
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 18 June 2021
Sec. Fungal Pathogenesis
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.648988
Immune cells can optimize the management of metabolic resources to balance their energy requirements in order to regulate immune responses. The interconnection between immunometabolism and fungal infections is becoming increasingly apparent. Using proteome and metabolome assays, we found that stimulation of primary human monocytes by Candida albicans was accompanied by upregulation of glucose transporter 3 (GLUT3) and activation of the glycerophospholipid metabolism pathway. Upregulated GLUT3 expression has been preliminarily confirmed in monocytes from patients with C. albicans bloodstream infection. Our findings support the importance of GLUT3 in the complex network of glycerophospholipid metabolism and the innate immune responses against C. albicans. In summary, this study might contribute to decipher the regulatory mechanism between the monocyte metabolic reprogramming and innate immune response and reveal potential targets for the antifungal treatments.
The human immune system is inextricably linked to metabolic systems. These two systems share common regulatory mechanisms that allow for coordination and extensive bidirectional communication (Odegaard and Chawla, 2013). Increased evidence supports the notion that metabolic reprogramming in immune cells is required for the response to microbial infections (Arts et al., 2016; Lachmandas et al., 2016; O’neill and Pearce, 2016; Domínguez-Andrés and Arts, 2017). Candida albicans is an opportunistic pathogen, which represents a severe threat to human life in immunocompromised individuals. Especially when C. albicans causes invasive fungal diseases, morbidity and mortality may exceed 50% even with antifungal therapy (Schultz et al., 2020).
The innate immune system is typically the first-line defense against C. albicans infection, and monocytes play a pivotal role in the process of antifungal immunity. (Qin et al., 2016; Grondman et al., 2019). Monocyte deficiency is associated with higher susceptibility to fungal infections in both mice and humans (Ngo et al., 2014; Ceesay et al., 2015; Domínguez-Andrés and Arts, 2017). When monocytes are stimulated and activated by pathogen-associated molecular patterns (PAMPs), glucose metabolism associated with ATP production is altered from aerobic oxidation to glycolysis (Palmer et al., 2016). While ensuring the energy supply for basic cellular processes, aerobic glycolysis fuels the production of nucleic acids and lipids required for the proliferation and differentiation of monocytes during the immune response (Raulien et al., 2017). However, not all the metabolic changes promote adequate immune responses. Previous studies have indicated that metabolic reprogramming after immune stimulation may be a major mechanism driving immune cell tolerance (Cheng et al., 2016; Kan and Michalski, 2018; Zhu et al., 2019). Moreover, the loss of metabolic plasticity affects the antifungal immune functions of monocytes (Grondman et al., 2019). Therefore, monocyte metabolic remodeling acts as a “double-edged sword” in regulation of the innate immune function.
In order to understand the role of cellular metabolism in the innate immune function of monocytes, human primary monocytes were stimulated with heat-killed C. albicans and proteomic and metabolomic analyses were performed. We found that stimulation of monocytes by C. albicans was accompanied by upregulation of glucose transporter 3 (GLUT3) and activation of the glycerophospholipid metabolism pathway. Upregulated GLUT3 expression has been preliminarily confirmed in monocytes from patients with C. albicans bloodstream infection (BSI) and GLUT3 knockdown by siRNA significantly reduced production of IL-1β in THP-1 cells. In addition, the glycerophospholipid metabolites could promote the localization and glucose uptake function of GLUT3 (Hresko et al., 2016). Our findings support the importance of GLUT3 in the complex network of glycerophospholipid metabolism and the innate immune responses against C. albicans.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood diluted in PBS (1:1) by differential density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare). Cells were washed twice in PBS (HyClone) and resuspended in RPMI 1640 culture medium (HyClone). Monocytes were isolated from the PBMCs using magnetic beads labeled with anti-CD14 without CD16 depletion (STEMCELL).
C. albicans SC5314 (ATCC MYA-2876) was grown on Sabouraud dextrose agar plates to generate yeast cells at 29°C for 36 h. The cells were collected by centrifugation, washed twice with PBS, and heat-killed for 30 min at 95°C. Monocytes (5×106 cells/mL) were stimulated with the heat-killed C. albicans yeast (MOI = 1) for 12 h and 24 h. Stimulated monocytes, including heat-killed yeast, were washed twice with PBS, harvested by centrifugation in sterile centrifuge tubes, snap frozen in liquid nitrogen, and shipped to perform proteomic and metabolomic tests (BIOMS).
Liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis was conducted using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific) combined with an EASY-nLC 1000 nanoflow system (Thermo Fisher Scientific). The MS data were analyzed by MaxQuant software (version 1.5.3.8). The false discovery rate (FDR) was set to 0.01 for both peptide and protein levels based on the q-value. The human Uniprot database (version 05/2019) with 73,939 protein entries was used for the proteomics search. Evaluation of regulatory events between different sample groups was achieved using two-sided Student’s t-tests, and the differentially expressed proteins were defined as P < 0.05, with a fold change ≥ 1.5, or ≤ 0.7. For differential proteins, principal component analysis (PCA) was performed through dimension reduction analysis of the data to detect the differences between the experimental groups and the repeatability within the groups. PCA was performed using Perseus software (version 1.5.5.1), and the diagram was generated using ATP-BioCloud analysis software (http://cloud.aptbiotech.com/#/). Venn diagrams and heat maps representing the normalized abundance of sample groups were drawn using TBTools software (https://github.com/CJ-Chen/TBtools/releases). The raw datasets are available in the ProteomeXchange Consortium (iPROX) repository under accession number PXD024515.
The samples were vortexed for 30 s. After three cycles of liquid nitrogen freezing and thawing, the samples were centrifuged at 12,000 rpm at 4°C for 20 min, and the supernatant was collected for freezing. Methanol was added for refluxing, and the samples were sonicated for 5 min. Finally, the samples were centrifuged at 12,000 rpm at 4°C for 20 min, the supernatant was collected and stored for LC-MS/MS analysis. A quality control (QC) sample was prepared and injected three times at the beginning of the run to ensure equilibration of the system. The QC sample was re-applied between every 10 samples during the run to ensure consistency of the analysis.
Sample analysis was performed on an ExionLC (SCIEX) coupled with a TripleTOF 5600+ (SCIEX) in both positive and negative ionization modes. The raw metabonomic data was imported to Progenesis QI (Waters) for peak alignment to obtain the retention time, molecular mass data (m/z), and peak area of each sample on the peak list. As the first step of EZinfo software analysis, PCA was mainly used to reduce the dimension of the data and to detect the difference between the experimental groups and the repeatability within the groups. Then PCA was performed by Progenesis QI software and the diagram was drawn by ATP-BioCloud analysis software. Differential metabolites were screened using the EZinfo software built-in statistical analysis method. Metabolites selected for further statistical analysis were identified on the basis of a coefficient of variation < 30%, variable importance in the projection (VIP) > 1, and P value < 0.05. The Human Metabolome Database (HMDB) (http://www.hmdb.ca), Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/), and National Institute of Standards and Technology (NIST) database (https://www.nist.gov/srd) were used to align the m/z values to identify metabolites. MetaboAnalyst (https://www.metaboanalyst.ca) was used to identify the metabolic pathways.
We collected samples from age-, sex-, and basic disease-matched populations, excluding C. albicans BSI patients and healthy donors with hyperthyroidism and a history of insulin injection, because these conditions may affect the expression of GLUT3. The demographic information and clinical characteristics of the subjects are listed in Table 1. PBMCs from patients and healthy controls were extracted as described above. Isolated cells were stained with PerCP-conjugated anti-CD3, PE-conjugated anti-CD14 (BioLegend) and FITC-conjugated anti-GLUT3 (R&D Systems) to detect the expression of GLUT3 in monocytes and analyze using FlowJo 10.0 software.
THP-1 human acute monocyte leukemia cells were cultured in RPMI 1640 supplemented with 10% FBS (Gibco). GLUT3 siRNA or negative control (Invitrogen) was transfected to THP-1 cells for 24 h by HiPerFect Transfection Reagent (Qiagen) and incubated with heat-killed C. albicans yeast (MOI = 1) for a further 24 h. The cells were used for RNA extraction, and culture supernatants were collected for analysis of in vitro IL-1β, IL-6, and TNF-α production using the ELISA kit (Elabscience). The siRNA control was represented by a non-specific Stealth RNAi® Negative Control.
