
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 23 February 2018
Sec. Molecular Bacterial Pathogenesis
Volume 8 - 2018 | https://doi.org/10.3389/fcimb.2018.00021
 Jin-xin Zheng1,2†
Jin-xin Zheng1,2† Zhi-wei Lin1,2†
Zhi-wei Lin1,2† Chen Chen3†
Chen Chen3† Zhong Chen1,2†
Zhong Chen1,2† Fo-jun Lin1
Fo-jun Lin1 Yang Wu4
Yang Wu4 Si-yu Yang3
Si-yu Yang3 Xiang Sun1
Xiang Sun1 Wei-ming Yao1
Wei-ming Yao1 Duo-yun Li1
Duo-yun Li1 Zhi-jian Yu1,2
Zhi-jian Yu1,2 Jia-lin Jin3*
Jia-lin Jin3* Di Qu4*
Di Qu4* Qi-wen Deng1,2*
Qi-wen Deng1,2*Klebsiella pneumoniae bacteremia biofilm traits and distribution characteristics have not been clarified. This study aimed to determine the prevalence and characteristics of K. pneumoniae bacteremia biofilm formation (BF) and to explore the virulence factors associated with K. pneumoniae BF. A total of 250 K. pneumoniae bacteremia isolates were collected from patients in Shenzhen and Shanghai, China. Virulence genes in their genomes were detected by PCR. The isolates were subjected to multilocus sequence typing (MLST) and clonal complex (CC) classification based on housekeeping genes. Biofilms were detected by crystal violet staining. Greater BF was observed in isolates from young adults (<40 years old) than in those from seniors (≥65 years old; P = 0.002). MLST yielded 65 different sequence types (STs), with the most represented STs being ST11, ST23, and ST65, and the main CCs were CC23 and CC65; CC23 isolates exhibited greater BF than CC65 or ST11 isolates (both P < 0.001). BF was more pronounced among magA(K1), aero+, rmpA+, rmpA2+, allS+, wcaG+, and iutA+ isolates than in isolates that were negative for these virulence factors. Multivariate regression analysis revealed only wcaG as an independent risk factor for BF (odds ratio 11.426, P < 0.001), and BF was decreased when wcaG was silenced by antisense RNA. In conclusion, BF in K. pneumoniae bacteremia isolates was found to be associated with CC23 classification and the presence of the wcaG virulence factor gene.
Klebsiella pneumoniae has been attracting increasing attention worldwide as an infectious microorganism due to the recent rise in the number of severe K. pneumoniae infections, antibiotic resistance, and growing difficulty with establishing effective treatments. K. pneumoniae is now the second most common cause of Gram-negative bacteremia and a major pathogen in hospital-acquired infection, particularly in immunocompromised patients (Candan and Aksöz, 2015; Paczosa and Mecsas, 2016). K. pneumoniae bacteremia has a high mortality rate (27.4–37.0%), especially when the strain is hypervirulent (Meatherall et al., 2009; Chetcuti-Zammit et al., 2014; Girometti et al., 2014; Li et al., 2014; Yu et al., 2016).
Relative to planktonic K. pneumoniae infections, infections with K. pneumoniae strains with the ability to form biofilms are more difficult to treat (Ribeiro et al., 2015). Diago-Navarro et al. (2014) found that nearly half of 40 examined carbapenem-resistant K. pneumoniae bacteremia strains were able to form obvious biofilms, including 13 that exhibited high levels of biofilm formation (BF). The antibiotic resistance of mature bacterial biofilm is 10–1,000 times that of planktonic bacteria, and bacteria in biofilms can resist phagocytosis, making them very challenging to eliminate (Lebeaux et al., 2014).
The phylogenetic relationships of bacterial pathogens can be described by multilocus sequence typing (MLST) and clonal complex (CC) classification (Urwin and Maiden, 2003). Virulence, drug resistance, and BF traits differ across bacterial isolates of different sequence types (STs) (Kozitskaya et al., 2005; Manning et al., 2009). ST27 of Staphylococcus epidermidis occurs preferentially in hospitals, and those ST27 strains with prevalent BF appear to adapt easily to nosocomial environments (Kozitskaya et al., 2005). Relative to other STs, the ST17 and ST19 lineages of group B Streptococcus strains isolated from invasive disease cases were found to be significantly more likely to form weak biofilms (Parker et al., 2016). ST23 isolates from carbapenem-resistant K. pneumoniae bacteremia samples were found to have higher BF than ST258 isolates (Diago-Navarro et al., 2014). However, the distributions of BF traits and STs among K. pneumoniae, especially in bacteremia, are still unknown in China.
