- 1Department of Animal Genetics, Breeding and Reproduction, College of Animal Science, South China Agricultural University, Guangzhou, China
- 2Guangdong Provincial Key Lab of AgroAnimal Genomics and Molecular Breeding and Key Lab of Chicken Genetics, Breeding and Reproduction, Ministry of Agriculture and Rural Affairs, Guangzhou, China
- 3Division of Immunology, Virginia-Maryland Regional College of Veterinary Medicine, University of Maryland, College Park, MD, United States
Avian leukosis virus subgroup J (ALV-J) is an oncogenic retrovirus that causes immunosuppression and neoplastic diseases in poultry. Cytokine signal-transduction inhibitor molecule 3 (SOCS3) is an important negative regulator of the JAK2/STAT3 signaling pathway and plays certain roles in ALV-J infection. It is of significance to confirm the roles of SOCS3 in ALV-J infection and study how this gene affects ALV-J infection. In this study, we assessed the expression of the SOCS3 gene in vivo and in vitro, and investigated the roles of SOCS3 in ALV-J infection using overexpressed or interfered assays with the SOCS3 in DF-1 cells. The results showed that the SOCS3 expression of ALV-J infected chickens was different from uninfected chickens in the spleen, thymus and cecal tonsil. Further, SOCS3 is mainly expressed in the nucleus as determined by immunofluorescence assay. Overexpression of SOCS3 in DF-1 cells promoted the replication of ALV-J virus, and the expression of interferons (IFNα and INFβ), inflammatory factors (IL-6 and TNFα) along with interferon-stimulating genes (CH25H, MX1, OASL, and ZAP). Conversely, interference of SOCS3 showed the opposite results. We also observed that SOCS3 promoted ALV-J virus replication by inhibiting JAK2/STAT3 phosphorylation. In conclusion, SOCS3 promotes ALV-J replication via inhibiting the phosphorylation of the JAK2/STAT3 signaling pathway. These results would advance further understanding of the persistent infection and the viral immune evasion of the ALV-J virus.
Introduction
Avian leukemia virus subgroup J was first isolated and identified from commercial broilers in 1988, which mainly causes myeloid leukosis (Payne et al., 1991; Payne et al., 1992). Infection with ALV-J causes immunosuppression and decreases chickens production performance, leading to serious economic losses in the poultry industry. ALV-J virus can transmit vertically and horizontally, and the viral genome is highly variable. There are no treatments or vaccines to prevent ALV-J infection, and eradication of ALV-J can be achieved only by chicken population cleansing (Payne and Nair, 2012). Therefore, it is necessary to study the immune response mechanisms of hosts after ALV-J virus infection.
Previously, we performed RNA-seq on chicken primary monocyte-derived macrophages (MDM) cells infected with ALV-J. The results revealed that the cytokine signal transduction inhibitor 3 (SOCS3) was a differentially expressed gene in the JAK/STAT signaling pathway (Feng et al., 2019). Also, we further found that the SOCS3 gene was one of the differentially expressed genes based on the RNA-seq data (PRJNA552417) of spleen tissues from 7 days-old chicken infected with ALV-J (data unpublished). Furthermore, overexpression of SOCS3 in MDM cells promotes ALV-J virus replication (Feng et al., 2019). These observations imply that SOCS3 may play an important role in ALV-J virus replication in chickens.
SOCS3 is an inhibitor of signaling pathways initiating cytokines, which belongs to the SOCS family (Chen et al., 2000). It is mainly involved in the negative feedback regulation of the tyrosine-protein kinase/signal transduction pathway and transcriptional activator signal transduction pathway. Furthermore, SOCS3 is closely related to inflammatory response, oxidative stress, cell injury, and apoptosis (Nicholson et al., 2000; Rawlings et al., 2004; Kershaw et al., 2013). The critical role of SOCS3 is manifested by its binding to both the JAK kinase and the cytokine receptor, which further inhibits STAT3 phosphorylation (Harrison, 2012). The JAK/STAT pathway is an evolutionarily conserved signaling pathway that transduces signals from extracellular to the nucleus (Rawlings et al., 2004). JAK/STAT pathway activation stimulates cell proliferation, differentiation, cell migration, and immune challenge (Rawlings et al., 2004; Harrison, 2012; Coskun et al., 2013). The JAK/STATs signaling pathway consists of three main components: 1. tyrosine kinase associated receptor; 2. JAK kinases; 3. STAT proteins (Gao et al., 2018). In recent years, many studies have reported that the JAK/STAT signaling pathway plays an important role in viral infection (Cherng et al., 2021; Leite et al., 2021; Wang et al., 2021; Ye et al., 2021).
Newcastle disease virus (NDV) infection activates the expression of SOCS3 at the mRNA and protein level through a mechanism that depends on the MEK/ERK signaling pathway, which is conducive to the virus replication (Wang et al., 2019a). Similarly, overexpression of SOCS3 in DF-1 cells promotes infectious bursal disease (IBD) virus replication (Duan et al., 2020). Porcine reproductive and respiratory syndrome virus (PRRSV) infection also induces SOCS3 expression through p38/AP-1 signaling pathway to enhance PRRSV replication during infection (Luo et al., 2021). In addition, SOCS3 also plays an important role in bacterial infections, inflammatory responses, and cancer (Gao et al., 2018; Fukuta et al., 2021; Matsumura et al., 2021). However, the mechanism of SOCS3 on ALV-J virus replication remains unclear. Thus, in this study, we focused on analyzing the expression of SOCS3 after ALV-J infection and the mechanism of effects of SOCS3 on ALV-J replication.
Materials and Methods
Ethics Statement
All animal experiments in this study were conducted following the protocols approved by the Institutional Animal Care and Use Committee of South China Agriculture University (No: SCAU 2018c008) and following the Animal Protection Law of the People’s Republic of China.
Tissue Samples Source
The 280 days-old chickens were from a local farm in Guangdong. They showed telltale symptoms of infection, such as a pale cockscomb, listlessness, a thin body, and obvious hemangiomas on their skin and digits. Zhang et al. (2021) have verified that the chickens were only infected with the ALV-J virus. We collected the spleen, thymus, and cecal tonsils from chickens infected with ALV-J (n = 3) and uninfected with ALV-J (n = 3), and stored them at −80°C till their later use.
Virus and Cells
The laboratory ALV-J strain SCAU-HN06 was kindly provided by Prof. Weisheng Cao (South China Agricultural University, Guangzhou, China). The ALV-J strain SCAU-HN06 was isolated from commercial layer hens with spontaneous hemangiomas in China (Zhang et al., 2011). Chicken embryo fibroblast (DF-1) cells were obtained from ATCC (Manassas, VA, USA) and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, USA) and 0.1% penicillin/streptomycin (Invitrogen, USA).
Overexpression and siRNA Knockdown Assay
The SOCS3 gene of chicken (GenBank ID. NM_204600) was cloned into a pcDNA3.1 vector (Invitrogen) with the primers sequences to generate the vector named pcDNA3.1-SOCS3. The forward primer sequence (5′ - 3′): CCGGAATTCATGGTCACCCACAGCAAG and the reverse sequence (5′-3′): TGCTCTAGAGATTTCCCTCTGCCAGCCT was used to generate the pcDNA3.1-SOCS3 vector. Blue letters represent enzyme cutting sites. In our preliminary experiments, we designed three siRNAs for each gene to interfere with SOCS3, JAK2, STAT3 and the siRNA with the highest interference efficiency was chosen. The results of knockdown and overexpression efficiency are show in Supplementary Figure S1. The specific small interfering RNA (siRNAs) were designed and constructed by Genewiz (Nanjing, China), and the sequences of siRNAs were as follows: si-SOCS3 5′- GCUUCUACUGGAGCACGGUTT-3′, si-JAK2 5′-GTCATGTCTTACCTATTTG-3′, si-STAT3 5′-GGACATCAGTGGAAAGACT-3′, a scrambled negative control RNA (si-NC) 5′-UUCUCCGAACGUGUCACGUTT-3′.
A total of 1 × 105 DF-1 cells/well were cultured in DMEM with 10% FBS and 0.1% penicillin/streptomycin overnight. According to the manufacturer’s instructions, the DF-1 cells were transfected with pcDNA3.1-SOCS3 plasmid or siRNAs by Lipofectamine 3000 reagent (Invitrogen, USA). pcDNA3.1 or si-NC was used as a control, respectively. After 24 h transfection, the DF-1 cells were infected with ALV-J strain SCAU-HN06 (105 TCID50/mL). Total protein, RNA, and cell supernatants samples were collected at 24 h and 48 h post-infection (hpi). Subsequently, qRT-PCR, ELISA, or western blotting (WB) were used to assess the roles of SOCS3 in ALV-J infection.
RNA Isolation and cDNA Synthesis
Following the manufacturer’s protocol, total RNA was extracted from tissues and DF-1 cells using the RNAiso reagent (Takara, Japan). RNA integrity and concentration were determined using 1% agarose gel electrophoresis and a Nanodrop 2000c spectrophotometer (Thermo, USA). cDNA was synthesized using MonAmp™ RTIII All-in-One Mix (Monad, Guangzhou, China), following the manufacturer′s protocol. Synthesized cDNA was stored at -20°C until subsequent analysis using qRT-PCR.
