- Department of Rheumatology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Numerous studies have demonstrated that gut microbiota is essential for the host’s health because it regulates the host’s metabolism, endocrine, and immune systems. In recent years, increasing evidence has shown that gut microbiota plays a role in the onset and progression of gout. Changes in the composition and metabolism of the gut microbiota, result in abnormalities of uric acid degradation, increasing uric acid generation, releasing pro-inflammatory mediators, and intestinal barrier damage in developing gout. As a result, gout therapy that targets gut microbiota has drawn significant interest. This review summarized how the gut microbiota contributes to the pathophysiology of gout and how gout affects the gut microbiota. Additionally, this study explained how gut microbiota might serve as a unique index for the diagnosis of gout and how conventional gout treatment medicines interact with it. Finally, prospective therapeutic approaches focusing on gut microbiota for the prevention and treatment of gout were highlighted, which may represent a future avenue in gout treatment.
1. Introduction
Gout is a common disease characterized by the deposition of monosodium urate (MSU) crystals in joint and non-joint structures (Dalbeth et al., 2021). The inflammatory response of host tissue to deposit monosodium urate (MSU) crystals induces clinical symptoms (Dalbeth et al., 2019). Globally, gout is highly prevalent. Adults in China have a gout prevalence rate of 1.1%, compared to 3% to 4% in the United States and 1% to 4% in Europe (Dehlin et al., 2020). Genetic diversity, environmental exposure, gene-environment interaction, and intrinsic risk factors (including age, gender and weight) contribute to the risk of developing gout (Major et al., 2018). In addition, gout and hyperuricemia have many common comorbidities, such as cardiovascular disease, chronic kidney disease, diabetes, metabolic syndrome and neurodegenerative diseases (Bardin and Richette, 2017).
The human digestive system contains trillions of species, including bacteria, fungi, archaea, viruses, and protozoa, which comprise the gut microbiota, a complex ecological community (Human Microbiome Project, 2012). The taxonomic diversity of the gut microbiota impacts the integrity of the epithelial barrier, preservation of intestinal metabolism, and immunological homeostasis (Parker et al., 2020). The gut microbiota influences healthy physiological function and disease susceptibility through its collective metabolic activity and host interaction (Lozupone et al., 2012). With the advancement of sequencing technology and the creation of new bioinformatics, it has been discovered that gut microbiota composition changes and metabolism disruptions are connected to the pathogenesis of numerous diseases, such as autoimmune disease (Jiao et al., 2020), mental illness (Jarbrink-Sehgal and Andreasson, 2020), cerebrovascular disease (Xu et al., 2020), and disorders of the central nervous system (Vuotto et al., 2020).
Emerging evidence revealed a link between gut microbiota and arthritis diseases, including gout (Chu et al., 2021). Therefore, this review aims to summarize gut microbiota function in gout pathogenesis and illustrate gut microbiota as a potential target of gout treatment.
2. Gut microbiota and physiologic acid uric metabolism
In humans and higher primates, urate is the final oxidation product of purine catabolism (Cabau et al., 2020). It is synthesized mainly in the liver, intestines and tissues, such as muscles, kidneys, and vascular endothelium (El Ridi and Tallima, 2017). Purine synthesis begins with 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP) leading to hypoxanthine nucleotide formation (Dewulf et al., 2022). Hypoxanthine is converted to xanthine, which is then transformed into uric acid (UA) by either xanthine oxidase (XO) or xanthine dehydrogenase (Sun et al., 2022). Approximately 700 mg of UA is produced daily by such processes (Yanai et al., 2021). Renal and gut excretions accounts for around two-thirds and one-third of urate excretion, respectively (Mandal and Mount, 2015). Urate homeostasis is primarily influenced by renal proximal tubule cells, which express several transporters that either reabsorb urate or are involved in urate excretion (Eckenstaler and Benndorf, 2021).
During the evolution of humanoid primates, the pseudogenization of the uricase gene caused humans and other mammals to lose uricase activity. It left them unable to oxidize further urate to the more water-soluble compound, allantoin (Chang, 2014).Therefore, serum uric acid (sUA) levels in humans are three- ten times higher than in organisms that preserve uricase (Kratzer et al., 2014).
Unlike humans, bacteria can degrade uric acid by urate oxidase (uricase), and specific bacterial strains also exhibit xanthine dehydrogenase (XDH) inhibitory activity (Alam et al., 2011). Lactobacillus species break down inosine and guanosine to inhibit uric acid biosynthesis during purine metabolism (Alvarez-Lario and Macarron-Vicente, 2010; Wu et al., 2020). Recently, Lactobacillus gallinii has been shown to reduce purine levels in the gut, and its fermentation products have urate-lowering effects (Li et al., 2014). In addition, Lactobacillus gasseri strains can reduce purine absorption in the gut (Yamada et al., 2016). However, not all gut microbiota is protective. Xi et al. demonstrated that Escherichia-Shigella secretes xanthine deaminase, converting hypoxanthine and xanthine into uric acid and elevating serum uric acid levels (Xi et al., 2000).
The gut microbiota also plays a role in uric acid excretion. Studies have shown that two short-chain fatty acids (SCFAs) (propionate and butyrate) provide adenosine triphosphate (ATP) to the intestinal wall cells and promote UA excretion (Nieuwdorp et al., 2014). In addition, a recent study found higher Escherichia coli levels in greater uric acid decomposition (Liu et al., 2020).
3. Dysbiosis and gout
3.1. Description of the microbiome in gout patients
The abnormal secretion of interleukin-1β (IL-1β) stimulated by MSU causes the acute onset of gout, which occurs by activating the innate immune system through the recognition of Toll-like receptor (TLR) or NOD-like receptor (NLR) (Dalbeth et al., 2021). The release of a large amount of IL-1β by activating of NLRP3 (NOD-, LRR-, and pyrin domain-containing 3 inflammasome) is the central process of MSU-mediated gout acute attack (Martinon et al., 2006).
A recent study reported that Phascolarctobacterium and Bacteroides were enriched in gout patients and identified a “core microbiota” for the gout group encompassing three Bacteroides species (Mendez-Salazar et al., 2021). Bacteroides is a gut enterotype reported to promote urate conversion into allantoin, and might be involved in serum urate level regulation in humans (Lim et al., 2014).
GUO et al. observed that the gut microbiota of gout was characterized by significantly-impaired butyric acid synthesis (Guo et al., 2016). Vieira et al. found that SCFAs were necessary to assemble inflammasome and produce IL-1β (Vieira et al., 2015). Another study explored the effects of a high-fiber diet and acetate on inflammation in gout mice models. The regression of neutrophil inflammation was found to be related to a decrease of nuclear factor κ B (NF-κ B) activity and an increase of anti-inflammatory mediators (including interleukin-10, tumor necrosis factor-β and Annexin A1). Acetate controlled the inflammatory response to the MSU lens by promoting the regression of the inflammatory response (Vieira et al., 2017). The species that make SCFAs have a protective effect on inflammation, and are more abundant in the healthy group (Sheng et al., 2021). These results collectively showed that SCFAs and gut microbiota play a role in controlling inflammatory response to MSU crystals (Cleophas et al., 2017). Additionally, by up-regulating TLR2/4/5 and promoting the release of IL-1β and tumor necrosis factor-α (TNF-α), the increase in the number of inflammation-related microbiota causes immunological diseases and intestinal barrier dysfunction. Increased intestinal permeability, positively linked with serum uric acid level, results from decreased levels of the epithelial tight junction proteins occludin and claudin-1 (Lv et al., 2020).
