- Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Objective: Probiotics may offer cancer-prevention benefits, based on experimental investigation results. This study aimed to determine the potential association between probiotics and hepatocellular carcinoma (HCC) in patients with hepatitis B-related cirrhosis (HBC) receiving antiviral therapy.
Design: This retrospective study included 1267 patients with HBC treated with entecavir or tenofovir between January 2013 and December 2017. The risk of developing HCC was compared between two cohorts of 449 probiotic users (taking a cumulative defined daily doses [cDDD] of ≥ 28) and 818 non-probiotic users (< 28 cDDD). To eliminate the bias caused by confounding factors, propensity score matching (PSM) was used.
Results: On multivariate regression analysis, probiotic consumption was an independent protective factor for HCC occurrence. After PSM, the incidence of HCC was significantly lower in the probiotic users than that in the nonusers (adjusted hazard ratio [aHR]: 0.70, 95% confidence interval: 0.59–0.83, P < 0.001). The aHRs for probiotics with 28–89, 90–180, and >180 cDDD were 0.58, 0.28, and 0.12, respectively, indicating a dose-response pattern. In 28–89, 90–180, and >180 cDDD, the 3-year cumulative incidence of HCC was 8.7%, 4.7%, and 3.0%, respectively. A multivariate stratified analysis confirmed that the administration of probiotics could help patients.
Conclusion: Adjuvant probiotic therapy may reduce the risk of HCC in patients receiving antiviral medication for HBC. However, further clinical research is required to confirm these findings.
Introduction
Hepatocellular carcinoma (HCC) is a severe health problem and has become the second leading cause of cancer mortality globally, accounting for 782,000 deaths annually (Bray et al., 2018). The hepatitis B virus (HBV) is the leading cause of cirrhosis and HCC worldwide (El-Serag, 2012). Cirrhosis affects approximately 80% of patients with a subsequent diagnosis of HCC. However, most patients are diagnosed at an advanced stage, with limited treatment options and a low survival rate (Balogh et al., 2016). Current antiviral therapies, such as nucleos(t)ide analogs [NA(s)], are efficacious in inhibiting viral replication, improving liver inflammation, and reducing HCC incidence (Chang et al., 2010; Marcellin et al., 2013). Several studies have reported that antiviral therapy can attenuate, but not completely eliminate the risk of HCC in patients with hepatitis B-related cirrhosis (HBC) (Lee and Ahn, 2016). Despite HBV replication decrease, antiviral therapy alone may be insufficient to prevent HCC. Because of the growing incidence and high mortality rate of HCC, new treatments or prophylactic strategies are urgently needed to lower the risk of HCC.
Probiotics, as live microorganisms, provide health benefits to the host when administered in adequate amounts (Wan and El-Nezami, 2018). Recent studies have reported that probiotics could be used as an alternative for cancer prevention and treatment (Yu and Li, 2016; Singh et al., 2021). Probiotics can regulate the intestinal microflora and maintain the balance in the microbiota ecosystem (Mendoza, 2019). Probiotics influence various biological processes associated with cancer, such as inflammation, oxidative stress, apoptosis, proliferation, and metastasis (Thilakarathna et al., 2021). In addition, probiotics are reported to play an important role in cancer prevention by regulating the microbiome and immune response (Feng et al., 2018). Therefore, clinical trials on the modulation of microbiota dysbiosis by probiotics will contribute to the clinical application of probiotics in the future management of several cancer types.
Multiple researchers have reported that patients with HBC have an altered gut microbiota and significantly greater plasma endotoxin levels than healthy individuals (Qin et al., 2014; Chen et al., 2021). Microbial dysbiosis and endotoxemia are important contributing factors to cirrhosis in HCC (Wan and El-Nezami, 2018). Probiotics have an antiviral activity that slows HCC progression by preventing chronic HBV infection (Lee et al., 2013; Thilakarathna et al., 2021). In an animal study, VSL#3 treatment reduced liver inflammation and restored intestinal homeostasis, preventing cirrhosis progression to HCC (Zhang et al., 2012; Li et al., 2016). Considering the increasing use of probiotics and incidence of HCC, the potential link between probiotics and HCC is an important issue to investigate. However, few studies have reported the effects of adjuvant probiotic therapy on the risk of HCC in patients with HBC in clinical practice. Propensity score matching (PSM) has been used to reduce bias caused by potential confounding factors between groups (Rusthoven et al., 2016; Tian et al., 2022). PSM is an effective statistical method to explore the efficacy of probiotics and HCC occurrence in patients with HBC.
Therefore, we used PSM to investigate the association between probiotic therapy and the risk of HCC among patients with HBC and to provide clinical evidence for probiotics as adjuvant therapy in these populations.
Materials and methods
Participants
We examined 1932 patients treated for HBC at Beijing Ditan Hospital, Capital Medical University, between January 2013 and December 2017. Patients with HBC aged 20–75 years were enrolled in this study. Exclusion criteria were being infected with human immunodeficiency virus; having other hepatitis infections, malignancies, or liver failure; having undergone liver transplantation; having alcoholic liver and severe fatty liver; having obtained a probiotic prescription before the index date; and having died or had less than 3 years of follow-up. After applying the exclusion criteria, 1267 patients were enrolled in the study.
Patients were divided into two cohorts based on the clinically effective proportion of probiotic therapy they received: probiotics ≥ 28 cumulative defined daily doses (cDDD) based on antiviral therapy or no probiotic at all (Marlicz et al., 2016). Ultimately, 449 patients received probiotics ≥ 28 cDDD, and 818 patients did not receive probiotics. Thus, 442 probiotic users and 442 non-users were randomly paired after a 1:1 PSM (Figure 1). The index date was the date of the diagnosis of cirrhosis at our hospital. The outcome of this study was the occurrence of HCC or the end of the 3-year follow-up period. The study protocol was performed in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of Beijing Ditan Hospital.
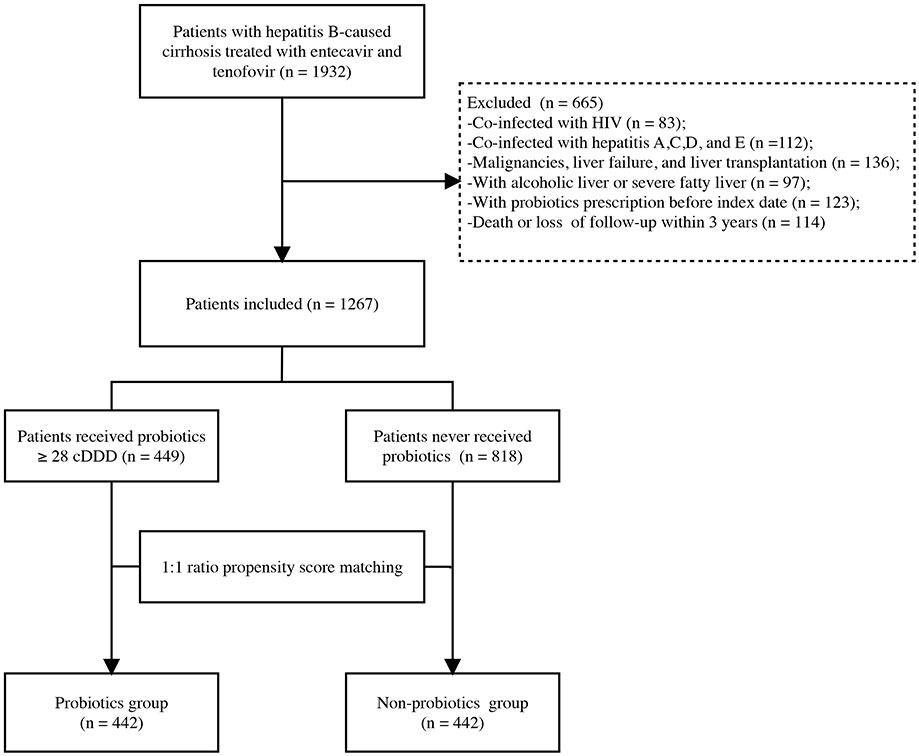
Figure 1 Flowchart of the enrollment of patients in the study. HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; cDDD, cumulative defined daily doses.
