
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 05 January 2021
Sec. Parasite and Host
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.526997
This article is part of the Research Topic Early Events During Host Cell - Parasite Interactions View all 11 articles
 Chao Yan1,2*†
Chao Yan1,2*† Jing Wu1,3†
Jing Wu1,3† Na Xu1
Na Xu1 Jing Li1
Jing Li1 Qian-Yang Zhou1
Qian-Yang Zhou1 Hui-Min Yang1
Hui-Min Yang1 Xiao-Dan Cheng1
Xiao-Dan Cheng1 Ji-Xin Liu1
Ji-Xin Liu1 Xin Dong1
Xin Dong1 Stephane Koda1
Stephane Koda1 Bei-Bei Zhang1,2
Bei-Bei Zhang1,2 Qian Yu1,2
Qian Yu1,2 Jia-Xu Chen4
Jia-Xu Chen4 Ren-Xian Tang1,2
Ren-Xian Tang1,2 Kui-Yang Zheng1,2*
Kui-Yang Zheng1,2*Mice with different genetic backgrounds have various susceptibilities to infection with Clonorchis sinensis, although the mechanisms underlying are largely unknown. Toll-like receptor 4 (TLR4) as one of the most important pattern recognition receptors (PPRs) is essential for the invasion, survival, pathogenesis, and elimination of worms. The roles played by TLR4 in C. sinensis infection may vary due to the different genetic backgrounds of mice. In the present study, a relatively resistant mouse strain-C57BL/10 to C. sinensis was used for investigation on the possible roles of TLR4 in the biliary injuries and peribiliary fibrosis. TLR4 wild type (TLR4wild) and TLR4 defective (TLR4def) mice were orally infected with 45 metacercariae of C. sinensis, and all C. sinensis-infected mice and non-infected groups were anesthetized on day 28 post-infection. The liver and serum from each mouse were collected for assessment of the biliary injuries and biliary fibrosis. Meanwhile, hepatic leukocytes were isolated and detected for the activation of M1 or M2 macrophage using flow cytometry. The hepatic type 1 immune response and type 2 immune responses -relative molecules were also evaluated using ELISA and quantitative PCR. The data showed that TLR4def aggravated liver inflammatory cell infiltrations, bile duct proliferation, biliary and hepatocellular injuries, and ECM deposition in C. sinensis-infected mice, compared with TLR4wild mice when they were intragastrically administered with the same amounts of C. sinensis metacercaria. Furthermore, the M2-like macrophages and type 2 immune responses were significantly predominant induced in TLR4def mice, compared with that of TLR4wild mice following C. sinensis infection. But the type 1 immune response were significantly decreased in TLR4def mice, compared with TLR4wild mice after C. sinensis infection. These data demonstrate that TLR4 deficiency exacerbates biliary injuries and peribiliary fibrosis caused by C. sinensis in C57BL/10 strain mice, which is contributed by augments of type 2 immune responses and decrease pro-inflammatory responses.
Clonorchis sinensis is a zoonotic food-borne parasite which infects human or other mammals via ingestion of raw or undercooked fresh fish and shrimp containing metacercaria (Tang et al., 2016). The adult worm mainly dwells in the intrahepatic bile duct, common bile duct, or gall bladder, although it can be also accidentally found in the pancreatic duct (Kim, 1999). Infection with C. sinensis caused 5,591 deaths every year and 275,370 disability-adjusted Life Year (DALYs) (Sripa, 2012), of which ranges from cholangitis, obstructive jaundice, gallstones to hepatic fibrosis due to its mechanical stimulation and production and secretion antigen (Hong and Fang, 2012; Zheng et al., 2015). In addition, a long-term infection by C. sinensis can potently induce bile duct carcinoma and C. sinensis is considered as a type I Biological carcinogen (Tyson and El-Serag, 2011; Zheng et al., 2015).
Toll-like receptors (TLRs) are an important family of pattern recognition receptors (PRRs), which play a very important role in the innate immune response as well as adaptive immune responses (Akira et al., 2001). It is widely expressed in immune cells (such as macrophages, dendritic cells, NK cells, lymphocytes, granulocytes) and non-immune cells (such as epithelial cells, endothelial cells, fibroblasts, cancer cells, etc.), and is involved in almost all human disease processes (Ciferska et al., 2020). Among the 13 TLRs in human or 11 TLRs in mice, TLR4 has been the first to be identified and has a relatively broad ligand specificity. TLR4 can interact with LPS, peptidoglycan, glycoprotein, and so on, and ultimately produce pro-inflammatory cytokines and inflammatory chemokines through MyD88 or TRIF adaptor protein to regulate immunity, which play critical roles in fighting against pathogenic insults (Iwasaki and Medzhitov, 2004). For example, upon binding to the ligand, TLR4 can activate nuclear transcription factors NF-κB or MAPK to induce the release of pro-inflammatory cytokines and regulate activation of macrophage by MyD88 dependent signaling pathway and TRIF-dependent signaling pathway (Li and Cherayil, 2003; Jiang et al., 2015; Molteni et al., 2016). However, recent studies also showed that the TLR4-induced signaling pathway participates in the homeostasis of the tissue and orchestrated tissues repair after damages caused by various insults. Studies have confirmed that TLR4 plays an important role in liver injury (Cengiz et al., 2015; Seki and Schwabe, 2015; Gandhi, 2020).
Our previous study also showed that TLR4 promoted peribiliary fibrosis by orchestrating the TGF-β signaling pathway in a susceptible mouse model (C3H/HeN mice) (Yan et al., 2017). However, the roles of TLR4 might vary in the different genetic backgrounds of the mouse following the encounter with different stimuli. In the present study, we used TLR4wild (C57BL/10JNju, TLR4wild) and TLR4def (C57BL/10ScN, TLR4def) mice to explore the roles of TLR4 in the pathogenesis of biliary injury within a resistant strain mice by C. sinensis. Surprisingly, different from the roles of TLR4 in a susceptible mouse model demonstrated by our previous study, in the present study, we found that TLR4 def mice showed an aggravation of the biliary injuries and peribiliary fibrosis caused by C. sinensis, which was associated with the increased type 2 immune responses in C. sinensis-resistant mice.
Animal care and all experimental perform in this study were strictly conformed to the guidelines of the National Laboratory Animal Center. The main procedures and protocol were reviewed and approved by the Animal Care and Use Committee of Xuzhou Medical University License (2017-SK-05).
Six- to 8-week-old TLR4wild mice (C57BL/10JNju) and TLR4def mice (C57BL/10ScN, TLR4def) were purchased from the Model Animal Research Center of Nanjing University and maintained under specific non-pathogenic conditions in the model animal research center of Xuzhou Medical University (Xuzhou, Jiangsu, China). The mice were housed in an air-conditioned room at 24°C with a 12-h dark/light cycle and permitted free access to food and water.
Metacercariae of C. sinensis from Pseudorasbora parva were collected by digestion with a pepsin-HCl (0.6%) artificial gastric juice (Yan et al., 2015). In each infected group, 45 metacercariae were intragastrically administrated to the individual, and the irrigating solution was observed under the microscope to ensure that all the metacercariae were completely intragastrically administrated; the mice of the non-infected received the same volume of normal saline. On day 28th post-infection all the mice were sacrificed and the serum and liver tissues were collected for further study.
The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), alkaline phosphatase (ALP), total bile acid (TBA) were assayed in the Department of Laboratory Medicine, Affiliated Hospital of Xuzhou Medical University, China to indicate for hepatocellular and biliary injuries in infected mice and uninfected mice.
Partial liver tissue (about 10 mm × 10 mm × 1 mm) was immersed in 4% paraformaldehyde for 48 h. The embedded tissue wax blocks were serially sectioned at 4 μm for hematoxylin and eosin (H&E) staining according to the manufacturer’s instructions (Jiangsu Beyotime biotechnology research institute, China). After sealing the slides with neutral adhesive, the pathological changes of stained histological sections were observed by microscope (Olympus, Japan).
Four percent paraformaldehyde was used to fix the liver tissue from each strain of mice, then the liver was embedded in paraffin. Three- to 4-μm thick sections were prepared and stained with Masson’s trichrome according to the manufacturer’s instructions (Jiancheng, Nanjing, Jiangsu, China). The sections were observed under the microscope and digitized using an imaging system (Olympus, Japan). Five high-power visual fields (×400 magnifications, Olympus, Japan) were randomly selected from the staining sections of each mouse, and Image Pro Plus6.0 software was used to calculate the Integral optical density (IOD) of fibrous tissue. A higher IOD value means stronger positive expression.
