
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 16 November 2021
Sec. Clinical Microbiology
Volume 11 - 2021 | https://doi.org/10.3389/fcimb.2021.636999
 Zeming Liu1†
Zeming Liu1† Di Hu2†
Di Hu2† Jinpeng Li3†
Jinpeng Li3† Qing Xia4†
Qing Xia4† Yan Gong5†
Yan Gong5† Zhengwei Li6
Zhengwei Li6 Qian Wu6
Qian Wu6 Meilin Yi7
Meilin Yi7 Yihui Huang2
Yihui Huang2 Meng Wu8*
Meng Wu8* Liang Guo2*
Liang Guo2* Xiaohui Wu6*
Xiaohui Wu6*Background: Coronavirus disease 2019 (COVID-19) has evolved into a pandemic. We hypothesized that biochemical indicators of liver function may help determine the prognosis of COVID-19 patients.
Methods: Patient information was collected from the Wuhan-Leishenshan hospital. Logistic and Cox regression analyses, Kaplan-Meier curves, and Curve fitting were used to determine the correlation between elevated levels of aspartate transaminase (AST), alanine transaminase (ALT), and AST/ALT and severity of disease/mortality.
Results: Logistic and Cox regression analyses and Kaplan-Meier survival curves showed that COVID-19 progression correlated with elevated levels of AST and AST/ALT. The odds ratios for elevated levels of AST and AST/ALT in patients were 0.818 (95% confidence interval [CI]: 0.274-2.441, P = 0.035) and 2.055 (95% CI: 1.269-3.327, P = 0.003), respectively; the hazard ratios were 4.195 (95% CI: 1.219-14.422, P = 0.023) and 3.348 (95% CI: 1.57-7.139, P = 0.002), respectively. The Kaplan-Meier survival curves demonstrated that patients with elevated AST and AST/ALT levels had a higher risk of developing severe COVID-19.
Conclusion: Elevated AST and AST/ALT levels correlated with severity of COVID-19 and mortality. Liver function tests may help clinicians in determining the prognosis of patients undergoing treatment for COVID-19.
Since its first reported occurrence in Wuhan, coronavirus disease 2019 (COVID-19) has become an alarming cause of morbidity and mortality worldwide. This can be attributed to the median incubation time, ranging from 4 to 7 days, between SARS-CoV-2 infection and the appearance of symptoms. This delays timely treatment and allows the transmission of viral particles across the population (Huang et al., 2020; Khan et al., 2020; Vardhana and Wolchok, 2020). More than five million patients have received COVID-19 diagnose, among which >355,000 deaths have been attributed to the disease (Worldometer COVID-19 Data).
The lack of targeted therapy, and factors affecting disease progression and prognosis of COVID-19 have also complicated the outcome of patients (Hui et al., 2020; Pan et al., 2020). Previous studies have shown that patient age, comorbidities, lymphocytopenia, elevated levels of D-dimer, creatine kinase, high-sensitivity cardiac troponin I, and higher prothrombin time correlate with disease severity in patients with COVID-19 in the intensive care unit. Liver function tests have recently been shown to be an important diagnostic tool for COVID-19. The liver function tests of patients show abnormalities; however, a study has refuted the relevance of impaired liver function in patients with COVID-19 and states that liver activity may not have serious clinical consequences (Zhang et al., 2020). Conversely, there are also researchers have different opinions. About 14-53% of patients with COVID-19 were reported to hepatic dysfunction, particularly in those with severe disease (Jothimani et al., 2020). Considering these contradictory findings, it is imperative to determine the effect of liver function in the pathogenesis of COVID-19. In this study, we aimed to examine the correlation between prognosis of patients with COVID-19 and aspartate transaminase (AST), alanine transaminase (ALT), and AST/ALT levels. This will help elucidate the role of impaired liver function in the progression of COVID-19 and devise novel therapeutic strategies for clinical intervention.
This retrospective study comprised 1,788 patients who were clinically diagnosed with COVID-19 between February 8 and March 19, 2020 at the Leishenshan Hospital in Wuhan. Their demographic characteristics, medical history and treatment, laboratory test results, and imaging data were obtained from the original patient medical records. Two physicians independently reviewed this data. This study was approved by the Research Ethics Commission of the Zhongnan Hospital, Wuhan University (approval number: 2020074), and patient consent was waived by the ethics committee as COVID-19 is a rapidly evolving infectious disease.
