- 1Division of Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
- 2Doctor of Veterinary Sciences, Veterinary Clinic, Eterinary Directorate, Qena, Egypt
- 3Department of Parasitology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 4Agricultural Research Center (ARC), Animal Health Research Institute-Mansoura Provincial Lab, (AHRI-Mansoura), Cairo, Egypt
- 5Agricultural Research Center (ARC), Animal Health Research Institute-Al Shalateen Provincial Lab (AHRI-Al Shalateen), Giza, Cairo, Egypt
- 6Division of Infectious Diseases, Department of Animal Medicine, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
- 7Department of Pathology, Parasitology and Microbiology, College of Veterinary Medicine, Sudan University of Science and Technology, Khartoum, Sudan
- 8Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Qassim, Saudi Arabia
- 9Institute of Parasitology, Department of Infectious Diseases and Pathobiology, Vetsuisse-Faculty, University of Bern, Bern, Switzerland
Introduction: Toxoplasma gondii and Neospora caninum are closely related intracellular protozoan parasites of medical and veterinary concern by causing abortions and systemic illness. Limited or ambiguous data on the prevalence of T. gondii and N. caninum in camels triggered us to conduct this study.
Methods: Camels (n = 460) recently imported from Sudan and destined mainly for human consumption, were tested for specific antibodies against these protozoans using commercially available ELISAs. From the two only quarantine stations for camels from Sudan, 368 camels were sampled between November 2015 and March 2016 in Shalateen, Red Sea governorate, and 92 samples were collected between September 2018 and March 2021 from Abu Simbel, Aswan governorate.
Results & Discussion: Overall, seropositive rates in camels were 25.7%, 3.9% and 0.8% for T. gondii, N. caninum and mixed infection, respectively. However, marked differences were found between the two study sites and/or the two sampling periods: For T. gondii, a higher rate of infection was recorded in the Red Sea samples (31.5%, 116/368; odds ratio 20.7, 5.0-85.6; P<0.0001) than in those collected in Aswan (2.2%, 2/92). The opposite was found for N. caninum with a lower rate of infection in the Red Sea samples (0.82%, 3/368; odds ratio 23.7, 6.7-83.9; P<0.0001) than in the samples from Aswan (16.3%, 15/92). Additionally, our systematic review revealed that the overall published seroprevalence of T. gondii and N. caninum was 28.6% and 14.3% in camels worldwide, respectively. To the best of our knowledge, this study provides the first record of seroprevalence of both T. gondii and N. caninum in recently imported camels kept under quarantine conditions before delivery to other Egyptian cities and regions. In addition, our review provides inclusive data on the prevalence of T. gondii and N. caninum in camel globally. This knowledge provides basic data for the implementation of strategies and control measures against neosporosis and toxoplasmosis.
Introduction
Globally, the population size of large camelids (dromedary, Camelus dromedarius, and Bactrian camel, C. bactrianus) is estimated at over 35.5 million heads; dromedaries constitute 95% of them with the largest populations being reared in Africa and the Middle East (Zhu et al., 2019; FAOSTAT, 2020; Faye, 2020; Khalafalla and Hussein, 2021). Camels are vital to many countries’ economies, primarily those in the Arabian Peninsula, Sudan, Somalia and Ethiopia, wherein they are being used to produce milk, meat, wool, and hides, and as draught and racing animals (Zarrin et al., 2020; Khalafalla and Hussein, 2021). However, camels have been well documented to transmit a number of zoonotic diseases to humans, among others the protozoan parasite Toxoplasma gondii (Sazmand et al., 2019; Zhu et al., 2019; Hughes and Anderson, 2020; Mohammadpour et al., 2020). Transmission of T. gondii to humans may occur by eating raw or undercooked camel meat or offal, such as the liver, which is widely consumed by pastoralists (Gebremedhin et al., 2014). Another source of transmission might be unpasteurized camel milk (Boughattas, 2017; Sazmand et al., 2019), which is consumed for its higher vitamin C and iron content than cow’s milk, and for attributed important therapeutic effects for type 1 diabetes as well as allergy reduction in children.
Toxoplasma gondii and Neospora caninum are obligate intracellular protozoan parasites that infect a wide variety of domestic and wild animals as well as humans in case of T. gondii (Dubey, 2003; Dubey et al., 2007; Dubey, 2010). Toxoplasma gondii affects most warm-blooded animals and is implicated in abortion cases in women, ewes and sows. Similarly, N. caninum is an abortifacient agent in many mammalian species, particularly in cattle. Natural T. gondii and/or N. caninum infections of livestock are mainly acquired through the consumption of oocysts contaminating food and/or water (Elamin et al., 1992; Dubey, 2003; Dubey et al., 2007; Moore and Venturini, 2018), or through intrauterine infection.
Clinical and congenital toxoplasmosis in camels is limited to a few reports and likely underestimated in dromedaries; clinical manifestation was described as hemorrhagic enterocolitis and toxoplasmic peritonitis (Hagemoser et al., 1990; Riley et al., 2017; Sazmand et al., 2019), and more recently, abortion related to T. gondii was documented in a Bactrian Camel (Komnenou et al., 2022). Despite instances of anti-N. caninum antibodies in camels’ sera, clinical illness in large camelids has not yet been reported (Sazmand and Joachim, 2017).
In Egypt, some reports have investigated the seroprevalence of T. gondii and N. caninum in camels using different serological tests and on animals selected from different regions with special interest for those in Greater Cairo and Nile Delta regions (reviewed by Rouatbi et al., 2019; Abbas et al., 2020). Reported seroprevalence rates varied widely between 3.3% and 96.4% (Kuraa and Malek, 2016; Saad et al., 2018). Only two studies detected anti-N. caninum antibodies in camels in Egypt so far; Hilali et al. (1998) in Cairo using Neospora agglutination test found a seroprevalence of 3.7%, while Selim and Abdelhady (2020) in various Egyptian governorates using ELISA determined 11% seropositive animals, respectively. Nothing is known about the seroprevalence of these protozoans in camels imported to Egypt and destined for human consumption.
The global pooled seroprevalence of T. gondii infection in the Camelidae family was found to be 28.16% by a meta-analysis based on 42 studies that included large camelids (dromedary and Bactrian camels) and small camelids (guanaco, llamas, vicunas, and alpacas) (Maspi et al., 2021). As there was no particular focus on large camelids, and as some articles on T. gondii seroprevalence have been published in regional journals, we performed a systematic search using different databases for a comprehensive assessment of infections. Furthermore, there was no literature review of N. caninum prevalence in camels. Thus, we aimed to review the studies conducted on T. gondii and N. caninum infections in large camelids globally. This work had thus two aims: First, to establish the seroprevalence of T. gondii and N. caninum in recently imported camels from Sudan and kept at Shalateen quarantine, Red Sea governorate and Abu Simbel quarantine, Aswan governorate, Southern Egypt. Second, to conduct a systematic review including all published prevalence and genotyping data in large camels worldwide. The extending cross-comparisons between our results and resources from Egyptian and global studies can be used to address this serious public health issue in order to better understand the parasite epidemiology in large camelids.
Materials and methods
Ethical statement
This study was conducted according to instructions established by the “Research Board” of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. The protocols were approved by Research Code of Ethics at South Valley University number 36 (RCOE-36). Blood samples were collected by a group of highly trained veterinarians and staff after consultation with the officials and animal owners.
Animal population and geographic locations
A total of 460 blood samples were randomly collected from recently imported camels at the only two Egyptian quarantine stations for camels imported from Sudan. Shalateen quarantine station belongs to the Red Sea governorate and is situated in southeastern Egypt, while Abu Simbel quarantine station, Aswan governorate, is situated in central south Egypt (Figure 1). Camels arriving at these quarantine points are usually imported in a way known as Dabuka journey, in which about 100-200 camels led by an expert man are walking for several days through the Sudanese and Egyptian desert. These camels are usually collected from different regions in Sudan, with camels of Eastern Sudan arriving at Shalateen and those of Western Sudan arriving at Abu Simbel (Figure 1B). At the Egyptian-Sudanese border, camels pass Argeen port before being sent to Abu Simbel, or Ras Hadarba port before arriving in Shalateen, respectively, where they are quarantined for 14 days or less (Figure 1B). During this period, camels are routinely checked for Rift valley fever and Corona virus infections by specific laboratory tests, and examined for apparent clinical abnormalities before permitting entrance to different Egyptian cities. Screening for T. gondii or N. caninum infection is not part of the mandated protocol. Most if not all of the imported camels are adult males, and they are primarily destined for human consumption and some for use as transport animals. In Shalateen, 368 samples were collected from November 2015 to March 2016 with two separate visits, one from November to December 2015 (n = 100 samples) and another from February to March 2016 (n = 268 samples). In Abu Simbel, 92 samples were collected from September 2018 to March 2021.