Three- to 4-μm serial thick sections of embedded tissue from each mouse were used for immunohistochemical staining of cytokeratin 19 (CK-19), ki67, alpha-smooth muscle Actin (α-SMA). The liver tissue was deparaffinized, hydrated, and heated in citric acid buffer at 95°C for 10 min and then blocked with 5% BSA for 30 min. The slides were then incubated overnight with primary Anti-Cytokeratin 19(1:500, ab52625, Abcam, Cambridge, US), ki67 (1:400, ab15580, Abcam, Cambridge, US), alpha-smooth muscle Actin (α-SMA) (1:400, ab124964, Abcam, Cambridge, US). After washing with PBS, DAB (1:200, ZSGB–BIO, Beijing, China) as an enzyme-substrate was added. Five high-power fields (×100 magnifications, Olympus, Japan) were randomly selected from each mouse staining section. CK19, ki67, α-SMA positive expression was calculated by the software of Image J (NIH, Bethesda, USA).
Intrahepatic leukocytes were obtained as descript elsewhere with minor modification (Blom et al., 2009). Partial liver tissue from each mouse was minced and grinded gently through a 40 µm-gauge nylon strainer using a sterile syringe plunger, the preparation was then centrifuged at 1,500 rpm for 10 min, the cells were resuspended using 4 ml 40% Percoll (GE Healthcare, catalog number: 17-0891-01) and then transferred into a new tube containing 5 ml 80% Percoll slowly. Subsequently, the gradient solution was centrifuged at 2,500 rpm for 25 min, the leukocytes were obtained from the interphase between 40% Percoll and 80% Percoll. Red blood cells in cell pellets were removed using ACK Lysis Buffer (BD Biosciences, catalog number: 555899, USA). Approximate 107 cells were subjected to cell surface phenotyping by flow cytometry. A panel of antibodies used as markers for detection as follows: anti-CD45 was labeled with PE, anti-CD11b was labeled with PerCP-Cy5.5, anti-CD86 was labeled with PE-Cy™7, anti-CD206 was labeled with Alexa Fluor 647 (all antibodies from BD PharMingen, San Diego, CA, USA). The cells were incubated with antibodies directed toward various cell surface antigens and in the dark on ice for 30 min, following the manufacturer’s recommendations (BD PharMingen, San Diego, CA, USA). Subsequently, the cells were washed by centrifugation at 1500 rpm for 10 min, resuspended in 400 µl FACS buffer, and analyzed utilizing FACSCantoII flow cytometer (Becton Dickinson, San Jose, CA, USA).
Serum and mouse liver homogenate from each mouse was immediately subjected to evaluate the concentration of IgE, IL-4, IL-6, IL-10, IL-13, MCP-1, and TNF-α by commercial Enzyme-linked Immunosorbent Assay (ELISA) Kits (Thermo Scientific, US). All procedures were performed according to the instructions provided by the kit. Concentrations of cytokine in the sera were calculated using standard curves as references.
Total RNA was isolated from the liver by the use of Trizol (Vazyme, Nanjing, China). According to the manufacturer’s instruction, RNA was reverse transcribed into cDNA by use of the FastQuant RT First Kit With gDNase (TIANGEN, Beijing, China). Primers for mouse: NOS2 F: GGCAGCCTCTTGTCTTTGACC; R: GGGAATCTTGGAGCGAGTTGT; Tnfa F: CTCCTCCACTTGGTGGTTTGT; R: GGTGCCTATGTCTCAGCCTCT; Arg1 F: GTCAGTCCCTGGCTTATGGTT; R: CAGCAGAGGAGGTGAAGAGTA; Ym1 F: GGATGGCTACACTGGAGAAA; R: AGAAGGGTCACTCAGGATAA; Fizzl F: CCCTCCACTGTAACGAAG; R: GTGGTCCAGTCAACGAGTAA; ILIb F: TGTGTTTTCCTCCTTGCCTCTGAT; R: TGCTGCCTAATGTCCCCTTGAAT; IL6 F: TCACAGAAGGAGTGGCTAAGGACC; R: ACGCACTAGGTTTGCCGAGTAGAT; Tgfb F: CCACCTGCAAGACCATCGAC; R: CTGGCGAGCCTTAGTTTGGAC;Col-1a F: CAGGGTATTGCTGGACAACGTG; R: GGACCTTGTTTGCCAGGTTCA
The real-time quantitative PCR was performed using a Roche 480 detection system using SYBR Green PCR master mix solution (Roche Diagnostics Ltd, Shanghai, China). Fold changes of each gene to that of control mice were calculated using the formula of 2−ΔΔCt, which was normalized by β-actin. The specificity of each PCR was monitored by Roche 480 detection system with melt-curve analysis.
The hydroxyproline content of the mouse liver were assayed to indicate for collagen deposition of liver in the infected mice and uninfected mice using a commercial kit (Nanjing JianCheng Technology Co. LTD, China). The assay was performed according to the instructions provided in the kit.
All values were expressed as mean ± SEM. The data were analyzed by SPSS19.0 software (SPSS Inc, Chicago, IL, USA). The student t-test was used for comparison between the two groups. One-way ANOVA with Tukey’s post hoc test was used for comparison for more than two groups. If the test level was α=0.05, the difference was statistically significant when P< 0.05.
There were no obvious changes in the liver between TLR4wild and TLR4def mice without infection of C. sinensis (Figure 1A). However, following C. sinensis infection, gross changes of the liver in TLR4 wild-type infected mice were scarce. In contrast, the liver of TLR4def mice infected by C. sinensis had a dark color, tough texture, and uneven edges. Multiple white sesame-sized nodules were visible and most of them were lesions distributed on the left lobe of the liver (Figure 1A). Histological changes of the liver collected from each mouse were observed by H&E staining (Figure 1B). HE staining showed that the hepatocytes in the normal group were arranged neatly, the hepatic lobule structure was complete, and there was no inflammatory cell infiltration in the portal area, bile duct expansion, or hyperplasia (Figure 1B). Following C. sinensis infection, the histological analysis of the liver of wild-type infected mice showed an infiltration of inflammatory cells, with thickened bile duct hyperplasia and a small amount of fibrous tissue hyperplasia (Figure 1B). However, the overall hepatic lobule structure was orderly, the damaged area was relatively limited, and the hepatocytes were orderly arranged. Worm bodies in the bile ducts were not observed within the staining of liver tissues. However, TLR4def infected mice had very serious liver damage: a large number of inflammatory cells were infiltrated around the bile duct, the bile duct epithelium was disrupted, the hepatic lobule structure was damaged, and the liver cells were arranged in disorder (Figure 1B, as indicated by arrows). Liver inflammation was increased as indicated by the mHAI score (Figure 1B, P<0.05). For the bile duct, it could be seen that the bile duct had a complete structure with single-layer epithelium in the non-infected groups of mice. However, when the mice were infected by C. sinensis, TLR4wild mice showed an irregular bile duct and mild proliferation of BECs accompanied by only a few inflammatory cells infiltrated. Compared with TLR4wild mice infected by C. sinensis, TLR4def infected mice showed that the bile duct was more severe and irregular in shape, where mature worms bodies could be observed (indicated as triangle, Figure 1B). Furthermore, we evaluated hepatic damages as indicated by the activities of ALT and AST. It was found that the levels of ALT and AST in TLR4def infected mice were significantly higher than those of TLR4def uninfected mice. Meanwhile, compared with TLR4wild infection group, the activities of ALT and AST were significantly increased in TLR4def infection group (Figures 1C, D, P<0.05).