We used patient survival, severity of COVID-19 during patient hospitalization, and computed tomography (CT) images to determine the primary outcomes of infected patients. Survival status was the most important indicator. According to the seventh interim guidance for diagnosis and treatment of COVID-19 published by the Chinese National Health Commission (National Health Commission of the People’s Republic of China), COVID-19 severity was divided into four degrees: mild, common, severe, and critical. As there was only one patient with mild COVID-19, we combined the first two groups to form the mild/common group.
The chest CT images were examined and scored independently by two experienced radiologists using common guidelines based on previous studies and symptoms of COVID-19. A score of 1 included characteristic like ground-glass opacities, reticulations or cord-like changes, consolidations, and pleural effusions. Each feature was assigned one point, comprising score 1. Score 2 was generated based on the area of lung lobe involvement, from 0 to 4: no involvement, 0; <25% involvement, 1; 26%-50% involvement, 2; 51%-75% involvement, 3; and 76%-100% involvement, 4. The total score was the sum of scores 1 and 2.
Continuous variables that exhibited normal distribution were presented as mean ± standard deviation (SD) or interquartile range (IQR). Categorical variables were described as frequency rates and percentages. Differences between continuous variables were analyzed using independent t-tests or Mann-Whitney tests in patients with normal and elevated levels of ALT and AST. Chi-square tests were used to analyze differences between categorical variables in patients with normal and elevated levels of ALT and AST. Fisher’s exact test were used for expected counts of less than 5 patients.
We used univariate logistic and Cox regression analyses to determine whether elevated levels of ALT, AST, and AST/ALT influenced the prognosis of patients with COVID-19. During logistic regression analysis, we divided all patients into two groups based on disease severity. Group 1 included patients who were diagnosed with mild/common or severe disease, and group 2 comprised patients who were critically ill. Subsequently, we adjusted the logistic and Cox regression analyses for age, history of cardiovascular disease, and white blood cell, platelet, and lymphocyte counts.
We used Kaplan-Meier survival analyses with log-rank tests to understand patient survival. We used curve fitting analysis for patients with normal or elevated levels of AST and ALT to examine the changes in chest cavities for CT scores 1, and 2, and for total score, in a time-dependent manner. All statistical analyses were performed using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA). Two-tailed P values < 0.05 were considered statistically significant.
Table 1 lists the demographic characteristics and symptoms of 1,788 patients with COVID-19. The numbers of male and female patients were approximately the same. The median and interquartile range for patient age were 59 and 49-68 years, respectively. Patients with normal and elevated levels of AST were of similar age. The median age of patients with normal levels of ALT was higher than that of patients with elevated ALT.
There was no significant difference between comorbidities (Table 1), such as pulmonary disease, endocrine disease, malignancy, digestive system disease, and neurological disorders, in patients with normal or elevated levels of ALT. However, differential AST function in patients correlated with the presence of endocrine disease and abnormalities of the digestive system. Fever or fatigue was a differentiating factor between patients with normal and elevated levels of ALT and AST (Table 1). Furthermore, among the remaining physiological anomalies analyzed, symptoms of respiratory failure influenced disease progression in patients with normal and elevated levels of AST (Table 1).
Tables 2, 3 shown the correlation between biochemical laboratory results in patients with normal and elevated levels of ALT and AST. The majority of the parameters showed significant differences between patient groups. Moreover, treatment with antiviral drugs, corticosteroids, and traditional Chinese medicines affected patients with normal and elevated levels of ALT (Table 4). Anticoagulants, corticosteroids, vitamin C, and oxygen support (positive pressure nasal cannula, high-flow nasal cannula, and invasive mechanical ventilation) showed significant differences in patients with normal and elevated levels of AST (Table 4). Compared to differential levels of ALT, those of AST in patients correlated with various parameters.
Tables 5, 6 shown the correlation between elevated levels of AST, ALT, and AST/ALT and disease severity/mortality in patients with COVID-19. Logistic and Cox regression analyses revealed that elevated levels of AST and AST/ALT, but not ALT, correlated with poor prognosis in patients with COVID-19 relative to the prognosis of patients with normal levels of AST and AST/ALT. After adjusting for age, history of cardiovascular disease, and white blood cell, platelet, and lymphocyte counts, the odds ratios for patients with COVID-19 with elevated levels of AST, ALT, and AST/ALT were 0.818 (95% confidence interval [CI]: 0.274-2.441, P = 0.035), 1.106 (95% CI: 0.777-1.575, P = 0.719), and 2.055 (95% CI: 1.269-3.327, P = 0.003), respectively. Hazard ratios for these patients were 4.195 (95% CI: 1.219-14.422, P = 0.023), 1.885 (95% CI: 0.450-7.904, P = 0.382), and 3.348 (95% CI: 1.57-7.139, P = 0.002), respectively.