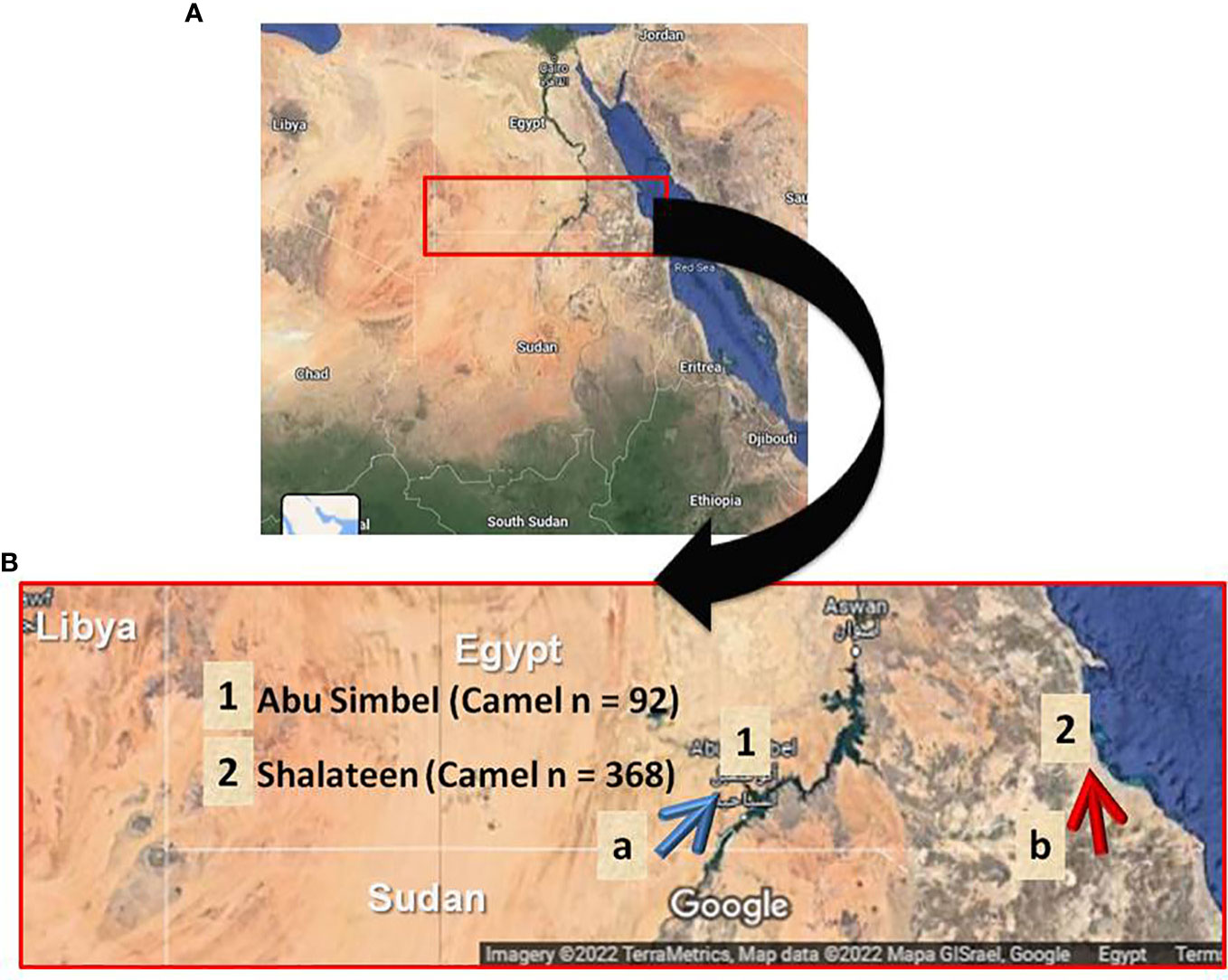
Figure 1 Geographical location of sample collection. (A) Map of Egypt illustrating the collection sites for camel samples in Southern Egypt. (B) Landscape enlarged area of testing samples. Blue arrow indicates the journey route of camel herds after crossing the Sudanese-Egyptian border at Argeen checking point (a) until reaching Abu Simbel quarantine (1). Red arrow shows the journey path of camel herds after crossing the Sudanese-Egyptian border at Ras Hadarba checking point (b) until reach Shalateen quarantine (2).
Serum sample collection and preparation
Blood samples were collected via puncture of the jugular vein using glass tubes without anticoagulant. All blood samples were kept in an icebox during transportation until separation of serum at Shalateen Laboratory for those collected at Shalateen quarantine, and our laboratory at South Valley University for those collected from Abu Simbel. Serum samples from Shalateen laboratory were sent in an icebox to our laboratory and all samples were stored at −20°C at the Faculty of Veterinary Medicine, South Valley University, Qena, until use in ELISA testing.
ELISA testing and interpretation of results
Serum samples of camels were tested for anti-T. gondii and anti-N. caninum antibodies, respectively, using commercial Multi-species ELISA kits (ID Screen® Toxoplasmosis Indirect Multi-species and ID Screen® Neospora caninum Competition, both ID Vet, Grables, France). Positive and negative control sera were provided in the kits and the tests were done following the manufacturer’s instructions. The optical density (OD) of ELISA results was read at 450 nm measured with an Infinite® F50/Robotic ELISA reader (Tecan Group Ltd., Männedorf, Switzerland).
The Toxoplasmosis kit detects specific immunoglobulin G (IgG) antibodies against the P30 T. gondii protein using a peroxidase-conjugated anti-multi-species secondary antibody. The percentage sample (S) to positive (P) ratio (S/P %) for each of the test samples was calculated according to the following formula:
The samples with S/P% values greater than 50% were considered to be positive, those between 40 and 50% were classified as doubtful, and measurements less than or equal to 40% were considered to be negative as per the manufacturer.
The N. caninum kit detects specific antibodies against a purified N. caninum extract, using an anti-N. caninum- peroxidase-conjugated competing antibody. The percentage sample (S) to negative (N) ratio (S/N %) for each of the test samples was calculated according to the following formula:
The samples with S/N% values less than or equal to 50% were considered to be positive, those greater than 50% and less than or equal to 60% were classified as doubtful, and measurements greater than 60% were considered to be negative as per the manufacturer.
Statistical analysis
The significance of the differences in the prevalence rates was analyzed with Chi-square (Pearson) test, 95% confidence intervals (including continuity correction) and odds ratios using an online statistical website www.vassarstats.net (accession dates; 01-02 July, 2022) as described previously (Fereig et al., 2016a; Fereig et al., 2016b). P-values and odds ratio were confirmed also with GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA). The results were considered significant when the p-value was< 0.05 (*) or highly significant when p-value was< 0.0001 (**).
Data searching strategy
PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar were searched for studies on camel toxoplasmosis and neosporosis published in English up to 2022 (May, 2022). In addition, the Egyptian knowledge bank’s website (http://www.ekb.eg) was searched to collect papers from Egypt published in local journals. Toxoplasma gondii and Neospora caninum were used as search terms, along with the keyword “camel(s).” Studies were considered eligible for inclusion if they found positive samples for toxoplasmosis and neosporosis in both the one-humped dromedary camels (Camelus dromedarius) and the two-humped Bactrian camels (Camelus bactrianus).
Articles on both serodiagnosis and molecular investigations of either parasite using serum, milk and meat samples were eligible. Data from eligible studies on infections of camels was organized in a database, and the following information was extracted: sub-region/country, sample size, number of positives (%), detection methods, study year (date of samples collection), cut-off values, genetic markers and revealed genotypes (where recorded), and references with publication date. Different serological tests were included to study the prevalence of both parasites. Even in a single article, two tests may have been used, all of which were included in our literature review. Studies with more than one test were also combined with others after selection of the test with highest number of positives for estimating the pooled prevalence of both parasites either in Egypt or worldwide.