GraphPad Prism 8.0 software was used to conduct statistical analyses, and the comparison between protein and metabolite levels was performed using the Student’s t-test. The non-parametric Mann-Whitney U test was used to determine the differences between C. albicans BSI patients and normal controls. Correlations between variables were evaluated using the Spearman rank correlation test. The comparisons between the mRNA level and IL-1β, IL-6, TNF-α secretion were performed using the paired t-test and all P values < 0.05 were considered statistically significant.
Monocytes are innate immune cells that play a pivotal role in antifungal immunity. To better understand the mechanism of monocyte metabolic remodeling and immune regulation, we generated proteomic and metabolomic profiles for primary monocytes from healthy volunteers (n = 5) stimulated with C. albicans for 12 h (Y-12 h) and 24 h (Y-24 h). Proteins were digested and analyzed by label-free LC-MS/MS. In parallel, polar metabolites were extracted from monocytes at each time point and analyzed using non-targeted metabolomics. Furthermore, we employed a bioinformatics analytical approach to identify and analyze the immunometabolic pathways among the different experimental groups (Figure 1).
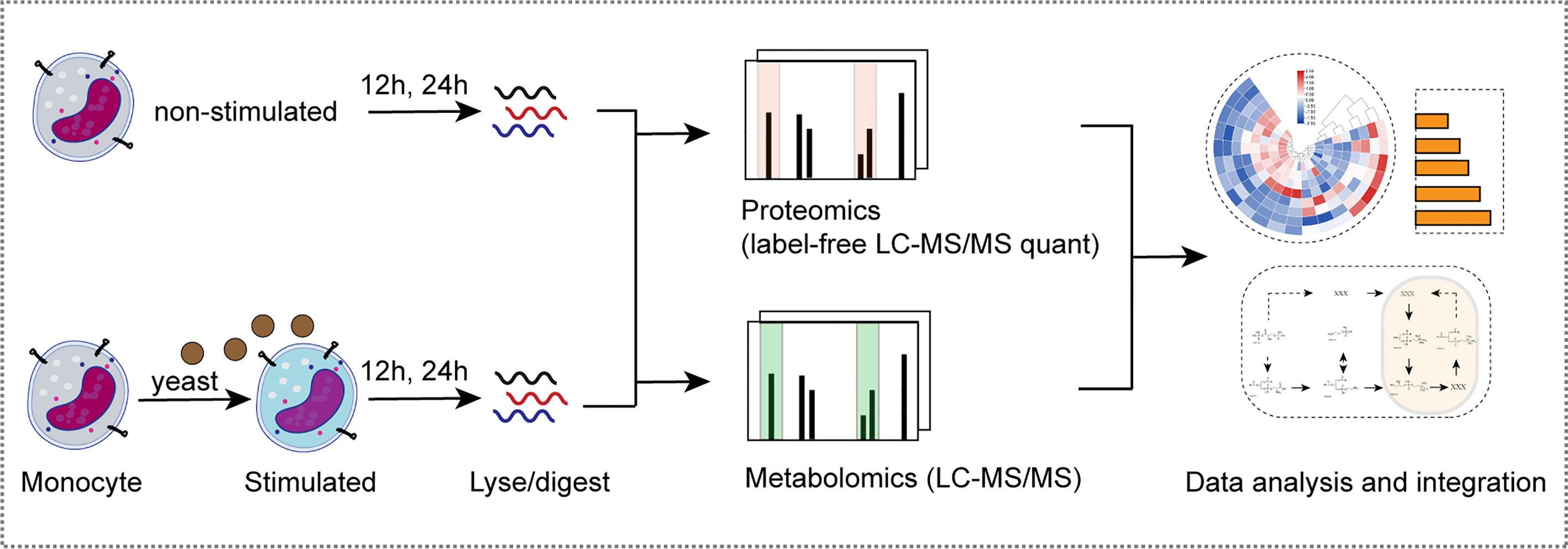
Figure 1 Multi-omics analysis of monocytes stimulated by C. albicans. Schematic view of the experimental investigation of monocytes stimulated by C. albicans.
The PCA of the proteome data, as depicted in Figure 2A, clearly demonstrated the separation of the three different groups. As shown in the Venn diagram, after stimulation with yeast, comparisons of Y-12 h versus unstimulated monocytes (M) and Y-24 h versus M shared 25 differentially expressed proteins (12 upregulated and 13 downregulated), with 48 (20 upregulated and 28 downregulated) and 76 differentially expressed proteins (35 upregulated and 41 downregulated), respectively. The bar graph showed the upregulated and downregulated proteins in monocytes after 12 h and 24 h of stimulation (Figure 2B). Using hierarchical cluster analysis, the differentially expressed proteins for mapping the landscape of the Y-12 h or Y-24 h groups could be distinguished from the unstimulated group (Figures 2C, D). The signaling pathways in monocytes were further selected using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) by filtering for differentially expressed proteins (n ≥ 2), and the FDR was < 0.05. Reactome Pathway and KEGG analyses showed that the differentially expressed proteins were specifically enriched in the innate immune system for both 12 h and 24 h C. albicans-stimulated monocytes (Figures 2E, F).
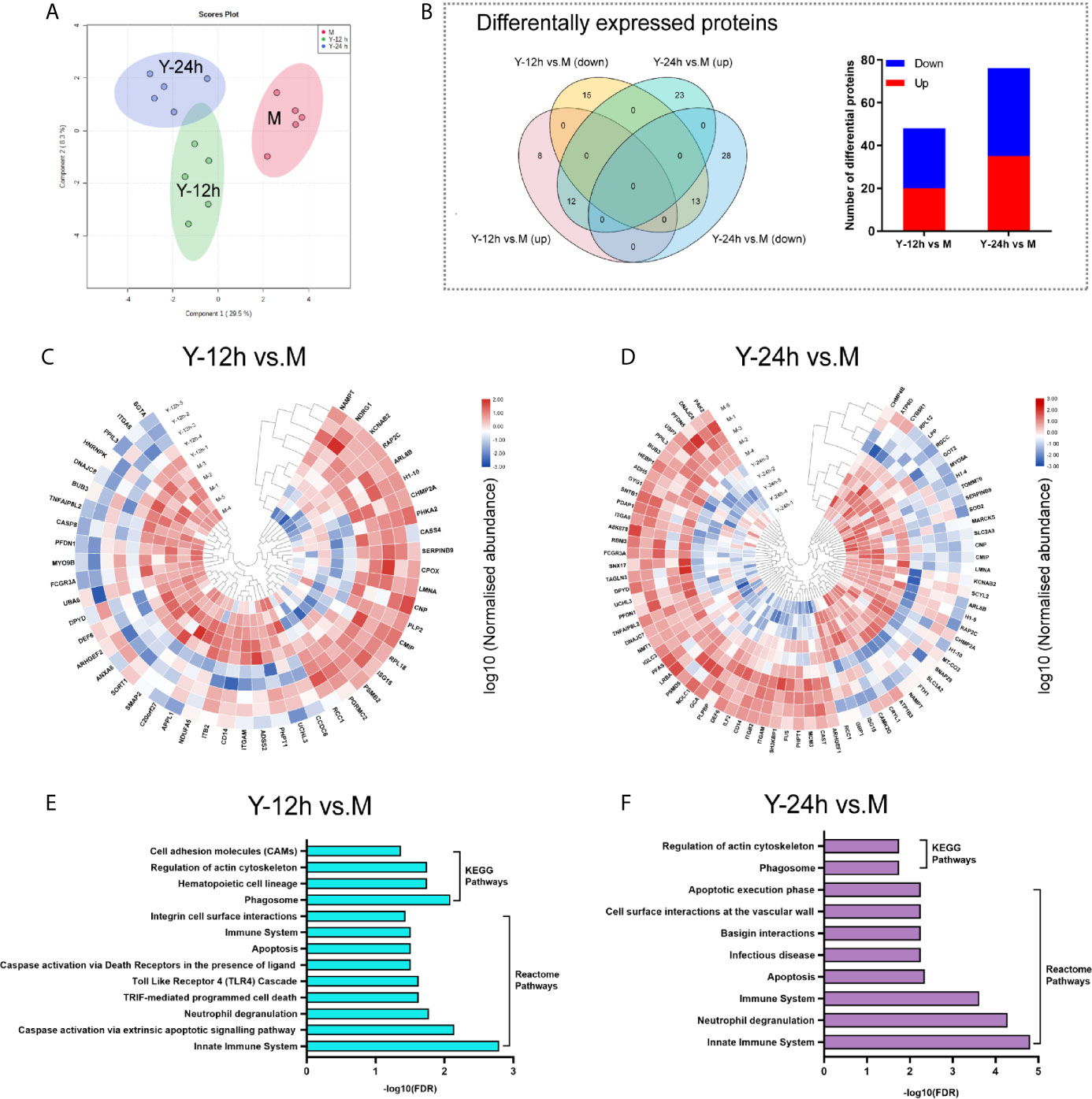
Figure 2 Landscape of proteome analyses. (A) Principal component analysis (PCA) of unstimulated monocytes (M) and monocytes stimulated with heat-killed C. albicans for 12 h (Y-12 h) and 24 h (Y-24 h) (n = 5). (B) Proteins differentially expressed in monocytes after 12 h and 24 h stimulation by heat-killed C. albicans versus M group were displayed in Venn diagrams. Bar graph shows the number of up- (red) and down-regulated (blue) proteins. (C, D) Hierarchical clustering analysis of differential proteins between the Y-12 h or Y-24 h and M group with a P < 0.05 (Student’s t-test). Normalized abundances of proteins were logarithmic transformed. (E, F) Pathway analysis of the differentially expressed proteins in monocytes stimulated by heat-killed C. albicans.