In one study, type 1 and type 3 fimbriae were found to enhance K. pneumoniae BF on urinary catheters in a catheterized bladder model (Stahlhut et al., 2012). However, in another study, type 1 fimbriae were found not to influence BF, whereas expression of type 3 fimbriae was found to strongly promote BF and to favor the development of catheter-associated K. pneumoniae infections (Schroll et al., 2010). In a study examining pyogenic liver abscess K. pneumoniae, Wu et al. (2011) found that the genes treC and sugE affect BF by modulating capsular polysaccharide production and that treC facilitates gastrointestinal tract colonization, indicating that BF contributes to the establishment and persistence of K. pneumoniae infection.
The characteristics of K. pneumoniae that are associated with BF, including virulence factor expression, have not been clarified. Thus, the aim of the present study was to explore the prevalence and characteristics of K. pneumoniae bacteremia BF and to identify virulence factors associated with BF.
A total of 250 unique K. pneumoniae bacteremia isolates were collected from patients at Shenzhen Nanshan People's Hospital, Shenzhen University and Huashan Hospital, Fudan University in China between January 2010 and August 2017. The strains were identified with a Phoenix 100 automated microbiology system (BD, Franklin Lakes, NJ, USA), and then after two subcultured generations re-identified with matrix-assisted laser desorption ionization-time of flight mass spectrometry (IVD MALDI Biotyper, Bruker, Bremen, Germany). All procedures involving human participants were performed in accordance with the ethical standards of Shenzhen University and Fudan University, and the 1964 Helsinki declaration and its later amendments. For this type of study, formal consent is not required.
Clinical data were collected for each case from an electronic medical records database. Bacteremia origin was determined, based on bacteriological sampling at the suspected origin and clinical examination reports (Picot-Guéraud et al., 2015). In cases that were unclear, a second physician was consulted. We sought to determine the infection acquisition setting in each case. If this could not be confirmed and bacteremia occurred >48 h after hospital admission, hospital-acquired infection was presumed (Picot-Guéraud et al., 2015). If the bacteremia began <48 h after admission and at least one of Friedman's criteria (i.e., intravenous chemotherapy or hemodialysis in the last 30 days; home intravenous therapy or wound care in the last 30 days; hospitalization for ≥2 days in the last 90 days; or residence in a long-term care facility) was met, then the bacteremia was considered to be healthcare associated (Culshaw et al., 2014; Picot-Guéraud et al., 2015). In all other cases, the bacteremia was considered to be community acquired.
The susceptibilities of the isolates to clinically relevant antibiotics (amikacin, cefotaxime, ceftazidime, cefepime, cefoperazone-sulbactam, chloramphenicol, ciprofloxacin, levofloxacin, gentamicin, piperacillin-tazobactam, tetracycline, imipenem, and meropenem) and extended-spectrum β-lactamase (ESBL) production were detected with a Phoenix 100 automated microbiology system (BD) (Saffert et al., 2011) or by the disk diffusion test (Chang et al., 2015). The minimal inhibitory concentrations (MICs) of imipenem, meropenem, tigecycline, and eravacycline were determined by the agar dilution method according to Clinical and Laboratory Standards Institute guidelines (Michail et al., 2013).
Strains with the hypermucoviscosity phenotype (as revealed by a positive string test result) were defined as hypervirulent K. pneumoniae; those with a negative result were defined as classic K. pneumoniae. A positive string test result was defined as the formation of a mucoviscous string of >5 mm on a bacteriology inoculation loop used to stretch a colony grown overnight on an agar plate at 37°C (Shon et al., 2013).
Bacterial DNA was extracted from isolates and purified with a DNeasy Blood and Tissue Kit (Qiagen China Co., Ltd, Shanghai, China) according to the manufacturer's protocol. MLST was conducted by the method of Diancourt et al. (2005). All primer sequences used in MLST are listed in Table S1. Briefly, seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) were PCR-amplified and sequenced from all isolates according to the K. pneumoniae MLST protocol (www.pasteur.fr/mlst). Alleles and sequence types (STs) were assigned by the MLST database (www.pasteur.fr/mlst/Kpneumoniae.html). K. pneumoniae CCs were identified by the eBURST v 3.0 program as described by Feil et al. (2004). CCs were defined as groups of two or more independent isolates that shared identical alleles at six or more loci; each complex was named after the putative founder ST (Wang et al., 2013).
K. pneumoniae BF was detected by the method of Wu et al. (2011). Briefly, 1 μl of an overnight culture was inoculated into 100 μl of fresh Luria-Bertani (LB) broth in each well of a 96-well polystyrene plate (Costar3599, Corning, New York, USA). After 5 h of static incubation at 37°C, bacteria were stained with 25 μl of 0.5% crystal violet for 20 min. The supernatant was discarded, and plates were washed three times with deionized water to remove unattached cells. The biofilm-bound dye was then eluted with 95% ethanol, and the optical density at 550 nm (OD550) was determined. The NTUH-K2044 strain, which exhibits strong BF, served as a positive control (Wu et al., 2011). Each assay was performed in triplicate on at least three occasions.