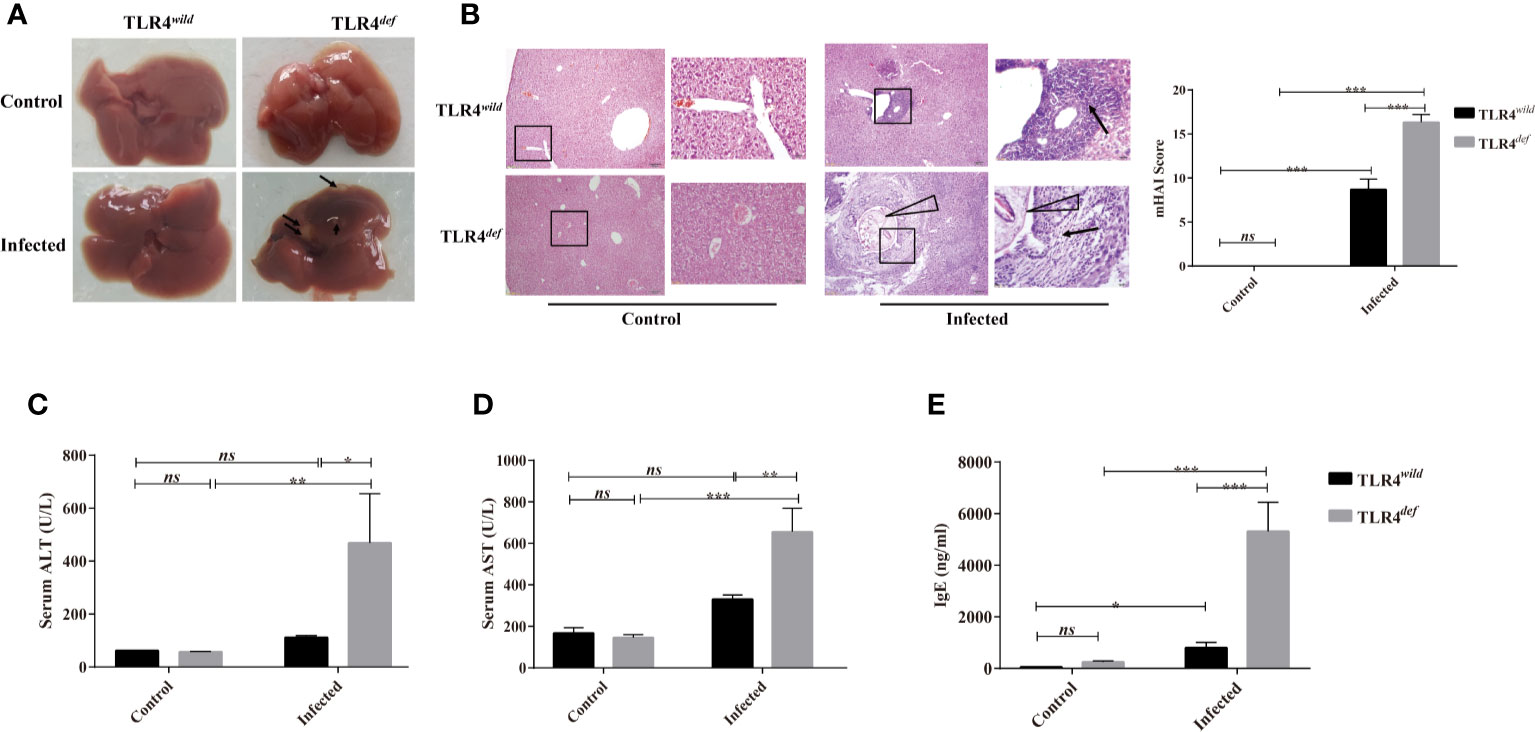
Figure 1 Toll-like receptor 4 (TLR4) deficiency aggravated liver injuries in C57BL/10 mice infected by Clonorchis sinensis. (A) Gross lesions in liver tissue of TLR4wild and TLR4def mice infected with C. sinensis. The arrow indicates the lesion nodule. (B) Histological changes of liver in TLR4wild and TLR4def mice infected with C. sinensis under 100× and 400× microscope were indicated by HE staining and liver histopathological changes were assessed by the mHAI score. An arrow indicates the infiltrated immune cells; triangle indicates worm bodies in the bile ducts. (C) The activities of alanine aminotransferase (ALT) and (D) aspartate aminotransferase (AST) were assessed in the sera of TLR4wild and TLR4def mice infected with C. sinensis. (E) The secretion of IgE in sera of TLR4wild and TLR4def mice infected with C. sinensis were determined using ELISA. The values were expressed as mean ± SEM. Compared with indicated groups, *P < 0.05, **P < 0.01, ***P < 0.001.
It has been reported that the increased production of IgE during the worms infection is related to tissue damages (Hamid et al., 2015). The data showed that the serum IgE secretion by TLR4wild infected mice was slightly higher than that of TLR4wild non-infected mice (Figure 1E, P<0.05). However, the serum IgE secretion in TLR4def infected mice was higher than that of the non-infected group, as well as that of TLR4wild infected mice (Figure 1E, P<0.001).
To observe and evaluate the liver fibrosis caused by C. sinensis, we used Masson’s trichrome staining and the expression of α-SMA was used for assessment of the degree of liver fibrosis. Masson staining showed that no obvious collagen deposition was observed in the non-infected groups. However, following C. sinensis infection, the hyperplasia of fibrous tissue in TLR4wild infected mice was mainly deposited around the bile duct, although the overall damage was relatively limited. In contrast, TLR4def infected mice had a large amount of fibrous tissue hyperplasia around the diseased bile duct (indicated as arrows, Figure 2A). The depositions of collagen in TLR4def infected mice were increased compared with the non-infected group (Figure 2A, P<0.05), whereas the mice in the TLR4def infected group had more fibrosis than those in the TLR4wild infected group (Figure 2A, P<0.05). IHC showed that the expression of α-SMA was slightly increased after C. sinensis-infected TLR4wildmice, compared with non-infected mice (Figure 2B). However, the expression of α-SMA was significantly increased in C. sinensis-infected TLR4def mice and there were statistical differences in the expression of α-SMA between TLR4wild and TLR4def mice that were infected with the same amount of C. sinensis (positive areas indicated as arrows, Figure 2B, P<0.05). Furthermore, the concentrations of hydroxyproline in TLR4def infected mice was increased compared with the non-infected group (Figure 2C, P<0.001), and the mice in the TLR4def infected group had more fibrosis than those in the TLR4wild infected group (Figure 2C, P<0.001). We further detected the relative hepatic expression of Tgfb and Col1a transcripts in the livers of TLR4wild as well as TLR4def mice infected by C. sinensis by qRT-PCR. It was found that the expression of Tgfb and Col1a mRNA transcripts in the liver of TLR4def mice were significantly increased compared with TLR4wild mice after C. sinensis infection (Figures 2D, E, P<0.001). Taken together, our data suggested that TLR4 deficiency aggravated hepatic fibrosis in C57BL/10 mice infected by C. sinensis.
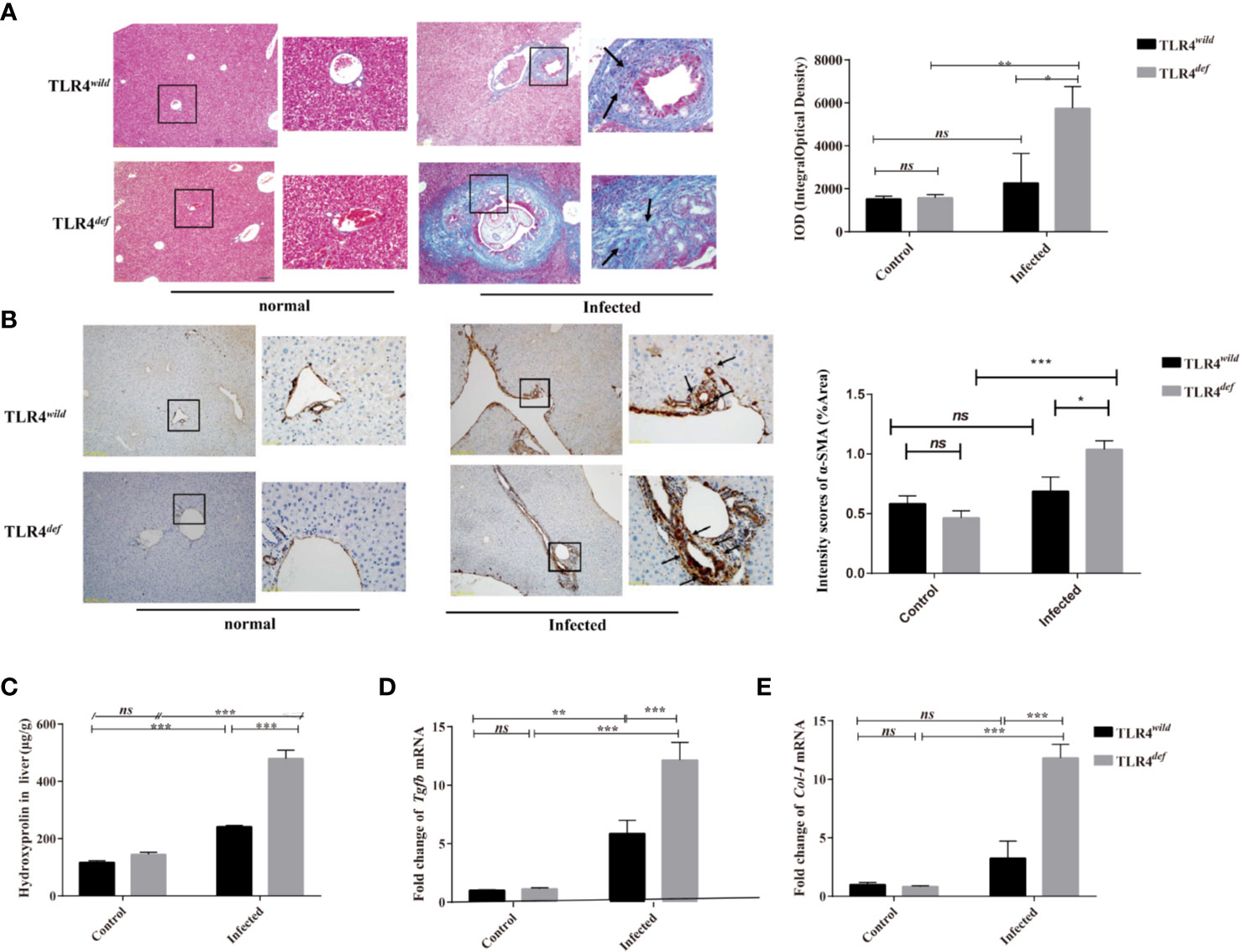
Figure 2 TLR4def led to an increased Clonorchis sinensis-caused pathogen-associated liver fibrosis in C57BL/10 mice. (A) Masson’s staining showed collagen depositions of the liver in TLR4wild and TLR4def mice infected with C. sinensis under 100× and 400× microscope. The integral optical density (IOD) of collagen fibers indicated by Masson’s trichrome staining was digitized and quantitated in the liver of non-infected and infected mice by on Image-Pro Plus software. (B) The expression of α-SMA of the liver in mice infected with C. sinensis under 100× and 400× microscope. Arrow indicates the positive cells. The positive areas in the liver of non-infected and infected mice were quantitated by Image J. (C) Detection of hydroxyproline in mice liver after C. sinensis infection. (D, E) Fold expression of fibrotic related molecules in Tgfb (D), Col1a (E) in the liver of mice infected with C. sinensis. The values were expressed as mean ± SEM. Compared with indicated groups, *P < 0.05, **P < 0.01, ***P < 0.001.