3.2. Changes of gut microbiota composition and hyperuricemia
3.2.1. Diversity and abundance of gut microbiota in hyperuricemia
The variety and richness of gut microbiota have changed in hyperuricemic individuals and rats, indicating that gut microbiota may have a possible involvement in gout (Liu et al., 2020; Chu et al., 2021). Uric acid is the final product of purine metabolism and alterations in uric acid production or excretion can lead to abnormal serum uric acid levels (Balakumar et al., 2020). The changes in the abundance and composition of gut microbiota increase the serum uric acid level through the dysfunction of uric acid degradation and increased uric acid production (Sheng et al., 2021).
Gout has higher relative abundances of Prevotella and Bacteroides while lower relative abundances of Enterobacteriaceae, which might cause uric acid degradation dysfunction and the buildup of uric acid in gout (Chu et al., 2021). Additionally, the greater serum urate (SU) level was closely connected to the lower relative abundance of Faecalibacterium in hyperuricemia (Wei et al., 2021).
A shotgun metagenomic study revealed the microbiota with the allantoinase gene, which can convert uric acid into urea was deficient in gout. In contrast, the microbiota with the xanthine dehydrogenase gene was abundant. The buildup of uric acid may be caused by excessive xanthine dehydrogenase and a relative lack of allantoinase (Guo et al., 2016; Gong et al., 2020).
An increase in UA in blood circulation affects the intestinal environment, causing changes in the gut microbiota (Wang et al., 2022). A metagenomic study reveals that hyperuricemia causes an imbalance in the gut microbiota and alters its composition. It might induce the gut microbiota to translocate into other tissues, particularly the kidney, causing inflammation (Xu et al., 2019). In addition, a recent study has demonstrated that the abundance of inflammation-related microbiota in hyperuricemia and increased uric acid levels are associated with the impairment of intestinal barrier, which disrupts the host-microbiota crosstalk (Lv et al., 2020). While luminal UA can play a protective role in intestinal injury, some studies have shown that the changes in gut microbiota caused by uric acid are beneficial to the body (Wada et al., 2022). However, a study exploring the relationship between blood uric acid levels and gut microbiota in diabetic patients, found that fluctuations in uric acid within the normal range were not associated with changes in gut microbiota (Zhang et al., 2021). Therefore, further studies are needed to explore the causal relationship between the alteration of gut microbiota and hyperuricemia (Figure 1).
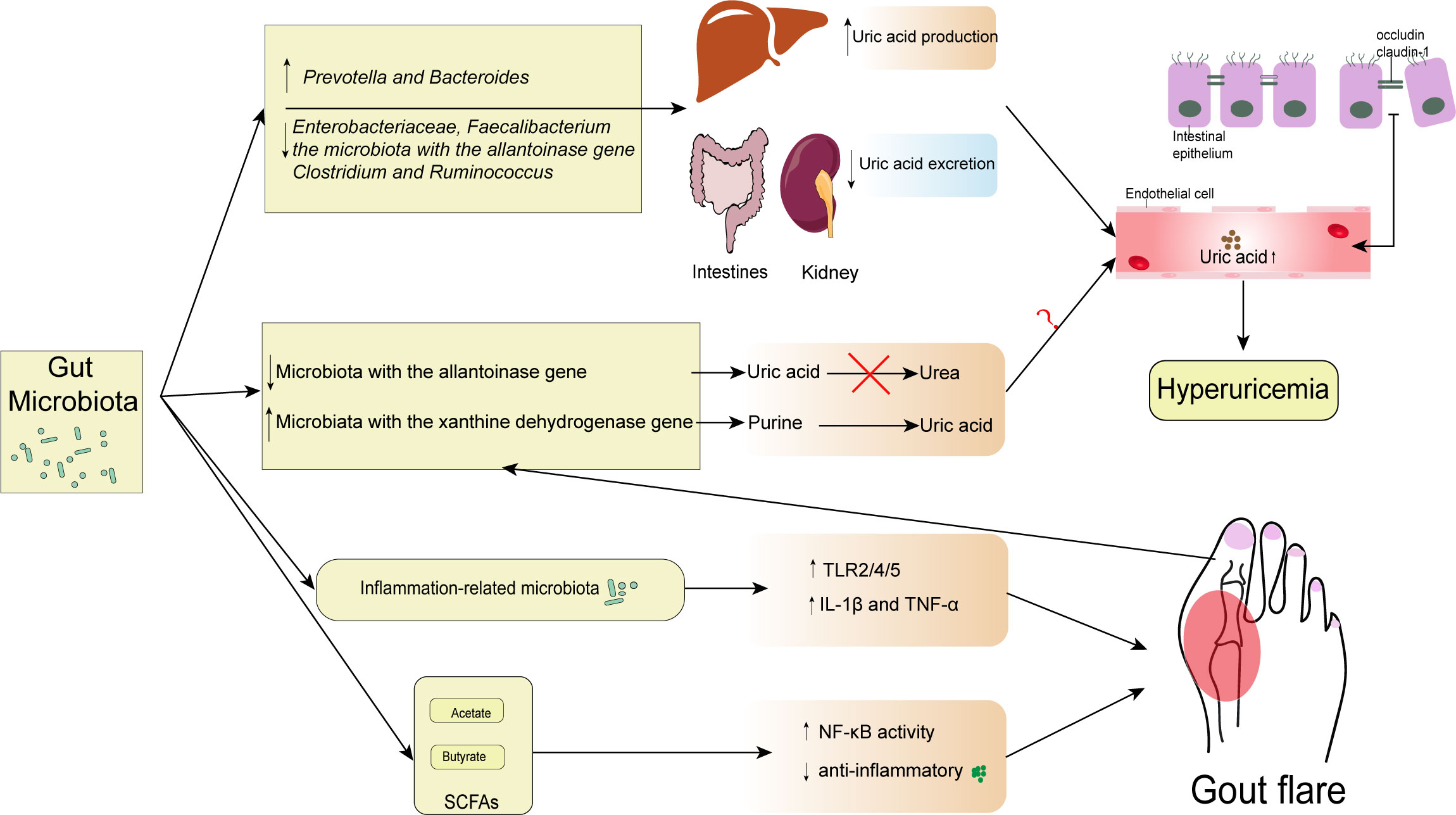
Figure 1 Interactions between gut microbiota and gout. The diversity and abundance of gut microbiota change include the increase of Prevotella and Bacteroides and the decrease of Enterobacteriaceae, Faecalibacterium, the microbiota with the allantoinase gene, Clostridium, and Ruminococcus. These changes result in excessive uric acid production in the liver and insufficient uric acid excretion in the kidney and intestine, raising serum uric acid levels above normal. In addition, some microbiota with the allantoinase and the xanthine dehydrogenase gene changed in gout can directly regulate the intestinal uric acid levels. However, the contribution of elevated intestinal uric acid levels to elevated serum uric acid levels remains unknown. Consequently, occludin and claudin-1 levels at tight epithelial junctions can drop when serum uric acid levels rise. Gout is caused by inflammation-related bacteria that upregulate TLR2/4/5 and encourage the release of IL-1 β and TNF- α. However, some SCFAs may have a protective role in inflammation. SCFAs, especially butyrate, are associated with the increased expression of Inhibitory-κκBα (I-κBα), which inhibits the phosphorylation and nuclear translocation of NF-κB p65, and the downstream inflammatory cytokine, MCP-1, and IL-1β expression.
