- Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Cryptococcosis in the central nervous system (CNS) can present with motor declines described as Parkinsonism. Although several lines of evidence indicate that dopaminergic (DA) neuron degeneration and α-synuclein accumulation contribute to the hallmark of Parkinsonism and Parkinson’s disease (PD), little is known about cryptococcal infections associated with neuronal degeneration. In this study, the effects of Cryptococcus neoformans and C. gattii infections on dopaminergic neuron degeneration, α-synuclein accumulation, and lifespan in Caenorhabditis elegans were investigated. The results showed that cryptococcal infections significantly (P<0.05) induced DA neuron degeneration similar to a selective cathecholamine neurotoxin 6-hydroxydopamine (6-OHDA) in C. elegans (BZ555 strain) when compared to mock infected controls. Cryptococcal infections also significantly (P< 0.05) induced α-synuclein aggregation in C. elegans (NL5901 strain). Moreover, lifespan of the infected worms was significantly decreased (P<0.0001). In conclusion, DA neurodegeneration and α-synuclein accumulation are associated with lifespan reduction during cryptococcal infection in C elegans.
Introduction
Cryptococcosis, is caused by infections of encapsulated basidiomycetous fungi of the genus Cryptococcus, is increasingly reported among acquired immunodeficiency syndrome (AIDS) patients and other immunosuppressed patients as well as in immunocompetent populations worldwide (Firacative et al., 2021). Environmental exposure to cryptococci is common as these pathogenic yeasts are ubiquitously found in soil, water, certain contaminated foods, and pigeon droppings (Malik et al., 2003). Currently, most cases are caused by Cryptococcus neoformans, however, infections of C. gattii are also reported (Olszewski et al., 2010). Infections of cryptococci are primarily caused by inhalation of infectious propagules (e.g., poorly encapsulated yeast cells and/or basidiospores) from contaminated reservoirs to the pulmonary alveoli, however, severe meningoencephalitis is the commonest clinical presentation (Stott et al., 2021). Cryptococci process a unique ability to penetrate through the blood brain barrier (BBB) and colonized the brain, which conferred a strong neurotropism (Esher et al., 2018). Cryptococcal infections that damage central nervous system (CNS) are highly lethal and cause several neurological disorders depending on the site of cerebral infections (Colombo and Rodrigues, 2015). Several clinical studies in humans and animal models have revealed the pattern of neurological disorders after cryptococcal infections include meningitis, encephalitis, meningoencephalitis, ventriculitis, increased intracranial pressure (ICP), and Parkinsonism (Camargos et al., 2006; Colombo and Rodrigues, 2015). Interestingly, Parkinsonian features during cryptococcal meningoencephalitis have been reported over the last three decades (Wszolek et al., 1988). Nevertheless, understanding the pathogenesis of cryptococcosis-induced CNS damage, particularly Parkinsonian symptoms, is limited and poorly investigated.
Patients with cryptococcal-induced Parkinsonism are often presented with prominent clinical characteristics including bradykinesia, tremor, rigidity, and postural instability. Postmortem brain examination has confirmed the fungal burden located at the substantia nigra and in basal ganglia suggesting the root-cause of Parkinsonism (Wszolek et al., 1988; Pedroso and Barsottini, 2012; Nelles et al., 2022). Degeneration of dopaminergic (DA) neurons of the substantia nigra and α-synucleinopathies are the common denominators of Parkinsonism during aging (Dickson, 2012). However, pathological investigation of the infectious yeast cryptococci to the degeneration of DA neurons,α-synuclein accumulation, and longevity is still poorly determined.
It is well known that fungal contamination in routine laboratory of cell culture can rapidly kill mammalian cells in vitro. However, cryptococcal yeast cells have little cytotoxic effect when co-cultured with animal cells in vitro suggesting that cryptococcal infections are not involved with tissue necrosis as seen in those infections caused by Aspergillus spp. (Casadevall et al., 2018) or Scedosporium spp. (Cortez et al., 2008). Moreover, it is even more difficult to isolate DA neurons from mammalian brains for studying cryptococcosis in vitro (Park et al., 2020). Murine models of Cryptococcus meningoencephalitis have been successfully developed but do not mimic to cryptococcal-induced Parkinsonism (Thompson et al., 2012; Sabiiti et al., 2012). Several alternative cryptococcosis invertebrate models have been introduced including Caenorhabditis elegans (Mylonakis et al., 2002; Mylonakis et al., 2004), Drosophila melanogaster (Apidianakis et al., 2004), and Galleria mellonella (Mylonakis et al., 2005). In a previous study, our laboratory showed that both C. neoformans ATCC#32045 and C. gattii ATCC#56992 can reduce the lifespan of C. elegans by regulating the insulin/insulin-like growth factor-1 (IGF-1) signaling (IIS) pathway and DAF-16 has been shown to play a central role during cryptococcal infections (Kitisin et al., 2022). Although C. elegans has been extensively used as a model to screen targeted drugs for anti-Parkinson effects (Chalorak et al., 2018; Malaiwong et al., 2019), however, the study of C. elegans neurodegeneration caused by pathogenic fungal infections has been limited.
