int. J. Infect. Dis. 84, 48–53. doi: 10.1016/j.ijid.2019.04.021
Doganay, M. (2003). Listeriosis: Clinical Presentation. FEMS Immunol. Med. Microbiol. 35 (3), 173–175. doi: 10.1016/S0928-8244(02)00467-4
Finkbeiner, S. R., Hill, D. R., Altheim, C. H., Dedhia, P. H., Taylor, M. J., Tsai, Y. H., et al. (2015). …Transcriptome-Wide Analysis Reveals Hallmarks of Human Intestine Development and Maturation In Vitro and In Vivo. Stem Cell Rep. 4 (6), 1140–1155. doi: 10.1016/j.stemcr.2015.04.010
Gahan, C. G. M., Hill, C. (2014). Listeria Monocytogenes: Survival and Adaptation in the Gastrointestinal Tract. Front. Cell. Infect. Microbiol. 4. doi: 10.3389/fcimb.2014.00009
Hill, D. R., Spence, J. R. (2017). Gastrointestinal Organoids: Understanding the Molecular Basis of the Host-Microbe Interface. Cell. Mol. Gastroenterol. Hepatol. 3 (2), 138–149. doi: 10.1016/j.jcmgh.2016.11.007
Huang, J., Zhou, C., Zhou, G. H., Li, H. K., Ye, K. P. (2021). Effect of Listeria Monocytogenes on Intestinal Stem Cells in the Co-Culture Model of Small Intestinal Organoids. Microbial Pathogenesis 153, 104776. doi: 10.1016/j.micpath.2021.104776
Jamshidi, A., Zeinali, T. (2019). Significance and Characteristics of Listeria Monocytogenes in Poultry Products. Int. J. Food Sci. 3, 1–7. doi: 10.1155/2019/7835253
Mokkala, K., Pellonperä, O., Röytiö, H., Pussinen, P., Rönnemaa, T., Laitinen, K. (2017). Increased Intestinal Permeability, Measured by Serum Zonulin, is Associated With Metabolic Risk Markers in Overweight Pregnant Women. Metabolism 69, 43–50. doi: 10.1016/j.metabol.2016.12.015
Pelaseyed, T., Bergstrom, J. H., Gustafsson, J. K., Ermund, A., Birchenough, G. M. H., Schutte, A., et al. (2014). The Mucus and Mucins of the Goblet Cells and Enterocytes Provide the First Defense Line of the Gastrointestinal Tract and Interact With the Immune System. Immunol. Rev. 260 (1), 8–20. doi: 10.1111/imr.12182
Pian, Y. Y., Chai, Q., Ren, B. Y., Wang, Y., Lv, M. J., Qiu, J., et al. (2020). Type 3 Innate Lymphoid Cells Direct Goblet Cell Differentiation via the LT-LT Beta R Pathway During Listeria Infection. J. Immunol. 205 (3), 853–863. doi: 10.4049/jimmunol.2000197
Putman, P. J., Burger-van Paassen, N., Schaart, M. W., de Bruijn, A. C. J. M., de Krijger, R. R., Tibboel, D., et al. (2011). Paneth Cell Hyperplasia and Metaplasia in Necrotizing Enterocolitis. Pediatr. Res. 69 (3), 217–223. doi: 10.1203/PDR.0b013e3182092a9a
Rajan, A., Vela, L., Zeng, X. L., Yu, X., Shroyer, N., Blutt, S. E., et al. (2018). Novel Segment- and Host-Specific Patterns of Enteroaggregative Escherichia Coli Adherence to Human Intestinal Enteroids. MBio 9, 1. doi: 10.1128/mBio.02419-17. J. m.
Robine, S., Fre, S., Huyghe, M., Artavanis-Tsakonas, S., Louvard, D. (2005). Notch Signals Control the Fate of Immature Progenitor Cells in the Intestine. M S-Med. Sci. 21 (8-9), 780–781. doi: 10.1051/medsci/2005218-9780
Ruch, T. R., Engel, J. N. (2017). Targeting the Mucosal Barrier: How Pathogens Modulate the Cellular Polarity Network. Cold Spring Harbor Perspect. Biol. 9 (6), a027953. doi: 10.1101/cshperspect.a027953
Scoville, D. H., Sato, T., He, X. C., Li, L. H. (2008). Current View: Intestinal Stem Cells and Signaling. Gastroenterology 134 (3), 849–864. doi: 10.1053/j.gastro.2008.01.079
Suzuki, K., Fukui, H., Kayahara, T., Sawada, M., Seno, H., Hiai, H., et al. (2005). Hes1-Deficient Mice Show Precocious Differentiation of Paneth Cells in the Small Intestine. Biochem. Biophys. Res. Commun. 328 (1), 348–352. doi: 10.1016/j.bbrc.2004.12.174
Umar, S. (2002). Intestinal Stem Cells. Curr. Gastroenterol. Rep. 12 (5), 340–348. doi: 10.1007/s11894-010-0130-3340-348
van der Flier, L. G., Clevers, H. (2009). Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 71, 241–260. doi: 10.1146/annurev.physiol.010908.163145
Vandussen, K. L., Marinshaw, J. M., Shaikh, N., Miyoshi, H., Stappenbeck, T. S. (2014). Development of an Enhanced Human Gastrointestinal Epithelial Culture System to Facilitate Patient-Based Assays. Gut 64 (6), 911–920. doi: 10.1136/gutjnl-2013-306651. J. G.
