- 1Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria
- 2Clinical Sciences Department, National Institute for Medical Research, Lagos, Nigeria
- 3Department of Radiology, College of Medicine, University of Lagos, Lagos, Nigeria
- 4Manchester Centre for Health Psychology, Division of Psychological Science and Mental Health, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom
- 5Manchester Fungal Infection Group, Core Technology Facility, The University of Manchester, Manchester, United Kingdom
Background: Pulmonary tuberculosis (PTB) often results in residual anatomical and functional changes despite microbiological cure and may be complicated by chronic pulmonary aspergillosis (CPA). In this study, we determined the perceived health-related quality of life (HRQoL) of patients during and after PTB therapy and compared it with their quantitative Aspergillus-specific IgG positivity rates.
Methodology: We conducted a longitudinal study among TB patients attending two directly observed therapy short-course (DOTS) clinics in Lagos, Nigeria. Two hundred and four confirmed TB patients were recruited over 9 months, with five visits at baseline and 3, 6, 9, and 12 months. They were all acid-fast bacilli smear, GeneXpert, or culture positive for Mycobacterium tuberculosis. Two HRQoL questionnaires translated into Yoruba were self-administered. Chest X-ray and Aspergillus IgG were collected at each visit.
Results: A total of 204 participants were recruited into this study. Most (70.6%) were age 18–39 years, and only 3.9% were above 60 years; 66.7% of all participants were males. A total of 189 (92.6%) participated in the 3-month assessment, 174 (85.3%) at 6 months, 139 (68.1%) at 9 months, and 99 (48.5%) at 12 months. At baseline, only 60.9% scored “good” or “very good” QoL and health on the WHOQOL-Bref, which improved to 77% at 6 months. At baseline, 10.4% had positive Aspergillus IgG levels, 15.1% at 3 months, 11.5% at 6 months, 16.7% at 9 months, and 19.3% at 12 months. Those with a positive Aspergillus IgG at 6 months had worse physical health (p = 0.001), psychological state (p = 0.002), social relationships (p = 0.006), and environmental QoL (p = 0.001) domains of the WHOQOL-Bref. Probable CPA was 10.4% at baseline and 19.3% at 6 months post-PTB therapy. Thirty-eight (18.6%) relocated after 6 months of treatment, 16 (7.8%) were lost to follow-up, and 11 (5.4%) died.
Conclusion: Our findings reveal a significant relationship between the QoL and Aspergillus IgG levels of TB patients. Further follow-up studies and additional imaging are required to determine when patients develop CPA and its clinical impact.
Introduction
Globally, tuberculosis (TB) is a major public health problem and remains one of the world’s deadliest communicable diseases. The World Health Organization (WHO) launched the “End TB Strategy” aimed at reducing TB incidence by 90% and mortality by 95% by 2030 (Uplekar et al., 2015). Each year, nearly 6 million people are diagnosed with pulmonary tuberculosis (PTB), of whom about 55% have their diagnosis confirmed with laboratory testing (World Health Organization, 2020). The recent WHO global TB report 2020 ranked Nigeria as the first in Africa and sixth globally among the 30 high TB burden countries, with a TB incidence of 219/100,000 population and 120,266 cases notified. Nigeria is on the list of 14 countries with the triple burden of TB, HIV-associated TB, and multidrug-resistant (MDR) TB with a population incidence of MDR-TB of 11/100,000 (World Health Organization, 2020).
Tuberculosis patients face various challenges such as physical restrictions, psychological and emotional issues, and economic and social problems. These known challenges impact on the quality of life of the patients (Hansel et al., 2004; Guo et al., 2009). Clinically, the therapeutic modalities, the side effects of the drugs, and the sequelae of the disease also impact on the quality of life (QoL) of the patients (Hansel et al., 2004; Muniyandi et al., 2007). PTB also results in residual anatomical and functional changes, despite microbiological cure (Pasipanodya et al., 2007). These changes are associated with post-TB lung disease (Pasipanodya et al., 2007). PTB patients often have pulmonary cavities, which can become colonized and infected by inhaled Aspergillus spores resulting in chronic pulmonary aspergillosis (CPA) (Denning et al., 2018). Other structural sequelae include fibrosis, chronic obstructive pulmonary disease (COPD) (Denning et al., 2018), and bronchiectasis, as well as sensitization to Aspergillus (IgE response), itself linked to worse lung function, independently of CPA (Dhooria et al., 2014). Worsening clinical status can be assumed to be MDR-TB.
TB services and clinical research in Nigeria have focused on the outcomes of mortality and microbiologic cure and have neglected the preferences of the patient, such as the quality of life of the patient, a crucial component of outcome. Health-related quality of life (HRQoL) involves assessing the perceptions of a person of his or her physical and mental health. Both physical and mental distress are common in TB patients leading to decreased compliance with medical care (Liefooghe et al., 1995; Rajeswari et al., 1999). The impact of PTB on HRQoL has been reported in a systematic literature review (Kastien-Hilka et al., 2016). This systematic review found that PTB had a negative impact on the HRQoL and overall wellbeing of the patients, even after PTB cure.
Quality of life assessment tools can be used in both high resource settings and in LMICs. Despite the availability of these standard instruments for assessing health-related quality of life, the feasibility, reliability, and validity of such instruments among TB patients in different populations of sub-Saharan Africa, where the burden of TB is of concern, are still limited.
This longitudinal study aimed to evaluate patient-reported HRQoL in pulmonary TB in Lagos, Nigeria, using two QoL assessment tools, namely, the generic WHOQOL-Bref (The WHOQOL Group, 1998; Skevington et al., 2004) and the specific St. George’s Respiratory Questionnaire (SGRQ) (American Thoracic Society (ATS); Welling et al., 2015). The study sought to identify persons with post-TB lung disease following “microbiological cure” 6 months post-therapy and understand the overall impact of TB on the QoL and important domains of qualities of life identified by the WHO.
Materials and Methods
Study Design
We conducted a longitudinal study among TB patients attending two directly observed treatment short-course (DOTS) clinics (National Institute of Medical Research and Lagos University Teaching Hospital) in Lagos, Nigeria, from March 2016 to February 2018. Ethical approval for this research was obtained from the HREC of both institutions. Confirmed (smear and/or Xpert (PCR) and/or culture positive for Mycobacterium tuberculosis) TB patients were recruited. Patients were excluded if they were diagnosed with multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) and/or had HIV co-infection. Informed written consent was obtained from each study participant. Eligible participants received a 6-month standard TB treatment with rifampicin, isoniazid, ethambutol, and pyrazinamide. Excluded from the study were patients with extrapulmonary TB and patients with any significant associated pulmonary diseases such as lung cancer, chronic obstructive pulmonary disease, and asthma and other chronic diseases likely to affect quality of life including diabetes mellitus and cardiovascular diseases.