The C. elegans model of neurodegeneration exhibits several biological advantages, as its simple anatomy, microscopic transparency, short lifespan, facile genetics, and eased ethical constraints, which are a great challenge when using vertebrate models (Teschendorf and Link, 2009). In general, C. elegans has a total of 959 somatic cells including 302 neuronal cells (Chalorak et al., 2018). Interestingly, C. elegans has exactly 8 DA neurons (ADEL, ADER, CEPDL, CEPDR, CEPVL, CEPVR, PDEL, and PDER), which are structurally and functionally similar to human DA neurons (Sulston et al., 1975; Maulik et al., 2017). Six DA neurons are located at the anterior part of the body as 4 anterior cephalic (CEP) and 2 anterior deirid (ADE) neurons and the 2 remaining posterior DA neurons are known as posterior deirid (PDE) neurons (Harrington et al., 2010). In the present study, we used transgenic C. elegans (BZ555 and NL5901 strains) to investigate the degeneration of DA neurons caused by cryptococcal infections. The C. elegans strain BZ555 has been created to localize the DA neurons that express green fluorescent protein (GFP) specifically tagged to the C. elegans dat-1 gene (DA transporter gene) (Nass et al., 2002). By chemically exposing the C. elegans DA neurons to neurotoxin 6-hydroxydopamine (6-OHDA), DA degeneration can be demonstrated (Offenburger et al., 2018). In addition, α-synucleinopathy can be observed using C. elegans strain NL5901, which express yellow fluorescent protein (YFP) under the specific and strong promotion of unc-54 gene (van Ham et al., 2008). Moreover, the lifespan of worms after cryptococcal infections was also determined. Thus, the present study demonstrates the degeneration of DA neurons caused by cryptococcal infections and provides an alternative model of C. elegans for studying the pathogenesis of infectious etiologies of Parkinsonism.
Materials and methods
Strains and culture conditions
C. neoformans (ATCC#32045) and C. gattii (ATCC#56992) strains were obtained from the American Type Culture Collection (ATCC). Fungal stock cultures were stored at −80°C in 25% glycerol until use. Yeasts were maintained on Yeast-Peptone-Dextrose (YPD) agar (Oxoid, Hampshire, UK) at 30°C unless otherwise specified (Tang et al., 2005). C. elegans transgenic BZ555 [egIs1 [dat-1p::GFP]] and NL5901 [pkIs2386 [unc-54p::α-synuclein:YFP + unc-119(+)]] strains were obtained from the Caenorhabditis Genetics Center (CGC, USA) of the University of Minnesota. C. elegans were maintained as described previously (Kitisin et al., 2022). Escherichia coli OP50 was obtained from the CGC and used as a food source for C. elegans. E. coli OP50 were grown in Luria Broth (LB, BD) by shaking overnight at 37°C, collected by centrifugation at 5,000 × g for 10 min, and diluted to OD = 0.1 using sterile M9 buffer. An alkaline hypochlorite treatment (12% NaClO and 10% 1 M NaOH) of gravid hermaphrodites was used to obtain synchronized L1 larvae and maintained on solid nematode growth media (NGM) fed with E. coli OP50 at 25°C until the L4 stage (3-day-old worms). All experiments used the L4 worms as day 0 of the experiments to control the reproductive systems in C. elegans (Oh et al., 2022).
Infections of C. elegans by Cryptococcus species and 6-OHDA treatment
C. neoformans and C. gattii strains were inoculated into 2 ml of YPD broth and grown at 30°C for 48 h. Then, 10 μl of fungal yeast cells was spread on 35-mm tissue-culture plate containing Brain Heart Infusion (BHI, Difco) agar supplemented with 150 μM of 5-fluoro-2′-deoxyuridine (FDdR, Sigma Aldrich) to inhibit the development of worm’s progeny (Mylonakis et al., 2002). Moreover, 50 μg/ml of kanamycin (Sigma Aldrich) was added to the BHI plates to prevent the growth of E. coli OP50 that might have been carried over during the transfer of worms to the yeast-containing BHI plates (Pukkila-Worley et al., 2009). To perform an infection assay, age-synchronized L4 worms (BZ555 strain) were transferred from a lawn of E. coli OP50 on NGM plates to the prepared yeast cells on BHI plates and incubated at 25°C for 72 h. Worms that fed with E. coli OP50 were used as mock-infected controls. To chemically ablate the worms’ dopaminergic neurons, control BZ555 worms grown for 24 h on E. coli OP50/NGM/FUdR plates were collected and washed with M9 buffer. Control worms were incubated with 50 mM of 6-hydroxy-dopamine (6-OHDA, Sigma Aldrich) and 10 mM ascorbic acid (Sigma Aldrich) (Nass et al., 2002). The assay solution (1 ml) was gently mixed in every 10 min at 20°C for 1 h. Then, the 6-OHDA treated worms were washed three times with M9 buffer and transferred to the new E. coli OP50/NGM/FUdR plates and incubated at 25°C for 48 h and served as a positive control for DA degeneration. Control BZ555 worms incubated on E. coli OP50/NGM/FUdR plates without 6-OHDA treatment were used as a positive control. Each experimental condition was performed in triplicate.
Quantification of C. elegans DA degeneration
Degeneration of DA neurons was observed in BZ555 worms after infection with either C. neoformans, C. gattii or treated with 6-OHDA as described previously. The worms were then washed three times with M9, immobilized with a few drops of 100 mM sodium azide (NaN3) on 1% agarose pads, and finally enclosed by a cover slip. Fifty of worms were photographed using a Zeiss Axio Imager fluorescence microscope (ZEISS, Germany) using an LED source and a GFP filter. The fluorescence intensity was measured using ImageJ software (National Institute of Health, NIH, Bethesda, MD, USA).
