
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 02 February 2022
Sec. Parasite and Host
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.767576
This article is part of the Research TopicNovel Insights Into the Immune Mechanisms Associated With the Pathogenesis of Chagas DiseaseView all 22 articles
 Roberto Rodrigues Ferreira1,2*
Roberto Rodrigues Ferreira1,2* Mariana Caldas Waghabi2
Mariana Caldas Waghabi2 Sabine Bailly3
Sabine Bailly3 Jean-Jacques Feige3
Jean-Jacques Feige3 Alejandro M. Hasslocher-Moreno4
Alejandro M. Hasslocher-Moreno4 Roberto M. Saraiva4
Roberto M. Saraiva4 Tania C. Araujo-Jorge1*
Tania C. Araujo-Jorge1*The anti-inflammatory cytokine transforming growth factor beta (TGF-β) plays an important role in Chagas disease (CD), a potentially life-threatening illness caused by Trypanosoma cruzi. In this review we revisited clinical studies in CD patients combined with in vitro and in vivo experiments, presenting three main sections: an overview of epidemiological, economic, and clinical aspects of CD and the need for new biomarkers and treatment; a brief panorama of TGF-β roles and its intracellular signaling pathways, and an update of what is known about TGF-β and Chagas disease. In in vitro assays, TGF-β increases during T. cruzi infection and modulates heart cells invasion by the parasite fostering its intracellular parasite cycle. TGF-β modulates host immune response and inflammation, increases heart fibrosis, stimulates remodeling, and slows heart conduction via gap junction modulation. TGF-β signaling inhibitors reverts these effects opening a promising therapeutic approach in pre-clinical studies. CD patients with higher TGF-β1 serum level show a worse clinical outcome, implicating a predictive value of serum TGF-β as a surrogate biomarker of clinical relevance. Moreover, pre-clinical studies in chronic T. cruzi infected mice proved that inhibition of TGF-β pathway improved several cardiac electric parameters, reversed the loss of connexin-43 enriched intercellular plaques, reduced fibrosis of the cardiac tissue, restored GATA-6 and Tbox-5 transcription, supporting cardiac recovery. Finally, TGF-β polymorphisms indicate that CD immunogenetics is at the base of this phenomenon. We searched in a Brazilian population five single-nucleotide polymorphisms (-800 G>A rs1800468, -509 C>T rs1800469, +10 T>C rs1800470, +25 G>C rs1800471, and +263 C>T rs1800472), showing that CD patients frequently express the TGF-β1 gene genotypes CT and TT at position -509, as compared to noninfected persons; similar results were observed with genotypes TC and CC at codon +10 of the TGF-β1 gene, leading to the conclusion that 509 C>T and +10 T>C TGF-β1 polymorphisms are associated with Chagas disease susceptibility. Studies in genetically different populations susceptible to CD will help to gather new insights and encourage the use of TGF-β as a CD biomarker.
More than 100 years ago, Trypanosoma cruzi was identified as the etiological agent of Chagas disease (CD), which remains an important social and health problem in Brazil and Latin America, endemic in 21 countries according to the World Health Organization (WHO) (Araujo-Jorge et al., 2017; World Health Organization - WHO, 2021). CD is still considered a neglected tropical disease (NTD) and was inserted in the WHO road map for “ending the neglected to attain the sustainable development goals” as a major economic and public health problem in most Latin American countries (World Health Organization, 2021). Nowadays, around 6-7 million people are estimated to be infected by T. cruzi worldwide leading to a mortality near to 10,000 patients per year (World Health Organization - WHO, 2021) Approximately 22 million people live in areas at risk of contamination in Brazil (Pan American Health Organization, 2006), where the most recent Health Minister Bulletin (Brasil, 2021) indicates the current CD epidemiological profile: 1.36 - 3.2 million estimated infected persons for 2020, a mean of 4.663 deaths/year (2007-2017), with more than 320 new cases/year (2017-2019). Until 2020 only acute cases were mandatorily reported in but the new guidelines for notification of chronic cases (Brasil, 2021) and for treatment (CONITEC and Ministério da Saúde, 2018) are still challenges to be implemented as public health policies. No biomarkers are available to predict the risk of CD progression (CONITEC and Ministério da Saúde, 2018).
The net global amount used for medical care for individuals affected by CD is currently 24-73 billion dollars (Lee et al., 2013). Thus, the annual amount spent with CD results in an expense of US$ 4,660/person, and over the course of life an individual can generate US$27,684 in public expenses, including spraying insecticide to control vectors (Lee et al., 2013). The economic cost of CD is very similar or even higher compared to other diseases in the world, such as: rotavirus (US$ 2.0 billion), cervical cancer (US$ 4.7 billion), Lyme disease ($2.5 billion). These data reinforce the economic relevance for more attention and effort to CD control. The globalization process brought challenges and changes in the public health scenario in different countries (Gascon et al., 2010). Due to the migratory movement from the 1980s onwards CD became a concern in the developed world (Rodari et al., 2018). Therefore, we currently observe a large number of cases of the CD in non-endemic countries, such as Australia, Canada Spain and, US (Ribeiro et al., 2012; Forsyth et al., 2021), leading to a broad organization of affected person’s Associations (Brasil, 2021). About 59,000 to 108,000 individuals are estimated to be infected by T. cruzi on the European continent. Since CD is not transmitted by direct contact with the infected person, in non-endemic countries the relevant transmission mechanisms of T. cruzi are transfusion with infected blood, organ donation and congenital transmission (Martelli et al., 2017).
The natural history of CD includes two distinct and successive phases (CONITEC and Ministério da Saúde, 2018; Simões et al., 2018). The acute phase is characterized by a high parasite load in the bloodstream of the infected individual (parasitemia), a short period of time, starting between 6 to 10 days after infection and lasting on average 1-2 months in humans, with intense inflammatory response and parasitism of several cell types. Most acute cases through vector transmission are not reported, as the clinical symptoms are nonspecific, such as fever, malaise, headache, which are typical of many infections. In some cases, the presence of edema is observed at the site of the T. cruzi entry, the inoculation chagoma (skin inflammation causing an edematous swelling), which is called the Romaña’s sign when located on the eyelids (Araujo-Jorge et al., 2017). Severe acute Chagas disease is not common, but in cases of symptomatic patients at this stage, they include generalized adenopathy, hepatosplenomegaly, lymphadenopathy, meningoencephalitis, and myocarditis (CONITEC and Ministério da Saúde, 2018). However, serious symptoms such as mortality from encephalomyelitis or severe heart failure can occur, but these symptoms represent 5% of cases, in which most infected individuals are children from endemic regions (Ribeiro et al., 2012). Most acute cases in Brazil are currently related to oral transmission and present a different clinical scenario with a higher prevalence of symptomatic cases, acute myopericarditis and death (Pinto et al., 2008).
