- Aix-Marseille Université, CNRS, Laboratoire de Chimie Bactérienne, Institut de Microbiologie de la Méditerranée, Marseille, France
Over the last decade, an increasing number of reports presented Galleria mellonella larvae as an important model to study host-pathogen interactions. Coherently, increasing information became available about molecular mechanisms used by this host to cope with microbial infections but few of them dealt with oxidative stress. In this work, we addressed the role of reactive oxygen species (ROS) produced by the immune system of G. mellonella to resist against Salmonella enterica, an intracellular pathogen responsible for a wide range of infections. We confirmed that Salmonella was pathogen for G. mellonella and showed that it had to reach a minimal bacterial load within the hemolymph to kill the larvae. ROS production by G. mellonella was revealed by the virulence defects of Salmonella mutants lacking catalases/peroxiredoxins or cytoplasmic superoxide dismutases, both strains being highly sensitive to these oxidants. Finally, we used bacterial transcriptional fusions to demonstrate that hydrogen peroxide (H2O2) was produced in the hemolymph of Galleria during infection and sensed by S. enterica. In line with this observation, the H2O2-dependent regulator OxyR was found to be required for bacterial virulence in the larvae. These results led us to conclude that ROS production is an important mechanism used by G. mellonella to counteract bacterial infections and validate this host as a relevant model to study host-pathogen interactions.
Introduction
The oxidative burst is one of the major mechanisms of the host innate immune system and the ability of pathogens to cope with this stress is often correlated with their virulence; that’s why studying such resistance mechanism is of primary importance to assess bacterial pathogenicity. In vivo assays are generally carried out in mammalian models, which are expensive, require significant expertise and a secure experimental environment. They also become ethically and socially controversial, leading to the emergence of alternative models. Among them, Galleria mellonella larva is of increasing interest. This lepidopteran was first used in the 1960s to test the virulence of a wide variety of microorganisms, from bacteria to fungi and viruses (Kurstak and Vega, 1968; Lysenko and Kucera, 1968; Younghusband and Lee, 1969). Over the last decade, it has become an attractive model in the field of host-pathogen interactions (Repizo et al., 2015; Cools et al., 2018; Pereira et al., 2018; Sciuto et al., 2018; Candela et al., 2019; Barros et al., 2019). And more recently, a high-throughput screening was carried out in G. mellonella to evaluate the synergy between antibiotics, human drugs and food additives on a various bacteria (Brochado et al., 2018).
Despite increasing information dealing with Galleria mellonella antibacterial mechanisms, the characterization of ROS production by the immune system remains elusive. In Lepidoptera, two pathways were proposed to produce free radicals: the humoral response through the production of melanin and the cellular response through phagocytosis and assembly of the NADPH oxidase (Sugumaran, 2002; Bergin et al., 2005). Oxidation reactions were first detected into free cells of Galleria hemolymph by Electron Spin Resonance spectroscopy but neither exogenous superoxide dismutase (SOD), nor phagocytosis activators were found to change the oxidation level (Slepneva et al., 1999). The Kavanagh group also demonstrated that the kinetics of phagocytosis and microbial killing were similar in Galleria hemocytes and human neutrophils (Bergin et al., 2005). Superoxide production and microbial killing were inhibited in the presence of an NADPH oxidase inhibitor, and immunoblotting of G. mellonella hemocytes with antibodies raised against human neutrophil phox proteins revealed the presence of proteins homologous to gp91phox and p67phox (Bergin et al., 2005). Nevertheless, these studies were conducted ex vivo and led to two open questions: (i) Does Galleria immune system produces ROS during bacterial infection and (ii) is this mechanism efficient to kill pathogens?
To answer these questions, we used Salmonella enterica serovar Typhimurium as a bacterial model. This facultative intracellular bacterium causes a wide range of infections and exhibits a broad host spectrum for various living organisms in which its virulence can be easily tested. Despite the fact that Salmonella is not a natural pathogen for Galleria, this bacteria was used for the first time in the host model Galleria mellonella in 1968 and showed to be pathogen for the larvae (Kurstak and Vega, 1968). After a gap of 45 years in literature, new sets of experiments using Galleria were conducted in the 2010s to characterize or confirm the role of Salmonella virulence factors, such as PhoQ activity and LPS (Bender et al., 2013), Rnases E and III (Viegas et al., 2013) or AcrB efflux function (Wang-Kan et al., 2017).
In the present work, we took advantage of the facile genetics approach and extensive literature of Salmonella enterica to address the implication of ROS produced by the immune system of G. mellonella. S. enterica produces an arsenal of detoxifying enzymes, which differ by their cellular location and substrate specificity. SODs allow the dismutation of superoxide into hydrogen peroxide (H2O2). SodA and SodB are located in the bacterial cytoplasm whereas SodCI and SodCII are located the periplasm (Tsolis et al., 1995; Fang et al., 1999; Krishnakumar et al., 2004). Furthermore, three catalases and two peroxidases are involved in H2O2 degradation within the cytoplasm (Hébrard et al., 2009). Inactivation of these five genes yielded the HpxF mutant, which exhibits a severe survival defect within macrophages and mice (Hébrard et al., 2009). Therefore, Salmonella relies on its capacity to metabolize and to degrade ROS produced by the host to cope with oxidative stress.
In this study, we confirmed that Salmonella was a pathogen for G. mellonella and we showed that it had to reach a threshold inside the hemolymph to kill the host. Virulence defects of Salmonella mutants lacking either antioxidant defences or redox-activated regulators suggest that Galleria immune system has the capacity to produce ROS. This hypothesis was validated by in vivo biosensors assays which allowed us to conclude that H2O2 was produced in the hemolymph of Galleria during Salmonella infection.
Method
Bacterial Strains
The strains and plasmids used in this study are listed in Table S1. Salmonella enterica serovar Typhimurium ATCC 14028 and Escherichia coli MG1655 were used in this study. Deletions of genes were carried out using one-step λ Red recombinase chromosomal inactivation system (Datsenko and Wanner, 2000). In Salmonella enterica, deletions were transferred to the WT strain using P22 transduction procedures and verified by PCR. Heat inactivated bacteria were incubated 15 min at 65°C. Strains were grown at 37°C in lysogeny broth (LB) medium.
