
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 02 November 2020
Sec. Fungal Pathogenesis
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.601834
This article is part of the Research TopicFungal Respiratory Infections in Cystic FibrosisView all 13 articles
Aspergillus fumigatus (Af) frequently colonizes the airways of patients with cystic fibrosis (CF) and can cause severe diseases, such as allergic bronchopulmonary aspergillosis, Af bronchitis or even Af pneumonia. However, risk factors, including environmental factors, for acquiring Af in the respiratory tract of patients with CF are rarely studied and described. The aim of this study was to investigate whether urban or rural life could affect colonization with Af in the respiratory tract of patients with CF. Due to privacy policy, registry data are usually not linked to patients´ home addresses. It is therefore very difficult to analyze the influence of the patient´s residential environment. This prospective questionnaire survey was carried out in 31 German CF centers in 2018. Only completed surveys, including a clearly assigned type of residential area were included. Statistical analysis was performed by chi-squared test and logistic regression models. A total of 1016 questionnaires were analyzed (Patients` age: 23 ± 13; 0–88 years; female gender: n=492; 48%). The majority of patients with CF live in large cities (n =314; 30.9%) or urban districts (n=461; 45.4%). Prevalence of 30.2% was found for Af, within the 12 months of investigation period. Af colonization was significantly associated with urban life (p=0.004). Urban live should be considered as possible new risk factor for colonization with Af in the respiratory tract of patients with CF. These new results may raise the awareness of the influence of environmental factors on patient outcomes and should be included in patient guidance and preventive measures.
The life-limiting autosomal recessive disorder Cystic fibrosis (CF) is characterized by abnormal viscous secretions in the lower airways leading to obstruction, inflammation, tissue damage and destruction of the lung (Elborn, 2016).In patients with CF, fungal colonization of the respiratory tract is frequently found (Pihet et al., 2009). Aspergillus fumigatus (Af), the most common filamentous fungus in CF patients with a prevalence of 30 %, is becoming more frequently isolated (Ziesing et al., 2016; Brandt et al., 2018; Schwarz et al., 2018; Hong et al., 2020). The airways are constantly exposed to fungal spores, yet which are only a part of the airway pathogens in the environment. Hundreds of Aspergillus spores are inhaled every day, and due to their small size, they directly enter the small airways (Latge, 1999). In CF airways, these spores are able to persist and germinate, leading to immune responses with leukocyte infiltration and mucus accumulation (Singh et al., 2018). Aspergillus species are associated with negative patient outcome and can cause severe diseases, like allergic bronchopulmonary aspergillosis (ABPA) (Singh et al., 2018). About 1–15 % of the CF patients suffer from this serious Aspergillus-related disease (Stevens et al., 2003). ABPA is caused by hypersensitivity to allergens of Af (Janahi et al., 2017), is characterized by a variety of clinical and immunological responses (Stevens et al., 2003) and is associated with a negative effect on lung function (Kraemer et al., 2006). Until now, diagnosis of ABPA in patients with CF remains challenging (Janahi et al., 2017). In future, approximately 70 % of the world´s population will live in urban areas and modern urbanites will spend approximately 90 % of their times indoors (Rydin et al., 2012; Leung and Lee, 2016; Flies et al., 2019). If the patient’s environment poses a potential risk to Aspergillus colonization, preventive measures could be identified. Limited work has been done examining Af colonization in CF patients regarding their type of residential area in Germany. As registry data of the patient are not linked with their home address, this questionnaire survey was conducted to gain evidence of an association between aspergillosis and urban or rural life.
This questionnaire survey was conducted in German CF centers in 2018. The target population was people with CF independent of age and living in Germany. Participation was voluntary. Ethical aspects were considered and approval for the study was gained by the Ethics Committee of the Charité – Universitätsmedizin Berlin (EA2/057/18).
The questionnaire included several items, which are not documented in the medical data base German Cystic Fibrosis Registry. Therefore, beside patients’ age and sex and their history of Af colonization and ABPA diagnosis within the last 12 months, the postal code of the place of residence, were asked for in the questionnaire. For a positive of Af colonization within the 12 month of observation period, at least one positive microbiological indication was required. The ABPA diagnosis was determined in every single CF center and was based on the minimal 2003 Cystic Fibrosis Foundation (CFF) consensus criteria (Stevens et al., 2003). No other Af associated phenotypes were considered. Besides Af, no other Aspergillus spp. were included. The questionnaire was sent to 94 CF centers in Germany in 2018 and data of 1,477 patients have been received from 31 CF centers. Postal code was correlated to rural and urban area with data from the German Federal Institute for Building, Urban and Spatial Research (BBSPR). The population density was correlated from the postal code with data from the German Statistic Federal Office (DESTATIS).