Clinical definitions and data collection
Chronic hepatitis B is defined as the persistent presence of the hepatitis B surface antigen for > 6 months (Sarin et al., 2016). Cirrhosis was diagnosed based on the following criteria: liver biopsy; endoscopic or ultrasound abnormal findings; cirrhotic elastography; and complications related to portal hypertension, such as ascites, hepatic encephalopathy (HE), and esophagogastric variceal bleeding (Shiha et al., 2009). Histological or radiological evidence (computed tomography or magnetic resonance imaging) was used to diagnose HCC (Bruix and Sherman, 2011). DDD was used to measure the prescribed dose of probiotics as recommended by the World Health Organization (WHO) (WHO Collaborating Center for Drugs Statistics Methodology, 2003). For example, the average daily maintenance dose of probiotics for an adult is 1 DDD. The cDDD, which indicates the duration of administration of probiotics, is the sum of all cDDDs administered during the 3-year follow-up. The probiotic group received at least 28 cDDD of probiotics after diagnosis of HBC (based on data from the medical records of probiotics used at our hospital). Virological response (VR) was defined as an undetectable HBV DNA load at the end of the study.
Baseline demographic variables and laboratory measurements, including hemogram; liver, renal, and coagulation tests; HBV DNA; and alpha-fetoprotein (AFP), were recorded from a computerized database during the first week of enrollment. The model for end-stage liver disease (MELD) and Child-Turcotte-Pugh (CTP) scores were used to estimate the severity of the liver disease (Wiesner et al., 2003; Botero and Lucey, 2003). Every 3–6 months, routine laboratory tests and radiological examinations were performed. The probiotic dosage, frequency, total number of prescriptions, and duration were recorded during the study period.
Treatments
Patients with HBC received first-line antiviral therapy, such as entecavir (ETV) or tenofovir (TDF). Moreover, according to the doctor’s diagnosis, some patients received probiotics as an additional medication to balance the gut flora. Prescription drugs are included in our database by name, dose, and course of treatment. Bacillus licheniformis capsule; live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules; live combined Bifidobacterium, Lactobacillus, and Enterococcus capsules; and live combined Bifidobacterium, Lactobacillus, Enterococcus, and Bacillus cereus tablets were included in this study. Table S1 shows more information on the probiotics used in this study.
Statistical analysis
The t-test was used to compare normally distributed continuous variables, whereas the Mann–Whitney U test was used for non-normally distributed variables. The chi-square or Fisher’s exact test was used to evaluate categorical variables. To identify independent risk factors influencing 3-year HCC occurrence, univariate and multivariate Cox proportional hazard regression models were used. The propensity score is the probability of treatment assignment conditional on baseline characteristics (Austin, 2011). Using the propensity score, the distribution of baseline characteristics between exposed and unexposed patients is independent of the therapy received, which allows for the estimation of unbiased treatment effects (Austin, 2011). A logistic regression model was used to calculate PSM based on variables related to outcome to balance the potential bias and minimize confounding variables. The probiotics users and nonusers were matched randomly by age, sex, ascites, HE, alanine aminotransferase (ALT), platelet count, and NA(s) treatment before ETV/TDF. The nearest neighbor matching algorithm with a caliper width of 0.05 was used to undertake one-to-one matching without replacement. The caliper width was 0.05 times the standard deviation of the logit of the propensity score (Austin, 2011), which eliminate at least 99% of the bias due to confounding factors (Rusthoven et al., 2016). The performance of the propensity score model was assessed by evaluating whether any important relationships between the exposure groups and the covariates remained after adjustment. SPSS 25.0 (IBM Corp., Armonk, New York, USA) was used for the analyses.
To examine the dose-response relationship, we divided patients into four groups according to probiotics use: 28–89, 90–180, and >180 cDDD. The Kaplan-Meier method was employed to estimate the cumulative incidence of HCC, and the log-rank test was utilized to assess the differences between the curves. The process was generated using the packages of survival via R software (version 4.1.3; The R Foundation, Vienna, Austria). The Cox proportional hazards regression model was used to calculate the multivariate-adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) of HCC associated with probiotic administration. Additionally, as a sensitivity analysis, a multivariate stratified analysis was performed to investigate the difference and consistency between probiotic therapy and the risk of HCC in the patient subgroups. A two-sided P < 0.05 was considered statistically significant. The supporting data and related scripts are available on GitHub (https://github.com/Shike130909/Probiotic-therapy-and-the-risk-of-HCC).
Results
Baseline characteristics
Of the 1267 patients with HBC, 134 (10.6%) developed HCC during the 3-year follow-up. Table 1 shows the baseline characteristics and laboratory variables for both the unmatched and matched cohorts. Before PSM, patients in the probiotics group had higher total bilirubin, platelet levels, and longer prothrombin time than those in the non-probiotics group (all P < 0.05). In the probiotic group, patients had a significant percentage of ascites and HE. Moreover, probiotic users had higher MELD and CTP scores than nonusers (P < 0.001). The VR was achieved in 97% and 98.7% of the probiotic and non-probiotics groups at the end of 1 year after antiviral therapy, respectively. After PSM, the two groups’ demographic and clinical baseline characteristics were consistent, and a matched cohort of 884 patients was included in the analysis.