Table 5 The odds ratio associated with elevated levels of AST, ALT, and AST/ALT and COVID-19 severity.
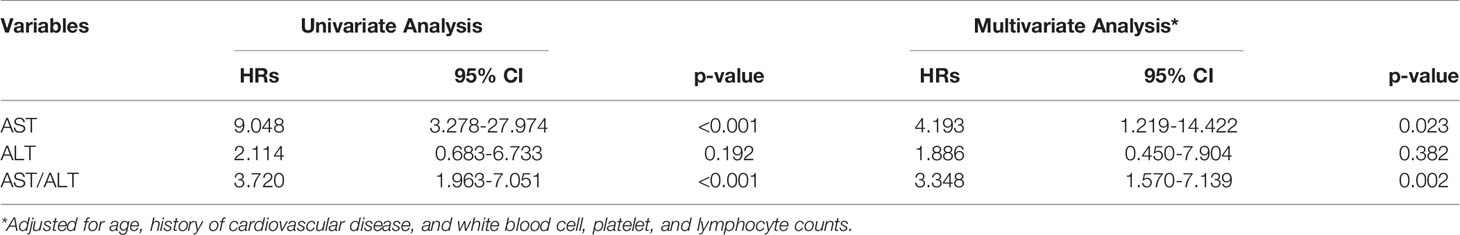
Table 6 The hazards ratio associated with elevated levels of AST, ALT, and AST/ALT and COVID-19 induced mortality.
Kaplan-Meier curves also demonstrated that patients with elevated levels of AST and AST/ALT were at a high risk of developing severe COVID-19. However, the ALT levels did not correlate with patient survival (Figure 1). Using the fitted curves, we observed rising trends in patients with varying levels of AST, ALT, and AST/ALT (Figures 2A–C, E, F and 3A–C, E), excluding score 1 patients with elevated levels of AST (Figure 2D) and ALT (Figure 3D), patients with changes in the total score (Figure 3F), and patients with elevated levels of ALT.
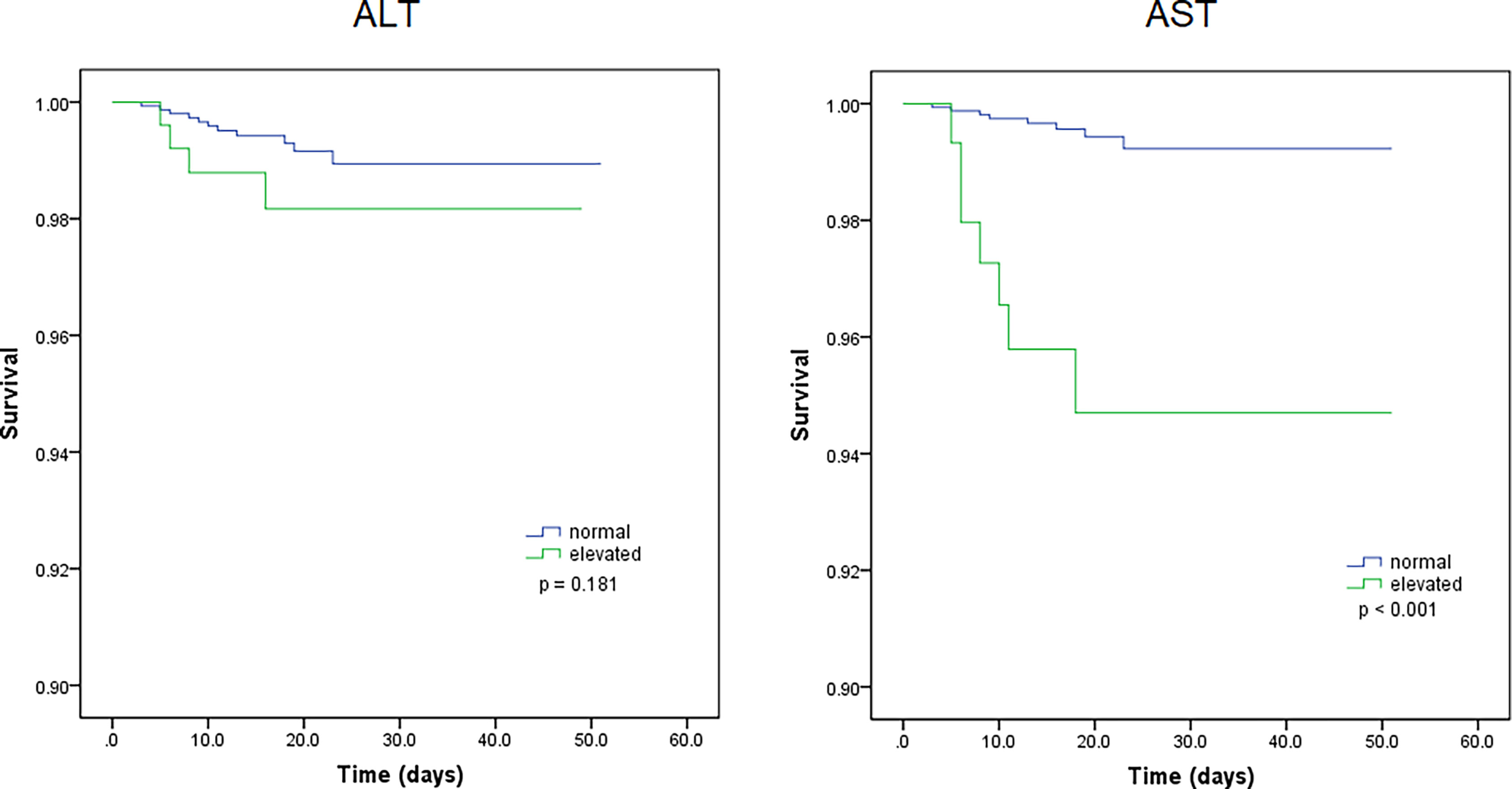
Figure 1 Kaplan-Meier survival curves for patients with normal and elevated levels of aspartate transaminase (AST) and alanine aminotransferase (ALT).
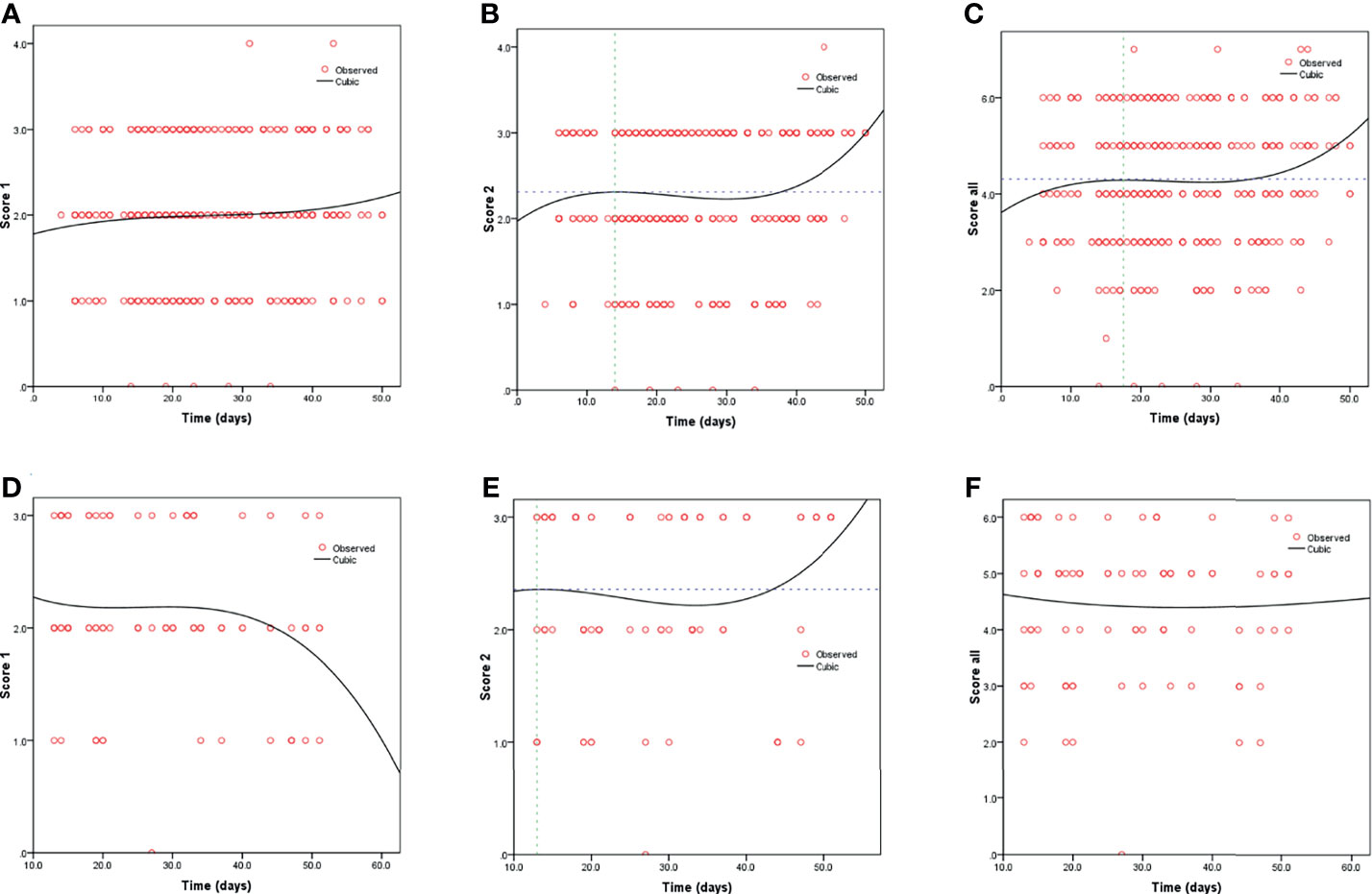
Figure 2 Fitting curves for COVID-19 patients with normal/elevated levels of AST based on CT score. Dynamic changes in patients with (A) CT score 1 and normal AST; (B) CT score 2 and normal AST; (C) total CT score and normal AST; (D) CT score 1 and elevated AST; (E) CT score 2 and elevated AST; and (F) total CT score and elevated AST.
