
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 21 January 2019
Sec. Microbiome in Health and Disease
Volume 9 - 2019 | https://doi.org/10.3389/fcimb.2019.00002
Fecal microbiota transplantation (FMT) has become a highly effective bacteriotherapy for recurrent Clostridium difficile infection. Meanwhile the efficacy of FMT for treating chronic diseases associated with microbial dysbiosis has so far been modest with a much higher variability in patient response. Notably, a number of studies suggest that FMT success is dependent on the microbial diversity and composition of the stool donor, leading to the proposition of the existence of FMT super-donors. The identification and subsequent characterization of super-donor gut microbiomes will inevitably advance our understanding of the microbial component of chronic diseases and allow for more targeted bacteriotherapy approaches in the future. Here, we review the evidence for super-donors in FMT and explore the concept of keystone species as predictors of FMT success. Possible effects of host-genetics and diet on FMT engraftment and maintenance are also considered. Finally, we discuss the potential long-term applicability of FMT for chronic disease and highlight how super-donors could provide the basis for dysbiosis-matched FMTs.
The human gut harbors an abundant and diverse microbial community that is as unique to an individual as a fingerprint (Human Microbiome Project Consortium, 2012). Despite the variability between individuals, it is clear that the composition and functionality of the gut microbiota associates with the health of the host, having specialized functions in nutrition, energy metabolism, immune development, and host defense (Thursby and Juge, 2017). The composition of the gut microbiota is shaped by both genetic and environmental influences, through a continual process that may begin in utero (Perez-Muñoz et al., 2017) and fluctuates throughout an individual's lifetime (Odamaki et al., 2016). In a healthy adult, the bacterial population within the gut predominantly consists of members from the strictly anaerobic Firmicutes and Bacteroidetes phyla, with minor representations from members of the Proteobacteria and Actinobacteria phyla (Eckburg et al., 2005; Ley et al., 2008).
Because no two gut microbiomes are identical, the definition of what comprises a healthy gut microbiome from an inventory standpoint remains unclear (Human Microbiome Project Consortium, 2012). Despite this, it is generally accepted that having a stable and diverse gut community correlates with a healthy intestinal state (Lloyd-Price et al., 2016). An alteration to the microbiota that is associated with negative functional outcomes on gut physiology, such as localized inflammation or disturbed metabolic processing, is known as gut dysbiosis (Petersen and Round, 2014). Typically, gut dysbiosis is characterized by a low microbial diversity (Kriss et al., 2018).
Observations of microbial dysbioses are increasingly being associated with a broad range of human diseases, including allergies (Penders et al., 2007; Bunyavanich et al., 2016), asthma (Arrieta et al., 2015), inflammatory bowel disease (IBD) (Fujimoto et al., 2013; Gevers et al., 2014; Takahashi et al., 2016; Nishino et al., 2018), irritable bowel syndrome (IBS) (Liu et al., 2017), obesity (Schwiertz et al., 2010), and cardiovascular disease (Cui et al., 2017; Jie et al., 2017). However, evidence that the dysbiosis is causal in the development of these conditions remains difficult to establish, in all but a few cases. The use of germ-free mice, which are born and raised in a sterile environment, play a pivotal role in demonstrating causative associations between the gut microbiota and disease (Balish and Warner, 2002; Bäckhed et al., 2004; Berer et al., 2011). In the case of obesity, the metabolic phenotype of the donor, be it lean or obese, can be recapitulated by a fecal microbiota transfer into germ-free mice (Ridaura et al., 2013). Germ-free conditions have also been found to be protective against the development of colitis and ileitis in IBD-like mouse models with disease transmission only occurring after transfer of a dysbiotic gut microbiota (Sellon et al., 1998; Schaubeck et al., 2016).
The mounting evidence of a causal role of the gut microbiota in multiple disease conditions has led to the development of targeted therapeutic approaches designed to alter the microbial composition. Among these, fecal microbiota transplantation (FMT) has consistently demonstrated a capability to overcome dysbiosis associated with a number of conditions through a profound sustained effect on the gut microbiome (e.g., Weingarden et al., 2015; Broecker et al., 2016; Kumar et al., 2017; Moss et al., 2017). FMT is considered an unrefined form of bacteriotherapy that utilizes the diverse microbial gut community of a healthy donor. Typical routes of administration to FMT recipients include endoscopic delivery (Mattila et al., 2012), naso-intestinal tube delivery (Tian et al., 2017), retention enemas (Lee et al., 2014), or capsule ingestion (Youngster et al., 2014).
The definition of FMT success is primarily based on a positive clinical response in the recipient. However, from a microbiological perspective, FMT success can also be defined by a shift in the gut microbiome profile of an individual toward that of the donor. We argue that FMT success can be considered to be a two-step process; first requiring the transplanted microbiome to engraft within the new host and augment the local commensal community, after which clinical improvement may be observed.
The selection of an appropriate stool donor is a key component in FMT success (Vermeire et al., 2016). Donors are clinically screened to ensure they do not harbor any transmissible pathogens or disease (Kelly et al., 2015). A detailed list of donor selection guidelines can be found in the recently published evidence-based report on FMT in clinical practice (Cammarota et al., 2017). Donors are typically described as being either effective or ineffective with regards to their ability to contribute to FMT success. Comparing the gut microbiota profiles of different donors has revealed that microbial diversity is a reliable predictor for FMT success (Kump et al., 2018). However, a variety of additional factors, both genetic and environmental, are also known to influence FMT success (Figure 1).
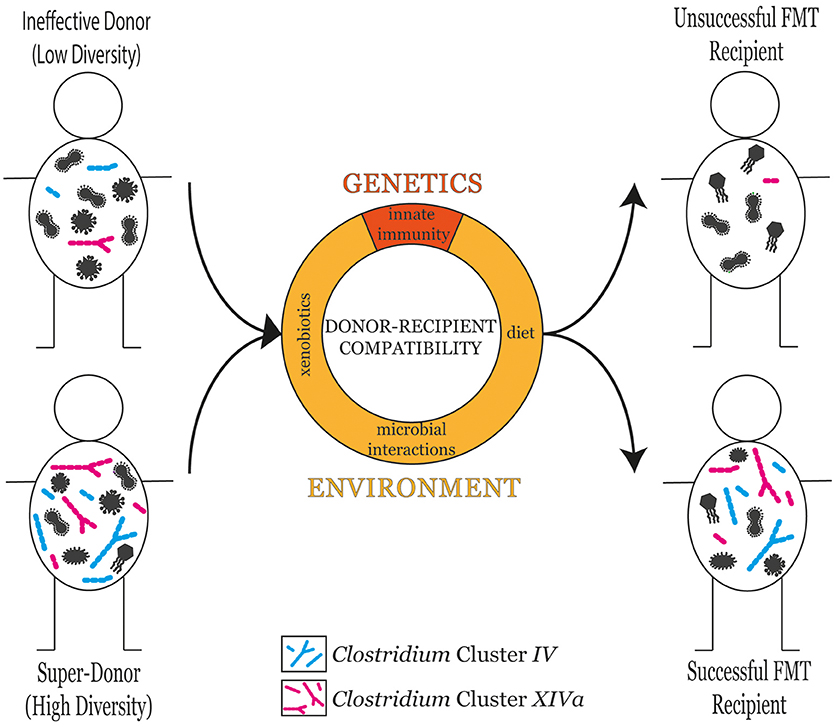
Figure 1. The microbial diversity of the donor is a good predictor of FMT success in the recipient. However, donor-recipient compatibility also plays an influential role in determining FMT success. Donor-recipient compatibility can stem from genetic factors such as differences in innate immune responses, or environmental factors including diet, xenobiotic exposure, and microbial interactions.
