- 1Department of Microbiology, Guru Nanak Dev University, Amritsar, India
- 2Department of Molecular Biology and Biochemistry, Guru Nanak Dev University, Amritsar, India
Diarrheal disease caused by Vibrio cholerae is endemic in developing countries including India and is associated with high rate of mortality especially in children. V. cholerae is known to form biofilms on the gut epithelium, and the biofilms once formed are resistant to the action of antibiotics. Therefore agents that prevent the biofilm formation and disperse the preformed biofilms are associated with therapeutic benefits. The use of antibiotics for the treatment of cholera is associated with side effects such as gut dysbiosis due to depletion of gut microflora, and the increasing problem of antibiotic resistance. Thus search for safe alternative therapeutic agents is warranted. Herein, we screened the lactobacilli spp. isolated from the fecal samples of healthy children for their abilities to prevent biofilm formation and to disperse the preformed biofilms of V. cholerae and V. parahaemolyticus by using an in vitro assay. The results showed that the culture supernatant (CS) of all the seven isolates of Lactobacillus spp. used in the study inhibited the biofilm formation of V. cholerae by more than 90%. Neutralization of pH of CS completely abrogated their antimicrobial activities against V. cholera, but had negligible effects on their biofilm inhibitory potential. Further, CS of all the lactobacilli isolates caused the dispersion of preformed V. cholerae biofilms in the range 62–85%; however, pH neutralization of CS reduced the biofilm dispersal potential of the 4 out of 7 isolates by 19–57%. Furthermore, the studies showed that CS of none of the lactobacilii isolates had antimicrobial activity against V. parahaemolyticus, but 5 out of 7 isolates inhibited the formation of its biofilm in the range 62–82%. However, none of the CS dispersed the preformed biofilms of V. parahaemolyticus. The ability of CS to inhibit the adherence of Vibrio spp. to the epithelial cell line was also determined. Thus, we conclude that the biofilm dispersive action of CS of lactobacilli is strain-specific and pH-dependent. As Vibrio is known to form biofilms in the intestinal niche having physiological pH in the range 6–7, the probiotic strains that have dispersive action at high pH may have better therapeutic potential.
Introduction
Cholera is an acute diarrheal infection caused by the ingestion of food or water contaminated with the cholera toxin-producing Gram-negative pathogen Vibrio cholerae. It has been the cause of 7 pandemics since 1,871, and still remains a major public health issue in more than one-third of the countries due to poor sanitation facilities and lack of safe drinking water (Morris and Acheson, 2003). The annual occurrence of cholera in 69 cholera-endemic countries is estimated to be 2.9 million that results in 95,000 deaths each year (Ali et al., 2015). It is an endemic disease in India and can be fatal if remained unmanaged. V. parahaemolyticus that secrets haemolysin is also known to cause gastroenteritis if consumed at high doses. The extensive use of antibiotics for the treatment of diarrhea has led to the emergence of drug resistance in V. cholerae (Kitaoka et al., 2011). Also the use of antibiotics can disrupt the homeostasis of the gut by killing the normal gut flora. Therefore, there is a need for safe alternative therapeutics to combat gut-bacterial infections. One of the safe alternative therapeutic agents is probiotics. Probiotics are live microorganisms which confer health benefits on the host when administered in adequate amounts (FAO/WHO, 2001). Lactobacillus spp. is the most widely accepted probiotic because of their GRAS (generally regarded as safe) status. Lactobacilli are rod-shaped Gram-positive, facultative anaerobes, comprising roughly 0.01% of the microbiome in the gastrointestinal tract (GIT) of humans (Harmsen et al., 2002) and range between 107-108 cells/gm of feces (Rinttilä et al., 2004). In GIT, they are known to possess health-promoting effects such as maintaining normal intestinal homeostasis by inhibiting the colonization of pathogens and modulating the immune responses (Lebeer et al., 2008; Kemgang et al., 2014). The use of probiotics for the treatment of gut-associated health disorders has yielded positive results as demonstrated by various clinical trials (Culligan et al., 2009). Meta-analysis of clinical trials showed the efficacy of lactobacilli probiotics for the treatment of antibiotic-induced diarrhea (D'Souza et al., 2002; McFarland, 2006), antibiotic-induced Clostridium difficile infection (McFarland, 2006), and reduction in the duration of rotavirus diarrhea (Huang et al., 2002; Ahmadi et al., 2015). However, the human clinical trial of probiotics against cholera was not successful (Mitra and Rabbani, 1990). V. cholerae is a biofilm-forming pathogen. It is known to form strong biofilms on the epithelial lining of gut in both mice (Millet et al., 2014) and humans (Yamamoto and Yokota, 1988). The biofilms play an important role in the pathogenesis of cholera (Fong et al., 2010; Almagro-Moreno et al., 2015). Also once the biofilms are formed, they resist the action of both immune defenses and antibiotics, and are also responsible for the recurrent nature of the infection. Therefore the probiotic strain having both antimicrobial and anti-biofilm properties may be expected to be therapeutically more effective. Some of the anti-biofilm agents effective against Vibrio spp. have been reported in the literature (Sambanthamoorthy et al., 2011; Sayem et al., 2011; Warner et al., 2015), but they do not have any antimicrobial properties against planktonic cells and thus are administered along with the conventional antibiotics. The antimicrobial probiotics having biofilm-dispersive properties can yield better clinical benefits for the treatment of diarrhea due to Vibrio spp as they may be used as stand-alone therapeutic agents. The in vitro antimicrobial activity of lactobacilli strains against different Vibrio spp. (Koga et al., 1998), V. parahaemolyticus (Shokryazdan et al., 2014; Chimchang et al., 2015) and V. cholerae (Petrova and Petrov, 2011) has been demonstrated by various workers. However the ability of lactobacilli in inhibiting the biofilm of Vibrio spp. has not been reported. With this in the background, we isolated lactobacilli from the fecal samples of healthy children and studied the antimicrobial and anti-biofilm activities of cell free culture supernatant (CS) of lactobacilli. The probiotic properties of the selected isolates were also studied with an idea to develop them as indigeneous probiotic strain.
Materials and Methods
Microorganisms and Growth Conditions
For the isolation of lactobacilli, fecal samples were collected from 32 healthy children of age group ranging from 2 to 13 yrs after taking the written informed consent of their parents. The study was approved by the Institutional Human Ethics Committee. The stool sample weighing approximately 1 g was collected in thioglycollate broth (HiMedia laboratories, Mumbai, India) and incubated for 4 h at 37°C in anaerobic jars having 5% carbon dioxide (CO2). Thereafter, 10-fold serial dilutions of the broth were plated onto De Man Rogosa and Sharpe (MRS; HiMedia) agar plates and incubated at 37°C under anaerobic conditions in anaerobic gas jars. Bacterial colonies with different morphologies were selected and preserved in 20% (v/v) glycerol (HiMedia)-containing MRS broth at −80°C. The lactobacilli were identified by Gram-positive staining and catalase-negative phenotype. For experimental purposes, the lactobacilli were cultured in MRS medium from the frozen stocks and propagated twice before use.
The various pathogenic indicator strains used in this study were V. cholerae strain 0139 MTCC 3906, Salmonella enterica Typhimurium MTCC 733, Listeria monocytogenes MTCC 657, Escherichia coli MTCC 119, Shigella flexeri MTCC 1457, V. parahaemolyticus MTCC 451, and Staphylococcus aureus MTCC 96. These strains were procured from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India. The fungal indicator strain Candida spp. was procured from the Department of Microbiology, Government Medical College, Amritsar. All the pathogenic bacteria were cultured in brain heart infusion (BHI) broth (HiMedia) and Candida spp. was cultured in Sabouroud dextrose broth (HiMedia) at 37°C under aerobic conditions. All the cultures were stored at −80°C in broth supplemented with 20% glycerol.
Characterization of the Lactobacilli Isolates
The lactobacilli isolates were characterized by using 16S rDNA sequencing and biochemical tests. For 16S rDNA sequencing, the genomic DNA of lactobacilli was isolated according to the method described by Moore et al. (2004). Following DNA isolation, 16S rDNA was amplified by PCR using universal primers-27F Forward: 5′-AGAGTTGATCCTGGCTCAG-3′ and 1492P Reverse: 5′-TACGGCTACCTTGTTACGACTT-3′. DNA amplification was carried out in 0.2 ml PCR tubes by using master cycle personal (Eppendorf Hamburg, Germany). The PCR reaction mixture (50 μl) consisted of 25 μl of 2X PCR master mix (3B Black Bio Biotech India, Ltd.,), 1 μl of each primer (Bioserve Biotechnologies Pvt. Ltd., India), 5 μl of template DNA, and 18 μl of nuclease free water. Initial denaturation of DNA was done at 95°C for 4 min, followed by 32 cycles of amplification comprising a denaturation step for 1 min at 95°C, annealing at 56°C for 1 min 30 s and extension at 72°C for 1 min. Reactions were completed with 10 min elongation at 72°C followed by cooling to 4°C. The PCR products were analyzed by electrophoresis on a 1.5% agarose gel at 100 V for 45 min against 100 bp step ladder. The bands were visualized with bio imaging system (Gene Genius gel imaging System, Syngene Bioimaging Private Ltd., India). The partial sequences of 16S rDNA were obtained and the isolate identified by aligning in the software BLAST version 2. The 16s rDNA sequences obtained have been submitted to NCBI and their accession no. were obtained (Table 1).