Two QoL assessment tools were collected: the WHOQOL-Bref and SGRQ questionnaires. Sociodemographic factors including age, gender, educational status, and work status were captured. Mean transformed QoL scores were calculated from the 26 items of the WHOQOL-Bref, for its four QoL domains (physical, psychological, social, and environment) and for general overall QoL and health (Skevington and Epton, 2018). High scores mean very good QoL. Domain scores are sensitive to change during interventions (Skevington and Epton, 2018). SGRQ is a disease-specific instrument designed to assess patients with respiratory tract and immune system diseases, especially asthma, pulmonary diseases, and COPD (American Thoracic Society (ATS); Welling et al., 2015). SGRQ comprises 50 items in three domains (symptoms, activity, and impacts on daily life). Both scores are scaled from 0 to 100, with higher scores indicating worse QoL. A minimally important difference (MID) for SGRQ was defined as an improvement of 4 points in the domain scores and the total score (American Thoracic Society (ATS); Welling et al., 2015).
Questionnaires were administered five times altogether, at the start of TB treatment and then at 3-month intervals until 6 months post-therapy (Supplementary Table S1). The questionnaires were incorporated into clinical care visits for the convenience of the participants and were self-administered although supervised. Patients completed the questionnaire themselves in a quiet area, free from distraction, but a research nurse was available to give explanations, if required. The questionnaire was designed to elicit the perceptions of the patient of his/her health, not others views, so family, friends, or healthcare staff did not influence the responses of the patient. An accompanying spouse or partner was asked to wait in a separate area. Similarly, patients were not allowed to take the questionnaires home. It was emphasized that they should complete the questionnaire as honestly as possible and that there are no right or wrong answers: simply the answer that best applies to them. They were asked to answer all questions. Since literacy rates in Nigeria are relatively low, translation of the questionnaires into the local dialect Yoruba was available and a nurse was available to read it aloud for those who could not read Yoruba either.
Five milliliters of blood was collected at each visit and serum was separated for Aspergillus IgG levels testing using ELISA (Bordier affinity products SA, Switzerland, Bordier® Kit), with a cutoff of 1.0. Sputum was not collected for culture. Chest X-ray (CXR) was done at recruitment, then again at 3, 6, 9, and 12 months. Two independent radiologists, who had no immediate access to the Aspergillus IgG results, interpreted the CXR. CT scans were not performed and so CPA was not definitively diagnosed or confirmed. As it was highly likely in many patients, we refer to it as “probable CPA”.
Data Analysis
The first analysis assessed measurement (psychometric properties) in the sample to find out whether the WHOQOL-Bref is a valid, reliable measure to use in this population. The second step compared the QoL of people at different intervals after starting treatment for TB. The third step compared those with and without positive Aspergillus antibodies, especially those with documented chronic pulmonary aspergillosis. Descriptive statistics were applied to sociodemographic data and furthermore to all HRQoL data at baseline and in all follow-up visits, to understand the HRQoL impairment of TB patients. Descriptive statistics tested the following: frequencies (N, N missing, %), central tendency (mean, median), and confidence interval (set at 95%). The distribution of data was examined by standard deviation (SD), minimum and maximum values, and frequency plots.
Significance testing (paired-samples t-test), multivariable analysis, and repeated measures analysis of variance (ANOVA) were based on observations that contained all data points required for a specific analysis. Overall changes in HRQoL between baseline and 6-month treatment (visit 4) were calculated as frequencies (%). Longitudinal changes were determined by the change in mean scores between all follow-up visits and baseline. The change in mean scores was examined by paired-samples t-test, with a statistical significance (two-tailed) set a priori at p <0.05. Changes in mean scores in the intensive treatment phase (baseline to visit 2) were compared with changes in the continuous treatment phase (visit 2 to visit 5) based on paired-samples t-test with a statistical significance (two-tailed) set a priori at p <0.05. The change in mean scores at each time point from baseline was also compared with the reported MID for each measure, to understand if longitudinal changes in HRQoL were clinically meaningful. A paired-samples t-test was applied to examine the difference between changes in mean scores and MID at a significance level p <0.05.
Differences in QoL mean scores among all these measures over time (baseline and follow-up visits 1–4) were examined by repeated measures ANOVA with a Bonferroni correction applied to adjust for multiple tests. Responsiveness over time for each HRQoL dimension was measured as an effect size partial eta-squared, providing information of the effect of time on changes in QoL. The resulting candidate factors were further assessed in multivariable models to understand the impact of sociodemographic factors over time [change from baseline to 6 months post-treatment (visit 5)]. The univariate and multivariate analyses included a general linear model and ANOVA.
Results
Sociodemographic Data
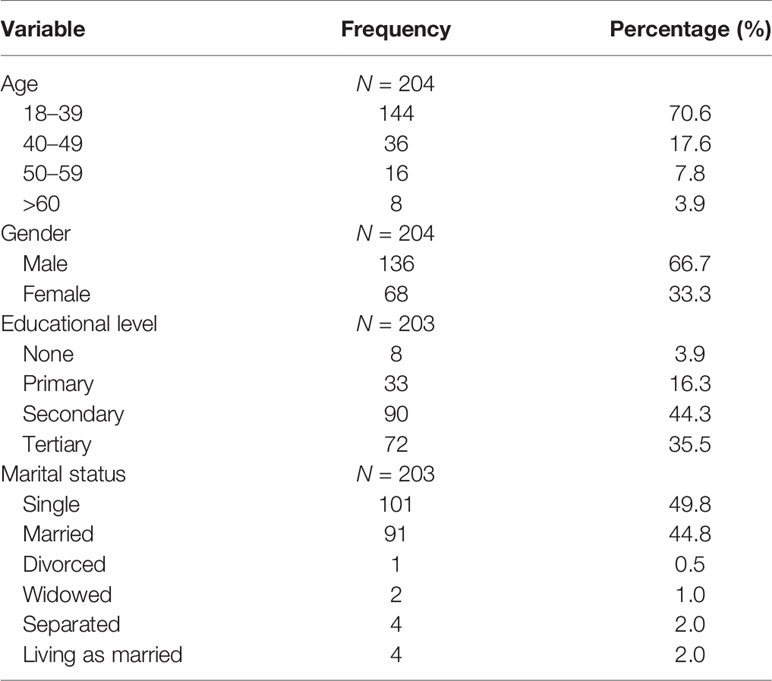
A total of 204 participants with confirmed PTB were recruited into this study, of which 144 (70.6%) were aged 18–39 years (Table 1). One hundred and thirty-six (66.7%) were men giving a male:female ratio of 2:1. Educational levels varied substantially (from illiteracy to tertiary education levels), with 3.9% having no formal education. About half (49.8%) of the participants were single, and 44.8% were married (Table 1).