Evaluation of C. elegans lifespan
The mean lifespan of BZ555 worms was determined by feeding on E. coli OP50, E. coli OP50/6-OHDA, C. neoformans or C. gattii as described (Mylonakis et al., 2002; Chalorak et al., 2018). In brief, fifty synchronized L4 of BZ555 were transferred onto yeast-containing BHI/FUdR plates and incubated at 25°C. Worms cultured on E. coli OP50 alone were used as a control, whereas worms incubated with 6-OHDA were used as a positive control. The animals remained on the experimental plates for the remainder of their life spans. Number of live and dead worms were counted every 24 h until all the worms died. Failure to respond a stimulation using a platinum wire pick or no sign of pharyngeal pumping movement were considered dead worms. Alternatively, worms cultured on BHI plates were transferred to new plates every 24 - 48 h to remove the presence of progeny. Worms that suffered from developmental defects, internal hatching, or those that burrowed, or crawled off the plates were censored from the analysis. Summation of worms in triplicates were used to statistically calculate the lifespan excluding the censored worms.
Quantification of C. elegans α-synuclein accumulation
Accumulation of α-synuclein was observed in NL5901 worms after infection with C. neoformans and C. gattii as described previously with some modifications (Chalorak et al., 2018; Kitisin et al., 2022). Briefly, age-synchronized L4 NL5901 worms were transferred onto yeast-containing BHI/FUdR plates and incubated at 25°C for 72 h. Worms cultured on E. coli OP50 were used as a control. After 72 h, the worms were washed with M9 buffer and immobilized with NaN3 on an agar pad. The fluorescence intensity of fifty worms in each condition was determined using fluorescence microscope and quantified by using ImageJ software.
Statistical analysis
Data were expressed as mean ± SD. All the experiments were performed in triplicate. The Kaplan-Meier lifespan analysis and log-rank test were used to calculate the mean lifespan and P-values, respectively. In the other experiments, one way or two-way ANOVA was conducted to test the differences. Statistical analyses were determined using GraphPad Prism software (GraphPad Software, Inc., San Diego, California, USA). Levels of significance were indicated as *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; NS, not significant (P > 0.05).
Ethical statement
This study was performed under the Animals for Scientific Purposes Act, B.E. 2558 (A.D. 2015), Thailand. C. elegans strains were provided by the CGC. C. elegans protocols were approved by the Faculty of Tropical Medicine – Animal Care and Use Committee (FTM‐ACUC), Mahidol University, Thailand, No. FTM024-2020. Safe handling of C. neoformans (ATCC#32045) and C. gattii (ATCC#56992) strains were approved by Institutional Biosafety Committee, Faculty of Tropical Medicine, Mahidol University, Thailand, Submission No. FTM-IBC-20-09.
Results
Infections of C. neoformans and C. gattii induced DA neuronal degradation in C. elegans
The DA neurons were evaluated by analyzing the mean fluorescence intensity of GFP expressions of BZ555 strain. The ADE and CEP neurons were highly degenerated after treating with 6-OHDA and showed partial loss of GFP to approximately 63.52 ± 3.54% (P < 0.05) when compared to controls (100 ± 1.49%, Figures 1A, B). Upon cryptococcal infection, we found an accumulation of C. neoformans and C. gattii yeast cells and the worms exhibited abnormally distended gastrointestinal tracts, which were surrounded by 6 DA neurons 4 anterior cephalic (CEP) and 2 anterior deirid (ADE) neurons (Figure 1A) (Harrington et al., 2010). Interestingly, GFP expression of DA neurons (ADE and CEP) in BZ555 worms infected with C. neoformans or C. gattii were significantly reduced to 77.88 ± 1.39% (P < 0.05) and 75.90 ± 1.30% (P < 0.05), respectively (Figures 1A, B). Degeneration of DA neurons caused by 6-OHDA treatment reduced the lifespan of BZ555 worms from 9.26 ± 1.79 days to 5.48 ± 1.96 days (-40.82%, P < 0.0001, Figure 1C, Table 1). Moreover, degeneration of DA neurons caused by the infections of C. neoformans or C. gattii also reduced the lifespan to 5.51 ± 1.47 days (-40.49%, P < 0.0001) or 5.76 ± 1.58 days (-37.80%, P < 0.0001) (Figure 1C, Table 1). Thus, cryptococci-induced DA degenerations may reduce lifespan in C. elegans.