Untreated acute CD progresses to the chronic phase, which has two clinical forms: indeterminate and determined (Dias et al., 2016). In the indeterminate form, individuals have positive serology, but there are no symptoms or signs of target organ involvement confirmed by complementary tests such as X-ray and electrocardiogram. Most patients remain in the indeterminate chronic stage until the end of their lives, without developing symptoms of the determined chronic phase. However, after a period of 10-30 years, 15-30% of infected individuals evolve to the determined forms of the disease, associated with tissue damage that includes cardiac, digestive, and mixed forms (Dias et al., 2016).
From the patients who move on to the chronic phase, 20-30% develop cardiac form, up to 10% exhibit digestive form, and it is still possible to identify mixed form (cardiac and digestive), in less than 5% (Gascón et al., 2007). The chronic CD cardiac form (CCC) is the most important cause of morbidity and mortality among people affected by CD, also resulting in a significant medical and social impact (Rassi et al., 2010). The most important CCC manifestations are arrhythmias, including sudden cardiac arrest, heart failure, and stroke. However, many patients may present stroke or sudden cardiac arrest as the first clinical presentation of the cardiac form. Regarding the digestive form, the most important presentations are megaesophagus and megacolon (Gascón et al., 2007; Henao-Martínez et al., 2012; Dias et al., 2016).
A variety of structural changes in the cardiovascular system have been described in patients with CCC (Rassi et al., 2017). The main change is observed in the reparative and fibrotic process, with a diffuse and dense accumulation of interstitial collagen that involves individual fibers or even an entire group of fibers. Thus, heart fibrosis is the most important histopathological outcome in CCC, both in humans and in experimental models (Rassi et al., 2010). Fibrosis is defined as an excessive deposition of extracellular matrix components in organs and tissues, because of the fibroblasts´ proliferation and activation, triggered by pro-inflammatory cytokines produced by cells of the innate and adaptive immune system (Wick et al., 2013). Basically, this is a process where the damaged tissue is replaced by connective tissue resulting in remodeling and in the functional impairment of the organ (Pohlers et al., 2009). The fibrosis pattern varies from focal to diffusely distributed fibrosis (Simões et al., 2018). Furthermore, the fibrotic reaction molded by the connective tissue after the inflammatory response is mainly characterized by an increase of extracellular matrix components production and by proliferation, migration, and accumulation of mesenchymal cells. When the inflammatory process ceases, the fibrotic process is sustained, and the installed fibrosis compromises the correct functionality of damaged tissues and organs (Pohlers et al., 2009). Some molecules influence these processes but transforming growth factor beta cytokine (TGF-β) plays a key role in this process by inducing the synthesis of extracellular matrix components and decreasing its degradation and turn-over (Leask, 2004).
In the early 1980s, it became evident that cell growth was controlled by different polypeptides and hormones (Sporn and Todaro, 1980) and a new polypeptide called TGF-β was identified in neoplastic cells (Roberts et al., 1981; Eisinger et al., 1988): a growth factor that induced proliferative phenotype in fibroblasts and collagen production in vivo and in vitro. Shortly after its discovery, a dual role of this cytokine was described, also showing the inhibition of cell proliferation. In these cells, TGF-β acted synergistically with another platelet-derived growth factor and inhibited colony formation (Roberts et al., 1985). TGF-β is a homodimeric protein that is part of the TGF-β superfamily and is found in most eukaryotic organisms, including C. elegans, Drosophila, Xenopus, rats, and humans (Harada et al., 2015). It is expressed in all cell types and in almost all developmental stages of organisms, playing an important role in the regulation of various biological and cellular responses, including cell proliferation and differentiation, extracellular matrix production, embryonic development, epithelial cell growth, carcinogenesis, and apoptosis (Massagué, 2012). In mammalian cells, there are three TGF-β subtypes: 1, 2 and 3. These isoforms are well characterized as small (25 kDa) homodimeric secreted proteins (Wilson, 2021) that are encoded by distinct genes located on different chromosomes. These molecules present significant homology (80%) and are 100% conserved across species, consisting of two monomers with a cysteine core linked by a disulfide bond (Huang et al., 2014). Although the three-dimensional structures of the isoforms are similar, intrinsic differences contribute to the activity and existence of each of these isoforms in vivo (Huang et al., 2014).
TGF-β synthesis is widespread in all cell types (Saharinen et al., 1996). However, due to its pleiotropic activity TGF-β is under strict control in organisms and is secreted in its latent, biologically inactive form (Figure 1). This latent inactive TGF-β is synthesized in a dimeric precursor form associated with the latency-associated protein (LAP), forming the small latent complex (Saharinen et al., 1996; Massagué and Chen, 2000). To perform its biological activities TGF-β needs to be activated to further interact with type I (TβRI), type II (TβRII) and type III (TβRIII) surface receptors, identified in virtually all cell types (Massagué and Gomis, 2006). Thus, activated TGF-β is recognized by its surface receptors following a time sequence (Figure 1): (i) initially, TβRIII acts by modulating the interaction between TGF-β with the appropriate signaling receptors (TβRI and TβRII). Therefore, TβRIII binds to TGF-β and directs it to TβRII; (ii) the binding of TGF-β to TβRII induces the dimerization and formation of a TβRI and TβRII complex, in which TβRII phosphorylates the serine/threonine domain of the TβRI resulting in the activation of an intracellular signaling cascade (Massagué, 1998; Massagué and Chen, 2000; Massagué and Gomis, 2006; Massague, 2008; Massagué, 2012) (Figure 1) Two types of signaling pathway triggered by active TGF-β have been described (Figure 1): the classic pathway, when the TβRI phosphorylates and activates the SMADs proteins (considered as important markers for the activation of the TGF-β signaling pathway) and the alternative pathway, when TβRI phosphorylates and activates other proteins such as: mitogen-activated protein kinases (ERK), C-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (Massague and Wotton, 2000; Massagué and Gomis, 2006). This process results in the recruitment of transcriptional cofactors or corepressors that lead to transcriptional activation or repression of TGF-β responsive genes (Shi and Massague, 2003).