3.2.2. Metabolism of gut microbiota in hyperuricemia
Various metabolites, including SCFAs, trimethylamine, amino acid derivatives, and vitamins, are produced by the gut microbiota from dietary components, including significant amounts of micronutrients, fiber, and polyphenols (Parker et al., 2020). Acetate (C2), propionate (C3), and butyrate (C4) are the most prevalent SCFAs in the human body. SCFAs are most extensively researched (Macfarlane and Macfarlane, 2003). The human body relies heavily on SCFAs. Butyric acid protects the human gut by nourishing the mucosa, fosters the development and repair of intestinal villi, boosts intestinal immunity, encourages the growth of good bacteria, and prevents the colonization of pathogens (Louis and Flint, 2009). A study through the Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathway analysis revealed significant differences in amino acid metabolism (phenylalanine, tyrosine and tryptophan biosynthesis, D-glutamine and D-glutamate metabolism, and phosphate and phosphonate metabolism) and nucleotide metabolism (purine metabolism) between hyperuricemia and healthy controls. The gut microbiota’s metabolic dysfunction may influence serum uric acid levels through its impact on host metabolites (Wei et al., 2021). The production of SCFAs (concentrations of acetate, propionate, and butyrate) derived from the gut microbiota in mice positively correlates with the effectiveness of treating hyperuricemia. This finding demonstrates that some beneficial bacteria decrease in HUA mice, including bacteria that produce SCFAs, such as Clostridium and Ruminococcus (Yu et al., 2018; Xu et al., 2019; Guo et al., 2021).
4. Gout diagnosis based on gut microbiota
Due to the causative relationship between the gut microbiota and gout development, the gout- specific gut microbiota may be a diagnostic marker. Lin et al. made a classification model using significantly-enriched bacterial genera between healthy individuals and gout patients. The result showed a high mean area under the working curve (AUC) of up to 0.973 by the receiver operating characteristic (ROC) analysis (Lin et al., 2021). Likewise, a cohort study established a diagnostic model based on 17 kinds of gout-related bacteria and reached 88.9% accuracy (Guo et al., 2016). The metagenomic analysis of gut microbiota by Chu et al. found three genes significantly enriched in the cohort gout. The AUC of the development and validation cohort were 0.91 and 0.80, respectively (Chu et al., 2021).
Furthermore, Yang et al. verified that several bacteria, including unclassified Enterobacteriaceae, Roseburia, and Faecalibacte-rium, have excellent diagnostic value for asymptomatic hyperuricemia (Yang et al., 2021). Therefore, the gut microbiota imbalance characterized by gout may become a non-invasive diagnostic tool for gout and asymptomatic hyperuricemia. It has a promising target for future prevention and intervention.
5. Treatment of gout
5.1. Traditional treatment and gut microbiota changes
Non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and colchicine are the first-line drugs for acute gout (Kiltz et al., 2016). International guidelines describe xanthine oxidase inhibitors and uricosuric drugs as the first- and second-line treatments, respectively, in uric acid-lowering therapy (Engel et al., 2017). Gut microbiota has become an important factor in hyperuricemia and has been shown to affect the response to disease treatment (Yu et al., 2018). A recent study found substantial alterations in the gut microbiota composition and promotion of SCFA formation, particularly acetate, in gouty arthritis patients after treatment (Park and Lee, 2022).
5.1.1. Non-steroidal anti-inflammatory drugs
NSAIDs are classic drugs for treating acute gout that affect pain relief and inflammation (Bindu et al., 2020). However, NSAIDs have several side effects, including gastrointestinal damage. Studies have indicated that NSAIDs can disrupt the gut microbiota equilibrium, multiplying gram-negative bacteria and decreasing gram-positive bacteria. Subsequently, pathogens activate inflammatory pathways through TLR4 and release inflammatory cytokines (Wang et al., 2021). In addition, NSAIDs can enhance intestinal permeability, making bacteria enter the mucosa (Montalto et al., 2010), leading to further changes in gut microbiota composition.
5.1.2. Colchicine
Colchicine (COL) is a traditional drug for gout that can block tubulin polymerization and prevent inflammasome activatin (Tristan Pascart, 2018). However, colchicine has potential toxicity to human health. Gastrointestinal discomfort is the most common symptom of COL toxicity, including nausea, vomiting and diarrhea (Akodad et al., 2020). Shi et al. found that acute oral COL in mice significantly affected the gastrointestinal structure and substantially changed the gut microbiota’s diversity, composition, and function. This is closely related to the down-regulation of intestinal proinflammatory cytokines and the destruction of intestinal integrity in mice. Therefore, it supports the destruction of homeostasis in the intestinal microbiome and might increase the toxic burden of COL (Shi et al., 2020). Another study in the same group identified bacterial biomarkers associated with diarrhea, indicating that the adverse reactions caused by COL were closely related to the gastric microbiological disturbance. By understanding the microbiome’s role in adverse COL reactions, the gut microbiota can be targeted, and the effectiveness of COL treatment can be increased (Shi et al., 2021).
5.1.3. Allopurinol
Allopurinol, an inhibitor of xanthine oxidase, is one of the most widely-used uric acid-lowering drugs (Mackenzie et al., 2020). Yu et al. found that the gut microbiota in the allopurinol treatment group changed compared with the control group. The treatment group had increased bifidobacterium and decreased anaerobes, which may be related to the decrease in UA. In addition, Bilophila, the only reduced genus in the allopurinol treatment group (Yu et al., 2018), has been shown to cause systemic inflammation (Feng et al., 2017).
5.1.4. Benzbromarone
Benzbromarone decreases blood uric acid levels and reabsorption by blocking the dominant apical (luminal) uric acid exchanger in the human proximal tubule, URAT-1 (Azevedo et al., 2019). Similar findings were made in another study, which showed that treating with benzbromarone altered the gut microbiota in the group that received it. It led to an increase in Bifidobacterium and a decrease in anaerobes Butyricimonas. In addition, benzbromarone repaired the lipid metabolism disorder in hyperuricemia rats through gut microbiota intervention (Yu et al., 2018).
5.1.5. Febuxostat
Febuxostat, a nonpurine inhibitor of xanthine oxidase, treats hyperuricemia in gout patients. It inhibits oxidized and reduced forms of xanthine oxidase, reducing uric acid formation (White et al., 2018). Lin et al. detected a restriction of gut microbiota biodiversity in untreated gout patients and febuxostat partially restored this change. Functional analysis revealed that the gut microbiome of gout patients was functionally-enriched for carbohydrate metabolism but had a lower potential for purine metabolism, which was relatively enhanced in gout patients treated with febuxostat (Lin et al., 2021). Another animal study verified that febuxostat could reshape gut microbiota dysbiosis in an animal model, regulate gut-derived metabolites, and inhibit microinflammation in vivo (Tu et al., 2020).