Quantitative Real-Time PCR
qRT-PCR primers specific for SOCS3, JAK2, STAT3, IFNα, IFNβ, IL-6, TNFα, ZAP, CH25H, MX1, and OASL were designed using Oligo 7.0 software. All primers were synthesized by Tsingke Biotech Technology Co., Ltd. (Guangzhou, China). The MonAmp™ SYBR® Green qPCR Mix (Monad, Guangzhou, China) was used for qPCR in an ABI 7500 Real-Time Detection instrument (Applied Biosystems, USA) following the manufacturer’s protocol. Relative gene expression was measured by qRT-PCR three for each reaction, and the nuclear GAPDH gene was used as a control. The gp85 of ALV-J virus copy was performed as previously described (Dai et al., 2015). The primers used in qRT-PCR were shown in Table S1.
Immunofluorescence Assay
Immunofluorescence assay was performed as previously described (Zhang et al., 2020). Rabbit anti-SOCS3 antibodies were used (XY-10R-5878; Fitzgerald, USA; 1:500) and were incubated at 4° for 8 h. The goat anti-rabbit IgG H&L/FITC (bs-0295G-FITC; Bioss, China; 1:500) was used to combine the antibody/antigen complex at room temperature for 1 h. The nuclei were dyed by 4′,6-diamidino-2-phenylindole (DAPI) for 5 min, and a fluorescence microscope (Nikon, Tokyo, Japan) was utilized to capture the immunofluorescence pictures.
Western Blotting Assay
Western Blotting (WB) assays were performed as previously described (Zhang et al., 2020). The antibodies and their dilutions used for WB were as follows: anti-ALV-J envelope protein-specific monoclonal antibody JE9 (kindly provided by Prof. Aijian Qin, Yangzhou University, 1:1000), Rabbit Anti-JAK2 antibody (bs-0908R; Boss, China; 1:1000), Rabbit Anti-phospho-JAK2 (Tyr1007+Tyr1008) (bsm-52171R; Boss, China; 1:1000), Rabbit Anti-STAT3 antibody (bs-1141R; Boss, China; 1:1000), Rabbit Anti-phospho-STAT3 (Ser727) antibody (bs-3429R; Boss, China; 1:1000), Rabbit Anti-beta-Actin antibody (bs-0061R; Boss, China; 1:1000), Goat Anti-Rabbit IgG H&L/HRP antibody (bs-40295G-HRP; Boss, China; 1:5000).
ELISA
According to the manufacturer’s protocol, the p27 (the main protein component of the ALV viral capsid) level was measured using an avian leukosis virus antigen test kit (IDEXX, USA). The IFNα, IFNβ, IL-6, and TNFα levels were measured using a Chicken Interferon α (IFNα) ELISA Kit (ML760024; mlbio, Shanghai, China), Chicken Interferon β (IFNβ) ELISA Kit (ML760024; mlbio, Shanghai, China), Chicken Interleukin 6 (IL-6) ELISA Kit (ML760041; mlbio, Shanghai, China), and Chicken Tumor necrosis factor α (TNFα) ELISA Kit (ML760161; mlbio, Shanghai, China) according to the manufacturer′s protocol, respectively. In brief, cells and medium were collected from tissue culture dishes, subjected to three freeze-thaw cycles, and centrifuged at 3000 × g at 4°C for 10 min. The supernatants were collected and subjected to ELISA according to the manufacturer′s protocols.
Statistical Analysis
Statistical comparisons were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). The data are presented as means ± standard error of the mean (SEM). The date differences in data were evaluated by the Student′s t-test. A P value of < 0.05 was considered statistically significant (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Results
The Expression of SOCS3 in Immune Organs and DF-1 cells
First, we evaluated the expression of SOCS3 in the spleen, thymus, and cecal tonsil of ALV-J infected and uninfected chickens by qRT-PCR. The chickens were only infected with ALV-J, as previously demonstrated by Zhang et al. (2021). The expression of SOCS3 in the cecal tonsil (P < 0.05) and thymus (P > 0.05) of ALV-J infected individuals was higher than that of uninfected individuals (Figure 1A). Next, we collected and detected DF-1 cells after ALV-J infection at different time points to investigate the SOCS3 expression in vitro. The expression of SOCS3 in DF-1 cells infected with ALV-J was higher than that of uninfected cells, except at 12 h post-infection (hpi) (Figure 1B). Besides, immunofluorescence assay results showed that SOCS3 was expressed in the nucleus and cytoplasm, but mainly in the nucleus (Figure 1C).
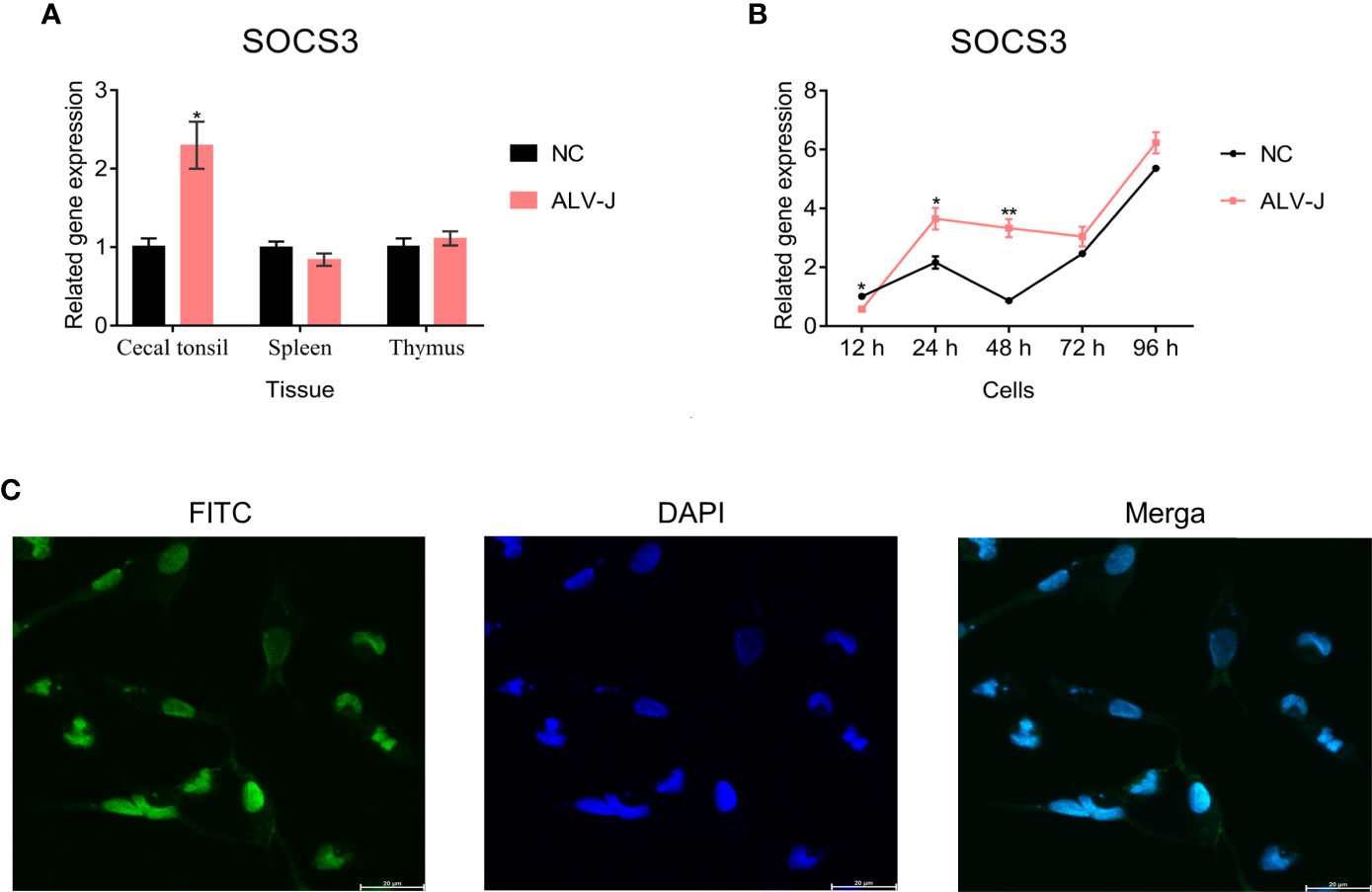
Figure 1 The expression of SOCS3 in tissues and cells. (A) The expression of SOCS3 in immune organs of ALV-J infected chickens (n = 3) and uninfected chickens (n = 3) was quantified by qRT-PCR. The chickens were only infected with ALV-J, as previously demonstrated by Zhang et al. (2021). (B) After DF-1 cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06, the expression of SOCS3 in cells was measured from 12 hpi to 96 hpi by qRT-PCR. (C) Immunofluorescence analysis of SOCS3 in DF-1 cells. Scale bar: 20 μm. A special antibody against SOCS3 was modified by FITC (green). The nucleus was stained by DAPI (blue). These experiments were performed independently at least three times independently. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, and **P ≤ 0.01).
Overexpression and Knockdown of SOCS3 Could Influence ALV-J Virus Replication
To explore the functions of SOCS3, we overexpressed and interfered with SOCS3 in DF-1 cells to evaluate its effect on ALV-J virus replication. The gp85, the major viral envelope protein, is the most variable of the structural proteins in the genome of ALV-J and exhibits high diversity (Venugopal et al., 1998; Pan et al., 2012). The capsid protein ALV p27 is encoded by the gag gene and acts as a major group-specific antigen (Weiss, 2006; Yun et al., 2013). Therefore, we evaluate the effect of SOCS3 on ALV-J virus replication by qRT-PCR (gp85) and ELISA (p27). The qRT-PCR, ELISA, and WB results showed that the overexpression of SOCS3 in DF-1 cells promoted the expression of gp85 and p27, suggesting that the SOCS3 benefits the replication of ALV-J virus (Figures 2A–C). In contrast, knockdown of SOCS3 in DF-1 cells inhibited the ALV-J virus replication (Figures 2D–F).