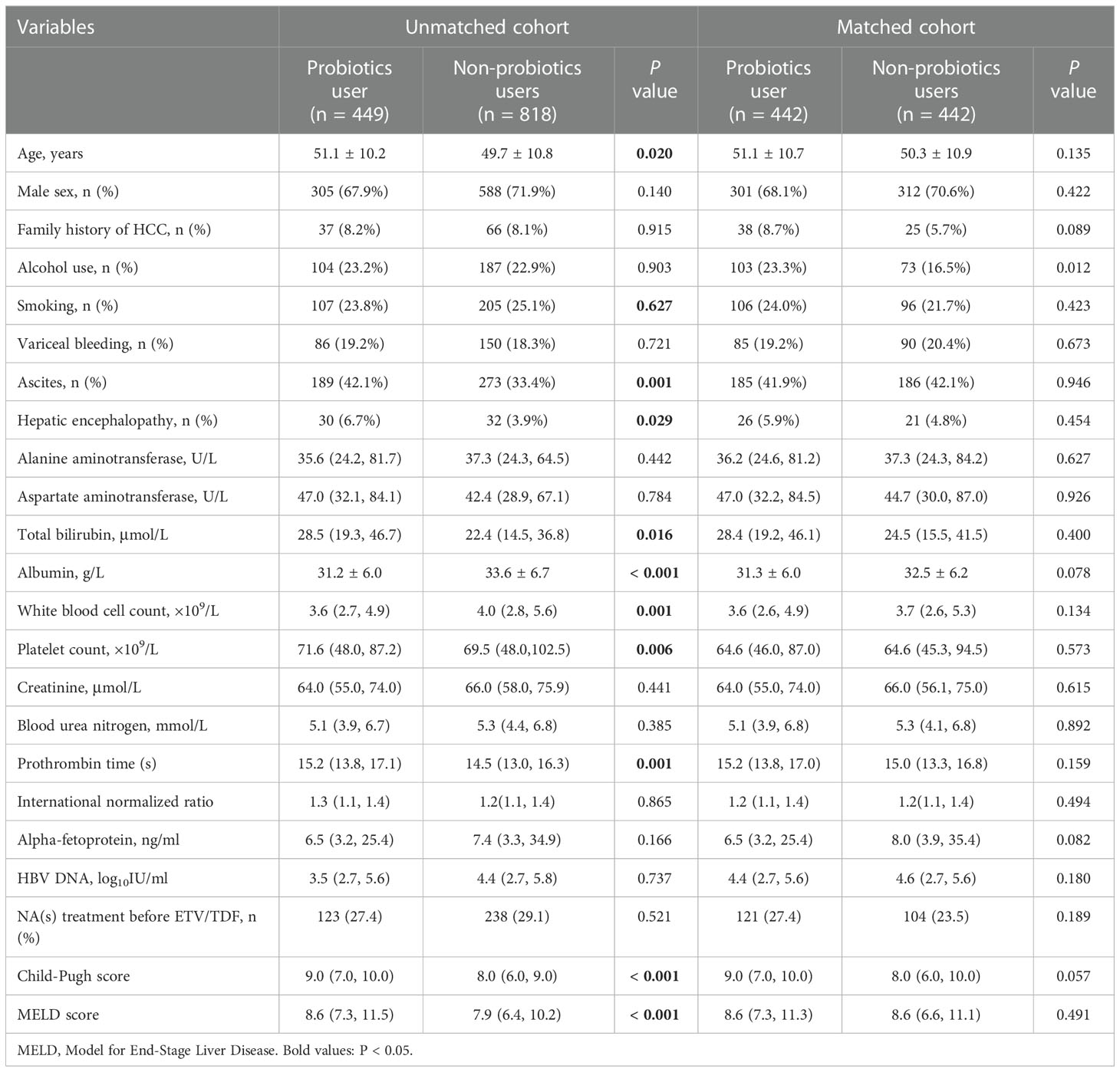
Table 1 Clinical characteristics of patients with hepatitis B-related cirrhosis in unmatched and matched cohorts.
Analysis of risk factors
As presented in Table 2, in the multivariate Cox regression analysis that was adjusted for age, sex, family history of HCC, ascites, HE, ALT, albumin, platelets, blood urea nitrogen, HBV DNA, and NA(s) treatment before ETV/TDF, probiotics use was still an independent factor against HCC occurrence (aHR = 0.43, 95% CI 0.28–0.65; P < 0.001). Older age (aHR = 1.04, 95% CI 1.02–1.06; P < 0.001), family history of HCC (aHR = 1.83, 95% CI 1.13–2.96; P = 0.014), ascites (aHR = 1.76, 95% CI 1.06–2.92; P = 0.028), HE (aHR = 2.62, 95% CI 1.45–4.71; P = 0.001), and higher blood urea nitrogen (aHR = 1.05, 95% CI 1.00–1.10; P = 0.048) also significantly increased HCC risk. However, using NA(s) treatment before ETV/TDF was related to a lower HCC risk (aHR = 0.50, 95%CI 0.32–0.78; P = 0.002).
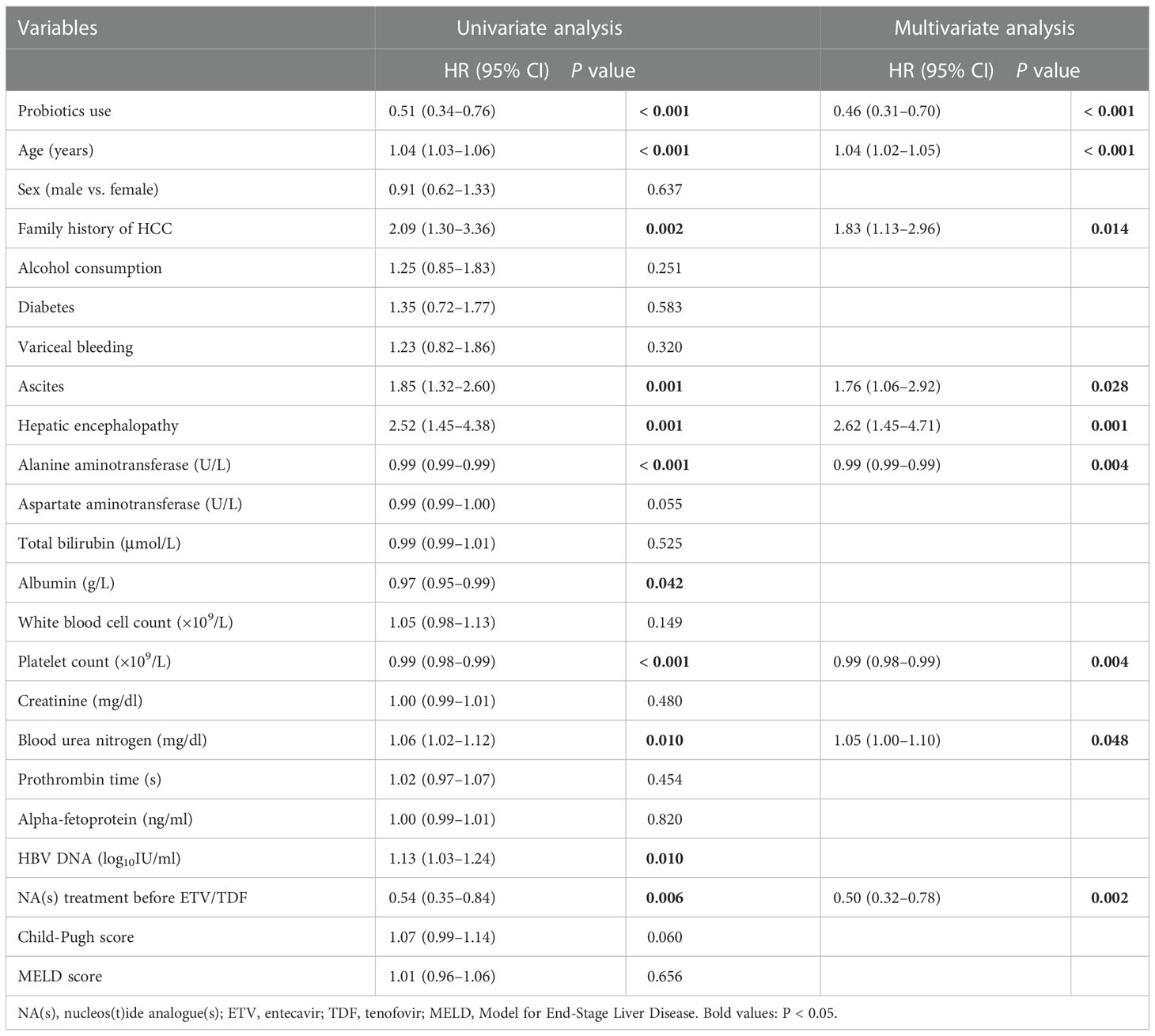
Table 2 Cox proportional hazards regression model analysis for risk of HCC in patients with hepatitis B-related cirrhosis.
