
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 13 February 2020
Sec. Fungal Pathogenesis
Volume 10 - 2020 | https://doi.org/10.3389/fcimb.2020.00034
This article is part of the Research Topic Advances in Understanding Innate Immunity to Fungi View all 7 articles
Monocytes and their derivatives, including macrophages and dendritic cells, play diverse roles in the response to fungal pathogens. Sensing of fungi by monocytes triggers signaling pathways that mediate direct effects like phagocytosis and cytokine production. Monocytes can also present fungal antigens to elicit adaptive immune responses. These monocyte-mediated pathways may be either beneficial or harmful to the host. In some instances, fungi have developed mechanisms to evade the consequences of monocyte activation and subvert these cells to promote disease. Thus, monocytes are critically involved in mediating the outcomes of these often highly fatal infections. This review will highlight the roles of monocytes in the immune response to some of the major fungi that cause invasive human disease, including Aspergillus, Cryptococcus, Candida, Histoplasma, Blastomyces, and Coccidioides, and discuss potential strategies to manipulate monocyte responses in order to enhance anti-fungal immunity in susceptible hosts.
Monocytes are innate immune cells that may be generated in the bone marrow from two different precursors, either a granulocyte-monocyte progenitor (GMP) or a monocyte-dendritic cell progenitor (MDP) (Yanez et al., 2017; Wolf et al., 2019; Figure 1). Monocytes derived from either of these lineages consist of two main types: (1) classical “inflammatory” monocytes that are CCR2+ Ly6Chi in mice and CD14+ CD16− in humans, and (2) non-classical “patrolling” monocytes that are CCR2lo Ly6Clo in mice and CD14lo CD16+ in humans. Lineage tracing studies suggest that non-classical monocytes develop directly from classical monocytes (Yona et al., 2013). During homeostatic conditions, non-classical monocytes patrol the circulation to engage in tissue repair and clearance of dead cells (Auffray et al., 2007). During inflammation or infection, classical monocytes are mobilized from bone marrow reserves in response to chemokines that bind to the CCR2 receptor, such as CCL2 and CCL7 (Shi and Pamer, 2011). Upon entering affected tissues, classical monocytes can further differentiate into macrophages and monocyte-derived dendritic cells (MoDCs). Macrophages that differentiate from monocytes in the adult bone marrow are distinguished from tissue resident macrophages (e.g., alveolar macrophages, glial cells) that originally derive from fetal yolk sac progenitor cells or monocytes from the fetal liver (Hoeffel and Ginhoux, 2018). However, there is evidence that bone marrow-derived monocytes can help replenish the tissue-resident macrophages of specific organs including the gut, the skin, and the heart (Ginhoux and Guilliams, 2016). MoDCs are also distinct in origin from conventional dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs) that are derived from a common dendritic cell progenitor (CDP). The ontogeny of monocytes and ongoing controversies about their origins and development are reviewed in further detail elsewhere (Jakubzick et al., 2017; Murray, 2018; Wolf et al., 2019).
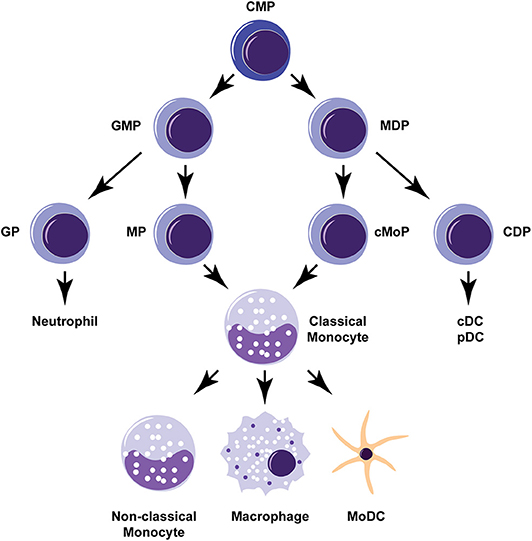
Figure 1. Monocyte development and differentiation pathways. Monocytes can develop from either a granulocyte-monocyte progenitor (GMP) or a monocyte-dendritic cell progenitor (MDP). Classical “inflammatory” monocytes can give rise to non-classical “patrolling” monocytes or further differentiate into macrophages or monocyte-derived dendritic cells (MoDCs). Common myeloid progenitor (CMP), granulocyte progenitor (GP), monocyte-committed progenitor (MP), common monocyte progenitor (cMoP), common dendritic cell progenitor (CDP), conventional dendritic cell (cDC), plasmacytoid dendritic cell (pDC).
Invasive fungal infections represent a significant cause of human disease, with an estimated 1.5 million people dying each year (Bongomin et al., 2017). Unfortunately, the incidence of these infections is increasing with the expanded use of immunosuppressive therapies, broad-spectrum antibiotics, and invasive medical devices (Pfaller and Diekema, 2010; Clark and Drummond, 2019). Invasive infections are commonly caused by fungi of the genera Aspergillus, Candida, Cryptococcus, Blastomyces, Coccidioides, and Histoplasma (Table 1). Except for Candida species, which are commensal organisms found on the skin and mucosal surfaces, these fungi are environmental microorganisms that are typically acquired after inhalation into the lungs. All of these fungi can cause invasive disease in a wide-spectrum of immunocompromised patients, such as those with genetic immunodeficiencies, HIV/AIDS, cancer, solid organ, and hematopoietic stem cell transplantation, autoimmune diseases, immunosuppressive treatments, and other predisposing states like diabetes and pregnancy. However, apparently immunocompetent patients can also be affected by many of these fungi, including Candida, Cryptococcus gattii, and the endemic fungi Blastomyces, Coccidioides, and Histoplasma. Unfortunately, the morbidity and mortality from invasive fungal infections remains quite high despite current drug regimens, some of which utilize multiple antifungal agents. Therefore, the development of novel therapeutic approaches to fungal infections is imperative to improving clinical outcomes.
Monocytes and their derivatives have been found to play critical roles in the outcomes of fungal infections (Table 1). For example, monocyte-deficient mice are more susceptible to infections with Aspergillus fumigatus (Hohl et al., 2009; Espinosa et al., 2014), Candida albicans (Ngo et al., 2014; Dominguez-Andres et al., 2017b), and Histoplasma capsulatum (Szymczak and Deepe, 2009). On the other hand, the absence of monocytes during Cryptococcus neoformans infection can either be detrimental or beneficial to host outcomes, depending on the infection model (Traynor et al., 2000; Osterholzer et al., 2008, 2009; Charlier et al., 2009; Heung and Hohl, 2019). This plasticity of monocytes in the regulation of immune responses to fungi makes these cells ideal targets for immunomodulatory therapies. Indeed, strategies to target monocyte development and function are already under investigation as potential cancer therapies given their roles in facilitating both pro-tumor and anti-tumor effects (Olingy et al., 2019). This review will highlight the key mechanisms by which monocytes regulate innate immunity to fungi, including fungal sensing, phagocytosis, cytokine production and cellular crosstalk, and antigen presentation and T cell priming. Recent developments in understanding the role of trained immunity in monocyte responses to fungal pathogens will also be discussed.