Only completed questionnaires with distinct classification for the type of residential area and population density were included in final analysis. The residential area was classified as large city, urban district, rural district and sparsely populated rural area. Population density was graduated in high, medium and low. Means and standard deviations were calculated for metrical variables. Frequency and percentage were used for categorical variables. Associations of categorical variables were analyzed using Pearson´s chi-square tests. In addition, all parameters were considered within binary logistic regression analysis. For multiple binary logistic regression analysis (only for Af colonization), the following parameters have been adjusted: age (years) and residential area (large city, urban district, rural district and sparsely populated rural area). In this exploratory study the level of significance (α=0.05) was not adjusted for multiple testing. Statistics were calculated using IBM SPSS Statistics 24.
A total of 1,465 patients with CF participated and a total of 1,016 completed questionnaires were analyzed. Of the included patients, 48.4% were female (n = 492; male: n = 524; 51.6 %). The mean age was 23 ± 13 years (range: 0–88 years). The majority of CF patients live in large cities (n = 314; 30.9 %) and urban districts (n = 461; 45.4 %; Table 1).
Prevalence of 30.2 % (n = 307) was found for Af colonization within the 12 months of observation period (Table 1). Af colonization was significantly associated with urban life (Tables 2, 4) including an increased prevalence of 36.6 % of Af colonization in large cities (Table 2). Binary logistic regression of Af colonization was performed including gender (male gender: p = 0.244), age (p < 0.001), different types of residential area (p = 0.022) and population densities (p = 0.918). Adjusted odds ratios for age and residential areas were shown in Table 4.
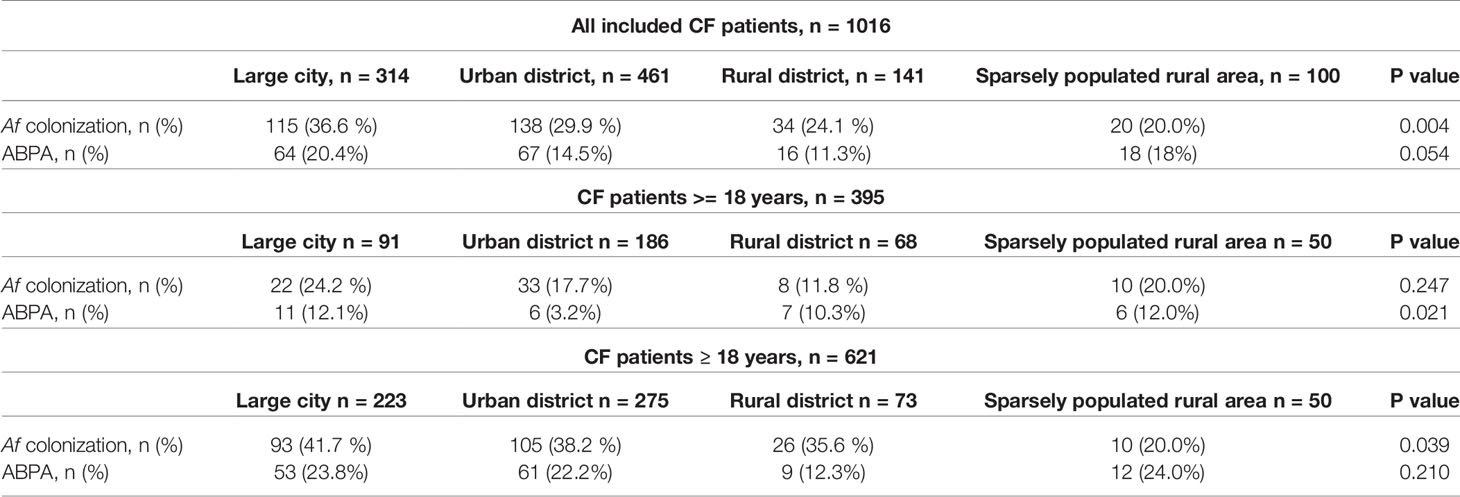
Table 2 Af colonization and ABPA diagnosis in different types of residential area for all included CF patients.