Overall Quality of Life and General Health (WHOQOL-Bref)
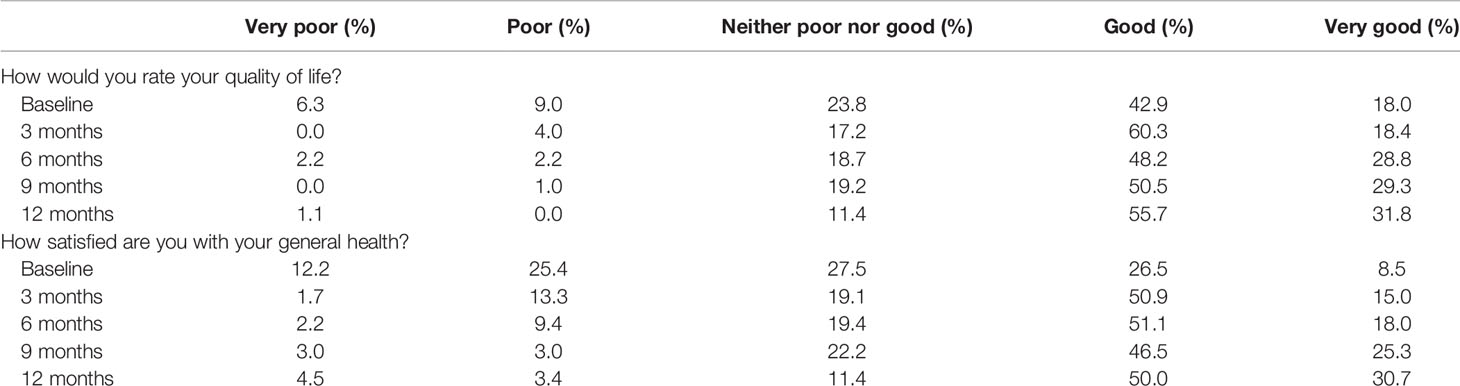
Of the 204 participants, 189 (92.6%) participated in the baseline assessment, 174 (85.3%) at 3 months, 139 (68.1%) at 6 months, 99 (48.5%) at 9 months, and 88 (43.1%) at 12 months. Of the participants, 60.9% had a good or very good perception of their QoL at baseline assessment, 78.7% at 3 months, 77% at 6 months, 79.8% at 9 months, and 87.5% at the 12-month assessment (Table 2). In contrast, at baseline, 15.3% described their QoL as very poor or poor, 4.0% at 3 months, 4.4% at 6 months, 1.0 at 9 months, and 1.1% at 12 months (Table 2). These response trends were mirrored by general health and overall QoL questions (Table 2).
Changes in the Dimensions of HRQOL Measures
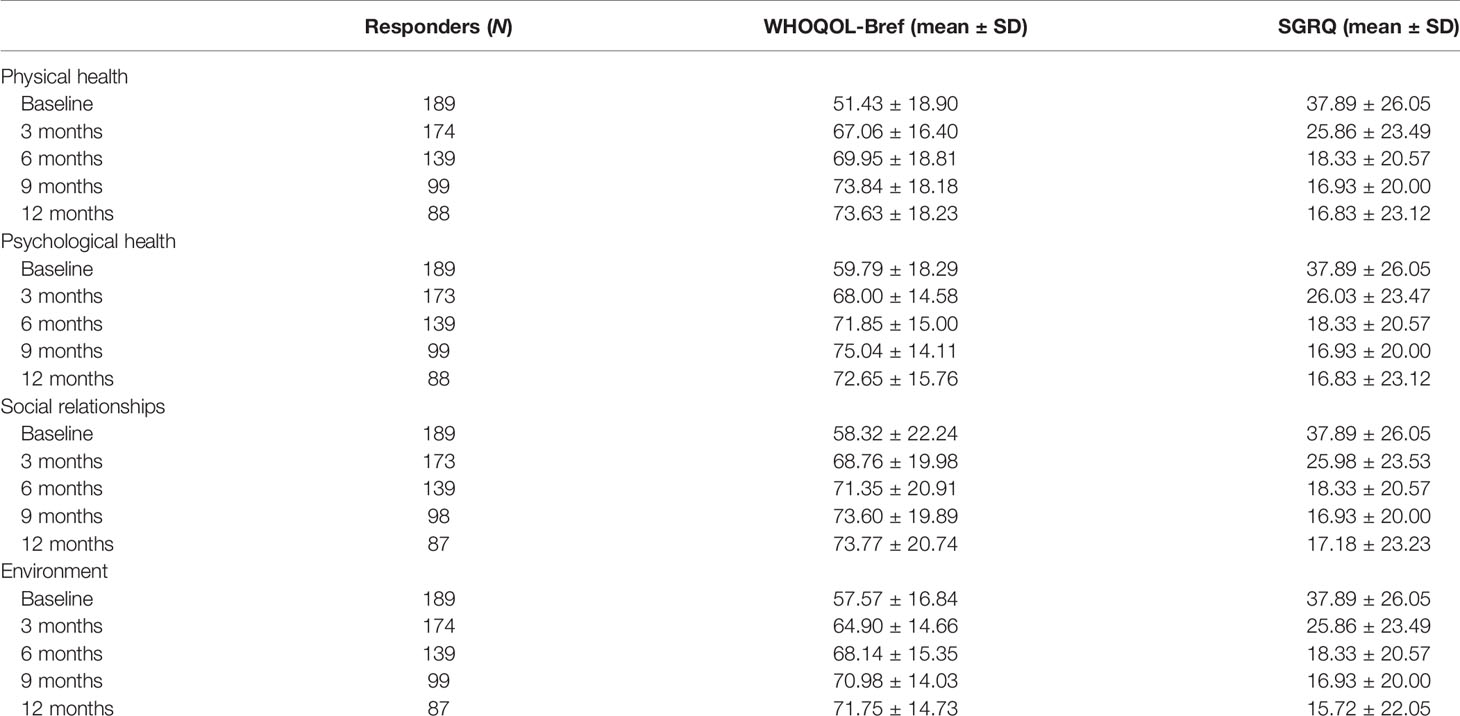
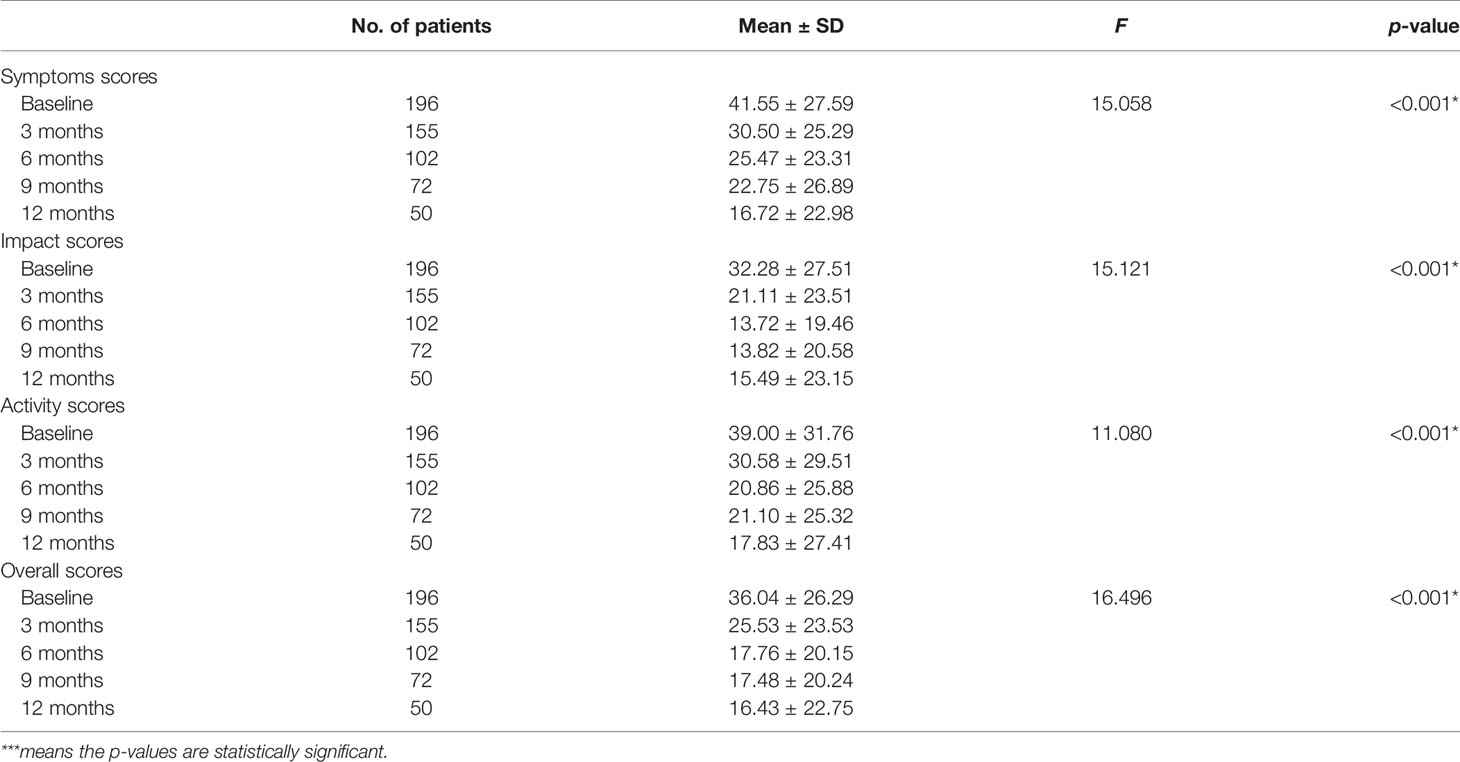
The mean scores increased with the length of TB therapy in the domains of the WHOQOL-Bref, with the highest at the 12-month assessment and the lowest during the baseline assessment (Table 3). Over the course of 12 months, there was significant improvement in quality of life as measured by both the WHOQOL-Bref and SGRQ. Scores decreased by 9–25 points in the SGRQ, while scores increased by 12–20 points depending on the domain of the WHOQOL-Bref.
Comparison of HRQoL Scores in Those With and Without a Positive Aspergillus IgG
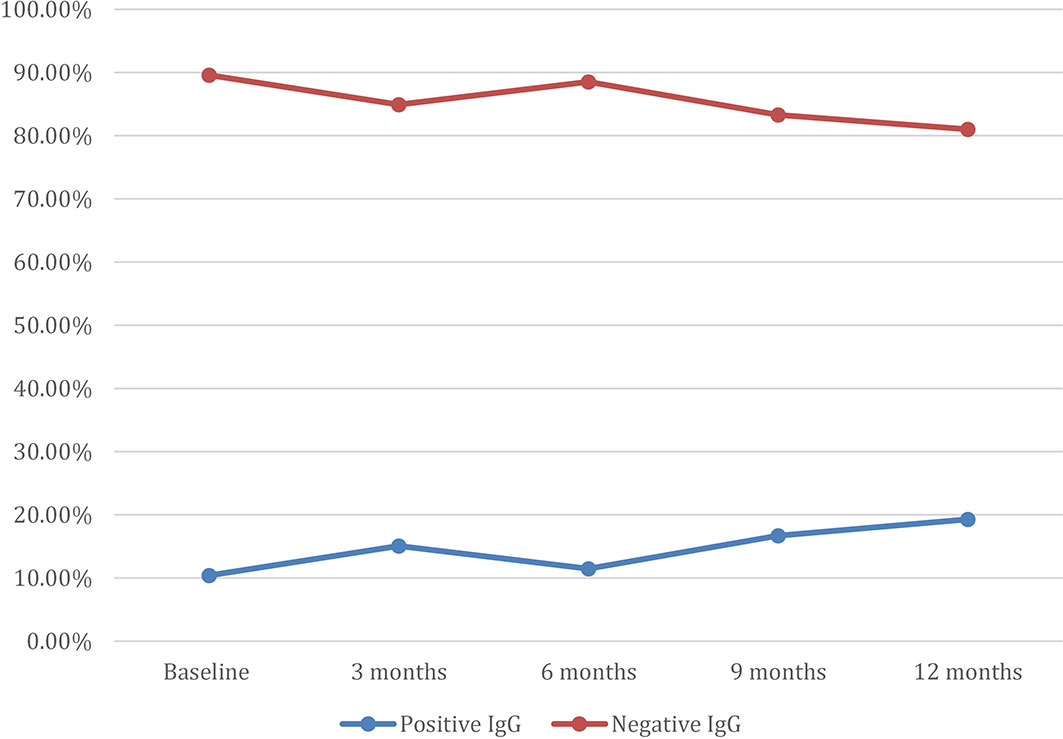
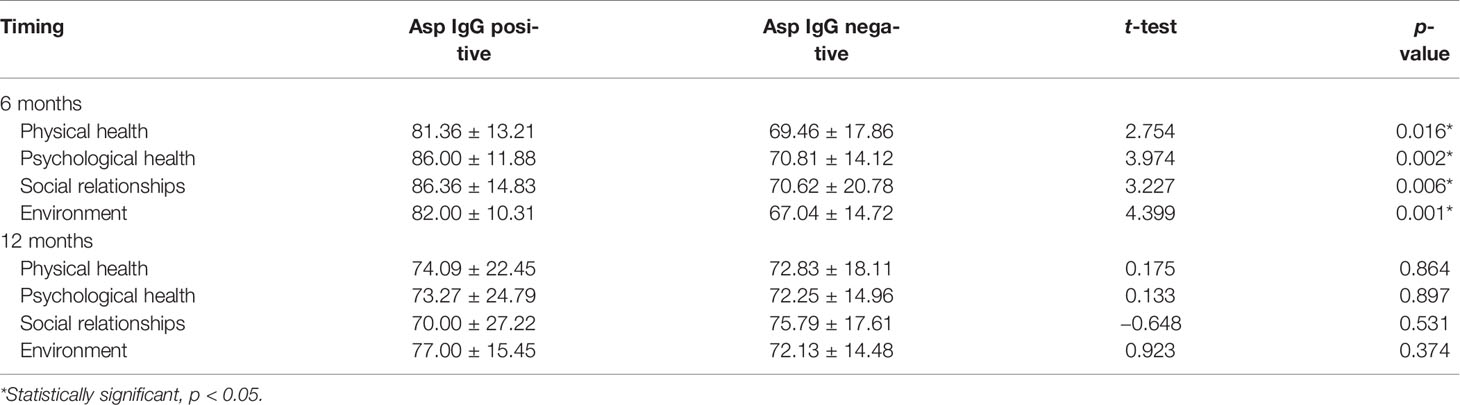