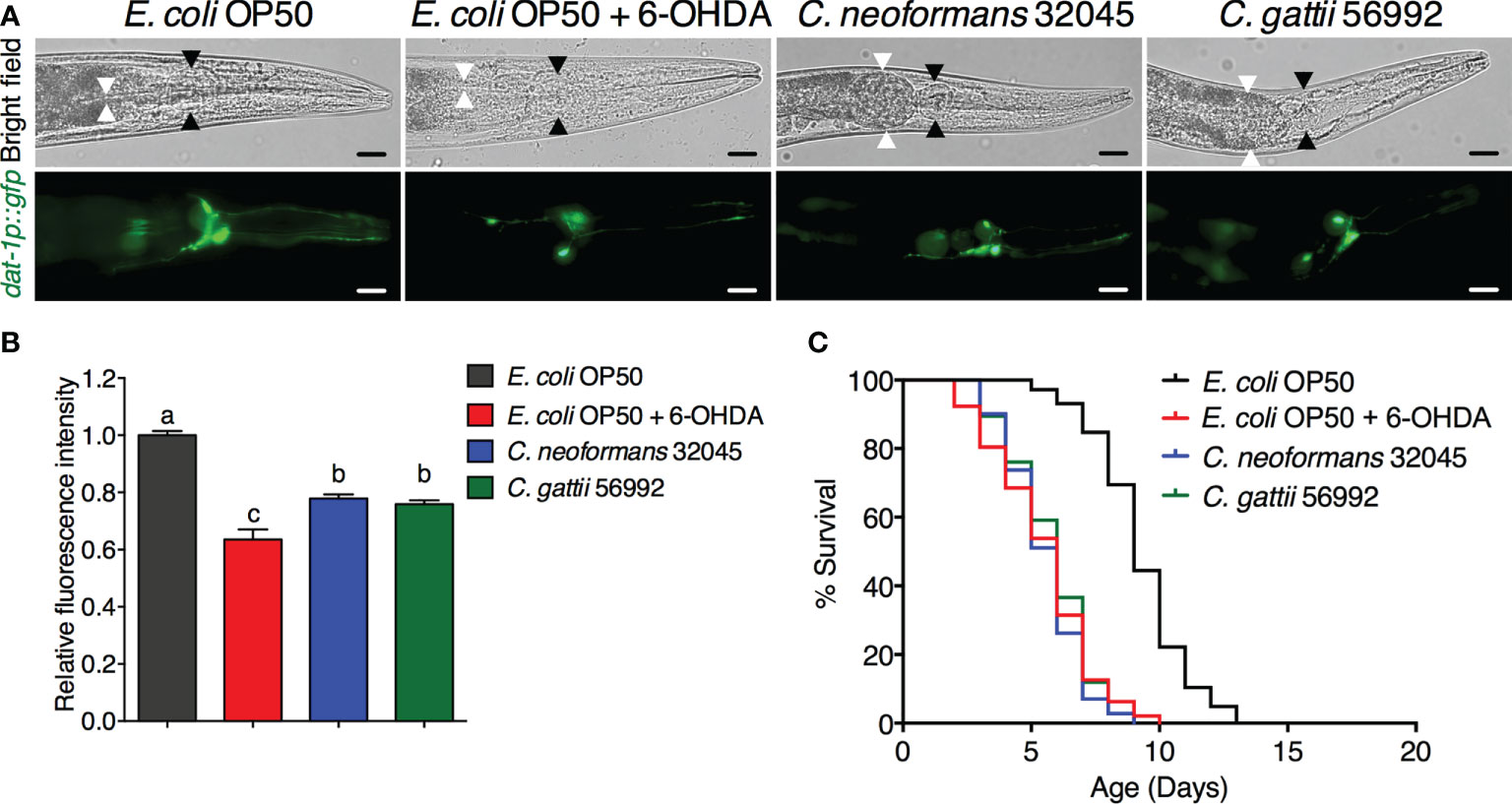
Figure 1 Infections of C neoformans and C gattii induced DA neuronal degradation are similar to 6-OHDA toxicity in C elegans. (A) The BZ555 worms that expressing GFP on DA neurons were exposed to either C neoformans (ATCC#32045), C gattii (ATCC#56992) or 6-OHDA. Upon infection, the cryptococcal yeast cells were seen at the distal area of the abnormally distended gastrointestinal tract of worms. Black arrowheads indicate the worm pharyngeal grinder. White arrowheads indicate the intestinal lumen of the nematode. Worms from all experiments were photographed alive. Scale bar = 20 μm. (B) Quantitation of GFP expression in DA neurons was measured by using ImageJ software. The data represented as mean ± SD (n = 30). a–cIndicated statistically significant differences between experimental conditions (P < 0.05) by two-way ANOVA. (C) Percent survival of worms fed with E coli OP50/6-OHDA, C neoformans or C gattii. Worms that fed with E coli OP50 alone were served as mock-infected controls (n = 50). Survival was analyzed using Kaplan-Meier lifespan analysis. P-value was calculated using log-rank test. Mean lifespan differences between each condition was summarized in Table 1.
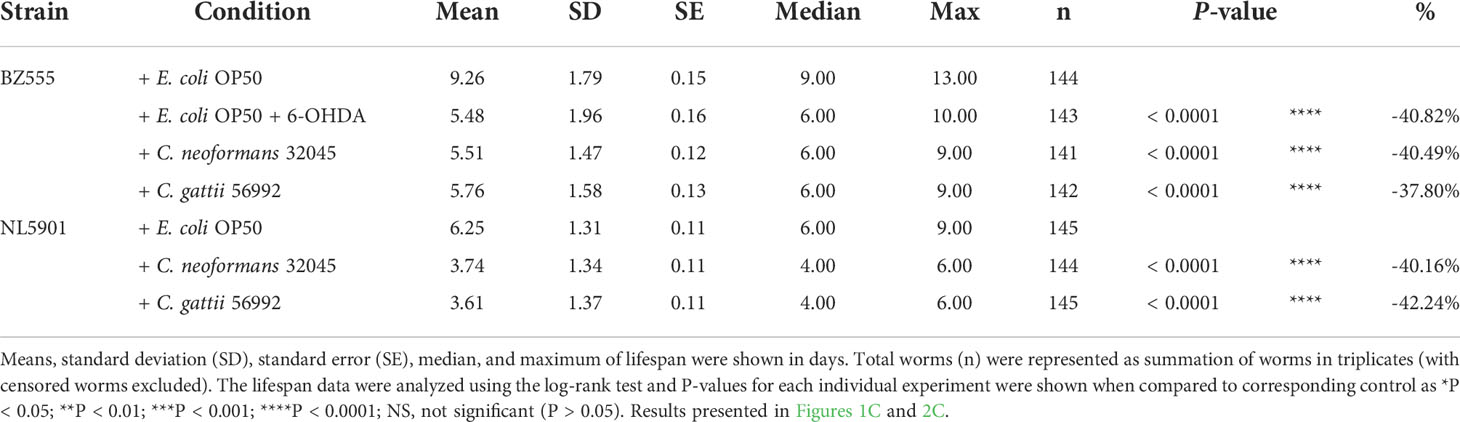
Table 1 Mean lifespan of C. elegans strain BZ555 and NL5901 upon C. neoformans 32045 or C. gattii 56992 infections.
