
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 31 August 2020
Sec. Parasite and Host
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.00431
 Anuradha Rajamanickam1
Anuradha Rajamanickam1 Saravanan Munisankar1
Saravanan Munisankar1 Pradeep A. Menon2
Pradeep A. Menon2 Chandrakumar Dolla2
Chandrakumar Dolla2 Thomas B. Nutman3
Thomas B. Nutman3 Subash Babu1,3,4*
Subash Babu1,3,4*Type 2 diabetes mellitus (T2DM) is characterized by heightened systemic inflammation and microbial translocation. Whether concomitant helminth infections can modulate this systemic response is unclear. We examined the presence of markers of systemic inflammation (levels of acute phase proteins) and of microbial translocation [levels of lipopolysaccharide (LPS) and its associated products] in T2DM individuals with (Ss+) or without (Ss−) Strongyloides stercoralis (Ss) infection. We also analyzed these parameters at 6 months following anthelmintic treatment in Ss+ individuals. Ss+ individuals exhibited significantly diminished levels of alpha-2 macroglobulin, C-reactive protein, haptoglobin and serum amyloid protein A1 compared to Ss− individuals and these levels increased significantly following therapy. Similarly, Ss+ individuals exhibited significantly diminished levels of LPS, sCD14, intestinal fatty acid binding protein, LPS binding protein and endotoxin IgG antibody and most of these levels increased significantly following therapy. Thus, helminth infection is associated with attenuation of systemic inflammation and microbial translocation in T2DM and its reversal following anthelmintic therapy.
Systemic inflammation and microbial translocation are typical characteristics of Type 2 diabetes mellitus (T2DM) and are known to drive T2DM-associated pathology (Donath and Shoelson, 2011; Donath et al., 2019; Tilg et al., 2020). Systemic inflammation in T2DM is often chronic and low grade and is labeled as metabolic inflammation or metaflammation (Hotamisligil, 2017). Several studies have associated T2DM with increased circulating levels of acute phase proteins such as C-reactive protein (CRP), serum amyloid protein A1 (SAA1), haptoglobin as well as cytokines and chemokines (Pickup et al., 1997; Ridker et al., 2000; Pradhan et al., 2001; Festa et al., 2002; Freeman et al., 2002; Thorand et al., 2003). Metaflammation is considered sterile, is driven by non-infectious factors such as nutrients and dietary lipid species, and characterized by endoplasmic reticulum stress and oxidative stress (Fu et al., 2012; Ertunc and Hotamisligil, 2016). Microbial translocation (with resultant metabolic endotoxemia) typically results from intestinal dysbiosis and is associated with increased intestinal permeability and translocation of microbial products into the circulation (Brenchley and Douek, 2012; Levy et al., 2017). It was demonstrated that mice fed a high fat diet exhibited a modified gut microbiome and an influx of bacterial derived lipopolysaccharides (LPS) into the systemic circulation, which, in turn, contributed to an increased susceptibility to T2DM (Cani et al., 2007). Subsequent studies have confirmed the elevation of microbial translocation markers in T2DM compared to healthy controls (Pendyala et al., 2012; Teixeira et al., 2012; Genser et al., 2018). Thus, systemic inflammation and microbial translocation with metabolic endotoxemia are key drivers of T2DM.
Interventions designed to control metabolic inflammation and endotoxemia are attracting a great deal of interest because of their ability to reduce the risk of metabolic diseases and their ability to reduce the pathogenic impact on already developed metabolic diseases (Donath et al., 2019; Tilg et al., 2020). We and others have previously shown that helminth infections are key factors in modulating T2DM-associated processes including insulin resistance, blood glucose and HbA1c levels, and dyslipidemias (Aravindhan et al., 2010; Chen et al., 2013; Hays et al., 2015; Wiria et al., 2015; Rajamanickam et al., 2018). Helminth infections can also modulate systemic inflammation seen in T2DM by down regulating pro-inflammatory cytokines and chemokines (Rajamanickam et al., 2018, 2020).
We postulate, therefore, that helminth infections could also influence systemic inflammation and metabolic endotoxemia by altering the levels of acute phase proteins and microbial translocation markers in T2DM. To this end, we measured the levels of these parameters in those with T2DM, with or without coincident infections with Strongyloides stercoralis (Ss), a common helminth parasite known to infect 50–100 million people worldwide. We also determined the effect of definitive anthelmintic treatment on the aforementioned parameters in Ss infected subjects.
