- 1Department of Respiratory Medicine, Shenzhen Children′s Hospital, Shenzhen, Guangdong, China
- 2Microbiology Laboratory, National Center for Children’s Health, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, Beijing, China
To investigate the clinical characteristics and treatment of septic arthritis caused by Streptococcus pyogenes(S. pyogenes) in children, we retrospectively analyzed the clinical data, laboratory results, treatments and outcomes of three pediatric cases of septic arthritis caused by S. pyogenes occurring from 2016–2018. The three cases of septic arthritis included 1 boy and 2 girls, aged from 2–7 years. Two patients experienced fever, and in all three cases, the affected joints showed redness, swelling, an increased local skin temperature, tenderness and restricted limb movement. At the first visit, all three cases showed a significantly increased white blood cell count [(27.68–32.02)×109/mL] and a significantly increased erythrocyte sedimentation rate (113–134 mm/h). The C-reactive protein level was significantly increased in two cases (67 mg/L, 147.7 mg/L) and normal in one case. The procalcitonin level was normal in 1 case, elevated in 1 case, and undetected in 1 case. S. pyogenes isolated from cases 1 and 2 were emm1/ST28 and from case 3 was emm12/ST36. All patients were treated by abscess incision and drainage, and S. pyogenes was cultured in the abscess puncture fluid. All patients were treated with intravenous antibiotics after admission, and all patients were cured and discharged. The patients were followed up for 2 months, and their condition was improved and stable. No sequelae such as heart and kidney damage were detected. In conclusion, for children with septic arthritis, early diagnosis and timely treatment with incision and drainage followed by culture of the abscess puncture fluid are important. Once S. pyogenes infection is confirmed, β-lactam antibiotics provide effective treatment, avoiding use of broad-spectrum antibiotics.
Introduction
Group A Streptococcus (GAS, also known as Streptococcus pyogenes), is an important pathogen that most commonly colonizes the upper respiratory tract and skin epithelium (Rouchon et al., 2017). Its pathogenicity corresponds to a broad spectrum of conditions, including pharyngitis, impetigo, scarlet fever, nephritis, rheumatic fever, rheumatic heart disease, and many other diseases (Chaudhary et al., 2018) and can also lead to severe invasive infections such as sepsis, necrotizing fasciitis, and toxic shock syndrome, which are associated with high morbidity, mortality, and disability rates (McMillan et al., 2007). The pathogenic bacteria most commonly found to be responsible for septic arthritis are Staphylococcus aureus (Jin et al., 2015), Haemophilus influenzae (Zhang et al., 2019), and Streptococcus agalactiae (Dhekane et al., 2020), and although less common, septic arthritis caused by S. pyogenes infection has been reported. In the present case series, we retrospectively analyzed the clinical data of children treated for septic arthritis due to S. pyogenes infection in our hospital to provide a basis for the diagnosis and treatment of septic arthritis cases due to S. pyogenes infection.
Data and methods
General data
Three cases with a clinical diagnosis of septic arthritis for which pus culture in the microbiology laboratory identified Streptococcus pyogenes between May 2016 and January 2018 were selected as the study subjects. The diagnosis of septic arthritis was made with reference to the literature criteria (Xu et al., 2016), which include: (1) signs and symptoms of infection, such as fever, local pain, swelling, and limitation of activity; (2) imaging examinations (such as ultrasound, computed tomography [CT] or magnetic resonance imaging [MRI]) suggesting local soft tissue swelling and joint cavity effusion; (3) X-ray examination suggesting joint dislocation or subluxation or local bone destruction; (4) significant elevation of inflammatory indexes such as white blood cell (WBC) count, C-reactive protein (CRP) level, and erythrocyte sedimentation rate (ESR); and (5) extraction of pus by puncture of the joint cavity. The diagnosis of septic arthritis is made only when three or more of the above criteria are met.
Methods
This retrospective study analyzed the clinical characteristics, laboratory results and pathological findings for three pediatric cases of septic arthritis, and the patients were followed up by telephone for more than 2 months. For bacterial culture detection, pus specimens were inoculated in 5% defibrinated sheep blood culture dishes. According to the morphological characteristics of the colony in the blood plate and its β- hemolytic ring, strains were further identified as GAS with Lancefield group specific antisera. Genomic DNA was obtained from freshly grown S. pyogenes using a Chelex-based DNA extraction kit for genetic analysis, according to the instructions of the DNA extraction kit (Beijing Sangong Bioengineering Co.). The emm sequence types were determined using a previously reported protocol (https://www2.cdc.gov/vaccines/biotech/strepblast.asp). Amplicons were sequenced by Guangzhou BGI Genomics Co. Ltd., and emm type was determined based on the sequence identity (>95%) of the first 180 bp of the emm gene between the tested sequence and the reference emm gene.