5.2. Treatment of gout based on gut microbiota changes
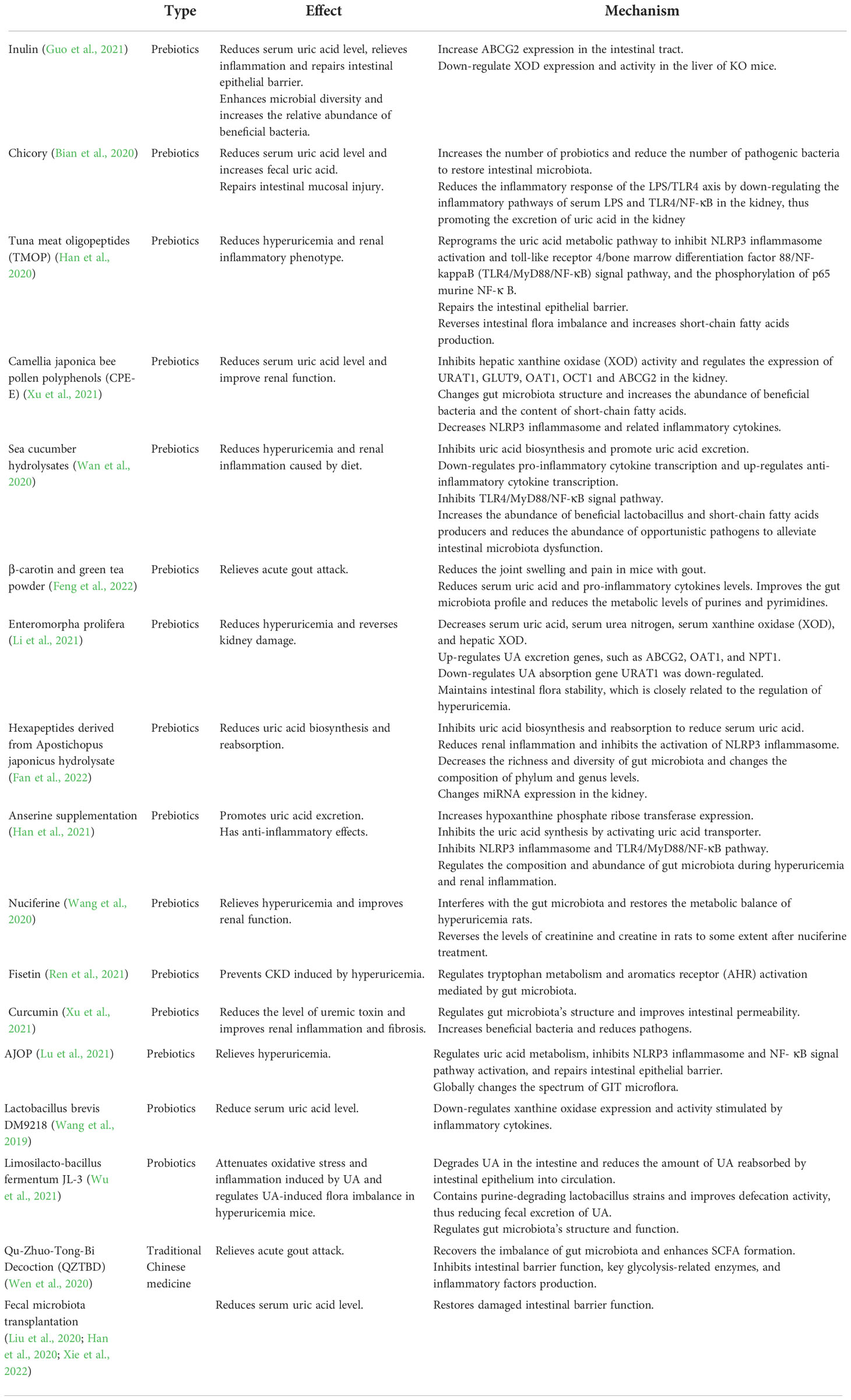
5.2.1. Probiotics and prebiotics
Nowadays, the low rates of urate-lowering therapy initiation and continuation and the side effects of traditional drugs remain challenges for gout treatment. These side effects include gastrointestinal toxicity, tolerance, allopurinol hypersensitivity syndrome, nephrotoxicity, and contraindications of other common comorbidities (Khanna et al., 2012; Becker et al., 2015; Vargas-Santos and Neogi, 2017; Rai et al., 2018). About 40% of gout patients suffer from chronic kidney disease (CKD) (at least stage II) and decreased GFR (Gaffo and Saag, 2008). Non-steroidal anti-inflammatory drugs, colchicine and uricosuric medicine use also are limited (Aslam and Michet, 2017). Therefore, therapies or drugs which are safer and can intervene in gout development are greatly needed.
In recent years, a better understanding of gut microbiota in the pathogenesis of gout and applying natural products in the prevention and treatment of gout have attracted widespread attention. Natural products, probiotics, probiotics and fecal microbiota transplantation (FMT) have been widely studied by new therapeutic methods acting on gut microbiota (Wu et al., 2021; Zhao et al., 2022; Xie et al., 2022). These play a role in treating gout by inhibiting the metabolism of purine and inflammatory factors and bodies, regulating the expression of transporters, and protecting the integrity of intestinal barrier. It can also increase the abundance of intestinal bacteria related to the production of SCFA and promote SCFA production, thus inhibiting the activity of XOD in serum and liver (Ni et al., 2021).
Probiotics are “live microorganisms that, when administered adequately, confer a health benefit on the host (Hill et al., 2014). Bifidobacterium and Lactobacillus strains are the most widely-used probiotics in functional food and dietary supplements. However, the next generation of probiotics, such as Faecalibacterium prausnitzii, Akkermansia muciniphila, or the genus Clostridium, have shown promising results (Vallianou et al., 2020).
Some in vitro experiments have proven that diets containing probiotics can prevent hyperuricemia by regulating the structure and function of intestinal flora. For example, Lactobacillus fermentans JL-3 can regulate hyperuricemia-induced intestinal microbiota dysbiosis and effectively reduce the UA level in mice (Wu et al., 2021). DM9218, as a probiotic strain, has the potential to ameliorate fructose-induced hyperuricemia. Animal experiments showed that DM9218 could reduce serum UA level and hepatic xanthine oxidase activity and regulate intestinal dysbiosis induced by high fructose in fructose-fed mice (Wang et al., 2019). In addition, Garcia-Arroyo et al. demonstrated that probiotics containing urate-decomposing bacteria could reduce serum uric acid in hyperuricemic animals. It could also beneficially affect hypertension and kidney disease (Garcia-Arroyo et al., 2018).
A prebiotic published is “a substrate that is selectively utilized by host microorganisms conferring a health benefit (Swanson et al., 2020). Recent studies employing a variety of probiotic molecules have consistently demonstrated an increase in the relative numbers of Lactobacillus and Bifidobacterium spp. as well as changes in bacterial metabolism, as evidenced, in particular, by increased production of short-chain fatty acids, such as butyrate and propionate (Holscher, 2017). The research of prebiotics in treating gout has become a promising direction.
An animal experiment by Ren et al. found that fisetin reversed changes in Bacteroides, and Firmicutes in hyperuricemic mice, suggesting that fisetin reduced serum uric acid levels by modulating hyperuricemia-induced changes in gut microbiota. In addition, fisetin could improve renal function in hyperuricemia-induced CKD mice by regulating intestinal microbiota-mediated tryptophan metabolism (Ren et al., 2021). In addition, relevant metabolomics studies have shown that nuciferine may inhibit the pathological process of hyperuricemia by regulating the disturbed metabolic pathways. Furthermore, nuciferine can restore the metabolic changes caused by hyperuricemia by regulating intestinal microbiota composition in rats (Wang et al., 2020). E. prolifera polysaccharides (EPP), one of the most widely distributed green algae belonging to the family Ulvaceae, showed beneficial effects on the serum levels of UA and significantly improved the diversity of gut microbiota, especially the proportions of Alistipes and Parasutterella. Further, correlation analysis revealed that the presence of Parasutterella might be negatively associated with increased UA (Li et al., 2021).
In addition, it has been shown that co-feeding of β-carotene and green tea powder in gouty mice significantly increased the positive interaction between gut microbes, which may positively in relieve gout symptoms (Feng et al., 2022). Dietary administration of tuna meat oligopeptides (TMOP) alleviates hyperuricemia and renal inflammatory phenotypes. Furthermore, it reprograms the uric acid metabolism pathway. TMOP treatment repairs the intestinal epithelial barrier, reverses the dysbiosis of the gut microbiota, and increased the production of SCFAs. Furthermore, the antihyperuricemic effect of TMOP was transmitted by transplanting fecal microbiota from TMOP-treated mice, mediating the protective effect, at least in part, by the gut microbiota (Han et al., 2020).
In recent years, there have been more studies on the mechanism of various probiotics and prebiotics in treating gout. Table 1 shows a summary of gout treatment targeting intestinal microorganisms. However, most studied conducted animal experiments, and no testing has been done on humans. Future research should focus more on human experiments to explore whether these new treatments, such as prebiotics and probiotics, can relieve the symptoms of gout and achieve the purpose of treatment.