K. pneumoniae virulence genes were detected by PCR. The extracted genomic DNA from planktonic isolates served as a template for the amplification of virulence genes and for determining capsular serotypes. All PCR primer sequences and corresponding references are listed in Table S2. PCR amplification was performed in a total volume of 50 μl, containing 2 × PCR Master Mix (Tiangen Biotech Beijing Co., Ltd, Beijing, China), 0.5 mM of each primer, and 1 μl template DNA. The cycling conditions were as follows: 95°C for 3 min, followed by 30 cycles at 95°C for 30 s, 49°C−58°C for 30 s, 72°C for 60 s, and a final 10-min extension step at 72°C. Each PCR set included a no-template control and a positive control [NTUH-K2044 strain for magA(K1) and wcaG]. Amplification products were analyzed by electrophoresis in 1.0% agarose gels.
We used the antisense RNA gene silencing technique to construct wcaG antisense RNA silencing K. pneumoniae strains (Nakashima et al., 2006). The wcaG antisense RNA, which included the predicted Shine-Dalgarno sequence plus ~100 bp following the start codon, was amplified by PCR. PCR fragments were purified and digested with HindIII and BamHI endonucleases and then inserted into the hairpin structure of plasmid pHN680 for gene silencing. Correct cloning was verified by PCR and sequencing. Verified loaded plasmids were introduced into three K. pneumoniae bacteremia isolates: Kp25, Kp57, and Kp63. All strains, plasmids, and primers used for RNA silencing are listed in Tables S3, S4. RNA silencing was induced with 1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). BF was detected by crystal violet as mentioned above. Growth of planktonic bacteria was measured with an OD600 assay. All assays were performed at least in triplicate.
The expression levels of the wcaG were determined by quantitative real-time PCR (qRT-PCR). The primers used for qRT-PCR are listed in Table S4. Briefly, the total bacterial RNA was extracted by using an RNeasy Mini Kit (Qiagen China Co., Ltd, Shanghai, China). cDNA was then synthesized using a PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). Finally, qRT-PCR was performed with a SYBR Premix Ex Taq II Kit (TaKaRa, Dalian, China) on the Mastercycler ep realplex System (Eppendorf, Hamburg, Germany), with an initial incubation at 95°C for 2 min, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. Each reaction was carried out in triplicate.
For all samples, a housekeeping gene (16S rRNA gene, rrsE) was used to normalize the expression of wcaG. The threshold cycle (CT) numbers were confirmed by the detection system software, and the data were analyzed based on the 2−ΔΔCt method. The expression levels of wcaG were determined and compared with those of wildtype strains (expression = 1).
The data, which are reported as means ± standard deviations (SDs), were analyzed with Student's t-test, one-way factorial analysis of variance, or the nonparametric Mann–Whitney U-test. Virulence factor association with BF was determined with a multivariate logistic regression model constructed based on the Wald statistic. Odds ratios (ORs) are reported with 95% confidence intervals (CIs). P < 0.05 were regarded as statistically significant. All data were analyzed in SPSS version 14.0 (Chicago, IL, USA).
OD550 microplate readings of isolates after crystal violet staining ranged from 0.05 to 3.5. The median OD550 value for NTUH-K2044 (control strain) was 2.180, a value indicative of strong BF as expected (Wu et al., 2011). As reported in Table 1, the average OD550 value of the 250 examined K. pneumoniae bacteremia isolates was 1.23 ± 0.52. Biofilm-forming K. pneumoniae prevalence was similar between male and female patients and was unrelated to various coexisting conditions, but was greater among young adults under 40 years old than among seniors over 65 years old (P = 0.002, Table 1). Biofilm-forming K. pneumoniae prevalence was unrelated to infection acquisition source (community, hospital, intensive care unit, medical unit, or surgical unit), but was more common among isolates from bacteremia cases that persisted beyond 72 h despite treatment than from bacteremia cases that were cleared within 72 h of commencing treatment (P < 0.001, Table 1).
BF prevalence did not differ between antibiotic-resistant and -susceptible K. pneumoniae isolates for any of the tested antibiotic drugs (Table 2). MIC levels (MIC < 1 mg/L, 1 ≤ MIC mg/L < 4 or MIC ≥ 4 mg/L) for imipenem, meropenem, tigecycline, and eravacycline were not significantly related to BF among K. pneumoniae isolates (Table 2). The capacity for ESBL production was also unrelated to BF.
MLST of the 250 K. pneumoniae bacteremia strains yielded clear results for 187 isolates, including 65 different STs. The biofilm characteristics and numbers of isolates assigned to each ST are shown in Table S5. Because the STs widely variation among these K. pneumoniae isolates, CCs were analyzed based on STs in eBURST (Figure 1). The main CCs of the K. pneumoniae bacteremia isolates were CC23 and CC65. This study also identified ST11 as the main ST among the K. pneumoniae bacteremia isolates. Thus, further analysis of the BF characteristics of CC23, CC65, and ST11 isolates (Figure 2) indicated that BF tended to be stronger among CC23 isolates than among CC65 isolates (P < 0.001) or ST11 isolates (P < 0.001).