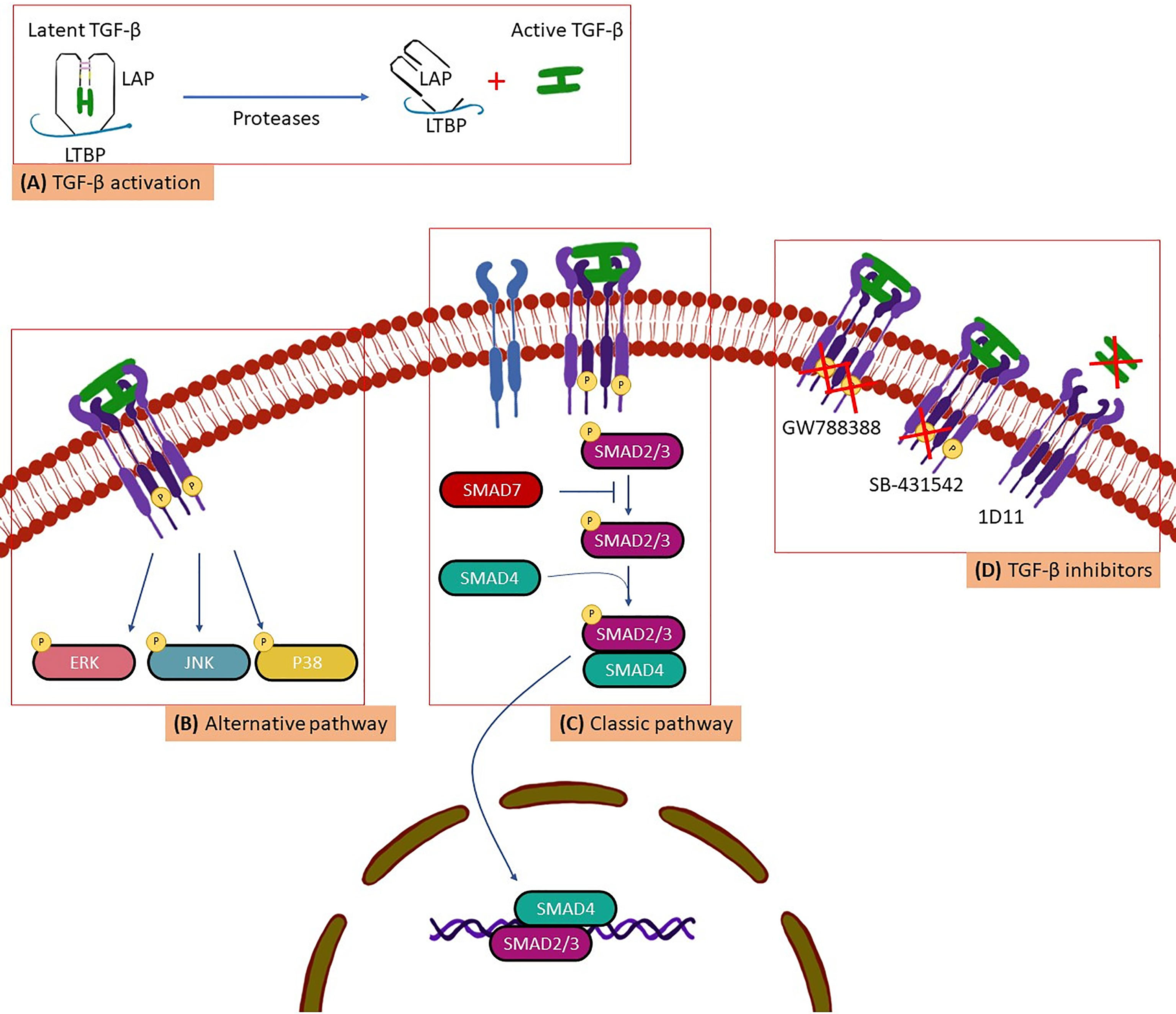
Figure 1 A scheme for (A) TGF-β activation, (B) alternative pathway, (C) Classic pathway and, (D) TGF-β inhibitors.
The first study involving the role of TGF-β in the development of CD was carried out in 1991, observing that, when peritoneal macrophages from mice and humans were treated with TGF-β, the trypanocide capacity of IFN-γ was inhibited (Silva et al., 1991). Furthermore, the treatment of human macrophages with TGF-β resulted in increased parasite replication. The effect of TGF-β on T. cruzi infection in vivo was also investigated. Mice infected by T. cruzi and treated with TGF-β developed higher parasitemia with decreased survival. The authors then proposed that TGF-β could play an important role in the regulation of the T. cruzi infection (Silva et al., 1991).
In 1996, Zhang and Tarleton (1996) observed increased TGF-β levels in inflammatory and regulatory cells in the spleen from different in vivo experimental models, at the beginning of the acute phase of T. cruzi infection. They also described that the peak of TGF-β production in the spleen of infected animals was concomitant with the peak of parasitemia in the three experimental models analyzed (Zhang and Tartelon, 1996). In addition, Zhang and Tarleton observed many TGF-β-producing cells in the heart tissue of infected animals during acute phase and persisting through the chronic phase (Zhang and Tartelon, 1996). A study developed with T. cruzi-infected primates also showed that TGF-β was produced in the first week of the acute phase and was constantly and systemically expressed during the chronic phase of the infection (Samudio et al., 1999). The gene expression of TGF-β increased in the heart of primates from two weeks after infection, remaining increased up to 10 years after infection (Samudio et al., 1999).
In 2002, Araújo-Jorge et al. (Araujo et al., 2002) observed that CCC patients had on average 10-20 times higher levels of circulating TGF-β1 when compared to healthy individuals (Araujo et al., 2002). These same patients were re-evaluated, and we demonstrated the predictive value of TGF-β1 as a biomarker of clinical progression in Chagas disease: patients in the early stages of the chronic phase, who presented high levels of circulating TGF-β1, evolved with worse prognosis after 10 years of follow-up (Saraiva et al., 2013). T. cruzi infection in different cell lines (human fibroblasts and epithelial cells) also resulted in a significant increase in the production of TGF-β in the supernatant of these cultures (Martello et al., 2013). An intriguing data was obtained in a further study that described low levels of TGF in stages C and D (Curvo et al., 2018). These results were justified because of different treatment schemes with carvedilol or spironolactone, since these drugs improved the survival of heart failure, but decreased TGF-β transcription.
We also demonstrated another important TGF-β role in Chagas disease: its involvement in cardiac tissue homeostasis, acting as a regulator of cell proliferation and death, extracellular matrix remodeling, electric coupling, and angiogenesis (Araujo-Jorge et al., 2012). Therefore, this cytokine is a key molecule to be studied in infectious diseases that damage the heart tissue, such as CD. Immunohistochemical analyzes of cardiac biopsies from patients with CCC presenting moderate or severe cardiac dysfunction showed intense staining for fibronectin in the extracellular matrix, as well as the presence of phosphorylated Smad2 (Araujo et al., 2002).
In addition to participating in the different processes already described in the progression of the CD, studies have shown that TGF-β also acts in the control of the different stages of T. cruzi life cycle (Waghabi et al., 2005; Waghabi et al., 2007). Waghabi et al. (2005) demonstrated that T. cruzi directly activates latent TGF-β, as a necessary strategy for host cell invasion. Ten years later, it was described that this activation is performed by a cysteine peptidase produced by T. cruzi, called cruzipain (Ferrão et al., 2015). These results represented the first example in the literature of a new mechanism by which a protozoan uses a host cell molecule, TGF-β, to control its own intracellular life cycle (Waghabi et al., 2005).