Plasmids Construction
The cloning vector used to monitor gene expression was pFPV25, carrying promotorless gfpmut3a gene. The inserts carrying 300 bp upstream ahpC or soxS start codon were PCR-amplified from S. enterica 12023 by using the primers listed in Table S2. PCR products were digested using XbaI and NdeI, and cloned into pFPV25 vector, yielding PahpC-gfp and PsoxS-gfp plasmids (Table S1). All the inserts were verified by DNA sequencing. The cloning vector used to detect Salmonella cells within the hemolymph was pGBM2, carrying promotorless mCherry gene. An insert carrying 300 bp upstream rpsM was digested using KpnI and HindIII, and cloned into pGBM2 vector, yielding the PrpsM-mCherry plasmid (Table S1).
Insects
Galleria mellonella larvae (Lepidoptera: Pyralidae, the Greater Wax Moth) (Sud-Est Appats, Queige, France) were stored in wood chips in the dark at 22°C. All larvae were 5 to 6 weeks old and weighed between 300 and 500 mg. They were used within 1 week.
Bacterial Infection of Galleria mellonella
Bacterial strains were grown during 16 h in LB under microaerobic conditions (screwed tubes, 37°C without shaking). The cultures were washed and immediately diluted in phosphate-buffered saline (PBS, NaCl 137 mM, KCl 2.7 mM, Na2HPO4 10 mM, KH2PO4 1.8 mM, pH 7.4) to a final concentration of 106 CFU/ml. G. mellonella larvae were incubated 16 h at 37°C before injection. 10 μl of the suspension containing 104 CFU of bacteria was injected into the last proleg of the larvae. Injected larvae were incubated at 37°C and death was assessed 24, 48, and 72 h post-injection. Experiments were repeated three times using at least 20 larvae per group. Differences in survival between larvae injected with the WT strain and Salmonella mutants were determined by Kaplan-Meier analysis with log-rank test.
Bacterial Viability in Galleria mellonella
Injections were carried out as described above and the hemolymph was collected as previously described (Candela et al., 2019). Briefly, 6, 12, 18, and 24 h post-injection, larvae were washed once in 70% ethanol and twice in PBS to minimize surface contaminants. The abdomen of the injected larvae was pricked with a sterile needle. 10 µl of hemolymph was collected with a pipette and incubated 5 min with Triton X-100 0.5% to release intracellular bacteria and to bring together the whole bacterial population (intracellular and free bacteria). Bacterial suspensions obtained after centrifugation were washed with PBS, serial-diluted in PBS, and spotted on LB agar plates. CFU were counted after 18 h at 37°C.
Bacterial Viability In Vitro
Bacterial strains were grown overnight in LB, washed, and serial-diluted in PBS. After that, 5 µl of the different dilutions were spotted on LB agar plates with or without bovine liver catalase (2,000 U/plate; Sigma-Aldrich), hydrogen peroxide (20 or 50 µM), or paraquat (50 or 100 µM), a redox cycler which stimulates superoxide production. Catalase, hydrogen peroxide, and paraquat were purchased from Sigma-Aldrich (Lyon, France). CFU were counted after 18 h at 37°C.
Flow Cytometry Analysis of Bacteria Extracted From Hemolymph
G. mellonella larvae were injected with 105 bacteria carrying the constitutive PrpsM-mCherry plasmid (to identify Salmonella cells within the hemolymph) and either PahpC-gfp, or PsoxS-gfp inducible plasmids (to monitor oxidative stress). In addition, 20 µl of hemolymph were collected 30 and 60 min post-injection, diluted in an anticoagulant solution, lysed with Triton X-100 0.5% and fixed in paraformaldehyde 3.2% during 20 min (Stoepler et al., 2012). Bacteria were pelleted at 6.500 g for 5 min and resuspended in 500 µl Dulbecco’s phosphate-buffered saline (DPBS, NaCl 138 mM, KCl 2.7 mM, Na2HPO4 8.1 mM, KH2PO4 1.5 mM, pH 7.0–7.3). For flow cytometric analysis, bacteria were first gated for their size/granulosity (FSCxSSC), for singlets in order to remove multiple events, then for the mCherry fluorescence (FL3 615 ± 25 nm) and finally analysed for the expression level of GFP (FL1 525 ± 30 nm). A compensation was applied on the mCherry signal due to the GFP overlapping signal. Samples were run in the low-pressure mode (about 10,000 particles/s). Data were acquired with an S3e cells sorter (Biorad) using 488 and 561 nm lasers and data were analyzed and plotted using FlowJo v10.6.
Results
Salmonella enterica Proliferation and Virulence in Galleria mellonella
To assess S. enterica’s capacity to infect G. mellonella, injections of 104 or 105 bacteria per larva were carried out. Injecting 104 bacteria/larva led to the survival of 40% of the population 24 h post-injection whereas injecting 105 bacteria/larva killed almost all the larvae in the same period (Figure S1A). This result is in accordance with the DL50 previously found by others (Bender et al., 2013). No significant killing was observed with Escherichia coli K12 and S. enterica heat inactivated strains, leading to the conclusion that Salmonella, and not E. coli, exhibited an active virulence mechanism against Galleria (Figure S1A). To decipher the early stages of Salmonella invasion within G. mellonella, we measured bacterial colony-forming units (CFU) in the hemolymph 6, 12, 18 and 24 h post-injection of 104 bacteria/larva. Killed larvae were observed from 12 h post-injection and the bacterial load upon death (BLUD) median value was found to be >108 CFU/larva (Figure S1B). This parameter, often used in host/pathogen interactions, allows to determine the ability of the host to resist infection. Twenty-four hours post-injection, a large majority of alive larvae cleared all bacteria (Figure S1B). In line with the results presented above, 60% of the larvae were killed 24 h post-injection. These data indicate that G. mellonella larvae injected with 104 Salmonella cells suffer a different fate and that a minimal bacterial load has to be reached in the hemolymph to kill the host.