5.2.2. Dietary habits
The microbiota composition can be modified by a variation in the individual’s dietary-nutritional habits, especially concerning the quality and quantity of fats, dietary fibers and carbohydrates consumed (Voreades et al., 2014). Dietary factors are also considered a risk factor for gout (Dehlin et al., 2020), thus, several well-established healthy eating patterns, such as the Mediterranean and Diet to Stop Hypertension (DASH) diets, can lower serum urate levels (Yokose et al., 2021). Cohort studies have shown that a typical western diet is associated with a higher risk of developing gout, while adherence to a Mediterranean diet is associated with a lower risk (Rai et al., 2017). Therefore, the Mediterranean and DASH diets have preventive effects on hyperuricemia (Sun et al., 2022).
In addition, studies have shown that an excessive fructose diet can affect gut microbiota composition through a series of damage to the intestinal barrier function of the inflammatory response. Therefore, a new method for gout treatment could be to limit the specific fructose intake and improve the composition of gut microbiota or targeted metabolite (Fang et al., 2022).
5.2.3. Fecal microbiota transplantation
FMT is the transfer of fecal microbial content from a healthy individual into the gastrointestinal tract of a diseased individual (Ooijevaar et al., 2019). The action mechanism is not entirely understood, but restoring a disturbed microbiota underlies the observed effect (Smits et al., 2016). Since gut microbiota imbalance is closely related to gout, FMT may become a new direction for treating gout.
Xie et al. found that the washed microbiota transplantation (WMT) effectively reduced serum uric acid levels, relieved gout symptoms, and improved impaired intestinal barrier function in gout patients (Xie et al., 2022). In addition, a previous study showed that fecal transplantation attenuated hyperuricemia and renal inflammatory phenotypes in mice (Han et al., 2020).
5.2.4. Traditional Chinese medicine
Traditional Chinese medicine (TCM) has been applied to treat gout since ancient China. Some chemical ingredients isolated from TCM herbs are multi-target and low toxicity, showing advantages and good prospects in gout prevention and treatment (Chi et al., 2020).
An animal study by Lin et al. demonstrated that Si Miao decoction improved gut microbiota dysbiosis associated with gouty arthritis by significantly reducing the abundance of Prevotella in the gut microbiota of mice, beneficial to relieve inflammation. In addition, some pathogenic bacteria positively correlated with intestinal inflammatory cytokines were reduced by Si Miao decoction, including Klebsiella, Brautia, Escherichia-Shigella, and Enterococcus (Lin et al., 2020).
Qu-Zhuo-Tong-Bi Decoction (QZTBD) has been shown to inhibit the growth of Larrelidae _A2, a bacterium enriched in gouty mice, and to improve the abundance of ranunculus (a bacterium closely related to SCFAs). QZTBD can exert its therapeutic effects by restoring the gut microbiota composition and SCFA production. QZTBD treatment attenuated intestinal mucosal barrier function and promoted intestinal uric acid excretion through these changes. Furthermore, it inhibited glycolysis and suppressed intestinal proinflammatory cytokines (Wen et al., 2020). Therefore, traditional Chinese medicine targeting intestinal flora in gout treatment may be a promising direction
6 Conclusions
Gut microbiota plays a key role in gout pathogenesis through the changes of diversity, abundance, metabolic pathway, and metabolites, such as SCFAs, resulting in hyperuricemia and gout flare. Hyperuricemia and gout can cause an imbalance in the microbiota in the gut, which can then trigger the development of other metabolic illnesses, creating a vicious cycle. In addition, drugs related to gout treatment can play a therapeutic role by changing the composition of gut microbiota. Gut microbiota examination provides a non-invasive, simple, sensitive, and reliable index in diagnosis. Developing novel and safe new drugs targeted at gut microbiota has become a research focus. Prebiotics, probiotics, traditional Chinese medicine and fecal transplantation therapy are expected to become new methods for gout treatment.
Author contributions
ST: Writing - Original Draft. PZ and MC: Visualization. QC and XC: Resources. ZW: Methodology. XL: Writing- Reviewing and Editing. HW: Conceptualization. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Research and Development Program of Zhejiang Province (No. 2020C3044) and the National Natural Science Foundation of China (No. 82071810).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akodad, M., Sicard, P., Fauconnier, J., Roubille, F. (2020). Colchicine and myocardial infarction: A review. Arch. Cardiovasc. Dis. 113, 652–659. doi: 10.1016/j.acvd.2020.04.007
Alam, N., Yoon, K. N., Lee, K. R., Kim, H Y., Shin, P. G., Cheong, J. C., et al. (2011). Assessment of antioxidant and phenolic compound concentrations as well as xanthine oxidase and tyrosinase inhibitory properties of different extracts of pleurotus citrinopileatus fruiting bodies. Mycobiology 39, 12–19. doi: 10.4489/MYCO.2011.39.1.012
Alvarez-Lario, B., Macarron-Vicente, J. (2010). Uric acid and evolution. Rheumatol. (Oxford) 49, 2010–2015. doi: 10.1093/rheumatology/keq204
Aslam, F., Michet, C., Jr. (2017). My treatment approach to gout. Mayo Clin. Proc. 92, 1234–1247. doi: 10.1016/j.mayocp.2017.05.026
Azevedo, V. F., Kos, I. A., Vargas-Santos, A. B., da Rocha Castelar Pinheiro, G., Dos Santos Paiva, E. (2019). Benzbromarone in the treatment of gout. Adv. Rheumatol 59, 37. doi: 10.1186/s42358-019-0080-x
Balakumar, P., Alqahtani, A., Khan, N. A., Mahadevan, N., Dhanaraj, S. A. (2020). Mechanistic insights into hyperuricemia-associated renal abnormalities with special emphasis on epithelial-to-mesenchymal transition: Pathologic implications and putative pharmacologic targets. Pharmacol. Res. 161, 105209. doi: 10.1016/j.phrs.2020.105209
Bardin, T., Richette, P. (2017). Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 15, 123. doi: 10.1186/s12916-017-0890-9
Becker, M. A., Fitz-Patrick, D., Choi, H. K., Dalbeth, N., Storgard, C., Cravets, M., et al. (2015). An open-label, 6-month study of allopurinol safety in gout: The LASSO study. Semin. Arthritis Rheum 45, 174–183. doi: 10.1016/j.semarthrit.2015.05.005
Bian, M., Wang, J., Wang, Y., Nie, A., Zhu, C., Sun, Z. (2020). Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. BioMed. Pharmacother. 131, 110719. doi: 10.1016/j.biopha.2020.110719
Bindu, S., Mazumder, S., Bandyopadhyay, U. (2020). Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 180, 114147. doi: 10.1016/j.bcp.2020.114147
