- 1College of Resources, Sichuan Agricultural University, Chengdu, China
- 2Department of Cardiovascular Surgery, Xijing Hospital, Medical University of the Air Force, Xi'an, China
A widespread epidemic of Zika virus (a mosquito-borne flavivirus) infection was reported from 2015 in South and Central America. A major concern associated with the infection is the significantly increased incidence of microcephaly in fetuses born to the mothers infected with Zika virus (Mlakar et al., 2016). Researchers studying monkeys have shown that one infection with Zika virus protects the animal against future infections. Neutralizing antibodies are detected at 21 days post-infection. Re-challenge at 10 weeks after the initial inoculation resulted in no detectable viral replication, indicating successfully protective immunity against the virus (Dudley et al., 2016). They also found that non-pregnant animals could clear the virus within 10 days post-infection, however the virus persisted in the blood of pregnant monkeys for 35–70 days (Dudley et al., 2016). One possible explanation for the persistence of the virus in pregnancy is that the immune system of the mother was compromised, and she simply was not able to clear the virus as fast as the non-pregnant one. However, the pregnant animal (woman) still has a certain level of immunity. Both type I interferons and type III interferons are apparently induced by Zika virus infections, and the interferons have an ability to restrict Zika virus replication in human trophoblast cells (Bayer et al., 2016; Quicke et al., 2016). The other explanation, more provocative hypothesis is that the persistence of the virus is indicative of the fetal infection, and what they observed in the maternal serum was the shedding of virus by the fetus back into the mother's blood (Driggers et al., 2016). We cannot conclusively claim that the persistence of the virus does not reflect the fetal infection or there is no persistence found in non-pregnant animals (people), as it is possible that Zika virus may persist in immune-privilege cells (Hazlett and Hendricks, 2010). However, an analysis of the clinical data implies that the virus may take ~5 weeks to reach the fetus for most cases (Noronha et al., 2016; Soares de Souza et al., 2016; Yuan et al., 2017a). Therefore, the virus detected in the pregnant monkey within 35 days of infection is unlikely the backflow from the fetus back into the mother's bloodstream.
Viral Secretion in the Exosome may be Central to its Immune Evasion
Then why was the long-time persistence of the virus only observed in pregnancy? We previously hypothesized that Zika virus may hide in the exosome, which forms a shield against the mother's immune system (Zhang et al., 2017). There is evidenced that Hepatitis C virus, another flavivirus, can be transmitted through exosomes and utilize the autophagy pathway for viral transmission thus evading antibody-mediated immune responses (Ramakrishnaiah et al., 2013; Longatti, 2015; Shrivastava et al., 2015). Like Hepatitis C virus, Zika virus may infect trophoblast cells by entering the endoplasmic reticulum of the trophoblast to become a sort of cargo of the placental exosome (Adibi et al., 2016; Zhang et al., 2017), which is closely linked with the “secretory autophagy” process. In contrast to degradative autophagy (fusion with the lysosome), the secretory autophagy may result in secretion or expulsion of viral particles instead of their degradation (Figure 1) (Chahar et al., 2015; Ponpuak et al., 2015; Carneiro and Travassos, 2016).
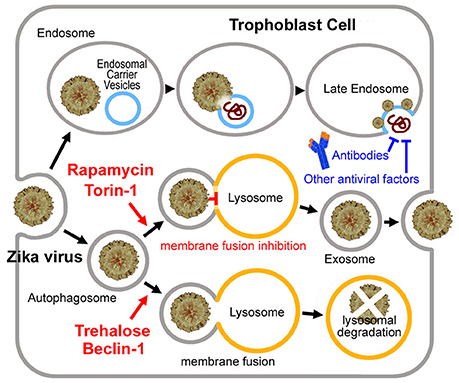
Figure 1. Putative Zika virus entry pathway and the exosome pathway in the trophoblast cell. For Zika virus, upon binding to the receptor, the virus enters the early endosome through the endocytic process. Several minutes later, the virus particle fuses with the endosomal carrier vesicle (ECV) membrane predominantly. However, for a short time, viral nucleocapsids remain trapped in the ECV lumen, until ECV fuses back with the late endosome membrane. Then the nucleocapsid is released. Alternatively, the virus may enter the autophagosome, and then to be degradated by fusing with the lysosome or become a sort of cargo of the placental exosome by blocking the autophagosome-lysosome fusion. Exosome may provide the virus with a shield from the mother's immune system. Neutralizing antibodies or other antiviral factors cannot work on the virus embedded in the exosome. Torin 1 or rapamycin induces exosome aggregation with the virus embedded in, if the lysosomal fusion step was blocked. While Beclin 1 or trehalose rescues the impaired fusion step, which results in lysosomal degradation of the virus.