All participants were examined as part of a natural history study protocol (12-I-073) approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (USA) and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
We recruited 118 individuals consisting of 60 clinically asymptomatic Ss infected individuals with T2DM (hereafter Ss+), and 58 individuals with T2DM and no Ss infection (hereafter Ss−) in Kanchipuram District, Tamil Nadu, South India (Table 1) and sample recruitment plan has been shown in Supplementary Figure 1. None had previous anthelmintic treatment, a history of helminth infections or of HIV. These individuals were all recruited from a rural population by screening of individuals for helminth infection by stool microscopy and serology. Follow up was performed at 6 months following treatment. The study groups were matched with respect to age and body mass index. We excluded those individuals who consumed antibiotic or probiotic within the prior 4 weeks, those who had gastrointestinal disease like irritable bowel syndrome, inflammatory bowel disease, history of gastrointestinal cancer or surgical resection, or acute, severe gastrointestinal symptoms and indication of hepatitis B or C virus infection. This was the same study population that was previously used for assessment of metabolic and immune parameters (Rajamanickam et al., 2018, 2020).
Ss infection was diagnosed by the presence of IgG antibodies to the recombinant NIE antigen as described previously (Bisoffi et al., 2014; Buonfrate et al., 2015). Stool microscopy was used to exclude the presence of other intestinal helminth infections. Filarial infection was excluded in all study participants by virtue of being negative in tests for circulating filarial antigen. All INF individuals were treated with a single dose of ivermectin (12 mg) and albendazole (400 mg) and follow-up blood draws were obtained 6 months later.
Diabetes was defined as an HbA1c reading of 6.5% or greater and a random blood glucose of >200 mg/dl, according to the American Diabetes Association criteria.
Plasma levels of alpha-2 macroglobulin (α-2M), C-reactive protein (CRP), haptoglobin, and Serum Amyloid A-1(SAA-1) were measured using a multiplex kit from R&D Systems, Minneapolis, MN, USA. according to the manufacturer's instructions.
To inactivate plasma proteins, plasma samples were heated to 75°C for 5 min. LPS levels were measured using a limulus amebocyte lysate assay (Cell Sciences Hycult Biotech, Canton, MA, USA) according to the manufacturer's protocol. Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure plasma levels of lipid-binding protein (LBP), endotoxin core antibodies IgG (EndoCAb), intestinal fatty acid binding protein (iFABP) (all Cell Sciences Hycult Biotech), and sCD14 (R&D Systems, Minneapolis, MN, USA).
Data analyses were performed using GraphPad PRISM 8 (GraphPad Software, Inc., San Diego, CA, USA). Geometric means (GM) were used for measurements of central tendency. Statistically significant differences were analyzed using the non-parametric Mann–Whitney U-test and Wilcoxon matched pair test. Multiple comparisons were corrected using the Holm's correction.
The baseline demographic characteristics and biochemical parameters are shown in Table 1. As shown and as described previously (Rajamanickam et al., 2018, 2020), there were no significant differences in age, sex, BMI or other biochemical parameters between the two groups.
To determine the effect of Ss infection on systemic inflammation in T2DM, we measured the levels of acute phase proteins (α-2M, CRP, haptoglobin and SAA-1) in Ss+ and Ss− individuals. As shown in Figure 1A, the levels of α-2M (GM of 868.6 ng/ml in Ss+ compared to 1,109 ng/ml in Ss−; p = 0.0016), CRP (GM of 3.962 ng/ml in Ss+ compared to 5.368 ng/ml in Ss−; p = 0.0021), haptoglobin (GM of 78.7 ng/ml in Ss+ compared to 134.1 ng/ml in Ss−; p = 0.0022) and SAA-1 (GM of 1.018 ng/ml in Ss+ compared to 3.593 ng/ml in Ss−; p = 0.0196) were significantly lower in Ss+ compared to Ss− individuals. Next, we wanted to determine the effect of anthelmintic treatment on the acute phase proteins in Ss+ individuals at 6 months following anthelmintic treatment. As shown in Figure 1B, the post-treatment levels of CRP (percentage increase of 30%; p = 0.0003), haptoglobin (percentage increase of 21%; p = 0.0002) and SAA-1 (percentage increase of 15%; p = 0.0001) were significantly increased when compared to pre-treatment levels. Thus, Ss infection is associated with diminished systemic inflammation in individuals with T2DM and a partial reversal following treatment.