Recently, the term “super-donor” has been proposed to describe donors whose stool results in significantly more successful FMT outcomes than the stool of other donors. The purpose of this review is to explore evidence for the phenomenon of FMT super-donors and other factors that can contribute to treatment success, particularly focusing on the gut microbiota characterization that has been performed on donors. We discuss the concept of keystone species as predictors of FMT success and consider the possible influence of host-genetics and diet on FMT engraftment and maintenance. Finally, we will suggest a rationale for abandoning the “one stool fits all” approach.
The ingestion of human stool for health-related purposes was first documented by Chinese herbal doctors in the fourth century (de Groot et al., 2017). In recent years, FMT has been widely and effectively used for the treatment of recurrent Clostridium difficile infections (CDI) in patients that are non-responsive to antibiotic therapy (van Nood et al., 2013; Cammarota et al., 2015; Lee et al., 2016; Kao et al., 2017). C. difficile is an opportunistic gut pathogen that is suppressed in healthy individuals by the commensal gut microbiota (Borriello, 1990). However, when the diversity of the gut microbiota is reduced, e.g., after a course of antibiotics, colonization resistance of the commensal microbiota is disturbed (Leffler and Lamont, 2015). C. difficile then has the potential to proliferate undeterred, producing enterotoxins leading to intestinal inflammation and diarrhea (Warny et al., 2005). Perhaps rather counterintuitively, CDI is treated in the first instance with antibiotics which cure around 80% of cases (Fekety et al., 1997). However, 20% of individuals will experience recurrent CDI after antibiotic therapy (Leffler and Lamont, 2015). FMT is a novel treatment approach for recurrent CDI that acts to restore the commensal gut microbiota and in turn re-establish colonization resistance to inhibit the growth of C. difficile (Eiseman et al., 1958).
A recent systematic review and meta-analysis of FMT for the treatment of CDI reported a primary cure rate of 92% across 30 case series and seven randomized control trials (Quraishi et al., 2017). Microbial analyses carried out on CDI patients before and after FMT have confirmed FMT is rapidly capable of restoring microbial diversity in patients to donor-like proportions (Song et al., 2013; Shankar et al., 2014; Kelly et al., 2016; Staley et al., 2016; Khanna et al., 2017; Kellingray et al., 2018). The choice of donor, be it a relative, spouse, or anonymous volunteer, does not appear to influence the clinical efficacy of FMT (Kassam et al., 2013). Similarly, no donor-specific effects were found in a large cohort study comprising 1999 CDI patients and 28 FMT donors (Osman et al., 2016). Overall, FMT appears to be a safe and effective treatment for microbial restoration in situations where the overgrowth of a particular pathogen has led to a reduction in the diversity and abundance of the commensal organism population (i.e., severe dysbiosis).
Encouraged by the overwhelming success of FMT in the resolution of recurrent CDI, researchers have begun to investigate the therapeutic potential of FMT for a broad range of other diseases associated with less severe forms of intestinal dysbiosis. Among these exploratory studies, FMT for the treatment of IBD has featured heavily (systematically reviewed Paramsothy et al., 2017b). However, FMT has also been trialed in several other gastrointestinal disorders [IBS (Pinn et al., 2014; Holvoet et al., 2017, 2018; Mizuno et al., 2017; Aroniadis et al., 2018; Halkjær et al., 2018; Johnsen et al., 2018), constipation (Tian et al., 2017; Ding et al., 2018), allergic colitis (Liu et al., 2017)] and for various liver (Kao et al., 2016; Bajaj et al., 2017; Philips et al., 2017; Ren et al., 2017), blood (Kakihana et al., 2016; Spindelboeck et al., 2017; DeFilipp et al., 2018), metabolic (Vrieze et al., 2012; Kootte et al., 2017), and neurological conditions (He et al., 2017a; Kang et al., 2017; Makkawi et al., 2018). Compared with CDI, the clinical efficacy of FMT for these more chronic diseases has so far been modest with a much higher variability in patient response which likely reflects the multi-faceted etiology of these disorders.
IBD encompasses both Crohn's disease and ulcerative colitis; two debilitating disorders characterized by chronic relapsing inflammation of the intestinal mucosa (Gajendran et al., 2018). In contrast to CDI, there is no evidence that IBD results from an overgrowth of one specific pathogen. Rather, the disease is likely brought on by complex interactions involving the host's genetics, immune system, and gut microbiota (Ni et al., 2017). Both Crohn's disease and ulcerative colitis are broadly characterized by a reduced diversity of the gut microbiota with lower relative abundances of the Bacteroidetes and Firmicutes phyla and higher proportions of Proteobacteria (Manichanh et al., 2006; Frank et al., 2007; Sokol et al., 2008; Walker et al., 2011; Gevers et al., 2014; Machiels et al., 2014; Nishino et al., 2018). A specific reduction in the abundance of butyrate-producing bacterial species, particularly Faecalibacterium prausnitzii, has been observed for both Crohn's disease and ulcerative colitis (Fujimoto et al., 2013; Lopez-Siles et al., 2015; Takahashi et al., 2016). Meanwhile, for Crohn's disease, an increase in a pro-inflammatory form of Escherichia coli has also been reported (Darfeuille-Michaud et al., 2004; Martin et al., 2004; Baumgart et al., 2007).
The first successful case report of an FMT for the treatment of IBD was published in 1989 when a male with refractory ulcerative colitis achieved clinical remission for 6 months following a retention enema with healthy donor stool (Bennet and Brinkman, 1989). Subsequently, a large number of FMT studies have been conducted on IBD patients with variable clinical outcomes, remission rates, and longevity of effect (Zhang et al., 2013; Cui et al., 2015; Moayyedi et al., 2015; Rossen et al., 2015; Suskind et al., 2015; Vaughn et al., 2016; Vermeire et al., 2016; Costello et al., 2017; He et al., 2017b; Nishida et al., 2017; Paramsothy et al., 2017a; Goyal et al., 2018; Kump et al., 2018). Recently, Paramsothy et al. performed a systematic review and meta-analysis of 53 studies (four RCT, 30 cohort, 19 case studies) of FMT in IBD patients (Paramsothy et al., 2017b). Avoiding publication bias, their analysis of cohort studies revealed FMT was more effective at inducing remission in Crohn's disease patients when compared to patients with ulcerative colitis (52 vs. 33%, respectively). With regard to ulcerative colitis, a larger number of FMT infusions and a lower gastrointestinal tract administration were associated with improved rates of remission.
In contrast to studies of CDI, FMT studies conducted on IBD patients have frequently identified differential recipient responses that have been associated with variability in the donor stool (Khanna, 2018). Currently, the stool used for FMT is not standardized in terms of donor selection (related vs. unrelated), preparation (fresh vs. frozen, aerobic vs. anaerobic), or the dose that is administered (single vs. multiple doses) (Kelly et al., 2015). While inconsistencies in FMT protocols make it difficult to compare different studies, there is a large degree of variability in clinical responses to FMT between recipients who have been subjected to the same study design. It is unfortunate that information on a recipient's genetic background or dietary intake is not yet routinely assessed, particularly given that some instances of IBD have an underlying genetic component (de Lange et al., 2017). Due to the lack of genetic information, investigators have instead focused on the donor-dependent effect and proposed the existence of so called super-donors to explain the variation in recipient responses.
The first study to record the super-donor effect was a randomized control trial that was investigating the efficacy of FMT for inducing clinical remission in patients with ulcerative colitis (Moayyedi et al., 2015). Moayyedi et al. assigned 75 patients with active disease to weekly enemas containing either fecal material or water (placebo) for a period of 6 weeks. FMT was shown to be superior to the placebo, resulting in significantly higher rates of endoscopic and clinical remission, albeit of modest effect (24 vs. 5%, respectively), after 7 weeks. Of the nine patients who entered remission, seven had received FMT from the same donor. Thus, it was argued that FMT success was donor-dependent.