The Roles of Autophagy in Flavivirus Entry and Replication
The roles of autophagy in flavivirus entry and replication have been well-studied. In general, the virus customizes autophagy proteins for efficient viral entry (Li et al., 2012; Dong and Levine, 2013; Jackson, 2015). For Hepatitis C virus, autophagy appears be required for initiation of, but not maintenance of, viral replication (Dreux et al., 2009). For Dengue virus, viral entry, replication, and translation have all been linked to the autophagic pathways (Panyasrivanit et al., 2009). Zika virus infection is accompanied with the observation of a lot of double membrane autophagosomes (Hamel et al., 2015; Souza et al., 2016; Lennemann and Coyne, 2017). While the co-detection of the virus envelope protein and the autophagy marker protein LC3 (cytosolic microtubule-associated light chain 3) has been reported (Hamel et al., 2015). Thus, some autophagosome formation inhibitors (such as 3-methyladenine and wortmannin) strongly reduced viral copy numbers in some cell lines (Nour et al., 2013; Hamel et al., 2015). However, some opposite reports suggest that the roles of autophagy in flavivirus infections are controversial (Li et al., 2016; Rolfe et al., 2016). ATG16L2 (Autophagy related 16-like 2) was identified among the top 30 down-regulated genes in human neural stem cells after the Zika virus infection (Rolfe et al., 2016). LC3 transcript was also repressed by the Zika virus infection in mouse brain cells (Li et al., 2016). However, in these two opposite reports, the gene expression analyses were performed at 56–72 h after the virus inoculation, which are late infection stages. Declines of some autophagy-related genes at the late infection stages do not mean that autophagy was inhibited at the early infection stages or at the entry steps.
Depletion of autophagy-related (ATG) protein ATG5 does not affect replication of West Nile virus in some cell lines (Vandergaast and Fredericksen, 2012). Some other reports even indicated that flavivirus replication levels were increased in autophagy-deficient cells. For example, West Nile virus replication was increased in mouse embryonic fibroblast cells depleted of ATG5 (Kobayashi et al., 2014). For Japanese Encephalitis virus, either depletion in ATG7 or deficiency in ATG5 would result in higher viral replication levels in mouse embryonic fibroblast cells (Sharma et al., 2014). However, these results do not mean that autophagy down-regulates viral replication directly. Autophagy may play a positive role in the early infection stages; however it becomes dysfunctional when the misfolded proteins accumulate at the late stages. Autophagy-deficient cells may be highly susceptible to virus-induced cell death (Sharma et al., 2014; Martín-Acebes et al., 2015). Therefore, higher viral loads were detected in these susceptible cells.
Flavivirus Infection Inhibits Autophagosome-lysosome Fusion
In the case of Dengue virus, early after the infection, basal, and activated autophagic fluxes were enhanced. However, during the established viral replication, basal, and Torin 1-activated autophagic fluxes were declined because of a block to autophagic vesicle formation and reduced autophagic degradation capacity (Metz et al., 2015). During the late stages of Dengue virus infection, autophagic vesicles increased as a result of inefficient fusion of autophagosomes with lysosomes, although the lysosomal activities were increased (Metz et al., 2015). Similar autophagosome-lysosome fusion defect may also occur in the Zika virus infection, since that a lot of genes required for the lysosomal fusion were down-regulated by the Zika virus infection (Li et al., 2016), such as RAB7 (a member of the Rab GTPase superfamily) (Pankiv et al., 2010) and genes of class C Vacuolar protein sorting (Vps)/HO motypic fusion and Protein Sorting (HOPS) tethering complex (Vps16, Vps18, Vps33) (Wurmser et al., 2000). If the lysosomal fusion step was blocked, up-regulating autophagosome formation may cause exosome aggregation with the virus embedded in Zhang et al. (2017). This assumption is supported by the fact that Torin 1 (a classic mechanistic target of rapamycin mTOR-dependent autophagy activator) greatly enhanced Zika virus replication (Hamel et al., 2015), and rapamycin treatment significantly enhanced Dengue virus replication (Lee et al., 2013; Chu et al., 2014). Because of the impaired autophagosome-lysosome fusion, the Zika virus may become a cargo of the placental exosome, other than to be degradated in the lysosome, which may be central to its immune evasion as observed in the pregnant animals (Figure 1).