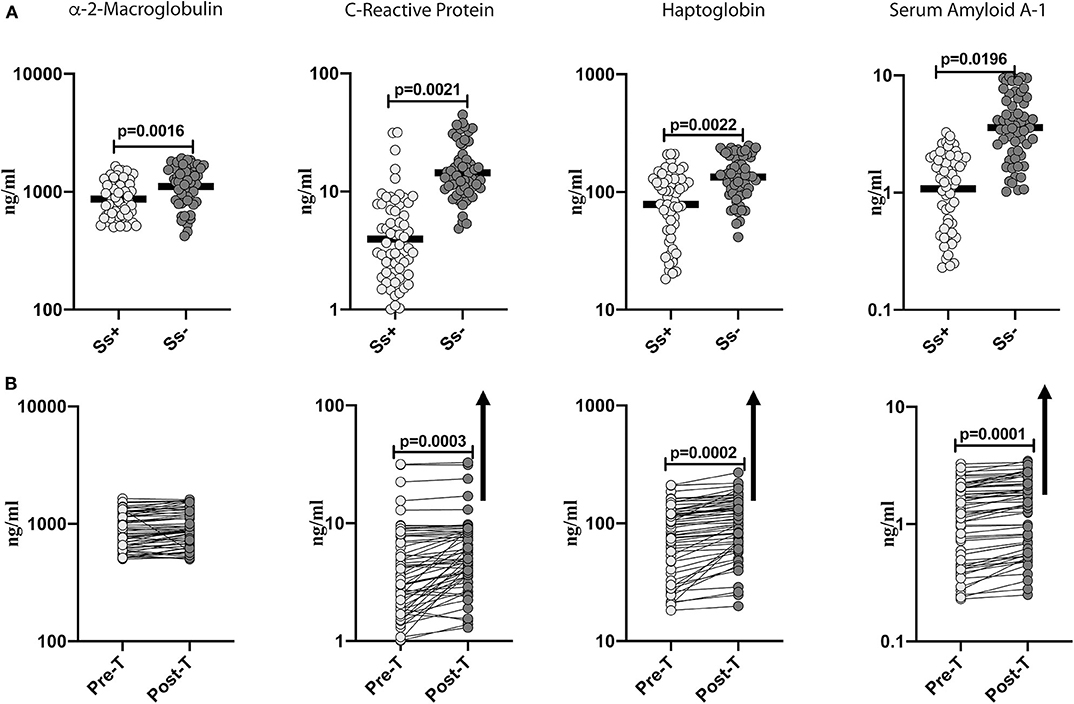
Figure 1. Diminished systemic levels of acute phase proteins in helminth-diabetes comorbidity and partial reversal after anthelmintic treatment. (A) Plasma levels of a-2 Macroglobulin, C-reactive protein (CRP), haptoglobin, and Serum Amyloid A-1 (SAA-1) from Ss infected [Ss+] (n = 60) or uninfected [Ss−] (n = 58) individuals were measured by multiplex assay. Data are shown as scatter plots with the bar representing the geometric mean. p-values were calculated using the Mann-Whitney U-test with Holms correction for multiple comparisons. (B) Plasma levels of a-2 Macroglobulin, C-reactive protein (CRP), haptoglobin, and serum amyloid proteins (SAA), from Ss-infected individuals at pre-treatment [pre-T] (n = 60) and at 6 months following treatment [post-treatment (post-T)] were measured by multiplex assay. The arrow shows the directionality of the significance. p-values were calculated using the Wilcoxon matched pair test.
To determine the effect of Ss infection on intestinal dysbiosis and metabolic endotoxemia in T2DM, we measured the levels of microbial translocation markers (LPS, sCD14, iFABP, LBP and EndoCAb) in Ss+ and Ss− individuals. As shown in Figure 2A, the levels of LPS (GM of 0.057 EU/ml in Ss+ compared to 0.076 EU/ml in Ss−; p = 0.0009), sCD14 (GM of 1,441 pg/ml in Ss+ compared to 1,879 pg/ml in Ss−; p = 0.0008), iFABP (GM of 170.7 pg/ml in Ss+ compared to 241.4 pg/ml in Ss−; p = 0.0007), LBP (GM of 353.8 ng/ml in Ss+ compared to 411.2 ng/ml in Ss−; p = 0.0006) and EndoCAb (GM of 282.1 GMU/ml in Ss+ compared to 399.6 GMU/ml in Ss−; p < 0.0001) were significantly lower in Ss+ compared to Ss− individuals. Next, we wanted to determine the effect of anthelmintic treatment on the microbial translocation markers in Ss+ individuals at 6 months following anthelmintic treatment. As shown in Figure 2B, the post-treatment levels of LPS (percentage increase of 24%; p = 0.0009), sCD14 (percentage increase of 6%; p = 0.0008), iFABP (percentage increase of 7%; p = 0.0007), and LBP (percentage increase of 5%; p = 0.0006) were significantly increased in Ss+ individuals when compared to pre-treatment levels. Thus, Ss infection is associated with diminished microbial translocation and metabolic endotoxemia in individuals with T2DM and a partial reversal following treatment.