Effects of probiotics on HCC occurrence
In the unmatched cohort, the probiotics group had a significantly lower incidence of HCC than the non-probiotics group (6.7% vs. 12.7%, P < 0.001; Figure 2A). After PSM, the 3-year HCC occurrence rates in probiotic users and nonusers were 6.1% and 14.0%, respectively (P < 0.001; Figure 2B). The probiotics group was categorized into three subgroups based on the probiotics dose used: 28–89 cDDD, 90–180 cDDD, and > 180 cDDD. Figures 2C, D show the Kaplan–Meier analysis results for the unmatched cohort and matched cohort. The probiotics group had a significantly reduced incidence of HCC in a dose-response manner than the non-probiotics group (both P < 0.005).

Figure 2 Cumulative incidence of HCC in patients with HBC. (A) Cumulative incidence of HCC among probiotics (n = 449) and nonusers (n = 818) in the unmatched cohort (6.7% vs. 12.7%, log-rank test P = 0.00091). (B) Cumulative incidence of HCC among probiotics (n = 442) and nonusers (n = 442) in the matched cohort (6.1% vs. 14.0%, log-rank test P < 0.0001). (C) Cumulative incidence of HCC in patients with < 28 (n = 818), 28–89 (n = 189), 90–180 (n = 194), and > 180 cDDD (n = 66) of probiotics use before matching (12.7% vs. 9.5% vs. 5.2% vs. 3.0%, log-rank test P = 0.0031). (D) Cumulative incidence of HCC in patients with with < 28 (n = 442), 28–89 (n = 184), 90–180 (n = 192), and > 180 cDDD (n = 66) of probiotics use after matching (14.0% vs. 8.7% vs. 4.7% vs. 3.0%, log-rank test P = 0.00039). HCC, hepatocellular carcinoma; HBC, hepatitis B-related cirrhosis; cDDD, cumulative defined daily doses.
Additionally, Table 3 shows the dose-response relationship between the administration of probiotics and the risk of HCC development. In the unmatched cohort, the aHRs were 0.67 (95% CI, 0.41–1.11, P = 0.120), 0.31 (95% CI, 0.16–0.58, P = 0.001), and 0.15 (95% CI, 0.04–0.61, P = 0.008) for patients that received 28–89, 90–180, and > 180 cDDD, probiotics respectively. In the matched cohort, the overall aHR for HCC development was 0.59 (95% CI, 0.34–0.81, P < 0.001) for probiotic users versus non-probiotic users. The aHRs for probiotic usage of 28–89 cDDD, 90–180 cDDD, and > 180 cDDD, were 0.58 (95% CI, 0.33–1.01, P = 0.053), 0.28 (95% CI, 0.14–0.56, P = 0.001), and 0.12 (95% CI, 0.03–0.52, P = 0.004), respectively.
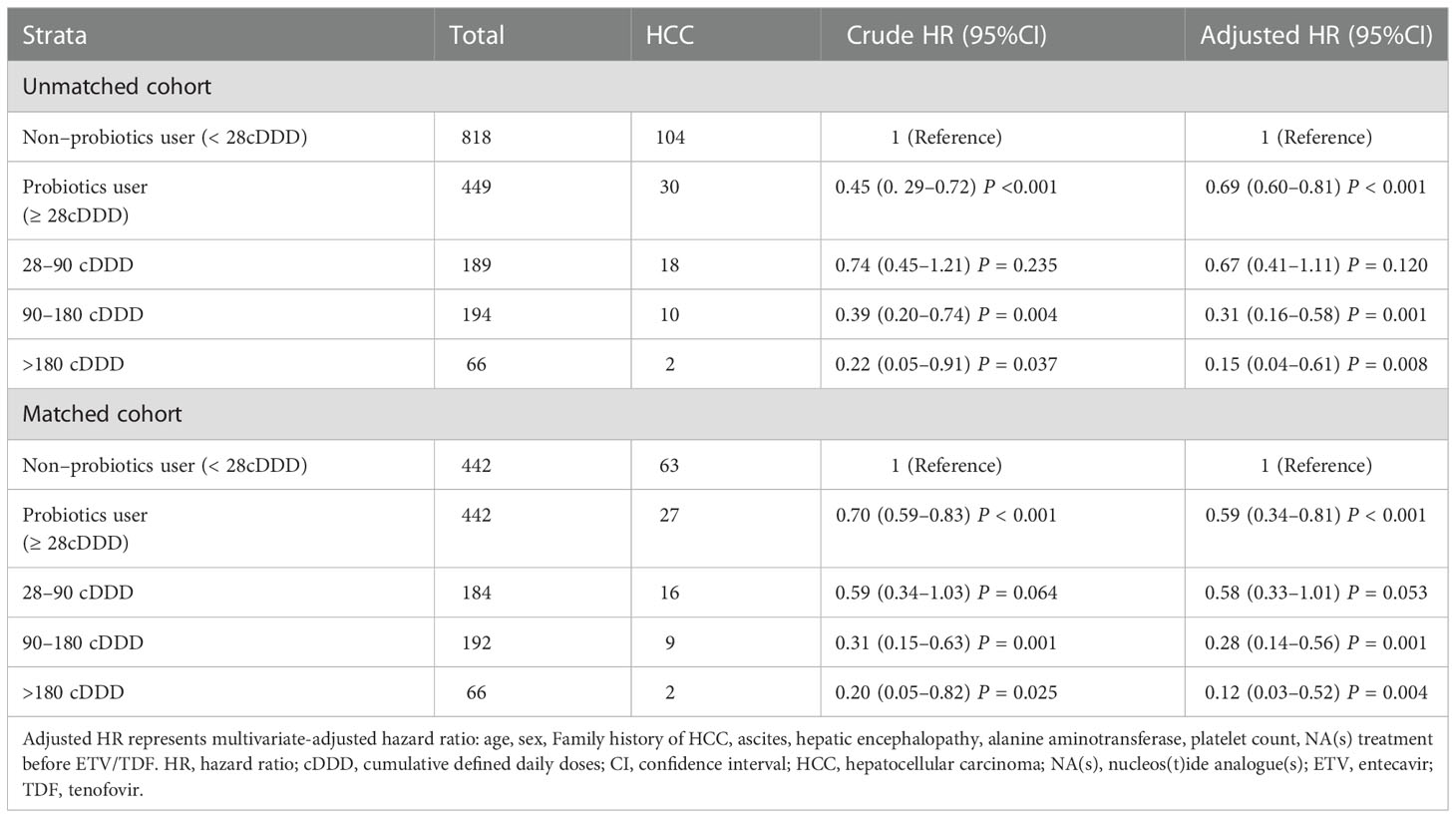
Table 3 Risk of of hepatocellular carcinoma according to probiotics use in unmatched and matched cohorts.