Among the participants, 10.4% had a positive Aspergillus IgG at baseline assessment, 15.1% at 3 months, 11.5% at 6 months, 16.7% at 9 months, and 19.3% at 12 months (Figure 1). There was a statistically significant difference in the mean WHOQOL-Bref domain scores between participants with positive and negative Aspergillus IgG at the 6-month assessment, in physical health (p = 0.001), psychological state (p = 0.002), social relationships (p = 0.006), and environmental (p = 0.001) quality of life domains (Table 4). At 6 months, WHOQOL-Bref scores were significantly higher in patients with positive Aspergillus IgG levels compared with those with negative Aspergillus IgG levels, in all four domains (p < 0.05). At 12 months, QoL scores were higher in those with elevated Aspergillus IgG levels, compared with low Aspergillus IgG levels in physical, psychological, and environmental QoL domains. Although social QoL scores were lower in probable CPA patients compared with those in non-CPA patients, the difference was not significant (p > 0.05). To remove the confounding effect, linear regression was done using Asp IgG levels and total QoL scores, and it was statistically significant (p-value 0.029) at 9 months.
Symptoms Measured by the SGRQ
Statistical difference was observed between mean symptoms scores from successive assessments over 12 months, using one-way ANOVA (F = 15.058, p-value < 0.001) (Table 4). A Tukey post-hoc test revealed that the mean baseline score was significantly higher than all the other months of assessment and significantly higher at 3 months than at 12 months (p < 0.05) (Table 5). A significant difference was also found between mean symptoms, impacts, activity, and overall score and between months of assessment (one-way ANOVA, p < 0.001) (Table 5).