To explore the metabolic changes in monocytes after stimulation by C. albicans, we used the same treatment protocol as that of the proteomics analyses to detect and analyze metabolomics. As shown in Figure 3A, a clear distinction between the M group and monocytes stimulated with C. albicans for 12 h and 24 h was observed in the PCA diagram, which was generated based on the metabolome data. After stimulation with C. albicans, the Y-12 h versus M and Y-24 h versus M comparisons shared 65 changed metabolites, including 52 upregulated and 11 downregulated, and the overlap between the two time points contained two metabolites that shifted from upregulated at 12 h to downregulated at 24 h. Comparisons of Y-12 h versus M and Y-24 h versus M individually showed 84 (68 upregulated and 16 downregulated) and 227 (183 upregulated and 44 downregulated) changed metabolites, respectively (Figure 3B, Venn diagram). The bar graph showed the upregulated and downregulated metabolites in monocytes after 12 h and 24 h of stimulation (Figure 3B). Using hierarchical cluster analysis of the changed metabolites to map a landscape, Y-12 h or Y-24 h monocytes could be clearly distinguished from the M group (Figures 3C, D). The differential metabolites were mainly enriched in the glycerophospholipid metabolic pathway (Figures 3E, F).
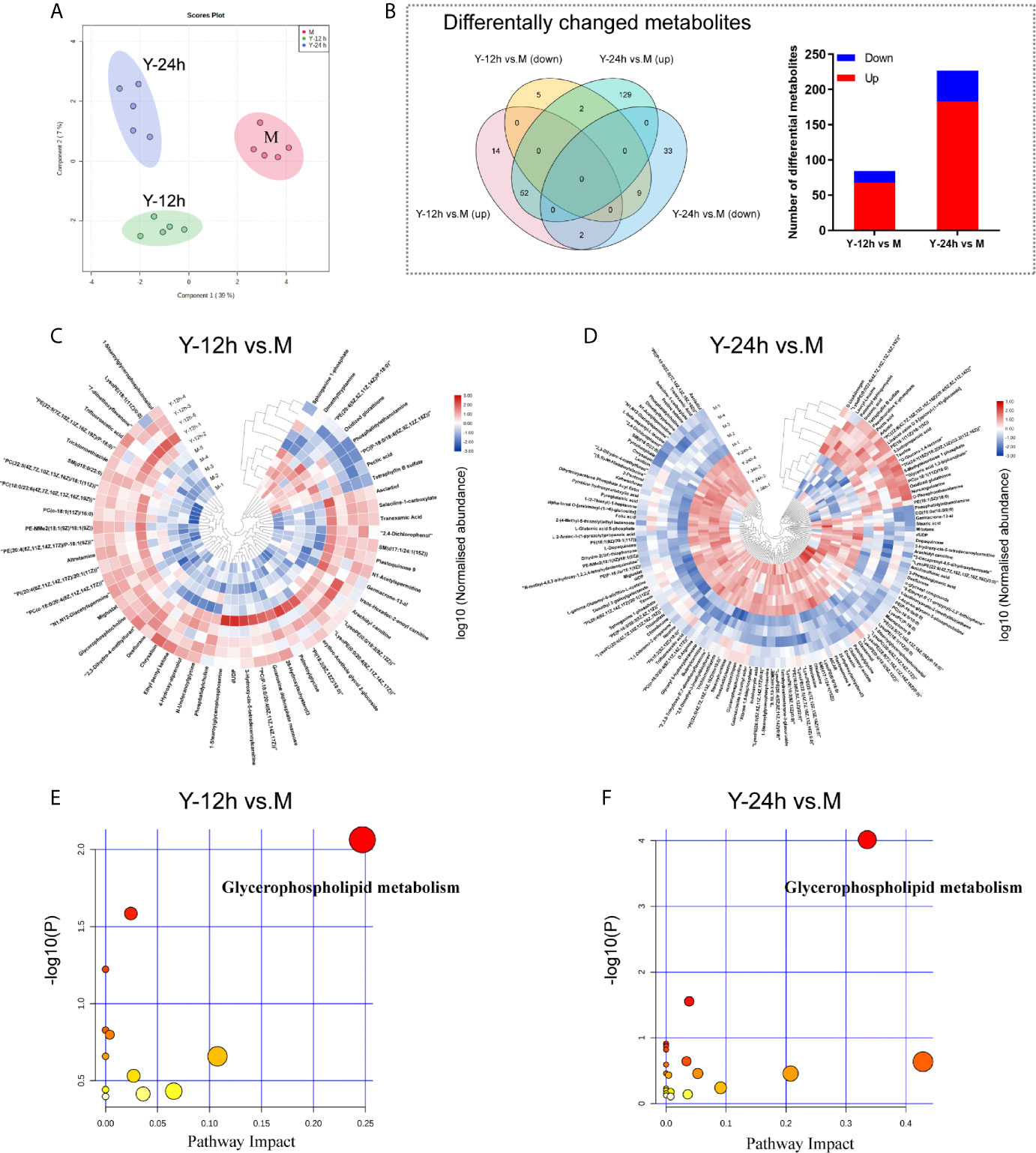
Figure 3 Data-dependent metabonomic analyses. (A) PCA of unstimulated monocytes (M) and monocytes stimulated with heat-killed C. albicans for 12 h (Y-12 h) and 24 h (Y-24 h) (n = 5). (B) Venn diagrams depict the differential metabolites of monocytes after 12 h and 24 h stimulation by heat-killed C. albicans versus M group. Bar graphs show the number of up- (red) and down-regulated (blue) metabolites. (C, D) Hierarchical clustering analysis of differential metabolites between monocytes stimulated by heat-killed C. albicans and the M group. Normalized abundance of metabolites was logarithmic transformed. (E, F) Pathway analysis of the differential metabolites in monocytes stimulated by C. albicans. The following criteria were used to identify relevant pathways: y axis, enrichment significance (P < 0.05, [log (P) > 1.3]) and x axis, pathway impact (> 0.2) for network topology.
Based on the results of the proteomics and metabolomics analyses, the relationship between the innate immune response and glycerophospholipid metabolism requires further exploration. The Y-12 h versus M and Y-24 h versus M groups shared 8 innate immune pathway-related proteins and individually had 10 and 18 differentially expressed proteins, respectively (Figure 4A). The heatmap shows the average fold changes of 20 differentially expressed proteins enriched in the innate immune response pathway. Compared to the M group, 8 differentially expressed proteins showed an increasing trend, while 12 differential proteins decreased (Figure 4B). Correlations between significantly regulated proteins in the innate immune pathway are shown in Figure 4C. Regarding the innate immune response pathway, the five proteins that we focused on are listed in Figure 4D, including four (namely CD14, ITGAM/CD11B, ITGB2/CD18, and CASP8) that were decreased, while GLUT3 was increased.