HCC risk analyses using MELD score and CTP class
After PSM, 577 patients had MELD scores <10, and 307 patients had MELD scores ≥10. When the MELD was <10, the 3-year HCC incidences were 12.6% and 6.8% in the non-probiotic and probiotics groups, respectively (P = 0.019, Figure 3A). When the MELD was ≥10, the 3-year HCC incidences were 16.5% and 4.7% in the non-probiotics and probiotics groups, respectively (P < 0.001, Figure 3B). Additionally, 237 patients belonged to CTP class A; 345, to CTP class B; and 302, to CTP class C. The 3-year HCC incidences were 11.4% and 6.2% in CTP class A (P = 0.17, Figure 3C), 13.4% and 5.5% in CTP class B (P = 0.012, Figure 3D), and 17.4% and 6.7% in CTP class C (P = 0.0025, Figure 3E) in the non-probiotics and probiotics groups, respectively.
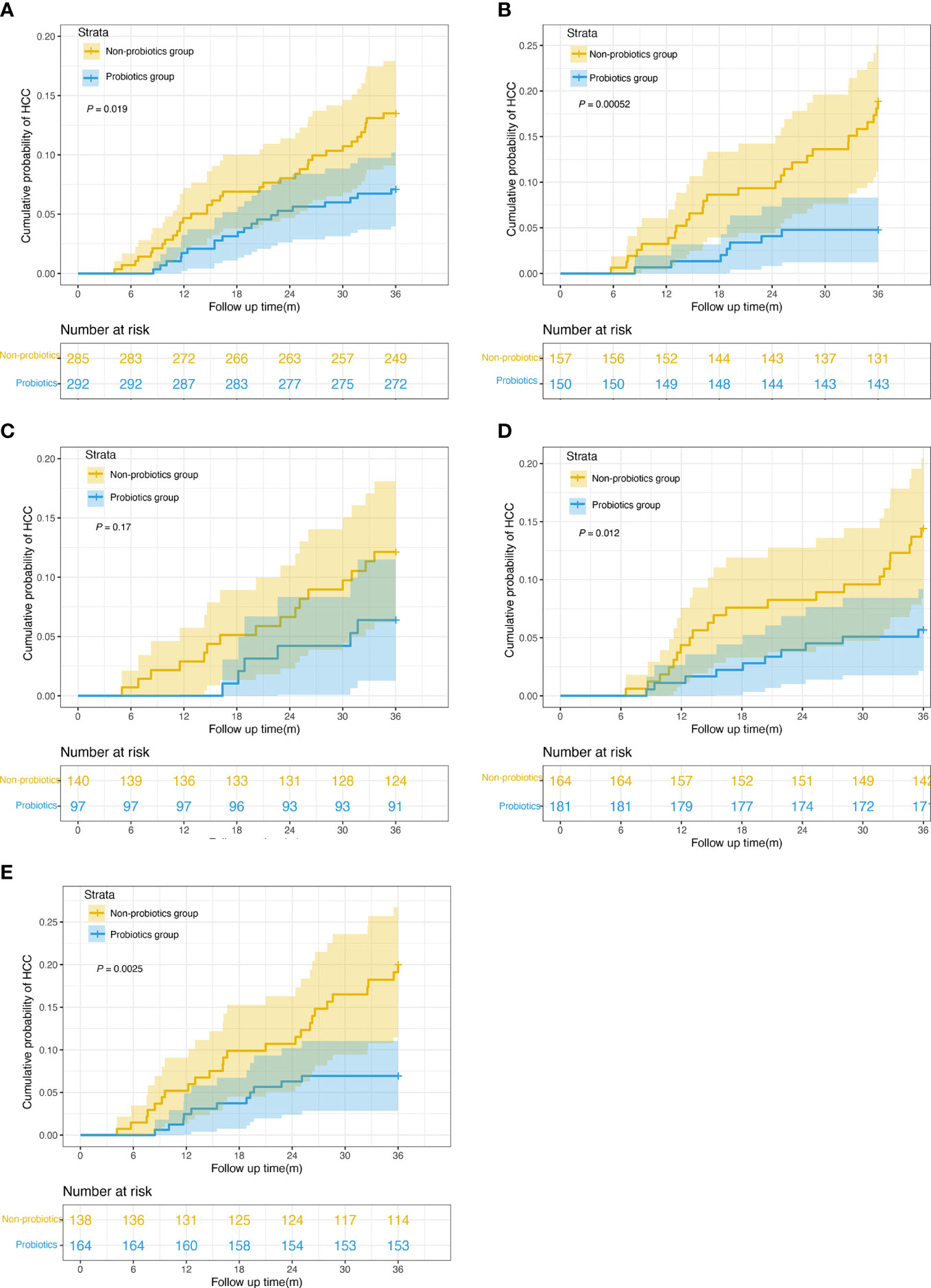
Figure 3 Kaplan-Meier survival curves of risk stratification according to MELD score and CTP classification. (A) Cumulative incidence of HCC in probiotics users and nonusers with MELD scores < 10 (n = 577, 6.8% vs. 12.6%, log-rank test P = 0.019). (B) Cumulative incidence of HCC in probiotics users and nonusers with MELD scores ≥ 10 (n = 307, 4.7% vs. 16.5%, log-rank test P = 0.00052). (C) Cumulative incidence of HCC in probiotics users and nonusers in CTP class A (n = 237, 6.2% vs. 11.4%, log-rank test P = 0.17). (D) Cumulative incidence of HCC in probiotics users and nonusers in CTP class A (n = 345, 5.5% vs. 13.4%, log-rank test P = 0.012). (E) Cumulative incidence of HCC in probiotics users and nonusers in CTP class A (n = 302, 6.7% vs. 17.4%, log-rank test P = 0.0025). MELD, Model for End-Stage Liver Disease. CTP,Child-Turcotte-Pugh.