Currently, it is not possible to predict the clinical efficacy of a donor before FMT in IBD patients. It has been suggested that remission rates could be improved by pooling donor's stool together, limiting the chances a patient will receive only ineffective stool (Kazerouni and Wein, 2017). This stool pooling approach was recently investigated on an Australian cohort of 85 mild to moderate ulcerative colitis patients, in the largest randomized control trial of FMT for IBD to date (Paramsothy et al., 2017a). Rather than receiving FMT from just one donor, patients in the treatment arm were administered a stool mixture that contained contributions from up to seven different donors with the hope that donor-dependent effects could be homogenized. In addition to this, a far more intensive dosing program was adopted with an initial FMT delivered by colonoscopy that was followed by fecal enemas, five times a week for 8 weeks. Despite the multi-donor and intensive dosing approach, Paramsothy et al. achieved post-FMT remission rates (FMT, 27% vs. placebo, 8%, p = 0.02) that were similar to those reported previously (Moayyedi et al., 2015; Rossen et al., 2015). Notably, however, both clinical and endoscopic remission were required for primary outcome achievement in this study (Paramsothy et al., 2017a), whereas previous studies have mostly focused on either endoscopic or clinical remission rates alone (Ishikawa et al., 2017; Nishida et al., 2017). The pooled stool mixture was demonstrated to have higher microbial diversity than individual stool alone based on OTU count and phylogenetic diversity measures. Subsequent analysis of the different stool batches discovered that one donor appeared to exhibit a super-donor effect. Specifically, patients that received FMT batches that contained stool from this one donor exhibited a higher remission rate than those whose FMT batches did not include the super-donor (37 vs. 18%, respectively) (Paramsothy et al., 2017a).
Evidence of FMT super-donors in other disorders outside of IBD is currently lacking. Case series and reports limit the capacity to identify super-donor effects because of limited sample sizes. However, despite the lack of large cohort studies, several studies have hinted at the possibility of a donor-dependent effect on FMT outcome (Vrieze et al., 2012; Kootte et al., 2017; Mizuno et al., 2017). For example, in a short-term FMT pilot trial on 18 middle-aged men with metabolic syndrome, FMTs from lean donors (allogenic FMT) were found to correspond with a 75% increase in insulin sensitivity and a greater diversity of intestinal bacteria in the recipient compared to autologous FMTs (recipient-derived) (Vrieze et al., 2012). It was later noted that the patients who experienced a more robust improvement of insulin sensitivity post-FMT had all been in receipt of the same donor. In a subsequent study on 38 Caucasian men with metabolic syndrome, lean donor FMT also resulted in a significant improvement in peripheral insulin sensitivity at 6 weeks. However, this effect was lost by the 18 week follow up (Kootte et al., 2017). For the allogenic FMT, 11 lean donors were used, seven of which were used for more than one recipient. Whilst donor-dependent effects were not reported, the authors noted that the “multiple fecal donors might explain the transient and variable effects seen in the allogenic group.” As FMT research in this field progresses from small-scale case series to larger-scale randomized placebo controlled clinical trials, it remains to be seen whether the super-donor phenomenon generalizes to other conditions outside of IBD.
To shed light on the varying patient responses to FMT and uncover any donor-dependent effects, a number of studies have carried out microbial profiling on donors and recipients before and after FMT (Vrieze et al., 2012; Moayyedi et al., 2015; Rossen et al., 2015; Vaughn et al., 2016; Vermeire et al., 2016; Bajaj et al., 2017; Fuentes et al., 2017; Mizuno et al., 2017; Paramsothy et al., 2017a; Kump et al., 2018). Despite a lack of large-cohort based studies, one key theme has begun to emerge: the donor's microbial diversity has an influential role in the therapeutic success of FMT (Vermeire et al., 2016; Kump et al., 2018).
It has been consistently shown that FMT recipients experience a significant increase in gut microbiota diversity, typically shifting in composition toward the profile of their respective stool donor (Vaughn et al., 2016; Paramsothy et al., 2017b). Those who achieve a clinical response to FMT (responders) typically exhibit a higher microbial diversity than those who do not (non-responders) (Vaughn et al., 2016; Vermeire et al., 2016) (Figure 1). In line with these observations, the microbial diversity of the stool donor has been shown to be one of the most significant factors influencing FMT outcome (Kump et al., 2018). In a Belgian IBD cohort, Vermeire et al. observed significantly higher bacterial richness in donors that produced a clinical response to FMT than those who did not (Vermeire et al., 2016).
A specific microbial signature that correlate with the clinical efficacy of FMT for IBD has also been explored (Moayyedi et al., 2015; Rossen et al., 2015; Vermeire et al., 2016; Fuentes et al., 2017; Nishida et al., 2017; Paramsothy et al., 2017a; Kump et al., 2018). Among the various taxa that have been reported, Clostridium clusters IV and XIVa have consistently been shown to be indicative of a positive patient response to FMT (Rossen et al., 2015; Fuentes et al., 2017; Paramsothy et al., 2017a). Clostridium clusters IV and XIVa are informal groups of bacteria that mostly include genera from the Ruminococcaceae and Lachnospiraceae family, respectively. Specific genera within these Clostridium clusters (e.g., Roseburia, Oscillibacter, Blautia, Dorea) have been shown to increase in relative abundance in responders following FMT (Moayyedi et al., 2015; Rossen et al., 2015; Vermeire et al., 2016; Paramsothy et al., 2017a). Likewise, stool donors that are rich in specific members of Clostridium clusters IV and XIVa have been found to be predictive of sustained FMT response in IBD patients (Rossen et al., 2015; Fuentes et al., 2017). Notably, the gut microbiome from the super-donor identified by Moayyedi et al. was enriched with Ruminococcaceae and Lachnospiraceae families (Moayyedi et al., 2015).
To further characterize FMT super-donors, metabolic differences between responders and non-responders have been investigated. In particular, an increased production of butyrate by key members within Clostridium clusters IV and XIVa has been associated with prolonged clinical remission in IBD in response to FMT therapy (Fuentes et al., 2017). Butyrate is an important short chain fatty acid (SCFA), produced by bacteria in the gut, with specialized functions in immune modulation and energy provision (Tan et al., 2014). An increased production of butyrate has also been associated with CDI resolution following FMT (Kellingray et al., 2018). Similarly, the butyrate-producing species Roseburia intestinalis was found to increase two and a half fold in obese participants given FMT from lean donors (Vrieze et al., 2012).
Collectively, published observations suggest that microbial restoration can lead to alterations in metabolic outputs, which may be responsible for resetting the gut homeostasis in dysbiotic individuals. This is consistent with the idea that the key to FMT success lies in the ability of the donor to transfer high levels of particular keystone species to recipients. For inflammatory conditions, such as IBD and metabolic syndrome, transfer of butyrate-producing taxa may be important for therapeutic restoration. By contrast, donors with high abundances of Bifidobacterium may be more effective at treating patients with IBS (Mizuno et al., 2017).