In the present study, we detected CK19 and ki67 as indicators of the hyperplasia of biliary epithelium cells (BECs) and the morphology of the bile duct by immunohistochemistry. For semi-quantitative analysis for CK19 and Ki67 expression, compared with non-infection and TLR4wild-infected mice, TLR4def infected mice showed higher expression of CK19 and Ki67 (as indicated by arrow), suggesting that TLR4def infected mice had more bile duct hyperplasia than TLR4wild infected mice (Figure 3A for CK19, and Figure 3B for Ki67, P<0.01). In addition, we also detected the serum activities of ALP, TBIL, and TBA which also indicated biliary injuries. The data showed that the levels of these indicators in TLR4def mice infected by C. sinensis were significantly increased, compared with those in TLR4wild mice when they were infected with the same dose of C. sinensis (Figures 3C–E, P<0.001). Taken together, our data indicated that TLR4 deficiency in mice with the C57BL/10 genetic background exacerbated hyperplasia and injuries of cholangiocytes caused by C. sinensis.
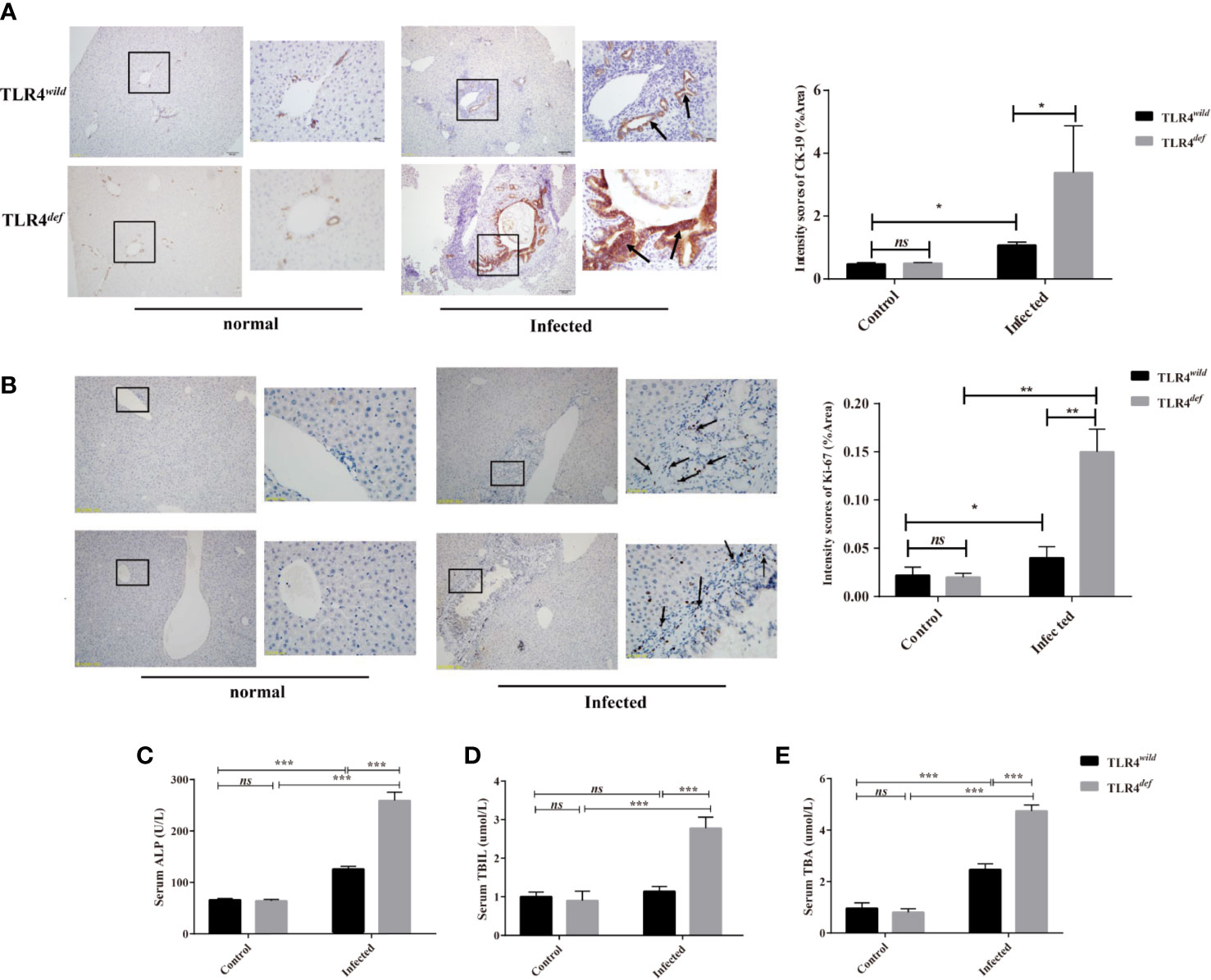
Figure 3 TLR4def promoted bile duct hyperplasia and biliary injuries in a resistant mouse strain of Clonorchis sinensis infection. (A, B) Hyperplasia of intrahepatic bile duct epithelial cells in mice with C. sinensis was indicated by immunohistochemical staining of CK19 (A) and ki67 (B), an arrow indicates the positive cells. (C–E) The activities of ALP (C), TBIL (D), and TBA (E) in the sera of TLR4wild and TLR4def mice were assessed for biliary injuries. The values were expressed as mean ± SEM. Compared with indicated groups, *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate the possible mechanisms that may contribute to the deteriorative clonorchiasis in TLR4def mice, we detected hepatic M1/M2-like macrophages in mice after C. sinensis infection. It was found that there were no significantly increased in percentages of CD86+ in CD11b+F4/80+ macrophages (M1-like macrophage) in TLR4def mice, compared with TLR4wild mice when they were infected with the same amounts of C. sinensis (Figure 4B, P>0.05), but the proportion of CD206+ in CD11b+F4/80+ macrophages (M2-like macrophage) in C. sinensis-infected TLR4def was significantly higher than that in TLR4wild mice infected by C. sinensis (Figure 4C, P<0.05).