Antioxidant Enzymes Support Salmonella Virulence in Galleria
To investigate G. mellonella superoxide production in antibacterial defence, we have constructed Salmonella mutants inactivated in cytoplasmic (sodA and sodB) or periplasmic (sodCI and sodCII) SOD-encoding genes. Galleria larvae injected with the ΔsodA ΔsodB mutant exhibited a survival rate two-fold higher than those injected with the WT strain, whereas no significant difference was observed between the ΔsodCI ΔsodCII mutant and the WT (Figure 1A). These results were in accordance with the sensitivity of these mutants to paraquat, a superoxide generator, i.e., the ΔsodA ΔsodB mutant was highly sensitive to paraquat whereas the ΔsodCI ΔsodCII mutant was not (Figure 1B). Surprisingly, we observed that the ΔsodB mutant was slightly more virulent than the WT strain (Figure 1A). Next, we observed that a Salmonella strain lacking all H2O2 degrading activities (catalases KatE, KatG, and KatN, and peroxiredoxins AhpC and TsaA), referred to as HpxF, exhibited an attenuated virulence in G. mellonella (Figure 2A). Larvae injected with catalase (ΔkatE ΔkatG ΔkatN) or peroxiredoxin (ΔahpC ΔtsaA) mutants showed a survival rate comparable to those injected with the WT strain (Figure 2A). Interestingly, co-injection of exogenous catalase with the HpxF mutant and the WT strain strongly decreased Galleria’s survival rate, indicating that oxidative stress fully participates to Galleria immune response (Figure 2B). As expected, the HpxF mutant was particularly sensitive to H2O2 whereas others were not (Figure 2C). Nevertheless, no growth defect was observed in liquid LB medium under microaerobic conditions for the HpxF and for the ΔsodA ΔsodB mutants compared to the wild-type strain (Figure S2). Together, these observations highlight the importance of ROS produced by Galleria immune system to counteract Salmonella infection.
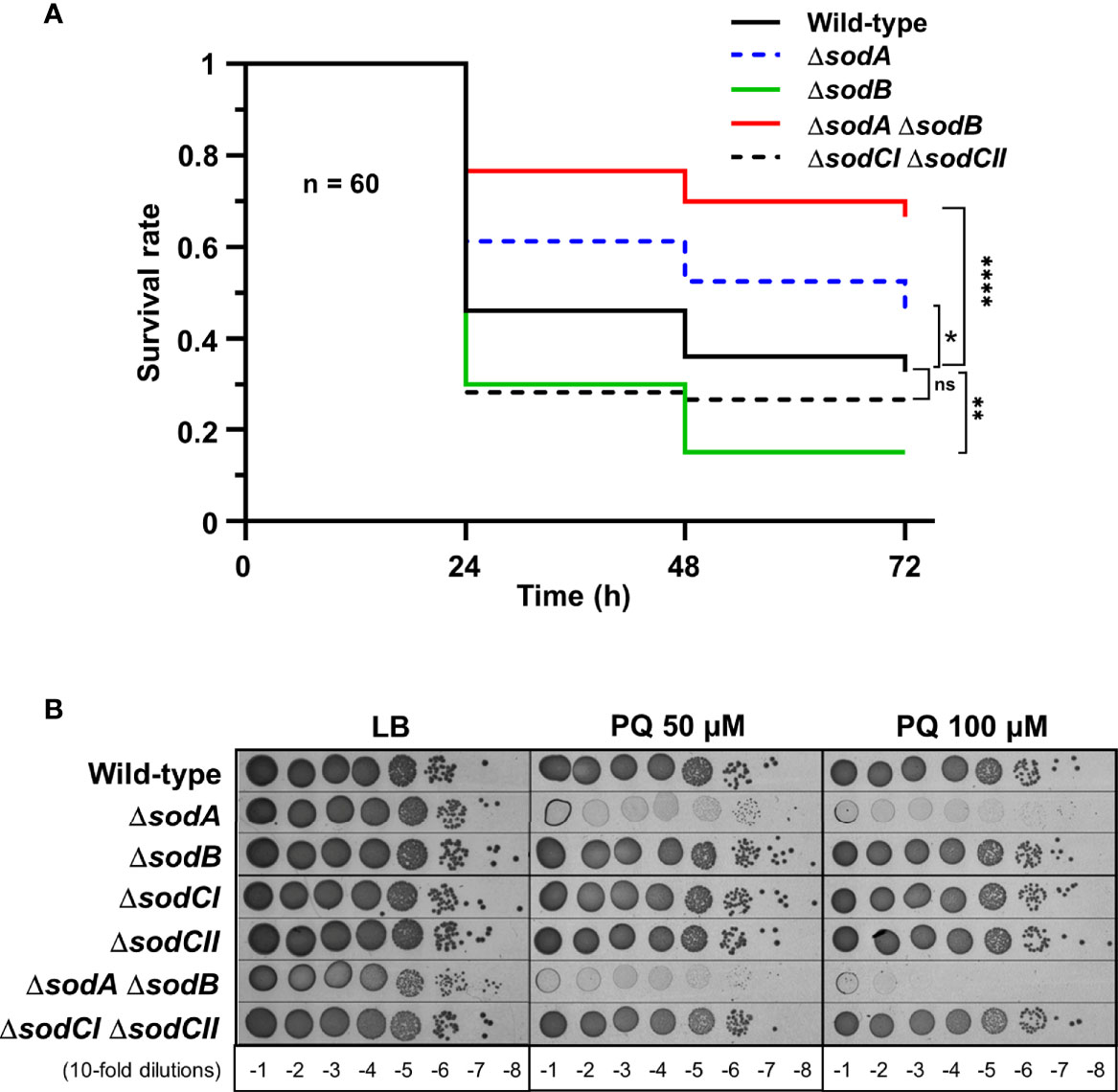
Figure 1 Cytoplasmic superoxide dismutases support Salmonella virulence in Galleria. (A) Galleria mellonella larvae were injected with 10 µl of solutions containing Salmonella WT, the singles mutants ΔsodA or ΔsodB, and the double mutants ΔsodA ΔsodB or ΔsodCI ΔsodCII at a concentration of 104 bacteria/larva. Infections were repeated three times using 20 larvae per group. The survival curves were compared by log-rank. ns, not significant; *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001 (Mantel-Cox test). (B) The WT, ΔsodA, ΔsodB, ΔsodCI, ΔsodCII, ΔsodA ΔsodB, and ΔsodCI ΔsodCII strains were grown in LB, serial diluted as indicated and spotted on LB agar plates or increasing concentrations of paraquat (PQ). The results are representative of three independent experiments.