Results
Clinical presentation
The three pediatric cases included in this retrospective analysis included two male patients and one female patient, between the ages of 2 and 7 years. All three children presented with inflammatory manifestations such as localized skin erythema, localized pain, increased local skin temperature, visible pus on puncture and identification of S. pyogenes by bacterial culture, confirming the diagnosis of septic arthritis. Details are shown in Table 1.
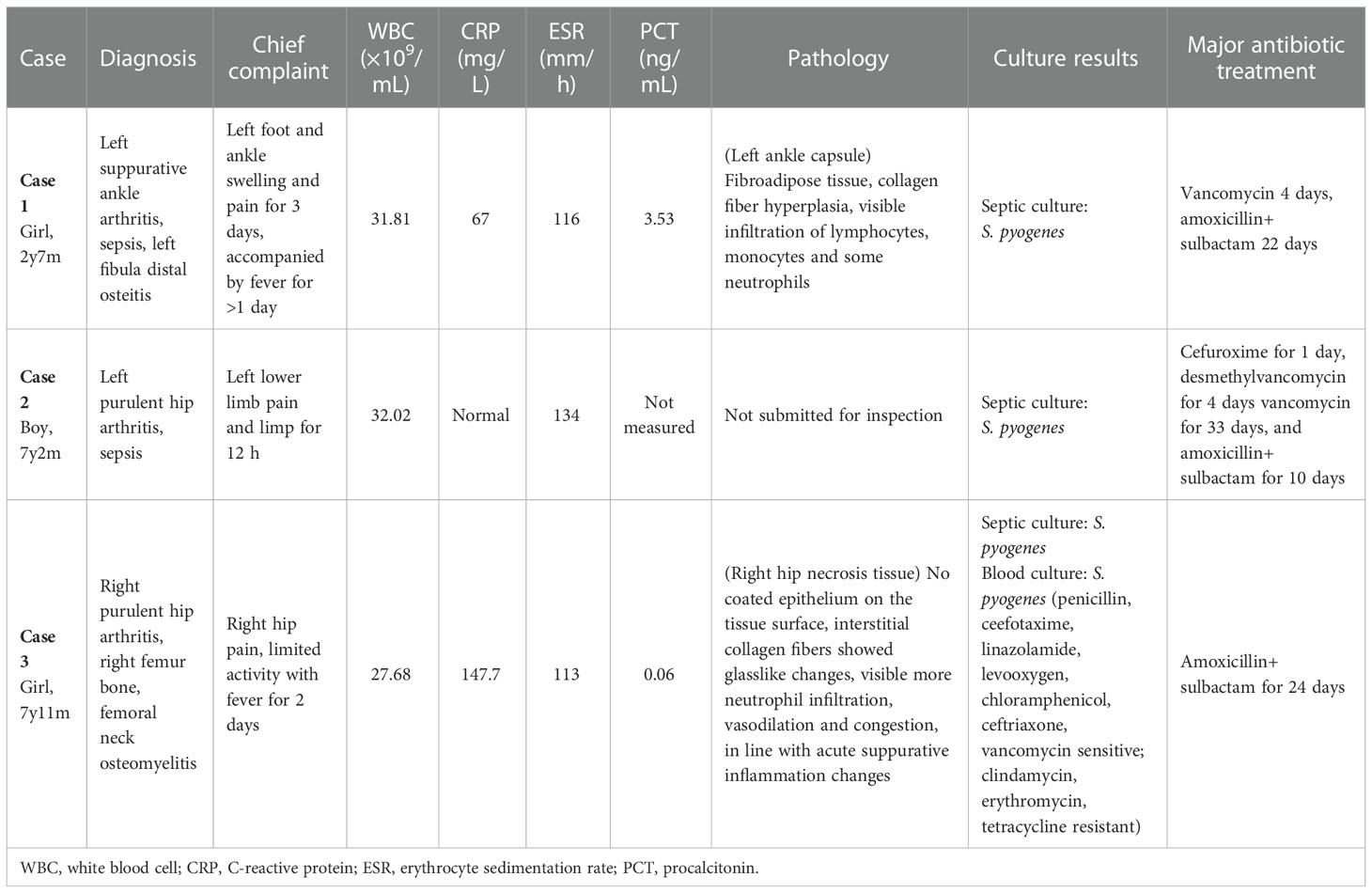
Table 1 Clinical data of three pediatric cases of septic arthritis caused by Streptococcus pyogenes.