Table 1. The GenBank accession numbers of fecal lactobacilli isolates characterized by using 16S rDNA sequencing.
Determination of the Antimicrobial Activity of CS of Lactobacilli Isolates
The agar well diffusion assay was used to determine the antimicrobial activities of CS of lactobacilli isolates grown in MRS broth (Reinheimer et al., 1990; Gonzalez et al., 2007). To prepare the CS, lactobacilli were cultured in MRS broth for 16 h at 37°C and the broth was centrifuged at 9,000 g for 10 min at 4°C. The supernatant was then filter sterilized using syringe filters having pore size of 0.2 μm and stored at 4°C till further use.
For performing agar well diffusion assay, the indicator strains were grown at 37°C in the appropriate medium till an optical density (OD595) of 0.2 is obtained. Following incubation, 100 μl of the indicator pathogen or commensal culture was spread onto suitable agar plates and 6 mm wells were cut into the agar plates by using sterile well-borer. An aliquot (100 μl) of CS was poured into wells and plates were placed at 4°C for 4 h to allow diffusion of CS into agar. Then the plates were incubated at 37°C for 24 h and the diameter of zone of inhibition around each well was measured in millimeters.
To nullify the effect of reduced pH on antimicrobial activity, the pH of CS was adjusted to 6.5 by using 1 N NaOH (HiMedia) and antimicrobial activity was similarly determined.
Growth Curve
The growth curves of V. cholerae and V. parahaemolyticus were made in BHI medium supplemented with lyophilized MRS (as control) or pH-neutralized/non neutralized CS of lactobacilli isolates at the concentrations of 45 mg/ml. The overnight Vibrio cultures were grown in BHI and diluted to 0.1 OD595 before inoculating in BHI supplemented with MRS or CS at the concentration 45 mg/ml and incubated at 37°C for 48 h in the total volume of 220 μl in 96-well polystyrene plates in triplicates. Absorbance at the wavelength 595 was measured after every 4 h on the microplate reader (Biorad) till 48 h.
The Effect of CS on the Biofilm Formation by Vibrio spp.
The effect of pH non-neutralized CS of lactobacilli isolates was determined on the biofilm formation by both V. cholerae and V. parahaemolyticus, whereas, the effects of pH neutralized CS (pH set to 6.5 by using 1 N NaOH) was tested only on the biofilm formation by V. cholerae. The biofilm formation was determined by using modified crystal violet assay (Sharma et al., 2015) in a 96-well polystyrene microtiter plate (Tarsons Product Pvt. Ltd., Kolkata). To initiate biofilm formation, 100 μl of sterile BHI broth was added to each well along with 100 μl of CS or MRS broth (at concentration 45 mg/ml) and 20 μl of overnight grown Vibrio spp. having OD595 of 0.1. The microtiter plate was incubated at 37°C for 48 h to allow biofilm formation. Following incubation, the non adherent cells were removed by washing the wells gently 3 times with sterile distilled water. The adherent cells were fixed by using 200 μl of methanol (HiMedia) for 15 min and then the plate was emptied and air dried. The fixed biofilms were stained by using 200 μl of 2% crystal violet (HiMedia) in distilled water for 5 min. Excess stain was removed by washing under running tap water till color fades away. The stain was extracted from the adherent cells by using 160 μl of 33% glacial acetic acid (HiMedia) in distilled water and OD595 was measured using microplate reader. The experiment was conducted in triplicates. The percentage inhibition was calculated as,
Percentage inhibition = 100–[(OD595 of wells in the presence of CS X 100)/ OD595 of wells in the presence of MRS].
Effect of CS of Lactobacilli on the Dispersal of Biofilms of Vibrio spp.
The effect of pH non-neutralized CS was determined on the dispersion of preformed biofilm of both V. cholerae and V. parahaemolyticus; whereas the effect of pH-neutralized CS (pH set to 6.5 by using 1 N NaOH) was studied only on the biofilm of V. cholera (Wu et al., 2013). Biofilm of Vibrio spp. was developed in 96-well microtiter plate by adding 100 μl of autoclaved BHI broth along with 20 μl of overnight grown Vibrio culture having OD595 of 0.1. After 24 h incubation at 37°C, non adherent cells were removed by gentle pipetting without disrupting biofilm. CS of lactobacilli (at concentration 45 mg/ml) were added to each well along with 100 μl BHI broth. In the control wells instead of CS 100 μl of autoclaved MRS broth was added. The plates were incubated at 37°C for 48 h. The experiment was conducted in triplicates. After specified incubation, quantification of biofilm formed was done as described previously.
Bacterial Adhesion Assays With HCT-15 Cell Line
The binding of Vibrio spp. to the intestinal cell line HCT-15 in the presence and absence of CS of lactobacilli isolates was determined. In separate set of experiments the binding of lactobacilli to HCT-15 was also determined. The intestinal cell line HCT-15 was cultured in Roswell Park Memorial Institute (RPMI)-1640 (Sigma-Aldrich) medium supplemented with 10% fetal calf serum (Biological Industries, USA) on the autoclaved glass coverslips kept in 60 mm petridishes and incubated at 37°C in 5% CO2-containing atmosphere. After the cells formed 50% confluent monolayer, they were washed twice with PBS (pH 7.2) and the viable overnight grown cells of Vibrio spp. or lactobacilli suspended in 4 ml of RPMI were added to the petridishes at the multiplicity of infection 1:100. For determining the effect of CS, the cells of vibrio suspended in 2 ml of RPMI along with 2 ml of CS of lactobacilli were added to the cell line-containing petridishes. After incubation for 1 h at 37°C, the dishes were washed four times with PBS (pH 7.2) to remove the unbound bacteria. The cells were fixed with 3 ml of methanol for 5–10 min at room temperature. The cells were air dried and stained with 3 ml of Giemsa stain solution (HiMedia) by incubating at room temperature for 30 min. The dishes were washed until no color was observed in the washing solution, dried in an incubator at 37°C overnight, and examined microscopically under oil immersion. The adherent lactobacilli in 25 random microscopic fields were counted for each test. Bacterial strains were scored as non-adhesive when fewer than 40 bacteria were present in 25 fields, adhesive with 41 to 100 bacteria in 25 fields, and strongly adhesive with more than 100 bacteria in 25 fields. The adhesion assay was performed in duplicate.
Simultaneously the other set of HCT-15-containing petridishes were subjected to the treatment with lysis solution (0.05% trypsin-EDTA) for 30 min at 37°C in order to lyse and detach the cells, and thereafter plated onto BHI agar plates for CFU counting.
Estimation of Lactic Acid Production
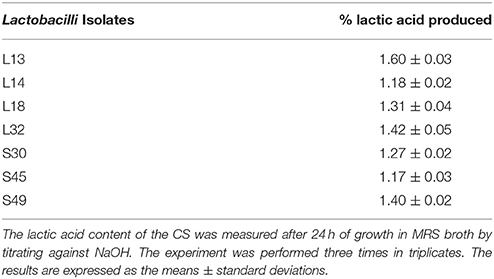
The percentage of lactic acid produced by Lactobacillus isolates in CS was determined by titration method. Briefly, the overnight grown lactobacilli culture in MRS broth was centrifuged at 9,000 g for 10 min at 4°C in cooling centrifuge. One ml of 0.5% phenolphthalein indicator dissolved in 50% ethanol was added to 9 ml CS and then the solution was titrated using 1N NaOH (SRL) till the appearance of light pink color. The percentage lactic acid in the CS was equal to the percent NaOH used to neutralize the acidity.
Probiotic Potential of Lactobacilli
Gastric Juice Tolerance and Bile Tolerance
The overnight grown lactobacilli were pelleted down by centrifugation at 9,000 g at 4°C for 10 min. The pellet was resuspended in simulated gastric juice having 2 g/l NaCl (HiMedia), 3.2 g/l pepsin and pH adjusted to 2.5 with conc. HCl (HiMedia). The suspension was incubated at 37°C for 3 h. Cells suspended in 1X PBS buffer (pH-7.2) was used as control. The viabilities of bacterial cells were evaluated by spreading onto MRS agar plates.
To determine bile tolerance, the serially diluted cultures were spread onto MRS agar plates supplemented with 0.3% oxgall (HiMedia). MRS agar plates without oxgall were used as control. The plates were incubated at 37°C in anaerobic jars. The colonies were counted after 24 h.