Multivariate stratified analysis for probiotic therapy
The multivariate stratified analysis was used to investigate the association between the administration of probiotics and the risk of HCC in patient subgroups (Table 4). We examined the outcome in groups based on age, sex, ascites, HE, ALT level, platelet counts, and NA(s) treatment before ETV/TDF. Among patients aged 50 years or younger (HR: 0.31, 95% CI 0.11–0.83), older than 50 years (HR: 0.42, 95% CI 0.25–0.69); males (HR: 0.40, 95% CI 0.23–0.66); and those with (HR: 0.36, 95% CI 0.19–0.67) or without ascites (HR: 0.48, 95% CI 0.24–0.93), without HE (HR: 0.43, 95% CI 0.27–0.68), with ALT levels < 40 U/L (HR: 0.49, 95% CI 0.28–0.85) or ≥ 40 U/L (HR: 0.29, 95% CI 0.13–0.65), with platelet counts < 80 ×109/L (HR: 0.39, 95% CI 0.22–0.67), and without NA(s) treatment before ETV/TDF (HR: 0.38, 95% CI 0.23–0.62), the positive effects of administration of probiotics continued. Although the HR was < 1.0 for most subgroups, statistical significance was not reached in several subgroups, including females (HR: 0.48, 95% CI 0.19–1.21), those with HE (HR: 0.24,95% CI 0.05–1.16) and platelet counts ≥ 80 ×109/L (HR: 0.46,95% CI 0.25–1.00), and those with NA(s) treatment before ETV/TDF (HR: 0.85, 95% CI 0.24–2.94).

Table 4 Multivariable stratified analyses of the association between probiotics use and HCC occurrence.
Discussion
HCC is commonly associated with cirrhosis, and early detection is an excellent way to enhance patients’ longevity. The gut microbiota is an essential intestinal ecosystem; its dysbiosis can disrupt intestinal homeostasis and lead to harmful bacterial overgrowth, causing liver fibrosis, cirrhosis, and HCC. Growing evidence suggests that microbial imbalance causes cancer via various pathways involving inflammation and immunological dysregulation (Plottel and Blaser, 2011; Zhang et al., 2020a). Probiotics are new adjuvant therapies to prevent and reduce the risk of HCC (Wan and El-Nezami, 2018). Endotoxemia and the number of bacterial species can be reduced by administering Bifidobacteria and Lactobacilli (Tandon et al., 2009; Rincón et al., 2014). In this study, we observed a significant inverse association between probiotics use and the risk of HCC in patients with HBC on antiviral therapy. Furthermore, the administration of probiotics reduced the incidence of HCC in a dose-dependent manner.
Several studies have found a close association between gut microbiota alterations and HBC and HCC. Studies have shown that probiotics reduce the length of hospital stay, improve portal hypertension and associated complications, and lower inflammatory factors while causing no adverse events (Dhiman et al., 2014; Rincón et al., 2014). Herein, probiotics were found to be an independent protective factor against HCC in patients with HBC. Probiotic use was associated with a significantly lower risk of HCC, regardless of whether PSM was performed (both P < 0.001). Additionally, the risk reduction of HCC showed a dose-response relationship in the unmatched and matched cohorts. The uncorrected HR for overall probiotic prescription after PSM was 0.70 (95% CI: 0.59–0.83), while the aHR was 0.59 (95% CI: 0.34–0.81). With non-probiotics users as controls, the aHRs were 0.58 (95% CI: 0.33–1.01), 0.28 (95% CI: 0.14–0.56), and 0.12 (95% CI: 0.03–0.52) for 28–89, 90–180, and >180 cDDD, respectively, In a recent study, the high-dose probiotic VSL#3 reduced the risk of lesions in mice as compared to low-dose probiotics or no therapy (Li et al., 2016), indicating that HCC risk decreased as the cumulative dose of probiotics increased. Therefore, probiotic therapy may improve prognosis by reducing HCC morbidity and mortality.
The association between microbiota and the host plays a crucial role in the pathogenesis of HCC. Altering the composition of the intestinal flora can limit bacterial translocation, promote gut microflora balance, and prevent HCC progression (Zhang et al., 2012b). Sandler et al. reported that liver disease severity is related to bacterial translocation and overgrowth (Sandler et al., 2011). In the present study, probiotic users benefited significantly, especially patients with CTP B and C classes. A similar effect of probiotics use was demonstrated in our previous study in patients with cirrhosis (Zhang et al., 2020b). The MELD score is a parameter of a liver function used to assess the prognosis of patients with cirrhosis (Bernardi et al., 2011). Probiotics users had a lower HCC risk than non-probiotics users, regardless of MELD score < 10 or ≥ 10. However, the statistical difference between the two groups was more pronounced when the MELD score was ≥ 10. Preveden et al. previously showed that changes in the gut microflora are affected by liver disease stage (Preveden et al., 2017). Patients with advanced MELD and CTP scores show greater perturbations in microbial toxins, lipopolysaccharide secretion, and proinflammatory cytokine levels (Rayes et al., 2002; Rayes et al., 2005). Therefore, these patients were positively correlated with the benefit.
Probiotic therapy is a novel regimen for chronic liver diseases complicated by gut dysbiosis. Hepatocarcinogenesis is aided by gut microbial dysbiosis. Accumulating evidence has suggested that the gut–liver axis plays a key role in the progression of cirrhosis and HCC (Wiest et al., 2017; Ohtani and Kawada, 2019). Progress has been made in understanding the relationship between microbiota dysbiosis and carcinogenesis. Several experimental studies have reported that probiotics positively impact hosts (Patel and DuPont, 2015; Elzouki, 2016). The mechanism by which probiotics increase HCC risk may be associated with the following: (1) Gut microbiota modulation. The production of short-chain fatty acids (SCFAs) such as butyrate may reverse intestinal dysbiosis and reduce the risk of HCC (Li et al., 2016). Some studies reported that probiotics prevent carcinogenesis, by producing SCFAs, controlling gut microbiota composition, and improving gut barrier integrity (LeBlanc et al., 2017). (2) Improvement of gut barrier integrity. Gut bacterial dysbiosis damages the intestinal barrier, promotes bacterial translocation, and, subsequently, induces inflammatory responses (Zhang et al., 2012; Bajaj et al., 2018). Probiotics can enhance the intestinal barrier and decrease inflammation, thereby inhibiting HCC progression (Zhang et al., 2012). (3) Immune regulation and anti-inflammatory role. Gut microbial dysbiosis triggers a pro-inflammatory immune response and cancer progression (Singh et al., 2021). A study has reported that probiotics promote the growth of beneficial microbes, producing anti-inflammatory metabolites with antitumor activity (Li et al., 2016). (4) Antimicrobial effect. Bacterial translocation leads to endotoxemia, which results in portal hypertension and liver cell damage, thus contributing to HCC occurrence (Tao et al., 2015). Probiotics can release antimicrobial molecules, reduce bacterial translocation and overgrowth, and improve endotoxemia (Seki and Schnabl, 2012; Petschow et al., 2013). Thus, these possible mechanisms explain how probiotics reduce the risk of HCC in patients with HBC.