Infections of C. neoformans and C. gattii induced the α-synuclein accumulation in C. elegans
The accumulation of human α-synuclein protein was evaluated by analyzing the mean fluorescence intensity of YFP expression in the muscle cells of the NL5901 strain. The level of α-synuclein was higher in C. neoformans or C. gattii infected worms (119.47 ± 1.98% or 119.75 ± 3.40%, respectively, P < 0.05) when compared to controls (100 ± 1.29%, Figures 2A, B). Accumulation of human α-synuclein protein caused by the infections of C. neoformans or C. gattii also reduced the lifespan from 6.25 ± 1.31 days to 3.74 ± 1.34 days (-40.16%, P < 0.0001) or 3.61 ± 1.37 days (-42.24%, P < 0.0001) (Figure 2C, Table 1). Thus, cryptococci-induced α-synuclein accumulation may reduce lifespan in C. elegans.
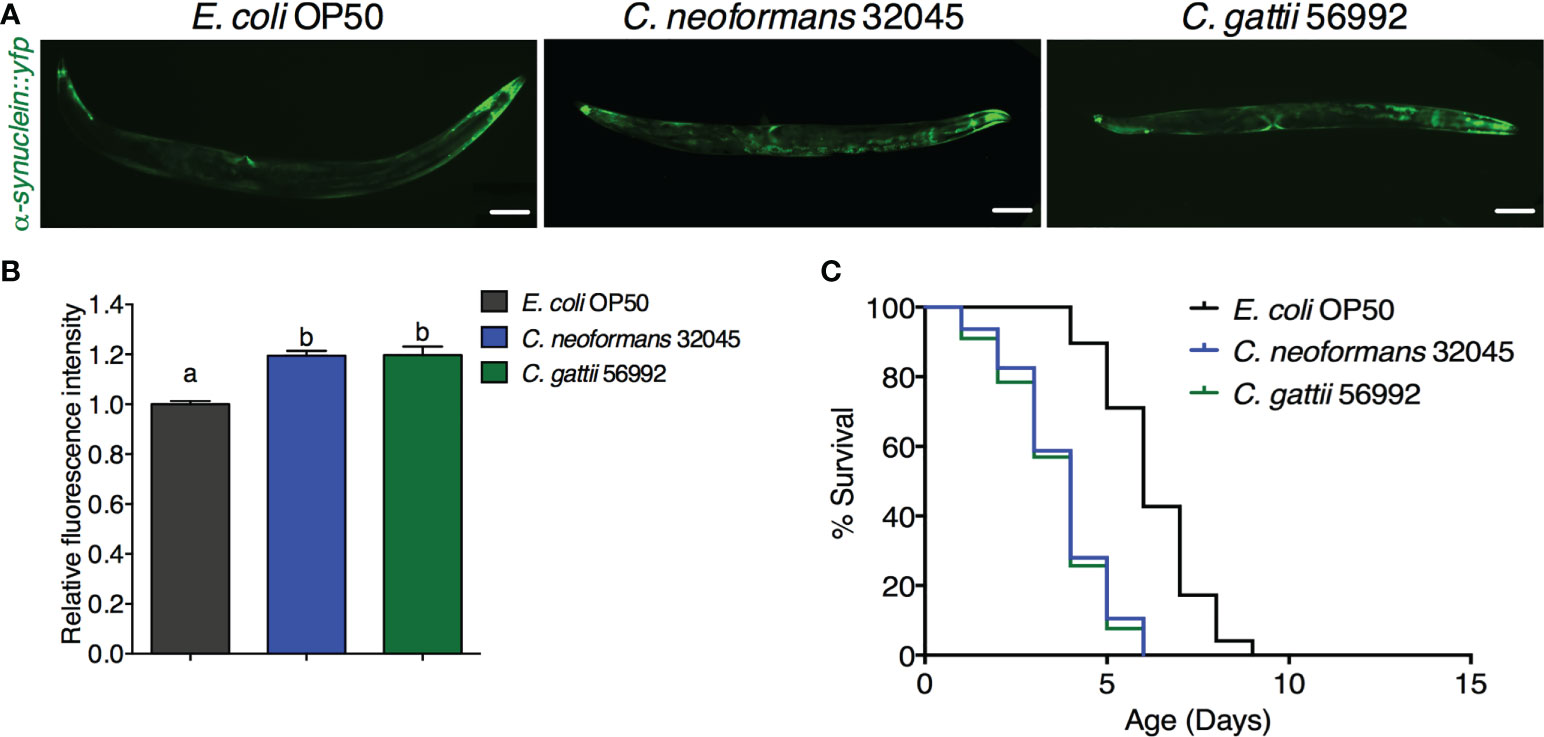
Figure 2 Infections of C neoformans and C gattii induced α-synuclein accumulation in C elegans. (A) C elegans strain NL5901 expressing YFP in muscle cells were exposed to either C neoformans (ATCC#32045) or C gattii (ATCC#56992) (scale bar = 200μm). (B) Quantitation of YFP expression in muscle cells was measured by using ImageJ software. The data represented as mean ± SD (n = 30). a–bIndicated statistically significant differences between experimental conditions (P < 0.05) by two-way ANOVA. (C) Percent survival of worms fed with C neoformans or C gattii. Worms that fed with E coli OP50 alone were served as mock-infected controls (n = 50). Survival was analyzed using Kaplan-Meier lifespan analysis. P-value was calculcated using log-rank test. Mean lifespan in each condition was summarized in Table 1.