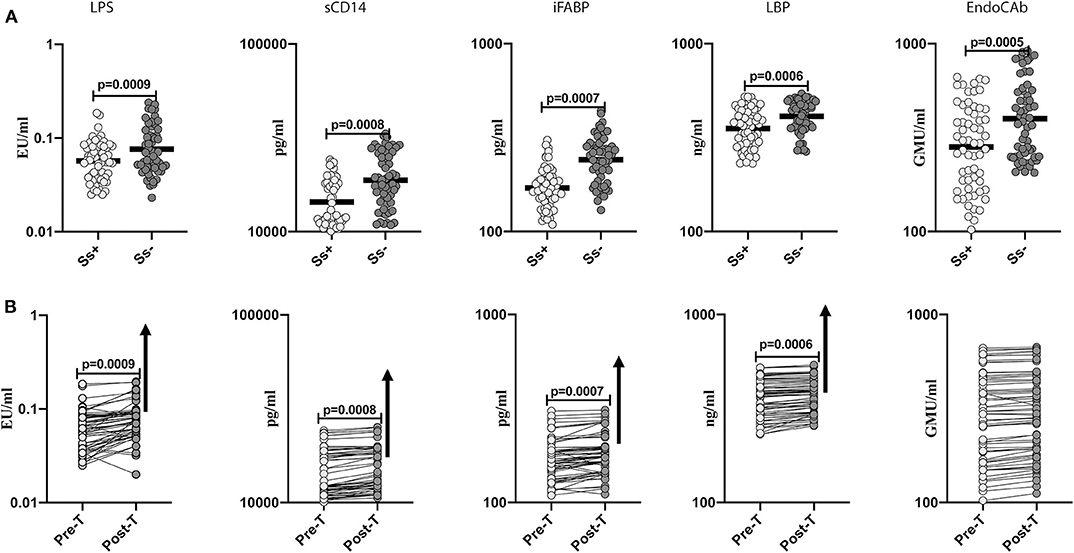
Figure 2. Diminished systemic levels of microbial translocation markers in helminth-diabetes comorbidity and partial reversal after anthelmintic treatment. (A) Plasma levels of lipopolysaccharide (LPS), soluble CD14 (sCD14), intestinal fatty acid binding protein (iFABP), lipid binding protein (LBP), and endotoxin core antibody IgG (EndoCAb), from Ss infected [Ss+] (n = 60) or uninfected [Ss−] (n = 58) individuals were measured by ELISA. Data are shown as scatter plots with the bar representing the geometric mean. p-values were calculated using the Mann-Whitney U-test with Holms correction for multiple comparisons. (B) Plasma levels of lipopolysaccharide (LPS), soluble CD14 (sCD14), intestinal fatty acid binding protein (iFABP), lipid binding protein (LBP), and endotoxin core antibody IgG (EndoCAb) from Ss-infected individuals at pre-treatment [pre-T] (n = 60) and at 6 months following treatment [post-treatment (post-T)] were measured by ELISA. The arrow shows the directionality of the significance. p-values were calculated using the Wilcoxon matched pair test.