The concept of keystone species was recently employed in a FMT study for recurrent hepatic encephalopathy (rHE) (Bajaj et al., 2017). Frequent exposure to antibiotics causes dysbiosis and decreased relative abundances of SCFA-producing families in rHE patients (Chen et al., 2011). Therefore, a rationalized donor selection approach was adopted in which microbiome data was used to select a donor with the highest relative abundance of families Lachnospiraceae and Ruminococcaceae from the universal stool donor bank, OpenBiome (Bajaj et al., 2017). In total, 10 patients received a 5-day course of broad-spectrum antibiotics followed by a single FMT enema from the selected donor. At 5 months post-FMT, none of the 10 FMT patients had experienced a recurrence of HE, compared to half (5/10) of the control patients who received the current standard of care (lactulose and rifaximin). Gut microbiome profiling revealed that the FMT patients had an enrichment for Ruminococcaceae but not Lachnospiraceae at 20 days post-FMT. Whilst rationalized donor selection is a step in the right direction, these results suggest that microbial enrichment in the donor does not completely guarantee enrichment in the FMT recipient. The forces governing FMT engraftment must therefore not solely be based on donor input.
FMT engraftment involves the integration or establishment of donor-derived microbial strains into the recipient's gut microbial community. Currently, it appears that the most important factors predicting strain engraftment in FMT are taxonomic identity and strain abundance in both the donor and the recipient prior to FMT (Smillie et al., 2018). Deep metagenomic sequencing enables strain level analysis for tracking microbial alterations and engraftment in the post-FMT gut microbiome. For example, Li et al. demonstrated that new microbial strains from the donor had a higher likelihood of engrafting if the recipient already possessed that species (Li et al., 2016). This led them to suggest that differences in microbiome engraftment between individuals of the same donor may stem from strain incompatibilities between the donor and the FMT recipient. Smillie et al. reported that strains of any given species engrafted in an “all or nothing” manner such that strains were either completely retained or completely replaced by donor strains in the recipient's post-FMT gut microbiome (Smillie et al., 2018). Meanwhile on a community level, FMT recipients harbored a complex mixture of both recipient-derived, donor-derived, and newly-acquired species post FMT (Smillie et al., 2018). Overall, it appears that microbial interactions have a significant part to play in FMT engraftment which helps to explain why dual recipients of a donor FMT do not exhibit identical gut microbiota profiles.
Genetics has been estimated to explain 5–10% of the variability in bacterial taxa observed between individuals(Willing et al., 2010; Goodrich et al., 2014, 2016; Wang et al., 2016; Xie et al., 2016; Hall et al., 2017). Among the taxa that have been found to be heritable, the majority have been linked to genes that are involved in innate immunity (Hall et al., 2017). The gut microbiota is known to be intricately connected to the host's immune system through a reciprocal developmental relationship. Specifically, the microbiome is critical for the appropriate development of the immune system, and in turn, the immune system helps modulate the microbiome community through a balance of pro- and anti-inflammatory pathways (Belkaid and Hand, 2014; Vatanen et al., 2016). Therefore, it remains possible that incompatibilities that arise from FMT may be attributable to a heightened immune response to the transplanted microbiota, possibly stemming from an underlying genetic difference between the donor and the recipient.
Taking this into consideration, an immune screening approach was recently investigated in a FMT case study for ulcerative colitis (Ponce-Alonso et al., 2017). To avoid FMT rejection by the immune system, a rectal biopsy was obtained from an ulcerative colitis patient in order to isolate a population of lymphoid cells. These patient-derived lymphoid cells were incubated with different gut microbiota samples isolated from three healthy stool donors. The donor microbiota that resulted in the lowest induction of pro-inflammatory interleukins was subsequently selected for FMT. The FMT proved to be a clinical success for the ulcerative colitis patient. Gut microbiome profiling revealed the ulcerative colitis patient's gut microbiome had become indistinguishable from that of the donor's, indicating highly successful FMT engraftment. Whilst immune screening in this instance led to a successful outcome for the FMT patient, the associated time and costs of running such a screen limit scalability to larger patient populations. Ideally, the development of a quick and simple rectal swab assay to assess immune response would be a much more feasible screening approach moving forward. In any case, the limited literature in this area must be supplemented by larger-scale studies to confirm the importance of immune screening prior to FMT.
It could be argued that a successful FMT not only requires the transplanted microbiota to engraft within the recipient's gut, but the newly acquired organisms must also be supported for therapeutic benefit to be maintained. Based on longitudinal analyses in patients who have received FMT for recurrent CDI, it is known that FMT-induced microbiota alterations can last anywhere from a few days to a few years after transfer (Weingarden et al., 2015; Broecker et al., 2016; Kumar et al., 2017; Moss et al., 2017). A recent FMT/CDI study by Moss et al. discovered that despite short-term similarity between donor and recipient gut microbiota profiles, concordance was significantly reduced after a year (Moss et al., 2017). In the FMT study by Moayeddi et al. eight of the nine ulcerative colitis patients who were in remission at week seven post-FMT were still in remission a year later with no instances of relapse (Moayyedi et al., 2015). Unfortunately, microbiome analyses were not carried out on these patients during follow-up so it can only be presumed that their transplanted microbiota remained stable.
In addition to underlying genetic differences between donor and the recipient, dietary selection pressures and subsequent antibiotic exposures are also likely to influence the long-term efficacy of FMT therapy. For recurrent CDI, the long-term stability of the FMT is less relevant because clearance of the pathogen and restoration of the commensal population is achieved rapidly. Thus, a gradual drift away from the donor's gut profile is unlikely to result in disease reoccurrence assuming there are no further insults to the commensal gut population. By contrast, the sustainability of the post-FMT microbiota in patients with chronic disease, such as IBD or obesity, may be much more pertinent. This is because the microbial dysbiosis associated with these conditions has not yet been proven in humans to be causal or consequential to disease (Ni et al., 2017). It is more likely that microbial dysbiosis is just one of several factors contributing toward disease progression in these individuals. If so, it may be that FMT provides only temporary relief to patient's symptoms and additional, “top-up FMTs” are required for continual disease management. The optimization of non-invasive FMT delivery approaches, such as capsule-based delivery, will thus be important moving forward.
Supporting the transplanted microbiome through diet could be a beneficial addition to FMT protocols (Thompson et al., 2016). Diet is known to play a significant role in shaping the developing gut microbiome in infancy as well as throughout adulthood (Singh et al., 2017). The diet provides the commensal microbes with substrates required for their proliferation and survival (Koh et al., 2016). It has been shown that a rapid change in diet, such as a switch from an animal-based to an exclusively plant-based diet, can alter the composition of the gut microbiota within 24 h (David et al., 2014). In inflammatory conditions, such as IBD and metabolic syndrome, FMT may act to overcome the initial hurdle in providing patients with therapeutic levels of anti-inflammatory bacteria. Subsequently, diet may be crucial in providing the necessary fiber required to support the growth of SCFA producing bacteria.
Microbial dysbiosis is a blanket term for an unhealthy or imbalanced gut community. As such, the population structure that is considered to represent microbial dysbiosis is variable between different disorders (Duvallet et al., 2017). Moreover, the microbiome deficit of one individual may not necessarily mirror that of another individual and therefore it is not surprising that patients respond differently to FMT. As more FMT-related clinical and microbial data are generated, it is becoming clear that “one stool does not fit all” in the context of treating chronic diseases with microbial dysbiosis. Equally so, the selection of donors based solely on clinical screening guidelines provides no guarantee of FMT success. It appears a patient's response to FMT predominantly depends on the capability of the donor's microbiota to restore the specific metabolic disturbances associated with their particular disease phenotype. If this is true, a donor-recipient matching approach, where a patient is screened to identify the functional perturbations specific to their microbiome, may be the best way forward. The patient could then be matched to a specific FMT donor known to be enriched in taxa associated with the metabolic pathway that needs to be restored. Immune tolerance screening would also be beneficial for reducing the impact of donor-recipient incompatibilities stemming from underlying differences in innate immune responses.