Discussion
Dopaminergic neurons (DA) can be identified in rodent and primate brain sections by using immunolabeling techniques or using positron emission tomography in vivo (Gaspar et al., 1992). In the laboratory, cell-specific markers of the presynaptic dopamine transporter (DAT) are frequently used to experimentally reveal DA neurons in vivo. However, expression of DATs in mammalian neurons are greatly distributed throughout genomic DNA. Furthermore, mammalian DA neurons are located deep in the ventral midbrain and basal ganglia of the CNS causing a great challenge to study these neurons only in fully developed, intact adult brain (Hegarty et al., 2013). To overcome these limitations, all eight DA neurons can be revealed in C. elegans strain BZ555 by using only ~ 0.7 kb of 5′ flanking DAT-1 genomic sequence targeted to the reporter genes e.g., GFP. A previous study has shown that C. elegans DA neurons can be chemically degenerated by the treatment of 6-OHDA and used as a C. elegans model of Parkinson’s disease (Nass et al., 2002). Thus, we employed this transgenic C. elegans strain (BZ555) to visualize the DA neurons in a living animal. We previously reported that C. neoformans and C. gattii can accumulate and kill the wildtype (N2) C. elegans (Kitisin et al., 2022). In the present study, we found that accumulation of C. neoformans and C. gattii yeast cells in the distended gastrointestinal tract was anatomically close to 6 DA neurons (Figures 1A, B) (Harrington et al., 2010). Moreover, we found that infections of C. neoformans and C. gattii induced the degeneration of DA neurons and accelerated aging similarly to the 6-OHDA treatment (Figure 1C). These observations have led to the suggestion that invasive cryptococcal infections can induce cellular cytotoxicity and CNS tissue compression leading to the death of DA neurons as seen our present study and in patients with cryptococcal meningoencephalitis associated with prominent features of Parkinsonism (Wszolek et al., 1988; Pedroso and Barsottini, 2012; Nelles et al., 2022). Therefore, C. elegans can be used as a model to investigate the pathogenesis of cryptococcal infection that induces DA neuronal degeneration related to Parkinsonian symptoms.
Another hallmark of Parkinson’s disease is the accumulation of α-synuclein in the aging brain (Stefanis, 2012). A previous study has demonstrated that expression of human α-synuclein fused YFP protein via unc-54 promoter in C. elegans strain NL5901 can be increasingly accumulated in age-dependent manner. Moreover, these inclusions of α-synuclein were structurally similar to human pathological inclusions in PD patients (Lakso et al., 2003). Therefore, we employed this transgenic C. elegans strain (NL5901) to visualize the accumulation of human α-synuclein in a living animal. We have previously reported that infections of C. neoformans and C. gattii can accelerate the aging process and reduce the lifespan of C. elegans involving with IIS/DAF-16 pathway (Kitisin et al., 2022). A recent study has also reported that accumulation of aggregated human α-synuclein inclusions was strongly associated with the activation of DAF-16 suggesting the relationship between the IIS pathway and PD (Haque et al., 2020). In the present study, we found that cryptococcal infections significantly increased human α-synuclein accumulation higher than mock infected controls, which associated with a reduction of infected worm’s lifespan (Figure 2). These observations suggest that chronic cryptococcal infections can induce the accumulation of pathological α-synuclein inclusions, which then accelerate neuronal loss as seen in patients with persistent Cryptococcal brain infection (Wingfield et al., 2014; Beardsley et al., 2015). Further study is required to elucidate the molecular mechanisms of cryptococcal induced DA degeneration and accumulation of α-synuclein during chronic infections in C. elegans.
Conclusion
Infections of C. neoformans and C. gattii robustly degenerated DA neurons, increased the accumulation of α-synuclein, and reduced the lifespan of C. elegans. Nevertheless, our study provides an alternative C. elegans infection model of cryptococcal-induced neurodegeneration, which may be beneficial for novel therapeutic screening to prevent CNS damage against cryptococcal infections.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
TK and PS: Designed the study. TK: Carried out the majority of the experiments, analyzed the data, and wrote the manuscript. WM: Assisted in C. elegans and cryptococcal cultures and maintenances. TK and PS: Reviewed and revised the manuscript, contributed to funding acquisitions. All authors read and approved the final version of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Mahidol University (Contract no. A22/2564) to TK; Mahidol University (Basic Research Fund: fiscal year 2021) and Health Systems Research Institute (Grant number: HSRI 64-051) to PS.