Laboratory findings
At the first visit, the peripheral WBC count was significantly elevated in all three cases [(27.68–32.02)×109/mL], as was the ESR for all three cases (113–134 mm/h). The CRP level was significantly elevated in two cases (67 mg/L and 147.7 mg/L) and normal in one case. The procalcitonin level was normal in one case, mildly elevated in one case, and not measured in 1 case. Joint X-ray and ultrasound showed local soft tissue swelling and fluid accumulation in the joint cavity in all three cases. For all three cases, treatment included incision and drainage, and pathological examination indicated septic inflammation. Subsequent microbiological culture of the puncture fluid indicated the growth of S. pyogenes, and for one patient (case 3), blood culture also identified infection by S. pyogenes strains isolated from case 1 and case 2 were emm1/ST28 genotype, and case 3 was emm12/ST36 genotype. The details are shown in Table 1.
Treatment and follow-up
All three children also received treatment with intravenous antibiotics. Patient 1 was treated with vancomycin intravenously upon initial presentation, and the antibiotic was changed to amoxicillin sodium + sulbactam delivered intravenously after the results of joint fluid culture were obtained. Patient 2 was treated with Cefuroxime for 1 day, desmethylvancomycin for 4 days, vancomycin intravenously for 33 days and then both vancomycin and amoxicillin sodium + sulbactam intravenously for 10 days. Patient 3 was treated with amoxicillin sodium + sulbactam intravenously for 24 days. All three cases showed improvement and were followed up by telephone. No sequelae such as nephritis, rheumatic fever or rheumatic heart disease were reported. Details are shown in Table 1.
Discussion
Septic arthritis is a rapidly progressive, highly destructive and life-threatening joint disease. A study in the UK found that the incidence of septic arthritis increased from 5.5/100,000 in 1998 to 7.8/100,000 in 2013 (Rutherford et al., 2016). According to the literature, the most predominant pathogen causing sepsis in joints is Staphylococcus, followed by Streptococcus pneumoniae and Klebsiella, but also Salmonella spp, Haemophilus influenzae type b, and group B streptococci (Wang et al., 2003; Frazee et al., 2009; Rutherford et al., 2016; Xu et al., 2016; Dernoncourt et al., 2019; van den Boom et al., 2021; Yagupsky, 2021). S. pyogenes can also cause septic arthritis and is easily overlooked. A case of septic arthritis caused by S. pyogenes infection was first reported in 1984, and in that case, a serious infection caused by S. pyogenes occurred in a nursing home patient and resulted in sepsis, necrotizing fasciitis, septic arthritis, and cellulitis (Ruben et al., 1984). Septic arthritis after toxic shock syndrome caused by S. pyogenes was reported in 1995 (González-Ruiz and Ridgway, 1995). S. pyogenes infection causing multifocal septic arthritis has also been reported (Marti and Anton, 2007). In contrast, a 10-year case summary in Hong Kong found that in 31 cases of septic arthritis in children, the predominant causative organism was S. aureus (42%), followed by S. pyogenes (23%) (Kuong et al., 2012). In another study in France, 1 of 26 cases of diffuse infection in infants younger than 3 months of age was septic arthritis due to S. pyogenes infection (Germont et al., 2020). Another study in France isolated bacteria from 377 children with suspected joint infections, of which S. pyogenes accounted for 15% (Brehin et al., 2020). Methicillin-resistant S. aureus infection and septic arthritis combined with osteomyelitis have been reported in the literature as risk factors for the development of sequelae (Wang et al., 2003). A study in Spain reported that the invasive diseases caused by S. pyogenes in the Spanish pediatric population include septic arthritis/osteomyelitis, and the common types of S. pyogenes are emm1/ST28, emm12/ST36-ST242, (Sanchez-Encinales et al., 2019). In the present pediatric case series, cases 1 and 2 were emm1/ST28 and case 3 was emm12/ST36, and these results were basically consistent with those of previous studies. Overall, our case serious combined with the literature reports show that S. pyogenes cannot be overlooked as a potential cause of septic arthritis.