In 2016, we analyzed the kinetics of the TGF-β signaling pathway during the acute phase of experimental CD, observing that the T. cruzi infection: (i) significantly increases the expression of receptors: TβRI and TβRII; (ii) stimulates the phosphorylation of both the classical signaling pathway proteins (Smad2/3) and the alternative one (JNK, p38, ERK); (iii) significantly increases the TGF-β1 mRNA levels; (iv) leads to a high expression of TGF-β-responsive proteins: CTGF and fibronectin and; (v) with increased collagen deposition (Ferreira et al., 2016). Once again, these data confirm the main role of TGF-β in the development and maintenance of cardiac damage in response to T. cruzi infection (Ferreira et al., 2016). Silva et al. (2019) also determined TGF-β regulatory mechanisms in CD. This study in Trypanosoma cruzi-infected cardiomyocytes and cardiac fibroblasts demonstrated that p38 and c-Jun pathways could participate in regulatory process of fibrosis mediated by TGF-β (Silva et al., 2019). A recent in-silico study showed the prediction and verification of nine potential genes that were strongly associated with the virulence mechanisms of T. cruzi and the host immune response. One of these target genes was a member of the Smad5 family, a protein involved in the classical TGF-β signaling pathway (Ballinas-Verdugo et al., 2021). Other in-silico study described that some known and novel PIWI-interacting RNA (piRNAs) from the host could be dysregulated and could target and potentially regulate the expression of genes including TGF-β and two other piRNAs target genes, with one degree of interaction to TGF-β1. However, the role of these piRNAs in the CD pathogenesis remains unknown (Rayford et al., 2020).
CD susceptibility and its clinical manifestations could be influenced by genetic factors of the host (Ayo et al., 2013). A review article updated the current knowledge of the genetic basis of Chagas disease. Acosta-Herrera at el. (2019) concluded that more than 50 polymorphisms are associated with the susceptibility to T. cruzi infection and to the chronic manifestation of Chagas disease (Acosta-Herrera et al., 2019). In 2009, a study carried out in Peru and Colombia identified the association of TGF-β1 gene polymorphisms with susceptibility to the development of CD. Calzada et al. (2009) observed that the T allele at codon 10, which is associated with low production of TGF-β1, was found more frequently in healthy individuals than in cardiac patients. Conversely, the frequency of the C allele, which is associated with high production of TGF-β1, was higher in the group of infected individuals, indicating that this allele is a risk factor for susceptibility to the development of the disease (Calzada et al., 2009).
In 2018, we evaluated the importance of the TGF-β1 polymorphism in Brazilian patients in the chronic phase, including the indeterminate form and the different stages of the cardiac form. We also correlated the expression of different TGF-β1 alleles with the susceptibility of CD development. We demonstrated that two TGF-β1 polymorphisms, -509 C>T (rs1800469) and codon 10 T>C (rs1800470), are associated with the susceptibility to the development of the disease in Brazilian cohort (Ferreira et al., 2018). Thus, TGF-β1 is a potential serological marker with predictive value in the clinic for patients in the early stages of the disease. Considering the extensive involvement of TGF-β in the development of infectious and genetic diseases, in cell proliferation and differentiation, extracellular matrix production and consequent fibrosis, inhibition of TGF-β activity could be a possibility for treatment or even cure of some disease and biological and cellular processes. In other infections such as HIV (Jiang et al., 2021) and COVID-19 (Carlson et al., 2020) the important role of TGF-β in the remodeling of the extracellular matrix is also proposed, contributing to fibrosis. TGF-β involvement in various diseases increased the efforts for the development of compounds that inhibit the activity and signaling pathway of this molecule. Thus, our group has intensively evaluated pre-clinically the therapeutic action of some pharmacological compounds that inhibit the activity of TGF-β in CD, such as SB-431542, GW788388 and 1D11 (Araujo-Jorge et al., 2012) (Figure 1).
In 2007, Waghabi et al. (2007) evaluated the role of SB-431542 (TβRI inhibitor) in an in vitro infection model. As shown in the Table 1, we observed that the compound: (i) inhibits the activation of the TGF-β pathway induced by the presence of the parasite in epithelial cells and cardiomyocytes; (ii) reduces T. cruzi invasion in cardiomyocyte; (iii) decreases the number of parasites per infected cell; (iv) impairs T. cruzi differentiation, and release of trypomastigote forms, and (v) induces the death of intracellular parasites. We also evaluated the effect of SB-431542 on Gap junction proteins connexin-43 (Cx-43) finding a reduction and disorganization profile in the expression of Cx-43 (Waghabi et al., 2009a). These changes contribute to the abnormal conduction of the electrical impulse, which was reserved after treatment with SB-431542, resulting in an increased expression of Cx-43 and reestablishment of the organization of its plaque structure in cardiomyocytes. Thus, for the first time in the literature, we demonstrated in animal models that inhibition of the TGF-β pathway could be tested as a possibility for the treatment of CD.