Our study examined the regulation of acute phase proteins and microbial translocation markers in helminth—diabetes co-morbidity and the effect of anthelmintic treatment on these markers. Helminth interactions with metabolic disorders has garnered attention recently due to the finding that coexistent helminth infections could mediate beneficial effects on the metabolic dysfunction in these diseases (De Ruiter et al., 2017; Van Der Zande et al., 2019). Thus, while there exists an inverse relationship between helminth infections and metabolic disorders such as T2DM, there are also reports that helminths might potentially confer protection against insulin resistance, dyslipidemias and overt diabetes pathology (Aravindhan et al., 2010; Chen et al., 2013; Hays et al., 2015; Wiria et al., 2015; Rajamanickam et al., 2018). In fact, a clinical trial has been proposed to examine the safety and tolerability of hookworm infection in individuals with metabolic disease (Pierce et al., 2019). Thus, it becomes important to unravel the mechanisms behind the helminth—metabolic disorder interaction. We have used the presence of Ss infection in T2DM individuals to study one aspect of this interaction (i.e., the effect on systemic inflammation and metabolic endotoxemia), both of which are hallmarks of T2DM.
Elevations in acute phase proteins is a prototypical finding in the chronic, systemic inflammation that characterizes metabolic disorders, including T2DM (Pickup et al., 1997; Ridker et al., 2000; Pradhan et al., 2001; Festa et al., 2002; Freeman et al., 2002; Thorand et al., 2003). Unresolved endoplasmic reticulum stress and oxidative stress are key drivers of an inflammatory responses in metabolic dysregulation (Fu et al., 2012; Ertunc and Hotamisligil, 2016). Various markers of inflammation especially CRP have been shown to be associated with a higher predisposition to T2DM and consequent cardiovascular disease (Esser et al., 2014). Thus, a meta-analysis of 22 studies showed that elevated CRP levels were associated with increased risk of T2DM (Wang et al., 2013), and another meta-analysis of 52 prospective studies showed that elevated CRP is a predictor of cardiovascular events (Emerging Risk Factors et al., 2012). α-2M, SAA1 and haptoglobin are markers associated with diabetic complications and duration of disease, and therefore reduction in these acute phase proteins might potentially be a mechanism of lowering the occurrence of complications in T2DM (Jonsson and Wales, 1976; McMillan, 1976; Mackellar and Vigerust, 2016). Our data clearly reveals an association of lower levels of acute phase proteins in the presence of Ss infection and a partial reversal following anthelmintic treatment. Thus, our study elucidates a novel mechanism by which concomitant helminth infection might potentially modulate pathology in T2DM.
Several studies have reported the presence of intestinal dysbiosis and increased intestinal barrier permeability in T2DM (Mooradian et al., 1986; Pendyala et al., 2012; Qin et al., 2012; Cotillard et al., 2013; Thaiss et al., 2016). Other studies have clearly revealed a role for endotoxemia as a driver of metabolic diseases (Gomes et al., 2017; Tilg et al., 2020). Endotoxemia and its associated biomarkers (elevated LPS and LBP) were linked to increased risk of obesity and T2DM and correlated with disease exacerbation in humans (Mehta et al., 2010; Sun et al., 2010; Pussinen et al., 2011; Camargo et al., 2019). Furthermore, LPS administration to mice and healthy humans results in systemic and adipose tissue inflammation and insulin resistance, confirming that LPS affects insulin sensitivity (Mehta et al., 2010). Other products of microbial translocation including iFABP, a marker of gut permeability (Cox et al., 2017), sCD14 (Shitole et al., 2019), LBP (Sun et al., 2010; Cox et al., 2017; Sakura et al., 2017), and EndoCAb (Barengolts et al., 2019) are also associated with pathology in T2DM. Our data reveal a novel association of diminished metabolic endotoxemia and intestinal permeability in the presence of Ss infection. Although, Ss infection by itself is known to promote microbial translocation (Rajamanickam et al., 2017), its influence in the presence of a diabetic environment appears to be one of downmodulation and this could be due to differential interaction of the helminth infection with the gut microbiota in the context of diabetes mellitus. More conclusive proof that Ss drives this downregulated microbial translocation comes from our data on the significant reversal of this downmodulated response following anthelmintic treatment. Thus, chronic helminth infection acts as an immunomodulator in T2DM by dampening metabolic endotoxemia.
While numerous studies have recently examined the epidemiological and clinical association of helminth infection and T2DM, very few studies have directly addressed the underlying mechanism behind this interaction. Modulation of the inflammatory milieu would be expected to be similar in other helminth infections since previous studies have clearly delineated an effect of hookworm and Schistosoma infections on metabolic syndrome/diabetes mellitus (Mishra et al., 2014; Tang et al., 2017; Toniolo et al., 2019). We have previously shown (using the same patient cohort) that concomitant Ss infection modulates systemic cytokine, chemokine, adipokine, and hormonal responses that favor protection from insulin resistance and pancreatic beta cell exhaustion. In the current study, we expand upon this to demonstrate a beneficial effect of helminth infection on the systemic inflammatory milieu, intestinal dysbiosis and metabolic endotoxemia, that is highly characteristic of T2DM. Further elucidation of the molecular pathways of this protective effect should provide greater insight into this interesting field of multi-morbidity.