Results
Seroprevalence for T. gondii and N. caninum infection in camels imported to Egypt
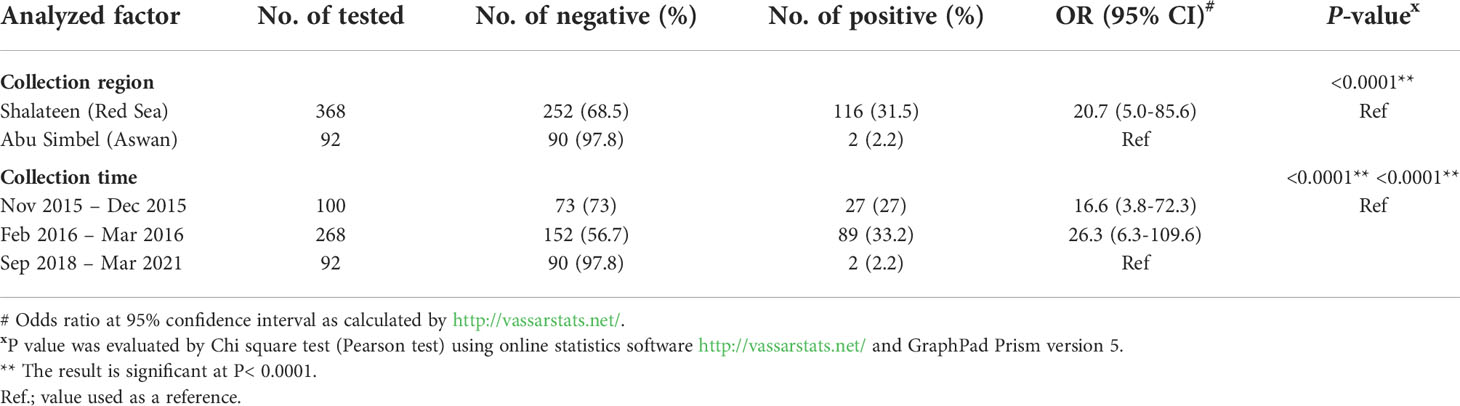
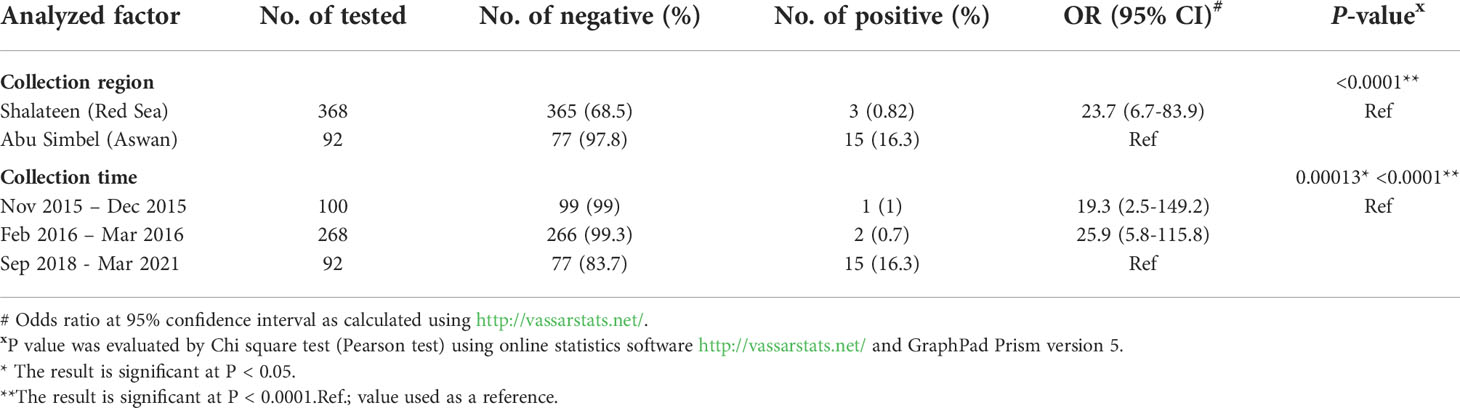
In this study, specific antibodies against T. gondii were detected in 118 of the 460 surveyed animals (25.7%; 95% CI: 21.8-29.9). Consistently, 18 camels tested positive for N. caninum antibodies (3.9%; 95% CI: 2.4-6.2), and mixed infection was determined in 3 animals (0.65%; 95% CI: 0.17-2.1) (Table 1).

Table 1 Seroprevalence of Toxoplasma gondii, Neospora caninum and mixed infection in camels in Egypt.
Based on available data, the location and period of sample collection were identified to have a significant influence on the presence of T. gondii and N. caninum antibodies in recently imported camels in Egypt. A significantly higher seroprevalence rate for T. gondii was recorded in animals sampled at Shalateen Quarantine, Red Sea governorate (31.5%; odds ratio = 20.7; P =<0.0001) compared to camel samples collected at Abu Simbel Quarantine, Aswan governorate (2.2%). Samples in Shalateen were collected between November to December 2015 and between February to March 2016, and those in Aswan between January 2018 to January 2021. Thus, the same effect was seen when univariable analysis of period of sample collection was performed. Samples collected between November to December 2015 and between February to March 2016 showed higher seropositive rates (27%; OR = 16.6; P =<0.0001, and 33.2%; OR = 26.3; P =<0.0001), respectively) than those collected between Jan 2018 to Jan 2021 (2.2%) set as a reference group (Table 2).
In case of N. caninum in camels, the seroprevalence rate recorded in animals sampled at Shalateen quarantine (0.82%; OR = 23.7; P =<0.0001) was significantly lower than that reported in camel samples collected at Abu Simbel Quarantine (16.3%). Again, this was also reflected when the collection periods were compared. The seropositive rates of samples collected between November to December 2015 and between February to March 2016 were lower (1%; OR = 19.3; P = 0.00013, and 0.7%; OR = 25.9; P =<0.0001), respectively) than in the samples collected between September 2018 to March 2021 (16.3%) set as a reference group (Table 3).
Global systematic review data
A total of 79 studies were included and reviewed in this article comprising 74 articles on large camels’ toxoplasmosis and 14 articles conducted on neosporosis, respectively, of which 9 articles investigated both parasites. For Egypt, a pooled prevalence rate of 38.5% for antibodies against T. gondii was found in 1,444 serum samples of dromedaries collected from various governorates and tested with various assays (Table 4). Furthermore, 71 milk samples from camels tested for T. gondii antibodies revealed a pooled prevalence of 18.3% in this matrix (Table 4). For antibodies against N. caninum, a pooled prevalence of 8.4% was found in 443 serum samples (Table 5). Globally, 12,092 serum samples collected from large camels were investigated for T. gondii antibodies, of which 3,457 were found to be positive giving an estimated overall prevalence of 28.6% (Table 4). Meanwhile, 2,654 serum samples were investigated for N. caninum antibodies, of which 380 samples were positive, resulting in an estimated pooled prevalence of 14.3% (Table 5). Toxoplasma gondii type I, II, and III were identified in meat, blood and milk samples from camels using different molecular markers (Table 6).
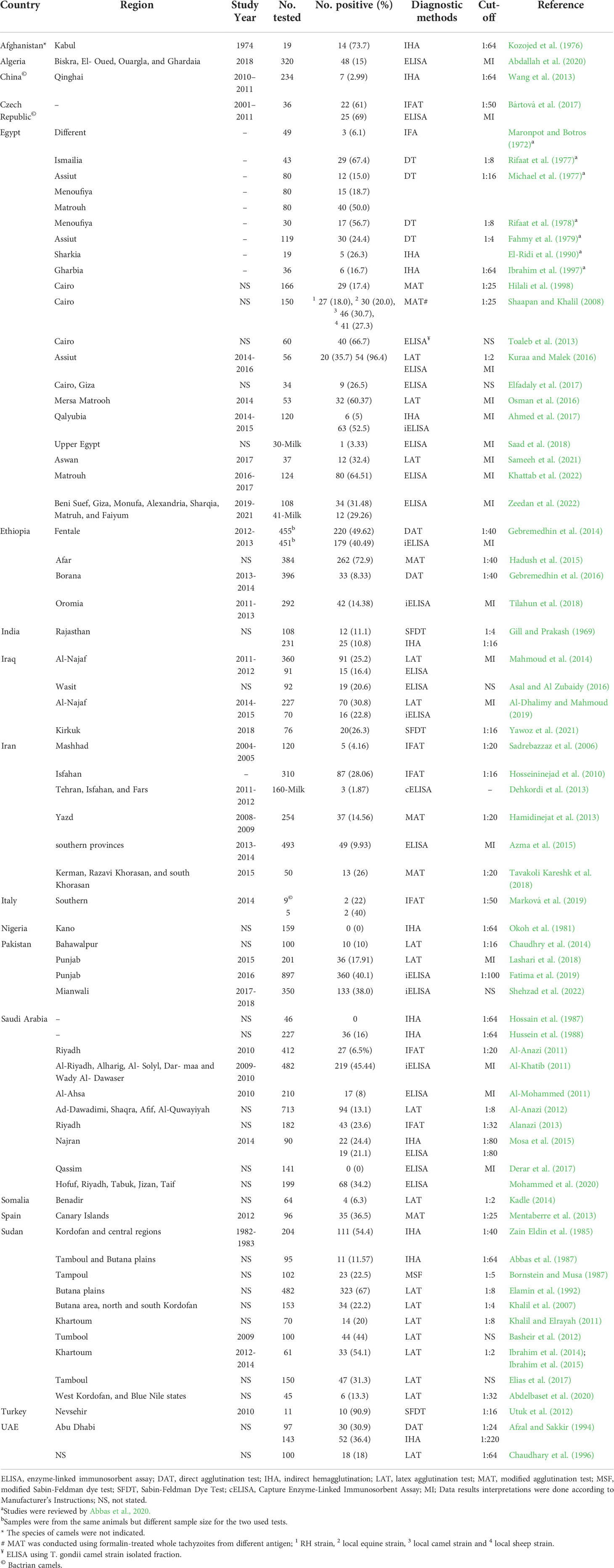
Table 4 Seroprevalence of anti-Toxoplasma gondii antibodies in camels (Camelus dromedarius and Camelus bactrianus) worldwide.