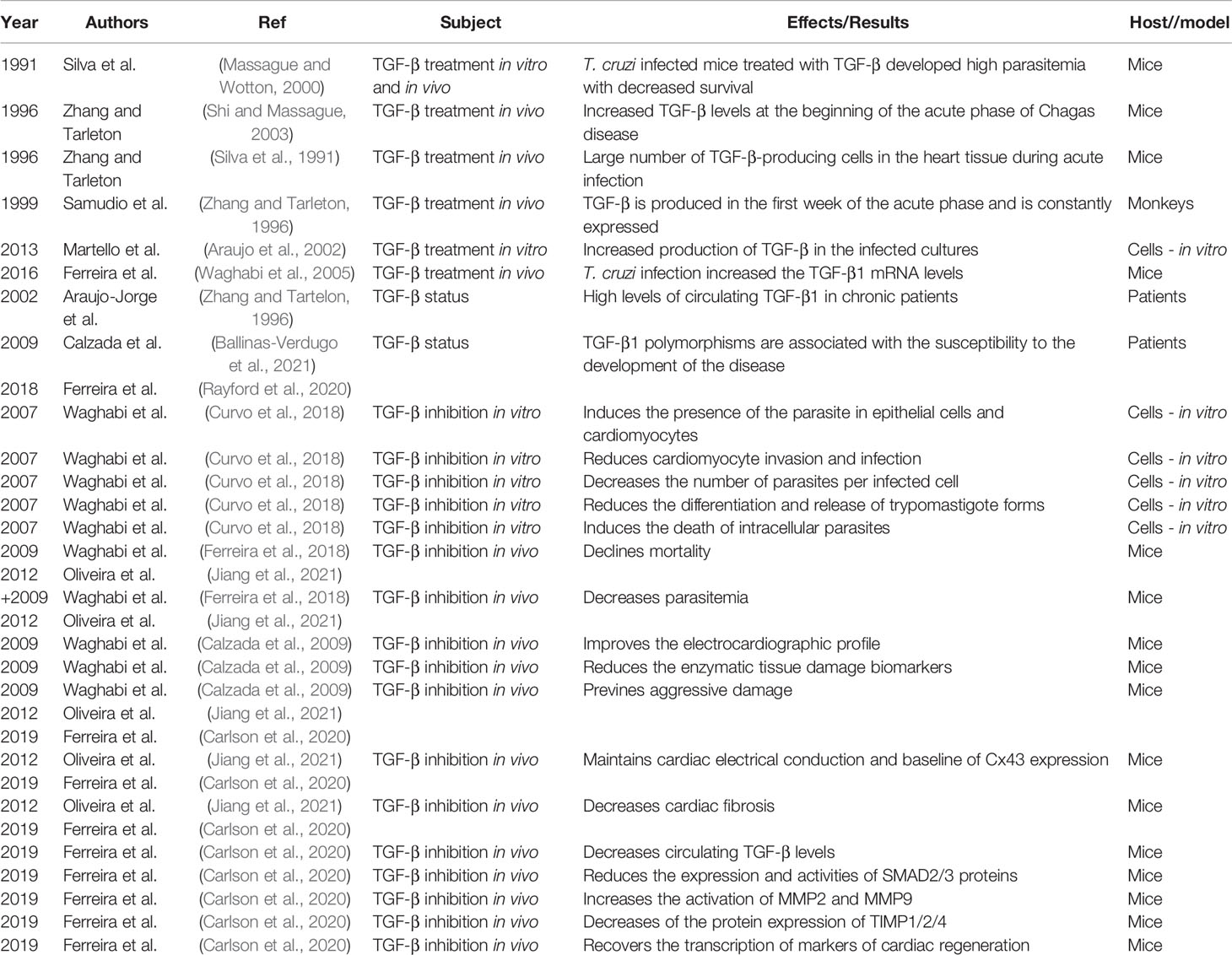
Table 1 Pre-clinical and clinical approaches implicating TGF-β in modulation of pathology in Chagas disease.
In 2009, Waghabi et al. (2009) demonstrated that male Swiss mice infected with the T. cruzi Y strain and treated with SB-431542: (i) reduces mice mortality; (ii) decrease in parasitemia; (iii) improve the electrocardiographic profile; and (iv) reduces the enzymatic tissue damage biomarkers (aspartate aminotransferase and creatine kinase). Thus, the inhibition of the TGF-β signaling pathway in vivo significantly attenuate the infection, preventing aggressive damage to the heart of infected animals (Waghabi et al., 2009b). The therapeutic activity of another TGF-β pathway inhibitor was also evaluated in an acute infection model of CD: male Swiss mice were infected with the T. cruzi Y strain and treated with GW788388. The compound was administered at the beginning of the infection and reduced parasitemia, increased animal survival, maintained cardiac electrical conduction and baseline of Cx43 expression. In addition, administration of GW788388 at the end of the acute phase increased animal survival and decreased cardiac fibrosis. These results reaffirm that inhibition of the TGF-β signaling pathway could be considered an important alternative strategy for the treatment of cardiomyopathy developed in CD (Oliveira et al., 2012). Another outstanding study established a therapeutic approach for controlling T. cruzi-driven fibroblast differentiation by poly [ADP-ribose] polymerase 1 inhibitors through modulation of the profibrotic macrophages signaling of the activator protein 1-MMP9 -TGF-pathway, thus controlling chronic fibrosis in CD by indirect inhibition of TGF-β’s functions (Choudhuri and Garg, 2020).
More recently, we also investigated the effect of GW788388 in a chronic CD experimental model (Ferreira et al., 2019). Ferreira et al. (2019) observed that treatment with GW788388 had a relevant therapeutic effect on the cardiac tissue of T. cruzi infected mice, resulting in: (1) decreased circulating TGF-β levels; (2) improved the electrocardiographic profile: decrease of the PR and QTc intervals, increase in heart rate, reversal of sinus arrhythmia and reversal of disturbances in atrial and atrioventricular conduction; (3) reversed the altered formation of intercellular plaques enriched with Cx-43; (4) decreased fibrosis in cardiac tissue; (5) decreased of the expression and activities of SMAD2/3 proteins, important proteins involved in TGF-β intracellular signaling pathway; (6) increased of the activity of MMP9, an enzyme important in the process of degradation of extracellular matrix proteins to decrease fibrosis; (7) decreased the protein expression of TIMP1/2/4, which are inhibitors of the activity of MMPs; and (8) recovered the transcription of GATA-6 and Tbox-5 genes, important markers of cardiac regeneration (Ferreira et al., 2019). Thus, the therapeutic effects of the TGF-β signaling pathway inhibition are promising and suggest a new possibility for the treatment of cardiac fibrosis in the chronic phase of CD.
Some studies evaluated the inhibition of the TGFβ-signaling pathway, and some molecules are under investigation in clinical trials for other diseases. AVID200, a potent TGF-beta 1 and 3 inhibitor, entered clinical trials phase I for the treatment of patients with advanced solid tumors. Weill Medical College of Cornell University New York, United States, is now recruiting patients for the investigation of safety and feasibility of Vactosertib, a small TGF-β type I receptor inhibitor molecule for the treatment of anemic chronic myeloproliferative neoplasms patients. Translation of the in vitro and in vivo result of TGF studies on Chagas disease development to patients into clinical trials is challenging but, as well as to other disease, could represent a new therapeutic strategy to be assessed.
Chagas disease affects approximately 8 million people in Latin America, and its prevalence in non-endemic countries has increased due to increasing international migration and non-vector transmission routes. The present drugs available for the treatment of CD in acute and chronic phases are first-generation trypanocidal drugs (benznidazol and nifurtimox) and only few new drugs are under evaluation in clinical trials, with no focus on cardiac physiopathological mechanisms of CD. Therefore, research to identify new treatments for CD is needed. To decrease the impact of CD on the population and the public health budget, governments must invest in treatment and health promotion policies as well as on innovation for new treatment strategies. The role of TGF-β as an inducer of several pathological processes leading to development and maintenance of cardiac damage in CD transforms this cytokine in a relevant target for the advancement of therapies for CCC patients. The therapeutic effects of inhibiting the TGF-β signaling pathway, in vitro and in vivo, are well described and promising. Thus, evaluation of the effect of the TGF-β signaling pathway inhibitors could be considered as: (i) a new strategy for the treatment of cardiac fibrosis in CD; (ii) a suggestion of effective and specific treatment for CCC. After 20-years of work in this line of research, we consider these conclusions as an important outcome.
All authors contributed to the concept and execution of the experiments that generated the ideas presented in this review. RF and TA-J wrote the original draft and all the co-authors reviewed and complemented the text. All authors contributed to the article and approved the submitted version.