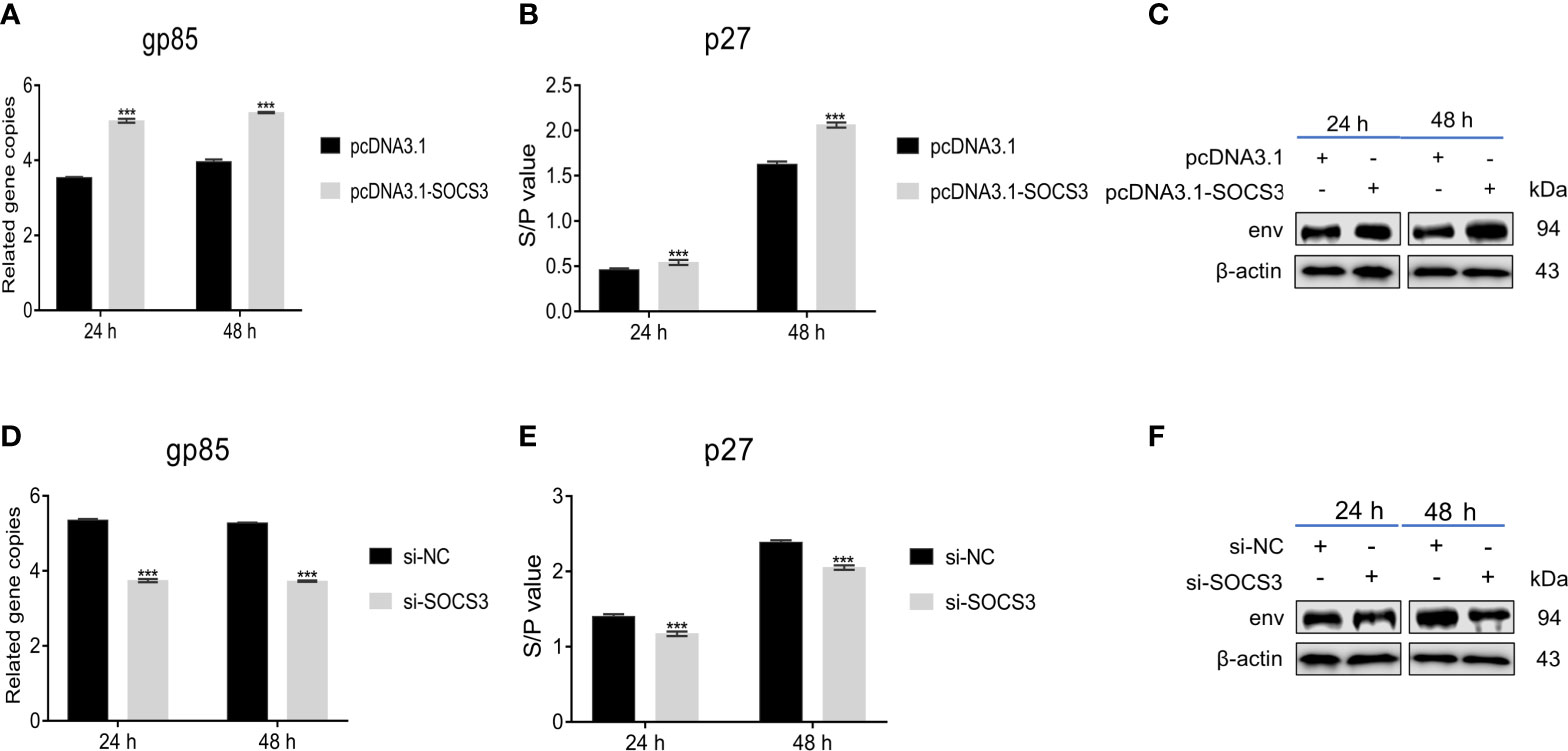
Figure 2 Overexpression and knockdown of SOCS3 in DF-1 cells could influence ALV-J virus replication. After 24 h of overexpression of SOCS3 in DF-1 cells, cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06. Samples were collected at 24 and 48 hpi, and qRT-PCR (A), ELISA (B), and WB (C) were performed for ALV-J virus content. After 24 h knockdown of SOCS3, DF-1 cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06. Samples were collected at 24 and 48 hpi, and qRT-PCR (D), ELISA (E), and WB (F) were performed for ALV-J virus content. These experiments were performed at least three times independently with similar results. Differences in data were evaluated by the Student’s t-test t test. The error bars are the standard error of the mean (SEMs) (***P ≤ 0.01).
SOCS3 Affects the Phosphorylation of the JAK2 and STAT3
Since SOCS3 plays an important role via the JAK/STAT signaling pathway (Chen et al., 2000; Rawlings et al., 2004), we further evaluated the expression of JAK2 and STAT3 genes in ALV-J infected and uninfected tissues and cells to study how SOCS3 promotes ALV-J virus replication. The expression of JAK2 was higher in the cecal tonsil but not in the thymus, since the difference was not significant in the thymus after ALV-J infection compared with uninfected chickens. In contrast, the expression of JAK2 in the spleen was lower than in the uninfected chickens (Figure 3A). The expression of STAT3 was higher in the cecal tonsil but lower in the spleens and thymus in chickens infected with ALV-J, compared to uninfected chickens (Figure 3B). Furthermore, after the DF-1 cells were infected with ALV-J, the expression of JAK2 and STAT3 were increased and higher than that of uninfected cells from 12 hpi to 96 hpi (Figures 3C, D).
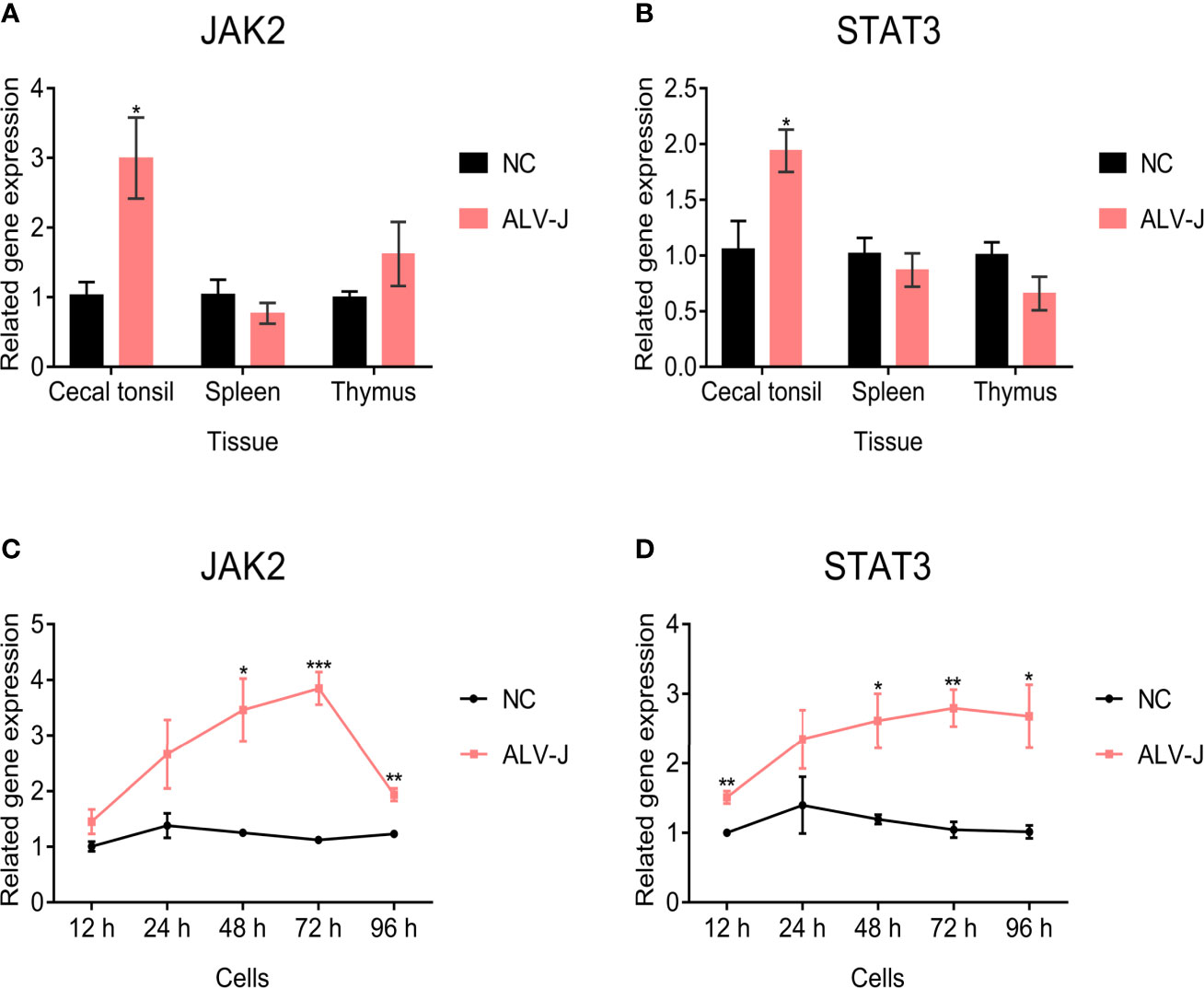
Figure 3 JAK2 and STAT3 gene expression in tissues and cells. The (A) JAK2 and (B) STAT3 expression in immune organs of ALV-J infected chickens (n = 3) and uninfected chickens (n = 3) were quantified by qRT-PCR. The chickens were only infected with ALV-J, as previously demonstrated by Zhang et al. (2021). After DF-1 cells were infected with ALV-J strain SCAU-HN06, the (C) JAK2 and (D) STAT3 genes expression was detected by qRT-PCR from 12 hpi to 96 hpi. These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Based on the above results, we speculated that SOCS3 might play an important role in ALV-J infection through JAK2/STAT3. Therefore, we analyzed the effect of SOCS3 on the JAK2/STAT3 signaling pathway. Overexpression of SOCS3 in DF-1 cells significantly increased the JAK2 and STAT3 mRNA expression, and significantly decreased the phosphorylation levels of JAK2 and STAT3 (Figures 4A–C). In contrast, knockdown of SOCS3 in DF-1 cells significantly decreased the JAK2 and STAT3 mRNA expression and significantly increased the phosphorylation levels of JAK2 and STAT3 (Figures 4D–F).