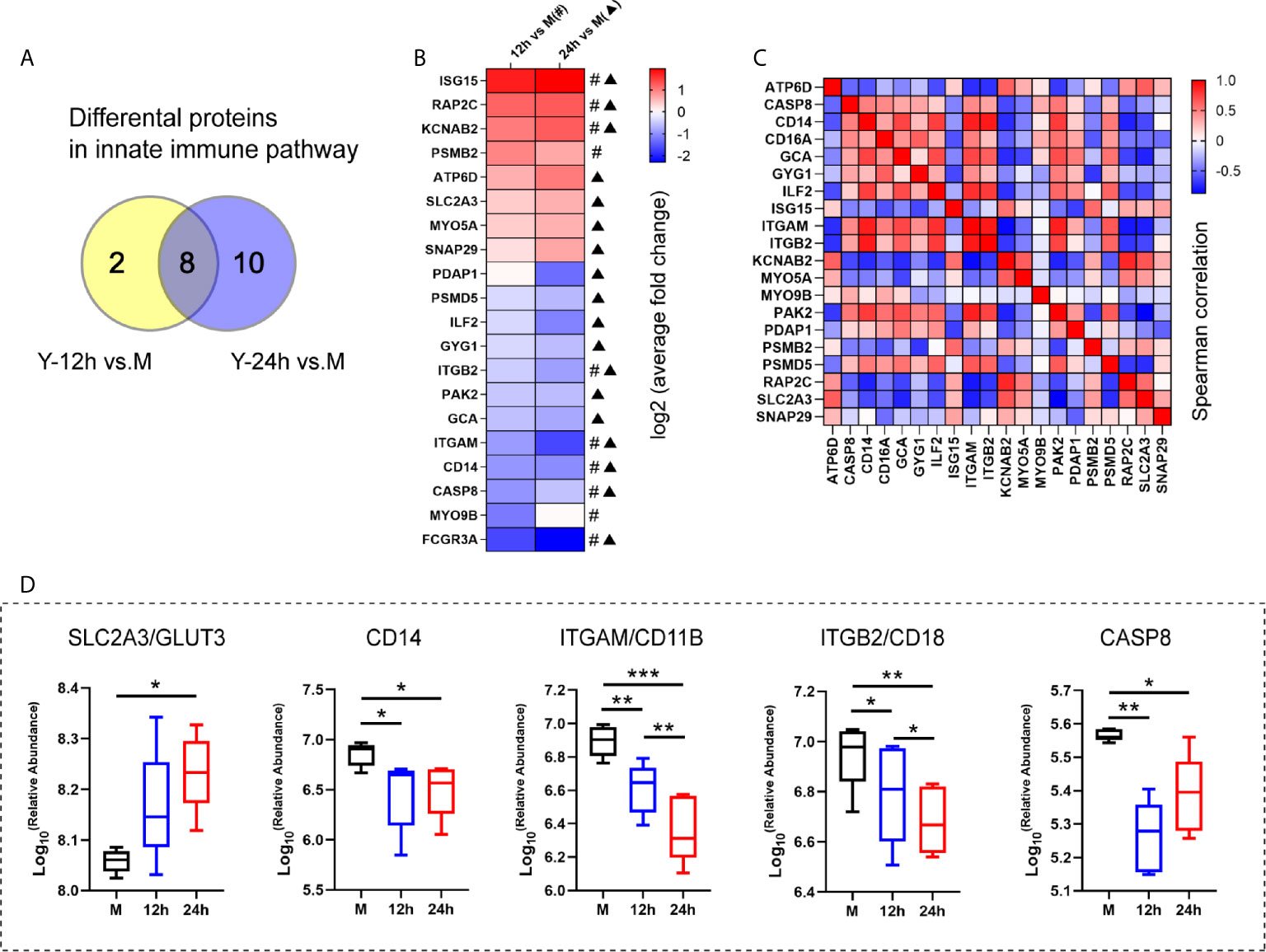
Figure 4 The analysis of the innate immune pathway-related proteins. (A) Venn diagrams depict the differential proteins in innate immune pathway after 12 h and 24 h stimulation by heat-killed C. albicans versus the M group. (B) The average fold changes of 20 differential proteins for those significantly regulated in at least one comparison group (Y-12 h versus M: # or Y-24 h versus M: ▲) were analyzed. The fold changes of proteins were logarithmic transformed. (C) Correlation matrix of innate immune pathway-related proteins. (D) Selected the up-regulated GLUT3 and down-regulated (ITGAM/CD11B, ITGB2/CD18, CD14 and CASP8) in the innate immune pathway are shown. ***P < 0.001; **P < 0.01; *P < 0.05.
To further characterize GLUT3 expression in the monocytes of patients with C. albicans BSI, CD3-CD14+GLUT3+ monocytes from patients (n = 9) and healthy donors (n = 10) were measured by flow cytometry. It was found that, compared to the healthy donors, the monocytes from patients showed upregulated expression of GLUT3 on the surface of the cell membrane (P < 0.0001; Figures 5A, B). To explore the effect of GLUT3 on the immune response of monocytes, siRNA-GLUT3 or negative control was delivered to THP-1 cells and the secretion of IL-1β, IL-6, and TNF-α was detected (Figures 5C–F). After successful knockdown of GLUT3 (P = 0.0035, Figure 5C), the si-GLUT3 and control THP-1 cells were treated with heat-killed C. albicans for 24 h. We found that the secretion of IL-1β in the supernatant decreased in si-GLUT3 group (P = 0.0364, Figure 5D), proving that expression of GLUT3 reductions would reduce the secretion of IL-1β.
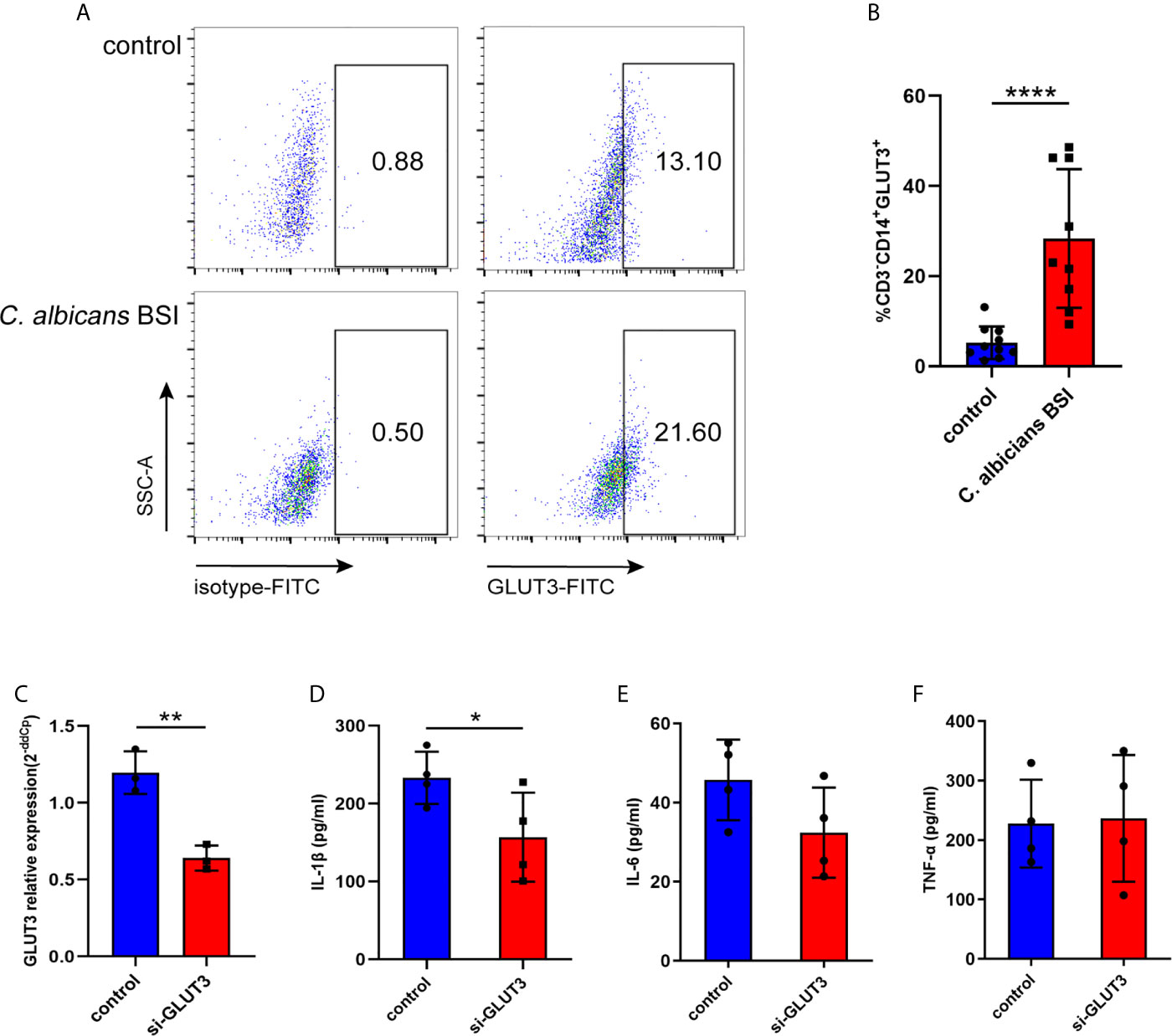
Figure 5 The GLUT3-expression in patients with C. albicans BSI was preliminarily confirmed in monocytes. PBMCs were isolated from patients (n = 9) and healthy controls (n = 10), and then CD3-CD14+GLUT3+ monocytes were separated by flow cytometer and compared using the non-parametric Mann-Whitney test (P < 0.0001). Representative flow cytometry dot plot (A) and summary data are shown (B). GLUT3 knockdown by siRNA significantly reduced production of IL-1β in the THP-1 cells. (C) The GLUT3 mRNA expression achieved by transfection of si-GLUT3 or negative control to THP-1 cells (n = 3). (D–F) Comparison of the secretion of IL-1β, IL-6, and TNF-α in THP-1 cells stimulated by C. albicans for 24 h (n = 4). ****P < 0.0001; **P < 0.01; *P < 0.05.