Biofilm Formation by Lactobacilli
Biofilm-forming abilities of lactobacilli isolates was determined in 96-well microtiter plate in MRS broth set at two different pH−4 and 6 by using crystal violet assay. Briefly, 135 μl of the autoclaved MRS broth was added to each well along with 15 μl of overnight grown lactobacilli culture having OD595 of 0.1. The microtiter plates were incubated at 37°C for different time periods−24, 48, and 72 h and the non adherent bacteria were removed by gently washing 3 times with the autoclaved distilled water. The biofilms were then fixed and stained as described previously. The sterile MRS broth was used as control. The experiment was performed in triplicates.
Based on obtained OD, strains were classified as –
(1) Non-biofilm producers OD ≤ ODc.
(2) Weak biofilm producers ODc < OD ≤ 2OD c.
(3) Moderate biofilm producers 2ODc < OD ≤ 4OD c.
(4) Strong biofilm producers 4ODc < OD.
OD: OD of experimental well having lactobacilli cells in MRS broth.
ODc: OD of control well having only MRS broth.
Autoaggregation Assay
Overnight grown bacterial cells were centrifuged at 9,000 g for 10 min at 4°C. The CS was discarded and pellet was diluted in PBS buffer (pH-7.2) to give final OD595 of 1. The suspension of lactobacilli was incubated at 37°C for 4 and 8 h. After incubation period, 1 ml of the suspension from the top of the tube was removed and its absorbance was determined at 595 nm. Autoaggregation percentage was determined using equation: (1–At/A0) × 100; where At is absorbance of suspension at different time points and A0 is absorbance at beginning of experiment (0 h). The experiment was performed in triplicates.
Antibiotic Susceptibility Test
The antibiotic susceptibility profiles of all the lactobacilli isolates was analyzed by using Kirby-Bauer diffusion test (Bauer et al., 1966). The antibiotic discs (HiMedia): tetracycline, streptomycin, ciprofloxacin, moxifloxacin, gentamycin, ampicillin, pencillin, vancomycin, clindamycin, kanamycin, and erythromycin were used in this study. The classification as “susceptible,” “intermediate,” or “resistant” was based on the European Food Safety Authority (EFSA)-recommended breakpoints for diameters of zone of inhibition (EFSA, 2012).
Effect of Commercially Available Drugs on the Growth of Lactobacilli
The agar well diffusion assay was used to study the effect of commercially available drugs on growth of lactobacilli isolates. The various tablet formulations used in this study were–paracetamol(500 mg/ml), diclofenac (10 mg/ml), nimugesic (20 mg/ml), ibuprofen (120 mg/ml), cetrizine HCl (2 mg/ml), and lansoprazole(4 mg/ml).
The lactobacilli were grown at 37°C for 18 h in MRS broth. Following the incubation period, 100 μl of culture was spread onto MRS agar plates (HiMedia). Using sterile well-borer, wells were cut onto agar plates. An aliquot (100 μl) of various drugs were poured in wells and plates were placed at 4°C for diffusion. After 4 h, plates were incubated at 37°C overnight. The diameter of zone of clearance was noted in millimeters.
Statistical Analysis
The results from at least three independent experiments represented as mean ± SD (standard deviation). Comparisons were performed by using unpaired Student's t-test in GraphPad Prism 5.04 software. In autoaggregation experiments, comparisons were performed by using paired Students's t-test.
Results
Characterization of the Lactobacilli Isolates by 16S rDNA Sequencing
On the basis of antimicrobial activity, 7 lactobacilli isolates were selected and characterized by 16S rDNA sequencing. BLAST analysis showed that L14 was 99% similar to L. plantarum, L32 and S45 was 98% similar to L. fermentum and L. pentosus respectively. L13, L18, S30, and S49 had <97% sequence matching with any known species and thus appear to be novel strains (Table 1).
Determination of Antimicrobial Activity of Lactobacilli Isolates Against Test Organisms
The well diffusion assay was used to determine the antimicrobial activity of Lactobacillus isolates. Out of 55 isolates, 7 isolates had antimicrobial activities against V. cholerae, E. coli and S. enterica. Five of the isolates had antimicrobial activity against L. monocytogenes, 4 of them against Sh. flexeri, and only 3 isolates (S30, S45, and S49) inhibited St. aureus (Table 2). None of the CS had any antimicrobial activities against the pathogens V. parahaemolyticus and C. albicans. Also, the CS of all the isolates had no antimicrobial activity against the gut commensal lactobacilli isolates when they were tested against each other (Table 2).
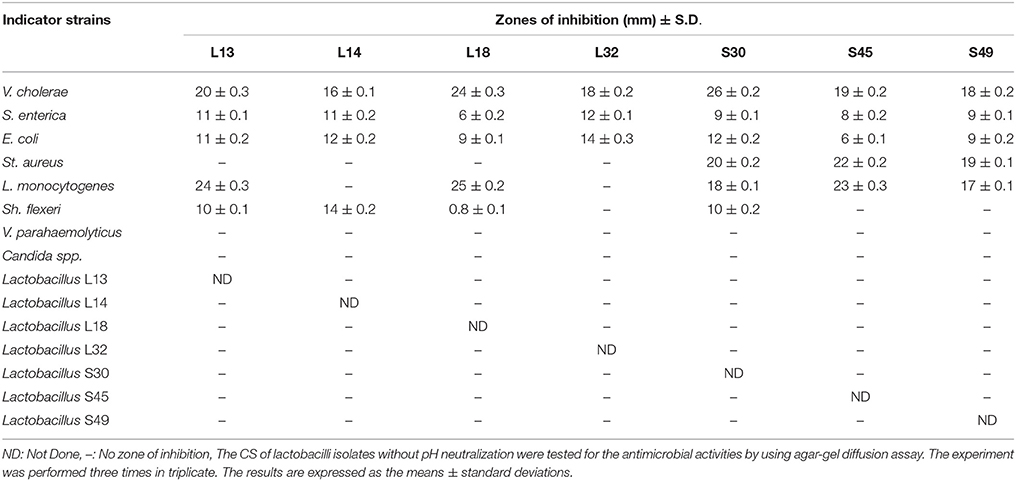
Table 2. The antagonistic activities of the cell-free culture supernatants of fecal lactobacilli against various pathogenic and commensal indicator strains.
The CS of all the 7 isolates after 16 h of growth in MRS broth had final pH in the range 3–4 (data not shown). Therefore in order to determine whether the antimicrobial property of CS is due to low pH, the pH of CS was set to 6.5 and its antimicrobial activity tested. The antimicrobial activities of all the CS against all the pathogens was completely abrogated after neutralizing the pH to 6.5 (data not shown). Further, Lactobacillus spp. is known to produce hydrogen peroxide that exhibits antimicrobial activity against various Gram-negative bacteria (Pridmore et al., 2008). Therefore, to negate the role of hydrogen-peroxide, we treated the CS of all the lactobacilli isolates with catalase before evaluating their antimicrobial activity. Our results showed that the catalase treatment had no effect on the antimicrobial activity of CS (data not shown).
Effect of CS on the Growth Kinetics of V. Cholerae and V. Parahaemolyticus
The growth kinetics of V. cholerae in BHI media supplemented with non-neutralized and pH neutralized CS was studied. The results showed that the supplementation of BHI growth medium with non-neutralized CS of all the 7 lactobacilli strains significantly (p < 0.001) inhibited the growth of V. cholerae till 12 h relative to that observed in BHI supplemented with MRS (Figure 1A). Beyond 12 h, no significant differences in the growth kinetics of the wells with and without CS was observed. On the other hand, the supplementation of BHI with pH-neutralized CS of all the seven lactobacilli strains had no significant (p < 0.001) effects on the growth kinetics of V. cholerae (Figure 1B) at all time points. Similarly the effect of non-neutralized CS of all the lactobacilli strains on the growth of V. parahaemolyticus was tested and the results showed that none of the CS inhibited its growth (Figure 1C).