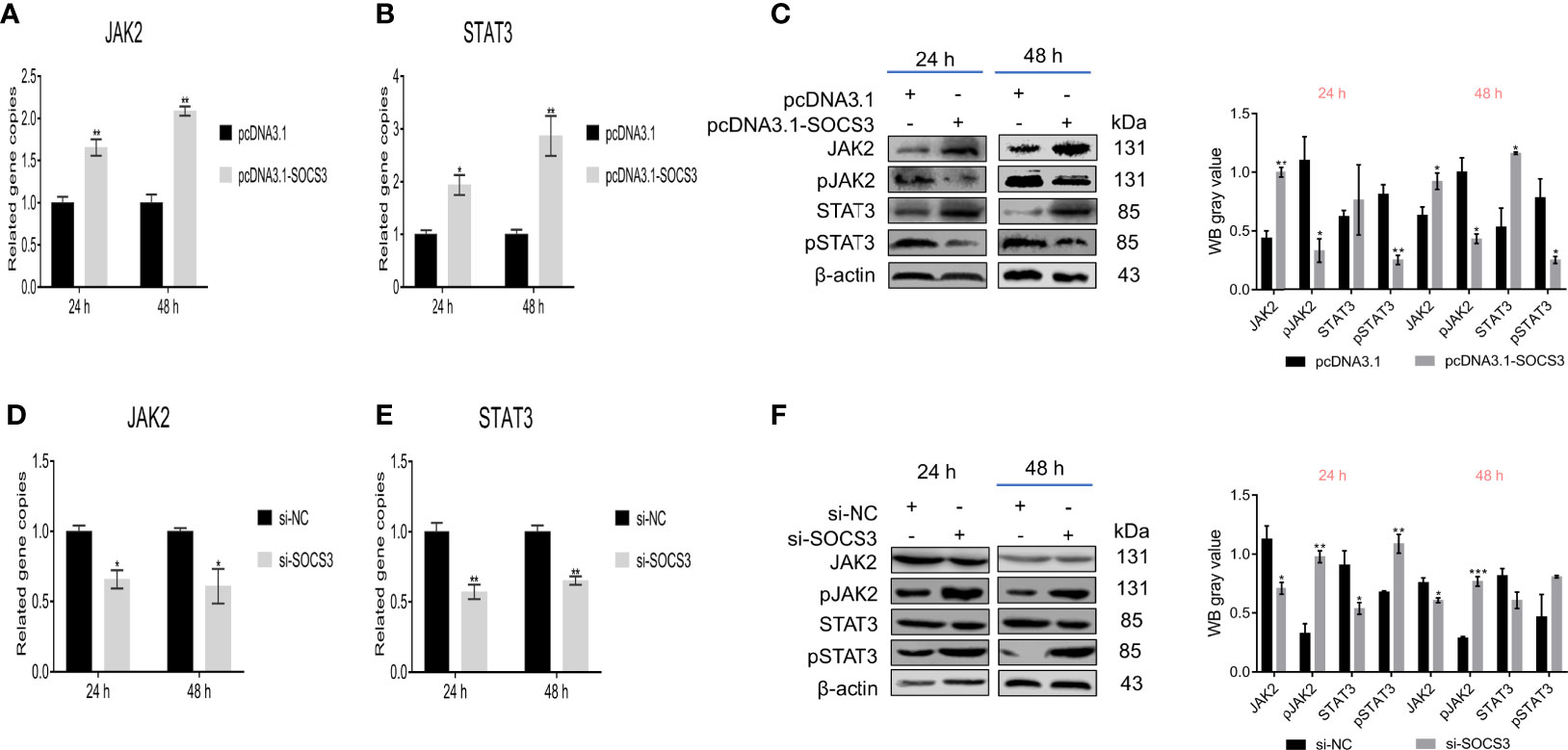
Figure 4 SOCS3 affects JAK2 and STAT3 phosphorylation. After overexpression of SOCS3 in DF-1 cells, the JAK2 and STAT3 expression and the phosphorylation levels were detected by qRT-PCR (A, B) and WB (C). After the knockdown of SOCS3 in DF-1 cells, the JAK2 and STAT3 expression and the phosphorylation levels were detected by qRT-PCR (D, E) and WB (F). These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Besides, we further verified the effect of JAK2/STAT3 on SOCS3. Knockdown of JAK2 in DF-1 cells significantly reduced the SOCS3 and STAT3 expression and the phosphorylation levels of JAK2 and STAT3 (Figures 5A–D). Also, knockdown of STAT3 in DF-1 cells significantly reduced the SOCS3 expression and the phosphorylation of STAT3, but did not affect the JAK2 expression and phosphorylation of JAK2 (Figures 5E–H). The above experimental data demonstrated that SOCS3 is a negative regulator of the JAK2/STAT3 signaling pathway.
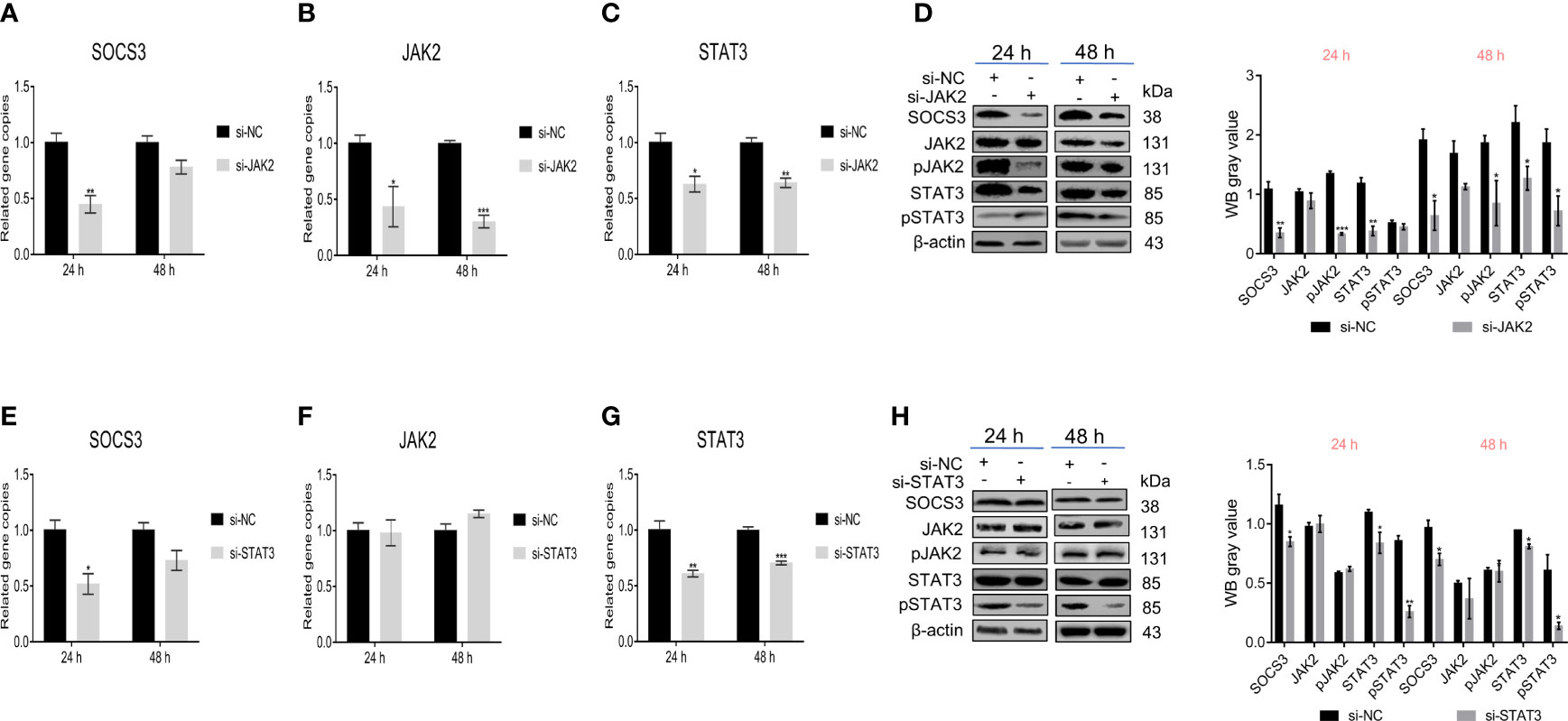
Figure 5 JAK2/STAT3 inhibited SOCS3 expression. After the knockdown of JAK2 in DF-1 cells, the SOCS3, JAK2, and STAT3 genes expression and the phosphorylation levels of JAK and STAT3 were detected by qRT-PCR (A–C) and WB (D). After knockdown of STAT3 in DF-1 cells, the SOCS3 and JAK2 genes expression and the phosphorylation levels of JAK and STAT3 were detected by qRT-PCR (E–G) and WB (H). These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
SOCS3 Affects ALV-J Virus Replication by Inhibiting JAK2/STAT3 Phosphorylation
To investigate how SOCS3 affects ALV-J virus replication, we conducted co-transfection experiments with pcDNA3.1-SOCS3, si-JAK2, and si-STAT3, respectively. After the knockdown of JAK2, the STAT3 expression decreased while the ALV-J virus content increased compared to the control. In pcDNA3.1-SOCS3 + si-JAK2 groups, the STAT3 expression was significantly increased, but the phosphorylation level of STAT3 was decreased. Both the qRT-PCR and ELISA results showed that the content of the ALV-J virus was significantly increased (Figures 6A–D). After the knockdown of STAT3, there was no significant difference in JAK2 expression, but the ALV-J virus’s content significantly increased compared to the control. In pcDNA3.1-SOCS3 + si-STAT3 groups, the STAT3 expression and ALV-J virus were significantly increased, but the phosphorylation level of JAK2 was decreased (Figures 6E–H). These results above indicated that SOCS3 inhibits the phosphorylation of JAK2 and STAT3, thereby affecting the replication of the ALV-J virus.