Comparison of SGRQ in Aspergillus IgG-Positive Versus Aspergillus IgG-Negative Participants
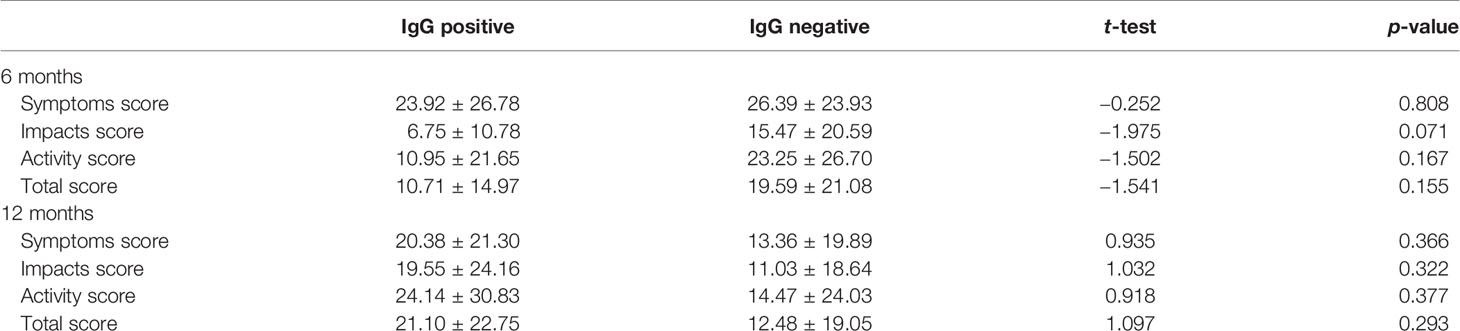
At 6 months, participants with positive Aspergillus-specific IgG levels had lower SGRQ scores indicative of worse health compared with those that had negative Aspergillus-specific IgG levels, but this difference was not statistically significant (p > 0.05) (Table 6). Scores were slightly higher in those with positive Aspergillus IgG at 12 months, but this was also not significant (p > 0.05).
Radiological Findings
At baseline, 69.5% of the participants had CXR features reported as PTB, while 14.7% had features suggestive of CPA or PTB/CPA co-infection, and 12.4% had normal CXR (Figure 2A). There was improvement at 3 months on anti-TB therapy, with 41.9% showing improvement in CXR findings with only 23.4% with classical features of PTB. At 6 months when anti-TB therapy was completed, both normal CXR features and features of improvement were found in 41.2% of the participants (Figure 2B). Further improvement was documented at 3 months after anti-TB therapy, with normal CXR features documented in 50% (Figure 2C). However, at 6 months after anti-TB therapy, normal CXR features were still found in only 50% of participants, but 20% had features suggestive of either PTB or CPA or both (Figure 2D).