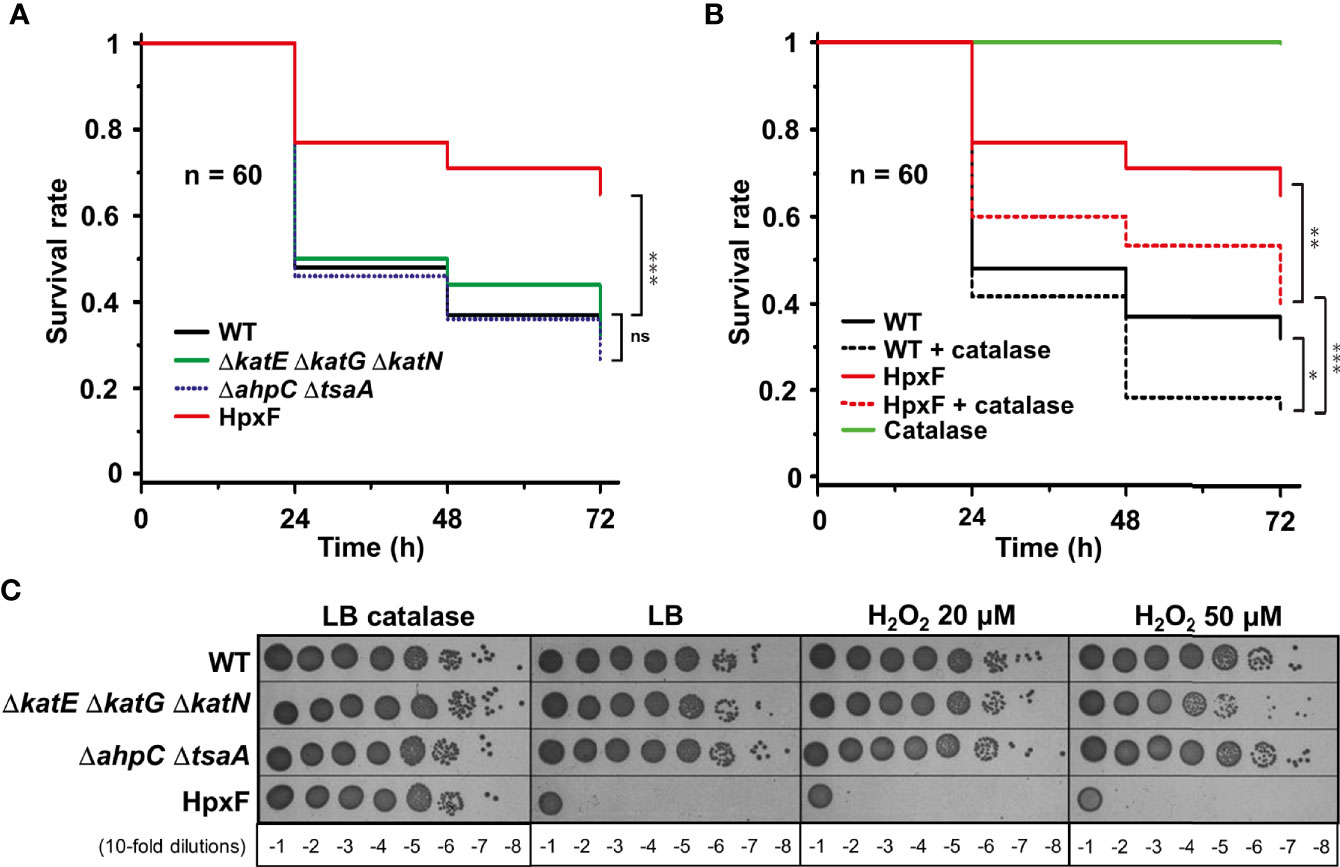
Figure 2 Sensitivity of Salmonella catalases/peroxiredoxins mutants reveal ROS production by Galleria. (A, B) Galleria mellonella larvae were injected with 10 µl of solutions containing Salmonella wild-type, ΔkatE ΔkatG ΔkatN, ΔahpC ΔtsaA, and HpxF strains at a concentration of 104 bacteria/larva. When indicated, catalase is used at a concentration of 4 mg/kg of larva. Infections were repeated three times using 20 larvae per group. The survival curves were compared by log-rank. ns, not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Mantel-Cox test). (C) The wild-type, ΔkatE ΔkatG ΔkatN, ΔahpC ΔtsaA, and HpxF strains were grown in LB, serial diluted as indicated and spotted on LB agar plates with or without catalase or increasing concentrations of hydrogen peroxide (H2O2).
Salmonella Sensed H2O2 Within Galleria Hemolymph
To characterize the ROS produced by Galleria and sensed by Salmonella, injection of larvae was carried out with bacterial strains carrying either the H2O2-inducible PahpC-gfp fusion or the -inducible PsoxS-gfp fusion. Bacteria were collected from the hemolymph and analysed by flow cytometry. They were first sorted for mCherry expression as all injected Salmonella cells carried the PrpsM-mCherry constitutive fusion (Figure 3A). GFP intensity was then measured as all injected Salmonella cells carried the PahpC-gfp or PsoxS-gfp fusions, reflecting the level of ROS experienced by Salmonella within the host. In vitro controls were first carried out and showed that addition of 10 to 100 µM H2O2 in the LB medium led to a dose-dependent induction of the PahpC-gfp fusion 30 min post-treatment (Figure 3B, left). A similar induction was observed with the PsoxS-gfp fusion treated with increasing concentrations of paraquat in LB (Figure 3C, left). These bacterial strains were next injected in G. mellonella and 30 min post-injection of larvae, a 2.5-fold induction of the PahpC-gfp fusion was measured whereas the level of the PsoxS-gfp fusion did not change significantly (Figures 3B, C, right). The fluorescence of the PahpC-gfp fusion recovered its basic level 60 min post-injection, indicating a possible decrease of H2O2 production by Galleria immune system and/or ROS degradation by Salmonella within this interval of time. Altogether, these results show that an H2O2 burst was generated by Galleria immune system and sensed by Salmonella just after infection.