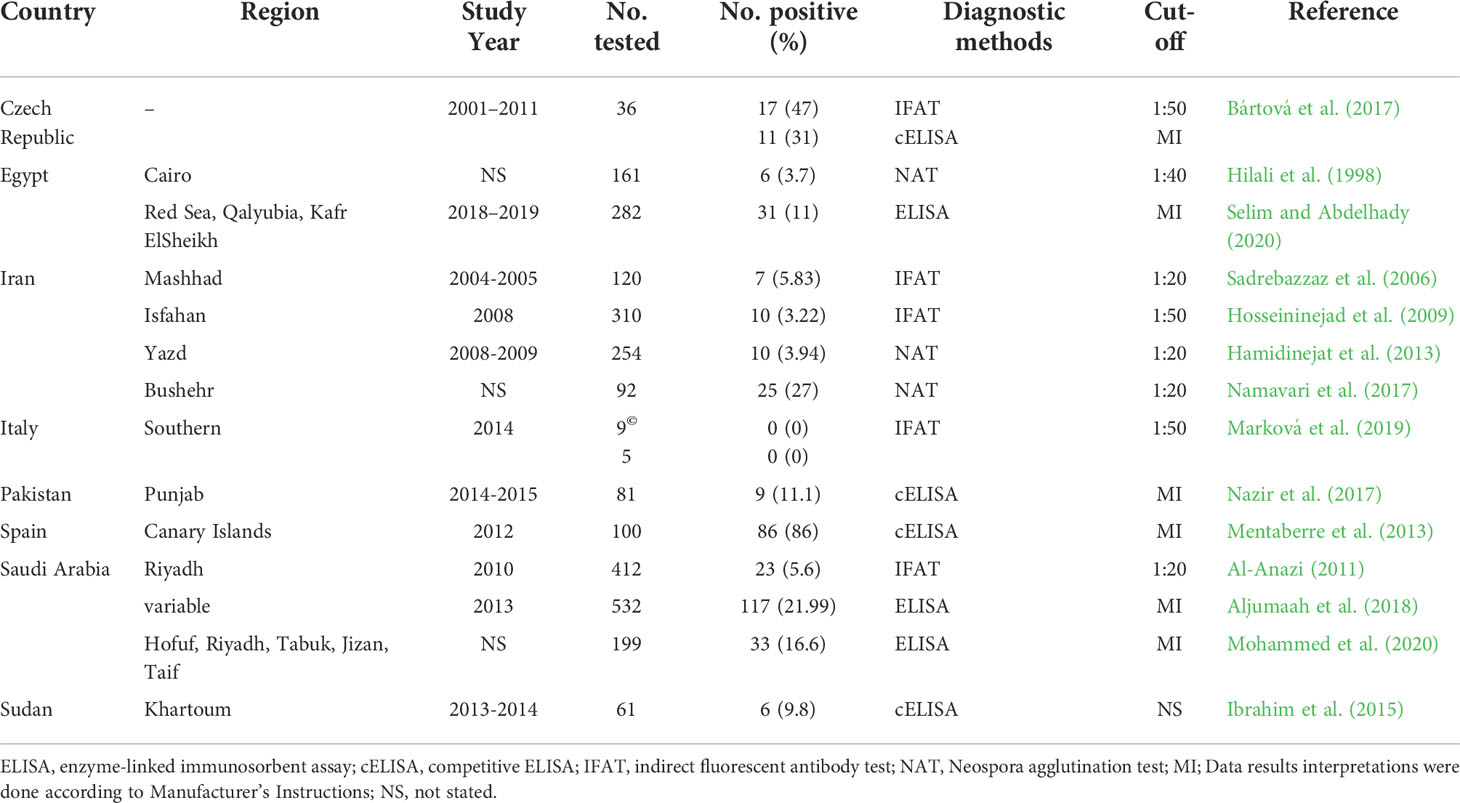
Table 5 Seroprevalence of anti-Neospora caninum antibodies in camels (Camelus dromedarius and Camelus bactrianus) worldwide.
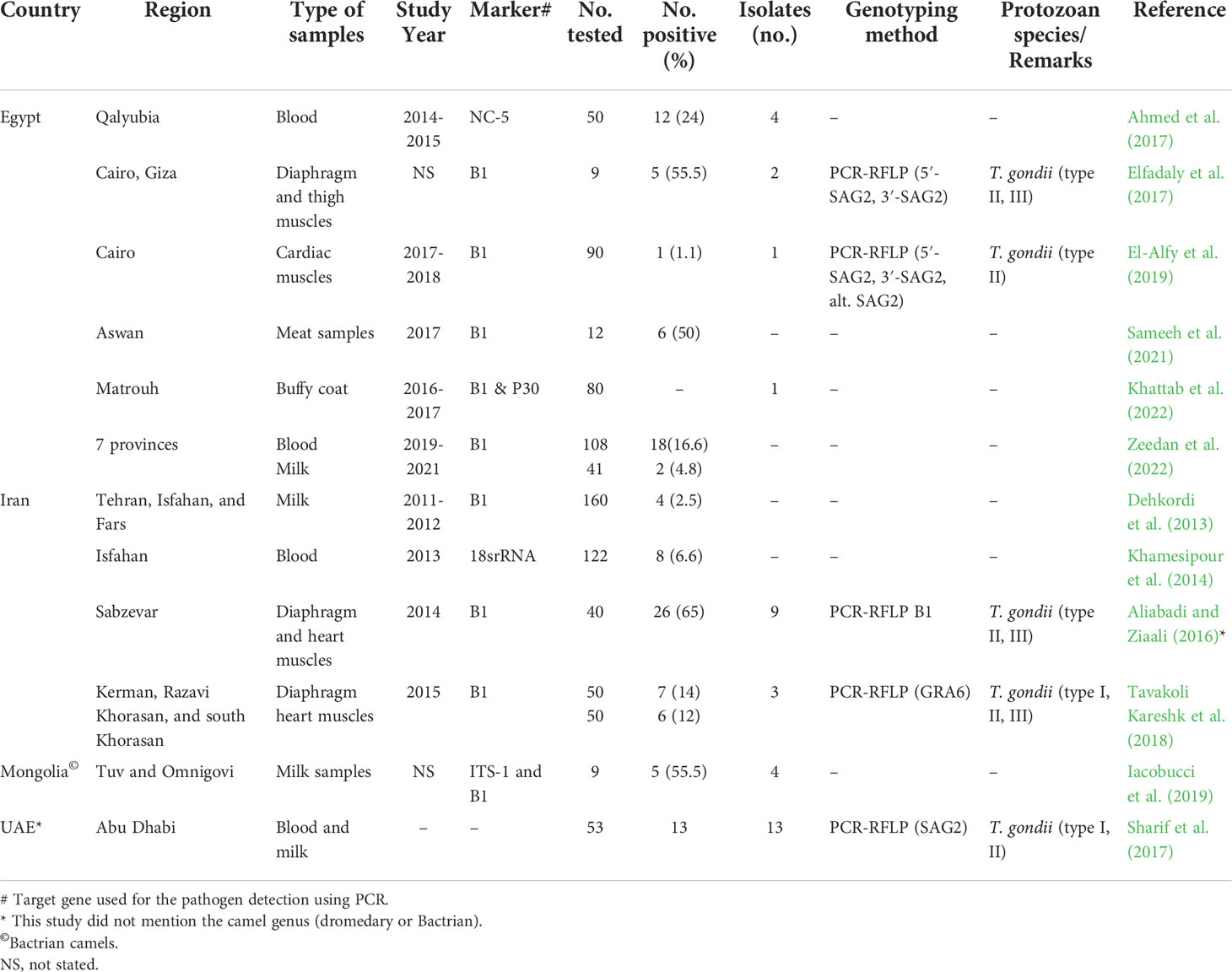
Table 6 Summary of molecular detection and genotyping reports for T. gondii and N. caninum infecting camels worldwide.
Discussion
In the present study, we investigated the seroprevalence of T. gondii and N. caninum in camels recently imported to Egypt from Sudan. The quarantine stations of Shalateen, Red Sea governorate, and Abu Simbel, Aswan governorate, are the only gates for importing camels to Egypt coming from Sudan. These animals are quarantined and subjected to numerous veterinary examinations including clinical and laboratory procedures, but not including T. gondii or N. caninum screening. Data on the seroprevalence of these important parasites is therefore missing, which is particularly concerning as these animals from Sudan are mainly destined for human consumption. We now determined an overall seroprevalence of T. gondii of 25.7%, which could represent a considerable infection risk for consumers. The seroprevalence for N. caninum and mixed infections was much lower with 3.9% and 0.65%, respectively. These results fall within the ranges of seroprevalence established in previous serological studies in large camels for T. gondii and/or N. caninum (Tables 4 and 5).