To the best of our knowledge, this is the first observational study to investigate the benefits of probiotics in reducing HCC risk in a population-based cohort of patients with HBC on antiviral therapy. However, this study had several limitations. First, this was an observational retrospective study; thus, potential confounding risk factors may have existed. Despite careful PSM, we cannot exclude the possibility of residual confounding resulting from unmeasured variables, such as diet, coffee, duration of antiviral therapy, and course of cirrhosis. Further prospective randomized controlled trials are required to determine the impact of these risk factors on developing HCC. Second, owing to the single-center study design, the number of patients was limited. Large-sample, multicenter studies need to be conducted in the future. Third, due to the cross-use of probiotics during the study period, we could not further compare the efficacy of different probiotics. We will examine the efficacy of different probiotics in preventing HCC in the future. In addition, the side effects of probiotics for patients were difficult to evaluate in this study. Before probiotic therapy is used to prevent HCC in practice, prospective trials should be conducted to assess its efficacy and safety. Lastly, gut microbiota data were not available in our database; therefore, we did not analyze the association between the microbiota population and HCC risk. We are currently conducting a prospective study using metabonomics, transcriptomics, and proteomic analyses to determine the relationship between the microbiome and carcinogenesis.
Conclusion
This study demonstrated that probiotics might reduce the risk of HCC occurrence in patients with HBC in a dose-dependent manner. However, further prospective studies are required to confirm the preventive effects of probiotics against HCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethical Review Committee of the Beijing Ditan Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
XW and KS conceived and designed the project. KS, QZ, YZ, and YB collected the data. KS and QZ analyzed and interpreted the data. KS drafted the manuscript. YZ, YB, and XZ was responsible for manuscript modification. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Beijing Municipal Science Technology Commission (No. Z191100006619033).
Acknowledgments
We are grateful to Ting Zhao for her constructive comments in improving the grammar and readability of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.1104399/full#supplementary-material
Abbreviations
ALT, alanine aminotransferase; AFP, alpha-fetoprotein; aHR, adjusted hazard ratio; cDDD, cumulative defined daily doses; CTP, Child-Turcotte-Pugh; CIs, confidence intervals; ETV, entecavir; HE, hepatic encephalopathy; HCC, hepatocellular carcinoma; HBC, hepatitis B-related cirrhosis; HBV, hepatitis B virus; MELD, model for end-stage liver disease; NA(s), nucleos(t)ide analogs; PSM, propensity score matching; SCFAs, short-chain fatty acids; TDF, tenofovir; VR, virological response; WHO, World Health Organization.
References
Austin, P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav. Res. 46, 399–424. doi: 10.1080/00273171.2011.568786
Bajaj, J. S., Idilman, R., Mabudian, L., Hood, M., Fagan, A., Turan, D., et al. (2018). Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 68, 234–247. doi: 10.1002/hep.29791
Balogh, J., Victor, D., 3rd., Asham, E. H., Burroughs, S. G., Boktour, M., Saharia, A., et al. (2016). Hepatocellular carcinoma: A review. J. Hepatocell Carcinoma. 3, 41–53. doi: 10.2147/JHC.S61146
Bernardi, M., Gitto, S., Biselli, M. (2011). The MELD score in patients awaiting liver transplant: strengths and weaknesses. J. Hepatol. 54, 1297–1306. doi: 10.1016/j.jhep.2010.11.008
Botero, R. C., Lucey, M. R. (2003). Organ allocation: Model for end-stage liver disease, child-Turcotte-Pugh, Mayo risk score, or something else. Clin. Liver Dis. 7, 715–727. doi: 10.1016/s1089-3261(03)00052-7
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Bruix, J., Sherman, M. (2011). American Association for the study of liver diseases. Management of hepatocellular carcinoma: An update. Hepatology 53, 1020–1022. doi: 10.1002/hep.24199
Chang, T. T., Liaw, Y. F., Wu, S. S., Schiff, E., Han, K. H., Lai, C. L., et al. (2010). Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis b. Hepatology 52, 886–893. doi: 10.1002/hep.23785
Chen, T., Ding, R., Chen, X., Lu, Y., Shi, J., Lu, Y., et al. (2021). Firmicutes and blautia in gut microbiota lessened in chronic liver diseases and hepatocellular carcinoma patients: A pilot study. Bioengineered. 12, 8233–8246. doi: 10.1080/21655979.2021.1982273
Dhiman, R. K., Rana, B., Agrawal, S., Garg, A., Chopra, M., Thumburuet, K. K., et al. (2014). Probiotic VSL3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 147, 1327–1337.e3. doi: 10.1053/j.gastro.2014.08.031
El-Serag, H. B. (2012). Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061
Elzouki, A. N. (2016). Probiotics and liver disease: Where are we now and where are we going? J. Clin. Gastroenterol. 50, suppl 2. doi: 10.1097/MCG.0000000000000712
Feng, P., Ye, Z., Kakade, A., Virk, A. K., Li, X., Liu, P. (2018). A review on gut remediation of selected environmental contaminants: possible roles of probiotics and gut microbiota. Nutrients 11, 22. doi: 10.3390/nu11010022
LeBlanc, J. G., Chain, F., Martín, R., Bermúdez-Humarán, L. G., Courau, S., Langella, P. (2017). Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 16, 79. doi: 10.1186/s12934-017-0691-z
Lee, H. W., Ahn, S. H. (2016). Prediction models of hepatocellular carcinoma development in chronic hepatitis b patients. World J. Gastroenterol. 22, 8314–8321. doi: 10.3748/wjg.v22.i37.8314
Lee, D. K., Kang, J. Y., Shin, H. S., Park, I. I., Ha, N. J. (2013). Antiviral activity of bifidobacterium adolescentis SPM0212 against hepatitis b virus. Arch. Pharm. Res. 36, 1525–1532. doi: 10.1007/s12272-013-0141-3
Li, J., Sung, C. Y., Lee, N., Ni, Y., Pihlajamäki, J., Panagiotou, G., et al. (2016). Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. U. S. A. 113, e1306–e1315. doi: 10.1073/pnas.1518189113
Marcellin, P., Gane, E., Buti, M., Afdhal, N., Sievert, W., Jacobson, I. M., et al. (2013). Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis b: A 5-year open-label follow-up study. Lancet 381, 468–475. doi: 10.1016/S0140-6736(12)61425-1
Marlicz, W., Wunsch, E., Mydlowska, M., Milkiewicz, M., Serwin, K., Mularczyk, M., et al. (2016). The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J. Physiol. Pharmacol. 67, 867–877.