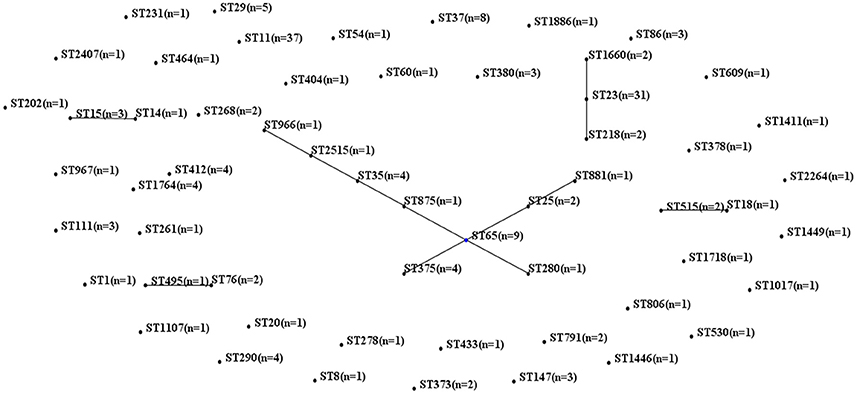
Figure 1. Characteristics of MLST and CC analysis of 187 K. pneumoniae isolates. Lines connecting STs indicate CCs, including CC23 (vertical line) and CC65 (crossing lines).
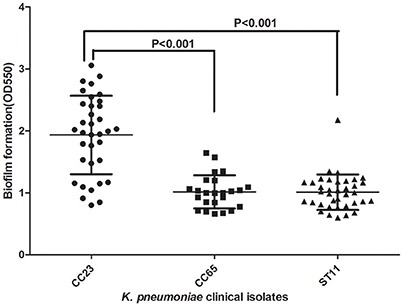
Figure 2. Comparison of BF between CC23, CC65, and ST11 isolates. A Student's t-test indicated significantly greater BF among the CC23 isolates than among the CC65 or ST11 isolates. Error bars show SD.
As reported in Table 3, analysis of PCR-amplified virulence factors showed that BF was more pronounced among magA(K1)-positive (+) isolates than among magA(K1)-negative (–) isolates. Additionally, BF was more pronounced among aero+, rmpA+, rmpA2+, allS+, wcaG+, and iutA+ isolates than among isolates that were negative (–) for these virulence factors. However, BF was not increased in hypermucoviscous or K2A(K2) serotype positive (+) isolates compared with isolates not expressing each of the two virulence factors.
In order to determine the independent contribution of each virulence factor to the K. pneumoniae bacteremia BF, multiple logistic regression analysis was conducted. As shown in Table 4, of eight independent variables [magA(K1), K2A(K2), wcaG, aero, rmpA, rmpA2, alls, and iutA] submitted to a logistic regression model constructed by a backward selection approach based on the Wald statistic (dependent variable: OD550-value ≥ 1.25), only one factor, wcaG, was found to be an independent risk factor for BF of K. pneumoniae bacteremia isolates (OR 11.426, P < 0.001). Notably, magA(K1) was not associated with the BF of K. pneumoniae bacteremia isolates.
In order to confirm the role of wcaG in the BF of K. pneumoniae bacteremia, wcaG was silenced by antisense RNA. As Figure 3A indicates, the expression levels of wcaG decreased by half following IPTG induction. RNA silencing of wcaG expression with pHN680 reduced growth, as indexed by OD600, of Kp25, Kp27, and Kp63 isolates relative to wildtype (no plasmid) controls, and the inducer IPTG (1.0 mM) had no apparent effect on the growth of the three pHN680-containing isolates (Figure 3B). Interestingly, the BF, as indexed by OD550, of Kp25 and Kp63 strains was decreased following IPTG induction, relative to controls not exposed to IPTG (Figure 3C).
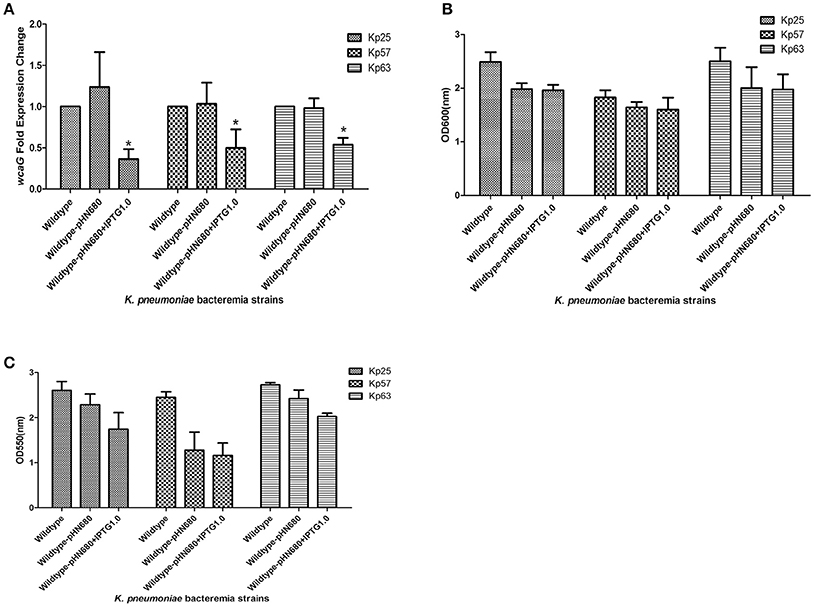
Figure 3. Silencing of wcaG expression by antisense RNA. Expression levels of wcaG were determined by qRT-PCR; *wildtype-pHN680+IPTG vs. wildtype-pHN680, P < 0.05 (Student's t-test) (A). Growth of planktonic bacteria was measured by optical density at 600 nm (B). Biofilm formation of K. pneumoniae bacteremia strains was detected by crystal violet and the optical density determined at 550 nm (C). Errors bars represent mean ± SD or three replicates.