Figure 2 Assessment of CXR findings in studied population. (A) Baseline, (B) 6 months, (C) 9 months, (D) 12 months.
Probable CPA
Integrating the combination of persistent symptoms (>3 months), CXR features of CPA and raised Aspergillus IgG enabled a diagnosis of probable CPA at different time points (Figure 2). Overall probable CPA was found in 20/193 (10.4%) at baseline, 17/148 (11.5%) at 6 months, and 17/88 (19.3%) at 6 months post-TB therapy. There was no statistically significant relationship between any of the domains of HRQoL and probable CPA.
Discussion
The WHO ascertains that patient involvement in their healthcare is a social, economic, and technical necessity (Guo et al., 2009; Kastien-Hilka et al., 2016). Disease-specific health status questionnaires have proven their ability to discriminate between different levels of disease severity (Hays et al., 1995; Kastien-Hilka et al., 2016). However, generic measures like the WHOQOL-Bref (Skevington et al., 2004) are designed to be completed by patients with almost any disease or condition and may be designed to be completed by healthy people, too. Such generic instruments provide a systematic way to usefully compare patient and proxy assessments of QoL and health status across different organ systems and geographies. They also provide information beyond those obtained from clinical and microbiological parameters. Patient experience is assessed using a patient-reported outcome measure (PRO). PROs for CPA have included SGRQ, but this can only be compared among other lung diseases, not other conditions, and is poorly validated in countries with English as a second or third language.
In this study, we observed that most participants were ill at enrollment, with the lowest WHOQO-Bref and the highest SGRQ scores. Scores improved in relation to the length of TB therapy in the different QoL domains, with the highest at 12 months. Moreover, most respondents at 3 months had good perceptions of their overall QoL and general health. However, during this same period, a significant number of respondents were found to have positive Aspergillus IgG (Figure 1). Overall QoL and general health demonstrated the highest elevation of scores from baseline to 6 months. This most likely reflects the known efficacy of anti-TB therapy and the compliance of the patients.
Research studies using the HRQoL among patients treated for TB in Africa are few (Adeyeye et al., 2014), and to our knowledge, none has been longitudinal. Furthermore, this study is the first to investigate both TB and Aspergillus IgG levels and CPA and its effect on HRQoL. Studies like these are urgently needed (Brown et al., 2015) to better manage these large populations.
The finding of progressive improvement in QoL due to anti-TB therapy accords with the findings from similar studies that used the WHOQOL-Bref, for <12 months (Dhuria et al., 2009; Balgude and Sontakke, 2012; Aggarwal et al., 2013; Aggarwal, 2019). This was also the trend in other studies that did not use the WHOQOL-Bref (Babikako et al., 2010; Kruijshaar et al., 2010; Atif et al., 2014). All these studies revealed a gradual increase in scores and improved QoL, as treatment progressed.
Some societal factors that could influence QoL were assessed in our study. Domain scores were generally better among men, urban residents, younger patients, and those with higher socioeconomic status, less severe disease, higher education, better social security, or close family support (Duyan et al., 2005; Babikako et al., 2010; Adeyeye et al., 2014). Independent predictors of low quality of life scores included depression, illiteracy, self-stigma, low monthly income, duration of illness, concomitant illnesses, unemployment, advancing age, no family support, and sociodemographic and other economic factors (Duyan et al., 2005; Adeyeye et al., 2014). Although some of our findings are comparable to other studies, the impact of gender is not clear. We recruited more men, and being male has been associated with good domain scores (Adeyeye et al., 2014; Brown et al., 2015), although not universally (Hays et al., 1995). As expected, treatment with antituberculosis drugs improves the HRQoL of patients (Deribew et al., 2009). From a programmatic perspective, it is important to ensure adherence to medications and avoid treatment default.
In our study, the 12-month means of physical and psychological QoL in this study were lower than the means at the 9-month visit. This finding was previously reported in a study by Mamani et al. in 2014 (Mamani et al., 2014) and may be attributed to a recurrence of symptoms. If so, these symptoms could be due to the re-emergence of TB or the presence of aspergillosis; this is further supported by CXR reports (Figures 2B, C). Alternatively, it could represent co-infection of TB and aspergillosis, bronchiectasis, or Aspergillus bronchitis complicating bronchiectasis. Further studies involving fungal culture and chest imaging would be helpful to measure the prevalence of pulmonary aspergillosis complicating tuberculosis among this cohort.
There was, however, a significant difference (p < 0.05) between respondents with positive and negative Aspergillus IgG at 6 months of assessment, in all the domains of the WHOQOL-Bref. This could result from the continuous presence of symptoms, as reported by a previous longitudinal study of CPA (Bongomin et al., 2018). A Ugandan study has reported that Aspergillus-specific IgG antibodies were elevated in 4% of HIV-infected adults at the start of TB treatment and in 9% at the end (Kwizera et al., 2017). This is likely due to colonization and either the development of CPA, a reflection of Aspergillus co-infection that needs to be resolved, or Aspergillus bronchitis complicating bronchiectasis among TB patients. Given the slow genesis of CPA, longitudinal follow-up and CT scanning are required, but our radiological data appear to supports this hypothesis (Figure 2). Reduced pulmonary function persists in patients cured of TB (Pasipanodya et al., 2007).
A drop in the number of respondents with each visit was observed in this study. Reasons include transfers of patients to sites closer to home, treatment default, death, and hospitalization. Other studies have reported that the dropout at the second and third follow-up visits was mostly among younger patients with no physical impairment as well as among male patients and due to poverty, severe psychological distress, or alcohol misuse (Louw et al., 2012; Atif et al., 2014; Olufemi et al., 2018). It is important to note that the treatment of TB was completed for these patients at 6 months. Thus, the need to return to the clinic may have not been vital to them visiting the facility again, as they may have achieved a complete cure to the disease. Also with improved QoL, there is a higher likelihood that treatment default may be high due to reduced motivation to improve it.
Some limitations of the study were the continuous drop in respondent numbers at 9 and 12 months, which may have had some impact on the results. A defined cutoff for Aspergillus IgG levels has not been established for Africans, although the European-derived cutoff (Wilopo et al., 2020) performs well in Vietnam (Nguyen et al., 2021). CT scan, which is a better representative imaging tool, was not used due to cost, and its inclusion would have allowed confirmation of the diagnosis of CPA. Also, patients underwent HIV testing when diagnosed with TB before starting treatment. HIV testing was not repeated during the treatment and any change in HIV status was therefore unknown. The severity of PTB was not accessed since it was not the focus of the study.