Our tested camels are short-lived in Egypt, and most if not all of them are adult males as the Sudanese government restricts the export of female camels for human consumption. The dromedaries are usually transported in a way known as “Dabuka journey” which means travelling from Sudan to Egyptian border ports by a long walk. This journey usually takes several days to weeks before arriving to Egypt, and thus Sudan might be suspicious as the original country of infection. Indeed, numerous reports revealed the high prevalence and endemicity of T. gondii and N. caninum among camels in various Sudanese regions (Zain Eldin et al., 1985; Abbas et al., 1987; Bornstein and Musa, 1987; Elamin et al., 1992; Khalil et al., 2007; Ibrahim et al., 2015; Elias et al., 2017; see Tables 4 and 5). However, we found marked differences in the seroprevalence of T. gondii and N. caninum between the two quarantine stations. While we cannot rule out that these differences were caused by the difference in sampling years, we would argue that the different origin in Sudan and differences in travel routes of the camels in the two quarantine stations played a crucial role. Camels arriving in Shalateen made a long journey from Eastern Sudan to Southeastern Egypt where wild cats such as leopard (Panthera pardus) were already reported (Soultan et al., 2017). Moreover, camels from Eastern Sudan, before being exported to Egypt, are quarantined in the Sudanese government quarantine near Kassala city, around which many stray domesticated cats are usually seen. In addition, in Eastern Sudan, wild cats (Civet cat; Civetticitis civetta and Serval cats; Leptailurus serval) are abundant (Khalid, 2016). As camels are probably mainly infected by ingestion of T. gondii oocysts, the presence of wild and stray cats could explain the higher seroprevalence for T. gondii in camels from Eastern Sudan. However, more studies are needed for the detection of Toxoplasma oocysts in the feces of the wild and stray cats in the area. On the other hand, the higher seroprevalence for N. caninum recorded in the camels that have entered Egypt through Abu Simbel region, could be explained by the fact that these animals are originally from Western Sudan and owned by nomad tribes. Common animal husbandry practice in that area is the use of guard dogs (at least 10 dogs per camel herd of 100 animals). This co-herding of camels and dogs can be considered as a major risk factor for many diseases including trypanosomosis (Mossaad et al., 2017), hydatidiosis (Ibrahim et al., 2011) and also N. caninum infection, as seen in the current study. It remains to be investigated whether N. caninum may also cause abortions in dromedaries, and the prevalence of N. caninum in dogs in the area should also be studied. In addition, camels with free access to pasture might have a greater opportunity of ingesting T. gondii or N. caninum oocysts compared to those raised in intensive and semi-intensive breeding systems (Venturoso et al., 2021).
As we explained in our systematic review, data on seroprevalence of T. gondii and N. caninum in camels is scarce and further studies are needed whether in Egypt or other countries. Our review also revealed a significant limitation regarding the overall number of camels (n = 443) that had been tested in all previous studies for N. caninum which is lower than the camels tested in this study (n = 460). Our seroprevalences for T. gondii and N. caninum in camels were lower than in the calculated pooled prevalence from previous studies Egypt for T. gondii (556/1144, 38.5%), and N. caninum (37/443, 8.4%), respectively. However, our positive rate for T. gondii in camel was similar to that reported globally from a pooled prevalence rate (3451/12047, 28.6%) but our positive rate for N. caninum was lower than that estimated worldwide (380/2654, 14.3%). These variable observations can be explained by the vast differences depending on the country, region, age, sex, season, breed of the animals, and type of serological test used (Dubey and Lindsay, 2006).
In conclusion, we provide novel data on the seroprevalence of T. gondii and N. caninum in recently imported camels from Sudan, quarantined in Shalateen and Abu Simbel, Southern Egypt. Our results demonstrated a high exposure of camels to T. gondii and N. caninum infection either in Egypt or in Sudan. Also, our study revealed the substantial lack of data on camel toxoplasmosis and neosporosis in Egypt and worldwide, demonstrating the need for further studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Research Code of Ethics at South Valley University number 36 (RCOE-36).
Author contributions
Conceptualization and design: RF, CF. Experiments, formal analysis, investigation: RF, HA, E-SE-A, ME-D. Resources and shared materials: RF, HA, E-SE-A, ME-D, AE, HM, AOA, AA, EM, AA, CF. Writing—original draft, RF, HA, E-SE-A, CF. Writing—review and editing: RF, HM, AOA, EM, AA, CF. Project administration: RF, CF. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all veterinarians and officials who helped in collection of samples of recently imported quarantined camels and the animal owners for their cooperation in providing animals and required data and information. We appreciate the great help of our colleagues at Department of Animal Medicine, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt, for their cooperation and technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, B., El Zubeir, A. E. A., Yassin, T. T. M. (1987). Survey for certain zoonotic diseases in camels in Sudan. Rev. Elev Med. Vet. Pays Trop. 40 (3), 231–233. doi: 10.19182/remvt.8635
Abbas, I. E., Villena, I., Dubey, J. P. (2020). A review on toxoplasmosis in humans and animals from Egypt. Parasitology 147 (2), 135–159. doi: 10.1017/S0031182019001367
Abdallah, M. C., Kamel, M., Karima, B., Samir, A., Hocine, B. M., Djamel, K., et al. (2020). First report of Toxoplasma gondii infection and associated risk factors in the dromedary camel (Camelus dromedarius) population in south East Algeria. Vet. Parasitol: Reg. Stud. Rep. 22, 100475. doi: 10.1016/j.vprsr.2020.100475
Abdelbaset, A. E., Mossaad, E., Ismail, A. A., Ibrahim, A. M., Xuan, X., Suganuma, K., et al. (2020). Seroprevalence of Toxoplasma gondii in farm animals in West kordofan, and blue Nile states, Sudan. J. Protozool Res. 30 (1-2), 31–37. doi: 10.32268/jprotozoolres.30.1-2_31
Afzal, M., Sakkir, M. (1994). Survey of antibodies against various infectious disease agents in racing camels in Abu Dhabi, united Arab Emirates. Rev. Scientifique Technique (International Office Epizootics) 13 (3), 787–792. doi: 10.20506/rst.13.3.794
Ahmed, N. E., Al–Akabway, L. M., Ramadan, M. Y., El-Gawad, A., Mohamed, S., Moustafa, M. (2017). Serological and PCR-sequencing assays for diagnosis of Toxoplasma gondii and Neospora caninum infecting camels in Egypt. Benha Vet. Med. J. 33 (2), 200–210. doi: 10.21608/bvmj.2017.30466
Al-Anazi, A. D. (2011). Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels (Camelus dromedarius) in Riyadh province, Saudi Arabia. J. Egypt Soc. Parasitol. 41 (2), 245–250.
Al-Anazi, A. D. (2012). Antibodies in sera from camels (Camelus dromedarius) in western and southern regions of central province, Saudi Arabia. J. Egypt Soc. Parasitol. 42 (3), 659–664. doi: 10.12816/0006349
Alanazi, A. D. (2013). Determination of seropositivity for Toxoplasma gondii in sheep, goats and camels slaughtered for food and human consumptions in Riyadh municipal abattoirs, Saudi Arabia. J. Egypt Soc. Parasitol. 43 (3), 569–576. doi: 10.12816/0006414
Al-Dhalimy, A. M. B., Mahmoud, M. H. S. (2019). Serological detection of infection rate with toxoplasmosis in camels at Al-najaf governorate, Iraq. Biochem. Cell Arch. 19 (1), 79–81.
Aliabadi, J., Ziaali, P. N. (2016). Survey of Toxoplasma gondii in livestocks’ meat (sheep, goat, camel), using nested PCR method in sabzavar district. Euro Online J. Nat. Soc. Sci. 5 (2), 368–376.
Aljumaah, R. S., Alshaikh, M. A., Jarelnabi, A., Abdelrahman, M. M., Hussein, M. F. (2018). Serological prevalence of Neospora caninum in indigenous dromedary camels (Camelus dromedarius) in Saudi Arabia. Pakistan J. Zool. 25 (46), 74–19. doi: 10.17582/journal.pjz/2018.50.4.1199.1203
Al-Khatib, R. M. (2011). Serological studies of Toxoplasma gondii infection in camels (Camelus dromedarius). Assiut Vet. Med. J. 57 (130), 1–10.
Al-Mohammed, H. I. (2011). Seroprevalence of Toxoplasma gondii infection in cats, dogs and ruminant animals in Al-ahsa area in Saudi Arabia. Res. J. Med. Sci. 5 (4), 190–192.
Asal, S. N., Al Zubaidy, I. A. (2016). Seroprevelance study of Toxoplasma gondii in horses and camels animal in wasit province. Iraq J. Vet. Med. 40 (1), 147–150. doi: 10.30539/iraqijvm.v40i1.152
Azma, F., Razavi, S. M., Nazifi, S., Rakhshandehroo, E., Sanati, A. R. (2015). A study on the status of inflammatory systems in camels naturally infected with Toxoplasma gondii. Trop. Anim. Health Prod. 47 (5), 909–914. doi: 10.1007/s11250-015-0807-6
Bártová, E., Kobédová, K., Lamka, J., Kotrba, R., Vodička, R., Sedlák, K. (2017). Seroprevalence of Neospora caninum and Toxoplasma gondii in exotic ruminants and camelids in the Czech republic. Parasitol. Res. 116 (7), 1925–1929. doi: 10.1007/s00436-017-5470-6
Basheir, H. M. E., Elias, S., Abdel-Aziz, B. E. (2012). Serosurveillance of Toxoplasma gondii antibodies in camels at tumbool slaughterhouse, central Sudan. Sudan J. Vet. Res. 27, 65–67.