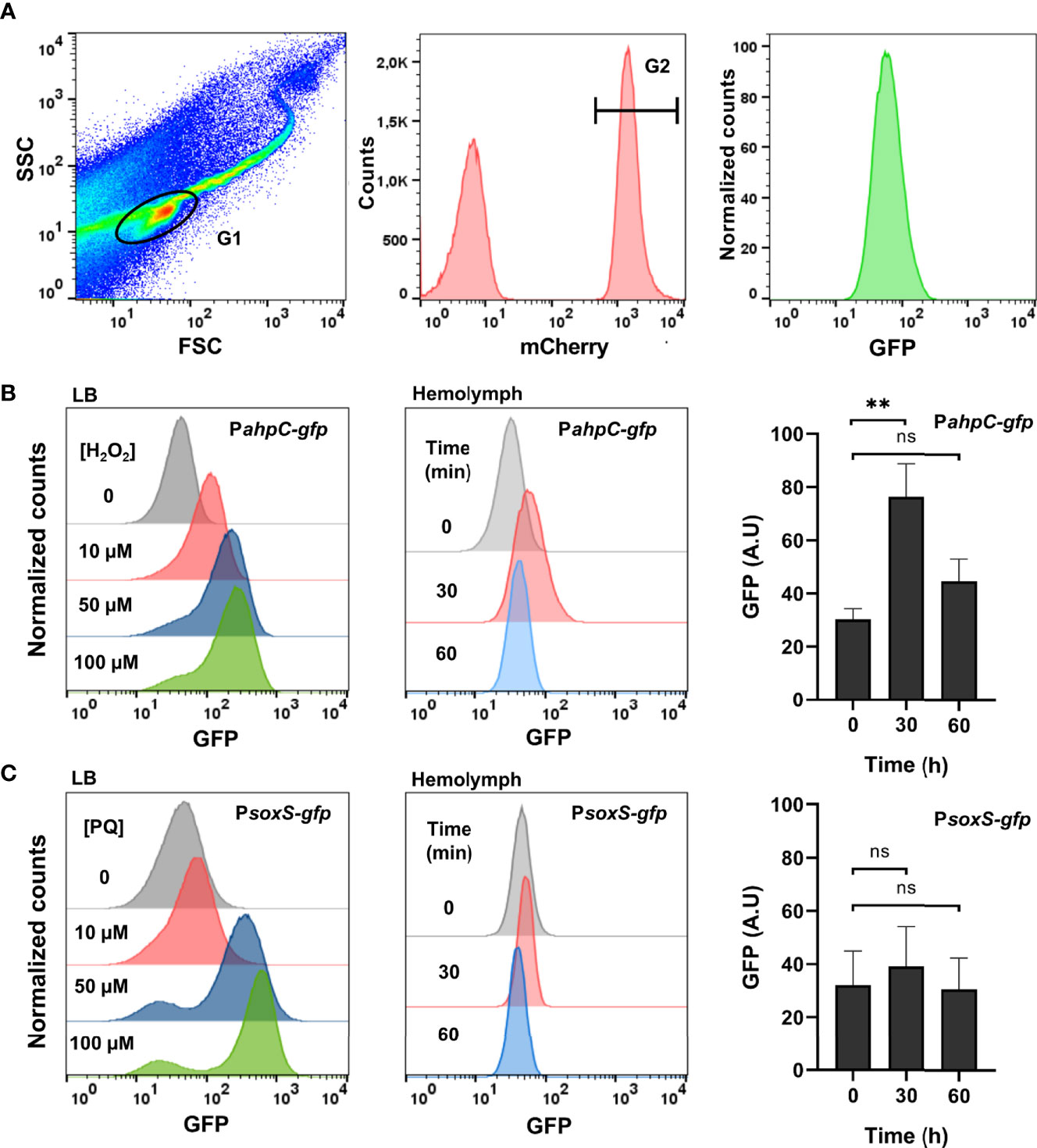
Figure 3 Salmonella experiences hydrogen peroxide within Galleria hemolymph. (A) Flow cytometry analysis. The first gate (G1) was based on size and granularity (FSC x SSC), outlining the bacterial population perimeter. The second gate (not shown) was intended to remove doublet events. Salmonella cells carried a constitutive mCherry plasmid and this population was gated in G2 by their red fluorescence. Finally, the GFP fluorescence of the bacterial population (G2) was measured. (B, C) A WT strain carrying the PahpC-gfp (B) and the PsoxS-gfp (C) fusions were grown in LB. Increasing concentrations of H2O2 (B), left and paraquat (C), left were added and the florescence was measured by flow-cytometry 30 min post-treatment. Galleria larvae were injected with a WT strain carrying the PahpC-gfp (B), center and right and the PsoxS-gfp (C), center and right fusions. Bacteria were extracted and analyzed from the hemolymph 30 and 60 min post-injection. The fluorescence was measured by flow cytometry and the raw data are shown on these panels where a representative experiment (center) and the quantification of three independent experiments (right) are presented. Asterisks indicate a statistically significant difference between two infection times. ns, not significant; **P ≤ 0.01 (Dunnett test).
The H2O2-Dependent Activator OxyR Is Required for Salmonella Full Virulence in G. mellonella
Next, we addressed the role of oxidative stress-dependent transcriptional regulators in Salmonella to trigger adaptive responses inside the host. We focused on three of them: OxyR dependent upon H2O2 concentration, HypT activated by hypochlorite acid (HOCl), and SoxR which responds to redox cycling drugs and (Figure 4A). Galleria larvae injected with the ΔoxyR mutant exhibited a survival rate two-fold higher than the WT strain (Figure 4B). Conversely, no virulence defects were observed for the ΔhypT and the ΔsoxR mutants (Figure 4B). These results did not suggest any major role for HypT and SoxR in Salmonella adaptive response during Galleria infection and highlight the importance of the H2O2-activated regulator OxyR in this process.