In conclusion, different domains of HRQoL can be a helpful tool for the assessment of the patient and outcome prediction. HRQoL is hindered in patients with PTB and improves significantly with program-based treatment. HRQoL-based disease appraisals in resource-limited countries have become an important instrument to grasp health outcomes and provide focused and empirically informed ways to manage care and treatment better. Aspergillus IgG levels were significantly raised in patients being managed for PTB, probably reflecting colonization by Aspergillus or early CPA, which alters treatment outcomes among TB patients. Routine screening of Aspergillus IgG at the DOTS facility would assist with improving the QoL of patients who may have been misdiagnosed as having PTB. Further follow-up studies and additional imaging, preferably CT scan, are required to determine when these patients develop CPA and its clinical impact.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee (HREC), Lagos University Teaching Hospital, Lagos, Nigeria; HREC, National Institute of Medical Research, Lagos, Nigeria; and HREC, The University of Manchester. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RO, SS, and DD conceptualized and designed the study. RO, TG, and NI collated the data. RO, SS, and DD analyzed and interpreted the data. All authors contributed to the writing and review of the manuscript.
Funding
This work was supported with funds from University Hospital South Manchester Charity Trust (Grant award number: TREG 006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Tina Nwosu for her support in the laboratory processes and the staff at the DOTS clinic for their support in the recruitment and follow-up of patients. We are indebted to Graham Atherton for setting up and hosting the online platform to capture and calculate the SGRQ data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.790134/full#supplementary-material
References
Adeyeye, O. O., Ogunleye, O. O., Coker, A., Kuyinu, Y., Bamisile, R. T., Ekrikpo, et al. (2014). Factors Influencing Quality of Life and Predictors of Low Quality of Life Scores in Patients on Treatment for Pulmonary Tuberculosis: A Cross Sectional Study. J. Public Health Afr. 5 (2), 88–92. doi: 10.4081/jphia.2014.366
Aggarwal, A. N. (2019). Quality of Life With Tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 17, 100121. doi: 10.1016/j.jctube.2019.100121
Aggarwal, A. N., Gupta, D., Janmeja, A. K., Jindal, S. K. (2013). Assessment of Health-Related Quality of Life in Patients With Pulmonary Tuberculosis Under Programme Conditions. Int. J. Tuberc. Lung Dis. 17 (7), 947–953. doi: 10.5588/ijtld.12.0299
Atif, M., Sulaiman, S. A., Shafie, A. A., Asif, M., Sarfraz, M. K., Low, H. C. (2014). Impact of Tuberculosis Treatment on Health-Related Quality of Life of Pulmonary Tuberculosis Patients: A Follow-Up Study. Health Qual. Life Outcomes 12, 19. doi: 10.1186/1477-7525-12-19
American Thoracic Society (ATS). St. George’s Respiratory Questionnaire (SGRQ). Available at: https://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php.
Babikako, H. M., Neuhauser, D., Katamba, A., Mupere, E. (2010). Feasibility, Reliability and Validity of Health-Related Quality of Life Questionnaire Among Adult Pulmonary Tuberculosis Patients in Urban Uganda: Cross-Sectional Study. Health Qual. Life Outcomes 8, 93. doi: 10.1186/1477-7525-8-93
Balgude, A., Sontakke, S. (2012). Study of Impact of Antitubercular Therapy on Quality of Life. Indian J. Med. Sci. 66 (3-4), 71–77. doi: 10.4103/0019-5359.110911
Bongomin, F., Harris, C., Hayes, G., Kosmidis, C., Denning, D. W. (2018). Twelve-Month Clinical Outcomes of 206 Patients With Chronic Pulmonary Aspergillosis. PloS One 13 (4), e0193732. doi: 10.1371/journal.pone.0193732
Brown, J., Capocci, S., Smith, C., Morris, S., Abubakar, I., Lipman, M. (2015). Health Status and Quality of Life in Tuberculosis. Int. J. Infect. Dis. 32, 68–75. doi: 10.1016/j.ijid.2014.12.045
Denning, D. W., Page, I. D., Chakaya, J., Jabeen, K., Jude, C. M., Cornet, M., et al. (2018). Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerging Infect. Dis. 24 (8). doi: 10.3201/eid2408.171312
Deribew, A., Tesfaye, M., Hailmichael, Y., Negussu, N., Daba, S., Wogi, A. (2009). Tuberculosis and HIV Co-Infection: Its Impact on Quality of Life. Health Qual. Life Outcomes 7, 105. doi: 10.1186/1477-7525-7-105
Dhooria, S., Kumar, P., Saikia, B., Aggarwal, A., Gupta, D., Behera, D., et al. (2014). Prevalence of Aspergillus Sensitisation in Pulmonary Tuberculosis-Related Fibrocavitary Disease. Int. J. Tuberc. Lung Dis. 18 (7), 850–855. doi: 10.5588/ijtld.13.0838
Dhuria, M., Sharma, N., Narender, P. S., Ram, C. J., Saha, R., Gopal, K. I. (2009). A Study of the Impact of Tuberculosis on the Quality of Life and the Effect After Treatment With DOTS. Asia Pac. J. Public Health 21 (3), 312–320. doi: 10.1177/1010539509336242
Duyan, V., Kurt, B., Aktas, Z., Duyan, G. C., Kulkul, D. O. (2005). Relationship Between Quality of Life and Characteristics of Patients Hospitalised With Tuberculosis. Int. J. Tuberc. Lung Dis. 9 (12), 1361–1366.
Guo, N., Marra, F., Marra, C. A. (2009). Measuring Health-Related Quality of Life in Tuberculosis: A Systematic Review. Health Qual. Life Outcomes 7, 14. doi: 10.1186/1477-7525-7-14
Hansel, N. N., Wu, A. W., Chang, B., Diette, G. B. (2004). Quality of Life in Tuberculosis: Patient and Provider Perspectives. Qual. Life Res. 13, 639–652. doi: 10.1023/B:QURE.0000021317.12945.f0
Hays, R. D., Wells, K. B., Sherbourne, C. D., Rogers, W., Spritzer, K. (1995). Functioning and Well-Being Outcomes of Patients With Depression Compared With Chronic General Medical Illnesses. Arch. Gen. Psychiatry 52 (1), 11–19. doi: 10.1001/archpsyc.1995.03950130011002
Kastien-Hilka, T., Abulfathi, A., Rosenkranz, B., Bennett, B., Schwenkglenks, M., Sinanovic, E. (2016). Health-Related Quality of Life and Its Association With Medication Adherence in Active Pulmonary Tuberculosis–a Systematic Review of Global Literature With Focus on South Africa. Health Qual. Life Outcomes 14 (1), 1–3. doi: 10.1186/s12955-016-0442-6
Kruijshaar, M. E., Lipman, M., Essink-Bot, M. L., Lozewicz, S., Creer, D., Dart, S. (2010). Health Status of UK Patients With Active Tuberculosis. Int. J. Tuberc. Lung Dis. 14 (3), 296–302.