In the present study, we found that BF was greater among isolates from young adults than among isolates from seniors. This differs somewhat from a previous study of sputum samples that found greater BF among isolates originating from patients over 70 years old than in those from patients under 70 years old (Yang and Zhang, 2008). This difference could be related to patients under 40 years old having stronger immune systems that put pressure on the bacteria to form biofilms in order to evade host immunity (Gunn et al., 2016). Our finding that BF was associated with persistent bacteremia infection after 72 h of treatment affirms that the bacteria in biofilms were very difficult to eliminate.
The relationship between K. pneumoniae BF and antibiotic resistance is uncertain. In the present study, we did not observe a significant association between BF and resistance to any one of the panel of antibiotics examined, nor between BF and ESBL production. However, Yang and Zhang (2008) reported that among 137 K. pneumoniae strains from sputum and urine, 85.0% (51/60) of biofilm-positive strains had the ability to produce ESBLs, while the rate was only 11.7% (9/77) for biofilm-negative strains, with the ESBL production rates being similar for isolates from blood and wound samples. Meanwhile, also differing from the present results, in a study of 100 urine samples, Subramanian et al. (2012) found 83.3 and 73.3% resistance rates to ampicillin and cefotaxime, respectively, among biofilm-forming isolates compared with only 60 and 35% resistance rates, respectively, among non-biofilm-forming isolates. Thus, the relationship between K. pneumoniae BF and antibiotic resistance remains controversial and needs to be further explored.
K. pneumoniae BF traits may vary among different geographical regions and STs. We observed a wide diversity of K. pneumoniae STs in our sample located in eastern and southern China. The most represented STs were ST11 (n = 37), ST23 (n = 31), and ST65 (n = 9), and the main CCs were CC23 and CC65. Prior research has shown a predominance of ST23 in east China (Wang et al., 2013; Qu et al., 2015). The ST11 type, as the most prevalent carbapenem-resistant K. pneumoniae type in China, was also recently found associated with hypervirulent or hypermucoviscous isolates of K. pneumonia (Bi et al., 2017; Zhan et al., 2017; Gu et al., 2018). However, the traits of BF among CC23, CC65, and ST11 are still unknown in China. Previously, BF ability has been reported to be higher for ST23 isolates than for ST258 isolates from carbapenem-resistant K. pneumoniae bacteremia samples (Diago-Navarro et al., 2014). The present study found that the CC23 isolates had a higher BF rate than the CC65 or ST11 isolates in China. Thus, those CC23 K. pneumoniae bacteremia isolates may be like S. epidermidis ST27 in terms of occurring preferentially in nosocomial environments and forming biofilms to better survive in adverse environmental conditions, including the presence of antibiotics and disinfectants (Kozitskaya et al., 2005; Lebeaux et al., 2014).
Currently, the role of virulence factors, such as magA(K1), K2A(K2), and hypermucoviscosity, in the BF of K. pneumoniae bacteremia remains poorly understood. The present study found that magA(K1)- or wcaG-positive isolates exhibited a greater BF ability than hypermucoviscous- or K2A(K2)-positive isolates. This is similar to previous research, which found that hypermucoviscosity was not associated with K. pneumoniae BF (Soto et al., 2017). Although BF was more pronounced in isolates that were positive for virulence factors, such as magA(K1), aero, rmpA, and rmpA2, than in isolates that were negative for those virulence factors, the present study found that only wcaG played an important role in K. pneumoniae bacteremia BF. The wcaG-positive genotype has been associated with the K1 and K54 capsular types, as well as with, to a lesser extent, the K16 and K58 capsular types (Turton et al., 2010). The isolation of K1 and K54 wcaG-positive strains from hospital origins and from patients with invasive and serious infections indicates that wcaG may contribute to the virulence of these strains. Indeed, the protein encoded by wcaG participates in the biosynthesis of fucose (Ho et al., 2011), whose inclusion as a component of the polysaccharide capsule of K. pneumoniae has been associated with bacterial virulence in mice (Wu et al., 2008). Interestingly, our study is the first to our knowledge to identify that wcaG is an important factor in K. pneumoniae bacteremia BF. However, the mechanism by which wcaG facilitates K. pneumoniae BF has not been elucidated. Prior work showing that wcaG deletion mutations affect a large portion (15/20) of capsule polysaccharide genes (Ho et al., 2011) suggests that wcaG may promote K. pneumoniae BF by altering the composition of the microbe's polysaccharide capsule.