An alternative approach to donor-recipient matching is to administer precision FMTs, which are more akin to a probiotic as opposed to a whole fecal transplant. In addition to the obvious regulatory and consumer advantages inherent in this approach, a precision FMT removes the donor-dependent effects by providing patients with a defined mixture of bacteria that have previously been shown to be beneficial for disease resolution (e.g., enhancing butyrate-production in inflammatory conditions). For example, providing IBD patients with a targeted microbiota-based formulation containing only butyrate-producers would be a logical, safer, and potentially patient preferable alternative to whole fecal transplantation. Precision approaches have so far been trialed in CDI treatment but with mixed results (Petrof et al., 2013; Emanuelsson et al., 2014). It may be that the microbial community structure as a whole plays a more influential role in FMT success than the isolation of critical species alone. Regardless, targeted bacteriotherapy approaches should be investigated for chronic diseases as a way of circumventing the risks associated with administering fecal material.
Whilst much of the FMT literature has focused on bacteria being the therapeutically active agent, the successful resolution of CDI using sterile fecal filtrates has suggested non-bacterial elements might play a more significant role than previously appreciated (Ott et al., 2017). In a preliminary case series, five patients with recurrent CDI were administered a stool solution that had been filtered to remove small particles and bacteria. The fecal filtrate was found to contain bacterial debris, proteins, DNA, antimicrobial compounds, metabolites, and viruses. Notably, a few days post-transfer, all five patients had achieved CDI resolution and remained symptom free for the duration of the study (up to 6 months). Although limited by the number of patients who were treated, this preliminary study demonstrates that resolution of CDI can be achieved by elements other than the live bacterial component of stool. Consistent with this, Zuo et al. recently reported that bacteriophage transfer during FMT was associated with CDI resolution outcomes (Zuo et al., 2018). Similarly, Conceição-Neto et al. suggested the eukaryotic virome was associated with the successful treatment of ulcerative colitis by FMT (Conceição-Neto et al., 2018).
Despite being reported in the literature as far back as the fourth century, FMT research is still in its infancy, particularly with regards to its mechanism of effect. The lack of large randomized controlled clinical trials of FMT for the treatment of chronic diseases has meant that many observations, such as the existence of FMT super-donors, are not yet robustly supported by empirical evidence. The growing number of small-scale studies, whilst difficult to compare with each other, do however suggest the donor plays an influential role in FMT outcomes for indications outside of CDI. Considerable effort has since been spent in identifying the various factors which contribute to FMT success. In a broad sense, high diversity of the gut microbiota, particularly in the donor, appears to best predict a patient's response to FMT. More specifically, the efficacy of FMT likely depends on the ability of the donor to provide the necessary taxa capable of restoring metabolic deficits in recipients that are contributing toward disease. Further characterization of super-donors will likely result in the development of more refined FMT formulations to help standardize therapy and reduce variability in patient response. In parallel, continued optimization of FMT protocols, including a shift toward capsule-based approaches, will help combat the longevity issues associated with FMT and create a more patient-friendly alternative to current disease management schemes.
BW wrote the manuscript. TV and WC commented on the manuscript. JOS directed and contributed to the writing of the manuscript.
The publishing fee for this review article was covered by the University of Auckland Foundation (grant number 3713937).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aroniadis, O. C., Brandt, L. J., Oneto, C., Feuerstadt, P., Sherman, A., Wolkoff, A. W., et al. (2018). 742 - A double-blind, randomized, placebo-controlled trial of fecal microbiota transplantation capsules (FMTC) for the treatment of diarrhea-predominant irritable bowel syndrome (IBS-D). Gastroenterology 154, S-154–S-155. doi: 10.1016/S0016-5085(18)30932-6
Arrieta, M. C., Stiemsma, L. T., Dimitriu, P. A., Thorson, L., Russell, S., Yurist-Doutsch, S., et al. (2015). Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7:307ra152. doi: 10.1126/scitranslmed.aab2271
Bäckhed, F., Ding, H., Wang, T., Hooper, L. V., Koh, G. Y., Nagy, A., et al. (2004). The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci.U.S.A. 101, 15718–15723. doi: 10.1073/pnas.0407076101
Bajaj, J. S., Kassam, Z., Fagan, A., Gavis, E. A., Liu, E., Cox, I. J., et al. (2017). Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 66, 1727–1738. doi: 10.1002/hep.29306
Balish, E., and Warner, T. (2002). Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 160, 2253–2257. doi: 10.1016/S0002-9440(10)61172-8
Baumgart, M., Dogan, B., Rishniw, M., Weitzman, G., Bosworth, B., Yantiss, R., et al. (2007). Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 1, 403–418. doi: 10.1038/ismej.2007.52
Belkaid, Y., and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. doi: 10.1016/j.cell.2014.03.011
Bennet, J. D., and Brinkman, M. (1989). Treatment of ‘ulcerative colitis by implantation of normal colonic flora. Lancet 1:164. doi: 10.1016/S0140-6736(89)91183-5
Berer, K., Mues, M., Koutrolos, M., Rasbi, Z. A., Boziki, M., Johner, C., et al. (2011). Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. doi: 10.1038/nature10554
Borriello, S. P. (1990). The influence of the normal flora on Clostridium difficile colonisation of the gut. Ann. Med. 22, 61–67. doi: 10.3109/07853899009147244
Broecker, F., Klumpp, J., Schuppler, M., Russo, G., Biedermann, L., Hombach, M., et al. (2016). Long-term changes of bacterial and viral compositions in the intestine of a recovered Clostridium difficile patient after fecal microbiota transplantation. Cold Spring Harb. Mol. Case Stud. 2:a000448. doi: 10.1101/mcs.a000448
Bunyavanich, S., Shen, N., Grishin, A., Wood, R., Burks, W., Dawson, P., et al. (2016). Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 138, 1122–1130. doi: 10.1016/j.jaci.2016.03.041
Cammarota, G., Ianiro, G., Tilg, H., Rajilić-Stojanović, M., Kump, P., Satokari, R., et al. (2017). European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66, 569–580. doi: 10.1136/gutjnl-2016-313017
Cammarota, G., Masucci, L., Ianiro, G., Bibbò, S., Dinoi, G., Costamagna, G., et al. (2015). Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 41, 835–843. doi: 10.1111/apt.13144
Chen, Y., Yang, F., Lu, H., Wang, B., Chen, Y., Lei, D., et al. (2011). Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54, 562–572. doi: 10.1002/hep.24423
Conceição-Neto, N., Deboutte, W., Dierckx, T., Machiels, K., Wang, J., Yinda, K. C., et al. (2018). Low eukaryotic viral richness is associated with faecal microbiota transplantation success in patients with UC. Gut 67, 1558–1559. doi: 10.1136/gutjnl-2017-315281
Costello, S. P., Soo, W., Bryant, R. V., Jairath, V., Hart, A. L., and Andrews, J. M. (2017). Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment. Pharmacol. Ther. 46, 213–224. doi: 10.1111/apt.14173
Cui, B., Feng, Q., Wang, H., Wang, M., Peng, Z., Li, P., et al. (2015). Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J. Gastroenterol. Hepatol. 30, 51–58. doi: 10.1111/jgh.12727
Cui, L., Zhao, T., Hu, H., Zhang, W., and Hua, X. (2017). Association study of gut flora in coronary heart disease through high-throughput sequencing. Biomed. Res. Int. 2017:3796359. doi: 10.1155/2017/3796359
Darfeuille-Michaud, A., Boudeau, J., Bulois, P., Neut, C., Glasser, A. L., Barnich, N., et al. (2004). High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 127, 412–421. doi: 10.1053/j.gastro.2004.04.061
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
de Groot, P. F., Frissen, M. N., de Clercq, N. C., and Nieuwdorp, M. (2017). Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes 8, 253–267. doi: 10.1080/19490976.2017.1293224
de Lange, K. M., Moutsianas, L., Lee, J. C., Lamb, C. A., Luo, Y., Kennedy, N. A., et al. (2017). Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 49, 256–261. doi: 10.1038/ng.3760
DeFilipp, Z., Peled, J. U., Li, S., Mahabamunuge, J., Dagher, Z., Slingerland, A. E., et al. (2018). Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2, 745–753. doi: 10.1182/bloodadvances.2018017731
Ding, C., Fan, W., Gu, L., Tian, H., Ge, X., Gong, J., et al. (2018). Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: results from a prospective study with long-term follow-up. Gastroenterol. Rep. 6, 101–107. doi: 10.1093/gastro/gox036
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A., and Alm, E. J. (2017). Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8:1784. doi: 10.1038/s41467-017-01973-8
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi: 10.1126/science.1110591
Eiseman, B., Silen, W., Bascom, G. S., and Kauvar, A. J. (1958). Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44, 854–859.