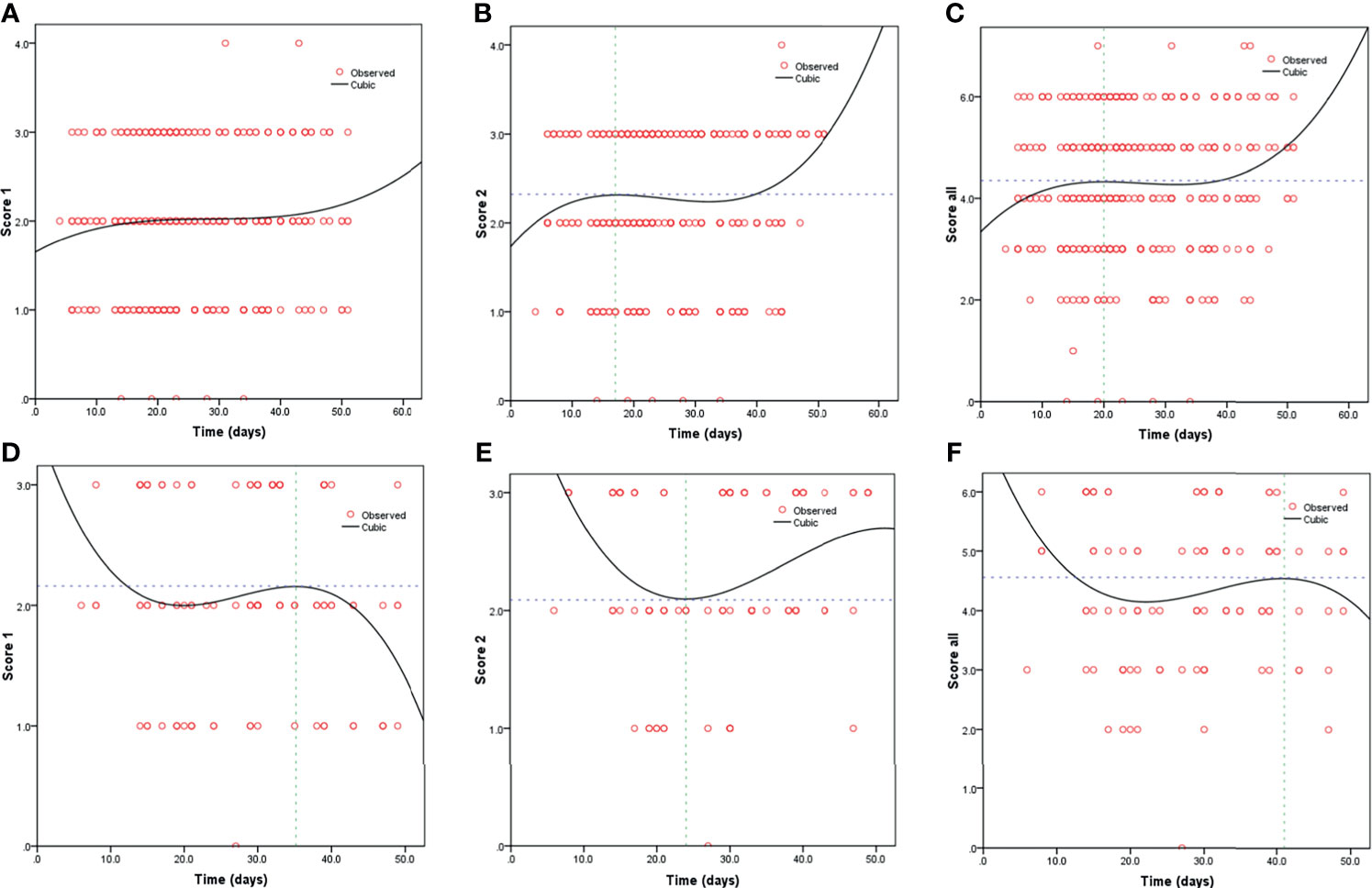
Figure 3 Fitting curves for patients with COVID-19 based on normal/elevated ALT levels and CT scores. Dynamic changes in patients with (A) CT score 1 and normal ALT; (B) CT score 2 and normal ALT; (C) total CT score and normal ALT; (D) CT score 1 and elevated ALT; (E) CT score 2 and elevated ALT; and (F) total CT score and elevated ALT.
In December 2019, we witnessed an outbreak of pneumonia (of unknown etiology) in Wuhan that rapidly spread through China and progressed into a pandemic. This disease was shown to affect multiple organs in patients, in addition to the lung, as research progressed (Huang et al., 2020; Li et al., 2020; Lu et al., 2020). In a pathology report, based on the minimal autopsies of three patients that died of COVID-19 in Chongqin, China, the authors indicated that the lungs of patients with COVID-19 manifested significant pathological lesions. Furthermore, moderate microvascular steatosis and mild lobular and portal lesions, were seen in liver biopsy specimens from COVID-19 cases (Yao et al., 2020). Thus, studies have discussed the involvement of liver function in determining the severity of COVID-19 (Xie et al., 2020; Zhao et al., 2020).