Mendoza, L. (2019). Potential effect of probiotics in the treatment of breast cancer. Oncol. Rev. 13, 422. doi: 10.4081/oncol.2019.422
Ohtani, N., Kawada, N. (2019). Role of the gut-liver axis in liver inflammation, fibrosis, and cancer: A special focus on the gut microbiota relationship. Hepatol. Commun. 3, 456–470. doi: 10.1002/hep4.1331
Patel, R., DuPont, H. L. (2015). New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 60, s108–s121. doi: 10.1093/cid/civ177
Petschow, B., Doré, J., Hibberd, P., Dinan, T., Reid, G., Blaser, M., et al. (2013). Probiotics, prebiotics, and the host microbiome: the science of translation. Ann. N Y Acad. Sci. 1306, 1–17. doi: 10.1111/nyas.12303
Plottel, C. S., Blaser, M. J. (2011). Microbiome and malignancy. Cell Host Microbe 10, 324–335. doi: 10.1016/j.chom.2011.10.003
Preveden, T., Scarpellini, E., Milic, N., Luzza, F., Abenavoli, L. (2017). Gut microbiota changes and chronic hepatitis c virus infection. Expert Rev. Gastroenterol. Hepatol. 11, 813–819. doi: 10.1080/17474124.2017.1343663
Qin, N., Yang, F., Li, A., Prifti, E., Chen, Y., Shao, L., et al. (2014). Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64. doi: 10.1038/nature13568
Rayes, N., Seehofer, D., Hansen, S., Boucsein, K., Müller, A. R., Serke, S., et al. (2002). Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation. 74, 123–127. doi: 10.1097/00007890-200207150-00021
Rayes, N., Seehofer, D., Theruvath, T., Schiller, R. A., Langrehr, J. M., Jonas, S., et al. (2005). Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation-a randomized, double-blind trial. Am. J. Transplant. 5, 125–130. doi: 10.1111/j.1600-6143.2004.00649.x
Rincón, D., Vaquero, J., Hernando, A., Galindo, E., Ripoll, C., Puerto, M., et al. (2014). Oral probiotic VSL3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 34, 1504–1512. doi: 10.1111/liv.12539
Rusthoven, C. G., Jones, B. L., Flaig, T. W., Crawford, E. D., Koshy, M., Sher, D. J., et al. (2016). Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J. Clin. Oncol. 34, 2835–2842. doi: 10.1200/JCO.2016.67.4788
Sandler, N. G., Koh, C., Roque, A., Eccleston, J. L., Siegel, R. B., Demino, M., et al. (2011). Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 141, 1220–1230. doi: 10.1053/j.gastro.2011.06.063
Sarin, S. K., Kumar, M., Lau, G. K., Abbas, Z., Chan, H., Chen, C. J., et al. (2016). Asian-Pacific clinical practice guidelines on the management of hepatitis b: A 2015 update. Hepatol. Int. 10, 1–98. doi: 10.1007/s12072-015-9675-4
Seki, E., Schnabl, B. (2012). Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 590, 447–458. doi: 10.1113/jphysiol.2011.219691
Shiha, G., Sarin, S. K., Ibrahim, A. E., Omata, M., Kumar, A., Lesmana, L. A., et al. (2009). Liver fibrosis: Consensus recommendations of the Asian pacific association for the study of the liver (APASL). Hepatol. Int. 3, 323–333. doi: 10.1007/s12072-008-9114-x
Singh, D., Khan, M. A., Siddique, H. R. (2021). Therapeutic implications of probiotics in microbiota dysbiosis: A special reference to the liver and oral cancers. Life Sci. 285, 120008. doi: 10.1016/j.lfs.2021.120008
Tandon, P., Moncrief, K., Madsen, K., Arrieta, M. C., Owen, R. J., Bain, V. G., et al. (2009). Effects of probiotic therapy on portal pressure in patients with cirrhosis: a pilot study. Liver Int. 29, 1110–1115. doi: 10.1111/j.1478-3231.2009.02020.x
Tao, X., Wang, N., Qin, W. (2015). Gut microbiota and hepatocellular carcinoma. Gastrointest. Tumors 2, 33–40. doi: 10.1159/000380895
Thilakarathna, W., Rupasinghe, H., Ridgway, N. D. (2021). Mechanisms by which probiotic bacteria attenuate the risk of hepatocellular carcinoma. Int. J. Mol. Sci. 22, 2606. doi: 10.3390/ijms22052606
Tian, F., Luo, M. J., Sun, M. Q., Lu, J., Huang, B. W., Guo, J. C. (2022). Staple line lockstitch reinforcement decreases clinically relevant pancreatic fistula following distal pancreatectomy: Results of a propensity score matched retrospective analysis. Front. Oncol. 21. doi: 10.3389/fonc.2022.999002
Wan, M. L. Y., El-Nezami, H. (2018). Targeting gut microbiota in hepatocellular carcinoma: Probiotics as a novel therapy. Hepatobiliary Surg. Nutr. 7, 11–20. doi: 10.21037/hbsn.2017.12.07
WHO Collaborating Center for Drugs Statistics Methodology (2003). ATC Index with DDDs 2003 (Oslo: WHO).
Wiesner, R., Edwards, E., Freeman, R., Harper, A., Kim, R., Kamath, P., et al. (2003). Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 124, 91–96. doi: 10.1053/gast.2003.50016
Wiest, R., Albillos, A., Trauner, M., Bajaj, J. S., Jalan, R. (2017). Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103. doi: 10.1016/j.jhep.2017.05.007
Yu, A. Q., Li, L. (2016). The potential role of probiotics in cancer prevention and treatment. Nutr. Cancer. 68, 535–544. doi: 10.1080/01635581.2016.1158300
Zhang, Q., Gao, F., Yang, X., Hu, Y., Liu, Y., Hou, Y., et al. (2020b). Protective effect of probiotics against esophagogastric variceal rebleeding in patients with liver cirrhosis after endoscopic therapy. Med. Sci. Monit. 26, e924040. doi: 10.12659/MSM.924040
Zhang, C., Yang, M., Ericsson, A. C. (2020a). The potential gut microbiota-mediated treatment options for liver cancer. Front. Oncol. 10. doi: 10.3389/fonc.2020.524205
Keywords: liver cancer, hepatitis B virus, probiotics, cirrhosis, propensity score matching, gut microbiota
Citation: Shi K, Zhang Q, Zhang Y, Bi Y, Zeng X and Wang X (2023) Association between probiotic therapy and the risk of hepatocellular carcinoma in patients with hepatitis B-related cirrhosis. Front. Cell. Infect. Microbiol. 12:1104399. doi: 10.3389/fcimb.2022.1104399
Received: 21 November 2022; Accepted: 30 December 2022;
Published: 13 January 2023.
Edited by:
Shicheng Guo, University of Wisconsin-Madison, United StatesReviewed by:
Haoyan Li, University of Texas MD Anderson Cancer Center, United StatesLei Huang, Microsoft, United States
Yi Liu, Stanford University, United States
Zhiping Hu, University of Pittsburgh, United States
Zeyuan Wang, Merck, United States
Wentao Liang, Wake Forest University, United States
Copyright © 2023 Shi, Zhang, Zhang, Bi, Zeng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianbo Wang, d2FuZ3hiQGNjbXUuZWR1LmNu
 Ke Shi
Ke Shi Qun Zhang
Qun Zhang Yi Zhang
Yi Zhang Yufei Bi
Yufei Bi Xuanwei Zeng
Xuanwei Zeng Xianbo Wang
Xianbo Wang