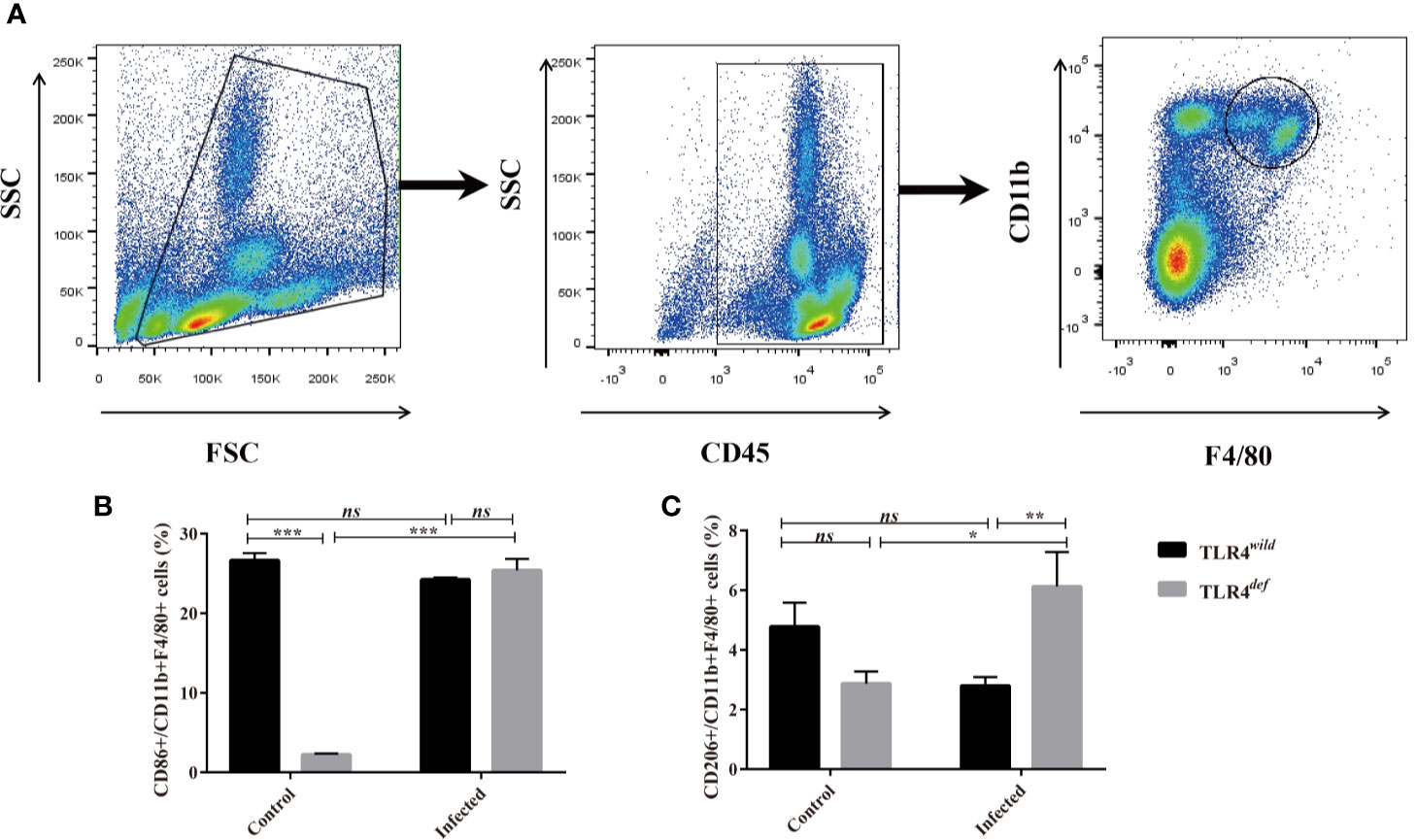
Figure 4 TLR4def mice infected with Clonorchis sinensis induced an predominant M2-like macrophage. (A) The strategies of gating hepatic macrophages. Hepatic leukocytes were isolated from TLR4def and TLR4wild mice, these cells were gated by CD45, and further gating for CD11b+F4/80+ cells using flow cytometer. (B) The percentages of CD86+cells in CD11b+F4/80+ cells from all the mice were calculated. (C) The percentages of CD206+cells in CD11b+F4/80+ cells from all the mice were calculated. The values were expressed as mean ± SEM. Compared with indicated groups, *P < 0.05, **P < 0.01, ***P < 0.001.
We next detected the levels of type 1 immune responses and type 2 immune responses of TLR4wild and TLR4def mice after C. sinensis infection. The data showed that there were significantly increases in IL-4, IL-10, and IL-13 cytokines in TLR4def mice, compared with TLR4wild mice when they were infected by the same amounts of C. sinensis (Figures 5A–C, P<0.05). Furthermore, we detected another type 2 immune molecules such as Arg1, Ym1 and Fizz1 using qPCR, it was found that the relative expression of Arg1, Ym1 and Fizz1 in the livers of C. sinensis-infected TLR4def mice were significantly higher than those in mice in C. sinensis-infected TLR4wild mice (Figures 5D–F, P<0.001).
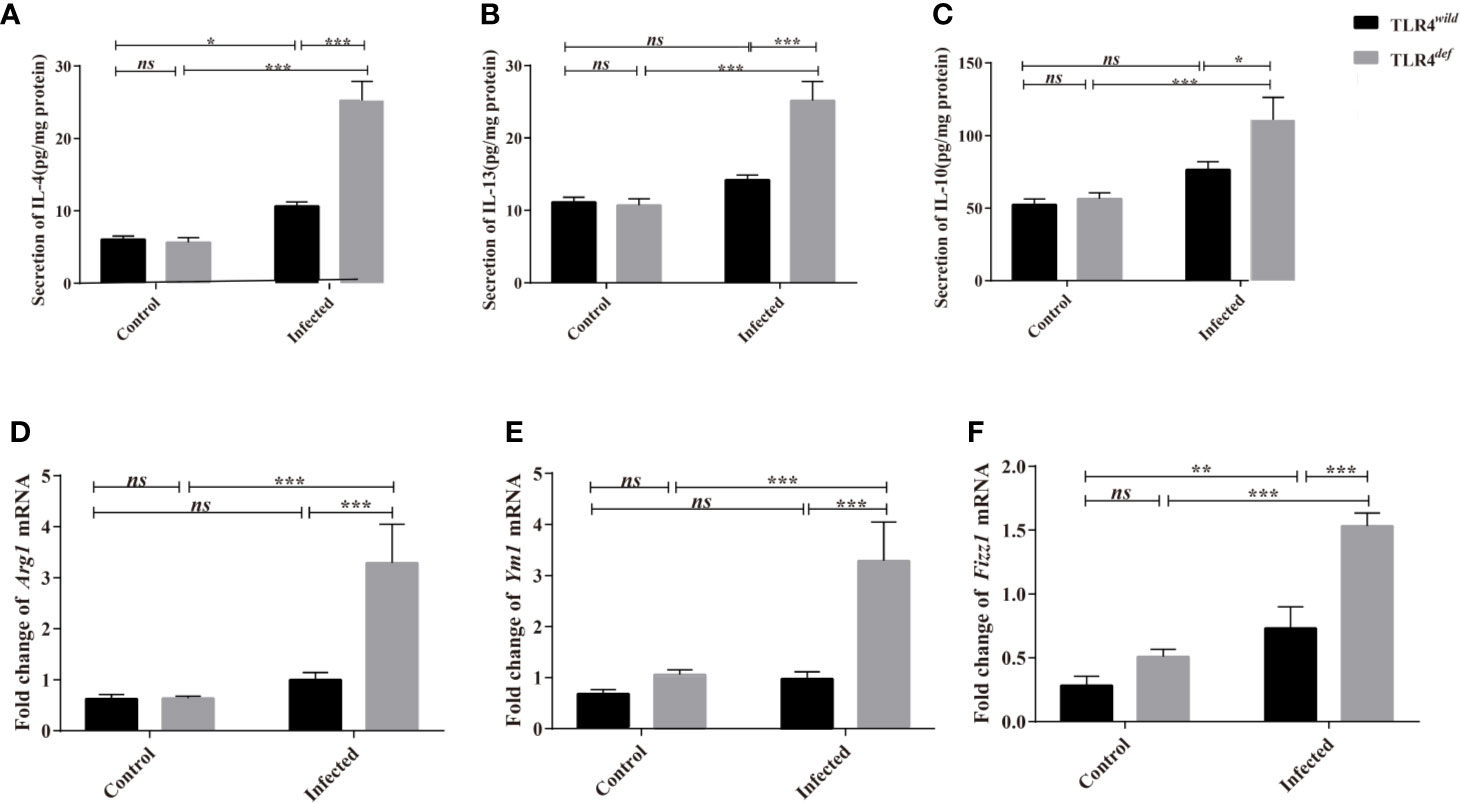
Figure 5 TLR4def enhanced type 2 immune response in C57BL/10 mice infected by Clonorchis sinensis. (A~C) The hepatic concentrations of IL-4 (A), IL-13 (B), and IL-10 (C) in the TLR4wild and TLR4def mice infected with C. sinensis were detected using ELISA kits. (D~F) The hepatic levels of Arg1 (D), Ym1 (E), Fizz1 (F) were determined by qPCR. The values were expressed as mean ± SEM. Compared with indicated groups, *P < 0.05, **P < 0.01, ***P < 0.001.