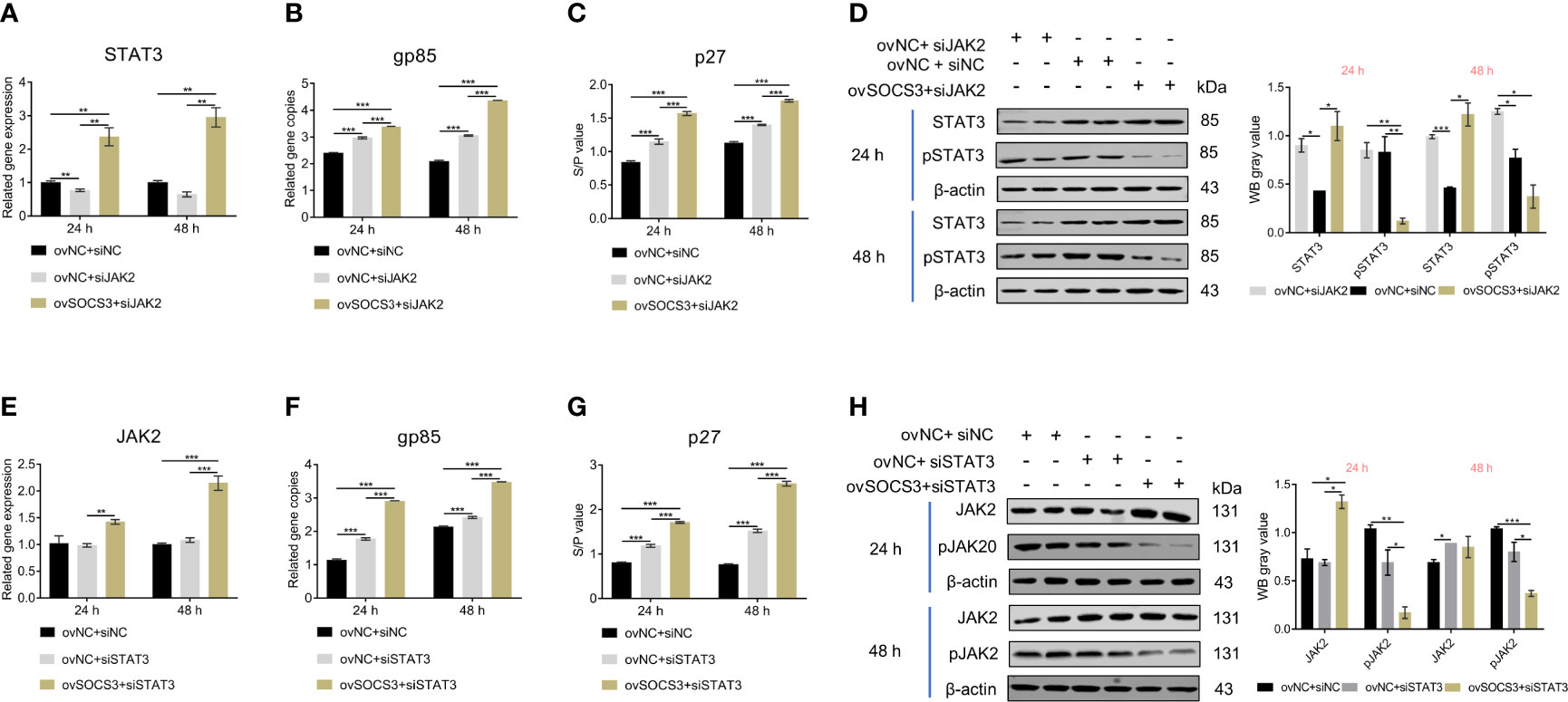
Figure 6 The effects of JAK2/STAT3 phosphorylation on ALV-J virus replication. The pcDNA3.1-SOCS3 and si-JAK2 were co-transfected into DF-1 cells. The transfected cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06 after 24 h. The (A) STAT3 gene and (B) ALV-J virus were detected by qRT-PCR. The (C) ALV-J virus (p27) and (D) STAT3 phosphorylation were detected by ELISA and WB, respectively. The pcDNA3.1-SOCS3 and si-STAT3 were co-transfected into DF-1 cells. The transfected cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06 after 24 h. The (E) STAT3 gene and (F) ALV-J virus were detected by qRT-PCR. The (G) ALV-J virus (p27) and (H) STAT3 phosphorylation were detected by ELISA and WB, respectively. pcDNA3.1+si-NC, ovNC + siNC; pcDNA3.1+si-JAK2, ovNC + si-JAK2; pcDNA3.1-SOCS3 + si-JAK2, ovSOCS3 + siJAK2; pcDNA3.1+si-STAT3, ovNC + si-STAT3; pcDNA3.1-SOCS3 + si-STAT3, ovSOCS3 + si STAT3. These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
SOCS3 Affects the Expression of Interferon, Inflammatory Factors, and Interferon-Stimulated Genes
In the present study, the ALV-J virus significantly increased the mRNA expression levels of IFNα, IFNβ, IL-6, and TNFα after overexpression of SOCS3 in DF-1 cells (Figures 7A–D). Furthermore, overexpression of SOCS3 significantly promoted the mRNA expression of interferon-stimulated genes ZAP, CH25H, Mx1, and OASL (Figures 7E–H). After three freeze-thaw cycles, DF-1 cells were detected by ELISA. The results were similar to those of qRT-PCR. Overexpression of SOCS3 significantly increased the content of IFNα, IFNβ, IL-6, and TNFα (Figures 7I–L). However, the expression of IFNα, IFNβ, IL-6, TNFα, ZAP, CH25H, MX1, and OASL genes significantly decreased after SOCS knockdown in DF-1 cells (Figure 8). It may be associated with ALV-J persistent infection.