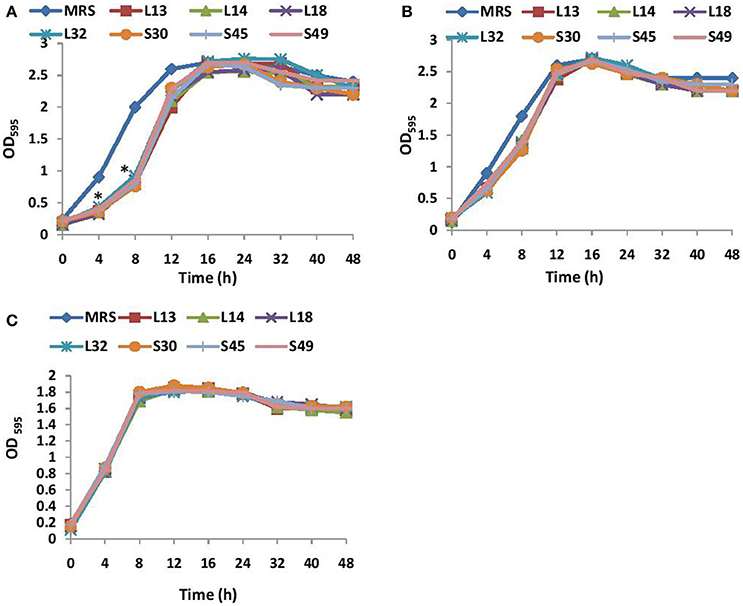
Figure 1. (A) Growth curves of V. cholera in BHI supplemented with non-neutralized CS and (B) pH neutralized CS of lactobacilli strains. (C) Growth curve of V. parahaemolyticus in BHI supplemented with non-neutralized CS of seven different Lactobacilli strains. *Significance (p < 0.001) was measured by using an unpaired Student's t-test relative to the growth in BHI supplemented with MRS at 45 mg/ml.
Effect of CS of Lactobacilli on Biofilm Formation of Vibrio Cholera
Before determining the effect of CS of Lactobacillus spp. on the biofilm-forming abilities of both V. cholerae and V. parahaemolyticus. the biofilm-forming potential of V. cholerae and V. parahaemolyticus in microtiter plates was determined. As shown in Figure 2, V. cholerae formed 2.5 times stronger (p < 0.001) biofilms as compared to V. parahaemolyticus after 24 h of growth.
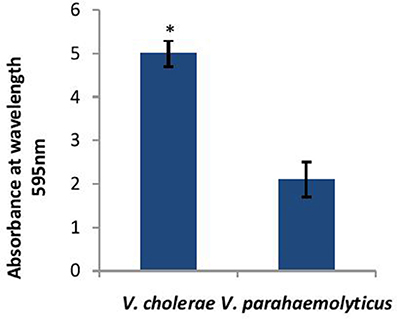
Figure 2. Biofilm forming abilities of V. cholerae and V. parahaemolyticus evaluated by crystal violet assay performed in microtiter plate. Bars represent the mean and error bars represent standard deviation of three independent experiments. *Significance (p < 0.001) was calculated by using an unpaired Student's t-test.
Next, the effect of CS of lactobacilli isolates on the formation of biofilm by V. cholerae was evaluated in an in vitro assay. The pH non-neutralized CS of all the seven isolates resulted in more than 90% inhibition (Figure 3) of the biofilm formation by V. cholerae. Maximum inhibition was observed in case of isolates L13 (96%), L14 (95%), and L32 (95.6%).
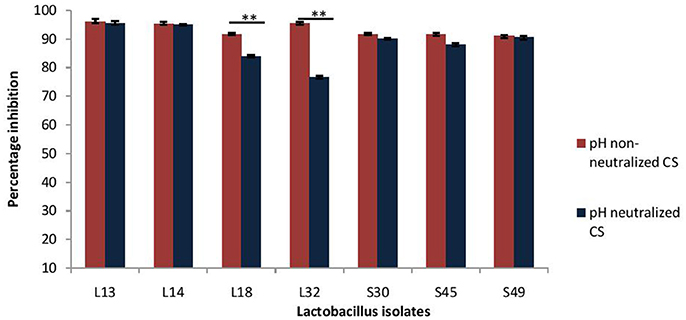
Figure 3. Percentage inhibition of biofilm formation of V. cholerae by pH neutralized and non-neutralized CS of fecal Lactobacillus isolates evaluated by modified crystal violet assay performed in a microtiter plate. Bars are representative of the mean and error bars are representative of standard deviation of three independent experiments. **Significance (p < 0.0001) was measured by using an unpaired Student's t-test.
Further, as the pH neutralization of CS abrogated its antimicrobial activity, we evaluated the effect of pH neutralized-CS on the biofilm formation by V. cholerae. The results showed that the pH neutralized-CS of all the isolates except L32 and L18 resulted in similar inhibition of the biofilm formation by V. cholerae. (Figure 3). In case of L32 and L18, the pH neutralization of CS significantly (p < 0.0001) reduced their potential to inhibit biofilm formation by 22 and 9%, respectively.
Effect of CS of Lactobacilli on the Dispersion of Biofilm of V. cholerae
As probiotic treatment are prescribed after the infection has established, thus dispersive action of probiotics on V. cholera biofilms may impart therapeutic benefits. Herein, the dispersion effect of CS at low pH (3.5) and after pH-neutralization was evaluated on the 24 h old preformed biofilms of V. cholerae. The results showed that CS of L14, S45, and S49 resulted in maximum dispersion of 85%, whereas the CS of the other lactobacilli isolates caused dispersion of V. cholera biofilms in the range 62–72% (Figure 4).
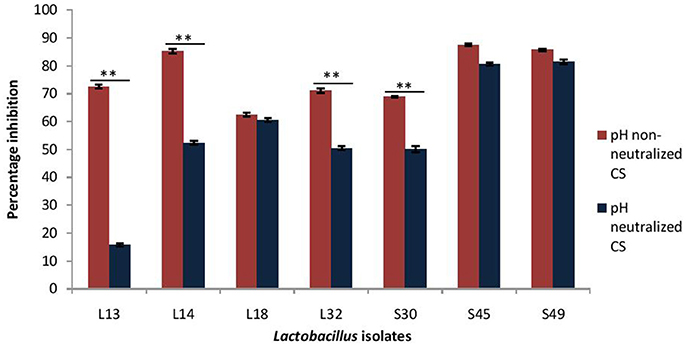
Figure 4. Percentage dispersion of pre-formed biofilm of V. cholerae by pH neutralized and non-neutralized CS of fecal Lactobacillus isolates evaluated by modified crystal violet assay performed in a microtiter plate. Bars are representative of the mean and error bars are representative of the standard deviation of three independent experiments. **Significance (p < 0.0001) was measured by using Student's t-test.
On adjusting the pH of CS to 6.5 the dispersive effect of the CS of the isolates S45, S49, and L18 was not affected; however, it was significantly (p < 0.0001) reduced in the case of four of the isolates viz. L13 (57% reduction), L14 (34%), L32 (19%), and S30 (19%). Thus, the dispersion effect of the CS is affected by pH neutralization in more than 50% of the isolates (Figure 4).
Effect of CS of Lactobacilli on the Formation and Dispersal of Biofilm by V. parahaemolyticus
As the CS of lactobacilli had no antimicrobial activity against V. parahaemolyticus, therefore the inhibitory effects of only pH non-neutralized CS of lactobacilli on the biofilm formation by V. parahaemolyticus and its dispersion was determined (Figure 5). The CS of all the isolates, except S45, inhibited the biofilm formation by V. parahaemolyticus in the range 47–82%. Maximum inhibition of 82% was observed with the CS of the isolate S49 and L14, followed by S30 (72%), L32 (67%), L18 (62%), and L13 (47%). Interestingly, the CS of all the lactobacilli isolates had no or negligible biofilm-dispersing abilities.
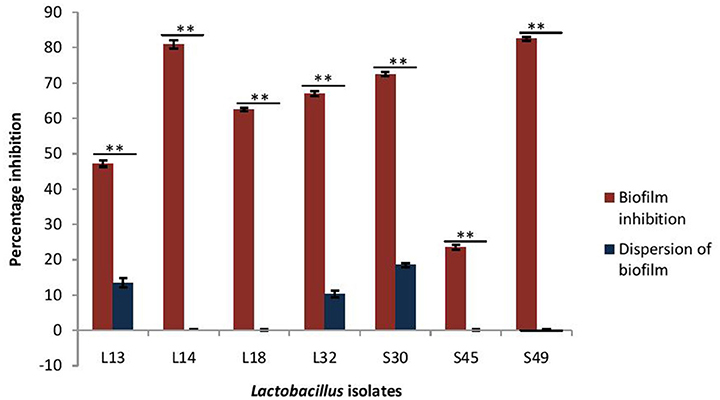
Figure 5. Effect of CS of lactobacilli isolates on the biofilm formation and dispersion of 24 h old performed biofilms of V. parahaemolyticus. Bars are representative of the means and error bars represent standard deviation of three independent experiments. **Significance (p < 0.0001) was measured by using Student's t-test.