In conclusion, the present study showed that many K. pneumoniae bacteremia strains form biofilms readily. BF tended to be greater in isolates from young adults than in those from seniors. MLST demonstrated substantial K. pneumoniae ST diversity in our sample group; the most represented STs were ST11, ST23, and ST65, and the main CCs were CC23 and CC65. BF was more strongly associated with CC23 isolates than with CC65 or ST11 isolates. We found that only wcaG was associated with K. pneumoniae bacteremia BF. This study may help to elucidate the epidemic characteristics of K. pneumoniae bacteremia biofilms and how biofilm traits are associated with virulence factors in K. pneumoniae bacteremia worldwide.
JZ: participated in the design of the study, carried out the biofilm assay and RNA silencing test, analyzed, and interpreted the data, and drafted the manuscript. ZL and ZC: performed antibiotic susceptibility testing, detected virulence genes by PCR, carried out the RNA silencing test, and participated in the data analysis. FL and YW: conducted the MLST and CC analysis, and biofilm assay, and provided a critical revision of the manuscript. XS,WY, and DL: participated in the acquisition of the samples, isolated DNA, conducted MLST and biofilm assay, and participated in the data analysis. ZY, DQ, and QD: designed the study, participated in the data analysis, and provided critical revisions of themanuscript for important intellectual content. CC: performed antibiotic susceptibility testing, detected virulence genes by PCR and participated in the data analysis. SY: conducted the MLST and CC analysis, and biofilm assay. JJ: participated in the data analysis, and provided critical revisions of the manuscript for important intellectual content.
This work was supported by grants from the National Natural Science Foundation of China (No. 81170370), Sanming Project of Medicine in Shenzhen (SMGC201705029), the Shenzhen Scientific Research Program (Nos. JCYJ20150402152130167, JCYJ20150402152130173, and JCYJ20170307153714512), the Scientific Research Project of the Shenzhen Health and Family Planning System (Nos. 201601058, 2016013, and B2017019), and the Shenzhen Nanshan District Scientific Research Program of the People's Republic of China (Nos. 2016010 and 2017015).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Prof. Jin-Town Wang and Ph.D. Zhi-Rong Lin (Department of Microbiology, National Taiwan University College of Medicine, Taipei, Taiwan, and Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan) for generously providing the K. pneumoniae NTUH-K2044 strain.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00021/full#supplementary-material
BF, biofilm formation; CI, confidence interval; CC, clonal complex; ESBL, extended spectrum β-lactamase; IPTG, isopropyl β-D-1-thiogalactopyranoside; MIC, minimal inhibitory concentration; MLST, multilocus sequence typing; OD, optical density; OR, odds ratio; PCR, polymerase chain reaction; SD, standard deviation; ST, sequence type.
Bi, W., Liu, H., Dunstan, R. A., Li, B., Torres, V. V. L., Cao, J., et al. (2017). Extensively drug-resistant Klebsiella pneumoniae causing nosocomial bloodstream infections in China: molecular investigation of antibiotic resistance determinants, informing therapy, and clinical outcomes. Front. Microbiol. 8:1230. doi: 10.3389/fmicb.2017.01230
Candan, E. D., and Aksöz, N. (2015). Klebsiella pneumoniae: characteristics of carbapenem resistance and virulence factors. Acta. Biochim. Pol. 62, 867. doi: 10.18388/abp.2015_1148
Chang, U. I., Kim, H. W., and Wie, S. H. (2015). Propensity-matched analysis to compare the therapeutic efficacies of cefuroxime versus cefotaxime as initial antimicrobial therapy for community-onset complicated nonobstructive acute pyelonephritis due to enterobacteriaceae infection in women. Antimicrob. Agents. Chemother. 59, 2488–2495. doi: 10.1128/AAC.04421-14
Chetcuti-Zammit, S., Azzopardi, N., and Sant, J. (2014). Mortality risk score for Klebsiella pneumoniae bacteraemia. Eur. J. Intern. Med. 25, 571–576. doi: 10.1016/j.ejim.2014.04.008
Culshaw, N., Glover, G., Whiteley, C., Rowland, K., Wyncoll, D., Jones, A., et al. (2014). Healthcare-associated bloodstream infections in critically ill patients: descriptive cross-sectional database study evaluating concordance with clinical site isolates. Ann. Intensive Care 4, 34. doi: 10.1186/s13613-014-0034-8