All datasets presented in this study are included in the article/Supplementary Material.
All participants were examined as part of a natural history study protocol (12-I-073) approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (USA) and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
SB: conceptualization, project administration, supervision, and writing and/or original draft. AR and SB: data curation, formal analysis, validation, and visualization. SB and TN: funding acquisition, software, and writing and/or review and editing. AR and SM: investigation and methodology. CD and PM: resources. All authors contributed to the article and approved the submitted version.
This work was supported by the Division of Intramural Research, NIAID, NIH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank M. Satiswaran, B. Suganthi, and Prabbu Balakrishnan for valuable assistance in collecting the clinical data for this study. We thank N. Pavan Kumar for technical assistance. We thank the staff of the Department of Epidemiology, NIRT, for valuable assistance in recruiting the patients for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.00431/full#supplementary-material
Supplementary Figure 1. Sample recruitment flowchart. Participants were screened for T2DM and T2DM positive participants were further tested for Strongyloides stercoralis (Ss) infection. Based on study inclusion criteria, participants were recruited for the study Ss+ (n = 60) and Ss− (n = 58).
Aravindhan, V., Mohan, V., Surendar, J., Muralidhara Rao, M., Pavankumar, N., Deepa, M., et al. (2010). Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83). PLoS Negl. Trop. Dis. 4:e707. doi: 10.1371/journal.pntd.0000707
Barengolts, E., Green, S. J., Chlipala, G. E., Layden, B. T., Eisenberg, Y., Priyadarshini, M., et al. (2019). Predictors of obesity among gut microbiota biomarkers in African American men with and without diabetes. Microorganisms 7:320. doi: 10.3390/microorganisms7090320
Bisoffi, Z., Buonfrate, D., Sequi, M., Mejia, R., Cimino, R. O., Krolewiecki, A. J., et al. (2014). Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl. Trop. Dis. 8:e2640. doi: 10.1371/journal.pntd.0002640
Brenchley, J. M., and Douek, D. C. (2012). Microbial translocation across the GI tract. Annu. Rev. Immunol. 30, 149–173. doi: 10.1146/annurev-immunol-020711-075001
Buonfrate, D., Formenti, F., Perandin, F., and Bisoffi, Z. (2015). Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin. Microbiol. Infect. 21, 543–552. doi: 10.1016/j.cmi.2015.04.001
Camargo, A., Jimenez-Lucena, R., Alcala-Diaz, J. F., Rangel-Zuniga, O. A., Garcia-Carpintero, S., Lopez-Moreno, J., et al. (2019). Postprandial endotoxemia may influence the development of type 2 diabetes mellitus: from the CORDIOPREV study. Clin. Nutr. 38, 529–538. doi: 10.1016/j.clnu.2018.03.016
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., et al. (2007). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. doi: 10.2337/db06-1491
Chen, Y., Lu, J., Huang, Y., Wang, T., Xu, Y., Xu, M., et al. (2013). Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J. Clin. Endocrinol. Metab. 98, E283–E287. doi: 10.1210/jc.2012-2517
Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., et al. (2013). Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588. doi: 10.1038/nature12480
Cox, A. J., Zhang, P., Bowden, D. W., Devereaux, B., Davoren, P. M., Cripps, A. W., et al. (2017). Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 43, 163–166. doi: 10.1016/j.diabet.2016.09.004
De Ruiter, K., Tahapary, D. L., Sartono, E., Soewondo, P., Supali, T., Smit, J. W. A., et al. (2017). Helminths, hygiene hypothesis and type 2 diabetes. Parasite Immunol. 39:e12404. doi: 10.1111/pim.12404
Donath, M. Y., Dinarello, C. A., and Mandrup-Poulsen, T. (2019). Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 19, 734–746. doi: 10.1038/s41577-019-0213-9
Donath, M. Y., and Shoelson, S. E. (2011). Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107. doi: 10.1038/nri2925