Effect of CS of Lactobacilli on Adhesion of Vibrio to HCT-15 Cell Line
Adherence of Vibrio spp. to the intestinal epithelial cells is the primary step involved in the biofilm formation and pathogenesis of gut infection. Therefore, the effect of CS of lactobacilli on the initial adherence of pathogens-V. cholerae and V. parahaemolyticus with the intestinal cell line HCT-15 was evaluated by both plating technique and by using light microscopy. The binding of V. cholera to HCT-15 cell line was almost one log higher as compared to that of V. parahaemolyticus. Further, in the presence of CS of L18, there was significant (p < 0.001) reduction by approximately 2.5 log10 CFUs that adhered to HCT-15. On the other hand, the adherence of V. parahaemolyticus in the presence of the CS of L18 was not significantly reduced (Figure 6). The light microscopic images also showed similar effects (Figure 7). The CS of other lactobacilli isolates similarly reduced the adherence of V. cholera in the range 1.5–2.2 log10CFUs; whereas the adherence of V. parahaemolyticus was reduced in the range 0.6–0.9 log10CFUs (data not shown).
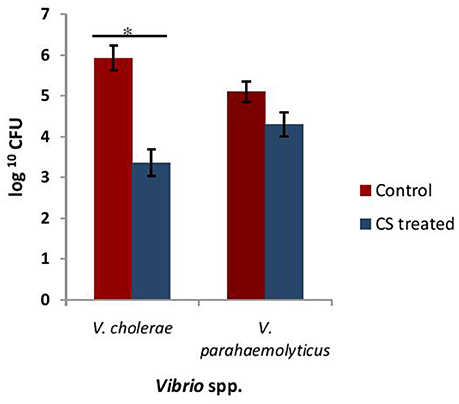
Figure 6. The numbers of CFU of Vibrio spp. that adhered to HCT-15 cell line in the absence and presence of CS of L18. Bars are representative of the mean, whereas, error bars are representative of standard deviation of three independent experiments. *Significance (p < 0.001) was measured by using unpaired Student's t-test.
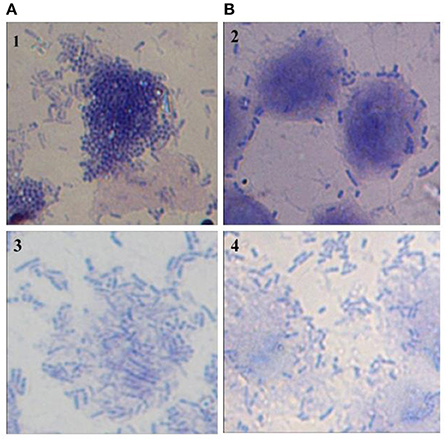
Figure 7. Microscopic (1000X) image of Giemsa-stained HCT-15 cells showing adhered V. Cholera (1 and 2) and V. parahaemolyticus (3 and 4) in the absence (A) and presence (B) of CS of lactobacilli isolate L18.
Lactic Acid Production Estimation
The lactic acid is the major organic acid produced by glucose fermentation by Lactobacillus spp. As biofilm-dispersive abilities in case of L13, L14, L32, and S30 is affected by pH neutralization, we evaluated the concentration of lactic acid in the CS. L13 produced highest amount of lactic acid followed by L32, S49, and L18 whereas S45 and L14 produced least amount of lactic acid (Table 3).
Probiotic Properties of Lactobacilli
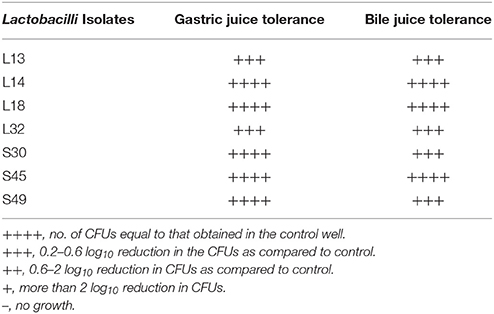
The probiotic properties of lactobacilli is known to be strain specific, therefore we evaluated the probiotic potential of all the seven lactobacilli isolates. One of the most important criteria for the strain to be good probiotic is its ability to resist the action of bile salts and gastric juice during its passage in the GIT. As expected for the lactobacilli of fecal origin, all the isolates were able to survive after 3 h of incubation in the simulated gastric juice and also showed growth in the presence of bile salts (Table 4).
The ability of lactobacilli to form strong biofilms has been correlated with their abilities to persist in vivo. Therefore the abilities of the lactobacilli to form biofilms was evaluated at two different pH 4 and 6, and at different time intervals −24, 48, and 72 h. At pH 4 all the isolates except S45 and S49, formed strong biofilms at 48 and 72 h. Whereas, at pH 6, all the lactobacilli isolates formed strong biofilms after 48 h, but by 72 h biofilms in all except L13 and L18 appeared to disperse and become moderate. L13 and L18 formed strong biofilms at all pH and time points (Table 5).
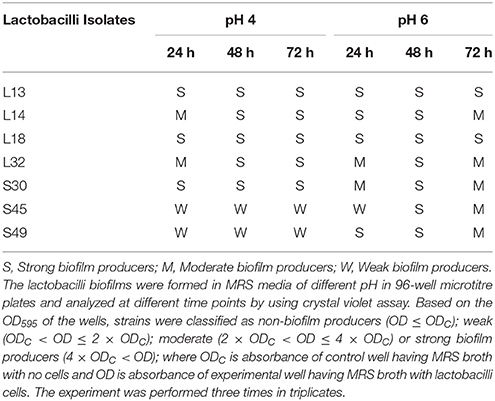
Table 5. Biofilm formation by lactobacilli isolates at different pH and time points using crystal violet microtiter plate assay.
The maturation of biofilms depends on the autoaggregation properties of the lactobacilli. Thus, we assessed their abilities to autoaggregate. All the isolates showed significantly (p < 0.0001) higher auto aggregation (more than 90%) after 8 h of incubation that at 4 h (Figure 8).
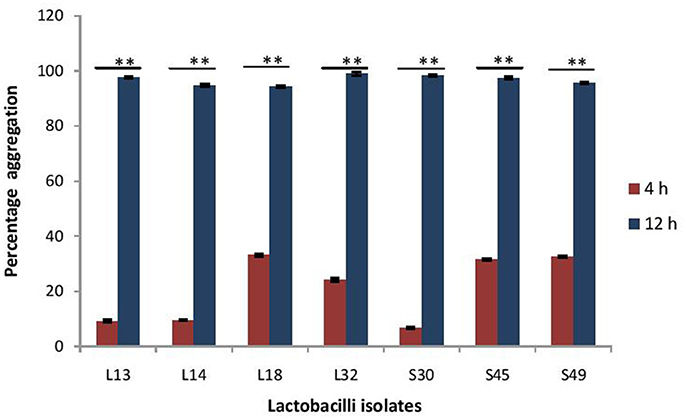
Figure 8. Percentage auto-aggregation exhibited by lactobacilli isolates. Bars represent the mean, error bars represent standard deviation of three independent experiments and **significance (p < 0.0001) was measured by using paired Student's t-test.
Further, the ability of the lactobacilli isolates to adhere to the intestinal epithelial cell line HCT-15 was determined. Cell adhesion assay demonstrated that all the selected lactobacilli isolates had strong adhesive ability to intestinal cell line – HCT-15 (Figure 9).
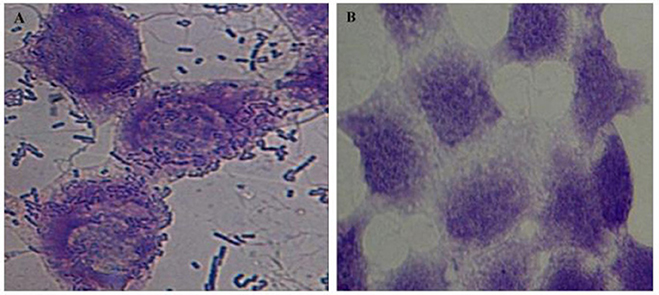
Figure 9. Microscopic (1000X) image of Giemsa-stained intestinal cell line HCT-15 cells with (A) and without (B) adhered lactobacilli isolate L32.
Antibiotic Susceptibility Test
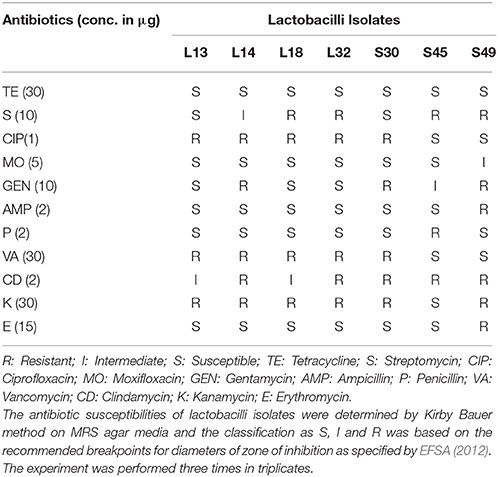
Kirby Bauer disc diffusion method was used to analyze the susceptibility profiles of the lactobacilli isolates. Eleven antibiotics belonging to different classes: aminoglycosides (streptomycin, gentamycin, kanamycin), fluoroquinolones (ciprofloxacin, moxifloxacin), beta-lactams (ampicillin, penicillin), macrolides (erythromycin), glycopeptides (vancomycin), lincomycin (clindamycin), and tetracycline (tetracycline), were used in this study. The antibiotic profiles of all the isolates are summarized in Table 6. All of them were susceptible to tetracycline. All, except S45, were susceptible to penicillin and all, except S49, were susceptible to ampicillin, moxifloxacin, and erythromycin.