Emanuelsson, F., Claesson, B. E., Ljungström, L., Tvede, M., and Ung, K. A. (2014). Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scand. J. Infect. Dis. 46, 89–97. doi: 10.3109/00365548.2013.858181
Fekety, R., McFarland, L. V., Surawicz, C. M., Greenberg, R. N., Elmer, G. W., and Mulligan, M. E. (1997). Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin. Infect. Dis. 24, 324–333. doi: 10.1093/clinids/24.3.324
Frank, D. N., St. Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., and Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci.U.S.A. 104, 13780–13785. doi: 10.1073/pnas.0706625104
Fuentes, S., Rossen, N. G., van der Spek, M. J., Hartman, J. H., Huuskonen, L., Korpela, K., et al. (2017). Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 11, 1877–1889. doi: 10.1038/ismej.2017.44
Fujimoto, T., Imaeda, H., Takahashi, K., Kasumi, E., Bamba, S., Fujiyama, Y., et al. (2013). Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn's disease. J. Gastroenterol. Hepatol. 28, 613–619. doi: 10.1111/jgh.12073
Gajendran, M., Loganathan, P., Catinella, A. P., and Hashash, J. G. (2018). A comprehensive review and update on Crohn's disease. Dis. Mon. 64, 20–57. doi: 10.1016/j.disamonth.2017.07.001
Gevers, D., Kugathasan, S., Denson, L. A., Vázquez-Baeza, Y., Van Treuren, W., Ren, B., et al. (2014). The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 15, 382–392. doi: 10.1016/j.chom.2014.02.005
Goodrich, J. K., Davenport, E. R., Beaumont, M., Jackson, M. A., Knight, R., Ober, C., et al. (2016). Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 19, 731–743. doi: 10.1016/j.chom.2016.04.017
Goodrich, J. K., Waters, J. L., Poole, A. C., Sutter, J. L., Koren, O., Blekhman, R., et al. (2014). Human genetics shape the gut microbiome. Cell 159, 789–799. doi: 10.1016/j.cell.2014.09.053
Goyal, A., Yeh, A., Bush, B. R., Firek, B. A., Siebold, L. M., Rogers, M. B., et al. (2018). Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm. Bowel Dis. 24, 410–421. doi: 10.1093/ibd/izx035
Halkjær, S. I., Christensen, A. H., Lo, B. Z. S., Browne, P. D., Günther, S., Hansen, L. H., et al. (2018). Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut 67, 2107–2115. doi: 10.1136/gutjnl-2018-316434
Hall, A. B., Tolonen, A. C., and Xavier, R. J. (2017). Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 18, 690–699. doi: 10.1038/nrg.2017.63
He, Z., Cui, B. T., Zhang, T., Li, P., Long, C. Y., Ji, G. Z., et al. (2017a). Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J. Gastroenterol. 23, 3565–3568. doi: 10.3748/wjg.v23.i19.3565
He, Z., Li, P., Zhu, J., Cui, B., Xu, L., Xiang, J., et al. (2017b). Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn's disease complicated with inflammatory mass. Sci Rep. 7:4753. doi: 10.1038/s41598-017-04984-z
Holvoet, T., Joossens, M., Boelens, J., Christiaens, E., Heyerick, L., Verhasselt, B., et al. (2018). 617 - fecal microbiota transplantation in irritable bowel syndrome with predominant abdominal bloating: results from a double blind, placebo-controlled clinical trial. Gastroenterology 154:S-130. doi: 10.1016/S0016-5085(18)30860-6
Holvoet, T., Joossens, M., Wang, J., Boelens, J., Verhasselt, B., Laukens, D., et al. (2017). Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 66, 980–982. doi: 10.1136/gutjnl-2016-312513
Human Microbiome Project Consortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Ishikawa, D., Sasaki, T., Osada, T., Kuwahara-Arai, K., Haga, K., Shibuya, T., et al. (2017). Changes in intestinal microbiota following combination therapy with fecal microbial transplantation and antibiotics for Ulcerative Colitis. Inflamm. Bowel Dis. 23, 116–125. doi: 10.1097/MIB.0000000000000975
Jie, Z., Xia, H., Zhong, S. L., Feng, Q., Li, S., Liang, S., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8:845. doi: 10.1038/s41467-017-00900-1
Johnsen, P. H., Hilpüsch, F., Cavanagh, J. P., Leikanger, I. S., Kolstad, C., Valle, P. C., et al. (2018). Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 3, 17–24. doi: 10.1016/S2468-1253(17)30338-2
Kakihana, K., Fujioka, Y., Suda, W., Najima, Y., Kuwata, G., Sasajima, S., et al. (2016). Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 128, 2083–2088. doi: 10.1182/blood-2016-05-717652
Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. doi: 10.1186/s40168-016-0225-7
Kao, D., Roach, B., Park, H., Hotte, N., Madsen, K., Bain, V., et al. (2016). Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 63, 339–340. doi: 10.1002/hep.28121
Kao, D., Roach, B., Silva, M., Beck, P., Rioux, K., Kaplan, G. G., et al. (2017). Effect of oral capsule– vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection. JAMA 318:1985. doi: 10.1001/jama.2017.17077
Kassam, Z., Lee, C. H., Yuan, Y., and Hunt, R. H. (2013). Fecal Microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am. J. Gastroenterol. 108, 500–508. doi: 10.1038/ajg.2013.59
Kazerouni, A., and Wein, L. M. (2017). Exploring the efficacy of pooled stools in fecal microbiota transplantation for microbiota-associated chronic diseases. PLoS ONE 12:e0163956. doi: 10.1371/journal.pone.0163956
Kellingray, L., Gall, G. L., Defernez, M., Beales, I. L. P., Franslem-Elumogo, N., and Narbad, A. (2018). Microbial taxonomic and metabolic alterations during faecal microbiota transplantation to treat Clostridium difficile infection. J. Infect. 77, 107–118. doi: 10.1016/j.jinf.2018.04.012
Kelly, C. R., Kahn, S., Kashyap, P., Laine, L., Rubin, D., Atreja, A., et al. (2015). Update on fecal microbiota transplantation : indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237. doi: 10.1053/j.gastro.2015.05.008
Kelly, C. R., Khoruts, A., Staley, C., Sadowsky, M. J., Abd, M., Alani, M., et al. (2016). Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection. Ann. Intern. Med. 165, 609–616. doi: 10.7326/M16-0271
Khanna, S. (2018). Microbiota replacement therapies: innovation in gastrointestinal care. Clin. Pharmacol. Ther. 103, 102–111. doi: 10.1002/cpt.923
Khanna, S., Vazquez-Baeza, Y., González, A., Weiss, S., Schmidt, B., Muñiz-Pedrogo, D. A., et al. (2017). Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 5:55. doi: 10.1186/s40168-017-0269-3
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kootte, R. S., Levin, E., Salojärvi, J., Smits, L. P., Hartstra, A. V., Udayappan, S. D., et al. (2017). Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619.e6. doi: 10.1016/j.cmet.2017.09.008
Kriss, M., Hazleton, K. Z., Nusbacher, N. M., Martin, C. G., and Lozupone, C. A. (2018). Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr. Opin. Microbiol. 44, 34–40. doi: 10.1016/j.mib.2018.07.003
Kumar, R., Yi, N., Zhi, D., Eipers, P., Goldsmith, K. T., Dixon, P., et al. (2017). Identification of donor microbe species that colonize and persist long term in the recipient after fecal transplant for recurrent Clostridium difficile. npj Biofilms Microb. 3:12. doi: 10.1038/s41522-017-0020-7
Kump, P., Wurm, P., Gröchenig, H. P., Wenzl, H., Petritsch, W., Halwachs, B., et al. (2018). The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 47, 67–77. doi: 10.1111/apt.14387
Lee, C. H., Belanger, J. E., Kassam, Z., Smieja, M., Higgins, D., Broukhanski, G., et al. (2014). The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1425–1428. doi: 10.1007/s10096-014-2088-9