van Es, J. H., van Gijn, M. E., Riccio, O., van den Born, M., Vooijs, M., Begthel, H., et al. (2005). Notch/Gamma-Secretase Inhibition Turns Proliferative Cells in Intestinal Crypts and Adenomas Into Goblet Cells. Nature 435 (7044), 959–963. doi: 10.1038/nature03659
Watson, C. L., Mahe, M. M., Munera, J., Howell, J. C., Sundaram, N., Poling, H. M., et al. (2014). …An In Vivo Model of Human Small Intestine Using Pluripotent Stem Cells. Nat. Med. 20 (11), 1310–1314. doi: 10.1038/nm.3737
Wilharm, A., Brigas, H. C., Sandrock, I., Ribeiro, M., Amado, T., Reinhardt, A., et al. (2021). Microbiota-Dependent Expansion of Testicular IL-17-Producing V Gamma 6(+) Gamma Delta T Cells Upon Puberty Promotes Local Tissue Immune Surveillance (Sep, 10.1038/S41385-020-00346-7, 2020). Mucosal Immunol. 14 (1), 278–278. doi: 10.1038/s41385-020-00346-7
Wilson, S. S., Tocchi, A., Holly, M. K., Parks, W. C., Smith, J. G. (2015). A Small Intestinal Organoid Model of non-Invasive Enteric Pathogen-Epithelial Cell Interactions. Mucosal Immunol. 8 (2), 352–361. doi: 10.1038/mi.2014.72
Wong, G. T., Manfra, D., Poulet, F. M., Zhang, Q., Josien, H., Bara, T., et al. (2004). Chronic Treatment With the Gamma-Secretase Inhibitor LY-411,575 Inhibits Beta-Amyloid Peptide Production and Alters Lymphopoiesis and Intestinal Cell Differentiation. J. Biol. Chem. 279 (13), 12876–12882. doi: 10.1074/jbc.M311652200
Wu, H. Q., Ye, L. L., Lu, X. X., Xie, S., Yang, Q., Yu, Q. H. (2018). Lactobacillus Acidophilus Alleviated Salmonella-Induced Goblet Cells Loss and Colitis by Notch Pathway. Mol. Nutr. Food Res. 62 (22), 1800552. doi: 10.1002/mnfr.201800552
Xie, S., Jiang, L., Wang, M. J., Sun, W. J., Yu, S. Y., Turner, J. R., et al. (2020). Cadmium Ingestion Exacerbates Salmonella Infection, With a Loss of Goblet Cells Through Activation of Notch Signaling Pathways by ROS in the Intestine. J. Hazardous Materials 391, 122262. doi: 10.1016/j.jhazmat.2020.122262
Yang, Q., Bermingham, N. A., Finegold, M. J., Zoghbi, H. Y. (2001). Requirement of Math1 for Secretory Cell Lineage Commitment in the Mouse Intestine. Science 294 (5549), 2155–2158. doi: 10.1126/science.1065718
Keywords: Listeria monocytogenes, intestine, goblet cell, Paneth cell, notch pathway
Citation: Zhou C, Zhang Y, Bassey A, Huang J, Zou Y and Ye K (2022) Expansion of Intestinal Secretory Cell Population Induced by Listeria monocytogenes Infection: Accompanied With the Inhibition of NOTCH Pathway. Front. Cell. Infect. Microbiol. 12:793335. doi: 10.3389/fcimb.2022.793335
Received: 12 October 2021; Accepted: 04 March 2022;
Published: 25 March 2022.
Edited by:
Qingli Dong, University of Shanghai for Science and Technology, ChinaReviewed by:
Qun Sun, Sichuan University, ChinaGeorge-John Nychas, Agricultural University of Athens, Greece
Copyright © 2022 Zhou, Zhang, Bassey, Huang, Zou and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keping Ye, eWVrZXBpbmcuYXJjQDE2My5jb20=
 Cong Zhou
Cong Zhou Yuanyuan Zhang
Yuanyuan Zhang Anthony Bassey
Anthony Bassey Jie Huang
Jie Huang Keping Ye
Keping Ye