Metabolomic analyses showed that seven metabolites contributing to glycerophospholipid metabolism, namely phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PtdCho), 1-acyl-sn-glycero-3- phosphocholine (1-acyl-GPC), glycerophosphocholine (GPC), O-phospho-ethanolamine (PEA), and glycericacid-1,3-biphosphate (PGAP), were mainly enriched after 24 h. Among these metabolites, PtdCho and GPC began to increase at 12 h after stimulation (Figure 6A). Our results revealed that the GPC pathway was activated in monocytes stimulated by heat-inactivated C. albicans, and all the metabolites listed above were directly or indirectly involved in the GPC pathway. We found a significant upregulation of the main metabolites involved in the GPC pathway, such as PtdCho, 1-acyl-GPC, and GPC. We also found that increased production of PS via PE was converted into GPC, followed by the conversion of GPC into Cho and transformation into PC and PtdCho (Figure 6B). Furthermore, previous studies have shown that PE promotes GLUT3 activation in monocytes, and PS is essential for GLUT3 function by activating and stabilizing its structure (Hresko et al., 2016). Consequently, these results support the idea that activation of the glycerophospholipid metabolism pathway and related metabolites could promote the function of GLUT3 during the innate immune response in monocytes stimulated with C. albicans.
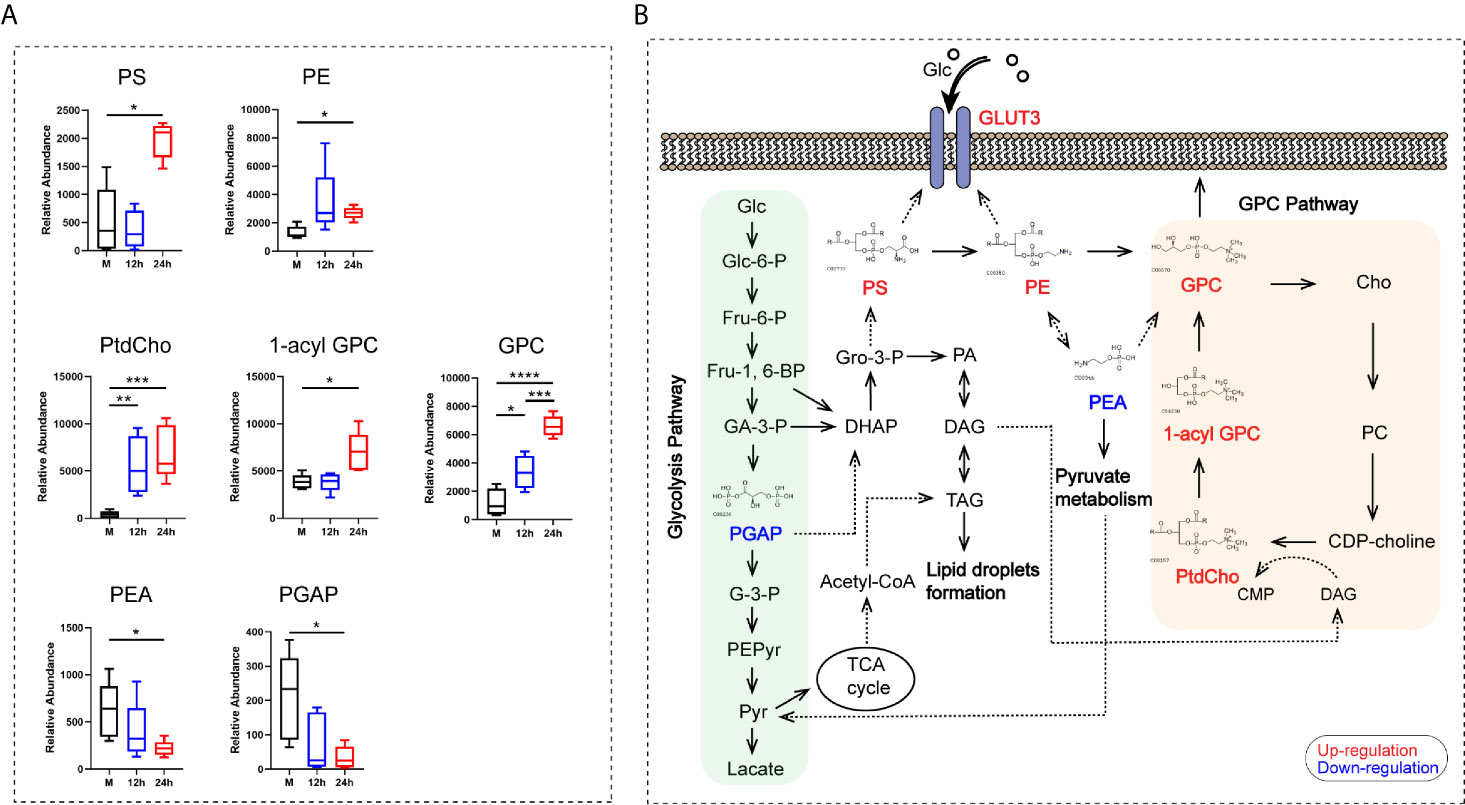
Figure 6 The analysis of the glycerophospholipid metabolism pathway-related metabolites. (A) Relative abundance of seven differential metabolites contributing to the GPC pathway of glycerophospholipid metabolism. (B) Schematic pathway map of the metabolites in the main metabolic pathways in monocytes stimulated with C. albicans. Glc, glucose; Glc-6-P, glucose-6-phosphate; Fru-6-P, fructose-6-phosphate; Fru-1,6-BP, fructose-1,6-bisphosphate; GA-3-P, Glyceraldehyde-3-phosphate; PGAP, glyceric acid 1, 3-biphosphate; G-3-P, 3-phosphoglycerate; PEPyr, phosphoenol- pyruvate; Pyr, pyruvate; DHAP, dihydroxyacetone phosphate; Gro-3-P, glycerol-3- phosphate; PA, phosphatidate; DAG, diacylglycerol; TAG, triacylglyceride; TCA, tricarboxylic acid cycle; PS, phosphatidylserine; PE, phosphatidylethanolamine; PEA, O-phospho-ethanolamine; GPC, glycerophosphocholine; Cho, choline; PC, phosphorcholine; CDP-choline, cytidine diphosphate choline; CMP, cytidine monophosphate; PtdCho, phosphatidylcholine; 1-acyl-GPC, 1-acyl-sn-glycero-3- phosphocholine; For all tests, significance values are denoted as follows: ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05.