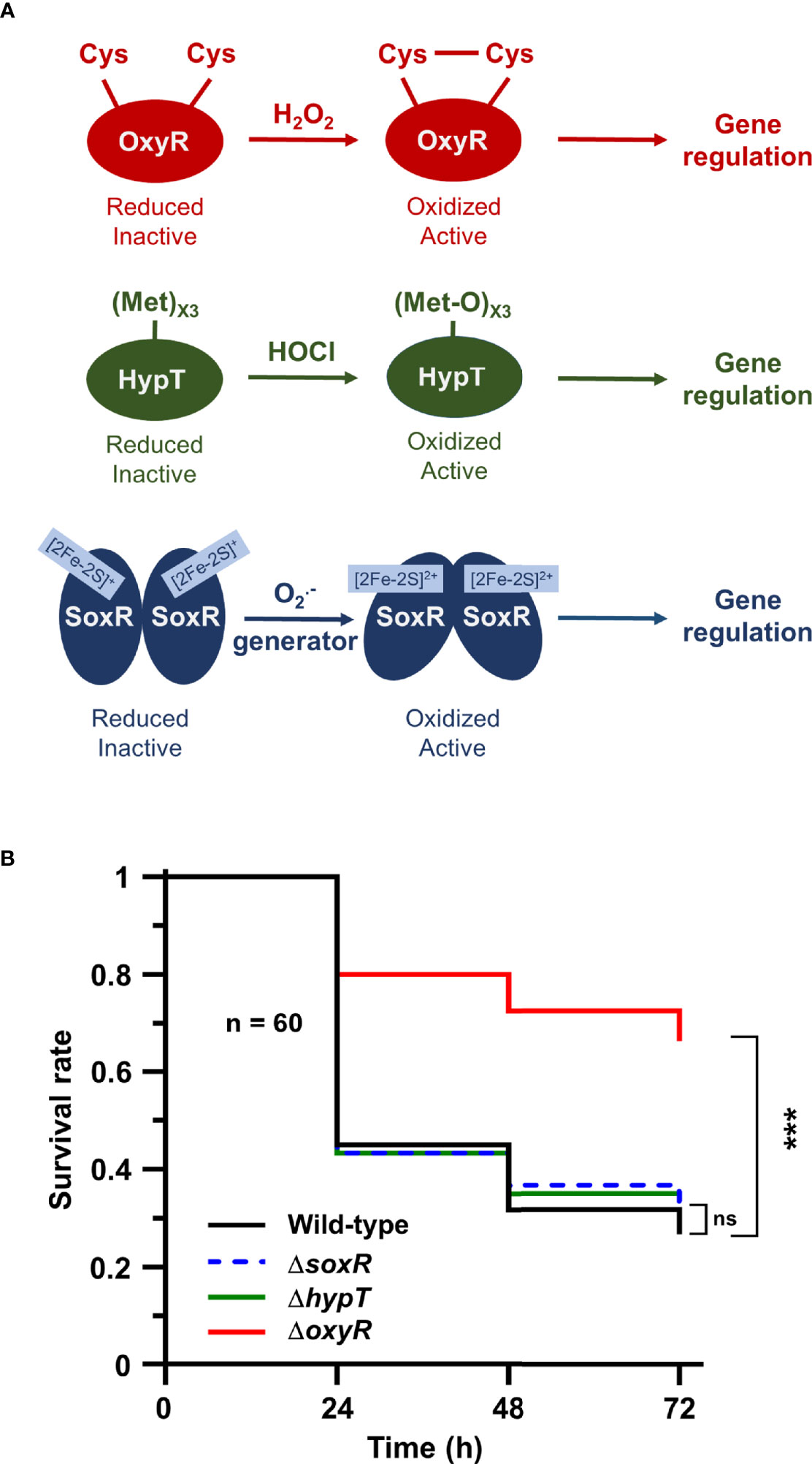
Figure 4 The OxyR regulator is required for Salmonella virulence in Galleria. (A) Models of OxyR, HypT, and SoxR activation by hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and redox-recycling drugs or superoxide generator , respectively. (B) Galleria mellonella larvae were injected with 10 µl of suspensions containing Salmonella WT and the ΔoxyR, ΔhypT and ΔsoxR mutants at a concentration of 104 bacteria/larva. Infections were repeated three times using 20 larvae per group. The survival curves were compared by log-rank. ns, not significant; ***P ≤ 0.001 (Mantel-Cox test).
Discussion
In this study, we showed that Salmonella strains inactivated for catalases/peroxiredoxins and cytoplasmic superoxide dismutases exhibited reduced virulence in G. mellonella, revealing oxidative stress production by the larvae. Moreover, induction of the PahpC-gfp fusion in the hemolymph during the first stage of the infection indicated that an H2O2 burst was generated by Galleria. These results demonstrate that oxidative stress is an important immune mechanism used by Galleria to resist microbial infections.
The periplasmic SodCI and SodCII enzymes were previously found to be important for virulence in mice whereas cytoplasmic SodA was shown to be dispensable (Tsolis et al., 1995; Fang et al., 1999; Krishnakumar et al., 2004). In the present study, we found that the cytoplasmic SODs are required for Salmonella full virulence in Galleria and that the periplasmic SODs are dispensable. Therefore, Galleria can be used as a particularly relevant model to highlight bacterial defence systems whose importance could have been underestimated so far.
Superoxide anion production by Galleria was previously documented by ex vivo experiments and one could postulate that it should be sensed by Salmonella (Bergin et al., 2005). Nevertheless, our in vivo experiments did not reveal any activation of the biosensor located in Salmonella cytoplasm. Charged molecules cannot cross bacterial membranes and is rapidly dismutated into H2O2, either enzymatically or spontaneously, explaining at least partially the modest activation of the PsoxS-gfp fusion. In addition, the half-life of superoxide anion was estimated to be about 5 s at physiological pH (Marklund, 1976). But we can’t exclude the presence of in the bacterial cytoplasm as the reduced virulence of the ΔsodA ΔsodB mutant strongly supports the importance of superoxide. We can hypothesize that Galleria produced oxidative stress in response to Salmonella invasion, being rapidly dismutated into H2O2 and targeted to the bacterial cytoplasm as demonstrated by the induction of the PahpC-gfp fusion 30 min post-injection. A pervious study has reported the production of H2O2 in the hemolymph of Galleria in the first hours after injection of Bacillus thuringiensis (Komarov et al., 2006). Interestingly, a similar observation was previously made in mouse macrophages where an H2O2 burst was detected 45 min after Salmonella infection (Aussel et al., 2011). We did not investigate the role of phagocytosis during the infection but we showed that an H2O2 burst was produced by Galleria and sensed by Salmonella 30 min post-infection. This correlation between Galleria mellonella and mice is an additional argument to validate this insect as a relevant alternative model to study host-pathogen interactions. Additional studies will be required to investigate the distribution of intracellular and free bacteria within the hemolymph.