Previous research has shown that S. pyogenes can invade the joint microenvironment, and step in the development of septic arthritis (Le Hello et al., 2009). Volzke et al (Volzke et al., 2020) inoculated mice intravenously with S. pyogenes and observed septic arthritis 3~20 days after infection with increased levels of interleukin (IL)-1β and IL-6 in the joints along with an increased amount of nuclear factor (NF)-κB receptor activator ligand (RANKL), which is a key cytokine for osteoclast formation. S. pyogenes infection can increase RANKL expression by increasing the production of activators to induce infectious arthritis.
Septic arthritis occurs in lower limb joints in 90% of cases, with the hip being the most commonly involved, followed by the knee (Wang et al., 2003; Xu et al., 2016). Sternoclavicular joint involvement has also been reported (Dhekane et al., 2020; Kwon et al., 2020). Joint pain (81%) is the most common presentation, followed by fever, swelling and limitation of movement, and 89% of patients show an increased ESR (≥20 mm/h) (Wang et al., 2003). The age at onset of septic arthritis due to S. pyogenes varies, with reports of onset in neonates and small infants less than 3 months of age (Umadevi et al., 2013; Germont et al., 2020). The age of onset in our pediatric case series ranged from 2–7 years. A study in Hong Kong reported that only 52% of 31 children with septic arthritis had a temperature below 38.5°C on admission; i.e., nearly half of their patients had a temperature above 38.5°C on admission. Additionally, 71% of their patients had a WBC count below 12×109/L, and the rate of positive blood culture was not high (negative in 74% of cases) (Kuong et al., 2012). In the present case series, all three children presented with a significantly elevated WBC count (30×109/L or more) and a significantly increased ESR. The CRP level was also significantly increased in two cases, while the procalcitonin level was mildly increased in only one case. Previous studies have suggested that procalcitonin is more sensitive than ESR and CRP level for the diagnosis of septic arthritis, and that the combination of these three indicators is beneficial for improving diagnostic sensitivity and specificity (Wang et al., 2014; Wei et al., 2015). In contrast, Chen (Chen et al., 2013) found that the CRP level is more sensitive than procalcitonin for the identification of local bacterial infection. The results in our pediatric case series are more consistent with the findings of Chen et al (Chen et al., 2013).
The standard treatment for septic arthritis in children is arthrocentesis combined with antibacterial drug therapy (Kwon et al., 2020). Studies have shown that adequate use of antimicrobial drugs and a single arthrocentesis is sufficient to treat septic arthritis in most pediatric cases, regardless of the infecting agent or site of infection, as long as the clinical response is good (Peltola et al., 2009). Additional research had indicated that early antibiotic treatment, incision and drainage, and combined non-pharmacological treatments such as drainage and early physiotherapy should be given (Couderc et al., 2020). More recent studies have suggested that targeted synovial cell therapy may be a promising treatment for septic arthritis (Volzke et al., 2020). Penicillin has been widely used worldwide for many years, and so far, no penicillin-resistant strains of S. pyogenes have been identified. The reasons for this are not yet known. In recent years, increased minimum inhibitory concentration (MIC) values of penicillin have been reported, and penicillin-nonsusceptible S. pyogenes strains have emerged. In 2006, Capoor et al. (Capoor et al., 2006) reported S. pyogenes with elevated MIC values to penicillin (0.19~0.25 µg/mL), and S. pyogenes with elevated MIC values to penicillin (0.25~0.75 µg/mL) were also found in Mexico (Ogawa et al., 2011). Additionally, several resistance surveillance networks in China have reported S. pyogenes “resistance” to β-lactam antimicrobials, but we confirmed that these strains are not truly resistant, and whether they carry a mutated gene for penicillin-binding protein 2X (pbp2x) needs to be confirmed by further studies (Vannice et al., 2020; Musser et al., 2020; Yu et al., 2020). In the present case series, S. pyogenes was cultured from the joint cavity pus of all three children, and they were considered sensitive to β-lactam antibiotics based on the outcomes in these cases. No antibiotic susceptibility testing was performed. However, in case 3, S. pyogenes was cultured from the blood, and antibiotic susceptibility testing suggested sensitive to β-lactam antibiotics such as penicillin and cephalosporin. For this case, amoxicillin sodium + sulbactam was selected and provided satisfactory treatment. Therefore, in clinical practice, once pus culture identifies S. pyogenes, the choice of β-lactam antibiotics is sufficient, and prompt step-down treatment should be given, as it is not advisable to continue advanced antibiotic therapy. In case 1 of our series, after the pus culture result was clear, vancomycin was promptly changed to amoxicillin sodium + sulbactam with good effect, while in case 2, vancomycin combined with β-lactams treatment was considered to be related to the doctor’s insufficient knowledge of S. pyogenes. In case 3, intravenous infusion of amoxicillin sodium + sulbactam was administered from the time of admission with good effect.