ABPA was documented in 16.2 % (n = 165) of the CF patients (Table 1), with a slightly pronounced documentation in large cities but without statistical significance compared to the other types of residential area (p = 0.054; Table 2). The individual associations between ABPA diagnosis and the investigated parameters were also examined using logistic regression (n = 1016; male gender: p = 0.913; residential area: p = 0.067; population density: p = 0.424). Odds ratio (1.021) and 95 % confidence interval (1.009–1.034) could only be determined for age (p = 0.001).
In addition to the residential area, population density was determined for analyzation. It shows that the majority of CF patients live in regions with a medium population density (n = 601; 59.2 %) and only 13.5 % (n = 137) in areas with high population density (Table 1). No statistical significance was calculated for Af (p = 0.357) nor ABPA (p = 0.871) documentation within the observation period in association with population density (Table 3).
Among the 1016 patients with CF included in this analysis, n = 395 (38.9 %) were less than 18 years of age. N = 197 (49.9 %) of them were female. Prevalence of 18.5 % (n = 73) was found for Af. ABPA was documented in 7.6 % (n = 30) in this cohort (Table 1). Both, Af colonization and ABPA diagnosis was significantly lower in CF patients <18 years compared to adult CF patients (p<0.001; Table 1). Due to small case numbers for CF patients < 18 years, examination of Af colonization and ABPA measures regarding the type of residential area (Table 2) and population density (Table 3), the results have to be carefully interpreted.
N = 621 (61.1 %) CF patients were ≥ 18 years of age and 47.5 % (n = 295) of them were female. Prevalence for Aspergillus was 37.7 % (n = 234; Table 1) Af colonization was significantly associated with urban life in large cities (p = 0.039; Table 2). ABPA was documented in n = 135 (21.7%) of the adult CF patients (Table 1). No statistical significance was calculated for Af colonization (p = 0.977) nor ABPA (p = 0.306) in association with population density (Table 3).
This nationwide questionnaire survey explored the association of aspergillosis documentation in different types of residential area and various population densities. Af colonization is significantly associated with urban life in large cities (Tables 2, 4). History of ABPA during the 12 months of observation period was not significantly associated with urban or rural life. ABPA tended to be elevated in large cities and sparsely populated rural areas (Table 2). In contrast to residential area types, population density measures showed no significance with regard to aspergillosis (except ABPA data for CF patients < 18 years; p = 0.017; Table 3). One could speculate that population density is not the decisive factor. This inconsistency may be related to different data origins (BBSPR vs. DESTATIS). In contrast to residential area types, population density was divided in only three categories (high, medium, low), not reflecting extreme values (e.g. large cities and sparsley populated rural area). Maybe other environmental parameters, better reflected by different residential area types, are more important, e.g. architecture, furnishing or exposure to air pollution including particulate matter. A French study previously described Af distribution: Urbanized areas and heterogeneous agricultural areas were mentioned to be positively related to Af (Rocchi et al., 2020). Particularly, large cities seem to have negative effects. But also, agricultural air pollutants contribute to human health problems (Aneja et al., 2009). Particulate air pollution was associated with an increased risk of pulmonary exacerbations in CF (Brugha et al., 2018), a decline in lung function (Goss et al., 2004) and can exert proinflammatory and immunomodulatory effects (Reinmuth-Selzle et al., 2017). Our findings may reflect that in urban as well as sparsely populated rural areas synergistic effects of more frequent Af acquisition and immunomodulatory effects of air pollution influence the CF patients Af outcome. It is postulated that, pollution exposure early in life might associate with an increased risk of early infection to certain microbes (Brugha et al., 2018). Furthermore, the exposure to environmental factors might be even more harmful in children than in adults, because of behavioural and physiological factors (Goldizen et al., 2016). This may partly explain the determined frequencies for ABPA in patients <18 years in the studied residential area types. In