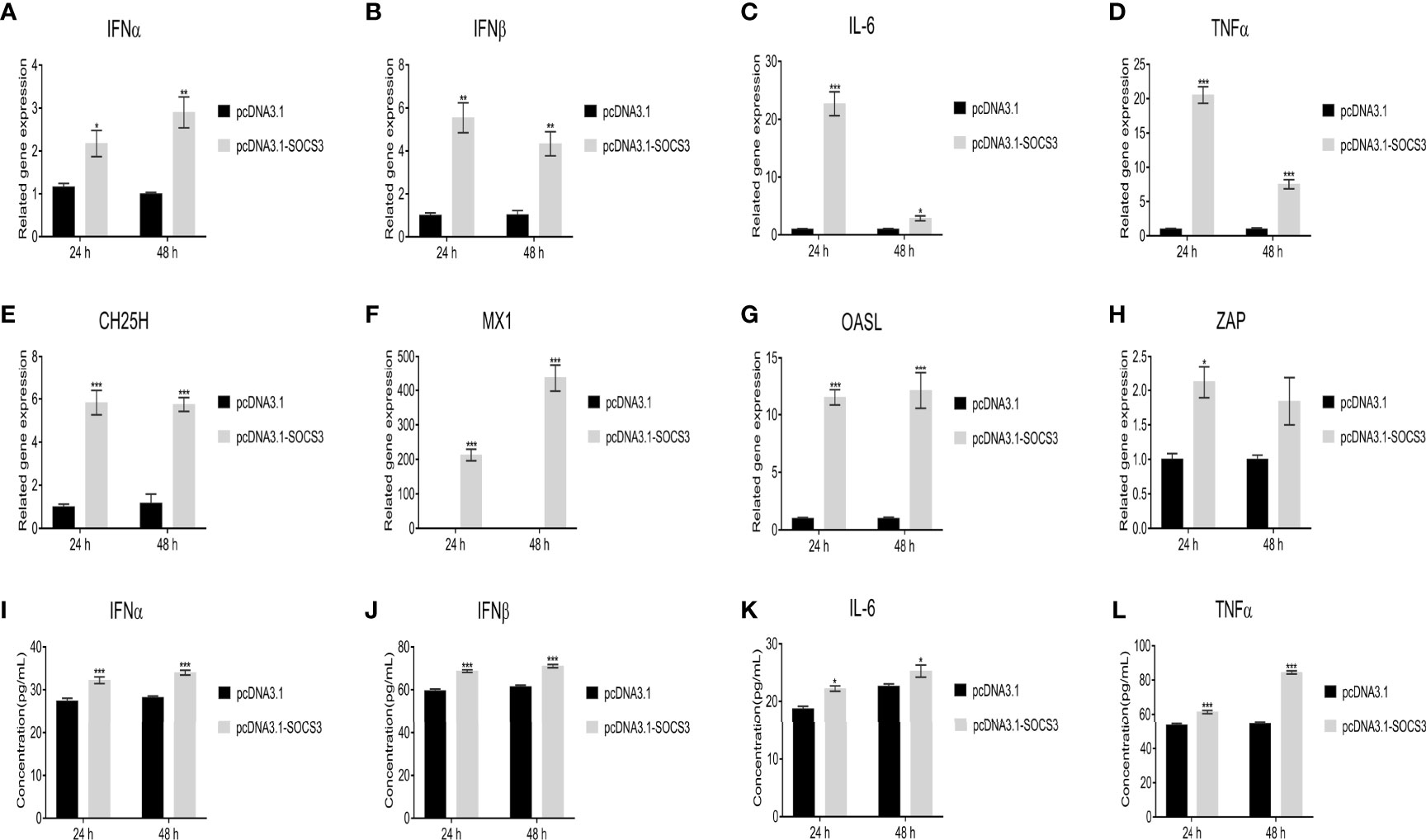
Figure 7 Overexpression of SOCS3 promotes the expression of interferons, inflammatory factors, and interferon-stimulated genes. After SOCS3 overexpression in DF-1 cells in 24h, the cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06. At 24 and 48 hpi, qRT-PCR was used to detect (A) IFNα, (B) IFNβ, (C) IL-6, (D) TNFα, (E) CH25H, (F) MX1, (G) OASL, and (H) ZAP mRNA levels. After three freeze-thaw cycles, the levels of the content of (I) IFNα, (J) IFNβ, (K) IL-6, and (L) TNFα in cells were detected by ELISA assay. These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
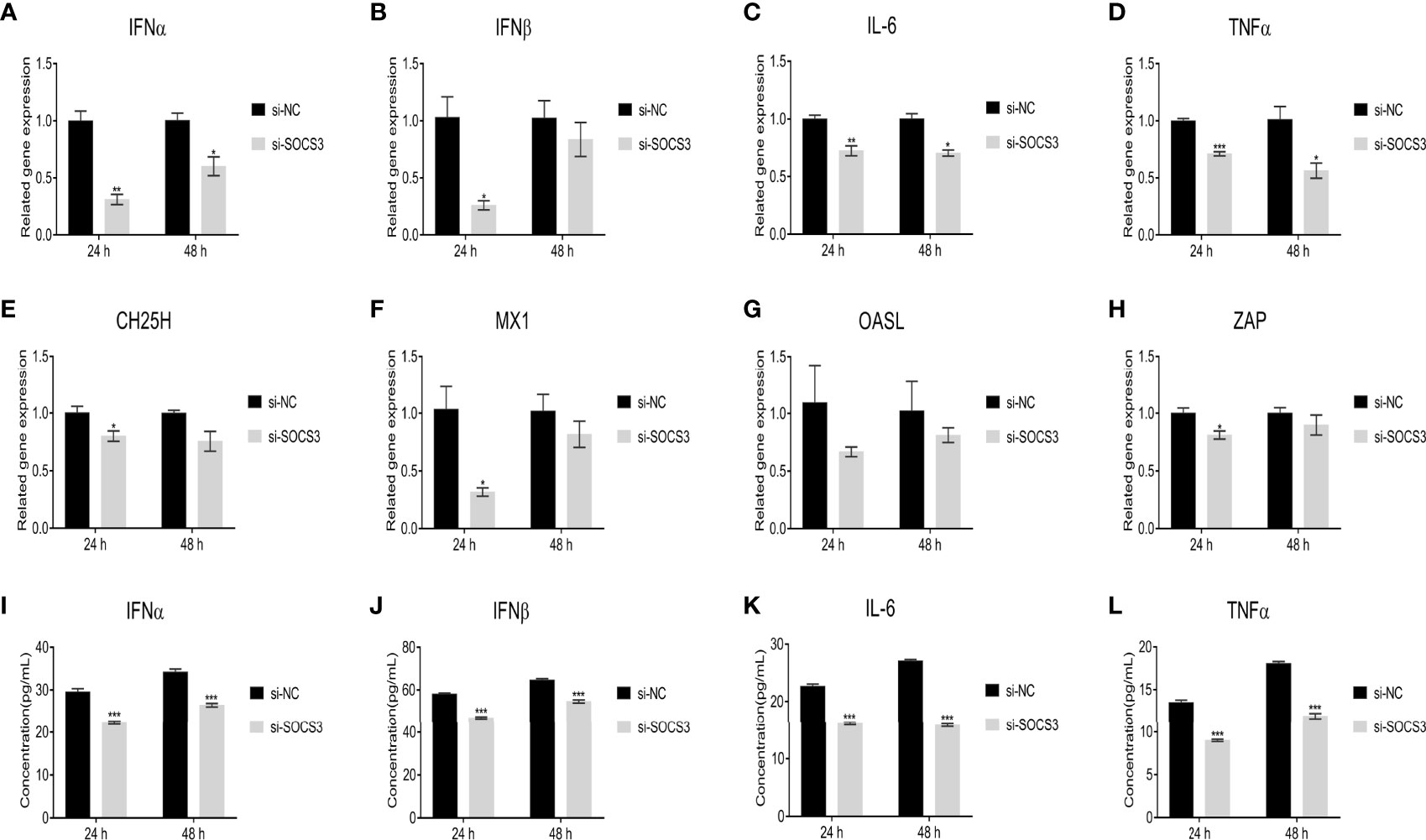
Figure 8 Knockdown of SOCS3 reduces the expression of interferons, inflammatory factors, and interferon-stimulated genes. SOCS3 knockdown in DF-1 cells in 24h, the cells were infected with 105 TCID50/mL ALV-J strain SCAU-HN06. At 24 and 48 hpi, qRT-PCR was used to detect (A) IFNα, (B) IFNβ, (C) IL-6, (D) TNFα, (E) CH25H, (F) MX1, (G) OASL, and (H) ZAP mRNA levels. After three freeze-thaw cycles, the levels of the content of (I) IFNα, (J) IFNβ, (K) IL-6, and (L) TNFα in cells were detected by ELISA assay. These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Discussion
Although researchers have learned a great deal about innate and adaptive immunity, several important questions need to be addressed. The virus could develop some strategies to escape the host immune responses. It can affect virus replication by neutralizing antibody (NAb) escape mutants (Pandiri et al., 2010) or by inhibiting/promoting the expression of certain genes (Feng et al., 2019). Some genes help the virus survive in the host; for example, the JAK/STAT pathway inhibitors (CISH, SOCS1, and SOCS3) are beneficial to ALV-J survival in MDM cells (Feng et al., 2019). In our previous data (RNA-seq, PRJNA552417), SOCS3 was highly expressed in slow-feathered chickens compared to late-feathered chickens (unpublished). Evidence shows that slow-feathered chickens are susceptible to ALV-J (Bacon et al., 1988; Fadly and Smith, 1997). These above results suggest that SOCS3 may play an important role in ALV-J virus infection.
Phosphotyrosine phosphatases (PTPs), protein inhibitors of activated STAT (PIAS) and SOCS proteins are negative regulators of the JAK/STAT signaling pathway (Gao et al., 2018). Among SOCS proteins, SOCS3 is a major regulator of JAK/STAT signaling (Chen et al., 2000). Also, SOCS3 is a host protein that can be employed by viruses. SOCS3 can be induced by various viruses, including NDV, DHAV-1, HIV-1 and Enterovirus 71 (EV71) (Akhtar et al., 2010; Wang et al., 2019a; Xie et al., 2019; Gao et al., 2020). SOCS3 also inhibits or promotes viral replication when acted upon by small RNA molecules (Ma et al., 2018; Wang et al., 2019b; Duan et al., 2020). Furthermore, SOCS3 inhibits the catalytic activity of JAK2 by occupying the receptor or blocking substrate association. SOCS3 uses a short motif (the kinase- inhibitory region, KIR) to restrain signaling transmission by directly inhibiting the catalytic activity of JAKs. Moreover, SOCS3, through binding to gp130, inhibits STAT3 phosphorylation, and it also regulates the response to cytokines and growth factors (Nicholson et al., 2000; Yoshimura et al., 2007; Gao et al., 2018). Studies indicate that the tyrosine phosphorylation of SOCS3 accelerates the degradation of SOCS3 protein, thereby regulating feedback inhibition of JAK/STAT signaling (Haan et al., 2003; Ke and Liu, 2003).
The JAK/STAT pathway constitutes the fulcrum in many important cellular processes, including growth, differentiation, proliferation, and immune functions (Coskun et al., 2013). The binding of a cytokine to its cell-surface receptor results in receptor dimerization, resulting in the activation of JAKs via cross-phosphorylation. Specific tyrosine residues on the receptor are then phosphorylated by activated JAKs and serve as docking sites for a family of latent cytoplasmic transcription factors known as STATs. STATs are phosphorylated by JAKs, then dimerize and subsequently leave the receptor and translocate to the nucleus, where they activate gene transcription (Ke and Liu, 2003; Gao et al., 2018). In addition, JKA proteins can bind to the multichain receptor, such as the IL-2R family (IL-2, IL-4, IL-7, IL-9), the IL-3R family (IL-3, IL-5), IL-6R family (IL-6, IL-11, IL-12), and IFN-R family (IFNs, IL-10, IL-19, IL-20) (Coskun et al., 2013). Genetic knockout studies have shown that JAKs and STATs have highly specific functions in controlling various immune responses (Ke and Liu, 2003). The mice experimental study showed that JAK- or STAT- deficiency is lethal or immunodeficiency (Coskun et al., 2013). Viruses can evade the host immune system by inactivating different adaptors of the IFN-activated JAK/STAT signaling pathway (Ke and Liu, 2003). Under normal conditions, STAT3 is low-expressed or not expressed in the signaling pathway. However, STAT3 is activated when the host is stimulated. It was found that overactivated STAT3 protein promotes viral replication (Koeberlein et al., 2010; Okemoto et al., 2013).