Diago-Navarro, E., Chen, L., Passet, V., Burack, S., Ulacia-Hernando, A., Kodiyanplakkal, R. P., et al. (2014). Carbapenem-resistant Klebsiella pneumoniae exhibit variability in capsular polysaccharide and capsule associated virulence traits. J. Infect. Dis. 210, 803–813. doi: 10.1093/infdis/jiu157
Diancourt, L., Passet, V., Verhoef, J., Grimont, P. A., and Brisse, S. (2005). Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43, 4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005
Feil, E. J., Li, B. C., Aanensen, D. M., Hanage, W. P., and Spratt, B. G. (2004). eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186, 1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004
Girometti, N., Lewis, R. E., Giannella, M., Ambretti, S., Bartoletti, M., Tedeschi, S., et al. (2014). Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine 93, 298–309. doi: 10.1097/MD.0000000000000111
Gu, D., Dong, N., Zheng, Z., Lin, D., Huang, M., Wang, L., et al. (2018). A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet. Infect. Dis. 18, 37–46. doi: 10.1016/S1473-3099(17)30489-9
Gunn, J. S., Bakaletz, L. O., and Wozniak, D. J. (2016). What's on the outside matters: the role of the extracellular polymeric substance of gramnegative biofilms in evading host immunity and as a target for therapeutic intervention. J. Biol. Chem. 291, 12538–12546. doi: 10.1074/jbc.R115.707547
Ho, J. Y., Lin, T. L., Li, C. Y., Lee, A., Cheng, A. N., Chen, M. C., et al. (2011). Functions of some capsular polysaccharide biosynthetic genes in Klebsiella pneumoniae NTUH K-2044. PLoS ONE 6:e21664. doi: 10.1371/journal.pone.0021664
Kozitskaya, S., Olson, M. E., Fey, P. D., Witte, W., Ohlse, K. N., and Ziebuhr, W. (2005). Clonal analysis of staphylococcus epidermidis isolates carrying or lacking biofilm-mediating genes by multilocus sequence typing. J. Clin. Microbiol. 43, 4751–4757. doi: 10.1128/JCM.43.9.4751-4757.2005
Lebeaux, D., Ghigo, J. M., and Beloin, C. (2014). Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 78, 510–543. doi: 10.1128/MMBR.00013-14
Li, W., Sun, G. Z., Yu, Y. H., Li, N., Chen, M., Jin, R. H., et al. (2014). Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin. Infect. Dis. 58, 225–232. doi: 10.1093/cid/cit675
Manning, S. D., Springman, A. C., Lehotzky, E., Lewis, M. A., Whittam, T. S., and Davies, H. D. (2009). Multilocus sequence types associated with neonatal group B streptococcal sepsis and meningitis in Canada. J. Clin. Microbiol. 47, 1143–1148. doi: 10.1128/JCM.01424-08
Meatherall, B. L., Gregson, D., Ross, T., Pitout, J. D., and Laupland, K. B. (2009). Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am. J. Med. 122, 866–873. doi: 10.1016/j.amjmed.2009.03.034
Michail, G., Labrou, M., Pitiriga, V., Manousaka, S., Sakellaridis, N., Tsakris, A., et al. (2013). Activity of tigecycline in combination with colistin, meropenem, rifampin, or gentamicin against KPC-producing enterobacteriaceae in a murine thigh infection model. Antimicrob. Agents. Chemother. 57, 6028–6033. doi: 10.1128/AAC.00891-13
Nakashima, N., Tamura, T., and Good, L. (2006). Paired termini stabilize antisense RNAs and enhance conditional gene silencing in Escherichia coli. Nucleic Acids Res. 34, e138. doi: 10.1093/nar/gkl697
Paczosa, M. K., and Mecsas, J. (2016). Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 80, 629–661. doi: 10.1128/MMBR.00078-15
Parker, R. E., Laut, C., Gaddy, J. A., Zadoks, R., Davies, H. D., and Manning, S. (2016). Association between genotypic diversity and biofilm production in group B Streptococcus. BMC Microbiol. 16:86. doi: 10.1186/s12866-016-0704-9
Picot-Guéraud, R., Batailler, P., Caspar, Y., Hennebique, A., and Mallaret, M. R. (2015). Bacteremia caused by multidrug-resistant bacteria in a French University Hospital Center: 3 years of collection. Am. J. Infect. Control 43, 960–964. doi: 10.1016/j.ajic.2015.05.004
Qu, T. T., Zhou, J. C., Jiang, Y., Shi, K. R., Li, B., Shen, P., et al. (2015). Clinical and microbiological characteristics of Klebsiella pneumoniae liver abscess in East China. BMC. Infect. Dis. 15:161. doi: 10.1186/s12879-015-0899-7