Conclusion
In conclusion, antimicrobial agents commonly used to treat S. pyogenes infections are highly active against clinical strains.S. pyogenes is an important pathogen causing septic arthritis, and WBC count, ESR, and CRP level are sensitive indicators for the diagnosis of septic arthritis. If S. pyogenes infection is confirmed upon culture of pus drained from an infection joint, β-lactam antibacterial therapy with antibiotics such as penicillin or cephalosporin can be selected for treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Shenzhen Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
WW and YY contributed to conception, design, and administrative support. DY, QL, WG, DG,YC, and YZ provided study materials and patients. DY contributed to the collection and assembly of data, data analysis, interpretation and the manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Shenzhen Key Medical Discipline Construction Fund (No. SZXK032), the Guangdong Medical Research Fund (No. A2021437), the Hospital Level Project of Shenzhen Children’s Hospital (No.ynkt2020-zz19), the Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No.SZGSP012), and the Project of the Expert Committee on Clinical Application and Drug Resistance Evaluation of Antimicrobial Drugs of the National Health Commission (No. KJYWZWH-OT-02-2021-06).
Acknowledgments
We wish to thank the help given by the physicians who participated in this study and the professionals involved in sample collection and culture maintenance, especially the healthcare workers, for their contribution to disease control.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MK is currently organizing a Research Topic with the authors DY, YZ, and YY.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brehin, C., Claudet, I., Dubois, D., Sales de Gauzy, J., Vial, J., Chaix, Y., et al. (2020). Assessing the management of pediatric bone and joint infections according to French guidelines. Med. Maladies Infection 50 (6), 515–519. doi: 10.1016/j.medmal.2019.07.016
Capoor, M. R., Nair, D., Deb, M., Batra, K., Aggarwal, P. (2006). Resistance to erythromycin and rising penicillin MIC in Streptococcus pyogenes in India. Jpn J. Infect. Dis. 59 (5), 334–336. doi: 10.1097/01.qai.0000246035.86135.c0
Chaudhary, P., Kumar, R., Sagar, V., Sarkar, S., Singh, R., Ghosh, S., et al. (2018). Assessment of cpa, Scl1 and Scl2 in clinical group a Streptococcus isolates and patients from north India: an evaluation of the host pathogen interaction. Res. Microbiol. 169 (1), 11–19. doi: 10.1016/j.resmic.2017.09.002
Chen, J., Zheng, Y., Wang, S., Ma, H., Wang, W., Bao, Y., et al. (2013). Diagnostic value of procalcitonin and c-reactive protein as markers of systemic and localized bacterial infections. Chin. J. Evidence Based Pediatr. 8 (2), 87–91. doi: 10.3969/j.issn.1673-5501.2013.02.002
Couderc, M., Bart, G., Coiffier, G., Godot, S., Seror, R., Ziza, J.-M., et al. (2020). 2020 French recommendations on the management of septic arthritis in an adult native joint. Joint Bone Spine 87 (6), 538–547. doi: 10.1016/j.jbspin.2020.07.012
Dernoncourt, A., El Samad, Y., Schmidt, J., Emond, J. P., Gouraud, C., Brocard, A., et al. (2019). Case studies and literature review of pneumococcal septic arthritis in adults. Emerg. Infect. Dis. 25 (10), 1824–1833. doi: 10.3201/eid2510.181695
Dhekane, P., Gopalakrishnan, R., Sree, V., Devaprasad, V. (2020). Streptococcus dysgalactiae septic arthritis of sternoclavicular joint with bacteraemia. Indian J. Med. Microbiol. 38, 481–484. doi: 10.4103/ijmm.IJMM_20_257
Frazee, B. W., Fee, C., Lambert, L. (2009). How common is MRSA in adult septic arthritis? Ann. Emerg. Med. 54 (5), 695–700. doi: 10.1016/j.annemergmed.2009.06.511
Germont, Z., Bidet, P., Plainvert, C., Bonacorsi, S., Poyart, C., Biran, V., et al. (2020). Invasive Streptococcus pyogenes infections in <3-month-old infants in France: clinical and laboratory features. Front. Pediatr. 8204. doi: 10.3389/fped.2020.00204
González-Ruiz, A., Ridgway, G. L. (1995). Septic arthritis due to local spread of necrotizing fasciitis caused by Streptococcus pyogenes. Clin. Infect. Dis. 22, 394–395. doi: 10.1093/clinids/22.2.394
Jin, T., Li, L., Jin, H., Xu, L., Zhou, F. (2015). Clinical study of pyogenic arthritis caused by Staphylococcus aureus infection. Chin. J. Nosocomiol 25 (19), 4418–4420. doi: 10.11816/cn.ni.2015‐150987
Kuong, E., To, M., Yuen, M., Choi, A., Fong, C., Chow, W. (2012). Pitfalls in diagnosing septic arthritis in Hong Kong children: ten years' experience. Hong Kong Med. J. 18 (6), 482–487.