future, the majority of people will live in urban areas, spending most of their time indoors (Rydin et al., 2012; Leung and Lee, 2016; Flies et al., 2019). Many issues are connected with this progress and the influence of urban environment associated factors will increase. The association between urban environments and disease is complex and context specific. Urban environments pose potential health risks and offer potential health benefits, such as improved access to health care, education, employment, or infrastructure (Flies et al., 2019). These health care aspects could lead to possible bias in the present work. One could speculate, that more severely affected CF patients tend to move to larger cities to benefit from better medical care. Infections require inhalation of fungal spores from the environment (Harun et al., 2010). Aspergillus is among the most common life-threatening airborne opportunistic fungus found indoors (Rocchi et al., 2020). The presence of Af in CF sputum is associated with worse respiratory quality of life in CF (Hong et al., 2020) and chronic Af is associated with more frequent pulmonary exacerbation dependent hospitalizations (Hong et al., 2018). CF patient registry data has already been used for risk factor analysis and macrolides, inhaled antibiotics and inhaled corticosteroids were associated with persistent Af isolation (Hong et al., 2018). In contrast, this questionnaire survey focused on urban and rural areas or population density items. Therefore, the impact of simultaneous exposure to multiple contaminants (other fungi, bacteria) has not been observed. Some species showed differences in concentrations according to geographic locations and the distribution of fungal spores is not homogeneous in the atmosphere (Rocchi et al., 2020). Regional or geographical patterns as well as different outdoor parameters or indoor characteristics (thermo-isolation, ventilation, architecture) were not considered in this study. Furthermore, no information on the frequency of microbiological examinations in the individual CF centres was available and questionnaire associated bias have to be considered. The possible influence of contact with pets will be discussed in detail in a separate article of this thematic special issue (see “Frequent pet contact as risk factor for allergic bronchopulmonary aspergillosis in cystic fibrosis”). Due to the 12 months observation period, the importance of seasonality on Af distribution can be excluded. The aim of the study was to investigate whether urban or rural life could affect the appearance of a colonization with Af in the respiratory tract of patients with CF. There is increasing evidence that there are adverse effects associated with the environment in large cities. The understanding of potential risk factors for aspergillosis permits the implementation of patient guidance and preventive measures.
Urban live should be considered as possible risk factor for colonization with Af in the respiratory tract of patients with CF. These new results may raise the awareness of the influence of environmental factors on patient outcomes and should be included in patient guidance and preventive measures.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Charité—Universitätsmedizin Berlin. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
CG, PE, ST, UD, KN, and CS contributed to the conception and design of the study. UD and CG organized the database. KN and CG performed the statistical analysis. CG, PE, ST, UD, KN, and CS wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the German Federal Ministry of Education and Research (BMBF)—Project InfectControl 2020 (“Art4Fun”, 03ZZ0813E).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all participating patients and members of the CF centers involved in this study and the Art4Fun working group. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Aneja V. P., Schlesinger W. H., Erisman J. W. (2009). Effects of agriculture upon the air quality and climate: research, policy, and regulations. Environ. Sci. Technol. 43 (12), 4234–4240. doi: 10.1021/es8024403
Brandt C., Roehmel J., Rickerts V., Melichar V., Niemann N., Schwarz C. (2018). Aspergillus Bronchitis in Patients with Cystic Fibrosis. Mycopathologia 183 (1), 61–69. doi: 10.1007/s11046-017-0190-0
Brugha R., Edmondson C., Davies J. C. (2018). Outdoor air pollution and cystic fibrosis. Paediatr. Respir. Rev. 28, 80–86. doi: 10.1016/j.prrv.2018.03.005
Elborn J. S. (2016). Cystic fibrosis. Lancet 388 (10059), 2519–2531. doi: 10.1016/S0140-6736(16)00576-6