C. albicans is the most common opportunistic fungal pathogen that causes a variety of human diseases, ranging from potential oral infections to life-threatening candidemia (Hasim et al., 2018). The development of immunotherapeutic approaches to boost host defense is urgently needed to reduce the mortality of C. albicans BSI (Pfaller and Diekema, 2007). Although increased evidence supports the interconnection between immunometabolism and fungal infections, we are just beginning to understand the metabolic reprogramming associated with the monocyte immune response and its impact on host defense against fungal infections (Tucey et al., 2018; Weerasinghe and Traven, 2020). Here, we describe the changes in proteins and metabolites in human monocytes after stimulation with heat-inactivated C. albicans. Briefly, we observed that monocytes stimulated with C. albicans for 24 h exhibited more significant changes in protein expression and metabolic activity than those stimulated for 12 h. Among these differentially expressed proteins in monocytes, the upregulation of GLUT3 may promote glucose transport and provide the energy needed for the innate immune response. Meanwhile, the structural stability and biological function of GLUT3 requires the participation of various glycerophospholipid metabolites, and concomitant with the activation of glycerophospholipid metabolism. Therefore, we speculate that GLUT3 is a critical intersection that participates in the innate immune response and glycerophospholipid metabolism.
Cellular glucose uptake is mediated by a family of GLUTs, which participate in immune and inflammatory responses and are critical for host defense against pathogens (Lang et al., 2020). GLUT3 is expressed and translocated to the cell membrane in response to a substantial increase in the energy demand associated with cell activation (Fu et al., 2004; Humphrey et al., 2004; Jones et al., 2006; Simpson et al., 2008). During infection, increased GLUT3 expression may help to redistribute glucose as a potential source of energy away from peripheral tissues and toward cells that mediate the immune response, and it is also essential for cell survival (Maratou et al., 2007). In addition, the differentiation of THP-1 monocytes into macrophages was associated with a significant induction of GLUT3 (Fu et al., 2004). GLUT3 is essential for monocytes to absorb energy for immune response function. However, few studies have focused on the relationship between GLUT3 expression and metabolic transformation in monocytes, especially glycerophospholipid metabolism.
Monocytes change their metabolic phenotype from oxidative to glycolytic metabolism when activated in humans (Palmer et al., 2016). However, based on our findings, there was no obvious activation of the pathways associated with glucose metabolism. Instead, the glycerophospholipid metabolism pathway, particularly the GPC pathway, was activated. Here, we briefly describe the basic features of these metabolites in the GPC pathway (Figure 6B). PtdCho is the most abundant phospholipid in mammalian cell membranes (Mountford and Wright, 1988; Sonkar et al., 2019). The first step in the GPC catabolic pathway is the hydrolysis of PtdCho to remove one fatty acid and produce 1-acyl GPC, which is subsequently converted to GPC by removing the second fatty acid, which is then converted to free choline (Cho) (Morash et al., 1988). In the synthesis pathway, free Cho is phosphorylated to phosphocholine (PC) and then converted to cytidinediphosphate (CDP)-choline to produce PtdCho, thus completing the GPC cycle (Sonkar et al., 2019).
The GPC metabolic pathway interacts with multiple biochemical pathways (Sonkar et al., 2019). PGAP is not only involved in glycerophosphate metabolism, but also is an important metabolite in glycolysis. Decreased expression of PGAP indicates that glycerol-3-phosphate (GA-3-P) tends to produce glycerol-3-phosphate (Gro-3-P) via dihydroxyacetone phosphate (DHAP), which promotes the synthesis of PS, PE, and GPC. As a product of fructose-1,6-bisphosphate (Fru-1,6-BP) in glycolysis, Gro-3-P is converted to phosphatidate (PA), which reversibly gets converted to diacylglycerol (DAG) or triacylglyceride (TAG), and then promotes the formation of lipid droplets. The DAG formed in this pathway was subsequently incorporated into the de novo synthesis of PtdCho (Sonkar et al., 2019). In addition, PEA participates in the pyruvate metabolism pathway and in the synthesis of PE and GPC. However, PEA and PGAP are indirectly involved in the synthesis of PS and PE, respectively, resulting in a decrease in their abundance (Figure 6B).
Previous studies have demonstrated that PE promoted GLUT3 activation in monocytes, and PS was found to be essential for GLUT3 function by activating and stabilizing its structure (Hresko et al., 2016). In this study, we found that PE and PS increased in monocytes after stimulation by heat-inactivated C. albicans, and both PE and PS were involved in the synthesis of GPC, promoting GPC cycling. These results suggest that PE and PS participate in the activation of GLUT3 via the GPC pathway, which contributes to the expression of GLUT3, localized at the cell membrane, thus facilitating immune response function. This prompted us to investigate the role of GLUT3 in the antifungal effector functions of monocytes against C. albicians infection. We found that downregulated GLUT3 significantly reduced the secretion of IL-1β in THP-1 cells stimulated with C. albicans. Pro-inflammatory cytokines IL-1β have been identified as integral components of human monocytes anti-fungal immune defenses (Ganesan et al., 2014; Gaidt et al., 2016).
The metabolic changes that we observed may correlate with the innate immune response after C. albicans stimulation. The downregulation proteins, such as ITGAM/CD11B, ITGB2/CD18, and CD14, involved in the innate immune pathway of monocytes, participate in the Toll-like receptor 4 (TLR4) signaling pathway. TLR4 is one of the receptors involved in the recognition of C. albicans and promotes the innate immune response (Ferwerda et al., 2008). TLR4 has been shown to be involved in proinflammatory responses in monocytes, macrophages, and dendritic cells, specifically, these immune cells combat C. albicans and require the release of cytokines that tend to rely on TLR4 pathway (Blasi et al., 2005; Qin et al., 2016; Wang et al., 2019). CD11B/CD18 (Mac-1/Complement Receptor 3, CR3) is an integrin molecule that is expressed on the surface of monocytes and plays an important role in their trafficking ability (Panni and Herndon, 2019). CD11B can modulate a variety of biological processes in innate immune cells, including the generation of type I interferon and proinflammatory cytokines (Khan et al., 2018). Notably, CD11b has also been implicated in the recognition of fungal β-glucan, which is a key PAMP located at the surface of C. albicans (Ballou et al., 2016). However, the downregulation of the inflammatory proteases caspase-8 (CASP8) levels may contribute to a reduction in the receptor-mediated apoptosis process (Mandal et al., 2020). A strong requirement for CASP8 and CR3 was also found for NLRP3 dependent IL-1β production in response to heat killed C. albicans (Ganesan et al., 2014).
Among the upregulated proteins that function in the innate immune response (Figure S1 in the Supplementary Materials), ISG15 is known as an immunomodulator that controls fungal infection and triggers the expression of proinflammatory cytokines (Dong et al., 2017; Liu and Lee, 2021). RAP2C is a member of the Ras GTPase superfamily, which acts as a molecular switch to regulate cellular movement, proliferation, differentiation, and apoptosis (Wang et al., 2020; Zhu et al., 2020). KCNAB2 is a voltage-gated potassium channel subunit beta-2 that promotes expression of the pore-forming alpha subunits at the cell membrane and plays an important role in the regulation of membrane potential, therefore, it is important for the absorption of glucose, amino acids, and long-chain fatty acids (Mcdaniel et al., 2001; Wongdee et al., 2009). Both KCNAB2 and GLUT3 are membrane channel proteins that are important for glucose absorption. These results indicate that elevated levels of ISG15, RAP2C, and KCNAB2 are beneficial to the immune response to resist C. albicans infection, however, whether these proteins are related to the metabolites of glycerophospholipids requires further study.