Finally, we have assayed the importance of adaptive responses in Salmonella through the involvement of different oxidative stress-activated regulators. During the infection of Galleria, the HOCl-sensing regulator HypT appeared to be dispensable. This result is in accordance with the absence in Galleria of the major HOCl-producing enzyme, the myeloperoxidase (MPO). Indeed, cytochemistry and immunodetection analysis failed to detect MPO in the hemocytes of the larvae (Chain and Anderson, 1983; Fallon et al., 2011). All these observations suggest that Galleria’s hemocytes don’t produce HOCl. Moreover, insects were shown to synthesize a dual oxidase (DUOX) able to catalyse HOCl production in the epithelial cells of its gut (Kim and Lee, 2014). But Salmonella has never been in contact with epithelial cells in any of our experiments. Future work using dedicated HOCl reporters (Stocker et al., 2017) might solve this issue.
OxyR was identified as an important regulator to allow the success of Salmonella infection. This transcriptional activator is dependent upon H2O2 and can activate a regulon composed of more than 30 genes, most of them encoding antioxidant enzymes such as catalases, peroxiredoxins or thioredoxins (Zheng et al., 2001). We showed that an oxyR mutant was poorly virulent during Galleria infection, conferring to the regulator encoded by this gene a key role in detecting low H2O2 levels and triggering bacterial adaptive mechanisms to cope with oxidative stress. Moreover, a mutant unable to degrade H2O2 was found to be attenuated during the larvae infection. We also showed that Salmonella experienced H2O2 produced by Galleria. Taken together, our results highlight the importance of H2O2 in the hemolymph of Galleria to eradicate pathogens.
Like mammalian models, insects developed a wide variety of mechanisms to resist against pathogens, among which oxidative stress. Our findings showed that Galleria mellonella produces ROS to defend against Salmonella enterica infection and might be useful to complete the characterization of the immune system of this host, which appears to be a suitable model to study bacterial pathogenicity.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Conceptualization: BE and LA. Methodology: HB, GB, BE, and LA. Investigation: HB, GB, BE, and LA. Writing-original draft preparation: HB, GB, BE, and LA. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Agence Nationale de la Recherche (ANR) (#ANR-16-CE11-0012-02 METOXIC), the Centre National de la Recherche Scientifique (CNRS) (#PICS-PROTOX), and Aix-Marseille Université.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the members of the Ezraty and Py groups for advices and discussions throughout the work. We thank N. Typas (EMBL Heidelberg), L. Bossi (Université Paris-Saclay), and Shoshi Altuvia (The Hebrew University of Jerusalem) for providing strains as well as R. Stevens (GAFL, INRAE Avignon) for critical reading of the manuscript. We are grateful to C. Nielsen-Leroux, J. Viala, and R. Voulhoux for helpful suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.640112/full#supplementary-material
References
Aussel L., Zhao W., Hébrard M., Guilhon A.-A., Viala J. P. M., Henri S., et al. (2011). Salmonella detoxifying enzymes are sufficient to cope with the host oxidative burst. Mol. Microbiol. 80, 628–640. doi: 10.1111/j.1365-2958.2011.07611.x
de Barros P. P., Rossoni R. D., de Ribeiro F. C., Silva M. P., de Souza C. M., Jorge A. O. C., et al. (2019). Two sporulated Bacillus enhance immunity in Galleria mellonella protecting against Candida albicans. Microb. Pathog. 132, 335–342. doi: 10.1016/j.micpath.2019.05.023
Bender J. K., Wille T., Blank K., Lange A., Gerlach R. G. (2013). LPS structure and PhoQ activity are important for Salmonella Typhimurium virulence in the Galleria mellonella infection model [corrected]. PloS One 8, e73287. doi: 10.1371/journal.pone.0073287
Bergin D., Reeves E. P., Renwick J., Wientjes F. B., Kavanagh K. (2005). Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect. Immun. 73, 4161–4170. doi: 10.1128/IAI.73.7.4161-4170.2005
Brochado A. R., Telzerow A., Bobonis J., Banzhaf M., Mateus A., Selkrig J., et al. (2018). Species-specific activity of antibacterial drug combinations. Nature 559, 259–263. doi: 10.1038/s41586-018-0278-9
Candela T., Fagerlund A., Buisson C., Gilois N., Kolstø A.-B., Økstad O. A., et al. (2019). CalY is a major virulence factor and a biofilm matrix protein. Mol. Microbiol. 111, 1416–1429. doi: 10.1111/mmi.14184
Chain B. M., Anderson R. S. (1983). Observations on the cytochemistry of the hemocytes of an insect, Galleria mellonella. J. Histochem. Cytochem. 31, 601–607. doi: 10.1177/31.5.6188780
Cools F., Torfs E., Vanhoutte B., de Macedo M. B., Bonofiglio L., Mollerach M., et al. (2018). Streptococcus pneumoniae galU gene mutation has a direct effect on biofilm growth, adherence and phagocytosis in vitro and pathogenicity in vivo. Pathog. Dis. 76, 1–10. doi: 10.1093/femspd/fty069
Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
Fallon J. P., Troy N., Kavanagh K. (2011). Pre-exposure of Galleria mellonella larvae to different doses of Aspergillus fumigatus conidia causes differential activation of cellular and humoral immune responses. Virulence 2, 413–421. doi: 10.4161/viru.2.5.17811