Cabau, G., Crisan, T. O., Kluck, V., Popp, R. A., Joosten, L. A. B. (2020). Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 294, 92–105. doi: 10.1111/imr.12833
Chang, B. S. (2014). Ancient insights into uric acid metabolism in primates. Proc. Natl. Acad. Sci. U.S.A. 111, 3657–3658. doi: 10.1073/pnas.1401037111
Chi, X., Zhang, H., Zhang, S., Ma, K. (2020). Chinese Herbal medicine for gout: a review of the clinical evidence and pharmacological mechanisms. Chin. Med. 15, 17. doi: 10.1186/s13020-020-0297-y
Chu, Y., Sun, S., Huang, Y., Gao, Q., Xie, X., Wang, P., et al. (2021). Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 7, 66. doi: 10.1038/s41522-021-00235-2
Cleophas, M. C., Crisan, T. O., Joosten, L. A. (2017). Factors modulating the inflammatory response in acute gouty arthritis. Curr. Opin. Rheumatol 29, 163–170. doi: 10.1097/BOR.0000000000000366
Dalbeth, N., Choi, H. K., Joosten, L. A. B., Khanna, P. P., Matsuo, H., Perez-Ruiz, F., et al. (2019). Gout. Nat. Rev. Dis. Primers 5, 69. doi: 10.1038/s41572-019-0115-y
Dalbeth, N., Gosling, A. L., Gaffo, A., Abhishek, A. (2021). Gout. Lancet 397, 1843–1855. doi: 10.1016/s0140-6736(21)00569-9
Dehlin, M., Jacobsson, L., Roddy, E. (2020). Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol 16, 380–390. doi: 10.1038/s41584-020-0441-1
Dewulf, J. P., Marie, S., Nassogne, M. C. (2022). Disorders of purine biosynthesis metabolism. Mol. Genet. Metab. 136, 190–198. doi: 10.1016/j.ymgme.2021.12.016
Eckenstaler, R., Benndorf, R. A. (2021). The role of ABCG2 in the pathogenesis of primary hyperuricemia and gout-an update. Int. J. Mol. Sci. 22 (13), 6678. doi: 10.3390/ijms22136678
El Ridi, R., Tallima, H. (2017). Physiological functions and pathogenic potential of uric acid: A review. J. Advanced Res. 8, 487–493. doi: 10.1016/j.jare.2017.03.003
Engel, B., Just, J., Bleckwenn, M., Weckbecker, K. (2017). Treatment options for gout. Dtsch Arztebl Int. 114, 215–222. doi: 10.3238/arztebl.2017.0215
Fan, S., Huang, Y., Lu, G., Sun, N., Wang, R., Lu, C., et al. (2022). Novel anti-hyperuricemic hexapeptides derived from apostichopus japonicus hydrolysate and their modulation effects on the gut microbiota and host microRNA profile. Food Funct. 13, 3865–3878. doi: 10.1039/d1fo03981d
Fang, X.-Y., Qi, L.-W., Chen, H.-F., Gao, P., Zhang, Q., Leng, R.-X., et al. (2022). The interaction between dietary fructose and gut microbiota in hyperuricemia and gout. Front. Nutr. 9. doi: 10.3389/fnut.2022.890730
Feng, Z, Long, W., Hao, B., Ding, D., Ma, X., Zhao, L., et al. (2017). A human stool-derived bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. 9, 59. doi: 10.1186/s13099-017-0208-7
Feng, Y., Yu, Y., Chen, Z., Wang, L., Ma, J., Bai, X., et al. (2022). Effects of beta-carotin and green tea powder diets on alleviating the symptoms of gouty arthritis and improving gut microbiota in C57BL/6 mice. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.837182
Gaffo, A. L., Saag, K. G. (2008). Management of hyperuricemia and gout in CKD. Am. J. Kidney Dis. 52, 994–1009. doi: 10.1053/j.ajkd.2008.07.035
García-Arroyo, F. E., Gonzaga, E., Muñoz-Jiménez, G., Blas-Marron, I., Silverio, G., Tapia, O., et al. (2018). Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PloS One 13, e0202901. doi: 10.1371/journal.pone.0202901
Gong, M., Wen, S., Nguyen, T., Wang, C., Jin, J., Zhou, L. (2020). Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab. Syndr. Obes. 13, 943–962. doi: 10.2147/DMSO.S232377
Guo, Z., Zhang, J., Wang, Z., Ang, K. Y., Huang, S., Hou, Q. (2016). Intestinal microbiota distinguish gout patients from healthy humans. Sci. Rep. 6, 20602. doi: 10.1038/srep20602
Guo, Y., Yu, Y., Li, H., Ding, X., Li, X., Jing, X., et al. (2021). Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in uox-knockout mice. Eur. J. Nutr. 60, 2217–2230. doi: 10.1007/s00394-020-02414-x
Han, J., Wang, X., Tang, S., Lu, C., Wan, H., Zhou, J., et al. (2020). Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J. 34, 5061–5076. doi: 10.1096/fj.201902597RR
Han, J., Wang, Z., Lu, C., Zhou, J., Li, Y., Ming, T., et al. (2021). The gut microbiota mediates the protective effects of anserine supplementation on hyperuricaemia and associated renal inflammation. Food Funct. 12, 9030–9042. doi: 10.1039/d1fo01884a
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B. (2014). Expert consensus document. the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Holscher, H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184. doi: 10.1080/19490976.2017.1290756
Human Microbiome Project, C. (2012). A framework for human microbiome research. Nature 486, 215–221. doi: 10.1038/nature11209
Jarbrink-Sehgal, E., Andreasson, A. (2020). The gut microbiota and mental health in adults. Curr. Opin. Neurobiol. 62, 102–114. doi: 10.1016/j.conb.2020.01.016
Jiao, Y., Wu, L., Huntington, N. D., Zhang, X. (2020). Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00282
Khanna, D., et al (2012). 2012 American college of rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. (Hoboken) 64, 1431–1446. doi: 10.1002/acr.21772
Kiltz, U., Alten, R., Fleck, M., Krüger, K., Manger, B., Müller-Ladner, U., et al. (2016). [Full version of the S2e guidelines on gouty arthritis: Evidence-based guidelines of the German society of rheumatology (DGRh)]. Z Rheumatol 75 Suppl 2, 11–60. doi: 10.1007/s00393-016-0147-6
Kratzer, J. T., Lanaspa, M. A., Murphy, M. N., Cicerchi, C., Graves, C. L., Tipton, P. A., et al. (2014). Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. U.S.A. 111, 3763–3768. doi: 10.1073/pnas.1320393111
Li, M., Yang, D., Mei, L., Yuan, L., Xie, A., Yuan, J. (2014). Screening and characterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PloS One 9, e105577. doi: 10.1371/journal.pone.0105577
Li, X., Gao, X., Zhang, H., Liu, Y., Sarker, M. R., Wu, Y., et al. (2021). The anti-hyperuricemic effects of green alga enteromorpha prolifera polysaccharide via regulation of the uric acid transporters in vivo. Food Chem. Toxicol. 158, 112630. doi: 10.1016/j.fct.2021.112630
Lim, M. Y., Rho, M., Song, Y.-M., Lee, K., Sung, J., Ko, G., et al. (2014). Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci. Rep. 4, 7348. doi: 10.1038/srep07348
Lin, X., Shao, T., Huang, L., Wen, X., Wang, M., Wen, C., et al. (2020). Simiao decoction alleviates gouty arthritis by modulating proinflammatory cytokines and the gut ecosystem. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.00955
Lin, S., Zhang, T., Zhu, L., Pang, K., Lu, S., Liao, X., et al. (2021). Characteristic dysbiosis in gout and the impact of a uric acid-lowering treatment, febuxostat on the gut microbiota. J. Genet. Genomics 48, 781–791. doi: 10.1016/j.jgg.2021.06.009
Liu, H., Zhuang, J., Tang, P., Li, J., Xiong, X., Deng, H. (2020). The role of the gut microbiota in coronary heart disease. Curr. Atheroscler Rep. 22, 77. doi: 10.1007/s11883-020-00892-2