We received funding by the Department of the Industrial Complex and Innovation in Health from the Brazilian Health Ministry (IOC Fiotec IOC-001-LIV-11-2-1; 25380.001603/2017-89; www.saude.gov.br/sctie), the governmental agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 309545/2014-5; 313011/2018-4, 159947/2018-9);, Fundação de Amparo à Pesquisa no Rio de Janeiro Carlos Chagas Filho (FAPERJ E26/201.838/2017; 201.983/2020; www.faperj.br); and Oswaldo Cruz Institute (IOC/Fiocruz; www.ioc.fiocruz.br).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acosta-Herrera, M., Strauss, M., Casares-Marfil, D., Martín, J. (2019). Chagas Genetics CYTED Network. Genomic Medicine in Chagas Disease. Acta Trop. 197:105062. doi: 10.1016/j.actatropica.2019.105062
Araujo-Jorge, T., Telleria, J., Rios-Dalenz, J. (2017) Chapter I: History of the Discovery of Iomarbiomarkertrypanosomiasis: Chagas Disease - One Hundred Years of Research Elsevier, Amsterdam. Available at: https://www.elsevier.com/books/american-trypanosomiasis-chagas-disease/telleria/978-0-12-801029-7.
Araujo-Jorge, T. C., Waghabi, M. C., Bailly, S., Feige, J. (2012). The TGF-β Pathway as an Emerging Target for Chagas Disease Therapy. Clin. Pharmacol. Ther. 92 (5), 613–621. doi: 10.1038/clpt.2012.102
Araujo, T. C., Waghabi, M. C., Hasslocher-Moreno, A. M., Xavier, S., Higuchi, M. D. L., Keramidas, M., et al. (2002). Implication of Transforming Growth Factor – β1 in Chagas Disease Myocardiopathy. J. Infect. Dis. 186, 1823–1828. doi: 10.1086/345882
Ayo, C. M., Dalalio, M. M., Visentainer, J. E., Reis, P. G., Sippert, E. A., Jarduli, L. R., et al. (2013). Genetic Susceptibility to Chagas Disease: An Overview About the Infection and About the Association Between Disease and the Immune Response Genes. BioMed. Res. Int. 2013:284729. doi: 10.1155/2013/284729
Ballinas-Verdugo, M. A., Jiménez-Ortega, R. F., Martínez-Martínez, E., Rivas, N., Contreras-López, E. A., Carbó, R., et al. (2021). Circulating miR-146a as a Possible Candidate Biomarker in the Indeterminate Phase of Chagas Disease. Biol. Res. 54, 1–16. doi: 10.1186/s40659-021-00345-3
Brasil (2021). “Boletim Epidemiológico Doença De Chagas - 14 De Abril - Dia Mundial,” in Secr. Vigilância Em Saúde/Brasília: Ministério Da Saúde, 42. Available at: https://www.gov.br/saude/pt-br/media/pdf/2021/abril/14/boletim_especial_chagas_14abr21_b.pdf.
Calzada, J. E., Beraún, Y., González, C. I., Martín, J. (2009). Cytokine Transforming Growth Factor Beta 1 (TGFβ1) Gene Polymorphisms and Chagas Disease Susceptibility in Peruvian and Colombian Patients. Cytokine 45, 149–153. doi: 10.1016/j.cyto.2008.11.013
Carlson, F. R., Jr., Bosukonda, D., Keck, P. C., Carlson, W. D. (2020). Multiorgan Damage in Patients With COVID-19: Is the TGF- β/BMP Pathway the Missing Link? JACC Basic. Transl. Sci. 5, 1145–1148. doi: 10.1016/j.jacbts.2020.09.003
Choudhuri, S., Garg, N. J. (2020). Trypanosoma Cruzi Induces the PARP1/AP-1 Pathway for Upregulation of Metalloproteinases and Transforming Growth Factor β in Macrophages: Role in Cardiac Fibroblast Differentiation and Fibrosis in Chagas Disease. mBio 11 (6), e01853–e01820. doi: 10.1128/mBio.01853-20
CONITEC and Ministério da Saúde (2018). “Protocolo Clínico E Diretrizes Terapêuticas Da Doença De Chagas,” in Comissão Nac. Inc. Tecnol. No SUS, 1–144.
Curvo, E. O., Ferreira, R. R., Madeira, F. S., Alves, G. F., Chambela, M. C., Mendes, V. G., et al. (2018). Correlation of Transforming Growth Factor-β1 and Tumour Necrosis Factor Levels With Left Ventricular Function in Chagas Disease. Mem. Inst. Oswaldo. Cruz. 113 (4), e170440. doi: 10.1590/0074-02760170440
Dias, J. C. P., Ramos, A. N., Gontijo, E. D., Luquetti, A., Shikanai-Yasuda, M. A., Coura, J. R., et al. (2016). Aspectos Gerais Da Epidemiologia Da Doença De Chagas Com Especial Atenção Ao Brasil. Epidemiol. e Serv. Saude. Rev. Do. Sist. Unico. Saude. Do. Bras. 25, 7–86. doi: 10.5123/S1679-49742016000500002
Eisinger, M., Sadan, S., Silver, I., Flick, R. (1988). Growth Regulation of Skin Cells by Epidermal Cell-Derived Factors : Implications for Wound Healing. Proc. Natl. Acad. Sci. U.S.A. 85, 1937–1941.
Ferrão, P. M., D’Avila-Levy, C. M., Araujo-Jorge, T. C., Degrave, W. M., Gonçalves, A. D. S., Garzoni, L. R., et al. (2015). Cruzipain Activates Latent TGF-β From Host Cells During Trypanosoma Cruzi Invasion. PloS One 10, 1–15. doi: 10.1371/journal.pone.0124832
Ferreira, R. R., Abreu, R., da, S., Vilar-Pereira, G., Degrave, W., Meuser-Batista, M., et al. (2019). TGF-β Inhibitor Therapy Decreases Fibrosis and Stimulates Cardiac Improvement in a Pre-Clinical Study of Chronic Chagas’ Heart Disease. PloS Negl. Trop. Dis. 13, 1–27. doi: 10.1371/journal.pntd.0007602
Ferreira, R. R., de Souza, E. M., de Oliveira, F. L., Ferrão, P. M., Gomes, L. H. F., Mendonça-Lima, L., et al. (2016). Proteins Involved on TGF-β Pathway Are Up-Regulated During the Acute Phase of Experimental Chagas Disease. Immunobiology 221, 587–594. doi: 10.1016/j.imbio.2016.01.009
Ferreira, R. R., Madeira, S., Alves, G. F., Chambela, C., Oliveira, E., Curvo, V., et al. (2018). TGF- β Polymorphisms Are a Risk Factor for Chagas Disease. Dis. Markers 2018:4579198. doi: 10.1155/2018/4579198
Forsyth, C. J., Manne-Goehler, J., Bern, C., Whitman, J., Hochberg, N. S., Edwards, M., et al. (2021). U.S. Chagas Diagnostic Working Group. Recommendations for Screening and Diagnosis of Chagas Disease in the United States. J. Infect. Dis. jiab513. doi: 10.1093/infdis/jiab513
Gascón, J., Albajar, P., Cañas, E., Flores, M., Herrera, R. N. (2007). Diagnosis, Management, and Treatment of Chronic Chagas’ Heart Disease in Areas Where Trypanosoma Cruzi Infection is Not Endemic. Rev. Esp. Cardiol. 60, 285–293.