Of note, the GPC pathway was activated gradually over 24 h. Previous research based on transcriptomic analysis has confirmed that the expression levels of several main enzymes involved in the glycolysis pathways were significantly upregulated, but this enhancement occurred after stimulation with heat-inactivated C. albicans for 24 h (Domínguez-Andrés and Arts, 2017). In our study, the protein abundance of GLUT3 also increased significantly after 24 h of stimulation. Monocyte activation and immune responses are processes that require high energy consumption and the metabolic transformation of monocytes needs to go through an interim stage (Palmer et al., 2016). To allow cells to adapt to these changes, an energy deficiency period is necessary to reduce energy consumption and ensure cell survival, resulting in dampening of the innate immune response. The expression and localization of GLUT3 could provide a channel for increased glucose uptake by monocytes and mobilize the metabolism of glycerophospholipids as a part of the signal transduction pathway.
In this study, we used heat-killed C. albicans to avoid the growth of live C. albicans that would consume the monocyte growth medium and lead to erroneous results, thereby reducing interference and clarifying the metabolic state of monocytes. However, heat-killed C. albicans did not elicit the same response as the metabolically active fungal cells. Thus, our results may not reflect what occurs in vivo. Therefore, the key proteins of the innate immune response and metabolites in the glycerophospholipid pathway need to be verified in the early stage of monocyte stimulation by C. albicans in vivo (infection model of mice). The mechanism of metabolism and the innate immune response of monocytes stimulated by C. albicans requires further validation.
There is a complex regulatory network between the innate immune system and cell metabolism. Loss of cellular energy homeostasis may occur during the process of monocyte metabolic remodeling, eventually accounting for reduced antifungal immune response. Our findings suggest that GLUT3, as an intersection of glycerophospholipid metabolism and innate immunity, may promote energy absorption and restore the functional immune response. Clarifying the immunometabolic mechanism of the monocyte response to C. albicans infections is of great significance for exploring novel potential therapeutic targets.
The data presented in the study is deposited in ProteomeXchange, accession number: PXD024515.
The studies involving human participants were reviewed and approved by Ethics Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (JS-2782). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
XW conceived and designed the experiment. GZ and W-HY contributed reagents/materials/analysis tools. XW and J-TC performed the experiments. XW analyzed the data and wrote the manuscript. Y-CX, MX, and LZ revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81971979, 81802049) and Beijing Key Clinical Specialty for Laboratory Medicine-Excellent Project (No. ZK201000).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.648988/full#supplementary-material
Arts, R. J., Joosten, L. A., Netea, M. G. (2016). Immunometabolic Circuits in Trained Immunity. Semin. Immunol. 28, 425–430. doi: 10.1016/j.smim.2016.09.002
Ballou, E. R., Avelar, G. M., Childers, D. S., Mackie, J., Bain, J. M., Wagener, J., et al. (2016). Lactate Signalling Regulates Fungal β-Glucan Masking and Immune Evasion. Nat. Microbiol. 2, 16238. doi: 10.1038/nmicrobiol.2016.238
Blasi, E., Mucci, A., Neglia, R., Pezzini, F., Colombari, B., Radzioch, D., et al. (2005). Biological Importance of the Two Toll-Like Receptors, TLR2 and TLR4, in Macrophage Response to Infection With Candida Albicans. FEMS Immunol. Med. Microbiol. 44, 69–79. doi: 10.1016/j.femsim.2004.12.005
Ceesay, M. M., Desai, S. R., Berry, L., Cleverley, J., Kibbler, C. C., Pomplun, S., et al. (2015). A Comprehensive Diagnostic Approach Using Galactomannan, Targeted β-D-Glucan, Baseline Computerized Tomography and Biopsy Yields a Significant Burden of Invasive Fungal Disease in at Risk Haematology Patients. Br. J. Haematol. 168, 219–229. doi: 10.1111/bjh.13114
Cheng, S. C., Scicluna, B. P., Arts, R. J., Gresnigt, M. S., Lachmandas, E., Giamarellos-Bourboulis, E. J. (2016). Broad Defects in the Energy Metabolism of Leukocytes Underlie Immunoparalysis in Sepsis 17, 406–413. doi: 10.1038/ni.3398
Domínguez-Andrés, J., Arts, R. J. W. (2017). Rewiring Monocyte Glucose Metabolism Via C-type Lectin Signaling Protects Against Disseminated Candidiasis. PLoS Pathog. 13, e1006632. doi: 10.1371/journal.ppat.1006632
Dong, C., Gao, N., Ross, B. X., Yu, F. X. (2017). ISG15 in Host Defense Against Candida Albicans Infection in a Mouse Model of Fungal Keratitis. Invest. Ophthalmol. Vis. Sci. 58, 2948–2958. doi: 10.1167/iovs.17-21476
Ferwerda, G., Meyer-Wentrup, F., Kullberg, B. J., Netea, M. G., Adema, G. J. (2008). Dectin-1 Synergizes With TLR2 and TLR4 for Cytokine Production in Human Primary Monocytes and Macrophages. Cell Microbiol. 10, 2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x
Fu, Y., Maianu, L., Melbert, B. R., Garvey, W. T. (2004). Facilitative Glucose Transporter Gene Expression in Human Lymphocytes, Monocytes, and Macrophages: A Role for GLUT Isoforms 1, 3, and 5 in the Immune Response and Foam Cell Formation. Blood Cells Mol. Dis. 32, 182–190. doi: 10.1016/j.bcmd.2003.09.002
Gaidt, M. M., Ebert, T. S., Chauhan, D., Schmidt, T., Schmid-Burgk, J. L., Rapino, F., et al. (2016). Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 44, 833–846. doi: 10.1016/j.immuni.2016.01.012
Ganesan, S., Rathinam, V., Bossaller, L., Army, K., Kaiser, W. J., Mocarski, E. S., et al. (2014). Caspase-8 Modulates Dectin-1 and Complement Receptor 3-Driven IL-1β Production in Response to β-Glucans and the Fungal Pathogen, Candida Albicans. J. Immunol. 193, 2519–2530. doi: 10.4049/jimmunol.1400276
Grondman, I., Arts, R. J. W., Koch, R. M., Leijte, G. P., Gerretsen, J., Bruse, N., et al. (2019). Frontline Science: Endotoxin-Induced Immunotolerance Is Associated With Loss of Monocyte Metabolic Plasticity and Reduction of Oxidative Burst. J. Leukoc. Biol. 106, 11–25. doi: 10.1002/JLB.5HI0119-018R
Hasim, S., Vaughn, E. N., Donohoe, D., Gordon, D. M., Pfiffner, S., Reynolds, T. B. (2018). Influence of Phosphatidylserine and Phosphatidylethanolamine on Farnesol Tolerance in Candida Albicans. Yeast 35, 343–351. doi: 10.1002/yea.3297
Hresko, R. C., Kraft, T. E., Quigley, A., Carpenter, E. P., Hruz, P. W. (2016). Mammalian Glucose Transporter Activity Is Dependent Upon Anionic and Conical Phospholipids. J. Biol. Chem. 291, 17271–17282. doi: 10.1074/jbc.M116.730168
Humphrey, B. D., Stephensen, C. B., Calvert, C. C., Klasing, K. C. (2004). Glucose and Cationic Amino Acid Transporter Expression in Growing Chickens (Gallus Gallus Domesticus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 138, 515–525. doi: 10.1016/j.cbpb.2004.06.016