All the isolates were resistant to clindamycin. All except S45 were resistant to kanamycin and all except S45 and S49 were resistant to vancomycin, and ciprofloxacin. Similarly all, except L13 and S30 were resistant to streptomycin. In case of gentamycin, expect L13, L18, and L32, all were resistant.
Effect of Commercially Available Drugs on Growth of Lactobacilli
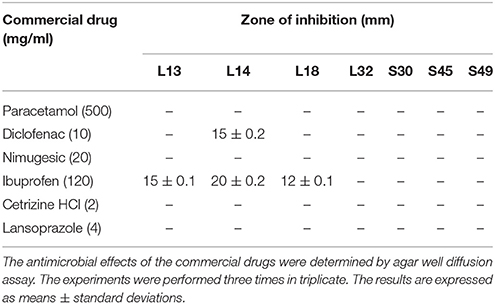
The effect of commercially available drugs on the growth of lactobacilli was evaluated. Paracetamol, nimugesic, cetrizine HCl, and lansoprazole had no antimicrobial activities against all the lactobacilli isolates. Ibuprofen inhibited the growth of L13, L14, and L18 whereas, diclofenac inhibited the growth of only L14 (Table 7).
Discussion
Inhibition of biofilm formation by pathogens is an attractive target for therapeutic intervention (Bjarnsholt et al., 2013) that has received significant attention in recent years, leading to the discovery of biofilm inhibitors for many of the commonly encountered bacterial pathogens including V. cholera (Melander and Melander, 2015; Rabin et al., 2015). V. cholerae is well known for its ability to form strong biofilms in vivo on the intestinal mucosa in rabbit model (Jones and Freter, 1976; Nelson et al., 1976) and in humans (Yamamoto and Yokota, 1988). The experiments conducted in our laboratory also demonstrated strong in vitro biofilm-forming abilities of both V. cholerae and V. parahaemolyticus both in 96-well microtite plates and their abilities to bind to HCT-15 colonic epithelial cell line. The ability of V. cholerae to develop biofilms is critical to intestinal colonization (Silva and Benitez, 2016) and virulence (Xu et al., 2003; Fong et al., 2010). This is because, biofilm-derived cells could be more effective in competing for limiting nutrients in the small intestine, as suggested by elevated expression of the phosphate uptake system compared to planktonic cells (Mudrak and Tamayo, 2012). Also, biofilms of V. cholerae are reported to resist the action of acid inactivation in the gut (Zhu and Mekalanos, 2003). The biofilms once formed become resistant to the action of antibiotics as most of the conventional antibiotics are active only against planktonic V. cholera cells and have no biofilm-dispersive action (Warner et al., 2015). Thus, probiotics strains having both antimicrobial and anti-biofilm activities against V. cholerae are expected to be clinically superior.
We screened 55 fecal lactobacilli isolates for their antimicrobial potential against Gram-negative gut pathogens, out of which seven isolates having broad-spectrum antimicrobial activities against V. cholerae, E. coli and S. enterica were selected. Interestingly, the CS appears to inhibit specifically the gut-associated pathogens only and had no antimicrobial action against the commensal gut lactobacilli. However, when pH of the CS was neutralized, the antimicrobial activity was completely abrogated as shown by disappearance of zones of inhibition. This showed that low pH due to lactic acid or other organic acids secreted by the lactobacilli in the CS are responsible for the antimicrobial activities. Similar reports showing abrogation of antimicrobial activities of CS on pH neutralization has been reported earlier also (De Keersmaecker et al., 2006; Zhang et al., 2011). Lactic acid solution at concentration of 0.5% has been shown to effectively kill both Gram-negative (Salmonella and E. coli) and Gram-positive (L. monocytogenes) pathogens by causing cell membrane damage that resulted in leakage of proteins (Qiao et al., 2008; Wang et al., 2015). Thus, lactic acid in the CS (concentrations ranging from 1.17–1.8%) of all the lactobacilli isolates used in the study may be responsible for the antimicrobial activity. However the role of other organic acids for the antimicrobial activity cannot be negated. Also, the catalase enzyme treatment of CS did not altered the zones of inhibition, thereby showing that hydrogen peroxide is not responsible for the antimicrobial activity of CS. Next, the effect of non-neutralized CS on the growth kinetics of V. cholerae showed that it had bacteriostatic effects that significantly inhibited the growth till 12 h, beyond which the growth was similar to the wells with MRS. pH neutralized CS on the other hand had no significant effects on the growth kinetics of V. cholerae. Similarly, CS of all the lactobacilli strains had no effects on the growth kinetics of V. parahaemolyticus.
Further the effects of lactobacilli CS at low pH (3.5) and high pH (after pH neutralization to 6.5) was evaluated on the biofilm-forming ability of V. cholera because on pH neutralization the antimicrobial activity of CS is completely abrogated. Results demonstrated that CS at both low and high pH similarly inhibited the biofilm-formation by V. cholera. Further, in case of V. parahaemolyticus the CS of 5 out of 7 lactobacilli isolates inhibited 62–82% of the biofilm formation despite having no antimicrobial activity. The growth kinetics study also show that both the non-neutralized and the pH neutralized CS did not affect the growth of Vibrio at 48 h. Thus, the inhibition of Vibrio spp. biofilm formation by lactobacilli CS was not due to its antimicrobial activity. The various components of CS of lactobacilli such as, exopolysaccharides (Kim et al., 2009) and biosurfactants (Walencka et al., 2008; Fracchia et al., 2010; Zakaria Gomaa, 2013) may inhibit the biofilm formation as reported against other pathogens. Purified EPS of L. acidophilus was shown to inhibit biofilm formation of a number of Gram-positive and Gram-negative pathogens. It was hypothesized that EPS interfered with the initial attachment of pathogen to small intestine cell line HT-29 (Kim et al., 2009). The physiological pH of mammalian stomach is acidic and that of intestine is toward neutral in the range 6–7. Thus, as the CS of all the lactobacilli isolates inhibited the biofilm formation at both low and high pH, these strains may have good prophylactic action against Vibrio-associated infection in both stomach and intestine. However, for the therapeutic action of lactobacilli spp., the dispersive action of CS against the preformed biofilms of Vibrio is important.
The CS at low pH dispersed the V. cholerae biofilm in the range 62–85%. Even on pH neutralization, the dispersal effect of CS of 6 isolates except L13 was in the range 50–75%; but it was reduced appreciably in the case of 4 isolates. However, none of the CS had any dispersal effects on the biofilm formation by V. parahaemolyticus. Thus, apparently the results showed that the biofilm dispersal effect of CS is dependent on its antimicrobial effect. But the role of CS components such as enzymes that caused the disintegration of V. cholera biofilm matrix but not that of V. parahaemolyticus cannot be negated. The V. cholera biofilm matrix is composed of exopolysaccharides containing glucose and galactose as the major components; whereas the biofilm matrix of V. parahaemolyticus is made up of capsular polysaccharides that contains many other sugar moieties apart from glucose and galactose (Yildiz and Visick, 2009). Thus, these differences may account for the differential dispersive effect. Further, CS may contain components that induced the secretion of quorum-sensing autoinducer and thereby caused dispersal of V. cholerae biofilms preferentially.
Further, we also assessed the abilities of CS of lactobacilli isolates to inhibit the adherence of both V. cholerae and V. parahaemolyticus to the intestinal epithelial cell line HCT-15. The CS of L18 was more effective at inhibiting the adherence of V. cholerae (2.5 log10 CFU reduction) to HCT-15 as compared to V. parahaemolyticus (0.7 log10 CFU reduction). Similar trend was observed with the CS of other isolates. The differences in the viable counts of V. cholera and V. parahaemolyticus on plating the lysed cell line can be due to the bactericidal action of CS against the V. cholera but not against V. parahaemolyticus. However, microscopic images of HCT-15 cells showed that L18 CS inhibited the binding of V. cholera more than V. parahaemolyticus.
Further, the probiotic potential of all the isolates were assessed. For an isolate to be a good oral probiotic candidate, it must have certain survival and adaptive characteristics such as resistance to the low pH, bile salts and various enzymes in the gut. All the Lactobacillus isolates showed more than 90% survival in the presence of bile salts and gastric juice. Probiotic bacteria having good biofilm-forming ability can prevent colonization of the gut epithelium by pathogenic bacteria (Jalilsood et al., 2015; Aoudia et al., 2016). Thus, the ability of probiotic bacteria to form biofilms and adhere to colonic cell line was evaluated. All the isolates showed strong adherence to the colonic cell line- HCT-15. Also all the isolates formed strong biofilms at pH-6 after 48 h; whereas, at pH 4 five of the isolates, except S45 and S49 formed strong biofilm. The maturation of biofilms is strongly dependent on the auto-aggregation properties of the probiotic bacteria as it helps the bacteria to form micro-colonies, which in turn secrete exopolysaccharides resulting in the maturation of biofilms. All the lactobacilli isolates used in this study exhibited more than 90% auto-aggregation after 8 h.