For type 1 immune responses, it was found that the secretion of MCP-1 and TNF-α in the liver of C. sinensis-infected TLR4def was significantly lower than that in TLR4wild mice infected by C. sinensis (Figures 6A, B, P<0.05). And the qPCR data showed that the transcripts of NOS2, Tnfa, and IL1b in the liver of TLR4def mice were significantly depressed, compared with TLR4wild mice after C. sinensis infection (Figures 6C–F, P<0.05). These data together suggested that TLR4def mice induced an augment of type 2 immune responses and decrease type 1 response, which might result in the exacerbation of biliary injuries and peribiliary fibrosis caused by C. sinensis in a resistant mice strain.
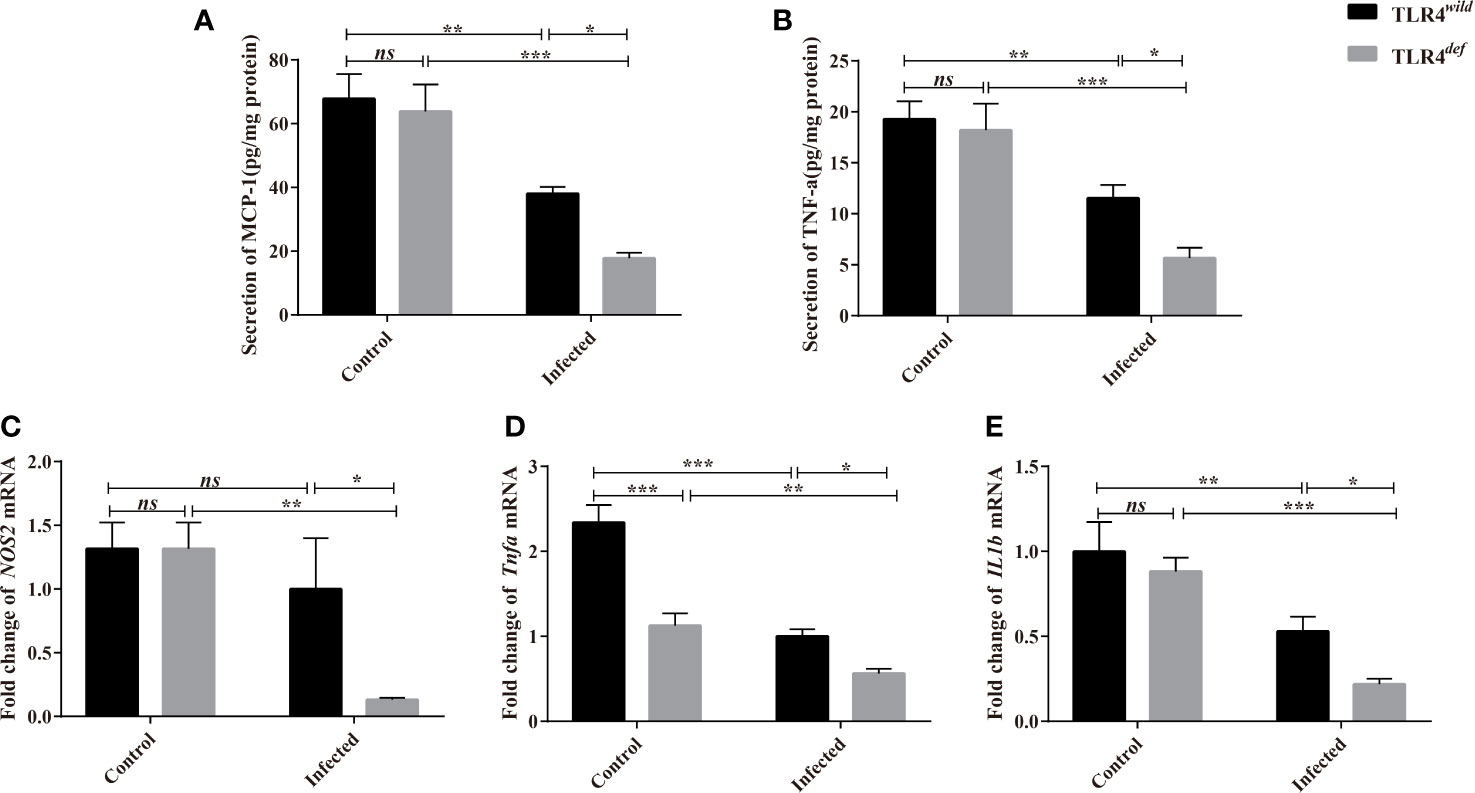
Figure 6 TLR4def decreased type 1 immune responses in C57BL/10 mice infected by Clonorchis sinensis. (A, B) The hepatic concentrations of MCP-1 (A) and TNF-e (B) in the TLR4wild and TLR4def mice infected with C. sinensis were detected using ELISA kits. (C, D) Fold changes of type 1 immunity-related molecules in NOS2 (C), Tnfa (D), IL1b (F) in the liver of mice infected with C. sinensis from each group were determined by qPCR. The values were expressed as mean ± SEM. Compared with indicated groups, *P < 0.05, **P < 0.01, ***P < 0.01.
Mice are widely used to mimic pathogenesis in human-caused by C. sinensis since they have gall-bladder and the genetic background is clear enough and many reagents are commercially available. However, other and our previous studies demonstrated that mice with different genetic backgrounds showed distinct susceptibility to C. sinensis. For example, liver damage of C3H/HeN (with the normal function of TLR4), BALB/c and FVB mice infected mice were characterized by massive hyperplasia and disordered arrangement of bile duct epithelial cells, as well as deposition of extracellular matrix (Yoon et al., 2001; Zhang et al., 2017). Furthermore, adult worm bodies can be found in the bile ducts of these strains of mice. In contrast, Uddin et al. found that C57BL/6 mice had the lowest worm recovery at 4 weeks after infection and the worms were less developed, which induced mild histological changes in the liver (Uddin et al., 2012). The lesions of C57BL/10 or C57BL/6 infected mice were very limited, with only a small amount of inflammatory cell infiltration in the lesions when they were subjected to the same amount of C. sinensis (Zhang et al., 2017). In addition, mild fibrosis lesions indicating by Masson staining and hydroxyproline assay were also found in C. sinensis-infected C57BL/10 and C57BL/6, which further suggested that the susceptibility of different strains of mice to C. sinensis was different (Robey et al., 2015). The mechanisms accounting for these may be very complex and largely unknown. In our present study, in contrast with TLR4 wild-type mice, we found that the loss of TLR4 in C57BL/10 mice showed exacerbated biliary injuries and peribiliary fibrosis caused by C. sinensis, which may be associated with an increase of type 2 immune responses due to TLR4 deficiency. There were some similarities or disparities of our data from TLR4 deficiency mice with patients infected by C. sinensis, for example, TLR4 deficiency mice showed relatively high levels of liver function indexes (such as ALP, ALT, AST, TBIL, and TBA, etc), IgE antibodies subclass as well as type 2 immune responses, which were also elevated in human infected by C. sinensis (Cai et al., 2004; Han et al., 2015); however, we did not find an increase of type 1 immune responses in TLR4def mice infected by C. sinensis whereas human with C. sinensis infection showed high levels pro-inflammatory cytokines such as TNF-α (Yuan et al., 2013).
A high level of IgE production is a hallmark of infection by helminth. It is believed that IgE plays a central role in protective immunity against worm infection although the mechanism underlying it remains unclear (Cooper et al., 2008). It also suggested that IgE induced the activation of mast cells to recruit immune effector cells including Th2 cells, M2 macrophage, and eosinophils and trigger type II cytokines (i.e. IL-4, IL-5, and IL-13), leading to cell hyperplasia and tissue injuries due to the hypersensitivity reactions (Gurish et al., 2004; Kondo et al., 2008). Interestingly, treatment with monoclonal IgE antibody (omalizumab, Xolair) alleviates allergic reactions and airway damages in asthma by blockade of IgE binding with Fc εRI receptor on the mast cells and other effector cells (Corren et al., 2017). In our present study, we found that the relative higher IgE production in TLR4 deficiency mice was induced by C. sinensis, compared with TLR4 wild type mice, suggesting that IgE may be involved in the pathogenesis caused by C. sinensis in addition to expelling worms.