Ribeiro, S. M., Fuente-Nú-ez, C. D., Baquir, B., Faria-Junior, C., Octávio, L., Franco, O. L., et al. (2015). Antibiofilm peptides increase the susceptibility of carbapenemase-producing Klebsiella pneumoniae clinical isolates to β-lactam antibiotics. Antimicrob. Agents Chemother. 59, 3906–3912. doi: 10.1128/AAC.00092-15
Saffert, R. T., Cunningham, S. A., Ihde, S. M., Monson Jobe, K. E., Jayawant Mandrekar, J., and Patel, R. (2011). Comparison of bruker biotyper matrix-assisted laser desorption ionization–time of flight mass spectrometer to BD phoenix automated microbiology system for identification of gram-negative Bacilli. J. Clin. Microbiol. 49, 887–892. doi: 10.1128/JCM.01890-10
Schroll, C., Barken, K. B., Krogfelt, K. A., and Struve, C. (2010). Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 10:179. doi: 10.1186/1471-2180-10-179
Shon, A. S., Bajwa, R. P., and Russo, T. A. (2013). Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4, 107–118. doi: 10.4161/viru.22718
Soto, E., Dennis, M. M., Beierschmitt, A., Francis, S., Sithole, F., Halliday-Simmons, I., et al. (2017). Biofilm formation of hypermucoviscous and non-hypermucoviscous Klebsiella pneumoniae recovered from clinically affected African green monkey (Chlorocebus aethiops sabaeus). Microb. Pathog. 107, 198–201. doi: 10.1016/j.micpath.2017.03.034
Stahlhut, S. G., Struve, C., Krogfelt, K. A., and Reisner, A. (2012). Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol. Med. Microbiol. 65, 350–359. doi: 10.1111/j.1574-695X.2012.00965.x
Subramanian, P., Shanmugam, N., Sivaraman, U., Kumar, S., and Selvaraj, S. (2012). Antiobiotic resistance pattern of biofilm-forming uropathogens isolated from catheterized patients in Pondicherry, India. Australas Med. J. 5, 344–348. doi: 10.4066/AMJ.2012.1193
Turton, J. F., Perry, C., Elgohari, S., and Hampton, C. V. (2010). PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J. Med. Microbiol. 59, 541–547. doi: 10.1099/jmm.0.015198-0
Urwin, R., and Maiden, M. C. (2003). Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11, 479–487. doi: 10.1016/j.tim.2003.08.006
Wang, Q., Li, B., Tsang, A. K., Yi, Y., Woo, P. C., and Liu, C. H. (2013). Genotypic analysis of Klebsiella pneumoniae Isolates in a Beijing hospital reveals high genetic diversity and clonal population structure of drug-resistant isolates. PLoS ONE 8:e57091. doi: 10.1371/journal.pone.0057091
Wu, J. H., Wu, A. M., Tsai, C. G., Chang, X. Y., Tsai, S. F., and Wu, T. S. (2008). Contribution of fucose-containing capsules in Klebsiella pneumoniae to bacterial virulence in mice. Exp. Biol. Med. 233, 64–70. doi: 10.3181/0706-RM-170
Wu, M. C., Lin, T. L., Hsieh, P. F., Yang, H. C., and Wang, J. T. (2011). Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 6:e23500. doi: 10.1371/journal.pone.0023500
Yang, D., and Zhang, Z. (2008). Biofilm-forming Klebsiella pneumoniae strains have greater likelihood of producing extended-spectrum beta-lactamases. J. Hosp. Infect. 68, 369–371. doi: 10.1016/j.jhin.2008.02.001
Yu, W. L., Lee, M. F., Chen, C. C., Tang, H. J., Ho, C. H., and Chuang, Y. C. (2016). Impacts of hypervirulence determinants on clinical features and outcomes of bacteremia caused by extended-spectrum β-Lactamase-producing Klebsiella pneumoniae. Microb. Drug. Resist. 23, 376–383. doi: 10.1089/mdr.2016.0018
Keywords: Klebsiella pneumonia, bacteremia, biofilm formation, virulence genes, multilocus sequence typing
Citation: Zheng J, Lin Z, Chen C, Chen Z, Lin F, Wu Y, Yang S, Sun X, Yao W, Li D, Yu Z, Jin J, Qu D and Deng Q (2018) Biofilm Formation in Klebsiella pneumoniae Bacteremia Strains Was Found to be Associated with CC23 and the Presence of wcaG. Front. Cell. Infect. Microbiol. 8:21. doi: 10.3389/fcimb.2018.00021
Received: 17 September 2017; Accepted: 16 January 2018;
Published: 23 February 2018.
Edited by:
Jan Potempa, University of Louisville, United StatesReviewed by:
Haijian Zhou, National Institute for Communicable Disease Control and Prevention (China CDC), ChinaCopyright © 2018 Zheng, Lin, Chen, Chen, Lin, Wu, Yang, Sun, Yao, Li, Yu, Jin, Qu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-lin Jin, amluamlhbGluQGZ1ZGFuLmVkdS5jbg==
Di Qu, ZHF1QGZ1ZGFuLmVkdS5jbg==
Qi-wen Deng, cWl3ZW5kZW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.