Bornstein, S., Musa, B. E. (1987). Prevalence of antibodies to some viral pathogens, Brucella abortus and Toxoplasma gondii in serum from camels (Camelus dromedarius) in Sudan. J. Vet. Med. Ser. B 34 (1-10), 364–370. doi: 10.1111/j.1439-0450.1987.tb00409.x
Boughattas, S. (2017). Toxoplasma infection and milk consumption: Meta-analysis of assumptions and evidences. Crit. Rev. Food Sci. Nutr. 57 (13), 2924–2933. doi: 10.1080/10408398.2015.1084993
Chaudhary, Z. I., Iqbal, J., Raza, M., Kandeel, M. I. (1996). Haematological and biochemical studies on toxoplasmosis in racing camels-a preliminary report. J. Camel Pract. Res. 3 (1), 7–9.
Chaudhry, U. N., Ali, A. A., Ashraf, S., Khan, M. T., Nadeem, S. M., Ashraf, K. (2014). Seroprevalence of Toxoplasma gondii infection in camels (Camelus dromedarius) in and around bahawalpur region of Pakistan. J. Infect. Mol. Biol. 2, 16–18. doi: 10.14737/jimb.2307-5465/2.1.16.18
Dehkordi, F. S., Haghighi Borujeni, M. R., Rahimi, E., Abdizadeh, R. (2013). Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathog. Dis. 10 (2), 120–125. doi: 10.1089/fpd.2012.1311
Derar, D. R., Ali, A., Osman, S. A., Al-Sobayil, F. A., Saeed, E., Hassanein, K., et al. (2017). Potential pathogens in infertile male dromedary camels and their association with the spermiogram and clinical findings. Comp. Clin. Pathol. 26 (4), 965–970. doi: 10.1007/s00580-017-2461-z
Dubey, J. P. (2003). Review of Neospora caninum and neosporosis in animals. Kor J. Parasitol. 41 (1), 1. doi: 10.3347/kjp.2003.41.1.1
Dubey, J. P., Lindsay, D. S. (2006). Neosporosis, toxoplasmosis, and sarcocystosis in ruminants. Vet. Clin. N Am. - Food Anim. Pract. 22 (3), 645–671. doi: 10.1016/j.cvfa.2006.08.001
Dubey, J. P., Schares, G., Ortega-Mora, L. (2007). Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20 (2), 323–367. doi: 10.1128/CMR.00031-06
El-Alfy, E. S., Abu-Elwafa, S., Abbas, I., Al-Araby, M., Al-Kappany, Y., Umeda, K., et al. (2019). Molecular screening approach to identify protozoan and trichostrongylid parasites infecting one-humped camels (Camelus dromedarius). Acta Trop. 197, 105060. doi: 10.1016/j.actatropica.2019.105060
Elamin, E. A., Elias, S., Daugschies, A., Rommel, M. (1992). Prevalence of Toxoplasma gondii antibodies in pastoral camels (Camelus dromedarius) in the butana plains, mid-Eastern Sudan. Vet. Parasitol. 43 (3-4), 171–175. doi: 10.1016/0304-4017(92)90158-6
Elfadaly, H. A., Hassanain, N. A., Shaapan, R. M., Hassanain, M. A., Barakat, A. M., Abdelrahman, K. A. (2017). Molecular detection and genotyping of Toxoplasma gondii from Egyptian isolates. Asian J. Epidemiol. 10 (1), 37–44. doi: 10.3923/aje.2017.37.44
Elias, H. M., Tagwa, M. B., Abdalla, H. S. (2017). Seroprevalence of Toxoplasma gondii in camels in tamboul area, Sudan. J. Vet. Med. Anim. Prod. 8 (1), 74–75.
El-Ridi, A. M., Nada, S. M., Aly, A. S., Habeeb, S. M., Aboul-Fattah, M. M. (1990). Serological studies on toxoplasmosis in zagazig slaughterhouse. J. Egypt Soc. Parasitol. 20, 677–681.
Fahmy, M. A., Mandour, A. M., Arafa, M. S., Abdel Rahman, B. M. (1979). Toxoplasmosis of camels in assiut governorate. J. Egypt Vet. Med. Assoc. 39, 27–31.
FAOSTAT (2020). Available at: http://www.fao.org/faostat/en/#data/QA.
Fatima, T., Mehnaz, S., Wang, M., Yang, J., Sajid, M. S., Shen B. Zhao, J. (2019). Seroprevalence of Toxoplasma gondii in one-humped camels (Camelus dromedarius) of thal and cholistan deserts, punjab, Pakistan. Parasitol. Res. 118 (1), 307–316. doi: 10.1007/s00436-018-6124-z
Faye, B. (2020). How many large camelids in the world? a synthetic analysis of the world camel demographic changes. Pastoralism 10 (1), 1–20. doi: 10.1186/s13570-020-00176-z
Fereig, R. M., AbouLaila, M. R., Mohamed, S., Mahmoud, H., Ali, A. O., Ali, A. F., et al. (2016b). Serological detection and epidemiology of Neospora caninum and Cryptosporidium parvum antibodies in cattle in southern Egypt. Acta Trop. 162, 206–211. doi: 10.1016/j.actatropica.2016.06.032
Fereig, R. M., Mahmoud, H., Mohamed, S., AbouLaila, M. R., Abdel-Wahab, A., Osman, S. A., et al. (2016a). Seroprevalence and epidemiology of Toxoplasma gondii in farm animals in different regions of Egypt. Vet. Parasitol Reg. Stud. Rep. 3–4, 1–6. doi: 10.1016/j.vprsr.2016.05.002
Gebremedhin, E. Z., Dima, N., Beyi, A. F., Dawo, F., Feyissa, N., Jorga, E., et al. (2016). Toxoplasmosis in camels (Camelus dromedarius) of borana zone, oromia region, Ethiopia: seroprevalence and risk factors. Trop. Anim. Health Prod. 48 (8), 1599–1606. doi: 10.1007/s11250-016-1133-3
Gebremedhin, E. Z., Yunus, H. A., Tesfamaryam, G., Tessema, T. S., Dawo, F., Terefe, G., et al. (2014). First report of Toxoplasma gondii in camels (Camelus dromedarius) in Ethiopia: bioassay and seroepidemiological investigation. BMC Vet. Res. 10 (1), 1–12. doi: 10.1186/s12917-014-0222-7
Gill, H. S., Prakash, O. (1969). Toxoplasmosis in India: prevalence of antibodies in camels. Ann. Trop. Med. Parasitol. 63 (2), 265–267. doi: 10.1080/00034983.1969.11686626
Hadush, A., Gebru, M. U., Zeru, F., Hadush, T., Tesfamaryam, G., Feleke, A. (2015). Sero-epidemiology of camel toxoplasmosis and public awareness on its zoonotic importance in central afar region, north East Ethiopia. World Appl. Sci. J. 33 (12), 1880–1887. doi: 10.5829/idosi.wasj.2015.33.12.10192
Hagemoser, W. A., Dubey, J. P., Thompson, J. R. (1990). Acute toxoplasmosis in a camel. J. Am. Vet. Med. Assoc. 196 (2), 347–347.
Hamidinejat, H., Ghorbanpour, M., Rasooli, A., Nouri, M., Hekmatimoghaddam, S., Namavari, M. M., et al. (2013). Occurrence of anti-Toxoplasma gondii and Neospora caninum antibodies in camels (Camelus dromedarius) in the center of Iran. Turkish J. Vet. Anim. Sci. 37 (3), 277–281. doi: 10.3906/vet-1110-21
Hilali, M., Romand, S., Thulliez, P., Kwok, O. C. H., Dubey, J. P. (1998). Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels from Egypt. Vet. Parasitol. 75 (2-3), 269–271. doi: 10.1016/S0304-4017(97)00181-7
Hossain, A., Bolbol, A. S., Bakir, T. M. F., Bashandi, A. M. (1987). A serological survey of the prevalence of Toxoplasma gondii in slaughtered animals in Saudi Arabia. Ann. Trop. Med. Parasitol. 81 (1), 69–70. doi: 10.1080/00034983.1987.11812094
Hosseininejad, M., Pirali-Kheirabadi, K., Ebrahimi, A., Hosseini, F. (2010). Toxoplasma gondii infection in camels (Camelus dromedarius): a serologic assay in Iran. J. Camel Pract. Res. 17 (1), 35–36.
Hosseininejad, M., Pirali-Kheirabadi, K., Hosseini, F. (2009). Seroprevalence of Neospora caninum infection in camels (Camelus dromedarius) in isfahan province, center of Iran. Iran J. Parasitol. 4 (4), 61–64.