ALV infection can result immunological tolerance, intermittent viremia, and persistent viremia (Dai et al., 2019; Yu et al., 2019). In the present study, we found that when the amount of ALV-J virus increased, the expression of JAK2 and STAT3 was also increased, indicating that JAK2 and STAT3 may have a positive effect on ALV-J persistent infection. Furthermore, when SOCS3 was co-transfected with si-JAK2 or si-STAT3, SOCS3 affected JAK2 or STAT3 and promoted ALV-J virus replication. In addition, we also interfered with the expression of JAK2 and STAT3, and found that both reduced SOCS3 expression. These results verified that SOCS3 is a negative regulator in JAK/STAT signaling pathway.
Interferon, inflammatory factors, and interferon-stimulating genes play an important role in the host’s innate immune response to antiviral infection. After overexpression of SOCS3, NDV, DHAV-1 and Hepatitis C virus (HCV) can reduce interferon and interferon-stimulated genes to evade IFN-mediated antiviral responses (Collins et al., 2014; Wang et al., 2019a; Xie et al., 2019). However, in this study, overexpression of SOCS3 promoted the expression of those genes, while knockdown of SOCS3 inhibited the expression of those genes, which may result from the ALV-J infection. Compared with other viruses, the clinicopathological changes of ALV-J virus infection require a longer period to appear under certain factors. Besides, some individuals may even be infected for life without disease clinicopathological changes, suggesting that ALV-J infection may be more complex than previously thought.
Altogether, these results suggest that SOCS3 promotes ALV-J replication via inhibiting JAK2/STAT3 phosphorylation. We conclude that SOCS3 is an important negative regulator of the chicken innate immune signaling pathway. The finding was representing a substantial increase in our understanding of the mechanisms of persistent ALV-J infection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of South China Agriculture University.
Author Contributions
Conceptualization, GM. Data curation, HF. Formal analysis, GM and HF. Funding acquisition, QN and XZ. Investigation, MX and LL. Methodology, GM and HF. Project administration, XZ. Resources, XZ. Software, QZ, MX, ZZ, and LL. Supervision QN and XZ. Validation, GM and BH. Visualization, QZ and HF. Writing, original draft, GM. Writing, review, and editing, BH and MS. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (31970540) and the China Agriculture Research System of MOF and MARA (CARS-41).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.748795/full#supplementary-material
Supplementary Figure 1 | The efficiency of overexpression and knock-down of SOCS3, JAK2 and STAT3 in DF-1 cell. According to the manufacturer’s instructions, the DF-1 cells were transfected with pcDNA3.1-SOCS3 plasmid or siRNAs by Lipofectamine 3000 reagent (Invitrogen, USA). After 24 h and 48 h transfection, the expression of SOCS3 (A, B), was measured by qRT-PCR. After 24 h transfection, the expression of JAK2 (C), and STAT3 (D) was measured by qRT-PCR. These experiments were performed independently at least three times with similar results. Differences in data were evaluated by the Student’s t-test. The error bars are the standard error of the mean (SEMs) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
References
Akhtar, L. N., Qin, H., Muldowney, M. T., Yanagisawa, L. L., Kutsch, O., Clements, J. E., et al. (2010). Suppressor of Cytokine Signaling 3 Inhibits Antiviral IFN-β Signaling to Enhance HIV-1 Replication in Macrophages. J. Immunol. 185 (4), 2393–2404. doi: 10.4049/jimmunol.0903563
Bacon, L. D., Smith, E., Crittenden, L. B., Havenstein, G. B. (1988). Association of the Slow Feathering (K) and an Endogenous Viral (Ev21) Gene on the Z Chromosome of Chickens. Poult. Sci. 67 (2), 191–197. doi: 10.3382/ps.0670191
Chen, X. P., Losman, J. A., Rothman, P. (2000). SOCS Proteins, Regulators of Intracellular Signaling. Immunity 3 (13), 287–290. doi: 10.1016/s1074-7613(00)00028-5
Cherng, Y., Chu, Y. C., Yadav, V. K., Huang, T., Hsieh, M., Lee, K., et al. (2021). Induced Mitochondrial Alteration and DNA Damage via IFNGR-JAK2-STAT1-PARP1 Pathway Facilitates Viral Hepatitis Associated Hepatocellular Carcinoma Aggressiveness and Stemness. Cancers 13 (11), 2755. doi: 10.3390/cancers13112755
Collins, A. S., Ahmed, S., Napoletano, S., Schroeder, M., Johnston, J. A., Hegarty, J. E., et al. (2014). Hepatitis C Virus (HCV)-Induced Suppressor of Cytokine Signaling (SOCS) 3 Regulates Proinflammatory TNF-Alpha Responses. J. Leukoc. Biol. 96 (2), 255–263. doi: 10.1189/jlb.2A1211-608RRRR
Coskun, M., Salem, M., Pedersen, J., Nielsen, O. H. (2013). Involvement of JAK/STAT Signaling in the Pathogenesis of Inflammatory Bowel Disease. Pharmacol. Res. 76, 1–8. doi: 10.1016/j.phrs.2013.06.007
Dai, M., Feng, M., Liu, D., Cao, W., Liao, M. (2015). Development and Application of SYBR Green I Real-Time PCR Assay for the Separate Detection of Subgroup J Avian Leukosis Virus and Multiplex Detection of Avian Leukosis Virus Subgroups A and B. Virol. J. 12 (1), 52. doi: 10.1186/s12985-015-0291-7
Dai, M., Feng, M., Xie, T., Li, Y., Zhang, X. (2019). Fluctuations in Luteinizing Hormone, Follicle Stimulating Hormone, and Progesterone Might Affect the Disappearance of Avian Leukosis Virus Subgroup J Viremia in Chickens With Intermittent Viremia. Poult. Sci. 98 (9), 3533–3538. doi: 10.3382/ps/pez195
Duan, X., Zhao, M., Li, X., Gao, L., Cao, H., Wang, Y., et al. (2020). gga-miR-27b-3p Enhances Type I Interferon Expression and Suppresses Infectious Bursal Disease Virus Replication via Targeting Cellular Suppressors of Cytokine Signaling 3 and 6 (SOCS3 and 6). Virus Res. 281, 197910. doi: 10.1016/j.virusres.2020.197910
Fadly, A. M., Smith, E. J. (1997). Role of Contact and Genetic Transmission of Endogenous Virus-21 in the Susceptibility of Chickens to Avian Leukosis Virus Infection and Tumors. Poult. Sci. 76 (7), 968–973. doi: 10.1093/ps/76.7.968
Feng, M., Xie, T., Li, Y., Zhang, N., Lu, Q., Zhou, Y., et al. (2019). A Balanced Game: Chicken Macrophage Response to ALV-J Infection. Vet. Res. 50 (1), 20. doi: 10.1186/s13567-019-0638-y
Fukuta, M., Suzuki, K., Kojima, S., Yabe, Y., Suzuki, K., Iida, K., et al. (2021). Suppressor of Cytokine Signalling 3 (SOCS3) Expressed in Podocytes Attenuates Glomerulonephritis and Suppresses Autoantibody Production in an Imiquimod-Induced Lupus Model. Lupus. Sci. Med. 8 (1), e426. doi: 10.1136/lupus-2020-000426
Gao, W., Hou, M., Liu, X., Li, Z., Yang, Y., Zhang, W. (2020). Induction of SOCS Expression by EV71 Infection Promotes EV71 Replication. BioMed. Res. Int. 2020, 1–9. doi: 10.1155/2020/2430640
Gao, Y., Zhao, H., Wang, P., Wang, J., Zou, L. (2018). The Roles of SOCS3 and STAT3 in Bacterial Infection and Inflammatory Diseases. Scand. J. Immunol. 88 (6), e12727. doi: 10.1111/sji.12727
Haan, S., Ferguson, P., Sommer, U., Hiremath, M., Mcvicai, D. W., Heinrich, P. C., et al. (2003). Tyrosine Phosphorylation Disrupts Elongin Interaction and Accelerates SOCS3 Degradation. J. Biol. Chem. 278 (34), 31972–31979. doi: 10.1074/jbc.M303170200
Harrison, D. A. (2012). The JAK/STAT Pathway. Cold Spring Harb. Perspect. Biol. 4 (3), a11205. doi: 10.1101/cshperspect.a011205
Ke, S., Liu, B. (2003). Regulation of JAK-STAT Signalling in the Immune System. Nat. Rev. Immunol. 3 (11), 900–911. doi: 10.1038/nri1226