Liu, X., Lv, Q., Ren, H., Gao, L., Zhao, P., Yang, X. (2020). The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ 8, e8664. doi: 10.7717/peerj.8664
Louis, P., Flint, H. J. (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294, 1–8. doi: 10.1111/j.1574-6968.2009.01514.x
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. doi: 10.1038/nature11550
Lu, C., Tang, S., Han, J., Fan, S., Huang, Y., Zhang, Z., et al. (2021). Apostichopus japonicus oligopeptide induced heterogeneity in the gastrointestinal tract microbiota and alleviated hyperuricemia in a microbiota-dependent manner. Mol. Nutr. Food Res. 65, e2100147. doi: 10.1002/mnfr.202100147
Lv, Q., Xu, D., Zhang, X., Yang, X., Zhao, P., Cui, X., et al. (2020). Association of hyperuricemia with immune disorders and intestinal barrier dysfunction. Front. Physiol. 11. doi: 10.3389/fphys.2020.524236
Macfarlane, S., Macfarlane, G. T. (2003). Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62, 67–72. doi: 10.1079/PNS2002207
Mackenzie, I. S., Ford, I., Nuki, G., Hallas, J., Hawkey, C. J., Webster, J., et al. (2020). Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 396, 1745–1757. doi: 10.1016/s0140-6736(20)32234-0
Major, T. J., Dalbeth, N., Stahl, E. A., Merriman, T. R. (2018). An update on the genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol 14, 341–353. doi: 10.1038/s41584-018-0004-x
Mandal, A. K., Mount, D. B. (2015). The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 77, 323–345. doi: 10.1146/annurev-physiol-021113-170343
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A., Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. doi: 10.1038/nature04516
Méndez-Salazar, O. E., Vázquez-Mellado, J., Casimiro-Soriguer, C. S., Dopazo, J., Çubuk, C., Zamudio-Cuevas, Y., et al. (2021). Taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism. Mol. Med. 27, 50. doi: 10.1186/s10020-021-00311-5
Montalto, M., Gallo, A., Curigliano, V., D'Onofrio, F., Santoro, L., Covino, M. (2010). Clinical trial: the effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy - a randomized, double-blind, cross-over, placebo-controlled study. Aliment Pharmacol. Ther. 32, 209–214. doi: 10.1111/j.1365-2036.2010.04324.x
Ni, C., Li, X., Wang, L., Li, X., Zhao, J., Zhang, H. (2021). Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activity. via short-chain Fatty acid-dependent mechanism. Food Funct. 12, 7054–7067. doi: 10.1039/d1fo00198a
Nieuwdorp, M., Gilijamse, P. W., Pai, N., Kaplan, L. M. (2014). Role of the microbiome in energy regulation and metabolism. Gastroenterology 146, 1525–1533. doi: 10.1053/j.gastro.2014.02.008
Ooijevaar, R. E., Terveer, E. M., Verspaget, H. W., Kuijper, E. J., Keller, J. J. (2019). Clinical application and potential of fecal microbiota transplantation. Annu. Rev. Med. 70, 335–351. doi: 10.1146/annurev-med-111717-122956
Parker, A., Fonseca, S., Carding, S. R. (2020). Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11, 135–157. doi: 10.1080/19490976.2019.1638722
Park, H. K., Lee, S. J. (2022). Treatment of gouty arthritis is associated with restoring the gut microbiota and promoting the production of short-chain fatty acids. Arthritis Res. Ther. 24, 51. doi: 10.1186/s13075-022-02742-9
Rai, S. K., Fung, T. T., Lu, N., Keller, S. F., Curhan, G. C., Choi, H. K., et al. (2017). The dietary approaches to stop hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ 357, j1794. doi: 10.1136/bmj.j1794
Rai, S. K., Choi, H. K., Choi, S. H.J., Townsend, A. F., Shojania, K., De Vera, M. A., et al. (2018). Key barriers to gout care: a systematic review and thematic synthesis of qualitative studies. Rheumatol. (Oxford) 57, 1282–1292. doi: 10.1093/rheumatology/kex530
Ren, Q., Cheng, L., Guo, F., Tao, S., Zhang, C., Ma, L., et al. (2021). Fisetin improves hyperuricemia-induced chronic kidney disease via regulating gut microbiota-mediated tryptophan metabolism and aryl hydrocarbon receptor activation. J. Agric. Food Chem. 69, 10932–10942. doi: 10.1021/acs.jafc.1c03449
Shen, S., Chen, J., Zhang, Y., Qin, Q., Li, W., Yan, S., et al. (2021). Structural and functional alterations of gut microbiota in males with hyperuricemia and high levels of liver enzymes. Front. Med. (Lausanne) 8. doi: 10.3389/fmed.2021.779994
Shi, Y., Li, J., Yang, P., Niu, Z., Wei, L., Chen, L., et al. (2020). Colchicine increases intestinal permeability, suppresses inflammatory responses, and alters gut microbiota in mice. Toxicol. Lett. 334, 66–77. doi: 10.1016/j.toxlet.2020.09.018
Shi, Y., Cai, H., Niu, Z., Li, J., Pan, G., Tian, H., et al. (2021). Acute oral colchicine caused gastric mucosal injury and disturbance of associated microbiota in mice. Toxicology 461, 152908. doi: 10.1016/j.tox.2021.152908
Smits, W. K., Lyras, D., Lacy, D. B., Wilcox, M. H., Kuijper, E. J. (2016). Clostridium difficile infection. Nat. Rev. Dis. Primers 2, 16020. doi: 10.1038/nrdp.2016.20
Sun, L., Ni, C., Zhao, J., Wang, G., Chen, W. (2022). Probiotics, bioactive compounds and dietary patterns for the effective management of hyperuricemia: a review. Crit. Rev. Food Sci. Nutr. 1-16. doi: 10.1080/10408398.2022.2119934
Swanson, K. S., Gibson, G. R., Hutkins, R., Reimer, R. A., Reid, G., Verbeke, K., et al. (2020). The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687–701. doi: 10.1038/s41575-020-0344-2
Tristan Pascart, P. R. (2018). Colchicine in gout: An update. Curr. Pharm. Des. 24 (6), 684–689. doi: 10.2174/1381612824999180115103951
Tu, Y., Fang, Q.-J., Sun, W, Liu, B.-H., Liu, Y.-L., Wu, W., et al. (2020). Total flavones of abelmoschus manihot remodels gut microbiota and inhibits microinflammation in chronic renal failure progression by targeting autophagy-mediated macrophage polarization. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.566611
Vallianou, N., Stratigou, T., Christodoulatos, G. S., Tsigalou, C., Dalamaga, M. (2020). Probiotics, prebiotics, synbiotics, postbiotics, and obesity: Current evidence, controversies, and perspectives. Curr. Obes. Rep. 9, 179–192. doi: 10.1007/s13679-020-00379-w
Vargas-Santos, A. B., Neogi, T. (2017). Management of gout and hyperuricemia in CKD. Am. J. Kidney Dis. 70, 422–439. doi: 10.1053/j.ajkd.2017.01.055
Vieira, A. T., Macia, L., Galvão, I., Martins, F. S., Canesso, M. C.C., Amaral, F. A., et al. (2015). A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol 67, 1646–1656. doi: 10.1002/art.39107
Vieira, A. T., Galvão, I., Macia, L. M., Sernaglia, É. M., Vinolo, M. A.R., Garcia, C. C., et al. (2017). Dietary fiber and the short-chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J. Leukoc. Biol. 101, 275–284. doi: 10.1189/jlb.3A1015-453RRR
Voreades, N., Kozil, A., Weir, T. L. (2014). Diet and the development of the human intestinal microbiome. Front. Microbiol. 5. doi: 10.3389/fmicb.2014.00494