Hughes, E. C., Anderson, N. E. (2020). Zoonotic pathogens of dromedary camels in Kenya: a systematised review. Vet. Sci. 7 (3), 103. doi: 10.3390/vetsci7030103
Hussein, M. F., Bakkar, M. N., Basmaeil, S. M., El Nabi, A. G. (1988). Prevalence of toxoplasmosis in Saudi Arabian camels (Camelus dromedarius). Vet. Parasitol. 28 (1-2), 175–178. doi: 10.1016/0304-4017(88)90030-1
Iacobucci, E., Taus, N. S., Ueti, M. W., Sukhbaatar, L., Bastsukh, Z., Papageorgiou, S., et al. (2019). Detection and genotypic characterization of Toxoplasma gondii DNA within the milk of Mongolian livestock. Parasitol. Res. 118 (6), 2005–2008. doi: 10.1007/s00436-019-06306-w
Ibrahim, A. M., Ismail, A. A., Angara, T. E. E., Osman, M. O. (2014). Area-wide detection of anti-Toxoplasma gondii antibodies in dairy animals from the Khartoum state, Sudan. J. Life Sci. 8 (9), 723–730.
Ibrahim, A. M., Ismail, A. A., Angara, E., Osman, O. M. (2015). Detection of antibodies against Toxoplasma gondii and Neospora caninum in dairy camels from the Khartoum state, Sudan. Sudan J. Sci. Technol. 1, 19–28. Available at: http://repository.sustech.edu/handle/123456789/16391
Ibrahim, B. B., Salama, M. M., Gawish, N. I., Haridy, F. M. (1997). Serological and histopathological studies on Toxoplasma gondii among the workers and the slaughtered animals in tanta abattoir, gharbia governorate. J. Egypt Soc. Parasitol. 27, 273–278.
Ibrahim, K., Thomas, R., Peter, K., Omer, R. A. (2011). A molecular survey on cystic echinococcosis in sinnar area, blue Nile state (Sudan). Chin. Med. J. (Engl). 124 (18), 2829–2833. doi: 10.3760/cma.j.issn.0366-6999.2011.18.006
Kadle, A. A. H. (2014). Sero-prevalence of toxoplasmosis in domestic animals in benadir region, Somalia. Open J. Vet. Med. 4, 170–174. doi: 10.4236/ojvm.2014.48019
Khalafalla, A. I., Hussein, M. F. (2021). Infectious diseases of dromedary camels. (Cham, Switzerland: Springer International Publishing). doi: 10.1007/978-3-030-79389-0
Khalid, O. H. H. (2016). Parasitological and molecular detection of echinococcosis in some wild animal species (Animal Resources Research Council, Sudan Academy of Sciences Animal Resources Research Council).
Khalil, K. M., Elrayah, I. E. (2011). Seroprevalence of Toxoplasma gondii antibodies in farm animals (camels, cattle, and sheep) in Sudan. J. Vet. Med. Anim. Health 3 (3), 36–39.
Khalil, K. M., Gadir, A. E. A., Rahman, M. M. A., Yassir Mohammed, O., Ahmed, A. A., Elrayah, I. E. (2007). Prevalence of Toxoplasma gondii antibodies in camels and their herders in three ecologically different areas in Sudan. J. Camel Pract. Res. 14 (1), 11–13.
Khamesipour, F., Doosti, A., Mobarakeh, H. I., Komba, E. V. (2014). Toxoplasma gondii in cattle, camels and sheep in isfahan and chaharmahal va bakhtiary provinces, Iran. Jundishapur J. Microbiol. 7 (6), e17460. doi: 10.5812/jjm.17460
Khattab, R. A. H., Barghash, S. M., Mostafa, O. M. S., Allam, S. A., Taha, H. A. H., Ashour, A. A. E. B. (2022). Seroprevalence and molecular characterization of Toxoplasma gondii infecting ruminants in the north-West of Egypt. Acta Trop. 225, 106139. doi: 10.1016/j.actatropica.2021.106139
Komnenou, A. T., Giadinis, N. D., Kritsepi-Konstantinou, M., Angelos-Loris, T., Danika, S., Terpsidis, K., et al. (2022). Abortion related to Toxoplasma gondii infection in a bactrian camel (Camelus bactrianus) in Greece: A case report. Iran J. Parasitol. 17 (1), 111. doi: 10.18502/ijpa.v17i1.9033
Kozojed, V., Blazek, K., Amin, A. (1976). Incidence of toxoplasmosis in domestic animals in Afghanistan. Folia Parasitologica 23 (3), 273–275.
Kuraa, H. M., Malek, S. S. (2016). Seroprevalence of Toxoplasma gondii in ruminants by using latex agglutination test (LAT) and enzyme-linked immunosorbent assay (ELISA) in assiut governorate. Trop. Biomed. 33 (4), 711–725.
Lashari, M. H., Ghouri, M. T., Akhtar, M. S., Kamran, Z., Chaudhari, M. S., Ayaz, M., et al. (2018). Hematological and biochemical alterations associated with toxoplasmosis in dromedaries (Camelus dromedarius) habitating in cholistan desert of bahawalpur, punjab, pakistan. JAPS. J. Anim. Plant Sci. 28 (4), 1043–1048.
Mahmoud, M. H., Al-Rubaie, A. E. S., Al-Jeburii K.O and Taha, A. K. A. (2014). Serosurvillance on toxoplasmosis in camels (Camelus dromedarius) at Al-najaf province. Kufa J. Vet. Med. Sci. 5 (2), 204–210.
Marková, J., Machačová, T., Bártová, E., Sedlák, K., Budíková, M., Silvestre, P., et al. (2019). Toxoplasma gondii, Neospora caninum and Encephalitozoon cuniculi in animals from captivity (zoo and circus animals). J. Eukaryotic Microbiol. 66 (3), 442–446. doi: 10.1111/jeu.12688
Maronpot, R. R., Botros, B. A. M. (1972). Toxoplasma serologic survey in man and domestic animals in Egypt. J. Egypt Pub Health Assoc. 47, 58–67.
Maspi, N., Nayeri, T., Moosazadeh, M., Sarvi, S., Sharif, M., Daryani, A. (2021). Global seroprevalence of Toxoplasma gondii in camelidae: A systematic review and meta-analysis. Acta Parasitol. 66 (3), 733–744. doi: 10.1007/s11686-020-00333-9
Mentaberre, G., Gutiérrez, C., Rodríguez, N. F., Joseph, S., González-Barrio, D., Cabezón, O., et al. (2013). A transversal study on antibodies against selected pathogens in dromedary camels in the canary islands, Spain. Vet. Microbiol. 167 (3-4), 468–473. doi: 10.1016/j.vetmic.2013.07.029
Michael, S. A., El Reaii, A. H., Morsy, T. A. (1977). Incidence of Toxoplasma antibodies among camels in Egypt. J. Egypt Soc. Parasitol. 7, 129–132.
Mohammadpour, R., Champour, M., Tuteja, F., Mostafavi, E. (2020). Zoonotic implications of camel diseases in Iran. Vet. Med. Sci. 6 (3), 359–381. doi: 10.1002/vms3.239
Mohammed, O. B., Amor, N., Omer S.A and Alagaili, A. N. (2020). Seroprevalence of Toxoplasma gondii and Neospora caninum in dromedary camels (Camelus dromedarius) from Saudi Arabia. Rev. Bras. Parasitol. Vet. 29 (1), e019119. doi: 10.1590/s1984-29612020008
Moore, D. P., Venturini, M. C. (2018). “Neospora,” in Parasitic Protozoa of farm animals and pets (Cham: Springer), 125–148.
Mosa, M., El-Shahawy, I. S., Fawzi, E. M. (2015). Comparative analysis of toxoplasmosis in farm animals by indirect hemagglutination assay and enzyme linked immunosorbent assay. Alex J. Vet. Sci. 46, 90–94.
Mossaad, E., Satti, R. A., Fadul, A., Suganuma, K., Salim, B., Elamin, E. A., et al. (2017). The incrimination of three trypanosome species in clinically affected German shepherd dogs in Sudan. Parasitol. Res. 116 (11), 2921–2925. doi: 10.1007/s00436-017-5598-4
Namavari, M., Tavanaei, H. R., Abbasifar, A., Manavian, M., Niko, D. (2017). High seroprevalence of Neospora caninum antibodies in camels (Camelus dromedarius) in the south of Iran. Appl. Anim. Sci. Res. J. 6 (24), 57–62. doi: 10.22092/AASRJ.2017.115836
Nazir, M. M., Oneeb, M., Ayaz, M. M., Bibi, F., Ahmad, A. N., Waheed, A., et al. (2017). Prevalence of antibodies to Neospora caninum in the serum of camels (Camelus dromedarius) from central punjab, Pakistan. Trop. Anim. Health Prod. 49 (5), 1081–1084. doi: 10.1007/s11250-017-1300-1
Okoh, A. E. J., Agbonlahor, D. E., Momoh, M. (1981). Toxoplasmosis in Nigeria–a serological survey. Trop. Anim. Health Prod. 13 (1), 137–140. doi: 10.1007/BF02237910
Osman, A. O., EL-Metwaly, H. A., Wahba, A. A., Hefny, S. F. (2016). Studies on causes of abortion in maghrabian camels. Egypt J. Agric. Res. 94 (4), 955–967. doi: 10.21608/ejar.2016.153236
Rifaat, M. A., Morsy, T. A., Sadek, M. S. M., Khalid, M. L. M., Azab, M. E., Makled, M. K., et al. (1977). Incidence of toxoplasmosis among farm animals in Suez canal governorates. J. Egypt Soc. Parasitol. 7, 135–140.