Trehalose May be an Idea Drug with a High Safety
Trehalose induces autophagosome formation in an mTOR-independent pathway and rescues the impaired lysosomal fusion (Ejlerskov et al., 2013). The roles of trehalose on autophagy in some other diseases have been reported before. For example, the defect in autophagosome-lysosome fusion has been observed in the amyotrophic lateral sclerosis (ALS) model mice of motor neuron degeneration. Interestingly, like flavivirus infections, rapamycin showed adverse effects to the ALS disease progression (Zhang et al., 2011). On the contrary, trehalose rescued the impaired fusion step, which resulted in aggregated autophagic degradation in the motor neurons. Trehalose is able to attenuate the autophagic flux defect and improve ALS disease course (Zhang et al., 2014; Yuan et al., 2017b). Besides ALS, a similar block to the autophagy-lysosome degradative pathway has been also reported in the mouse model of human tauopathy. Stimulation of autophagy by trehalose reduced tau aggregates and improved neuronal survival in the cerebral cortex and the brainstem (Schaeffer et al., 2012). Trehalose also enhances the degradative capacity of macrophages and is considered as a therapy for atherosclerotic vascular disease (Sergin et al., 2017). Therapeutic effects of trehalose on virus infections have been proved recently that trehalose had a profound inhibitory effect on Human cytomegalovirus replication and strongly inhibited viral spread through activating degradative autophagy presumably (Belzile et al., 2015).
As discussed in our previous analysis (Yuan et al., 2017a), the placental transfer of Zika virus may be a time-consuming process. The virus may take about 5 weeks to reach the fetus (or over 12 weeks, if the infection occurs early in pregnancy). People could postulate that if most Zika virus was cleared before it reaches the fetus, the incidence of microcephaly may be largely decreased. Trehalose promotes the autophagosome-lysosome fusion and prevents the virus from entering the exosome, and therefore induces viral degradation or makes the virus exposed to the mother's immune system (Figure 1). Hence the trehalose treatment might help to clear the virus within 5 weeks. Trehalose therefore may be useful for early Zika virus infections. However, trehalose may not prevent Zika virus induced microcephaly in the late infection stages when the virus has been already reached the fetus. Considering that the mother's immune system would spend more than 10 days to clear the virus, trehalose treatment should be applied as soon as possible after the infection.
During pregnancy, when the number of candidate drugs is exceedingly limited and the bar for the clinical approval is extremely high, people must be very cautious when testing any potential therapies that could be used in human pregnancy. Trehalose is non-reducing disaccharide, with stable chemical property and multiple protective effects to organisms and biological macromolecules (Richards et al., 2002). It is a kind of food, but not a drug, and does not produce any significant side effects. Trehalose at effective intracellular concentrations does not impair of mouse or rat fetus development or show any teratogenic effect (Richards et al., 2002; Eroglu et al., 2003). However, clinical trials to assess its embryotoxicity or teratogenicity to humans are still lacking. Therefore, only after careful toxicological tests in humans, trehalose treatment could be used as a promising therapy for the pregnant women infected with Zika virus.
Future Clinical Applications
The optimal dosage of trehalose for humans needs clinical investigations. In the mouse experiments, 2% trehalose containing water was given to the mouse through ad-libitum consumption, and these oral administrations showed significant therapeutic effects (Schaeffer et al., 2012; Zhang et al., 2014). Whether similar trehalose treatments should be given for humans needs further studies. There is no recommendation from the FDA on this sugar. It would be well to follow the WHO guideline and restrict intake of all sugars to 50 g per day (46). The normal person daily potable water quantity is about 1,500–2,500 ml. Two percentages trehalose means 30–50 g per day. Thus, the WHO guideline may be a feasible one. However, pregnant women also intake other sugars from their diets. High maternal intake of free sugars during pregnancy is associated with increased risks of many diseases in the offspring, such as atopic asthma and allergic asthma (Bédard et al., 2017; Torjesen, 2017). Appropriate oral dose needs further studies.