Gascon, J., Bern, C., Pinazo, M. (2010). Chagas Disease in Spain, the United States and Other Non-Endemic Countries. Acta Trop. 115, 22–27. doi: 10.1016/j.actatropica.2009.07.019
Harada, S., Wei, S., Siegal, G. P. (2015). Molecular Pathology of Osteosarcoma. 2nd ed. (Cambridge, Massachusetts: Elsevier Inc). doi: 10.1016/B978-0-12-416721-6/00019-4
Henao-Martínez, A. F., Schwartz, D. A., Yang, I. V. (2012). Chagasic Cardiomyopathy, From Acute to Chronic: Is This Mediated by Host Susceptibility Factors? Trans. R. Soc Trop. Med. Hyg. 106, 521–527. doi: 10.1016/j.trstmh.2012.06.006
Huang, T., Schor, S. L., Hinck, A. P. (2014). Biological Activity Differences Between TGF-β1 and TGF-β3 Correlate With Differences in the Rigidity and Arrangement of Their Component Monomers. Biochemistry 16, 5737–5749.
Jiang, Y., Chai, L., Wang, H., Shen, X., Fasae, M. B., Jiao, J., et al. (2021). HIV Tat Protein Induces Myocardial Fibrosis Through TGF-Beta1-CTGF Signaling Cascade: A Potential Mechanism of HIV Infection-Related Cardiac Manifestations. Cardiovasc. Toxicol. 21 (12), 965–972. doi: 10.1007/s12012-021-09687-6
Leask, A. (2004). TGF- Signaling and the Fibrotic Response. FASEB J. 18, 816–827. doi: 10.1096/fj.03-1273rev
Lee, B. Y., Bacon, K. M., Bottazzi, M. E., Hotez, P. J. (2013). Global Economic Burden of Chagas Disease: A Computational Simulation Model. Lancet Infect. Dis. 13, 342–348. doi: 10.1016/S1473-3099(13)70002-1
Martelli, G., Girolamo, C., Zammarchi, L., Angheben, A., Morandi, M., Tais, S., et al. (2017). Seroprevalence of Five Neglected Parasitic Diseases Among Immigrants Accessing Five Infectious and Tropical Diseases Units in Italy: A Cross-Sectional Study. Clin. Microbiol. Infect. 23, 335.e1–335.e5. doi: 10.1016/j.cmi.2017.02.024
Martello, L. A., Wadgaonkar, R., Gupta, R., Tanowitz, H. B., Haseeb, M. A. (2013). Characterization of Trypanosoma Cruzi Infectivity , Proliferation , and Cytokine Patterns in Gut and Pancreatic Epithelial Cells Maintained In Vitro. Parasitol. Res. 112, 4177–4183. doi: 10.1007/s00436-013-3609-7
Massague, J. (2008). Previews a Very Private TGF- β Receptor Embrace. Mol Cell. 29 (2), 149–150. doi: 10.1016/j.molcel.2008.01.006
Massagué, J. (2012). TGF β Signalling in Context. Nat. Rev. Mol. Cell Biol. 13, 616–630. doi: 10.1038/nrm3434
Massagué, J., Gomis, R. R. (2006). The Logic of Tgfβ Signaling. FEBS Lett. 580, 2811–2820. doi: 10.1016/j.febslet.2006.04.033
Massague, J., Wotton, D. (2000). Transcriptional Control by the TGF-Beta/Smad Signaling System. EMBO J. 19, 1745–1754.
Oliveira, F. L., Souza, E. M., Oliveira, G. M., Araujo-Jorge, T. C., Degrave, W. M., Feige, J. J., et al. (2012). Oral Administration of GW788388 , an Inhibitor of Transforming Growth Factor Beta Signaling, Prevents Heart Fibrosis in Chagas Disease. PloS Negl. Trop. Dis. 6 (6), e16966. doi: 10.1371/journal.pntd.0001696
Pan American Health Organization (2006). Estimación Cuantitativa De La Enfermedad De Chagas En Las Américas. 26 pp, Ops/Hdm/Cd/425-06.
Pinto, A. Y., Valente, S. A., Valente, V. C., Ferreira Junior, A. G., Coura, J. R. (2008). Acute Phase of Chagas Disease in the Brazilian Amazon Region: Study of 233 Cases From Pará, Amapá and Maranhão Observed Between 1988 and 2005. Rev. Soc. Bras. Med. Trop. 41, 602–614.
Pohlers, D., Brenmoehl, J., Löf, I., Müller, C. K., Leipner, C., Schultze-mosgau, S., et al. (2009). TGF- β and Fibrosis in Different Organs - Molecular Pathway Imprints. Biochim. Biophys. Acta 1792, 746–756. doi: 10.1016/j.bbadis.2009.06.004
Rassi, J. A., Marin-Neto, J. A., Rassi, A. (2017). Chronic Chagas Cardiomyopathy : A Review of the Main Pathogenic Mechanisms and the Efficacy of Aetiological Treatment Following the BENznidazole Evaluation for Interrupting Trypanosomiasis ( BENEFIT ) Trial. Mem. Inst. Oswaldo. Cruz. 112, 224–235. doi: 10.1590/0074-02760160334
Rassi, A., Jr., Rassi, A., Marin-Neto, J. A. (2010). Chagas Disease. Lancet 375, 1388–1402. doi: 10.1016/S0140-6736(10)60061-X
Rayford, K. J., Cooley, A., Arun, A., Rachakonda, G., Kleschenko, Y., Villalta, F., et al. (2020). Trypanosoma Cruzi Modulates Piwi-Interacting Rna Expression in Primary Human Cardiac Myocytes During the Early Phase of Infection. Int. J. Mol. Sci. 21, 1–16. doi: 10.3390/ijms21249439
Ribeiro, A. L., Nunes, M. P., Teixeira, M. M., Rocha, M. O. C. (2012). Diagnosis and Management of Chagas Disease and Cardiomyopathy. Nat. Rev. Cardiol. 9, 576–589. doi: 10.1038/nrcardio.2012.109
Roberts, A. B., Anzano, M. A., Lamb, L. C., Smith, J. M., Sporn, M. B. (1981). New Class of Transforming Growth Factors Potentiated by Epidermal Growth Factor : Isolation From Non-Neoplastic Tissues. Proc. Natl. Acad. Sci. U.S.A. 78, 5339–5343.