Monocytes express a variety of receptors to facilitate detection of fungal cells. Pattern recognition receptors (PRRs), including C-type lectin receptors (CLRs), Toll-like receptors (TLRs), and NOD-like receptors (NLRs), can detect pathogen-associated molecular patterns (PAMPs) like β-glucan, chitin and mannose in the fungal cell wall and trigger downstream signaling pathways to coordinate the innate immune response (Lionakis et al., 2017). Complement receptors (CRs) and Fc receptors also assist in fungal sensing by detecting complement or antibody-bound fungal cells (Erwig and Gow, 2016). These receptors can have individual effects or work in collaboration with each other. For example, cytokine production by macrophages and DCs is regulated by the CLR Dectin-1 and complement receptor 3 (CR3) during H. capsulatum infection and by Dectin-1 and TLR2 in Coccidioides infection models (Viriyakosol et al., 2005, 2013; del Pilar Jimenez et al., 2008; Lin et al., 2010; Huang et al., 2015). The activation of the NLRP3 inflammasome during histoplasmosis is coordinated by Dectin-1 and Dectin-2 signaling (Chang et al., 2017). During C. albicans infection, Dectin-1, Dectin-2, and Mincle collectively contribute to host defenses by regulating monocyte cytokine production and phagocytosis of the fungus (Thompson et al., 2019). Additionally, the balance between Dectin-1 and TLR signaling in MoDCs can determine the Th1 and Th17 responses to A. fumigatus (Rivera et al., 2011).
An interesting facet of PRR expression by monocytes is the capacity to discern different morphologic forms of fungi. C. albicans exists in yeast and filamentous forms, which can be present at different stages of the infection process (Noble et al., 2017). The morphogenesis of C. albicans from yeast to hyphae at mucosal surfaces activates the NLRP3 inflammasome in macrophages, which can stimulate Th17 responses that are important for mucosal defense (Joly et al., 2009; Gow et al., 2011). Dectin-1 on macrophages can bind to β-glucan that is exposed at budding sites on the yeast form of C. albicans which triggers phagocytosis and reactive oxygen species (ROS) production (Gantner et al., 2005). Additionally, DCs exposed to C. albicans yeast can induce Th1 immune responses, while exposure to hyphal forms elicits Th2 responses (d'Ostiani et al., 2000). The mold A. fumigatus forms airborne spores called conidia. Under permissive conditions, these resting conidia can be induced to swell, germinate, and form hyphae that can invade underlying tissues. Germination involves shedding of the immunosuppressive outer rodlet layer of conidia and exposure of PAMPs in the fungal cell wall, including β-glucan and α-mannan (Aimanianda et al., 2009). These PAMPs are detected by Dectin-1 and Dectin-2, resulting in the activation of NF-κβ and pro-inflammatory cytokine production by macrophages and moDCs (Hohl et al., 2005; Steele et al., 2005; Gersuk et al., 2006; Carrion Sde et al., 2013). Different receptors also mediate phagocytosis of the different forms of A. fumigatus. Mannose receptor can regulate the uptake of conidia by DCs, while FcγRII and FcγRIII assist with uptake of hyphal forms (Bozza et al., 2002). Similar to C. albicans, the sensing of different morphotypes of A. fumigatus can affect the adaptive immune response. Metabolically active, live A. fumigatus conidia induce beneficial Th1 CD4+ T cell responses, while inactive, heat-killed conidia and hyphae can stimulate a Th2-skewed response (Bozza et al., 2002; Hohl et al., 2005; Rivera et al., 2005). Thus, the ability to sense different morphologies of fungi may enable monocytes and their derivatives to distinguish potentially invasive forms from non-invasive forms, as well as different stages of fungal infection, so that the immune system can respond accordingly.
Phagocytosis of fungal cells by monocytes and their derivative macrophages and DCs is another key element of the immune response. Fungi can be eliminated in these cells in the phagolysosome, an acidified compartment that can sequester nutrients and contains various enzymes, ROS generated by NADPH oxidase (NOX2), and reactive nitrogen species (RNS) produced by inducible nitric oxide synthase (iNOS or NOS2) in response to pro-inflammatory stimuli (Uribe-Querol and Rosales, 2017). This fungal killing may be sufficient to halt the progression of infection, but it can also provide fungal antigens that can be used to initiate the adaptive immune response to ensure sterilizing immunity. Fungal uptake is not always beneficial to the host, however, as some fungi have adapted to the harsh environment in the phagolysosome or can subvert monocytes to enable fungal persistence and proliferation.
Macrophages are the prototypical phagocyte and are conventionally described as polarizing into either pro-inflammatory, classically-activated (M1) macrophages or anti-inflammatory, alternatively-activated (M2) macrophages (Lawrence and Natoli, 2011). This M1/M2 classification is based on the expression of particular markers. For example, M1 macrophages typically express NOS2. M2 macrophages express markers like transglutaminase 2 (TGM2), arginase 1 (ARG1), resistin-like molecule-alpha (RETNLA/FIZZ1), chitinase-like 3 (CHIL3/YM1), and chitinase-like 4 (CHIL4/Ym2), the latter four being murine-specific. M1 macrophage polarization can be induced by IFNγ, GM-CSF, or lipopolysaccharide (LPS), while M2 polarization can be induced by IL-4 or IL-13. Despite this binary designation, macrophages are actually quite heterogenous along the spectrum from M1 to M2, so other classification schemes have been proposed but have not yet been used consistently in the literature (Mosser and Edwards, 2008; Martinez and Gordon, 2014; Murray et al., 2014).