Nevertheless, a large part of trehalose would be broken down into glucose by the trehalase on the intestinal mucosa (Richards et al., 2002), oral administration may not be a very effective method. Trehalose injection might be an alternative option (Echigo et al., 2012; Sergin et al., 2017), although the tests in the rhesus macaque model should be performed before the advancement to human clinical trials.
Author Contributions
SY coordinated the writing and wrote this manuscript together with inputs form all other listed co-authors. All authors made equal contributions in finalizing this manuscript.
Funding
This work was funded by the Preeminent Youth Fund of Sichuan Province (2015JQO045).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SR and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
Adibi, J. J., Marques, E. T. Jr., Cartus, A., and Beigi, R. H. (2016). Teratogenic effects of the Zika virus and the role of the placenta. Lancet 387, 1587–1590. doi: 10.1016/S0140-6736(16)00650-4
Bayer, A., Lennemann, N. J., Ouyang, Y., Bramley, J. C., Morosky, S., Marques, E. T. Jr., et al. (2016). Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19, 705–712. doi: 10.1016/j.chom.2016.03.008
Bédard, A., Northstone, K., Henderson, A. J., and Shaheen, S. O. (2017). Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur. Respir. J. 50:1700073. doi: 10.1183/13993003.00073-2017
Belzile, J. P., Sabalza, M., Craig, M., Clark, E., Morello, C. S., and Spector, D. H. (2015). Trehalose, an mTOR-independent inducer of autophagy, inhibits human cytomegalovirus infection in multiple cell types. J. Virol. 90, 1259–1277. doi: 10.1128/JVI.02651-15
Carneiro, L. A., and Travassos, L. H. (2016). Autophagy and viral diseases transmitted by Aedes aegypti and Aedes albopictus. Microbes Infect. 18, 169–171. doi: 10.1016/j.micinf.2015.12.006
Chahar, H. S., Bao, X., and Casola, A. (2015). Exosomes and their role in the lifecycle and pathogenesis of RNA viruses. Viruses 7, 3204–3225. doi: 10.3390/v7062770
Chu, L. W., Huang, Y. L., Lee, J. H., Huang, L. Y., Chen, W. J., Lin, Y. H., et al. (2014). Single-virus tracking approach to reveal the interaction of Dengue virus with autophagy during the early stage of infection. J. Biomed. Opt. 19:011018. doi: 10.1117/1.JBO.19.1.011018
Dong, X., and Levine, B. (2013). Autophagy and viruses: adversaries or allies? J. Innate Immun. 5, 480–493. doi: 10.1159/000346388
Dreux, M., Gastaminza, P., Wieland, S. F., and Chisari, F. V. (2009). The autophagy machinery is required to initiate hepatitis C virus replication. Proc. Natl. Acad. Sci. U.S.A. 106, 14046–14051. doi: 10.1073/pnas.0907344106
Driggers, R. W., Ho, C. Y., Korhonen, E. M., Kuivanen, S., Jääskeläinen, A. J., Smura, T., et al. (2016). Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142–2151. doi: 10.1056/NEJMoa1601824
Dudley, D. M., Aliota, M. T., Mohr, E. L., Weiler, A. M., Lehrer-Brey, G., Weisgrau, K. L., et al. (2016). A rhesus macaque model of asian-lineage Zika virus infection. Nat. Commun. 7:12204. doi: 10.1038/ncomms12204
Echigo, R., Shimohata, N., Karatsu, K., Yano, F., Kayasuga-Kariya, Y., Fujisawa, A., et al. (2012). Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J. Transl. Med. 10:80. doi: 10.1186/1479-5876-10-80
Ejlerskov, P., Rasmussen, I., Nielsen, T. T., Bergström, A. L., Tohyama, Y., Jensen, P. H., et al. (2013). Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion. J. Biol. Chem. 288, 17313–17335. doi: 10.1074/jbc.M112.401174
Eroglu, A., Lawitts, J. A., Toner, M., and Toth, T. L. (2003). Quantitative microinjection of trehalose into mouse oocytes and zygotes, and its effect on development. Cryobiology 46, 121–134. doi: 10.1016/S0011-2240(03)00018-X