Roberts, A. B., Anzano, M. A., Wakefield, L. M., Roche, N. S., Sternt, D. F., Sporn, M. B. (1985). Type Beta Transforming Growth Factor: A Bifunctional Regulator of Cellular Growth. Proc. Natl. Acad. Sci. U.S.A. 82, 119–123.
Rodari, P., Angheben, A., Gennati, G., Trezzi, L., Bargiggia, G., Maino, M., et al. (2018). Congenital Chagas Disease in a Non-Endemic Area: Results From a Control Programme in Bergamo Province, Northern Italy. Travel. Med. Infect. Dis. 25, 31–34. doi: 10.1016/j.tmaid.2018.04.011
Saharinen, J., Taipale, J., Keski-Oja, J. (1996). Association of the Small Latent Transforming Growth Factor-Beta With an Eight Cysteine Repeat of Its Binding Protein LTBP-1. EMBO J. 15, 245–253.
Samudio, M., Montenegro-James, S., Kasamatsu, E., Cabral, M. (1999). Local and Systemic Cytokine Expression During Experimental Chronic Trypanosoma Cruzi Infection in a Cebus Monkey Model. Parasit. Immunol. 21, 451–460.
Saraiva, R. M., Waghabi, M. C., Vilela, M. F., Madeira, F. S., da Silva, G. M. S., Xavier, S. S., et al. (2013). Predictive Value of Transforming Growth Factor-β1in Chagas Disease: Towards a Biomarker Surrogate of Clinical Outcome. Trans. R. Soc Trop. Med. Hyg. 107, 518–525. doi: 10.1093/trstmh/trt050
Shi, Y., Massague, J. (2003). Mechanisms of TGF-Beta Signaling From Cell Membrane to the Nucleus. Cell 113, 685–700.
Silva, T. A., de Ferreira, L. F. C., de Pereira, M. C. S., Calvet, C. M. (2019). Differential Role of TGF-B in Extracellular Matrix Regulation During Trypanosoma Cruzi-Host Cell Interaction. Int. J. Mol. Sci. 20, 1–21. doi: 10.3390/ijms20194836
Silva, J. S., Twardzik, D. R., Reed, S. G. (1991). Regulation of Trypanosoma Cruzi Infections In Vitro and In Vivo by Transforming Growth Factor (TGF-β). J. Exp. Med. 174, 539–545. doi: 10.1084/jem.174.3.539
Simões, M. V., Moreira, M., Romano, D., Schmidt, A., Suely, K., Martins, M., et al. (2018). Chagas Disease Cardiomyopathy. Int. J. Cardiovasc. Sci. 31, 173–189.
Sporn, M. B., Todaro, G. J. (1980). Autocrine Secretion and Malignant Transformation of Cells. N. Engl. J. Med. 9, 878–880.
Waghabi, M. C., Coutinho-silva, R., Feige, J., Higuchi, M. D. L., Becker, D., Burnstock, G., et al. (2009a). Gap Junction Reduction in Cardiomyocytes Following Transforming Growth Factor-Beta Treatment and Trypanosoma Cruzi Infection. Mem. Inst. Oswaldo. Cruz. 104, 1083–1090. doi: 10.1590/S0074-02762009000800004
Waghabi, M. C., Keramidas, M., Bailly, S., Degrave, W., Mendonça-Lima, L., Soeiro, M., et al. (2005). Uptake of Host Cell Transforming Growth Factor-β by Trypanosoma Cruzi Amastigotes in Cardiomyocytes. Am. J. Pathol. 167, 993–1003. doi: 10.1016/S0002-9440(10)61189-3
Waghabi, M. C., Keramidas, M., Calvet, C. M., Meuser, M., Nazare, M., Soeiro, C., et al. (2007). SB-431542, a Transforming Growth Factor β Inhibitor, Impairs Trypanosoma Cruzi Infection in Cardiomyocytes and Parasite Cycle Completion. Antimicrob. Agents Chemother. 51, 2905–2910. doi: 10.1128/AAC.00022-07
Waghabi, M. C., De Souza, E.M., De Oliveira, G.M., Keramidas, M., Feige, J., Araujo, T. C., et al. (2009b). Pharmacological Inhibition of Transforming Growth Factor β Signaling Decreases Infection and Prevents Heart Damage in Acute Chagas´Disease. Antimicrob. Agents Chemother. 53, 4694–4701. doi: 10.1128/AAC.00580-09
Wick, G., Grundtman, C., Mayerl, C., Wimpissinger, T., Feichtinger, J., Zelger, B., et al. (2013). The Immunology of Fibrosis. Annu. Rev. Immunol. 31, 107–135. doi: 10.1146/annurev-immunol-032712-095937
Wilson, S. E. (2021). TGF Beta –1, –2 and –3 in the Modulation of Fibrosis in the Cornea and Other Organs. Exp. Eye. Res. 207:108594. doi: 10.1016/j.exer.2021.108594
World Health Organization - WHO (2021) Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Available at: https://apps.who.int/iris/rest/bitstreams/1326801/retrieve.
World Health Organization (2021) Chagas Disease (American Trypanosomiasis). Available at: https://www.who.int/health-topics/chagas-diseasehttps://www.who.int/health-topics/chagas-disease#.
Zhang, L., Tarleton, R. (1996). Characterization of Cytokine Production in Murine Trypanosoma Cruzi Infection by in Situ Immunocytochemistry: Lack of Association Between Susceptibility and Type 2 Cytokine Production. Eur. J. Immunol. 26, 102–109.
Keywords: Chagas disease, TGF-beta, fibrosis, biomarker, polymorphism
Citation: Ferreira RR, Waghabi MC, Bailly S, Feige J-J, Hasslocher-Moreno AM, Saraiva RM and Araujo-Jorge TC (2022) The Search for Biomarkers and Treatments in Chagas Disease: Insights From TGF-Beta Studies and Immunogenetics. Front. Cell. Infect. Microbiol. 11:767576. doi: 10.3389/fcimb.2021.767576
Received: 31 August 2021; Accepted: 03 December 2021;
Published: 02 February 2022.
Edited by:
Christophe Chevillard, TAGC Theories and Approaches of Genomic Complexity, FranceReviewed by:
Ramendra Pati Pandey, SRM University (Delhi-NCR), IndiaCopyright © 2022 Ferreira, Waghabi, Bailly, Feige, Hasslocher-Moreno, Saraiva and Araujo-Jorge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tania C. Araujo-Jorge, dGFuaWFhakBpb2MuZmlvY3J1ei5icg==; Roberto Rodrigues Ferreira, cm9iZXJ0b2ZlcnJlaXJhQGlvYy5maW9jcnV6LmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.