M1 macrophages are typically fungicidal cells. For example, while H. capsulatum can replicate within resting (M0) macrophages and monocytes, activation of these cells with cytokines, including IFNγ and GM-CSF, restricts the intracellular growth of H. capsulatum, in part by sequestering nutrients like zinc ions that are needed for fungal growth (Howard, 1964; Wu-Hsieh and Howard, 1987; Newman et al., 1991; Subramanian Vignesh et al., 2013). Similarly, Coccidioides immitis arthroconidia (the spore form) can survive within unstimulated macrophages in vitro, but the addition of IFNγ or TNF enables fungal killing (Beaman et al., 1983). Human monocytes do have an innate ability to take up and kill C. immitis arthroconidia, however killing of endospores (the replicating form within the host) requires stimulation by pro-inflammatory cytokines (Ampel and Galgiani, 1991; Beaman, 1991; Ampel et al., 1992). A. fumigatus induces M1 polarization and ROS production by macrophages, and mice that lack NOX2 activity in monocytes and macrophages are highly susceptible to A. fumigatus infection (Gersuk et al., 2006; Grimm et al., 2013; Zhang et al., 2019). M1 macrophages are more fungicidal against C. neoformans than M2 macrophages in vitro (Davis et al., 2013). In vivo, a shift in macrophage polarization from M2 to M1 correlates with the fungal clearance phase in a murine model of chronic cryptococcosis, and M1 polarization has been associated with host protection against C. neoformans in vaccination models (Osterholzer et al., 2011; Hardison et al., 2012). Interestingly, C. neoformans has been able to take advantage of the dynamic process of macrophage polarization. In a fatal infection model of cryptococcosis, the fungus induces monocytes to assume an M2 macrophage phenotype that is permissive for fungal proliferation and dissemination (Heung and Hohl, 2019). However, disrupting IL-4 and IL-13 signaling can improve murine outcomes after C. neoformans challenge (Stenzel et al., 2009; Muller et al., 2013).
MoDCs can also have direct fungicidal effects. For instance, MoDCs take up and kill A. fumigatus conidia, a process mediated in part by NOX2 (Espinosa et al., 2014). They have been shown to engulf and kill both C. neoformans and C. gattii, although this leads to different outcomes in the adaptive immune responses to the two species (Wozniak and Levitz, 2008; Huston et al., 2013). Additionally, MoDCs kill and process H. capsulatum for subsequent antigen presentation to T cells (Gildea et al., 2001).
Fungi have developed counteractive mechanisms to avoid or survive within the phagolysosome. Cryptococcus species produce a large polysaccharide capsule to avoid phagocytosis in the first place, but they also can survive within the phagolysosome of monocytes and macrophages (Feldmesser et al., 2000; Alvarez and Casadevall, 2006; Ma et al., 2006; Alvarez et al., 2008, 2009; Heitman et al., 2010; Nicola et al., 2011). C. immitis endospores and arthroconidia have been shown to block the fusion of phagosomes with the lysosome in monocytes and macrophages (Beaman and Holmberg, 1980; Beaman et al., 1981). H. capsulatum blocks phagosome-lysosome fusion in macrophages and can inhibit acidification of the phagolysosome (Eissenberg et al., 1993; Newman et al., 1997). Aspergillus terreus, which can be more refractory to treatment than other Aspergillus species, persists as viable conidia in the phagolysosome of macrophages and MoDCs, in addition to dampening the expression of pro-inflammatory cytokines and markers of transmigration by DCs (Slesiona et al., 2012; Hachem et al., 2014; Hsieh et al., 2017). B. dermatitidis spores are readily taken up by lung macrophages, but this step promotes the conversion of spores into the yeast form of the fungus with subsequent proliferation (Sterkel et al., 2015). The yeast form of B. dermatitidis has also been found to reduce nitric oxide production by macrophages by inhibiting NOS2 activity (Rocco et al., 2011). These studies clearly indicate that the regulation of phagocytosis and maturation of the phagolysosome in monocytes and their derivatives play key roles in the outcomes of fungal infections and, therefore, may be important targets for enhancing host antifungal immunity.
The inflammatory milieu generated by monocyte-derived cytokine and chemokine secretion is important for the development of both the innate and adaptive immune response to fungal pathogens. Human susceptibility to blood stream infections with Candida has been correlated to single-nucleotide polymorphisms in monocyte-derived cytokines (Jaeger et al., 2019). Monocytes and their derivative cells can produce pro-inflammatory cytokines like TNF, IL-1, and IL-12, anti-inflammatory cytokines like IL-10 and TGF-β, pleiotropic cytokines like IL-6 and IL-15, and chemokines like CXCL1, CXCL2, CCL5, CXCL9, and CXCL10 (Carson et al., 1995; Arango Duque and Descoteaux, 2014). These molecules can influence the activation and recruitment of other immune cells and the polarization of the adaptive immune response.
The secretion of pro-inflammatory cytokines is typically associated with beneficial host responses. Monocytes and their derivatives are an important source of TNF and other pro-inflammatory cytokines and chemokines during infection with A. fumigatus and C. albicans (Kim et al., 2005; Cortez et al., 2006; Gersuk et al., 2006; Espinosa et al., 2014). In response to H. capsulatum, macrophages and DCs secrete pro-inflammatory TNF, IL-6, and IL-12 that inform the adaptive immune response (Lin et al., 2010; Huang et al., 2015). Anti-inflammatory cytokines are more often correlated with poor antifungal immunity. For instance, during Coccidioides infection, the susceptibility of C57BL/6 mice is strongly correlated with IL-10 secretion (Jimenez Mdel et al., 2006; Fierer, 2007). DBA/2 mice that are more resistant to Coccidioides have DCs that produce less IL-10 and both macrophages and DCs that produce more pro-inflammatory cytokines compared to C57BL/6 mice (del Pilar Jimenez et al., 2008). Additionally, IL-1RA is secreted by human whole blood samples and murine bone marrow-derived DCs upon stimulation with coccidioidal antigens, and it may play a role in blocking IL-1R1 signaling that is host protective (Ampel et al., 2018; Viriyakosol et al., 2018).
Monocyte-derived cytokines can also mediate crosstalk with other innate effector cells. During systemic C. albicans infection, IL-15 secreted by monocytes and IL-23p19 produced by DCs induce natural killer (NK) cells to secrete GM-CSF that is required for neutrophil fungicidal activity (Whitney et al., 2014; Dominguez-Andres et al., 2017b). Additionally, monocytes and moDCs promote the fungicidal activity of neutrophils against A. fumigatus conidia through the production of pro-inflammatory cytokines (Espinosa et al., 2014). Therefore, manipulation of cytokines produced by monocytes and their derivative cells can have a significant impact on both the innate and adaptive immune response.
Monocytes and their derivatives are capable of serving as antigen presenting cells (APCs) to prime T cells and induce adaptive immune responses that promote fungal clearance (Roy and Klein, 2012). DCs are the main professional APCs and can pick up fungal antigens by scavenging apoptotic infected cells and antigens shed by fungal cells or by ingesting and processing fungal pathogens directly (Bozza et al., 2002; Lin et al., 2005). After antigen acquisition, DCs mature, as evidenced by upregulation of surface markers including major histocompatibility complex I (MHC I) and II (MHCII) molecules that present antigen to CD8+ and CD4+ T cells, respectively, and co-stimulatory molecules such as CD80 and CD86. The mature DCs then migrate to lymphoid tissues where they encounter and prime T cells (Eisenbarth, 2019).