Kwon, H. Y., Cha, B., Im, J. H., Baek, J. H., Lee, J.-S. (2020). Medical management of septic arthritis of sternoclavicular joint. Med. (Baltimore) 99 (44), e22938. doi: 10.1097/md.0000000000022938
Le Hello, S., Doloy, A., Baumann, F., Roques, N., Coudene, P., Rouchon, B., et al. (2009). Clinical and microbial characteristics of invasive Streptococcus pyogenes disease in new caledonia, a region in Oceania with a high incidence of acute rheumatic fever. J. Clin. Microbiol. 48 (2), 526–530. doi: 10.1128/jcm.01205-09
Marti, J., Anton, E. (2007). Polyarticular septic arthritis caused by Streptococcus pyogenes in an immunocompetent woman. Eur. J. Intern. Med. 18 (1), 80. doi: 10.1016/j.ejim.2006.05.007
McMillan, D. J., Geffers, R., Buer, J., Vlaminckx, B. J., Sriprakash, K. S., Chhatwal, G. S. (2007). Variations in the distribution of genes encoding virulence and extracellular proteins in group a Streptococcus are largely restricted to 11 genomic loci. Microbes Infection 9 (3), 259–270. doi: 10.1016/j.micinf.2006.11.014
Musser, J. M., Beres, S. B., Zhu, L., Olsen, R. J., Vuopio, J., Hyyryläinen, H.-L., et al. (2020). Reduced in vitro susceptibility of Streptococcus pyogenes to beta-lactam antibiotics associated with mutations in the pbp2x gene is geographically widespread. J. Clin. Microbiol. 58 (4), e01993–e01919. doi: 10.1128/JCM.01993-19
Ogawa, T., Terao, Y., Sakata, H., Okuni, H., Ninomiya, K., Ikebe, K., et al. (2011). Epidemiological characterization of Streptococcus pyogenes isolated from patients with multiple onsets of pharyngitis. FEMS Microbiol. Lett. 318 (2), 143–151. doi: 10.1111/j.1574-6968.2011.02252.x
Peltola, H., Paakkonen, M., Kallio, P., Kallio, M. J., Osteomyelitis-Septic Arthritis Study, G. (2009). Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clin. Infect. Dis. 48 (9), 1201–1210. doi: 10.1086/597582
Rouchon, C. N., Ly, A. T., Noto, J. P., Luo, F., Lizano, S., Bessen, D. E. (2017). Incremental contributions of FbaA and other impetigo-associated surface proteins to fitness and virulence of a classical group a streptococcal skin strain. Infect. Immun. 85 (11), e00374–e00317. doi: 10.1128/IAI.00374-17
Ruben, F. L., Norden, C. W., Heisler, B., Korica, Y. (1984). An outbreak of Streptococcus pyogenes infections in a nursing home. Ann. Intern. Med., 101494–101496. doi: 10.7326/0003-4819-101-4-494
Rutherford, A. I., Subesinghe, S., Bharucha, T., Ibrahim, F., Kleymann, A., Galloway, J. B. (2016). A population study of the reported incidence of native joint septic arthritis in the united kingdom between 1998 and 2013. Rheumatology 55 (12), 2176–2180. doi: 10.1093/rheumatology/kew323
Sanchez-Encinales, V., Ludwig, G., Tamayo, E., Garcia-Arenzana, J. M., Munoz-Almagro, C., Montes, M. (2019). Molecular characterization of Streptococcus pyogenes causing invasive disease in pediatric population in Spain a 12-year study. Pediatr. Infect. Dis. J. 38 (12), 1168–1172. doi: 10.1097/INF.0000000000002471