Kershaw, N. J., Murphy, J. M., Liau, N. P. D., Varghese, L. N., Laktyushin, A., Whitlock, E. L., et al. (2013). SOCS3 Binds Specific Receptor–JAK Complexes to Control Cytokine Signaling by Direct Kinase Inhibition. Nat. Struct. Mol. Biol. 20 (4), 469–476. doi: 10.1038/nsmb.2519
Koeberlein, B., Hausen, A. Z., Bektas, N., Zentgraf, H., Chin, R., Toan, N. L., et al. (2010). Hepatitis B Virus Overexpresses Suppressor of Cytokine Signaling-3 (SOCS3) Thereby Contributing to Severity of Inflammation in the Liver. Virus Res. 148 (1-2), 51–59. doi: 10.1016/j.virusres.2009.12.003
Leite, T. H. J. F., Ferreira, Á. G. A., Imler, J., Marques, J. T. (2021). Distinct Roles of Hemocytes at Different Stages of Infection by Dengue and Zika Viruses in Aedes Aegypti Mosquitoes. Front. Immunol. 12, 660873. doi: 10.3389/fimmu.2021.660873
Luo, X., Chen, X., Qiao, S., Li, R., Lu, Q., Geng, R., et al. (2021). Porcine Reproductive and Respiratory Syndrome Virus Increases SOCS3 Production via Activation of P38/AP-1 Signaling Pathway to Promote Viral Replication. Vet. Microbiol. 257, 109075. doi: 10.1016/j.vetmic.2021.109075
Matsumura, S., Nakamori, M., Tsuji, T., Kato, T., Nakamura, M., Ojima, T., et al. (2021). Oncolytic Virotherapy With SOCS3 Enhances Viral Replicative Potency and Oncolysis for Gastric Cancer. Oncotarget 12 (4), 344–354. doi: 10.18632/oncotarget.27873
Ma, Y., Wang, C., Xue, M., Fu, F., Zhang, X., Li, L., et al. (2018). The Coronavirus Transmissible Gastroenteritis Virus Evades the Type I Interferon Response Through IRE1α-Mediated Manipulation of the microRNA miR-30a-5p/SOCS1/3 Axis. J. Virol. 92 (22), e00728–e00718. doi: 10.1128/JVI.00728-18
Nicholson, S. E., De Souza, D., Fabri, L. J., Corbin, J., Willson, T. A., Zhang, J. G., et al. (2000). Suppressor of Cytokine Signaling-3 Preferentially Binds to the SHP-2-Binding Site on the Shared Cytokine Receptor Subunit Gp130. Proc. Natl. Acad. Sci. U. S. A. 97 (12), 6493–6498. doi: 10.1073/pnas.100135197
Okemoto, K., Wagner, B., Meisen, H., Haseley, A., Kaur, B., Chiocca, E. A. (2013). STAT3 Activation Promotes Oncolytic HSV1 Replication in Glioma Cells. PLoS One 8 (8), e71932. doi: 10.1371/journal.pone.0071932
Pan, W., Gao, Y., Qin, L., Ni, W., Liu, Z., Yun, B., et al. (2012). Genetic Diversity and Phylogenetic Analysis of Glycoprotein GP85 of ALV-J Isolates From Mainland China Between 1999 and 2010: Coexistence of Two Extremely Different Subgroups in Layers. Vet. Microbiol. 156 (1–2), 205–212. doi: 10.1016/j.vetmic.2011.10.019
Pandiri, A. R., Mays, J. K., Silva, R. F., Hunt, H. D., Reed, W. M., Fadly, A. M. (2010). Subgroup J Avian Leukosis Virus Neutralizing Antibody Escape Variants Contribute to Viral Persistence in Meat-Type Chickens. Avian Dis. 54 (2), 848–856. doi: 10.1637/9085-092309-Reg.1
Payne, L. N., Brown, S. R., Bumstead, N., Howes, K., Frazier, J. A., Thouless, M. E. (1991). A Novel Subgroup of Exogenous Avian Leukosis Virus in Chickens. J. Gen. VirologyJ. Gen. Virol. 72 ( Pt 4), 801–807. doi: 10.1099/0022-1317-72-4-801
Payne, L. N., Howes, K., Gillespie, A. M., Smith, L. M. (1992). Host Range of Rous Sarcoma Virus Pseudotype RSV(HPRS-103) in 12 Avian Species: Support for a New Avian Retrovirus Envelope Subgroup, Designated J. J. Gen. Virol. 73 (Pt 11), 2995–2997. doi: 10.1099/0022-1317-73-11-2995
Payne, L. N., Nair, V. (2012). The Long View: 40 Years of Avian Leukosis Research. Avian Pathol. 41 (1), 11–19. doi: 10.1080/03079457.2011.646237
Rawlings, J. S., Rosler, K. M., Harrison, D. A. (2004). The JAK/STAT Signaling Pathway. J. Cell. Sci. 117 (8), 1281–1283. doi: 10.1242/jcs.00963
Venugopal, K., Smith, L. M., Howes, K., Payne, L. N. (1998). Antigenic Variants of J Subgroup Avian Leukosis Virus: Sequence Analysis Reveals Multiple Changes in the Env Gene. J. Gen. Virol. 79 (Pt 4), 757–766. doi: 10.1099/0022-1317-79-4-757
Wang, X., Jia, Y., Ren, J., Huo, N., Liu, H., Xiao, S., et al. (2019a). Newcastle Disease Virus Nonstructural V Protein Upregulates SOCS3 Expression to Facilitate Viral Replication Depending on the MEK/ERK Pathway. Front. Cell. Infect. Microbiol. 9, 317. doi: 10.3389/fcimb.2019.00317
Wang, X., Jia, Y., Ren, J., Liu, H., Xiao, S., Wang, X., et al. (2019b). MicroRNA gga-miR-455-5p Suppresses Newcastle Disease Virus Replication via Targeting Cellular Suppressors of Cytokine Signaling 3. Vet. Microbiol. 239, 108460. doi: 10.1016/j.vetmic.2019.108460
Wang, Y., Yang, F., Yin, H., He, Q., Lu, Y., Zhu, Q., et al. (2021). Chicken Interferon Regulatory Factor 7 (IRF7) Can Control ALV-J Virus Infection by Triggering Type I Interferon Production Through Affecting Genes Related With Innate Immune Signaling Pathway. Dev. Comp. Immunol. 119, 104026. doi: 10.1016/j.dci.2021.104026
Weiss, R. (2006). The Discovery of Endogenous Retroviruses. Retrovirology 3, 67. doi: 10.1186/1742-4690-3-67
Xie, J., Wang, M., Cheng, A., Zhao, X., Liu, M., Zhu, D., et al. (2019). DHAV-1 Inhibits Type I Interferon Signaling to Assist Viral Adaption by Increasing the Expression of SOCS3. Front. Immunol. 10, 731. doi: 10.3389/fimmu.2019.00731
Ye, H., Duan, X., Yao, M., Kang, L., Li, Y., Li, S., et al. (2021). USP18 Mediates Interferon Resistance of Dengue Virus Infection. Front. Microbiol. 12, 682380. doi: 10.3389/fmicb.2021.682380
Yoshimura, A., Naka, T., Kubo, M. (2007). SOCS Proteins, Cytokine Signalling and Immune Regulation. Nat. Rev. Immunol. 7 (6), 454–465. doi: 10.1038/nri2093
Yu, M., Bao, Y., Wang, M., Zhu, H., Wang, X., Xing, L., et al. (2019). Development and Application of a Colloidal Gold Test Strip for Detection of Avian Leukosis Virus. Appl. Microbiol. Biotechnol. 103 (1), 427–435. doi: 10.1080/03079457.2011.560142
Yun, B., Li, D., Zhu, H., Liu, W., Qin, L., Liu, Z., et al. (2013). Development of an Antigen-Capture Elisa for the Detection of Avian Leukosis Virus P27 Antigen. J. Virol. Methods 187 (2), 278–283. doi: 10.1016/j.jviromet.2012.11.027
Zhang, H., Lai, H., Qi, Y., Zhang, X., Ning, Z., Luo, K., et al. (2011). An ALV-J Isolate Is Responsible For Spontaneous Haemangiomas in Layer Chickens in China. Avian Pathol. 40, 261–267. doi: 10.1080/03079457.2011.560142
Zhang, J., Cai, B., Ma, M., Luo, W., Zhang, Z., Zhang, X., et al. (2020). ALDH1A1 Inhibits Chicken Preadipocytes' Proliferation and Differentiation via the Pparγ Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 21 (9), 3150. doi: 10.3390/ijms21093150
Keywords: ALV-J, chicken, immune, JAK2/STAT3, SOCS3
Citation: Mo G, Fu H, Hu B, Zhang Q, Xian M, Zhang Z, Lin L, Shi M, Nie Q and Zhang X (2021) SOCS3 Promotes ALV-J Virus Replication via Inhibiting JAK2/STAT3 Phosphorylation During Infection. Front. Cell. Infect. Microbiol. 11:748795. doi: 10.3389/fcimb.2021.748795
Received: 28 July 2021; Accepted: 26 August 2021;
Published: 10 September 2021.
Edited by:
Chunfu Zheng, University of Calgary, CanadaReviewed by:
Huaijun Zhou, University of California, Davis, United StatesLing Lian, China Agricultural University, China
Copyright © 2021 Mo, Fu, Hu, Zhang, Xian, Zhang, Lin, Shi, Nie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiquan Zhang, eHF6aGFuZ0BzY2F1LmVkdS5jbg==
†These authors have contributed equally to this work
 Guodong Mo
Guodong Mo Huali Fu1,2†
Huali Fu1,2† Bowen Hu
Bowen Hu Ling Lin
Ling Lin Meiqing Shi
Meiqing Shi Qinghua Nie
Qinghua Nie Xiquan Zhang
Xiquan Zhang