In a murine model of chronic cryptococcosis, monocytes can differentiate into DCs that mediate the generation of a Th1 adaptive response that aids in clearance of the fungus (Osterholzer et al., 2009). DCs have been shown to ingest C. neoformans or bind cryptococcal antigens, resulting in DC maturation and the subsequent activation and proliferation of T cells (Syme et al., 2002; Mansour et al., 2006; Wozniak et al., 2006; Wozniak and Levitz, 2008). MoDCs that present H. capsulatum antigen can dampen harmful Th2 responses by reducing IL-4 production by CD4+ T cells (Szymczak and Deepe, 2010). Additionally, MoDCs can cross-present H. capsulatum antigen acquired from apoptotic macrophages to promote CD8+ T cell cytotoxic responses under conditions where CD4+ T cells are absent or low, as might be found in HIV/AIDS patients (Lin et al., 2005). In vaccination models for B. dermatitidis and H. capsulatum, robust CD4+ T cell priming is dependent on monocyte recruitment to the immunization site (Wuthrich et al., 2012). Human MoDCs exposed to C. immitis antigen can induce T cell proliferation and IFNγ secretion (Richards et al., 2001). During respiratory aspergillosis, monocytes differentiate into DCs that traffic A. fumigatus conidia to the draining mediastinal lymph nodes and trigger beneficial CD4+ T cell responses (Bozza et al., 2002; Hohl et al., 2009). Interestingly, it appears that fungi can also subvert DC antigen presentation pathways. As noted earlier, MoDCs can kill C. gattii, but concurrently, the fungus is able to prevent further DC maturation that would lead to a robust adaptive immune response (Huston et al., 2013). Hence, there are multiple steps in antigen presentation by moDCs that can be optimized to generate more effective adaptive immune responses to fungi.
The adaptive immune response, which includes the generation of memory T and B cells, is the classic mechanism by which the immune system retains memory of foreign antigens to ensure a rapid and specific response upon re-exposure. However, recent studies indicate that monocytes and other innate immune cells can also contribute to immunological memory through the process of trained immunity (Netea et al., 2011, 2016). Trained immunity is established when innate immune cells exposed to microbial antigens undergo sustained epigenetic and metabolic modifications that can enhance their response to a subsequent non-specific stimulus. This innate immune memory is typically maintained over a shorter period of time (weeks to months) compared to adaptive immune memory (years). Notably, the life span of innate immune cells can be quite short, but there is evidence to suggest that hematopoietic stem cells and progenitors of innate immune cells can undergo trained immunity, thereby extending the duration of innate immune memory (Yanez et al., 2013).
Fungal antigens have been found to induce trained immunity in monocytes and their derivative cells. Exposure to β-glucan and to heat-killed or sublethal doses of the commensal fungus C. albicans can cause histone modifications and metabolic changes in monocytes and macrophages (Quintin et al., 2012; Saeed et al., 2014). Upon rechallenge with a lethal infection of C. albicans, these trained monocytes and macrophages had enhanced cytokine production and improved survival of the infected mice (Browder et al., 1984; Quintin et al., 2012). These host protective effects were confirmed to take place in the absence of T and B cells (Bistoni et al., 1986; Leonhardt et al., 2018). The trained cells also had stronger responses upon restimulation with other microbial antigens and pathogens, including lipopolysaccharide (LPS) and Staphylococcus aureus (Di Luzio and Williams, 1978; Quintin et al., 2012; Marakalala et al., 2013). Blocking epigenetic modifications or inhibiting glycolysis can disrupt trained immunity and these beneficial effects (Dominguez-Andres et al., 2017a, 2019). Fungal antigens other than β-glucan may also be able to induce trained immunity. For example, chitin isolated from the commensal yeast Saccharomyces cerevisiae can enhance monocyte responses to C. albicans as well as gram-positive and gram-negative bacteria (Rizzetto et al., 2016).
There is some evidence that DCs may also have memory-like capabilities. Studies using a vaccine strain of C. neoformans indicate that splenic DCs undergo histone modifications that enhance cytokine responses upon rechallenge with a virulent strain of C. neoformans (Hole et al., 2019). However, these DCs did not have a robust response to other secondary stimuli including LPS, S. aureus, and C. albicans. The fungal component of C. neoformans that may be involved in stimulating this DC memory also remains to be identified.
Strides are being made to further enhance the effects of trained immunity. For example, deleting SHIP-1 in trained macrophages increases their production of pro-inflammatory cytokines and improves their protection against lethal C. albicans infection (Saz-Leal et al., 2018). However, priming innate immune cells with microbial antigens does not always result in beneficial host responses. Trained immunity may play pathologic roles in conditions involving chronic inflammation, and LPS has previously been shown to induce tolerance in monocytes and macrophages to secondary stimuli (Dobrovolskaia and Vogel, 2002; Fan and Cook, 2004; Netea et al., 2016). Thus, as with any immunomodulatory strategies, it will be important to evaluate the full effects of trained immunity on the overall immune response.
The multiple roles of monocytes and their derivative cells in the host response to fungal pathogens highlight their importance in mediating the outcomes of infection. Dissecting the specific mechanisms by which monocytes carry out these functions may enable us to develop novel therapeutics that can target these pathways to improve the mortality rates from invasive fungal infections. With the current intense focus on the role of the microbiome in human health, it will be interesting to further uncover the roles that commensal organisms may play in the trained immunity of monocytes as a key defense mechanism against pathogenic fungi. There is ongoing work to determine whether the heterogeneity of monocyte responses may be tied to their origins in the hematopoietic tissues. For instance, there is evidence that the fate of monocytes is predetermined in the bone marrow and may originate from differences in expression of the transcription factor PU.1, which can dictate their eventual differentiation into iNOS+ macrophages vs. moDCs (Menezes et al., 2016). Whether the development of monocytes from different progenitors (i.e., from an MDP vs. GMP progenitor) can influence their ultimate role in the response to fungal pathogens also remains to be determined (Wolf et al., 2019). Besides the pathogens discussed in this review, there are other medically important fungi in which the role of monocytes and monocyte-derived cells is unknown or has only begun to be explored, such as Pneumocystis jirovecii, Fusarium spp., the Zygomycetes like Rhizopus spp. and Mucor spp., and emerging pathogens like Candida auris (Friedman and Schwartz, 2019). Therefore, the study of monocytes and immunity to fungal pathogens remains a burgeoning and critical area of research.
LH conceptualized, wrote, and edited the manuscript.
LH was supported by NIH/NIAID award K08 AI130366.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author thanks Fayyaz Sutterwala for helpful comments. The figure in this manuscript incorporates illustrations created by Motifolio (motifolio.com).