In this study, we studied the symptoms, laboratory test results after admission, treatment measures during hospitalization, and prognosis of 1,788 patients with COVID-19, including 15 fatal cases. Based on t-tests, the majority of biochemical laboratory results differed significantly between the patient groups. There was also a correlation between elevated AST levels and fever or fatigue; symptoms of respiratory failure influenced disease progression in patients with normal and elevated levels of AST. However, there were no significant differences between the patient groups in relation to other comorbidities. Furthermore, adjusted logistic and Cox regression analyses showed that levels of AST and AST/ALT, but not those of ALT, may serve as prognostic markers for increased disease severity and mortality in patients with COVID-19.
COVID-19 is caused by SARS-CoV-2, which similar to other coronaviruses (SARS-CoV-1 and MERS-CoV). Angiotensin-converting enzyme 2 (ACE2) is the receptor responsible for the internalization of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). ACE2 is primarily expressed in the heart, kidney, and testes and expressed at basal levels in many other tissues (Hoffmann et al., 2020; Yan et al., 2020). A recent study has shown the binding between SARS-CoV-2 particles and ACE2 expressed by cholangiocytes. This triggers a systemic inflammatory response that leads to liver injury (Chai et al., 2020) and elevated levels of AST in patients with COVID-19. Furthermore, patients with severe disease exhibit a steep increase in the levels of AST, relative to other indicators of liver injury, thereby increasing the mortality of patients during hospitalization (Lei et al., 2020).
While the AST/ALT levels do not correlate with the histological parameters of disease severity in the liver, changes in this ratio indicate specificity in differentiation between cirrhotic and non-cirrhotic patients with chronic hepatitis C (Sheth et al., 1998). Studies have also shown that the development of primary biliary cirrhosis is associated with changes in AST/ALT (Nyblom et al., 2006). A study by Park et al., revealed that AST/ALT levels were valid predictors of cirrhosis, and that the AST/ALT ratio correlated with the histological grade of necroinflammatory activity and fibrosis (Park et al., 2000). In our study cohort, we found significant differences between patients with elevated AST/ALT levels in terms of severity/mortality. This suggests that patients with COVID-19 and elevated levels of AST/ALT need long-term follow-up, owing to their increased susceptibility to liver cirrhosis.