Kwizera, R., Parkes-Ratanshi, R., Page, I. D., Sekaggya-Wiltshire, C., Musaazi, J., Fehr, J., et al. (2017). Elevated Aspergillus-Specific Antibody Levels Among HIV Infected Ugandans With Pulmonary Tuberculosis. BMC Pulm. Med. 17 (1), 1–9. doi: 10.1186/s12890-017-0500-9
Liefooghe, R., Michiels, N., Habib, S., Moran, M. B., De Muynck, A. (1995). Perception and Social Consequences of Tuberculosis: A Focus Group Study of Tuberculosis Patients in Sialkot, Pakistan. Soc. Sci. Med. 41 (12), 1685–1692. doi: 10.1016/0277-9536(95)00129-U
Louw, J., Peltzer, K., Naidoo, P., Matseke, G., Mchunu, G., Tutshana, B. (2012). Quality of Life Among Tuberculosis (TB), TB Retreatment and/or TB-HIV Co-Infected Primary Public Health Care Patients in Three Districts in South Africa. Health Qual. Life Outcomes 10, 77. doi: 10.1186/1477-7525-10-77
Mamani, M., Majzoobi, M. M., Ghahfarokhi, S. M., Esna-Ashari, F., Keramat, F. (2014). Assessment of Health-Related Quality of Life Among Patients With Tuberculosis in Hamadan, Western Iran. Oman Med. J. 29 (2), 102–105. doi: 10.5001/omj.2014.25
Muniyandi, M., Rajeswari, R., Balasubramanian, R., Nirupa, C., Gopi, P. G., Jaggarajamma, K., et al. (2007). Evaluation of Post-Treatment Health-Related Quality of Life (HRQoL) Among Tuberculosis Patients. Int. J. Tuberc. Lung Dis. 11, 887–892.
Nguyen, N. T., Le Ngoc, H., Nguyen, N. V., Dinh, L. V., Nguyen, H. V., Nguyen, H. T., et al. (2021). Chronic Pulmonary Aspergillosis Situation Among Post Tuberculosis Patients in Vietnam: An Observational Study. J. Fungi 7 (7), 532. doi: 10.3390/jof7070532
Olufemi, A. O., Chikaodinaka, A. A., Abimbola, P., Oluwatoyin, A. T., Oluwafunmilola, A., Fasanmi, K. T. (2018). Health-Related Quality of Life (HRQoL) Scores Vary With Treatment and may Identify Potential Defaulters During Treatment of Tuberculosis. Malawi Med. J. 30 (4), 283–290. doi: 10.4314/mmj.v30i4.12
Pasipanodya, J. G., Miller, T. L., Vecino, M., Munguia, G., Bae, S., Drewyer, G., et al. (2007). Using the St. George Respiratory Questionnaire to Ascertain Health Quality in Persons With Treated Pulmonary Tuberculosis. Chest 132, 1591–1598. doi: 10.1378/chest.07-0755
Rajeswari, R., Balasubramanian, R., Muniyandi, M., Geetharamani, S., Thresa, X., Venkatesan, P. (1999). Socio-Economic Impact of Tuberculosis on Patients and Family in India. Int. J. Tuberc. Lung Dis. 3 (10), 869–877.
Skevington, S. M., Epton, T. (2018). How Will the Sustainable Development Goals Deliver Changes in Wellbeing? A Systematic Review and Meta-Analysis to Investigate Whether WHOQOL-BREF Scores Respond to Change. Br. Med. J. Global Health 3, 1–13, 0e.000609. doi: 10.1136/bmjgh-2017-000609
Skevington, S. M., Lotfy, M., O’Connell, K. A. (2004). The World Health Organisation's WHOQOL-BREF Quality of Life Assessment: Psychometric Properties and Results of the International Field Trial- A Report From the WHOQOL Group. Qual. Life Res. 13 (2), 299–310. doi: 10.1023/B:QURE.0000018486.91360.00
The WHOQOL Group (1998). Development of the WHOQOL-BREF Quality of Life Assessment. Psychol. Med. 28, 551–558. doi: 10.1017/S0033291798006667
Uplekar, M., Weil, D., Lonnroth, K., Jaramillo, E., Lienhardt, C., Dias, H. M., et al. (2015). WHO's New End TB Strategy. Lancet 385 (9979), 1799–1801. doi: 10.1016/S0140-6736(15)60570-0
Welling, J. B., Hartman, J. E., Ten Hacken, N. H., Klooster, K., Slebos, D. J. (2015). The Minimal Important Difference for the St George's Respiratory Questionnaire in Patients With Severe COPD. Eur. Respir. J. 46 (6), 1598–1604. doi: 10.1183/13993003.00535-2015
Wilopo, B. A., Hunter, E. S., Richardson, M. D., Denning, D. W. (2020). Optimising the Cut-Off of the Bordier Aspergillus IgG ELISA for the Diagnosis of Chronic Pulmonary Aspergillosis. J. Microbiol. Methods 176, 106021. doi: 10.1016/j.mimet.2020.106021
Keywords: Aspergillus IgG, tuberculosis, quality of life, LMICs, Nigeria, chronic pulmonary aspergillosis
Citation: Oladele RO, Gbajabimiala T, Irurhe N, Skevington SM and Denning DW (2022) Prospective Evaluation of Positivity Rates of Aspergillus-Specific IgG and Quality of Life in HIV-Negative Tuberculosis Patients in Lagos, Nigeria. Front. Cell. Infect. Microbiol. 12:790134. doi: 10.3389/fcimb.2022.790134
Received: 06 October 2021; Accepted: 10 January 2022;
Published: 03 February 2022.
Edited by:
Mohammad T. Hedayati, Mazandaran University of Medical Sciences, IranReviewed by:
Felix Bongomin, Gulu University, UgandaRitesh Agarwal, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2022 Oladele, Gbajabimiala, Irurhe, Skevington and Denning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita O. Oladele, cm9sYWRlbGVAdW5pbGFnLmVkdS5uZw==
 Rita O. Oladele
Rita O. Oladele Titi Gbajabimiala2
Titi Gbajabimiala2 Nicholas Irurhe
Nicholas Irurhe Suzanne M. Skevington
Suzanne M. Skevington David W. Denning
David W. Denning