Fang F. C., DeGroote M. A., Foster J. W., Bäumler A. J., Ochsner U., Testerman T., et al. (1999). Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. U.S.A. 96, 7502–7507. doi: 10.1073/pnas.96.13.7502
Hébrard M., Viala J. P. M., Méresse S., Barras F., Aussel L. (2009). Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J. Bacteriol. 191, 4605–4614. doi: 10.1128/JB.00144-09
Kim S.-H., Lee W.-J. (2014). Role of DUOX in gut inflammation: lessons from Drosophila model of gut-microbiota interactions. Front. Cell. Infect. Microbiol. 3, 1–12. doi: 10.3389/fcimb.2013.00116
Komarov D. A., Slepneva I. A., Dubovskii I. M., Grizanova E. V., Khramtsov V. V., Glupov V. V. (2006). Generation of superoxide radical and hydrogen peroxide in insect hemolymph in the course of immune response. Dokl Biol. Sci. 411, 482–485. doi: 10.1134/S0012496606060160
Krishnakumar R., Craig M., Imlay J. A., Slauch J. M. (2004). Differences in enzymatic properties allow SodCI but not SodCII to contribute to virulence in Salmonella enterica serovar Typhimurium strain 14028. J. Bacteriol. 186, 5230–5238. doi: 10.1128/JB.186.16.5230-5238.2004
Kurstak E., Vega C. E. (1968). [Bacterial infection due to Salmonella typhimurium in an invertebrate, Galleria mellonella L]. Can. J. Microbiol. 14, 233–237. doi: 10.1139/m68-039
Lysenko O., Kucera M. (1968). The mechanism of pathogenicity of Pseudomonas aeruginosa. VI. The toxicity of preteinases for larvae of the greater wax moth, Galleria mellonella L. Folia Microbiol. (Praha) 13, 295–299. doi: 10.1007/BF02909617
Marklund S. (1976). Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J. Biol. Chem. 251, 7504–7507. doi: 10.1016/S0021-9258(17)32878-8
Pereira T. C., de Barros P. P., Fugisaki L. R., de O., Rossoni R. D., de Camargo Ribeiro F., et al. (2018). Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. J. Fungi (Basel) 4, 1–19. doi: 10.3390/jof4040128
Repizo G. D., Gagné S., Foucault-Grunenwald M.-L., Borges V., Charpentier X., Limansky A. S., et al. (2015). Differential Role of the T6SS in Acinetobacter baumannii Virulence. PloS One 10, e0138265. doi: 10.1371/journal.pone.0138265
Sciuto A. L., Martorana A. M., Fernández-Piñar R., Mancone C., Polissi A., Imperi F. (2018). Pseudomonas aeruginosa LptE is crucial for LptD assembly, cell envelope integrity, antibiotic resistance and virulence. Virulence 9, 1718–1733. doi: 10.1080/21505594.2018.1537730
Slepneva I. A., Glupov V. V., Sergeeva S. V., Khramtsov V. V. (1999). EPR detection of reactive oxygen species in hemolymph of Galleria mellonella and Dendrolimus superans sibiricus (Lepidoptera) larvae. Biochem. Biophys. Res. Commun. 264, 212–215. doi: 10.1006/bbrc.1999.1504
Stocker P., Cassien M., Vidal N., Thétiot-Laurent S., Pietri S. (2017). A fluorescent homogeneous assay for myeloperoxidase measurement in biological samples. A positive correlation between myeloperoxidase-generated HOCl level and oxidative status in STZ-diabetic rats. Talanta 170, 119–127. doi: 10.1016/j.talanta.2017.03.102
Stoepler T. M., Castillo J. C., Lill J. T., Eleftherianos I. (2012). A Simple Protocol for Extracting Hemocytes from Wild Caterpillars. J. Vis. Exp. 69, e4173. doi: 10.3791/4173
Sugumaran M. (2002). Comparative Biochemistry of Eumelanogenesis and the Protective Roles of Phenoloxidase and Melanin in Insects. Pigment Cell Res. 15, 2–9. doi: 10.1034/j.1600-0749.2002.00056.x
Tsolis R. M., Bäumler A. J., Heffron F. (1995). Role of Salmonella typhimurium Mn-superoxide dismutase (SodA) in protection against early killing by J774 macrophages. Infect. Immun. 63, 1739–1744. doi: 10.1128/IAI.63.5.1739-1744.1995
Viegas S. C., Mil-Homens D., Fialho A. M., Arraiano C. M. (2013). The virulence of Salmonella enterica Serovar Typhimurium in the insect model galleria mellonella is impaired by mutations in RNase E and RNase III. Appl. Environ. Microbiol. 79, 6124–6133. doi: 10.1128/AEM.02044-13
Wang-Kan X., Blair J. M. A., Chirullo B., Betts J., La Ragione R. M., Ivens A., et al. (2017). Lack of AcrB Efflux Function Confers Loss of Virulence on Salmonella enterica Serovar Typhimurium. mBio 8, e00968–e00917. doi: 10.1128/mBio.00968-17
Younghusband H. B., Lee P. E. (1969). Virus-cell studies of tipula iridescent virus in Galleria mellonella L. I. Electron microscopy of infection and synthesis of tipula iridescent virus in hemocytes. Virology 38, 247–254. doi: 10.1016/0042-6822(69)90366-3
Keywords: Galleria mellonella, Salmonella enterica, reactive oxygen species, host-pathogen interactions, biosensors
Citation: Bismuth HD, Brasseur G, Ezraty B and Aussel L (2021) Bacterial Genetic Approach to the Study of Reactive Oxygen Species Production in Galleria mellonella During Salmonella Infection. Front. Cell. Infect. Microbiol. 11:640112. doi: 10.3389/fcimb.2021.640112
Received: 10 December 2020; Accepted: 13 January 2021;
Published: 01 March 2021.
Edited by:
Xihui Shen, Northwest A and F University, ChinaReviewed by:
Irina Slepneva, Institute of Chemical Kinetics and Combustion (RAS), RussiaElisa Borghi, University of Milan, Italy
Agnieszka Zdybicka-Barabas, Maria Curie-Skłodowska University, Poland
Copyright © 2021 Bismuth, Brasseur, Ezraty and Aussel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Ezraty, ZXpyYXR5QGltbS5jbnJzLmZy; Laurent Aussel, YXVzc2VsQGltbS5jbnJzLmZy
 Hanna D. Bismuth
Hanna D. Bismuth Gaël Brasseur
Gaël Brasseur Benjamin Ezraty
Benjamin Ezraty Laurent Aussel
Laurent Aussel