Emerging Risk Factors, Kaptoge, S., Di Angelantonio, E., Pennells, L., Wood, A. M., White, I. R., et al. (2012). C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 367, 1310–1320. doi: 10.1056/NEJMoa1107477
Ertunc, M. E., and Hotamisligil, G. S. (2016). Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 57, 2099–2114. doi: 10.1194/jlr.R066514
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J., and Paquot, N. (2014). Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150. doi: 10.1016/j.diabres.2014.04.006
Festa, A., D'agostino, R. Jr, Tracy, R. P., Haffner, S. M., and Insulin Resistance Atherosclerosis. (2002). Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 51, 1131–1137. doi: 10.2337/diabetes.51.4.1131
Freeman, D. J., Norrie, J., Caslake, M. J., Gaw, A., Ford, I., Lowe, G. D., et al. (2002). C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 51, 1596–1600. doi: 10.2337/diabetes.51.5.1596
Fu, S., Watkins, S. M., and Hotamisligil, G. S. (2012). The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 15, 623–634. doi: 10.1016/j.cmet.2012.03.007
Genser, L., Aguanno, D., Soula, H. A., Dong, L., Trystram, L., Assmann, K., et al. (2018). Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 246, 217–230. doi: 10.1002/path.5134
Gomes, J. M. G., Costa, J. A., and Alfenas, R. C. G. (2017). Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism 68, 133–144. doi: 10.1016/j.metabol.2016.12.009
Hays, R., Esterman, A., Giacomin, P., Loukas, A., and Mcdermott, R. (2015). Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian aboriginal adults. Diabetes Res. Clin. Pract. 107, 355–361. doi: 10.1016/j.diabres.2015.01.012
Hotamisligil, G. S. (2017). Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185. doi: 10.1038/nature21363
Jonsson, A., and Wales, J. K. (1976). Blood glycoprotein levels in diabetes mellitus. Diabetologia 12, 245–250. doi: 10.1007/BF00422091
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A., and Elinav, E. (2017). Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232. doi: 10.1038/nri.2017.7
Mackellar, M., and Vigerust, D. J. (2016). Role of haptoglobin in health and disease: a focus on diabetes. Clin. Diabetes 34, 148–157. doi: 10.2337/diaclin.34.3.148
McMillan, D. E. (1976). Alpha2-macroglobulin in diabetes. Lancet 2, 1020–1021. doi: 10.1016/S0140-6736(76)90852-7
Mehta, N. N., Mcgillicuddy, F. C., Anderson, P. D., Hinkle, C. C., Shah, R., Pruscino, L., et al. (2010). Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59, 172–181. doi: 10.2337/db09-0367
Mishra, P. K., Palma, M., Bleich, D., Loke, P., and Gause, W. C. (2014). Systemic impact of intestinal helminth infections. Mucosal Immunol. 7, 753–762. doi: 10.1038/mi.2014.23
Mooradian, A. D., Morley, J. E., Levine, A. S., Prigge, W. F., and Gebhard, R. L. (1986). Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia 29, 221–224. doi: 10.1007/BF00454879
Pendyala, S., Walker, J. M., and Holt, P. R. (2012). A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142, 1100–1101. doi: 10.1053/j.gastro.2012.01.034
Pickup, J. C., Mattock, M. B., Chusney, G. D., and Burt, D. (1997). NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40, 1286–1292. doi: 10.1007/s001250050822
Pierce, D., Merone, L., Lewis, C., Rahman, T., Croese, J., Loukas, A., et al. (2019). Safety and tolerability of experimental hookworm infection in humans with metabolic disease: study protocol for a phase 1b randomised controlled clinical trial. BMC Endocr. Disord. 19:136. doi: 10.1186/s12902-019-0461-5
Pradhan, A. D., Manson, J. E., Rifai, N., Buring, J. E., and Ridker, P. M. (2001). C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286, 327–334. doi: 10.1001/jama.286.3.327
Pussinen, P. J., Havulinna, A. S., Lehto, M., Sundvall, J., and Salomaa, V. (2011). Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34, 392–397. doi: 10.2337/dc10-1676
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Rajamanickam, A., Munisankar, S., Bhootra, Y., Dolla, C., Nutman, T. B., and Babu, S. (2017). Microbial translocation associated with an acute-phase response and elevations in MMP-1, HO-1, and proinflammatory cytokines in Strongyloides stercoralis infection. Infect. Immun. 85:e00772-16. doi: 10.1128/IAI.00772-16