Aimanianda, V., Bayry, J., Bozza, S., Kniemeyer, O., Perruccio, K., Elluru, S. R., et al. (2009). Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460, 1117–1121. doi: 10.1038/nature08264
Alvarez, M., Burn, T., Luo, Y., Pirofski, L. A., and Casadevall, A. (2009). The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC Microbiol. 9:51. doi: 10.1186/1471-2180-9-51
Alvarez, M., and Casadevall, A. (2006). Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16, 2161–2165. doi: 10.1016/j.cub.2006.09.061
Alvarez, M., Saylor, C., and Casadevall, A. (2008). Antibody action after phagocytosis promotes Cryptococcus neoformans and Cryptococcus gattii macrophage exocytosis with biofilm-like microcolony formation. Cell. Microbiol. 10, 1622–1633. doi: 10.1111/j.1462-5822.2008.01152.x
Ampel, N. M., Bejarano, G. C., and Galgiani, J. N. (1992). Killing of Coccidioides immitis by human peripheral blood mononuclear cells. Infect. Immun. 60, 4200–4204. doi: 10.1128/IAI.60.10.4200-4204.1992
Ampel, N. M., and Galgiani, J. N. (1991). Interaction of human peripheral blood mononuclear cells with Coccidioides immitis arthroconidia. Cell. Immunol. 133, 253–262. doi: 10.1016/0008-8749(91)90195-h
Ampel, N. M., Robey, I., Nguyen, C. T., Roller, B., August, J., Knox, K. S., et al. (2018). Ex vivo cytokine release, determined by a multiplex cytokine assay, in response to coccidioidal antigen stimulation of whole blood among subjects with recently diagnosed primary pulmonary coccidioidomycosis. mSphere 3:e00065-18. doi: 10.1128/mSphere.00065-18
Arango Duque, G., and Descoteaux, A. (2014). Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5:491. doi: 10.3389/fimmu.2014.00491
Auffray, C., Fogg, D., Garfa, M., Elain, G., Join-Lambert, O., Kayal, S., et al. (2007). Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670. doi: 10.1126/science.1142883
Beaman, L. (1991). Effects of recombinant gamma interferon and tumor necrosis factor on in vitro interactions of human mononuclear phagocytes with Coccidioides immitis. Infect. Immun. 59, 4227–4229. doi: 10.1128/IAI.59.11.4227-4229.1991
Beaman, L., Benjamini, E., and Pappagianis, D. (1981). Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect. Immun. 34, 347–353. doi: 10.1128/IAI.34.2.347-353.1981
Beaman, L., Benjamini, E., and Pappagianis, D. (1983). Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect. Immun. 39, 1201–1207. doi: 10.1128/IAI.39.3.1201-1207.1983
Beaman, L., and Holmberg, C. A. (1980). In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect. Immun. 28, 594–600.
Bistoni, F., Vecchiarelli, A., Cenci, E., Puccetti, P., Marconi, P., and Cassone, A. (1986). Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 51, 668–674. doi: 10.1128/IAI.51.2.668-674.1986
Bongomin, F., Gago, S., Oladele, R. O., and Denning, D. W. (2017). Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi. 3:E57. doi: 10.3390/jof3040057
Bozza, S., Gaziano, R., Spreca, A., Bacci, A., Montagnoli, C., di Francesco, P., et al. (2002). Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168, 1362–1371. doi: 10.4049/jimmunol.168.3.1362
Browder, I. W., Williams, D. L., Kitahama, A., and Di Luzio, N. R. (1984). Modification of post-operative C. albicans sepsis by glucan immunostimulation. Int. J. Immunopharmacol. 6, 19–26. doi: 10.1016/0192-0561(84)90030-4
Carrion Sde, J., Leal, S. M. Jr., Ghannoum, M. A., Aimanianda, V., Latge, J. P., and Pearlman, E. (2013). The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 191, 2581–2588. doi: 10.4049/jimmunol.1300748
Carson, W. E., Ross, M. E., Baiocchi, R. A., Marien, M. J., Boiani, N., Grabstein, K., et al. (1995). Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Invest. 96, 2578–2582. doi: 10.1172/JCI118321
Chang, T. H., Huang, J. H., Lin, H. C., Chen, W. Y., Lee, Y. H., Hsu, L. C., et al. (2017). Dectin-2 is a primary receptor for NLRP3 inflammasome activation in dendritic cell response to Histoplasma capsulatum. PLoS Pathog. 13:e1006485. doi: 10.1371/journal.ppat.1006485
Charlier, C., Nielsen, K., Daou, S., Brigitte, M., Chretien, F., and Dromer, F. (2009). Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 77, 120–127. doi: 10.1128/IAI.01065-08
Clark, C., and Drummond, R. A. (2019). The hidden cost of modern medical Interventions: how medical advances have shaped the prevalence of human fungal disease. Pathogens 8:E45. doi: 10.3390/pathogens8020045
Cortez, K. J., Lyman, C. A., Kottilil, S., Kim, H. S., Roilides, E., Yang, J., et al. (2006). Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus. Infect. Immun. 74, 2353–2365. doi: 10.1128/IAI.74.4.2353-2365.2006
Davis, M. J., Tsang, T. M., Qiu, Y., Dayrit, J. K., Freij, J. B., Huffnagle, G. B., et al. (2013). Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 4, e00264–e00213. doi: 10.1128/mBio.00264-13
del Pilar Jimenez, A. M., Viriyakosol, S., Walls, L., Datta, S. K., Kirkland, T., Heinsbroek, S. E., et al. (2008). Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes. Immun. 9, 338–348. doi: 10.1038/gene.2008.23
Di Luzio, N. R., and Williams, D. L. (1978). Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect. Immun. 20, 804–810. doi: 10.1128/IAI.20.3.804-810.1978
Dobrovolskaia, M. A., and Vogel, S. N. (2002). Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes. Infect. 4, 903–914. doi: 10.1016/s1286-4579(02)01613-1
Dominguez-Andres, J., Arts, R. J. W., Ter Horst, R., Gresnigt, M. S., Smeekens, S. P., Ratter, J. M., et al. (2017a). Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog. 13:e1006632. doi: 10.1371/journal.ppat.1006632
Dominguez-Andres, J., Feo-Lucas, L., Minguito de la Escalera, M., Gonzalez, L., Lopez-Bravo, M., and Ardavin, C. (2017b). Inflammatory Ly6C (high) monocytes protect against candidiasis through IL-15-driven NK cell/neutrophil activation. Immunity 46, 1059–1072 e1054. doi: 10.1016/j.immuni.2017.05.009
Dominguez-Andres, J., Ferreira, A. V., Jansen, T., Smithers, N., Prinjha, R. K., Furze, R. C., et al. (2019). Bromodomain inhibitor I-BET151 suppresses immune responses during fungal-immune interaction. Eur. J. Immunol. 49, 2044–2050 doi: 10.1002/eji.201848081