Lee, C. H., Steiner, T., Petrof, E. O., Smieja, M., Roscoe, D., Nematallah, A., et al. (2016). Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA 315, 142–149. doi: 10.1001/jama.2015.18098
Leffler, D. A., and Lamont, J. T. (2015). Clostridium difficile infection. N Engl J Med. 372, 1539–1548. doi: 10.1056/NEJMra1403772
Ley, R. E., Hamady, M., Lozupone, C., Turnbaugh, P. J., Ramey, R. R., Bircher, J. S., et al. (2008). Evolution of mammals and their gut microbes. Science 320, 1647–1651. doi: 10.1126/science.1155725
Li, S. S., Zhu, A., Benes, V., Costea, P. I., Hercog, R., Hildebrand, F., et al. (2016). Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 352, 586–589. doi: 10.1126/science.aad8852
Liu, H. N., Wu, H., Chen, Y. Z., Chen, Y. J., Shen, X. Z., and Liu, T. T. (2017). Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig. Liver Dis. 49, 331–337. doi: 10.1016/j.dld.2017.01.142
Liu, S. X., Li, Y. H., Dai, W. K., Li, X. S., Qiu, C. Z., Ruan, M. L., et al. (2017). Fecal microbiota transplantation induces remission of infantile allergic colitis through gut microbiota re-establishment. World J. Gastroenterol. 23, 8570–8581. doi: 10.3748/wjg.v23.i48.8570
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Genome Med. 8:51. doi: 10.1186/s13073-016-0307-y
Lopez-Siles, M., Martinez-Medina, M., Abellà, C., Busquets, D., Sabat-Mir, M., Duncan, S. H., et al. (2015). Mucosa-associated Faecalibacterium prausnitzii phylotype richness is reduced in patients with inflammatory bowel disease. Appl. Environ. Microbiol. 81, 7582–7592. doi: 10.1128/AEM.02006-15
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., et al. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283. doi: 10.1136/gutjnl-2013-304833
Makkawi, S., Camara-Lemarroy, C., and Metz, L. (2018). Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 5:e459. doi: 10.1212/NXI.0000000000000459
Manichanh, C., Rigottier-Gois, L., Bonnaud, E., Gloux, K., Pelletier, E., Frangeul, L., et al. (2006). Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55, 205–211. doi: 10.1136/gut.2005.073817
Martin, H. M., Campbell, B. J., Hart, C. A., Mpofu, C., Nayar, M., Singh, R., et al. (2004). Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 127, 80–93. doi: 10.1053/j.gastro.2004.03.054
Mattila, E., Uusitalo-Seppälä, R., Wuorela, M., Lehtola, L., Nurmi, H., Ristikankare, M., et al. (2012). Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 142, 490–496. doi: 10.1053/j.gastro.2011.11.037
Mizuno, S., Masaoka, T., Naganuma, M., Kishimoto, T., Kitazawa, M., Kurokawa, S., et al. (2017). Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 96, 29–38. doi: 10.1159/000471919
Moayyedi, P., Surette, M. G., Kim, P. T., Libertucci, J., Wolfe, M., Onischi, C., et al. (2015). Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e6. doi: 10.1053/j.gastro.2015.04.001
Moss, E. L., Falconer, S. B., Tkachenko, E., Wang, M., Systrom, H., Mahabamunuge, J., et al. (2017). Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS ONE 12:e0182585. doi: 10.1371/journal.pone.0182585
Ni, J., Wu, G. D., Albenberg, L., and Tomov, V. T. (2017). Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584. doi: 10.1038/nrgastro.2017.88
Nishida, A., Imaeda, H., Ohno, M., Inatomi, O., Bamba, S., Sugimoto, M., et al. (2017). Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J. Gastroenterol. 52, 476–482. doi: 10.1007/s00535-016-1271-4
Nishino, K., Nishida, A., Inoue, R., Kawada, Y., Ohno, M., Sakai, S., et al. (2018). Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 53, 95–106. doi: 10.1007/s00535-017-1384-4
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J. Z., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16:90. doi: 10.1186/s12866-016-0708-5
Osman, M., Stoltzner, Z., O'Brien, K., Ling, K., Koelsch, E., Dubois, N., et al. (2016). Donor efficacy in fecal microbiota transplantation for recurrent clostridium difficile: evidence from a 1,999-patient cohort. Open Forum Infect. Dis. 3, 841. doi: 10.1093/ofid/ofw194.48
Ott, S. J., Waetzig, G. H., Rehman, A., Moltzau-Anderson, J., Bharti, R., Grasis, J. A., et al. (2017). Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 152, 799–811.e7. doi: 10.1053/j.gastro.2016.11.010
Paramsothy, S., Kamm, M. A., Kaakoush, N. O., Walsh, A. J., van den Bogaerde, J., Samuel, D., et al. (2017a). Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228. doi: 10.1016/S0140-6736(17)30182-4
Paramsothy, S., Paramsothy, R., Rubin, D. T., Kamm, M. A., Kaakoush, N. O., Mitchell, H. M., et al. (2017b). Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohn's Colitis 11, 1180–1199. doi: 10.1093/ecco-jcc/jjx063
Penders, J., Thijs, C., van den Brandt, P. A., Kummeling, I., Snijders, B., Stelma, F., et al. (2007). Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth Cohort study. Gut 56, 661–667. doi: 10.1136/gut.2006.100164
Perez-Muñoz, M. E., Arrieta, M.-C., Ramer-Tait, A. E., and Walter, J. (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5:48. doi: 10.1186/s40168-017-0268-4
Petersen, C., and Round, J. L. (2014). Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 16, 1024–1033. doi: 10.1111/cmi.12308
Petrof, E. O., Gloor, G. B., Vanner, S. J., Weese, S. J., Carter, D., Daigneault, M. C., et al. (2013). Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating' the gut. Microbiome 1:3. doi: 10.1186/2049-2618-1-3
Philips, C. A., Pande, A., Shasthry, S. M., Jamwal, K. D., Khillan, V., Chandel, S. S., et al. (2017). Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin. Gastroenterol. Hepatol. 15, 600–602. doi: 10.1016/j.cgh.2016.10.029
Pinn, D. M., Aroniadis, O. C., and Brandt, L. J. (2014). Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am. J. Gastroenterol. 109, 1831–1832. doi: 10.1038/ajg.2014.295
Ponce-Alonso, M., Garcia-Fernandez, S., Aguilera, L., Rodriguez de santiago, E., Foruny, J. R., Roy, G., et al. (2017). P782 A new compatibility test for donor selection for faecal microbiota transplantation in ulcerative colitis. J Crohn's Colitis 11, S480–S481. doi: 10.1093/ecco-jcc/jjx002.903
Quraishi, M. N., Widlak, M., Bhala, N., Moore, D., Price, M., Sharma, N., et al. (2017). Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 46, 479–493. doi: 10.1111/apt.14201
Ren, Y. D., Ye, Z. S., Yang, L. Z., Jin, L. X., Wei, W. J., Deng, Y. Y., et al. (2017). Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 65, 1765–1768. doi: 10.1002/hep.29008
Ridaura, V. K., Faith, J. J., Rey, F. E., Cheng, J., Duncan, A. E., Kau, A. L., et al. (2013). Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. doi: 10.1126/science.1241214
Rossen, N. G., Fuentes, S., van der Spek, M. J., Tijssen, J. G., Hartman, J. H., Duflou, A., et al. (2015). Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149, 110–118.e4. doi: 10.1053/j.gastro.2015.03.045
Schaubeck, M., Clavel, T., Calasan, J., Lagkouvardos, I., Haange, S. B., Jehmlich, N., et al. (2016). Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence. Gut 65, 225–237. doi: 10.1136/gutjnl-2015-309333
Schwiertz, A., Taras, D., Schäfer, K., Beijer, S., Bos, N. A., Donus, C., et al. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity 18, 190–195. doi: 10.1038/oby.2009.167
Sellon, R. K., Tonkonogy, S., Schultz, M., Dieleman, L. A., Grenther, W., Balish, E., et al. (1998). Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66, 5224–5231.