Flies E. J., Mavoa S., Zosky G. R., Mantzioris E., Williams C., Eri R., et al. (2019). Urban-associated diseases: Candidate diseases, environmental risk factors, and a path forward. Environ. Int. 133 (Pt A):105187. doi: 10.1016/j.envint.2019.105187
Goldizen F. C., Sly P. D., Knibbs L. D. (2016). Respiratory effects of air pollution on children. Pediatr. Pulmonol. 51 (1), 94–108. doi: 10.1002/ppul.23262
Goss C. H., Newsom S. A., Schildcrout J. S., Sheppard L., Kaufman J. D. (2004). Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am. J. Respir. Crit. Care Med. 169 (7), 816–821. doi: 10.1164/rccm.200306-779OC
Harun A., Gilgado F., Chen S. C., Meyer W. (2010). Abundance of Pseudallescheria/Scedosporium species in the Australian urban environment suggests a possible source for scedosporiosis including the colonization of airways in cystic fibrosis. Med. Mycol. 48 (Suppl 1), S70–S76. doi: 10.3109/13693786.2010.515254
Hong G., Psoter K. J., Jennings M. T., Merlo C. A., Boyle M. P., Hadjiliadis D., et al. (2018). Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J. Cyst. Fibros 17 (5), 624–630. doi: 10.1016/j.jcf.2018.01.008
Hong G., Alby K., Ng S. C. W., Fleck V., Kubrak C., Rubenstein R. C., et al. (2020). The presence of Aspergillus fumigatus is associated with worse respiratory quality of life in cystic fibrosis. J. Cyst. Fibros 19 (1), 125–130. doi: 10.1016/j.jcf.2019.08.008
Janahi I. A., Rehman A., Al-Naimi A. R. (2017). Allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Ann. Thorac. Med. 12 (2), 74–82. doi: 10.4103/atm.ATM_231_16
Kraemer R., Delosea N., Ballinari P., Gallati S., Crameri R. (2006). Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 174 (11), 1211–1220. doi: 10.1164/rccm.200603-423OC
Latge J. P. (1999). Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12 (2), 310–350. doi: 10.1128/CMR.12.2.310
Leung M. H., Lee P. K. (2016). The roles of the outdoors and occupants in contributing to a potential pan-microbiome of the built environment: a review. Microbiome 4 (1), 21. doi: 10.1186/s40168-016-0165-2
Pihet M., Carrere J., Cimon B., Chabasse D., Delhaes L., Symoens F., et al. (2009). Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis–a review. Med. Mycol. 47 (4), 387–397. doi: 10.1080/13693780802609604
Reinmuth-Selzle K., Kampf C. J., Lucas K., Lang-Yona N., Frohlich-Nowoisky J., Shiraiwa M., et al. (2017). Air Pollution and Climate Change Effects on Allergies in the Anthropocene: Abundance, Interaction, and Modification of Allergens and Adjuvants. Environ. Sci. Technol. 51 (8), 4119–4141. doi: 10.1021/acs.est.6b04908
Rocchi S., Reboux G., Scherer E., Laboissiere A., Zaros C., Rouzet A., et al. (2020). Indoor Microbiome: Quantification of Exposure and Association with Geographical Location, Meteorological Factors, and Land Use in France. Microorganisms 8 (3), 341. doi: 10.3390/microorganisms8030341
Rydin Y., Bleahu A., Davies M., Davila J. D., Friel S., De Grandis G., et al. (2012). Shaping cities for health: complexity and the planning of urban environments in the 21st century. Lancet 379 (9831), 2079–2108. doi: 10.1016/S0140-6736(12)60435-8
Schwarz C., Bouchara J. P., Buzina W., Chrenkova V., Dmenska H., de la Pedrosa E. G. G., et al. (2018). Organization of Patient Management and Fungal Epidemiology in Cystic Fibrosis. Mycopathologia 183 (1), 7–19. doi: 10.1007/s11046-017-0205-x
Singh A., Ralhan A., Schwarz C., Hartl D., Hector A. (2018). Fungal Pathogens in CF Airways: Leave or Treat? Mycopathologia 183 (1), 119–137. doi: 10.1007/s11046-017-0184-y
Stevens D. A., Moss R. B., Kurup V. P., Knutsen A. P., Greenberger P., Judson M. A., et al. (2003). Allergic bronchopulmonary aspergillosis in cystic fibrosis–state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 37 (Suppl 3), S225–S264. doi: 10.1086/376525
Keywords: cystic fibrosis, aspergillosis, Aspergillus fumigatus, allergic bronchopulmonary aspergillosis, urban life, rural life, respiratory infection
Citation: Grehn C, Eschenhagen P, Temming S, Düesberg U, Neumann K and Schwarz C (2020) Urban Life as Risk Factor for Aspergillosis. Front. Cell. Infect. Microbiol. 10:601834. doi: 10.3389/fcimb.2020.601834
Received: 01 September 2020; Accepted: 13 October 2020;
Published: 02 November 2020.
Edited by:
Andy Mark Borman, Public Health England, United KingdomReviewed by:
Stéphane Ranque, Aix-Marseille Université, FranceCopyright © 2020 Grehn, Eschenhagen, Temming, Düesberg, Neumann and Schwarz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Grehn, Y2xhdWRpYS5ncmVobkBjaGFyaXRlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.