The fitted curves showed rising trends with increases in the duration of hospital stay for various patient groups. Thus, we speculated that, except for virus-induced liver injury during hospitalization, the use of drugs would significantly increase the risks of liver damage (Chau et al., 2004). Cai et al. showed that the use of lopinavir/ritonavir increased the odds ratio associated with liver injury by four-fold, suggesting the need to closely monitor patients who have been administered lopinavir/ritonavir (especially in patients with abnormal findings in liver function tests during admission) (Cai et al., 2020).
There are also limitations in our study cohort. We found a correlation between the levels of AST and AST/ALT and the prognosis of patients with COVID-19. However, contrary to the results of published studies, ALT levels were not significantly correlated with the prognosis of patients with COVID-19 (Lei et al., 2020). Thus, the involvement of the ALT level as a prognostic marker for patients with COVID-19 remains to be understood in detail. Fang et al. indicated that elevations in the levels of lactate dehydrogenase and gamma-glutamyl transpeptidase reflect bile duct injury; another thesis reported that the binding of ACE2 expressed by cholangiocytes to SARS-CoV-2 affects the liver (Lei et al., 2020; Xu et al., 2020). Thus, it is imperative to delineate the mechanism(s) employed by SARS-CoV-2 to alter liver function.
In conclusion, we have demonstrated that elevated levels of AST and AST/ALT may serve as markers for disease progression, poor prognosis, and high mortality. Moreover, the correlation between elevated levels of AST/ALT and poor long-term prognosis indicates the development of liver cirrhosis. Thus, clinicians should provide special attention to patients with COVID-19 with abnormal live function and encourage long-term follow-up after their recovery and discharge.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Research Ethics Commission of the Zhongnan Hospital, Wuhan University (approval number: 2020074), and patient consent was waived by the ethics committee as COVID19 is a rapidly evolving infectious disease.
All authors contributed to the design of the study and writing of the manuscript. QX and YG undertook the research. ZWL, QW, MLY, and YH performed the analyses and interpretation of data. ZML, DH, and JL wrote the main manuscript text and prepared figures. MW, LG, and XW revised the article critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
COVID-19, coronavirus disease; AST, aspartate transaminase; ALT, alanine transaminase; CI, confidence interval; CT, computed tomography; SD, standard deviation; IQR, median and interquartile range; ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Cai, Q., Huang, D., Yu, H., Zhu, Z., Xia, Z., Su, Y., et al. (2020). COVID-19: Abnormal Liver Function Tests. J. Hepatol. 73 (3), 566–574. doi: 10.1016/j.jhep.2020.04.006
Chai, X., Hu, L., Zhang, Y., Han, W., Lu, Z., Ke, A., et al. (2020). Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv 2020.02.03.931766. doi: 10.1101/2020.02.03.931766
Chau, T.-N., Lee, K.-C., Yao, H., Tsang, T.-Y., Chow, T.-C., Yeung, Y.-C., et al. (2004). SARS-Associated Viral Hepatitis Caused by a Novel Coronavirus: Report of Three Cases. Hepatology 39 (2), 302–310. doi: 10.1002/hep.20111
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181 (2), 271–280. doi: 10.1016/j.cell.2020.02.052
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (London England) 395 (10223), 497–506. doi: 10.1016/S0140-6736(20)30183-5
Hui, D. S., Azhar E, I., Madani, T. A., Ntoumi, F., Kock, R., Dar, O., et al. (2020). The Continuing 2019-nCoV Epidemic Threat of Novel Coronaviruses to Global Health - The Latest 2019 Novel Coronavirus Outbreak in Wuhan, China. Int. J. Infect. Dis. 91, 264–266. doi: 10.1016/j.ijid.2020.01.009
Jothimani, D., Venugopal, R., Abedin, M. F., Kaliamoorthy, I., Rela, M. (2020). COVID-19 and the Liver. J. Hepatol. 73 (5), 1231–1240. doi: 10.1016/j.jhep.2020.06.006
Khan, S., Siddique, R., Shereen, M. A., Ali, A., Liu, J., Bai, Q., et al. (2020). Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options. J. Clin. Microbiol. 58 (5):e00187-20. doi: 10.1128/JCM.00187-20
Lei, F., Liu, Y.-M., Zhou, F., Qin, J.-J., Zhang, P., Zhu, L., et al. (2020). Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology 72 (2), 389–398. doi: 10.1002/hep.31301
Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., et al. (2020). Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl. J. Med. 382 (13), 1199–1207. doi: 10.1056/NEJMoa2001316
Lu, H., Stratton, C. W., Tang, Y.-W. (2020). Outbreak of Pneumonia of Unknown Etiology in Wuhan, China: The Mystery and the Miracle. J. Med. Virol. 92 (4), 401–402. doi: 10.1002/jmv.25678
National Health Commission of the People’s Republic of China. Prevention and Control Guideline for COVID-19 (7th Edition CiC).