Acknowledgments
Caenorhabditis elegans strains used in this study were provided by the Caenorhabditis Genetics Center (CGC) of the University of Minnesota, which is funded by the National Institutes of Health (NIH)—Office of Research Infrastructure Programs (P40 OD010440). The authors would like to thank the Central Instrument Facility Unit, Faculty of Tropical Medicine, Mahidol University, Thailand for the use of fluorescence microscopy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Apidianakis, Y., Rahme, L. G., Heitman, J., Ausubel, F. M., Calderwood, S. B., Mylonakis, E. (2004). Challenge of drosophila melanogaster with cryptococcus neoformans and role of the innate immune response. eukaryot. Cell 3, 413–419. doi: 10.1128/EC.3.2.413-419.2004
Beardsley, J., Thanh, L. T., Day, J. (2015). A model CNS fungal infection: Cryptococcal meningitis. Curr. Clin. Micro. Rpt. 2, 96–113. doi: 10.1007/s40588-015-0016-0
Camargos, S. T., Teixeira, A. L., Jr., Cardoso, F. (2006). Parkinsonism associated with basal ganglia cryptococcal abscesses in an immunocompetent patient. Mov. Disord. 21, 714–715. doi: 10.1002/mds.20789
Casadevall, A., Coelho, C., Alanio, A. (2018). Mechanisms of cryptococcus neoformans-mediated host damage. Front. Immunol. 9. doi: 10.3389/fimmu.2018.00855
Chalorak, P., Jattujan, P., Nobsathian, S., Poomtong, T., Sobhon, P., Meemon, K. (2018). Holothuria scabra extracts exhibit anti-Parkinson potential in c. elegans: A model for anti-Parkinson testing. Nutr. Neurosci. 21, 427–438. doi: 10.1080/1028415X.2017.1299437
Colombo, A. C., Rodrigues, M. L. (2015). Fungal colonization of the brain: Anatomopathological aspects of neurological cryptococcosis. An. Acad. Bras. Cienc. 87, 1293–1309. doi: 10.1590/0001-3765201520140704
Cortez, K. J., Roilides, E., Quiroz-Telles, F., Meletiadis, J., Antachopoulos, C., Knudsen, T., et al. (2008). Infections caused by scedosporium spp. Clin. Microbiol. Rev. 21, 157–197. doi: 10.1128/CMR.00039-07
Dickson, D. W. (2012). Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2, a009258. doi: 10.1101/cshperspect.a009258
Esher, S. K., Zaragoza, O., Alspaugh, J. A. (2018). Cryptococcal pathogenic mechanisms: A dangerous trip from the environment to the brain. Mem. Inst. Oswaldo. Cruz. 113, e180057. doi: 10.1590/0074-02760180057
Firacative, C., Trilles, L., Meyer, W. (2021). Recent advances in cryptococcus and cryptococcosis. Microorganisms 10, 13. doi: 10.3390/microorganisms10010013
Gaspar, P., Stepniewska, I., Kaas, J. H. (1992). Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J. Comp. Neurol. 325, 1–21. doi: 10.1002/cne.903250102
Haque, R., Shamsuzzama, Kumar, L., Sharma, T., Fatima, S., Jadiya, P., et al. (2020). Human insulin modulates α-synuclein aggregation via DAF-2/DAF-16 signalling pathway by antagonising DAF-2 receptor in c. elegans model of parkinson’s disease. Oncotarget 11, 634–649. doi: 10.18632/oncotarget.27366
Harrington, A. J., Hamamichi, S., Caldwell, G. A., Caldwell, K. A. (2010). C. elegans as a model organism to investigate molecular pathways involved with parkinson’s disease. Dev. Dyn. 239, 1282–1295. doi: 10.1002/dvdy.22231
Hegarty, S. V., Sullivan, A. M., O'Keeffe, G. W. (2013). Midbrain dopaminergic neurons: A review of the molecular circuitry that regulates their development. Dev. Biol. 379, 123–138. doi: 10.1016/j.ydbio.2013.04.014
Kitisin, T., Muangkaew, W., Sukphopetch, P. (2022). Caenorhabditis elegans DAF-16 regulates lifespan and immune responses to cryptococcus neoformans and cryptococcus gattii infections. BMC Microbiol. 22, 162. doi: 10.1186/s12866-022-02579-x
Lakso, M., Vartiainen, S., Moilanen, A. M., Sirviö, J., Thomas, J. H., Nass, R., et al. (2003). Dopaminergic neuronal loss and motor deficits in caenorhabditis elegans overexpressing human α-synuclein. J. Neurochem. 86, 165–172. doi: 10.1046/j.1471-4159.2003.01809.x
Malaiwong, N., Chalorak, P., Jattujan, P., Manohong, P., Niamnont, N., Suphamungmee, W., et al. (2019). Anti-Parkinson activity of bioactive substances extracted from holothuria leucospilota. Biomed. Pharmacother. 109, 1967–1977. doi: 10.1016/j.biopha.2018.11.063
Malik, R., Krockenberger, M. B., Cross, G., Doneley, R., Madill, D. N., Black, D., et al. (2003). Avian cryptococcosis. Med. Mycol. 41, 115–124. doi: 10.1080/mmy.41.2.115.124
Maulik, M., Mitra, S., Bult-Ito, A., Taylor, B. E., Vayndorf, E. M. (2017). Behavioral phenotyping and pathological indicators of parkinson’s disease in c. elegans models. Front. Genet. 8. doi: 10.3389/fgene.2017.00077
Mylonakis, E., Ausubel, F. M., Perfect, J. R., Heitman, J., Calderwood, S. B. (2002). Killing of caenorhabditis elegans by cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99, 15675–15680. doi: 10.1073/pnas.232568599
Mylonakis, E., Idnurm, A., Moreno, R., El Khoury, J., Rottman, J. B., Ausubel, F. M., et al. (2004). Cryptococcus neoformans Kin1 protein kinase homologue, identified through a caenorhabditis elegans screen, promotes virulence in mammals. Mol. Microbiol. 54, 407–419. doi: 10.1111/j.1365-2958.2004.04310.x