Vuotto, C., Battistini, L., Caltagirone, C., Borsellino, G. (2020). Gut microbiota and disorders of the central nervous system. Neuroscientist 26, 487–502. doi: 10.1177/1073858420918826
Wada, A., Higashiyama, M., Kurihara, C., Ito, S., Tanemoto, R., Mizoguchi, A., et al. (2022). Protective effect of luminal uric acid against indomethacin-induced enteropathy: Role of antioxidant effect and gut microbiota. Dig Dis. Sci. 67, 121–133. doi: 10.1007/s10620-021-06848-z
Wan, H., Han, J., Tang, S., Bao, W., Lu, C., Zhou, J., et al. (2020). Comparisons of protective effects between two sea cucumber hydrolysates against diet induced hyperuricemia and renal inflammation in mice. Food Funct. 11, 1074–1086. doi: 10.1039/c9fo02425e
Wang, H., Mei, L., Deng, Y., Liu, Y., Wei, X., Liu, M. (2019). Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition 62, 63–73. doi: 10.1016/j.nut.2018.11.018
Wang, L.-M., Wang, P., Teka, T., Zhang, Y.-C., Yang, W.-Z., Zhang, Y., et al. (2020). (1)H NMR and UHPLC/Q-Orbitrap-MS-Based metabolomics combined with 16S rRNA gut microbiota analysis revealed the potential regulation mechanism of nuciferine in hyperuricemia rats. J. Agric. Food Chem. 68, 14059–14070. doi: 10.1021/acs.jafc.0c04985
Wang, X., Tang, Q., Hou, H., Zhang, W., Li, M., Chen, D., et al. (2021). Gut microbiota in NSAID enteropathy: New insights from inside. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.679396
Wang, J., Chen, Y., Zhong, H., Chen, F., Regenstein, J., Hu, X., et al. (2022). The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 62, 3979–3989. doi: 10.1080/10408398.2021.1874287
Wei, J., Zhang, Y., Dalbeth, N., Terkeltaub, R., Yang, T., Wang, Y., et al. (2021). Association between gut microbiota and elevated serum urate in two independent cohorts. Arthritis Rheumatol. 74 (4), 682–691. doi: 10.1002/art.42009
Wen, X., Lou, Y., Song, S., He, Z., Chen, J., Xie, Z., et al. (2020). Qu-Zhuo-Tong-Bi decoction alleviates gouty arthritis by regulating butyrate-producing bacteria in mice. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.610556
White, W. B., Saag, K. G., Becker, M. A., Borer, J. S., Gorelick, P. B., Whelton, A., et al. (2018). Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl. J. Med. 378, 1200–1210. doi: 10.1056/NEJMoa1710895
Wu, J., Wei, Z., Cheng, P., Qian, C., Xu, F., Yang, Y., et al. (2020). Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 10, 10665–10679. doi: 10.7150/thno.43528
Wu, Y., Ye, Z., Feng, P., Li, R., Chen, X., Tian, X., et al. (2021). Limosilactobacillus fermentum JL-3 isolated from “Jiangshui” ameliorates hyperuricemia by degrading uric acid. Gut Microbes 13, 1–18. doi: 10.1080/19490976.2021.1897211
Xie, X.-Q., Geng, Y., Guan, Q., Ren, Y., Guo, L., Lv, Q., et al. (2021). Influence of short-term consumption of hericium erinaceus on serum biochemical markers and the changes of the gut microbiota: A pilot study. Nutrients 13. doi: 10.3390/nu13031008
Xi, H., Schneider, B. L., Reitzer, L. (2017). Purine catabolism in Escherichia coli and function of xanthine dehydrogenase in purine salvage. J. Bacteriol. 182, 5332–5341. doi: 10.1128/JB.182.19.5332-5341.2000
Xie, W.-R., Yang, X.-Y., Deng, Z.-H., Zheng, Y.-M., Zhang, R., Wu, L.-H., et al. (2022). Effects of washed microbiota transplantation on serum uric acid levels, symptoms, and intestinal barrier function in patients with acute and recurrent gout: A pilot study. Dig Dis. 40, 684–690. doi: 10.1159/000521273
Xu, D., Lv, Q., Wang, X., Cui, X, Zhao, P., Yang, X., et al. (2019). Hyperuricemia is associated with impaired intestinal permeability in mice. Am. J. Physiol. Gastrointest Liver Physiol. 317, G484–G492. doi: 10.1152/ajpgi.00151.2019
Xu, H., Wang, X., Feng, W., Liu, Q., Zhou, S., Liu, Q., et al. (2020). The gut microbiota and its interactions with cardiovascular disease. Microb. Biotechnol. 13, 637–656. doi: 10.1111/1751-7915.13524
Xu, Y., Cao, X., Zhao, H., Yang, E., Wang, Y., Cheng, N., et al. (2021). Impact of camellia japonica bee pollen polyphenols on hyperuricemia and gut microbiota in potassium oxonate-induced mice. Nutrients 13 (8), 2665. doi: 10.3390/nu13082665
Xu, X., Wang, H., Guo, D., Man, X., Liu, J., Li, J., et al. (2021). Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren Fail 43, 1063–1075. doi: 10.1080/0886022X.2021.1944875
Yamada, N., Iwamoto, C., Kano, H., Yamaoka, N., Fukuuchi, T., Kaneko, K., et al. (2016). Evaluation of purine utilization by lactobacillus gasseri strains with potential to decrease the absorption of food-derived purines in the human intestine. Nucleosides Nucleotides Nucleic Acids 35, 670–676. doi: 10.1080/15257770.2015.1125000
Yanai, H., Adachi, H., Hakoshima, M., Katsuyama, H. (2021). Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 22 (17), 9221. doi: 10.3390/ijms22179221
Yang, H.-T., Xiu, W.-J., Liu, J.-K., Yang, Y., Hou, X.-G., Zheng, Y.-Y., et al. (2021). Gut microbiota characterization in patients with asymptomatic hyperuricemia: probiotics increased. Bioengineered 12, 7263–7275. doi: 10.1080/21655979.2021.1976897
Yokose, C., McCormick, N., Choi, H. K. (2021). The role of diet in hyperuricemia and gout. Curr. Opin. Rheumatol 33, 135–144. doi: 10.1097/BOR.0000000000000779
Yu, Y., Liu, Q., Li, H., Wen, C., He, Z. (2018). Alterations of the gut microbiome associated with the treatment of hyperuricaemia in Male rats. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02233
Zhang, W., Wang, T., Guo, R., Cui, W., Yu, W., Wang, Z., et al. (2021). Variation of serum uric acid is associated with gut microbiota in patients with diabetes mellitus. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.761757
Keywords: gout, hyperuricemia, gut microbiota, probiotics, prebiotics
Citation: Tong S, Zhang P, Cheng Q, Chen M, Chen X, Wang Z, Lu X and Wu H (2022) The role of gut microbiota in gout: Is gut microbiota a potential target for gout treatment. Front. Cell. Infect. Microbiol. 12:1051682. doi: 10.3389/fcimb.2022.1051682
Received: 23 September 2022; Accepted: 11 November 2022;
Published: 24 November 2022.
Edited by:
Xin Zhou, Stanford University, United StatesReviewed by:
Gabriela Angélica Martínez-Nava, National Institute of Rehabilitation Luis Guillermo Ibarra Ibarra, MexicoMaite Casado-Bedmar, INSERM U1149 Centre de, Recherche sur l’Inflammation, France
Copyright © 2022 Tong, Zhang, Cheng, Chen, Chen, Wang, Lu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyong Lu, bHV4eXpqdTE4QHpqdS5lZHUuY24=; Huaxiang Wu, d3VoeDg4NTVAemp1LmVkdS5jbg==
†ORCID: Xiaoyong Lu, orcid.org/0000-0002-8975-1999
Huaxiang Wu, orcid.org/0000-0002-5052-7519
 Shuting Tong
Shuting Tong Peiyu Zhang
Peiyu Zhang Qi Cheng
Qi Cheng Mo Chen
Mo Chen Xin Chen
Xin Chen Xiaoyong Lu
Xiaoyong Lu Huaxiang Wu
Huaxiang Wu