TLR4, as an ancient and conservative pattern recognition receptor, plays an important role in the invasion, colonization, pathogenesis, and elimination of pathogens, and may display multiple effects at different stages of disease development (Shah et al., 2012; Cheng et al., 2015). Recently, it has been reported that the TLR4-mediated NF-κB signaling pathway which can cross-talk with the TGF-β/samds signaling pathway plays a very important role in the pathogenesis of hepatic fibrosis and cholestatic injuries (Seki et al., 2007). Our previous study has used C3H/HeN (TLR4 wild) and C3H/HeJ (TLR4 mutation) strain mice to establish the C. sinensis infection model and found that the biliary fibrosis of TLR4 mutant mice (TLR4mut) was ameliorative, compared with wild-type mice, which suggested that TLR4 promotes liver fibrosis caused by C. sinensis (Yan et al., 2017). In contrast, in the present study, TLR4 deficiency in a resistant mouse strain following C. sinensis infection deteriorated the biliary injuries and biliary fibrosis, as indicated by CK19 immunohistochemical staining and Masson staining, respectively, which was consistent with previous studies (Yang et al., 2017; Zhang et al., 2017). Furthermore, we also found that the liver function was also damped to some extent as the activities of ALT were significantly increased in the TLR4 deficient mouse infected by C. sinensis, compared with TLR4 wild type with the same background. These data suggested that TLR4 might play contradictory roles in pathogenesis caused by C. sinensis due to the different genetic backgrounds of mice (Zhang et al., 2018).
Type 1 immune responses are characterized with CD4+Th1, classically activated macrophages (M1 macrophages), group 1 innate lymphoid cells (ILC1s), and cytotoxic T cells, which protect against intracellular microbes and enhance cell-mediated immunity by secretion of cytokines such as TNF-α, IL-6, INF-γ, and MCP-1 and secretion of NO (Annunziato et al., 2015); Type 2 immune responses are mediated by CD4+Th2 cells, group 2 ILCs, eosinophils, basophils, mast cells, M2 macrophages by producing the cytokines IL−4, IL−5, IL−9, IL−13, and the IgE antibody subclass, which suppress the development of type 1-driven inflammation and contribute to the pathogenesis of allergy and tissue fibrosis (Wynn, 2015). In the healthy animals, the type 1 and 2 immune responses of the host are finely regulated; however, their balance might determine the occurrence, development, and outcome of the disease. For example, during worm infection, Type 2 immune responses were overwhelmed in the chronic infection to protect from such large extra−cellular helminth parasites infection as well as facilitate tissue fibrosis (Wynn, 2015). In our previous study, compared with BALB/c and FVB mice, we found that C57BL/6 mice showed stronger resistance to C. sinensis infection and the worm body was not completely developed or retarded and even eliminated (Zhang et al., 2017). It was speculated that these differences might be closely related to the high level of type 2 immune responses in the liver of BALB/c and FVB mice (Zhang et al., 2017). The type 1 immune response was predominant in C57BL/6 mice. For example, macrophages of C57BL/6 mice can effectively eliminate the bacterial infection in the model of cecal ligation and perforated peritonitis by producing high levels of TNF-α, IL-12 and NO, and other types I immune response molecules (Uddin et al., 2012; Pine et al., 2018). However, BALB/c mice have a dominating type II immune response and could not eliminate bacterial infections effectively (Watanabe et al., 2004; Jovicic et al., 2015). Studies also have shown that type 2 immune response can promote the development and progression of liver fibrosis, thereby promoting the activation of HSCs by producing IL-10, Arg-1, Ym1, and Fizz1 (Liu et al., 2004) whereas pro-inflammatory mediators such as iNOS could inhibit the activation or induce the apoptosis of HSCs (Allen and Sutherland, 2014; Pine et al., 2018). In our present study, we focused on the hepatic macrophages which play a critical role in the cholestatic liver injuries including pathogenesis caused by C. sinensis (Kim et al., 2017; Guicciardi et al., 2018). To address this issue, we used CD86+CD11b+F4/80+ defining as an activation marker of M1 macrophage whereas CD206+CD11b+F4/80+ were used to assess M2 activation, we found that TLR4 deficiency in C57BL/10 increase M2 macrophage producing the molecules of Arg-1, Ym1 and Fizz1 to possibly result in severe immunopathological damages (such as liver fibrosis), however, whether the biased type 2 immune responses induced by TLR4 deficiency may affect the development of the worms in the host or not, it remains to be further studied.
In conclusion, the present study found that biliary injuries and peribiliary fibrosis caused by C. sinensis had deteriorated when TLR4 was absent, suggesting that TLR4 might be involved in the resistance to C. sinensis in C57BL/10 mice. However, further studies on the mechanisms by which TLR4 deficiency in C57BL/10 mice induced pathogenesis of C. sinensis should be warranted.
The datasets generated for this study are available on request to the corresponding authors.
The animal study was reviewed and approved by Animal Care and Use Committee of Xuzhou Medical University.
CY and K-YZ conceived and designed the experiments. CY, JW, and NX performed the majority of experiments. JL, Q-YZ, H-MY, X-DC, J-XL, XD, SK, QY, B-BZ, J-XC, and R-XT contributed to the acquisition of data. JW and CY wrote the paper. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation of China (Grant Nos: 81572019 to K-YZ and 81702027 to QY), Natural Science Foundation of Jiangsu Province of China (Grant No. BK20171176 to CY and Grant No. BK20201011 for B-BZ), China Postdoctoral Science Foundation (Grant No. 2018M640525 to CY), Qian Lan Project of Jiangsu Province (to CY), Jiangsu Planned Projects for Postdoctoral Research Funds (No. 2018K053B to CY), the starting grants for young scientist of Xuzhou Medical University (No. D2019040 to B-BZ), Priority Academic Program Development of Jiangsu Higher Education Institutions of China (Grant No. 1506 to K-YZ) and Graduate research project of Jiangsu Province (Grant No. KYCX18-2172 to JW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akira S., Takeda K., Kaisho T. (2001). Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2 (8), 675–680. doi: 10.1038/90609
Allen J. E., Sutherland T. E. (2014). Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin. Immunol. 26 (4), 329–340. doi: 10.1016/j.smim.2014.06.003
Annunziato F., Romagnani C., Romagnani S. (2015). The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 135 (3), 626–635. doi: 10.1016/j.jaci.2014.11.001
Blom K. G., Qazi M. R., Matos J. B., Nelson B. D., DePierre J. W., Abedi-Valugerdi M., et al. (2009). Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization ofT and natural killer T cells obtained. Clin. Exp. Immunol. 155 (2), 320–329. doi: 10.1111/j.1365-2249.2008.03815.x
Cai L. S., Xiao J. Y., Xin H., Zhu L. X., Chen G., Zhang T., et al. (2004). Studies on the relationship between the level of cytokine and liver function in patients with clonorchiasis sinensis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 22 (1), 54–56. doi: 1000-7423(2004)-01-0054-03
Cengiz M., Ozenirler S., Elbeg S. (2015). Role of serum toll-like receptors 2 and 4 in non-alcoholic steatohepatitis and liver fibrosis. J. Gastroenterol. Hepatol. 30 (7), 1190–1196. doi: 10.1111/jgh.12924
Cheng Y., Zhu Y., Huang X., Zhang W., Han Z., Liu S. (2015). Association between TLR2 and TLR4 Gene Polymorphisms and the Susceptibility to Inflammatory Bowel Disease: A Meta-Analysis. PloS One 10 (5), e0126803. doi: 10.1371/journal.pone.0126803
Ciferska H., Honsova E., Lodererova A., Hruskova Z., Neprasova M., Vachek J., et al. (2020). Does the renal expression of Toll-like receptors play a role in patients with IgA nephropathy? J. Nephrol. 33(2):307–316. doi: 10.1007/s40620-019-00640-z