d'Ostiani, C. F., Del Sero, G., Bacci, A., Montagnoli, C., Spreca, A., Mencacci, A., et al. (2000). Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191, 1661–1674. doi: 10.1084/jem.191.10.1661
Eisenbarth, S. C. (2019). Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 19, 89–103. doi: 10.1038/s41577-018-0088-1
Eissenberg, L. G., Goldman, W. E., and Schlesinger, P. H. (1993). Histoplasma capsulatum modulates the acidification of phagolysosomes. J. Exp. Med. 177, 1605–1611. doi: 10.1084/jem.177.6.1605
Erwig, L. P., and Gow, N. A. (2016). Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 14, 163–176. doi: 10.1038/nrmicro.2015.21
Espinosa, V., Jhingran, A., Dutta, O., Kasahara, S., Donnelly, R., Du, P., et al. (2014). Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 10:e1003940. doi: 10.1371/journal.ppat.1003940
Fan, H., and Cook, J. A. (2004). Molecular mechanisms of endotoxin tolerance. J. Endotoxin. Res. 10, 71–84. doi: 10.1179/096805104225003997
Feldmesser, M., Kress, Y., Novikoff, P., and Casadevall, A. (2000). Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68, 4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000
Fierer, J. (2007). The role of IL-10 in genetic susceptibility to coccidioidomycosis on mice. Ann. N.Y. Acad. Sci. 1111, 236–244. doi: 10.1196/annals.1406.048
Friedman, D. Z. P., and Schwartz, I. S. (2019). Emerging fungal infections: new patients, new patterns, and new pathogens. J. Fungi 5:E67. doi: 10.3390/jof5030067
Gantner, B. N., Simmons, R. M., and Underhill, D. M. (2005). Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286. doi: 10.1038/sj.emboj.7600594
Gersuk, G. M., Underhill, D. M., Zhu, L., and Marr, K. A. (2006). Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176, 3717–3724. doi: 10.4049/jimmunol.176.6.3717
Gildea, L. A., Morris, R. E., and Newman, S. L. (2001). Histoplasma capsulatum yeasts are phagocytosed via very late antigen-5, killed, and processed for antigen presentation by human dendritic cells. J. Immunol. 166, 1049–1056. doi: 10.4049/jimmunol.166.2.1049
Ginhoux, F., and Guilliams, M. (2016). Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449. doi: 10.1016/j.immuni.2016.02.024
Gow, N. A., van de Veerdonk, F. L., Brown, A. J., and Netea, M. G. (2011). Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122. doi: 10.1038/nrmicro2711
Grimm, M. J., Vethanayagam, R. R., Almyroudis, N. G., Dennis, C. G., Khan, A. N., D'Auria, A. C., et al. (2013). Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J. Immunol. 190, 4175–4184. doi: 10.4049/jimmunol.1202800
Hachem, R., Gomes, M. Z., El Helou, G., El Zakhem, A., Kassis, C., Ramos, E., et al. (2014). Invasive aspergillosis caused by Aspergillus terreus: an emerging opportunistic infection with poor outcome independent of azole therapy. J. Antimicrob. Chemother. 69, 3148–3155. doi: 10.1093/jac/dku241
Hardison, S. E., Herrera, G., Young, M. L., Hole, C. R., Wozniak, K. L., and Wormley, F. L. Jr. (2012). Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J. Immunol. 189, 4060–4068. doi: 10.4049/jimmunol.1103455
Heitman, J., Kozel, T. R., Kwon-Chung, K. J., Perfect, J. R., and Casadevall, A. (2010). Cryptococcus: From Human Pathogen to Model Yeast. Washington, DC: ASM Press. doi: 10.1128/9781555816858
Heung, L. J., and Hohl, T. M. (2019). Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans. PLoS Pathog. 15:e1007627. doi: 10.1371/journal.ppat.1007627
Hoeffel, G., and Ginhoux, F. (2018). Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 330, 5–15. doi: 10.1016/j.cellimm.2018.01.001
Hohl, T. M., Rivera, A., Lipuma, L., Gallegos, A., Shi, C., Mack, M., et al. (2009). Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6, 470–481. doi: 10.1016/j.chom.2009.10.007
Hohl, T. M., Van Epps, H. L., Rivera, A., Morgan, L. A., Chen, P. L., Feldmesser, M., et al. (2005). Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1:e30. doi: 10.1371/journal.ppat.0010030
Hole, C. R., Wager, C. M. L., Castro-Lopez, N., Campuzano, A., Cai, H., Wozniak, K. L., et al. (2019). Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 10:2955. doi: 10.1038/s41467-019-10486-5
Howard, D. H. (1964). Intracellular behavior of Histoplasma capsulatum. J. Bacteriol. 87, 33–38. doi: 10.1128/JB.87.1.33-38.1964
Hsieh, S. H., Kurzai, O., and Brock, M. (2017). Persistence within dendritic cells marks an antifungal evasion and dissemination strategy of Aspergillus terreus. Sci. Rep. 7:10590. doi: 10.1038/s41598-017-10914-w
Huang, J. H., Lin, C. Y., Wu, S. Y., Chen, W. Y., Chu, C. L., Brown, G. D., et al. (2015). CR3 and Dectin-1 collaborate in macrophage cytokine response through association on lipid rafts and activation of Syk-JNK-AP-1 pathway. PLoS Pathog. 11:e1004985. doi: 10.1371/journal.ppat.1004985
Huston, S. M., Li, S. S., Stack, D., Timm-McCann, M., Jones, G. J., Islam, A., et al. (2013). Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J. Immunol. 191, 249–261. doi: 10.4049/jimmunol.1202707
Jaeger, M., Matzaraki, V., Aguirre-Gamboa, R., Gresnigt, M. S., Chu, X., Johnson, M. D., et al. (2019). A genome-wide functional genomics approach identifies susceptibility pathways to fungal bloodstream infection in humans. J. Infect. Dis. 220, 862–872. doi: 10.1093/infdis/jiz206
Jakubzick, C. V., Randolph, G. J., and Henson, P. M. (2017). Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 17, 349–362. doi: 10.1038/nri.2017.28
Jimenez Mdel, P., Walls, L., and Fierer, J. (2006). High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect. Immun. 74, 3387–3395. doi: 10.1128/IAI.01985-05
Joly, S., Ma, N., Sadler, J. J., Soll, D. R., Cassel, S. L., and Sutterwala, F. S. (2009). Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 183, 3578–3581. doi: 10.4049/jimmunol.0901323
Kim, H. S., Choi, E. H., Khan, J., Roilides, E., Francesconi, A., Kasai, M., et al. (2005). Expression of genes encoding innate host defense molecules in normal human monocytes in response to Candida albicans. Infect. Immun. 73, 3714–3724. doi: 10.1128/IAI.73.6.3714-3724.2005