As probiotics are commonly used as adjuncts to antibiotic therapy, therefore the inherent resistance of lactobacilli to common antibiotics (Egervärn et al., 2009) was evaluated. Further the antibiotic susceptibility profiles to various classes of antibiotics could provide the hint to the presence of transferable resistance elements that can potentially be transmitted to the gut microbiota (Morelli et al., 1988). All the isolates were susceptible to tetracycline, and all except one were susceptible to erythromycin, ampicillin, penicillin, and moxifloxacin. High ciprofloxacin, gentamycin, streptomycin, and vancomycin resistance were observed among the isolates. The Lactobacillus species are known to be intrinsically resistant to vancomycin which is chromosomally-encoded and non-transmissible (Ruoff et al., 1988). Ciprofloxacin resistance in lactobacilli has been reported earlier among lactobacilli of fermented food (Kaktcham et al., 2012) and fecal origin (Shazali et al., 2014). Similar high resistance to aminoglycosides has been reported in probiotic lactobacilli (Zhou et al., 2005) and from fermented food (Kaktcham et al., 2012).
The probiotics are often prescribed along with various drugs such as, analgesic, anti-pyretic, non-steriodal anti-inflammatory drugs (NSAIDs), anti-allergic, and proton pump inhibitor. Therefore, the effect of these commonly used drugs on the growth of lactobacilli isolates need to be evaluated. The drugs - paracetamol, nimugesic, cetrizine hydrochloride and lanzoprazole had no inhibitory effects on the growth of isolates except ibuprofen which inhibited the growth of lactobacilli isolates–L13, L14, and L18 and diclofenac inhibited L14. Previous study also demonstrated the inhibitory effects of sodium diclofenac on the growth of L. plantarum ST8KF and ST341LD (Todorov and Dicks, 2008).
All the seven Lactobacilli isolates used in this study had broad-spectrum antimicrobial and biofilm-inhibitory activities. Thus, they may have good prophylactic properties to inhibit the gut-associated infectious diseases. The isolates S45, S49, and L18 seemed to possess the best biofilm dispersion abilities at both low and high pH, therefore their therapeutic potential to treat V. cholera infection in the mouse model should be further tested.
Author Contributions
SukK conceived the idea and supervised the experiments. SumK and SukK designed the experiments and SumK performed almost all of the experiments, except those involving cell lines. PS, NK, and JS designed and performed the cell line studies. All authors discussed the results and contributed to the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by research grant (grant number: 42-478/2013 SR) sponsored by University Grants Commission (UGC), New Delhi, India. PS is thankful to University of Potential for Excellence scheme of UGC for the fellowship. SumK is thankful to UGC for the fellowship.
References
Ahmadi, E., Alizadeh-Navaei, R., and Rezai, M. S. (2015). Efficacy of probiotic use in acute rotavirus diarrhea in children: a systematic review and meta-analysis. Caspian J. Intern. Med. 6, 187–195.
Ali, M., Nelson, A. R., Lopez, A. L., and Sack, D. A. (2015). Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 9:e0003832. doi: 10.1371/journal.pntd.0003832
Almagro-Moreno, S., Pruss, K., and Taylor, R. K. (2015). Intestinal colonization dynamics of Vibrio cholerae. PLoS Pathog. 11:e1004787. doi: 10.1371/journal.ppat.1004787
Aoudia, N., Rieu, A., Briandet, R., Deschamps, J., Chluba, J., Jego, G., et al. (2016). Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol. 53, 51–59. doi: 10.1016/j.fm.2015.04.009
Bauer, A. W., Kirby, W. M., Sherris, J. C., and Turk, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496. doi: 10.1093/ajcp/45.4_ts.493
Bjarnsholt, T., Ciofu, O., Molin, S., Givskov, M., and Hoiby, N. (2013). Applying insights from biofilm biology to drug development—can a new approach be developed? Nat. Rev. Drug Discov. 12, 791–808. doi: 10.1038/nrd4000
Chimchang, J., Theparee, T., Ladda, B., Tanasupawat, S., Wongsatayanon, B. T., and Taweechotipatr, M. (2015). Antimicrobial properties of a potential probiotic Lactobacillus from Thai newborn feces. J. Med. Assoc. Thai. 98(Suppl. 9), S116–S122.
Culligan, E. P., Hill, C., and Sleator, R. D. (2009). Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut Pathog. 1:19. doi: 10.1186/1757-4749-1-19
De Keersmaecker, S. C., Verhoeven, T. L., Desair, J., Marchal, K., Vanderleyden, J., and Nagy, I. (2006). Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 259, 89–96. doi: 10.1111/j.1574-6968.2006.00250.x
D'Souza, A. L., Rajkumar, C., Cooke, J., and Bulpitt, C. J. (2002). Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ 324, 1361–1367. doi: 10.1136/bmj.324.7350.1361
EFSA (2012). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10:2740. doi: 10.2903/j.efsa.2012.2740
Egervärn, M., Roos, S., and Lindmark, H. (2009). Identification and characterization of antibiotic resistance genes in Lactobacillus reuteri and Lactobacillus plantarum. J. Appl. Microbiol. 107, 1658–1668. doi: 10.1111/j.1365-2672.2009.04352.x
FAO/WHO (2001). Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Report of a joint Food Agricultural Organisation/WHO expert consultation, Cordoba.
Fong, J. C., Syed, K. A., Klose, K. E., and Yildiz, F. H. (2010). Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology 156, 2757–2769. doi: 10.1099/mic.0.040196-0
Fracchia, L., Cavallo, M., Allegrone, G., and Martinotti, M. G. (2010). “A Lactobacillus-derived biosurfactant inhibits biofilm formation of human pathogenic Candida albicans biofilm producers,” in Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, eds A. Mendez-Vilas (Formatex Research Center), 827–837.
Gonzalez, L., Sandoval, H., Sacristan, N., Castro, J. M., Fresno, J. M., and Tornadijo, M. E. (2007). Identification of lactic acid bacteria isolated from Genestoso cheese throughout ripening and study of their antimicrobial activity. Food Control. 18, 716–722. doi: 10.1016/j.foodcont.2006.03.008
Harmsen, H. J. M., Raangs, G. C., He, T., Degener, J. E., and Welling, G. W. (2002). Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68, 2982–2990. doi: 10.1128/AEM.68.6.2982-2990.2002
Huang, J. S., Bousvaros, A., Lee, J. W., Diaz, A., and Davidson, E. J. (2002). Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig. Dis. Sci. 47, 2625–2634. doi: 10.1023/A:1020501202369
Jalilsood, T., Baradaran, A., Song, A. A.-L., Foo, H. L., Mustafa, S., Saad, W. Z., et al. (2015). Inhibition of pathogenic and spoilage bacteria by a novel biofilm-forming Lactobacillus isolate: a potential host for the expression of heterologous proteins. Microb. Cell Fact. 14, 1–14. doi: 10.1186/s12934-015-0283-8
Jones, G. W., and Freter, R. (1976). Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect. Immun. 14, 240–245.