Cooper P. J., Ayre G., Martin C., Rizzo J. A., Ponte E. V., Cruz A. A. (2008). Geohelminth infections: a review of the role of IgE and assessment of potential risks of anti-IgE treatment. Allergy 63 (4), 409–417. doi: 10.1111/j.1398-9995.2007.01601.x
Corren J., Kavati A., Ortiz B., Colby J. A., Ruiz K., Maiese B. A., et al. (2017). Efficacy and safety of omalizumab in children and adolescents with moderate-to-severe asthma: A systematic literature review. Allergy Asthma Proc. 38 (4), 250–263. doi: 10.2500/aap.2017.38.4067
Gandhi C. R. (2020). Pro- and Anti-fibrogenic Functions of Gram-Negative Bacterial Lipopolysaccharide in the Liver. Front. Med. (Lausanne) 7, 130. doi: 10.3389/fmed.2020.00130
Guicciardi M. E., Trussoni C. E., Krishnan A., Bronk S. F., Lorenzo Pisarello M. J., O’Hara S. P., et al. (2018). Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol. 69 (3), 676–686. doi: 10.1016/j.jhep.2018.05.018
Gurish M. F., Bryce P. J., Tao H., Kisselgof A. B., Thornton E. M., Miller H. R., et al. (2004). IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J. Immunol. 172 (2), 1139–1145. doi: 10.4049/jimmunol.172.2.1139
Hamid F., Amoah A. S., van Ree R., Yazdanbakhsh M. (2015). Helminth-induced IgE and protection against allergic disorders. Curr. Top. Microbiol. Immunol. 388, 91–108. doi: 10.1007/978-3-319-13725-4_5
Han X., Xu J. X., Wang B. L., Sun T. T., Sun Z. Y., Dai Y., et al. (2015). Role of cytokines(IL-6,IL-10,TNF-α) in liver dysfunction patients with Clonorchis sinensis. Chin. J. Microecology 27 (3), 263–263. doi: 10.13381/j.cnki.cjm.201503005
Hong S. T., Fang Y. (2012). Clonorchis sinensis and clonorchiasis, an update. Parasitol. Int. 61 (1), 17–24. doi: 10.1016/j.parint.2011.06.007
Iwasaki A., Medzhitov R. (2004). Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5 (10), 987–995. doi: 10.1038/ni1112
Jiang Q., Yi M., Guo Q., Wang C., Wang H., Meng S., et al. (2015). Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway. Int. Immunopharmacol. 29 (2), 370–376. doi: 10.1016/j.intimp.2015.10.027
Jovicic N., Jeftic I., Jovanovic I., Radosavljevic G., Arsenijevic N., Lukic M. L., et al. (2015). Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PloS One 10 (7), e0134089. doi: 10.1371/journal.pone.0134089
Kim E. M., Kwak Y. S., Yi M. H., Kim J. Y., Sohn W. M., Yong T. S. (2017). Clonorchis sinensis antigens alter hepatic macrophage polarization in vitro and in vivo. PloS Negl. Trop. Dis. 11 (5), e0005614. doi: 10.1371/journal.pntd.0005614
Kim Y. H. (1999). Pancreatitis in association with Clonorchis sinensis infestation: CT evaluation. AJR Am. J. Roentgenol. 172 (5), 1293–1296. doi: 10.2214/ajr.172.5.10227505
Kondo Y., Yoshimoto T., Yasuda K., Futatsugi-Yumikura S., Morimoto M., Hayashi N., et al. (2008). Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 20 (6), 791–800. doi: 10.1093/intimm/dxn037
Li Q., Cherayil B. J. (2003). Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 71 (9), 4873–4882. doi: 10.1128/iai.71.9.4873-4882.2003
Liu T., Dhanasekaran S. M., Jin H., Hu B., Tomlins S. A., Chinnaiyan A. M., et al. (2004). FIZZ1 stimulation of myofibroblast differentiation. Am. J. Pathol. 164 (4), 1315–1326. doi: 10.1016/S0002-9440(10)63218-X
Molteni M., Gemma S., Rossetti C. (2016). The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediators Inflamm. 2016, 6978936. doi: 10.1155/2016/6978936
Pine G. M., Batugedara H. M., Nair M. G. (2018). Here, there and everywhere: Resistin-like molecules in infection, inflammation, and metabolic disorders. Cytokine 110, 442–451. doi: 10.1016/j.cyto.2018.05.014
Robey R. B., Weisz J., Kuemmerle N. B., Salzberg A. C., Berg A., Brown D. G., et al. (2015). Metabolic reprogramming and dysregulated metabolism: cause, consequence and/or enabler of environmental carcinogenesis? Carcinogenesis 36 Suppl 1, S203–S231. doi: 10.1093/carcin/bgv037
Seki E., Schwabe R. F. (2015). Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61 (3), 1066–1079. doi: 10.1002/hep.27332
Seki E., De Minicis S., Osterreicher C. H., Kluwe J., Osawa Y., Brenner D. A., et al. (2007). TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 13 (11), 1324–1332. doi: 10.1038/nm1663
Shah J. A., Vary J. C., Chau T. T., Bang N. D., Yen N. T., Farrar J. J., et al. (2012). Human TOLLIP regulates TLR2 and TLR4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J. Immunol. 189 (4), 1737–1746. doi: 10.4049/jimmunol.1103541
Sripa B. (2012). Global burden of food-borne trematodiasis. Lancet Infect. Dis. 12 (3), 171–172. doi: 10.1016/S1473-3099(11)70321-8
Tang Z. L., Huang Y., Yu X. B. (2016). Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty 5 (1), 71. doi: 10.1186/s40249-016-0166-1
Tyson G. L., El-Serag H. B. (2011). Risk factors for cholangiocarcinoma. Hepatology 54 (1), 173–184. doi: 10.1002/hep.24351
Uddin M. H., Li S., Bae Y. M., Choi M. H., Hong S. T. (2012). Strain variation in the susceptibility and immune response to Clonorchis sinensis infection in mice. Parasitol. Int. 61 (1), 118–123. doi: 10.1016/j.parint.2011.07.002
Watanabe H., Numata K., Ito T., Takagi K., Matsukawa A. (2004). Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22 (5), 460–466. doi: 10.1097/01.shk.0000142249.08135.e9
Wynn T. A. (2015). Type 2 cytokines: mechanisms and therapeutic strategies. Nat. Rev. Immunol. 15 (5), 271–282. doi: 10.1038/nri3831
Yan C., Wang L., Li B., Zhang B. B., Zhang B., Wang Y. H., et al. (2015). The expression dynamics of transforming growth factor-beta/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis. Parasit. Vectors 8, 70. doi: 10.1186/s13071-015-0675-y
Yan C., Li B., Fan F., Du Y., Ma R., Cheng X. D., et al. (2017). The roles of Toll-like receptor 4 in the pathogenesis of pathogen-associated biliary fibrosis caused by Clonorchis sinensis. Sci. Rep. 7 (1), 3909. doi: 10.1038/s41598-017-04018-8
Yang Q. L., Shen J. Q., Jiang Z. H., Shi Y. L., Wan X. L., Yang Y. C. (2017). TLR2 signal influences the iNOS/NO responses and worm development in C57BL/6J mice infected with Clonorchis sinensis. Parasit. Vectors 10 (1), 379. doi: 10.1186/s13071-017-2318-y
Yoon B. I., Choi Y. K., Kim D. Y., Hyun B. H., Joo K. H., Rim H. J., et al. (2001). Infectivity and pathological changes in murine clonorchiasis: comparison in immunocompetent and immunodeficient mice. J. Vet. Med. Sci. 63 (4), 421–425. doi: 10.1292/jvms.63.421
Yuan J.-f., Yuan J., Luo Y. (2013). Influence of Albendazole Combined with Astragalus Injection on Th1/Th2 Cytokines of Patients Infected with Clonorchis Sinensis. Chin. Gen. Pract. 33, 119–121. doi: 10.3969/j.issn.1007-9572.2013.09.111
Zhang B. B., Yan C., Fang F., Du Y., Ma R., Li X. Y., et al. (2017). Increased hepatic Th2 and Treg subsets are associated with biliary fibrosis in different strains of mice caused by Clonorchis sinensis. PloS One 12 (2), e0171005. doi: 10.1371/journal.pone.0171005
Zhang R., Sun Q., Chen Y., Sun X., Gu Y., Zhao Z., et al. (2018). Ts-Hsp70 induces protective immunity against Trichinella spiralis infection in mouse by activating dendritic cells through TLR2 and TLR4. PloS Negl. Trop. Dis. 12 (5), e0006502. doi: 10.1371/journal.pntd.0006502
Keywords: TLR4, Clonorchis sinensis, cholangiocytes, fibrosis, C57BL/10 mice
Citation: Yan C, Wu J, Xu N, Li J, Zhou Q-Y, Yang H-M, Cheng X-D, Liu J-X, Dong X, Koda S, Zhang B-B, Yu Q, Chen J-X, Tang R-X and Zheng K-Y (2021) TLR4 Deficiency Exacerbates Biliary Injuries and Peribiliary Fibrosis Caused by Clonorchis sinensis in a Resistant Mouse Strain. Front. Cell. Infect. Microbiol. 10:526997. doi: 10.3389/fcimb.2020.526997
Received: 15 January 2020; Accepted: 13 November 2020;
Published: 05 January 2021.
Edited by:
Patricia Sampaio Tavares Veras, Gonçalo Moniz Institute (IGM), BrazilReviewed by:
Sharon DeMorrow, University of Texas at Austin, United StatesCopyright © 2021 Yan, Wu, Xu, Li, Zhou, Yang, Cheng, Liu, Dong, Koda, Zhang, Yu, Chen, Tang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Yan, eWFuY2hhbzY5NTdAeHpobXUuZWR1LmNu; Kui-Yang Zheng, emt5QHh6aG11LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.