Hamel, R., Dejarnac, O., Wichit, S., Ekchariyawat, P., Neyret, A., Luplertlop, N., et al. (2015). Biology of Zika virus infection in human skin cells. J. Virol. 89, 8880–8896. doi: 10.1128/JVI.00354-15
Hazlett, L. D., and Hendricks, R. L. (2010). Reviews for immune privilege in the year 2010: immune privilege and infection. Ocul. Immunol. Inflamm. 18, 237–243. doi: 10.3109/09273948.2010.501946
Jackson, W. T. (2015). Viruses and the autophagy pathway. Virology 479–480, 450–456. doi: 10.1016/j.virol.2015.03.042
Kobayashi, S., Orba, Y., Yamaguchi, H., Takahashi, K., Sasaki, M., Hasebe, R., et al. (2014). Autophagy inhibits viral genome replication and gene expression stages in West Nile virus infection. Virus Res. 191, 83–91. doi: 10.1016/j.virusres.2014.07.016
Lee, Y. R., Hu, H. Y., Kuo, S. H., Lei, H. Y., Lin, Y. S., Yeh, T. M., et al. (2013). Dengue virus infection induces autophagy: an in vivo study. J. Biomed. Sci. 20:65. doi: 10.1186/1423-0127-20-65
Lennemann, N. J., and Coyne, C. B. (2017). Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 13, 322–332. doi: 10.1080/15548627.2016.1265192
Li, C., Xu, D., Ye, Q., Hong, S., Jiang, Y., Liu, X., et al. (2016). Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120–126. doi: 10.1016/j.stem.2016.04.017
Li, J. K., Liang, J. J., Liao, C. L., and Lin, Y. L. (2012). Autophagy is involved in the early step of Japanese encephalitis virus infection. Microbes Infect. 14, 159–168. doi: 10.1016/j.micinf.2011.09.001
Longatti, A. (2015). The dual role of exosomes in Hepatitis, A., and C virus transmission and viral immune activation. Viruses 7, 6707–6715. doi: 10.3390/v7122967
Martín-Acebes, M. A., Blázquez, A. B., and Saiz, J. C. (2015). Reconciling West Nile virus with the autophagic pathway. Autophagy 11, 861–864. doi: 10.1080/15548627.2015.1037062
Metz, P., Chiramel, A., Chatel-Chaix, L., Alvisi, G., Bankhead, P., Mora-Rodriguez, R., et al. (2015). Dengue virus inhibition of autophagic flux and dependency of viral replication on proteasomal degradation of the autophagy receptor p62. J. Virol. 89, 8026–8041. doi: 10.1128/JVI.00787-15
Mlakar, J., Korva, M., Tul, N., Popović, M., Poljšak-Prijatelj, M., Mraz, J., et al. (2016). Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958. doi: 10.1056/NEJMoa1600651
Noronha, L. D., Zanluca, C., Azevedo, M. L., Luz, K. G., and Santos, C. N. (2016). Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem. Inst. Oswaldo Cruz 111, 287–293. doi: 10.1590/0074-02760160085
Nour, A. M., Li, Y., Wolenski, J., and Modis, Y. (2013). Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS Pathog. 9:e1003585. doi: 10.1371/journal.ppat.1003585
Pankiv, S., Alemu, E. A., Brech, A., Bruun, J. A., Lamark, T., Overvatn, A., et al. (2010). FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol. 188, 253–269. doi: 10.1083/jcb.200907015
Panyasrivanit, M., Khakpoor, A., Wikan, N., and Smith, D. R. (2009). Linking dengue virus entry and translation/replication through amphisomes. Autophagy 5, 434–435. doi: 10.4161/auto.5.3.7925
Ponpuak, M., Mandell, M. A., Kimura, T., Chauhan, S., Cleyrat, C., and Deretic, V. (2015). Secretory autophagy. Curr. Opin. Cell Biol. 35, 106–116. doi: 10.1016/j.ceb.2015.04.016
Quicke, K. M., Bowen, J. R., Johnson, E. L., McDonald, C. E., Ma, H., O'Neal, J. T., et al. (2016). Zika virus infects human placental macrophages. Cell Host Microbe 20, 83–90. doi: 10.1016/j.chom.2016.05.015
Ramakrishnaiah, V., Thumann, C., Fofana, I., Habersetzer, F., Pan, Q., de Ruiter, P. E., et al. (2013). Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. U.S.A. 110, 13109–13113. doi: 10.1073/pnas.1221899110