Kaktcham, P. M., Zambou, N. F., Tchouanguep, F. M., El-Soda, M., and Choudhary, M. I. (2012). Antimicrobial and safety properties of Lactobacilli isolated from two Cameroonian traditional fermented foods. Sci. Pharma. 80, 189–203. doi: 10.3797/scipharm.1107-12
Kemgang, T. S. S., Kapila, S., Shanmugam, V. P., and Kapila R. (2014). Cross-talk between probiotic lactobacilli and host immune system. J. Appl. Microbiol. 117, 303–319. doi: 10.1111/jam.12521
Kim, Y., Oh, S., and Kim, S. H. (2009). Released exoploysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherchia coli O157:H7. Biochem. Biophy. Res. Commun. 379, 324–329. doi: 10.1016/j.bbrc.2008.12.053
Kitaoka, M., Miyata, S. T., Unterweger, D., and Pukatzki, S. (2011). Antibiotic resistance mechanisms of Vibrio cholerae. J. Med. Microbiol. 60(Pt 4), 397–407. doi: 10.1099/jmm.0.023051-0
Koga, T., Mizobe, T., and Takumi, K. (1998). Antibacterial activity of Lactobacillus species against Vibrio species. Microbiol. Res. 153, 271–275. doi: 10.1016/S0944-5013(98)80011-6
Lebeer, S., Vanderleyden, J., and De Keersmaecker, S. C. J. (2008). Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72, 728–764. doi: 10.1128/MMBR.00017-08
McFarland, L. V. (2006). Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol.101, 812–822. doi: 10.1111/j.1572-0241.2006.00465.x
Melander, R. J., and Melander, C. (2015). Innovative strategies for combating biofilm-based infections. Adv. Exp. Med. Biol. 831, 69–91. doi: 10.1007/978-3-319-09782-4_6
Millet, Y. A., Alvarez, D., Ringgaard, S., von Andrian, U. H., Davis, B. M., and Waldor M, K. (2014). Insights into Vibrio cholerae intestinal colonization from monitoring fluorescently labeled bacteria. PLoS Pathog. 10:e1004405. doi: 10.1371/journal.ppat.1004405
Mitra, A. K., and Rabbani, G. H. (1990). A double-blind, controlled trial of bioflorin (Streptococcus faecium SF68) in adults with acute diarrhea due to Vibrio cholerae and enterotoxigenic Escherichia coli. Gastroenterology 99, 1149–1152. doi: 10.1016/0016-5085(90)90638-H
Moore, E., Arnscheidt, A., Kruger, A., Strompl, C., and Mau, M. (2004). “Simplified protocols for the preparation of genomic DNA from bacterial cultures,” in Molecular Microbial Ecology Manual, 2nd Edn., eds A. D. L. Akkrmans, J. D. V. Elsas, and F. J. De Bruijn (Netherlands: Kluwer Academic Publishers), 3–18.
Morelli, L., Sarra, P. G., and Bottazzi, V. (1988). In vivo transfer of pAMβ1 from Lactobacillus reuteri to Enterococcus faecalis. J. Appl. Bacteriol. 65, 371–375. doi: 10.1111/j.1365-2672.1988.tb01905.x
Morris, J. G., and Acheson, D. (2003). Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin. Infect. Dis. 37, 272–280. doi: 10.1086/375600
Mudrak, B., and Tamayo, R. (2012). The Vibrio cholerae Pst2 phosphate transport system is upregulated in biofilms and contributes to biofilm-induced hyperinfectivity. Infect. Immun. 80, 1794–1802. doi: 10.1128/IAI.06277-11
Nelson, E. T., Clements, J. D., and Finkelstein, R. A. (1976). Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect. Immun. 14, 527–547.
Petrova, P. M., and Petrov, K. K. (2011). Antimicrobial activity of starch-degrading Lactobacillus strains isolated from boza. Biotechnol. Biotechnol. Equip. 25, 114–116. doi: 10.5504/BBEQ.2011.0124
Pridmore, R. D., Pittet, A.-C., Praplan, F., and Cavadini, C. (2008). Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol. Lett. 283, 210–215. doi: 10.1111/j.1574-6968.2008.01176.x
Qiao, Z., Chen, Y., Lu, Z., Guan, L., and Li, L. (2008). Antibacterial and bactericide activity of lactic acid against three strains of food-borne pathogenic bacteria. Food Sci. Technol. 10, 187–190.
Rabin, N., Zheng, Y., Opoku-Temeng, C., Du, Y., Bonsu, E., and Sintim, H. O. (2015). Agents that inhibit bacterial biofilm formation. Future Med. Chem. 7, 647–671. doi: 10.4155/fmc.15.7
Reinheimer, J. A., Demkow, M. R., and Condioti, M. C. (1990). Inhibition of coliform bacteria by lactic cultures. Aust. J. Dairy Technol. 45, 5–9.
Rinttilä, T., Kassinen, A., Malinen, E., Krogius, L., and Palva, A. (2004). Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97, 1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x
Ruoff, K. L., Kuritzkes, D. R., Wolfson, J. S., and Ferraro, M. J. (1988). Vancomycin-resistant gram-positive bacteria isolated from human sources. J. Clin. Microbiol. 26, 2064–2068.
Sambanthamoorthy, K., Gokhale, A. A., Lao, W., Parashar, V., Neiditch, M. B., Semmelhack, M. F., et al. (2011). Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 55, 4369–4378. doi: 10.1128/AAC.00583-11
Sayem, S. M., Manzo, E., Ciavatta, L., Tramice, A., Cordone, A., Zanfardino, A., et al. (2011). Anti-biofilm activity of an exopolysaccharide from a sponge-associated strain of Bacillus licheniformis. Microb. Cell Fact. 10, 1–12. doi: 10.1186/1475-2859-10-74
Sharma, D., Saharan, B. S., Chauhan, N., Procha, S., and Lal, S. (2015). Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. Springerplus 4:4. doi: 10.1186/2193-1801-4-4
Shazali, N., Foo, H. L., Loh, T. C., Choe, D. W., and Abdul Rahim, R. (2014). Prevalence of antibiotic resistance in lactic acid bacteria isolated from the faeces of broiler chicken in Malaysia. Gut Pathog. 6, 1–7. doi: 10.1186/1757-4749-6-1
Shokryazdan, P., Sieo, C. C., Kalavathy, R., Liang, J. B., Alitheen, N. B., Jahromi, M. F., et al. (2014). Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res. Int. 927268, 1–16. doi: 10.1155/2014/927268
Silva, A. J., and Benitez, J. A. (2016). Vibrio cholerae biofilms and cholera pathogenesis. PLoS Negl. Trop. Dis. 10:e0004330. doi: 10.1371/journal.pntd.0004330
Todorov, S. D., and Dicks, L. M. T. (2008). Evaluation of lactic acid bacteria from kefir, molasses and olive brine as possible probiotics based on physiological properties. Annals Microbiol. 58, 661–670. doi: 10.1007/BF03175572
Walencka, E., Rozalska, S., Sadowska, B., and Rozalska, B. (2008). The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 53, 61–66. doi: 10.1007/s12223-008-0009-y
Wang, C., Chang, T., Yang, H., and Cui, M. (2015). Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control. 47, 231–236. doi: 10.1016/j.foodcont.2014.06.034
Warner, C. J. A., Cheng, A. T., Yildiz, F. H., and Linington, R. G. (2015). Development of benzo[1,4]oxazines as biofilm inhibitors and dispersal agents against Vibrio cholerae. Chem. Commun. 51, 1305–1308. doi: 10.1039/C4CC07003H
Wu, C., Labrie, J., Tremblay, Y. D. N., Haine, D., Mourez, M., and Jacques, M. (2013). Zinc as an agent for the prevention of biofilm formation by pathogenic bacteria. J. Appl. Microbiol. 115, 30–40. doi: 10.1111/jam.12197
Xu, Q., Dziejman, M., and Mekalanos, J. J. (2003). Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc. Natl. Acad. Sci. U.S.A. 100, 1286–1291. doi: 10.1073/pnas.0337479100
Yamamoto, T., and Yokota, T. (1988). Electron microscopic study of Vibrio cholerae O1 adherence to the mucus coat and villus surface in the human small intestine. Infect. Immun. 56, 2753–2759.
Yildiz, F. H., and Visick, K. L. (2009). Vibrio biofilms: so much the same yet so different. Trends Microbiol. 17, 109–118. doi: 10.1016/j.tim.2008.12.004
Zakaria Gomaa, E. (2013). Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J. Gen. Appl. Microbiol. 59, 425–436. doi: 10.2323/jgam.59.425
Zhang, Y., Zhang, L., Du, M., Yi, H., Guo, C., Tuo, Y., et al. (2011). Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol. Res. 167, 27–31. doi: 10.1016/j.micres.2011.02.006
Zhou, J. S., Pillidge, C. J., Gopal, P. K., and Gill, H. S. (2005). Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int. J. Food Microbiol. 98, 211–217. doi: 10.1016/j.ijfoodmicro.2004.05.011
Keywords: Vibrio, anti-biofilm, biofilm-dispersion, lactobacilli, probiotic, antimicrobial
Citation: Kaur S, Sharma P, Kalia N, Singh J and Kaur S (2018) Anti-biofilm Properties of the Fecal Probiotic Lactobacilli Against Vibrio spp. Front. Cell. Infect. Microbiol. 8:120. doi: 10.3389/fcimb.2018.00120
Received: 24 November 2017; Accepted: 03 April 2018;
Published: 24 April 2018.
Edited by:
Wilhelmina May Huston, University of Technology Sydney, AustraliaReviewed by:
Hongxia Wang, University of Alabama at Birmingham, United StatesFohad Mabood Husain, King Saud University, Saudi Arabia
Copyright © 2018 Kaur, Sharma, Kalia, Singh and Kaur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sukhraj Kaur, ZHJzdWtocmFqa2F1ckBnbWFpbC5jb20=
 Sumanpreet Kaur
Sumanpreet Kaur Preeti Sharma
Preeti Sharma Namarta Kalia
Namarta Kalia Jatinder Singh
Jatinder Singh Sukhraj Kaur
Sukhraj Kaur