Shankar, V., Hamilton, M. J., Khoruts, A., Kilburn, A., Unno, T., Paliy, O., et al. (2014). Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome 2:13. doi: 10.1186/2049-2618-2-13
Singh, R. K., Chang, H. W., Yan, D., Lee, K. M., Ucmak, D., Wong, K., et al. (2017). Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15:73. doi: 10.1186/s12967-017-1175-y
Smillie, C. S., Sauk, J., Gevers, D., Friedman, J., Sung, J., Youngster, I., et al. (2018). Strain tracking reveals the determinants of bacterial engraftment in the human gut following fecal microbiota transplantation. Cell Host Microbe. 23, 229–240.e5. doi: 10.1016/j.chom.2018.01.003
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U S A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Song, Y., Garg, S., Girotra, M., Maddox, C., von Rosenvinge, E. C., Dutta, A., et al. (2013). Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS ONE. 8:e81330. doi: 10.1371/journal.pone.0081330
Spindelboeck, W., Schulz, E., Uhl, B., Kashofer, K., Aigelsreiter, A., Zinke-Cerwenka, W., et al. (2017). Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica 102, e210–e213. doi: 10.3324/haematol.2016.154351
Staley, C., Kelly, C. R., Brandt, L. J., Khoruts, A., and Sadowsky, M. J. (2016). Complete microbiota engraftment is not essential for recovery from recurrent Clostridium difficile infection following fecal microbiota transplantation. MBio 7, e01965–e01916. doi: 10.1128/mBio.01965-16
Suskind, D. L., Brittnacher, M. J., Wahbeh, G., Shaffer, M. L., Hayden, H. S., Qin, X., et al. (2015). Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn's disease. Inflamm. Bowel Dis. 21, 556–563. doi: 10.1097/MIB.0000000000000307
Takahashi, K., Nishida, A., Fujimoto, T., Fujii, M., Shioya, M., Imaeda, H., et al. (2016). Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn's disease. Digestion 93, 59–65. doi: 10.1159/000441768
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., and Macia, L. (2014). The role of short-chain fatty acids in health and disease. Adv. Immunol. 121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
Thompson, S., Guetterman, H., Taylor, A., Bogner, A., Martin, D., Farrell, J. J., et al. (2016). Dietary predictors of fecal microbiota transplantation success. J. Acad. Nutr. Diet. 116:A76. doi: 10.1016/j.jand.2016.06.267
Thursby, E., and Juge, N. (2017). Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836. doi: 10.1042/BCJ20160510
Tian, H., Ge, X., Nie, Y., Yang, L., Ding, C., McFarland, L.V., et al. (2017). Fecal microbiota transplantation in patients with slow-transit constipation: a randomized, clinical trial. PLoS ONE 12:e0171308. doi: 10.1371/journal.pone.0171308
van Nood, E., Vrieze, A., Nieuwdorp, M., Fuentes, S., Zoetendal, E. G., de Vos, W. M., et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415. doi: 10.1056/NEJMoa1205037
Vatanen, T., Kostic, A. D., d'Hennezel, E., Siljander, H., Franzosa, E.A., Yassour, M., et al. (2016). Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853. doi: 10.1016/j.cell.2016.04.007
Vaughn, B. P., Vatanen, T., Allegretti, J. R., Bai, A., Xavier, R. J., Korzenik, J., et al. (2016). Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn's disease. Inflamm. Bowel Dis. 22, 2182–2190. doi: 10.1097/MIB.0000000000000893
Vermeire, S., Joossens, M., Verbeke, K., Wang, J., Machiels, K., Sabino, J., et al. (2016). Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohn's Colitis 10, 387–394. doi: 10.1093/ecco-jcc/jjv203
Vrieze, A., Van Nood, E., Holleman, F., Salojärvi, J., Kootte, R. S., Bartelsman, J. F., et al. (2012). Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e7. doi: 10.1053/j.gastro.2012.06.031
Walker, A. W., Sanderson, J. D., Churcher, C., Parkes, G. C., Hudspith, B. N., Rayment, N., et al. (2011). High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 11:7. doi: 10.1186/1471-2180-11-7
Wang, J., Thingholm, L. B., Skiecevičiene, J., Rausch, P., Kummen, M., Hov, J. R., et al. (2016). Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 48, 1396–1406. doi: 10.1038/ng.3695
Warny, M., Pepin, J., Fang, A., Killgore, G., Thompson, A., Brazier, J., et al. (2005). Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366, 1079–1084. doi: 10.1016/S0140-6736(05)67420-X
Weingarden, A., González, A., Vázquez-Baeza, Y., Weiss, S., Humphry, G., Berg-Lyons, D., et al. (2015). Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 3:10. doi: 10.1186/s40168-015-0070-0
Willing, B. P., Dicksved, J., Halfvarson, J., Andersson, A. F., Lucio, M., Zheng, Z., et al. (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139, 1844–1854.e1. doi: 10.1053/j.gastro.2010.08.049
Xie, H., Guo, R., Zhong, H., Feng, Q., Lan, Z., Qin, B., et al. (2016). Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 3, 572–584.e3. doi: 10.1016/j.cels.2016.10.004
Youngster, I., Russell, G. H., Pindar, C., Ziv-Baran, T., Sauk, J., and Hohmann, E. L. (2014). Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312:1772. doi: 10.1001/jama.2014.13875
Zhang, F. M., Wang, H. G., Wang, M., Cui, B. T., Fan, Z. N., and Ji, G. Z. (2013). Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn's disease. World J. Gastroenterol. 19, 7213–7216. doi: 10.3748/wjg.v19.i41.7213
Keywords: fecal microbiota transplantation (FMT), super-donor, microbial dysbiosis, clostridium difficile infection (CDI), inflammatory bowel disease (IBD)
Citation: Wilson BC, Vatanen T, Cutfield WS and O'Sullivan JM (2019) The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 9:2. doi: 10.3389/fcimb.2019.00002
Received: 15 October 2018; Accepted: 03 January 2019;
Published: 21 January 2019.
Edited by:
Omry Koren, Bar-Ilan University, IsraelReviewed by:
Stefano Fiorucci, University of Perugia, ItalyCopyright © 2019 Wilson, Vatanen, Cutfield and O'Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin M. O'Sullivan, anVzdGluLm9zdWxsaXZhbkBhdWNrbGFuZC5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.