Jones, K. S., Fugo, K., Petrow-Sadowski, C., Huang, Y., Bertolette, D. C., Lisinski, I., et al. (2006). Human T-Cell Leukemia Virus Type 1 (HTLV-1) and HTLV-2 Use Different Receptor Complexes to Enter T Cells. J. Virol. 80, 8291–8302. doi: 10.1128/JVI.00389-06
Kan, B., Michalski, C. (2018). Cellular Metabolism Constrains Innate Immune Responses in Early Human Ontogeny. Nat. Commun. 9, 4822. doi: 10.1038/s41467-018-07215-9
Khan, S. Q., Khan, I., Gupta, V. (2018). Cd11b Activity Modulates Pathogenesis of Lupus Nephritis. Front. Med. (Lausanne) 5, 52. doi: 10.3389/fmed.2018.00052
Lachmandas, E., Beigier-Bompadre, M., Cheng, S. C., Kumar, V., Van Laarhoven, A., Wang, X., et al. (2016). Rewiring Cellular Metabolism Via the AKT/mTOR Pathway Contributes to Host Defence Against Mycobacterium Tuberculosis in Human and Murine Cells. Eur. J. Immunol. 46, 2574–2586. doi: 10.1002/eji.201546259
Lang, F., Singh, Y., Salker, M. S., Ma, K., Pandyra, A. A., Lang, P. A., et al. (2020). Glucose Transport in Lymphocytes. Pflugers Arch. 472, 1401–1406. doi: 10.1007/s00424-020-02416-y
Liu, G., Lee, J. H., Parker, Z. M., Acharya, D., Chiang, J. J., van Gent, M., et al (2021). ISG15-Dependent Activation of the Sensor MDA5 Is Antagonized by the SARS-CoV-2 Papain-Like Protease to Evade Host Innate Immunity. Nat. Microbiol. 6, 467–478 doi: 10.1101/2020.10.26.356048
Mandal, R., Barrón, J. C., Kostova, I., Becker, S., Strebhardt, K. (2020). Caspase-8: The Double-Edged Sword. Biochim. Biophys. Acta Rev. Cancer 1873, 188357. doi: 10.1016/j.bbcan.2020
Maratou, E., Dimitriadis, G., Kollias, A., Boutati, E., Lambadiari, V., Mitrou, P., et al. (2007). Glucose Transporter Expression on the Plasma Membrane of Resting and Activated White Blood Cells. Eur. J. Clin. Invest. 37, 282–290. doi: 10.1111/j.1365-2362.2007.01786.x
Mcdaniel, S. S., Platoshyn, O., Yu, Y., Sweeney, M., Miriel, V. A., Golovina, V. A., et al. (2001). Anorexic Effect of K+ Channel Blockade in Mesenteric Arterial Smooth Muscle and Intestinal Epithelial Cells. J. Appl. Physiol. (1985) 91, 2322–2333. doi: 10.1152/jappl.2001.91.5.2322
Morash, S. C., Cook, H.W., Spence, M. W. (1988). Lysophosphatidylcholine as An Intermediate in Phosphatidylcholine Metabolism and Glycerophosphocholine Synthesis in Cultured Cells: An Evaluation of The Roles of 1-Acyl- and 2-Acyl-Lysophosphatidylcholine. Biochim. Biophys. Acta. 1004, 221–229.doi: 10.1016/0005-2760(89)90271-3
Mountford, C. E., Wright, L. C. (1988). Organization of Lipids in the Plasma Membranes of Malignant and Stimulated Cells: A New Model. Trends. Biochem. Sci. 13, 172–177. doi: 10.1016/0968-0004(88)90145-4
Ngo, L. Y., Kasahara, S., Kumasaka, D. K., Knoblaugh, S. E., Jhingran, A., Hohl, T. M. (2014). Inflammatory Monocytes Mediate Early and Organ-Specific Innate Defense During Systemic Candidiasis. J. Infect. Dis. 209, 109–119. doi: 10.1093/infdis/jit413
Odegaard, J. I., Chawla, A. (2013). The Immune System as a Sensor of the Metabolic State. Immunity 38, 644–654. doi: 10.1016/j.immuni.2013.04.001
O’neill, L. A., Pearce, E. J. (2016). Immunometabolism Governs Dendritic Cell and Macrophage Function. J. Exp. Med. 213, 15–23. doi: 10.1084/jem.20151570
Palmer, C. S., Cherry, C. L., Sada-Ovalle, I., Singh, A., Crowe, S. M. (2016). Glucose Metabolism in T Cells and Monocytes: New Perspectives in HIV Pathogenesis. EBioMedicine 6, 31–41. doi: 10.1016/j.ebiom.2016.02.012
Panni, R. Z., Herndon, J. M. (2019). Agonism of CD11b Reprograms Innate Immunity to Sensitize Pancreatic Cancer to Immunotherapies. Sci. Transl. Med. 11, eaau9240. doi: 10.1126/scitranslmed.aau9240
Pfaller, M. A., Diekema, D. J. (2007). Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 20, 133–163. doi: 10.1128/CMR.00029-06
Qin, Y., Zhang, L., Xu, Z., Zhang, J., Jiang, Y. Y., Cao, Y., et al. (2016). Innate Immune Cell Response Upon Candida Albicans Infection. Virulence 7, 512–526. doi: 10.1080/21505594.2016.1138201
Raulien, N., Friedrich, K., Strobel, S., Rubner, S., Baumann, S., Von Bergen, M., et al. (2017). Fatty Acid Oxidation Compensates for Lipopolysaccharide-Induced Warburg Effect in Glucose-Deprived Monocytes. Front. Immunol. 8, 609. doi: 10.3389/fimmu.2017.00609
Schultz, C. M., Goel, A., Dunn, A., Knauss, H., Huss, C., Launder, D., et al. (2020). Stepping Up to the Plate(let) Against Candida Albicans. Infect. Immun. 88, e00784–19. doi: 10.1128/IAI
Sonkar, K., Ayyappan, V., Tressler, C. M., Adelaja, O., Cai, R., Cheng, M., et al. (2019). Focus on The Glycerophosphocholine Pathway in Choline Phospholipid Metabolism of Cancer. NMR Biomed. 32, e4112. doi: 10.1002/nbm.4112
Simpson, I. A., Dwyer, D., Malide, D., Moley, K. H., Travis, A., Vannucci, S. J. (2008). The Facilitative Glucose Transporter GLUT3: 20 Years of Distinction. Am. J. Physiol. Endocrinol. Metab. 295, E242–E253. doi: 10.1152/ajpendo.90388.2008
Tucey, T. M., Verma, J., Harrison, P. F., Snelgrove, S. L., Lo, T. L., Scherer, A. K., et al. (2018). Glucose Homeostasis Is Important for Immune Cell Viability During Candida Challenge and Host Survival of Systemic Fungal Infection. Cell Metab. 27, 988–1006.e1007. doi: 10.1016/j.cmet.2018.03.019
Wang, W., Deng, Z., Wu, H., Zhao, Q., Li, T., Zhu, W., et al. (2019). A Small Secreted Protein Triggers a TLR2/4-Dependent Inflammatory Response During Invasive Candida Albicans Infection. Nat. Commun. 10, 1015. doi: 10.1038/s41467-019-08950-3
Wang, J. C., Lee, J. Y., Dang-Lawson, M., Pritchard, C. (2020). The Rap2c Gtpase Facilitates B Cell Receptor-Induced Reorientation of the Microtubule-Organizing Center. Small GTPases. 11, 402–412. doi: 10.1080/21541248.2018.1441626
Weerasinghe, H., Traven, A. (2020). Immunometabolism in Fungal Infections: The Need to Eat to Compete. Curr. Opin. Microbiol. 58, 32–40. doi: 10.1016/j.mib.2020.07.001
Wongdee, K., Teerapornpuntakit, J., Riengrojpitak, S., Krishnamra, N., Charoenphandhu, N. (2009). Gene Expression Profile of Duodenal Epithelial Cells in Response to Chronic Metabolic Acidosis. Mol. Cell. Biochem. 321, 173–188. doi: 10.1007/s11010-008-9931-1
Zhu, X., Meyers, A., Long, D., Ingram, B., Liu, T., Yoza, B. K., et al. (2019). Frontline Science: Monocytes Sequentially Rewire Metabolism and Bioenergetics During an Acute Inflammatory Response. J. Leukoc. Biol. 105, 215–228. doi: 10.1002/JLB.3HI0918-373R
Keywords: GLUT3, Candida albicans, innate immune response, glycerophospholipid metabolism, immunometabolic
Citation: Wu X, Zhang G, Yang W-H, Cui J-T, Zhang L, Xiao M and Xu Y-C (2021) GLUT3 as an Intersection of Glycerophospholipid Metabolism and the Innate Immune Response to Candida albicans. Front. Cell. Infect. Microbiol. 11:648988. doi: 10.3389/fcimb.2021.648988
Received: 03 January 2021; Accepted: 28 May 2021;
Published: 18 June 2021.
Edited by:
Salomé LeibundGut-Landmann, University of Zurich, SwitzerlandReviewed by:
Rebecca Anne Hall, University of Kent, United KingdomCopyright © 2021 Wu, Zhang, Yang, Cui, Zhang, Xiao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Chun Xu, eHljcHVtY2hAMTM5LmNvbQ==; Meng Xiao, Y2p0Y3hpYW9tZW5nQGFsaXl1bi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.