Rajamanickam, A., Munisankar, S., Bhootra, Y., Dolla, C., Thiruvengadam, K., Nutman, T. B., et al. (2018). Metabolic consequences of concomitant Strongyloides stercoralis infection in type 2 diabetes mellitus. Clin. Infect. Dis. 69, 697–704. doi: 10.1093/cid/ciy935
Rajamanickam, A., Munisankar, S., Dolla, C., Menon, P. A., Thiruvengadam, K., Nutman, T. B., et al. (2020). Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl. Trop. Dis. 14:e0008101. doi: 10.1371/journal.pntd.0008101
Ridker, P. M., Hennekens, C. H., Buring, J. E., and Rifai, N. (2000). C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl. J. Med. 342, 836–843. doi: 10.1056/NEJM200003233421202
Sakura, T., Morioka, T., Shioi, A., Kakutani, Y., Miki, Y., Yamazaki, Y., et al. (2017). Lipopolysaccharide-binding protein is associated with arterial stiffness in patients with type 2 diabetes: a cross-sectional study. Cardiovasc. Diabetol. 16:62. doi: 10.1186/s12933-017-0545-3
Shitole, S. G., Biggs, M. L., Reiner, A. P., Mukamal, K. J., Djousse, L., Ix, J. H., et al. (2019). Soluble CD14 and CD14 variants, other inflammatory markers, and glucose dysregulation in older adults: the cardiovascular health study. Diabetes Care 42, 2075–2082. doi: 10.2337/dc19-0723
Sun, L., Yu, Z., Ye, X., Zou, S., Li, H., Yu, D., et al. (2010). A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33, 1925–1932. doi: 10.2337/dc10-0340
Tang, C. L., Liu, Z. M., Gao, Y. R., and Xiong, F. (2017). Schistosoma infection and schistosoma-derived products modulate the immune responses associated with protection against type 2 diabetes. Front Immunol. 8:1990. doi: 10.3389/fimmu.2017.01990
Teixeira, T. F., Souza, N. C., Chiarello, P. G., Franceschini, S. C., Bressan, J., Ferreira, C. L., et al. (2012). Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin. Nutr. 31, 735–740. doi: 10.1016/j.clnu.2012.02.009
Thaiss, C. A., Itav, S., Rothschild, D., Meijer, M. T., Levy, M., Moresi, C., et al. (2016). Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540, 544–551. doi: 10.1038/nature20796
Thorand, B., Lowel, H., Schneider, A., Kolb, H., Meisinger, C., Frohlich, M., et al. (2003). C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch. Intern. Med. 163, 93–99. doi: 10.1001/archinte.163.1.93
Tilg, H., Zmora, N., Adolph, T. E., and Elinav, E. (2020). The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 20, 40–54. doi: 10.1038/s41577-019-0198-4
Toniolo, A., Cassani, G., Puggioni, A., Rossi, A., Colombo, A., Onodera, T., et al. (2019). The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev. Med. Microbiol. 30, 1–17. doi: 10.1097/MRM.0000000000000155P795-808
Van Der Zande, H. J. P., Zawistowska-Deniziak, A., and Guigas, B. (2019). Immune regulation of metabolic homeostasis by helminths and their molecules. Trends Parasitol. 35, P795–P808. doi: 10.1016/j.pt.2019.07.014
Wang, X., Bao, W., Liu, J., Ouyang, Y. Y., Wang, D., Rong, S., et al. (2013). Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36, 166–175. doi: 10.2337/dc12-0702
Keywords: helminths, type 2 diabetes mellitus, systemic inflammation, acute phase proteins, microbial translocation
Citation: Rajamanickam A, Munisankar S, Menon PA, Dolla C, Nutman TB and Babu S (2020) Helminth Mediated Attenuation of Systemic Inflammation and Microbial Translocation in Helminth-Diabetes Comorbidity. Front. Cell. Infect. Microbiol. 10:431. doi: 10.3389/fcimb.2020.00431
Received: 28 April 2020; Accepted: 14 July 2020;
Published: 31 August 2020.
Edited by:
Kenneth Pfarr, University Hospital Bonn, GermanyReviewed by:
Paul Giacomin, Centre for Molecular Therapeutics, Australian Institute of Tropical Health & Medicine, James Cook University, AustraliaCopyright © 2020 Rajamanickam, Munisankar, Menon, Dolla, Nutman and Babu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subash Babu, c2JhYnVAbWFpbC5uaWguZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.