Mylonakis, E., Moreno, R., El Khoury, J. B., Idnurm, A., Heitman, J., Calderwood, S. B., et al. (2005). Galleria mellonella as a model system to study cryptococcus neoformans pathogenesis. Infect. Immun. 73, 3842–3850. doi: 10.1128/IAI.73.7.3842-3850.2005
Nass, R., Hall, D. H., Miller, D. M., 3rd., Blakely, R. D. (2002). Neurotoxin-induced degeneration of dopamine neurons in caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 99, 3264–3269. doi: 10.1073/pnas.042497999
Nelles, R., Britton, S., John, G. T., Denaro, C. (2022). Parkinsonism and prolonged cognitive decline as a manifestation of cryptococcal meningitis in a renal transplant patient. BMJ Case Rep. 15, e245788. doi: 10.1136/bcr-2021-245788
Offenburger, S. L., Ho, X. Y., Tachie-Menson, T., Coakley, S., Hilliard, M. A., Gartner, A. (2018). 6-OHDA-induced dopaminergic neurodegeneration in caenorhabditis elegans is promoted by the engulfment pathway and inhibited by the transthyretin-related protein TTR-33. PLoS. Genet. 14, e1007125. doi: 10.1371/journal.pgen.1007125
Oh, M., Yeom, J., Schraermeyer, U., Julien-Schraermeyer, S., Lim, Y. H. (2022). Remofuscin induces xenobiotic detoxification via a lysosome-to-Nucleus signaling pathway to extend the caenorhabditis elegans lifespan. Sci. Rep. 12, 7161. doi: 10.1038/s41598-022-11325-2
Olszewski, M. A., Zhang, Y., Huffnagle, G. B. (2010). Mechanisms of cryptococcal virulence and persistence. Future Microbiol. 5, 1269–1288. doi: 10.2217/fmb.10.93
Park, T. I., Schweder, P., Lee, K., Dieriks, B. V., Jung, Y., Smyth, L., et al. (2020). Isolation and culture of functional adult human neurons from neurosurgical brain specimens. Brain Commun. 2, fcaa171. doi: 10.1093/braincomms/fcaa171
Pedroso, J. L., Barsottini, O. G. (2012). Acute parkinsonism in cryptococcus gattii meningoencephalitis: Extensive lesions in basal ganglia. Mov. Disord. 27, 1372. doi: 10.1002/mds.25074
Pukkila-Worley, R., Peleg, A. Y., Tampakakis, E., Mylonakis, E. (2009). Candida albicans hyphal formation and virulence assessed using a caenorhabditis elegans infection model. Eukaryot. Cell 8, 1750–1758. doi: 10.1128/EC.00163-09
Sabiiti, W., May, R. C., Pursall, E. R. (2012). Experimental models of cryptococcosis. Int. J. Microbiol. 2012, 626745. doi: 10.1155/2012/626745
Stefanis, L. (2012). α-synuclein in parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009399. doi: 10.1101/cshperspect.a009399
Stott, K. E., Loyse, A., Jarvis, J. N., Alufandika, M., Harrison, T. S., Mwandumba, H. C., et al. (2021). Cryptococcal meningoencephalitis: Time for action. Lancet Infect. Dis. 21, e259–e271. doi: 10.1016/S1473-3099(20)30771-4
Sulston, J., Dew, M., Brenner, S. (1975). Dopaminergic neurons in the nematode caenorhabditis elegans. J. Comp. Neurol. 163, 215–226. doi: 10.1002/cne.901630207
Tang, R. J., Breger, J., Idnurm, A., Gerik, K. J., Lodge, J. K., Heitman, J., et al. (2005). Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a caenorhabditis elegans progeny-based approach. Infect. Immun. 73, 8219–8225. doi: 10.1128/IAI.73.12.8219-8225.2005
Teschendorf, D., Link, C. D. (2009). What have worm models told us about the mechanisms of neuronal dysfunction in human neurodegenerative diseases? Mol. Neurodegener. 4, 38. doi: 10.1186/1750-1326-4-38
Thompson, G. R., 3rd, Wiederhold, N. P., Najvar, L. K., Bocanegra, R., Kirkpatrick, W. R., Graybill, J. R., et al. (2012). A murine model of cryptococcus gattii meningoencephalitis. J. Antimicrob. Chemother. 67, 1432–1438. doi: 10.1093/jac/dks060
van Ham, T. J., Thijssen, K. L., Breitling, R., Hofstra, R. M., Plasterk, R. H., Nollen, E. A. (2008). C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet. 4, e1000027. doi: 10.1371/journal.pgen.1000027
Wingfield, T., Baxter, J., Herwadkar, A., du Plessis, D., Blanchard, T. J., Javier Vilar, F., et al. (2014). Persistent cryptococcal brain infection despite prolonged immunorecovery in an HIV-positive patient. Case Rep. Neurol. Med. 2014, 164826. doi: 10.1155/2014/164826
Keywords: Parkinson’s disease, Parkinsonism, Cryptococcosis, dopaminergic neurons, α-synuclein accumulation, Caenorhabditis elegans
Citation: Kitisin T, Muangkaew W and Sukphopetch P (2022) Infections of Cryptococcus species induce degeneration of dopaminergic neurons and accumulation of α-Synuclein in Caenorhabditis elegans. Front. Cell. Infect. Microbiol. 12:1039336. doi: 10.3389/fcimb.2022.1039336
Received: 08 September 2022; Accepted: 11 October 2022;
Published: 26 October 2022.
Edited by:
Mark S. Gresnigt, Hans Knöll Institute, GermanyReviewed by:
Angie Gelli, University of California, Davis, United StatesMichael S. Price, Liberty University, United States
Copyright © 2022 Kitisin, Muangkaew and Sukphopetch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Passanesh Sukphopetch, bmF0dGhhbmVqLmx1cEBtYWhpZG9sLmFjLnRo
 Thitinan Kitisin
Thitinan Kitisin Passanesh Sukphopetch
Passanesh Sukphopetch