Lawrence, T., and Natoli, G. (2011). Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761. doi: 10.1038/nri3088
Leonhardt, J., Grosse, S., Marx, C., Siwczak, F., Stengel, S., Bruns, T., et al. (2018). Candida albicans beta-glucan differentiates human monocytes into a specific subset of macrophages. Front. Immunol. 9:2818. doi: 10.3389/fimmu.2018.02818
Lin, J. S., Huang, J. H., Hung, L. Y., Wu, S. Y., and Wu-Hsieh, B. A. (2010). Distinct roles of complement receptor 3, Dectin-1, and sialic acids in murine macrophage interaction with Histoplasma yeast. J. Leukoc. Biol. 88, 95–106. doi: 10.1189/jlb.1109717
Lin, J. S., Yang, C. W., Wang, D. W., and Wu-Hsieh, B. A. (2005). Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J. Immunol. 174, 6282–6291. doi: 10.4049/jimmunol.174.10.6282
Lionakis, M. S., Iliev, I. D., and Hohl, T. M. (2017). Immunity against fungi. JCI Insight 2:e93156. doi: 10.1172/jci.insight.93156
Ma, H., Croudace, J. E., Lammas, D. A., and May, R. C. (2006). Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16, 2156–2160. doi: 10.1016/j.cub.2006.09.032
Mansour, M. K., Latz, E., and Levitz, S. M. (2006). Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J. Immunol. 176, 3053–3061. doi: 10.4049/jimmunol.176.5.3053
Marakalala, M. J., Williams, D. L., Hoving, J. C., Engstad, R., Netea, M. G., and Brown, G. D. (2013). Dectin-1 plays a redundant role in the immunomodulatory activities of beta-glucan-rich ligands in vivo. Microbes. Infect. 15, 511–515. doi: 10.1016/j.micinf.2013.03.002
Martinez, F. O., and Gordon, S. (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6:13. doi: 10.12703/P6-13
Menezes, S., Melandri, D., Anselmi, G., Perchet, T., Loschko, J., Dubrot, J., et al. (2016). The heterogeneity of Ly6C(hi) monocytes controls their differentiation into iNOS(+) macrophages or monocyte-derived dendritic cells. Immunity 45, 1205–1218. doi: 10.1016/j.immuni.2016.12.001
Mosser, D. M., and Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. doi: 10.1038/nri2448
Muller, U., Stenzel, W., Piehler, D., Grahnert, A., Protschka, M., Kohler, G., et al. (2013). Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing T(h)2 response. Int. Immunol. 25, 459–470. doi: 10.1093/intimm/dxt003
Murray, P. J. (2018). Immune regulation by monocytes. Semin. Immunol. 35, 12–18. doi: 10.1016/j.smim.2017.12.005
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. doi: 10.1016/j.immuni.2014.06.008
Netea, M. G., Joosten, L. A., Latz, E., Mills, K. H., Natoli, G., Stunnenberg, H. G., et al. (2016). Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. doi: 10.1126/science.aaf1098
Netea, M. G., Quintin, J., and van der Meer, J. W. (2011). Trained immunity: a memory for innate host defense. Cell. Host. Microbe. 9, 355–361. doi: 10.1016/j.chom.2011.04.006
Newman, S. L., Gootee, L., Bucher, C., and Bullock, W. E. (1991). Inhibition of intracellular growth of Histoplasma capsulatum yeast cells by cytokine-activated human monocytes and macrophages. Infect. Immun. 59, 737–741. doi: 10.1128/IAI.59.2.737-741.1991
Newman, S. L., Gootee, L., Kidd, C., Ciraolo, G. M., and Morris, R. (1997). Activation of human macrophage fungistatic activity against Histoplasma capsulatum upon adherence to type 1 collagen matrices. J. Immunol. 158, 1779–1786.
Ngo, L. Y., Kasahara, S., Kumasaka, D. K., Knoblaugh, S. E., Jhingran, A., and Hohl, T. M. (2014). Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J. Infect. Dis. 209, 109–119. doi: 10.1093/infdis/jit413
Nicola, A. M., Robertson, E. J., Albuquerque, P., Derengowski Lda, S., and Casadevall, A. (2011). Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. MBio 2:e00167-11. doi: 10.1128/mBio.00167-11
Noble, S. M., Gianetti, B. A., and Witchley, J. N. (2017). Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 15, 96–108. doi: 10.1038/nrmicro.2016.157
Olingy, C. E., Dinh, H. Q., and Hedrick, C. C. (2019). Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 106, 309–322. doi: 10.1002/JLB.4RI0818-311R
Osterholzer, J. J., Chen, G. H., Olszewski, M. A., Curtis, J. L., Huffnagle, G. B., and Toews, G. B. (2009). Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J. Immunol. 183, 8044–8053. doi: 10.4049/jimmunol.0902823
Osterholzer, J. J., Chen, G. H., Olszewski, M. A., Zhang, Y. M., Curtis, J. L., Huffnagle, G. B., et al. (2011). Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. Am. J. Pathol. 178, 198–211. doi: 10.1016/j.ajpath.2010.11.006
Osterholzer, J. J., Curtis, J. L., Polak, T., Ames, T., Chen, G. H., McDonald, R., et al. (2008). CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J. Immunol. 181, 610–620. doi: 10.4049/jimmunol.181.1.610
Pfaller, M. A., and Diekema, D. J. (2010). Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 36, 1–53. doi: 10.3109/10408410903241444
Quintin, J., Saeed, S., Martens, J. H. A., Giamarellos-Bourboulis, E. J., Ifrim, D. C., Logie, C., et al. (2012). Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 12, 223–232. doi: 10.1016/j.chom.2012.06.006
Richards, J. O., Ampel, N. M., Galgiani, J. N., and Lake, D. F. (2001). Dendritic cells pulsed with Coccidioides immitis lysate induce antigen-specific naive T cell activation. J. Infect. Dis. 184, 1220–1224. doi: 10.1086/323664
Rivera, A., Hohl, T. M., Collins, N., Leiner, I., Gallegos, A., Saijo, S., et al. (2011). Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208, 369–381. doi: 10.1084/jem.20100906
Rivera, A., Van Epps, H. L., Hohl, T. M., Rizzuto, G., and Pamer, E. G. (2005). Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect. Immun. 73, 7170–7179. doi: 10.1128/IAI.73.11.7170-7179.2005
Rizzetto, L., Ifrim, D. C., Moretti, S., Tocci, N., Cheng, S. C., Quintin, J., et al. (2016). Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. J. Biol. Chem. 291, 7961–7972. doi: 10.1074/jbc.M115.699645
Rocco, N. M., Carmen, J. C., and Klein, B. S. (2011). Blastomyces dermatitidis yeast cells inhibit nitric oxide production by alveolar macrophage inducible nitric oxide synthase. Infect. Immun. 79, 2385–2395. doi: 10.1128/IAI.01249-10