Umadevi, S., Kali, A., Sreenivasan, S., Pramodhini, S., Charles, M. V. (2013). Septic arthritis caused by group a Streptococcus in newborn: an unusual presentation. J. Clin. Diagn. Res. 7 (6), 1143–1144. doi: 10.7860/jcdr/2013/4852.3034
van den Boom, M., Lennon, D., Crawford, H., Freeman, J., Castle, J., Mistry, R., et al. (2021). Microbiology of septic arthritis in young Auckland children. J. Paediatr. Child Health. 58(2), 326–331. doi: 10.1111/jpc.15716
Vannice, K., Ricaldi, J., Nanduri, S., Fang, F. C., Lynch, J., Bryson-Cahn, C., et al. (2020). Streptococcus pyogenes pbp2x mutation confers reduced susceptibility to β-lactam antibiotics. Clin Infec. Dis. 71(1):201–204. doi: 10.1093/cid/ciz1000
Volzke, J., Schultz, D., Kordt, M., Müller, M., Bergmann, W., Methling, K., et al. (2020). Inflammatory joint disease is a risk factor for streptococcal sepsis and septic arthritis in mice. Front. Immunol. 11579475. doi: 10.3389/fimmu.2020.579475
Wang, L., Lou, A., Guo, Y., Li, W., Zhang, Z., Yang, B., et al. (2014). Significance of serumprocalcitonin for discrimination between adult septic and aseptic arthritis. Guangdong Med. J. 35 (23), 3646–3649. doi: 10.13820/j.cnki.gdyx.2014.23.015
Wang, C., Wang, S., Yang, Y., Tsai, C., Liu, C. (2003). Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J. Microbiol. Immunol. Infection 36 (1), 41–46.
Wei, L., Luo, J., Yi, G., Chai, C., Wang, P. (2015). Diagnosis value of joint detection of serum procalcitonin,erythrocyte sedimentation rate,C-reactive protein for suppurative arthritis. Chin. J. Nosocomiol 25 (20), 4664–4666. doi: 10.11816/cn.ni.2015-150840
Xu, H., Li, Y., Zhou, Q., Liu, Y., Chen, W., Li, J., et al. (2016). Treatment strategies for septic arthritis with a neonatal onset. Chin. J. Pedia Surg. 37 (8), 582–588. doi: 10.3760/cma.J.issn.0253-3006.2016.08.006
Yagupsky, P. (2021). Changing aetiology of paediatric septic arthritis. J.Paediatr. Child Health. 57(10), 1560–1563. doi: 10.1111/jpc.15654
Yu, D., Zheng, Y., Yang, Y. (2020). Is there emergence of β-lactam antibiotic-resistant Streptococcus pyogenes in China? Infect. Drug Resist. 13, 2323–2327. doi: 10.2147/IDR.S261975
Keywords: Streptococcus pyogenes, child, septic arthritis, antibiotics, resistance
Citation: Yu D, Gao W, Guo D, Lu Q, Chen Y, Zheng Y, Wang W and Yang Y (2023) Case Report: Septic arthritis in children caused by Streptococcus pyogenes–rational use of antibiotics. Front. Cell. Infect. Microbiol. 12:1117217. doi: 10.3389/fcimb.2022.1117217
Received: 06 December 2022; Accepted: 29 December 2022;
Published: 18 January 2023.
Edited by:
Heinrich Korner, University of Tasmania, AustraliaReviewed by:
Mogens Kilian, Aarhus University, DenmarkYuanhai You, Chinese Center For Disease Control and Prevention, China
Copyright © 2023 Yu, Gao, Guo, Lu, Chen, Zheng, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Yang, eXloNjI4NjI4QHNpbmEuY29t; Wenjian Wang, d3dqeHhAMTI2LmNvbQ==
 Dingle Yu
Dingle Yu Waiwai Gao
Waiwai Gao Danchun Guo1
Danchun Guo1 Qinghua Lu
Qinghua Lu Yuejie Zheng
Yuejie Zheng Wenjian Wang
Wenjian Wang