Richards, A. B., Krakowka, S., Dexter, L. B., Schmid, H., Wolterbeek, A. P., Waalkens-Berendsen, D. H., et al. (2002). Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 40, 871–898. doi: 10.1016/S0278-6915(02)00011-X
Rolfe, A. J., Bosco, D. B., Wang, J., Nowakowski, R. S., Fan, J., and Ren, Y. (2016). Bioinformatic analysis reveals the expression of unique transcriptomic signatures in Zika virus infected human neural stem cells. Cell Biosci. 6, 42. doi: 10.1186/s13578-016-0110-x
Schaeffer, V., Lavenir, I., Ozcelik, S., Tolnay, M., Winkler, D. T., and Goedert, M. (2012). Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 135, 2169–2177. doi: 10.1093/brain/aws143
Sergin, I., Evans, T. D., Zhang, X., Bhattacharya, S., Stokes, C. J., and Song, E. (2017). Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 8:15750. doi: 10.1038/ncomms15750
Sharma, M., Bhattacharyya, S., Nain, M., Kaur, M., Sood, V., Gupta, V., et al. (2014). Japanese encephalitis virus replication is negatively regulated by autophagy and occurs on LC3-I- and EDEM1-containing membranes. Autophagy 10, 1637–1651. doi: 10.4161/auto.29455
Shrivastava, S., Devhare, P., Sujijantarat, N., Steele, R., Kwon, Y. C., Ray, R., et al. (2015). Knockdown of autophagy inhibits infectious hepatitis C virus release by the exosomal pathway. J. Virol. 90, 1387–1396. doi: 10.1128/JVI.02383-15
Soares de Souza, A., Moraes Dias, C., Braga, F. D., Terzian, A. C., Estofolete, C. F., Oliani, A. H., et al. (2016). Fetal infection by Zika virus in the third trimester: report of 2 cases. Clin. Infect. Dis. 63, 1622–1625. doi: 10.1093/cid/ciw613
Souza, B. S., Sampaio, G. L., Pereira, C. S., Campos, G. S., Sardi, S. I., Freitas, L. A., et al. (2016). Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci. Rep. 6:39775. doi: 10.1038/srep39775
Torjesen, I. (2017). Sugar intake in pregnancy is linked to child's allergy and allergic asthma. BMJ 358:j3293. doi: 10.1136/bmj.j3293
Vandergaast, R., and Fredericksen, B. L. (2012). West Nile Virus (WNV) replication is independent of autophagy in mammalian cells. PLoS ONE 7:e45800. doi: 10.1371/journal.pone.0045800
Wurmser, A. E., Sato, T. K., and Emr, S. D. (2000). New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J. Cell Biol. 151, 551–562. doi: 10.1083/jcb.151.3.551
Yuan, S., Luo, Q., Zhang, Z. W., and Li, Z. L. (2017a). Commentary: Teratogenic effects of the Zika virus and the role of the placenta. Front. Cell Infect. Microbiol. 7:62. doi: 10.3389/fcimb.2017.00062
Yuan, S., Zhang, Z. W., and Li, Z. L. (2017b). Cell death-autophagy loop and glutamate-glutamine cycle in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 10:231. doi: 10.3389/fnmol.2017.00231
Zhang, X., Chen, S., Song, L., Tang, Y., Shen, Y., Jia, L., et al. (2014). MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy 10, 588–602. doi: 10.4161/auto.27710
Zhang, X., Li, L., Chen, S., Yang, D., Wang, Y., Zhang, X., et al. (2011). Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy 7, 412–425. doi: 10.4161/auto.7.4.14541
Keywords: autophagy, exosome, lysosomal fusion, trehalose, Zika virus
Citation: Yuan S, Zhang Z-W and Li Z-L (2017) Trehalose May Decrease the Transmission of Zika Virus to the Fetus by Activating Degradative Autophagy. Front. Cell. Infect. Microbiol. 7:402. doi: 10.3389/fcimb.2017.00402
Received: 28 June 2017; Accepted: 25 August 2017;
Published: 06 September 2017.
Edited by:
Shelton S. Bradrick, University of Texas Medical Branch, United StatesReviewed by:
Ravi Jhaveri, University of North Carolina Hospitals, United StatesShannan Rossi, University of Texas Medical Branch, United States
Copyright © 2017 Yuan, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Yuan, cm91bmR0cmVlMzE4QGhvdG1haWwuY29t
 Shu Yuan
Shu Yuan Zhong-Wei Zhang1
Zhong-Wei Zhang1