Roy, R. M., and Klein, B. S. (2012). Dendritic cells in antifungal immunity and vaccine design. Cell. Host. Microbe. 11, 436–446. doi: 10.1016/j.chom.2012.04.005
Saeed, S., Quintin, J., Kerstens, H. H., Rao, N. A., Aghajanirefah, A., Matarese, F., et al. (2014). Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345:1251086. doi: 10.1126/science.1251086
Saz-Leal, P., Del Fresno, C., Brandi, P., Martinez-Cano, S., Dungan, O. M., Chisholm, J. D., et al. (2018). Targeting SHIP-1 in myeloid cells enhances trained immunity and boosts response to infection. Cell. Rep. 25, 1118–1126. doi: 10.1016/j.celrep.2018.09.092
Shi, C., and Pamer, E. G. (2011). Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774. doi: 10.1038/nri3070
Slesiona, S., Gressler, M., Mihlan, M., Zaehle, C., Schaller, M., Barz, D., et al. (2012). Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS ONE 7:e31223. doi: 10.1371/journal.pone.0031223
Steele, C., Rapaka, R. R., Metz, A., Pop, S. M., Williams, D. L., Gordon, S., et al. (2005). The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog, 1:e42. doi: 10.1371/journal.ppat.0010042
Stenzel, W., Muller, U., Kohler, G., Heppner, F. L., Blessing, M., McKenzie, A. N., et al. (2009). IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am. J. Pathol. 174, 486–496. doi: 10.2353/ajpath.2009.080598
Sterkel, A. K., Mettelman, R., Wuthrich, M., and Klein, B. S. (2015). The unappreciated intracellular lifestyle of Blastomyces dermatitidis. J. Immunol. 194, 1796–1805. doi: 10.4049/jimmunol.1303089
Subramanian Vignesh, K., Landero Figueroa, J. A., Porollo, A., Caruso, J. A., and Deepe, G. S. Jr. (2013). Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39, 697–710. doi: 10.1016/j.immuni.2013.09.006
Syme, R. M., Spurrell, J. C., Amankwah, E. K., Green, F. H., and Mody, C. H. (2002). Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcgamma receptor II for presentation to T lymphocytes. Infect. Immun. 70, 5972–5981. doi: 10.1128/iai.70.11.5972-5981.2002
Szymczak, W. A., and Deepe, G. S. Jr. (2009). The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J. Immunol. 183, 1964–1974. doi: 10.4049/jimmunol.0901316
Szymczak, W. A., and Deepe, G. S. Jr. (2010). Antigen-presenting dendritic cells rescue CD4-depleted CCR2-/- mice from lethal Histoplasma capsulatum infection. Infect. Immun. 78, 2125–2137. doi: 10.1128/IAI.00065-10
Thompson, A., Davies, L. C., Liao, C. T., da Fonseca, D. M., Griffiths, J. S., Andrews, R., et al. (2019). The protective effect of inflammatory monocytes during systemic C. albicans infection is dependent on collaboration between C-type lectin-like receptors. PLoS Pathog. 15:e1007850. doi: 10.1371/journal.ppat.1007850
Traynor, T. R., Kuziel, W. A., Toews, G. B., and Huffnagle, G. B. (2000). CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164, 2021–2027. doi: 10.4049/jimmunol.164.4.2021
Uribe-Querol, E., and Rosales, C. (2017). Control of phagocytosis by microbial pathogens. Front. Immunol. 8:1368. doi: 10.3389/fimmu.2017.01368
Viriyakosol, S., Fierer, J., Brown, G. D., and Kirkland, T. N. (2005). Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73, 1553–1560. doi: 10.1128/IAI.73.3.1553-1560.2005
Viriyakosol, S., Jimenez Mdel, P., Gurney, M. A., Ashbaugh, M. E., and Fierer, J. (2013). Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio 4, e00597–e00512. doi: 10.1128/mBio.00597-12
Viriyakosol, S., Walls, L., Okamoto, S., Raz, E., Williams, D. L., and Fierer, J. (2018). Myeloid Differentiation Factor 88 and Interleukin-1R1 signaling contribute to resistance to Coccidioides immitis. Infect. Immun. 86:e00028-18. doi: 10.1128/IAI.00028-18
Whitney, P. G., Bar, E., Osorio, F., Rogers, N. C., Schraml, B. U., Deddouche, S., et al. (2014). Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 10:e1004276. doi: 10.1371/journal.ppat.1004276
Wolf, A. A., Yanez, A., Barman, P. K., and Goodridge, H. S. (2019). The ontogeny of monocyte subsets. Front. Immunol. 10:1642. doi: 10.3389/fimmu.2019.01642
Wozniak, K. L., and Levitz, S. M. (2008). Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect. Immun. 76, 4764–4771. doi: 10.1128/IAI.00660-08
Wozniak, K. L., Vyas, J. M., and Levitz, S. M. (2006). In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect. Immun. 74, 3817–3824. doi: 10.1128/IAI.00317-06
Wu-Hsieh, B. A., and Howard, D. H. (1987). Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect. Immun. 55, 1014–1016. doi: 10.1128/IAI.55.4.1014-1016.1987
Wuthrich, M., Ersland, K., Sullivan, T., Galles, K., and Klein, B. S. (2012). Fungi subvert vaccine T cell priming at the respiratory mucosa by preventing chemokine-induced influx of inflammatory monocytes. Immunity 36, 680–692. doi: 10.1016/j.immuni.2012.02.015
Yanez, A., Coetzee, S. G., Olsson, A., Muench, D. E., Berman, B. P., Hazelett, D. J., et al. (2017). Granulocyte-monocyte progenitors and monocyte-dendritic cell progenitors independently produce functionally distinct monocytes. Immunity 47, 890–902.e894. doi: 10.1016/j.immuni.2017.10.021
Yanez, A., Hassanzadeh-Kiabi, N., Ng, M. Y., Megias, J., Subramanian, A., Liu, G. Y., et al. (2013). Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur. J. Immunol. 43, 2114–2125. doi: 10.1002/eji.201343403
Yona, S., Kim, K. W., Wolf, Y., Mildner, A., Varol, D., Breker, M., et al. (2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91. doi: 10.1016/j.immuni.2012.12.001
Keywords: monocyte, macrophage, dendritic cell, fungal infections, innate immunity
Citation: Heung LJ (2020) Monocytes and the Host Response to Fungal Pathogens. Front. Cell. Infect. Microbiol. 10:34. doi: 10.3389/fcimb.2020.00034
Received: 30 October 2019; Accepted: 17 January 2020;
Published: 13 February 2020.
Edited by:
Floyd Layton Wormley, Texas Christian University, United StatesReviewed by:
Daniel Alford Powell, University of Arizona, United StatesCopyright © 2020 Heung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lena J. Heung, bGVuYS5oZXVuZ0Bjc2hzLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.