Rifaat, M. A., Morsy, T. A., Sadek, M. S. M., Khalid, M. L. M., Azab, M. E., Safar, E. H. (1978). Prevalence of Toxoplasma antibodies among slaughtered animals in lower Egypt. J. Egypt Soc. Parasitol. 8, 339–345.
Riley, J., Garner, M. M., Kiupel M and Hammond, E. E. (2017). Disseminated toxoplasmosis in a captive adult dromedary camel (Camelus dromedarius). J. Zoo Wildl Med. 48 (3), 937–940. doi: 10.1638/2016-0057.1
Rouatbi, M., Amairia, S., Amdouni, Y., Boussaadoun, M. A., Ayadi, O., Al-Hosary, A., et al. (2019). Toxoplasma gondii infection and toxoplasmosis in north Africa: a review. infection par toxoplasma gondii et toxoplasmose en afrique du nord : synthèse. Parasite (Paris France) 26, 6. doi: 10.1051/parasite/2019006
Saad, N. M., Hussein, A. A., Ewida, R. M. (2018). Occurrence of Toxoplasma gondii in raw goat, sheep, and camel milk in upper Egypt. Vet. World 11 (9), 1262. doi: 10.14202/vetworld.2018.1262-1265
Sadrebazzaz, A., Haddadzadeh, H., Shayan, P. (2006). Seroprevalence of Neospora caninum and Toxoplasma gondii in camels (Camelus dromedarius) in mashhad, Iran. Parasitol. Res. 98 (6), 600–601. doi: 10.1007/s00436-005-0118-3
Sameeh, S., Mahmoud, A. E., Monib, M. M., M El-Salahy and Eldeek, H. E. (2021). Latex agglutation test and PCR assays for diagnosis of Toxoplasma gondii infection in red meat producing animals in Aswan governorate, southern Egypt. Slov Vet. Res. 58, 281–288.
Sazmand, A., Joachim, A. (2017). Parasitic diseases of camels in Iran, (1931–2017)–a literature review. Parasite 24, 1–15. doi: 10.1051/parasite/2017024
Sazmand, A., Joachim, A., Otranto, D. (2019). Zoonotic parasites of dromedary camels: so important, so ignored. Parasit Vectors 12 (1), 1–10. doi: 10.1186/s13071-019-3863-3
Selim, A., Abdelhady, A. (2020). Neosporosis among Egyptian camels and its associated risk factors. Trop. Anim. Health Prod 52 (6), 3381–3385. doi: 10.1007/s11250-020-02370-y
Shaapan, R. M., Khalil, A. F. (2008). Evaluation of different Toxoplasma gondii isolates as antigens used in the modified agglutination test for the detection of toxoplasmosis in camels and donkeys. Am-Eurasian J. Agr Environ. Sci. 3, 837–841.
Sharif, M., Amouei, A., Sarvi, S., Mizani, A., Aarabi, M., Hosseini, S. A., et al. (2017). Genetic diversity of Toxoplasma gondii isolates from ruminants: a systematic review. Int. J. Food Microbiol. 258, 38–49. doi: 10.1016/j.ijfoodmicro.2017.07.007
Shehzad, ,. A., Masud, A., Fatima, T., Khan, F. M., Rehman, S., Effendi, M. H., et al. (2022). Seroprevalence of Toxoplasma gondii and associated alterations in hematology and serum biochemistry of one-humped camels (Camelus dromedarius) in Pakistan. Vet. World 15 (1), 110–118. doi: 10.14202/vetworld.2022.110-118
Soultan, A., Attum, O., Hamada, A., Hatab, E., Ahmed, S. E., Eisa, A., et al. (2017). Recent observation for leopard Panthera pardus in Egypt. Mammalia 81, 115–117. doi: 10.1515/mammalia-2015-0089
Tavakoli Kareshk, A., Oliaee, R. T., Mahmoudvand, H., Keyhani, A., Mohammadi, M. A., Bamorovat, M., et al. (2018). The first survey of isolation and molecular typing of Toxoplasma gondii by bioassay and PCR method in BALB/c mice in camels (Camelus dromedarius) from eastern Iran. Iran J. Parasitol. 13 (3), 382.
Tilahun, B., Tolossa, Y. H., Tilahun, G., Ashenafi, H., Shimelis, S. (2018). Seroprevalence and risk factors of Toxoplasma gondii infection among domestic ruminants in East hararghe zone of oromia region, Ethiopia. Vet. Med. Int. 2018:4263470. doi: 10.1155/2018/4263470
Toaleb, N. I., Shaapan, R. M., Hassan, S. E., El Moghazy, F. M. (2013). High diagnostic efficiency of affinity isolated fraction in camel and cattle toxoplasmosis. World Med. Sci. J. 8 (1), 61–66. doi: 10.5829/idosi.wjms.2013.8.1.72161
Utuk, A. E., Kirbas, A., Babur, C., Balkaya, I. (2012). Detection of Toxoplasma gondii antibodies and some helminthic parasites in camels from nevsehir province of Turkey. Isr. J. Vet. Med. 67 (2), 106–108.
Venturoso, S., Venturoso, J., Silva, G., Maia, O. (2021). Risk factor analysis associated with Neospora caninum in dairy cattle in Western Brazilian Amazon. Braz. J. Vet. Parasitol. 30 (1), 1–11. doi: 10.1590/s1984-296120201088
Wang, M., Wang, Y. H., Meng, P., Ye, Q., Zhang, D. L. (2013). Toxoplasma gondii infection in bactrian camel (Camelus bactrianus) in China. Vet. Parasitol. 192 (1-3), 288–289. doi: 10.1016/j.vetpar.2012.09.028
Yawoz, M., Jaafar, S. E., Alali, F., Babur, C. (2021). Seroprevalence of camels listeriosis, brucellosis and toxoplasmosis from kirkuk province-Iraq. Pak Vet. J. 41 (3), 335–340. doi: 10.29261/pakvetj/2021.030
Zain Eldin, E. A., Elkhawad, S. E., Kheir, H. S. M. (1985). A serological survey for Toxoplasma antibodies in cattle, sheep, goats and camels (Camelus dromedarius) in the Sudan. Rev. d'Elevage Med. Vet. Des. Pays Tropicaux (France) 38 (3), 247–249.
Zarrin, M., Riveros, J. L., Ahmadpour, A., de Almeida, A. M., Konuspayeva, G., Vargas-Bello-Pérez, E., et al. (2020). Camelids: new players in the international animal production context. Trop. Anim. Health Prod. 52 (3), 903–913. doi: 10.1007/s11250-019-02197-2
Zeedan, G. S., Abdalhamed, A. M., Shaapan, R. M., El-Namaky, A. H. (2022). Rapid diagnosis of Toxoplasma gondii using loop-mediated isothermal amplification assay in camels and small ruminants. Beni-Suef Univ J. Basic Appl. Sci. 11 (1), 1–10. doi: 10.1186/s43088-021-00184-x
Keywords: toxoplasmosis, neosporosis, camel, dromedary, ELISA, Egypt
Citation: Fereig RM, Abdelbaky HH, El-Alfy E-S, El-Diasty M, Elsayed A, Mahmoud HYAH, Ali AO, Ahmed A, Mossaad E, Alsayeqh AF and Frey CF (2022) Seroprevalence of Toxoplasma gondii and Neospora caninum in camels recently imported to Egypt from Sudan and a global systematic review. Front. Cell. Infect. Microbiol. 12:1042279. doi: 10.3389/fcimb.2022.1042279
Received: 12 September 2022; Accepted: 31 October 2022;
Published: 14 November 2022.
Edited by:
William Harold Witola, University of Illinois at Urbana–Champaign, United StatesReviewed by:
Yurong Yang, Henan Agricultural University, ChinaDong-Hui Zhui, Fujian Agriculture and Forestry University, China
Copyright © 2022 Fereig, Abdelbaky, El-Alfy, El-Diasty, Elsayed, Mahmoud, Ali, Ahmed, Mossaad, Alsayeqh and Frey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline F. Frey, Y2Fyb2xpbmUuZnJleUB2ZXRzdWlzc2UudW5pYmUuY2g=; Ragab M. Fereig, UmFnYWIuZmVyYWVnMkB2ZXQuc3Z1LmVkdS5lZw==
 Ragab M. Fereig
Ragab M. Fereig Hanan H. Abdelbaky
Hanan H. Abdelbaky El-Sayed El-Alfy
El-Sayed El-Alfy Mohamed El-Diasty4
Mohamed El-Diasty4 Hassan Y. A. H. Mahmoud
Hassan Y. A. H. Mahmoud Ehab Mossaad
Ehab Mossaad Abdullah F. Alsayeqh
Abdullah F. Alsayeqh Caroline F. Frey
Caroline F. Frey