Nyblom, H., Björnsson, E., Simrén, M., Aldenborg, F., Almer, S., Olsson, R. (2006). The AST/ALT Ratio as an Indicator of Cirrhosis in Patients With PBC. Liver Int. 26 (7), 840–845. doi: 10.1111/j.1478-3231.2006.01304.x
Pan, F., Ye, T., Sun, P., Gui, S., Liang, B., Li, L., et al. (2020). Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology 295 (3), 715–721. doi: 10.1148/radiol.2020200370
Park, G. J., Lin, B. P., Ngu, M. C., Jones, D. B., Katelaris, P. H. (2000). Aspartate Aminotransferase: Alanine Aminotransferase Ratio in Chronic Hepatitis C Infection: Is it a Useful Predictor of Cirrhosis? J. Gastroenterol. Hepatol. 15 (4), 386–390. doi: 10.1046/j.1440-1746.2000.02172.x
Sheth, S. G., Flamm, S. L., Gordon, F. D., Chopra, S. (1998). AST/ALT Ratio Predicts Cirrhosis in Patients With Chronic Hepatitis C Virus Infection. Am. J. Gastroenterol. 93 (1), 44–48. doi: 10.1111/j.1572-0241.1998.044_c.x
Vardhana, S. A., Wolchok, J. D. (2020). The Many Faces of the Anti-COVID Immune Response. J. Exp. Med. 217 (6), e20200678. doi: 10.1084/jem.20200678
Worldometer COVID-19 Data. Available at: https://www.worldometers.info/coronavirus/about/.
Xie, H., Zhao, J., Lian, N., Lin, S., Xie, Q., Zhuo, H. (2020). Clinical Characteristics of non-ICU Hospitalized Patients With Coronavirus Disease 2019 and Liver Injury: A Retrospective Study. Liver Int. 40 (6), 1321–1326. doi: 10.1111/liv.14449
Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al. (2020). Pathological Findings of COVID-19 Associated With Acute Respiratory Distress Syndrome. Lancet Respir. Med. 8 (4), 420–422. doi: 10.1016/S2213-2600(20)30076-X
Yan, R., Zhang, Y., Li, Y., Xia, L., Guo, Y., Zhou, Q. (2020). Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 367 (6485), 1444–1448. doi: 10.1126/science.abb2762
Yao, X. H., Li, T. Y., He, Z. C., Ping, Y. F., Liu, H. W., Yu, S. C., et al. (2020). A Pathological Report of Three COVID-19 Cases by Minimal Invasive Autopsies. Zhonghua Bing Li Xue Za Zhi 49 (5), 411–417. doi: 10.3760/cma.j.cn112151-20200312-00193
Zhang, Y., Zheng, L., Liu, L., Zhao, M., Xiao, J., Zhao, Q. (2020). Liver Impairment in COVID-19 Patients: A Retrospective Analysis of 115 Cases From a Single Centre in Wuhan City, China. Liver Int. 40 (9), 2095–2103. doi: 10.1111/liv.14455
Keywords: AST - aspartate transaminase, ALT - alanine transaminase, AST/ALT, aspartate aminotransferase/alanine aminotransferase, COVID-19, pneumonia
Citation: Liu Z, Hu D, Li J, Xia Q, Gong Y, Li Z, Wu Q, Yi M, Huang Y, Wu M, Guo L and Wu X (2021) Prognostic Potential of Liver Enzymes in Patients With COVID-19 at the Leishenshan Hospital in Wuhan. Front. Cell. Infect. Microbiol. 11:636999. doi: 10.3389/fcimb.2021.636999
Received: 02 December 2020; Accepted: 25 October 2021;
Published: 16 November 2021.
Edited by:
Yang Zhang, University of Pennsylvania, United StatesReviewed by:
Ahmad Qasem, University of Central Florida, United StatesCopyright © 2021 Liu, Hu, Li, Xia, Gong, Li, Wu, Yi, Huang, Wu, Guo and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Wu, d3V4aWFvaHVpMTk3MUBzaW5hLmNvbQ==; Liang Guo, Z3VvbGlhbmd3aHpuQDE2My5jb20=; Meng Wu, d2IwMDA3MTNAd2h1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.