
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell. Infect. Microbiol. , 05 September 2017
Sec. Parasite and Host
Volume 7 - 2017 | https://doi.org/10.3389/fcimb.2017.00390
This article is part of the Research Topic Tick-host-pathogen Interactions View all 39 articles
Ticks transmit a greater variety of pathogenic agents that cause disease in humans and animals than any other haematophagous arthropod, including Lyme disease, Rocky Mountain spotted fever, human granulocytic anaplasmosis, babesiosis, tick-borne encephalitis, Crimean Congo haemorhagic fever, and many others (Gulia-Nuss et al., 2016). Although diverse explanations have been proposed to explain their remarkable vectorial capacity, among the most important are their blood feeding habit, their long term off-host survival, the diverse array of bioactive molecules that disrupt the host's natural hemostatic mechanisms, facilitate blood flow, pain inhibitors, and minimize inflammation to prevent immune rejection (Hajdušek et al., 2013). Moreover, the tick's unique intracellular digestive processes allow the midgut to provide a relatively permissive microenvironment for survival of invading microbes. Although tick-host-pathogen interactions have evolved over more than 300 million years (Barker and Murrell, 2008), few microbes have been able to overcome the tick's innate immune system, comprising both humoral and cellular processes that reject them. Similar to most eukaryotes, the signaling pathways that regulate the innate immune response, i.e., the Toll, IMD (Immunodeficiency) and JAK-STAT (Janus Kinase/ Signal Transducers and Activators of Transcription) also occur in ticks (Gulia-Nuss et al., 2016). Recognition of pathogen-associated molecular patterns (PAMPs) on the microbial surface triggers one or the other of these pathways. Consequently, ticks are able to mount an impressive array of humoral and cellular responses to microbial challenge, including anti-microbial peptides (AMPs), e.g., defensins, lysozymes, microplusins, etc., that directly kill, entrap or inhibit the invaders. Equally important are cellular processes, primarily phagocytosis, that capture, ingest, or encapsulate invading microbes, regulated by a primordial system of thioester-containing proteins, fibrinogen-related lectins and convertase factors (Hajdušek et al., 2013). Ticks also express reactive oxygen species (ROS) as well as glutathione-S-transferase, superoxide dismutase, heat shock proteins and even protease inhibitors that kill or inhibit microbes. Nevertheless, many tick-borne microorganisms are able to evade the tick's innate immune system and survive within the tick's body. The examples that follow describe some of the many different strategies that have evolved to enable ticks to transmit the agents of human and/or animal disease.
Borrelia burgdorferi (sensu latu), the causative agent of Lyme disease, is a spirochete, a Gram-negative helically coiled bacterium approximately 0.5 μm wide by 20 μm long, with a flagellum below the outer membrane that controls its whiplash-like movements.
These bacteria are transmitted by ticks of the genus Ixodes, especially I. scapularis in North America and I. ricinus and I. persulcatus in Europe and Asia. In its tick host, B. burgdorferi is an intercellular pathogen, i.e., it survives in the midgut lumen, then migrates between the midgut's epithelial cells into the hemolymph and then into the salivary gland ducts. Bacteria are acquired by the tick host during blood feeding. Larval ticks ingest spirochetes while feeding on small mammals, especially white-footed mice. During and after feeding, the spirochetes remain within the midgut lumen. Those spirochetes trapped outside of the rapidly developing peritrophic membrane are unlikely to survive. Others between the membrane and midgut epithelial cells use an external surface lipoprotein, OspA, to bind to a species-specific receptor, TROSPA, located on the luminal surfaces of the midgut epithelial cells (Pal et al., 2004). Although surviving spirochetes may undergo an initial phase of multiplication, these populations decline during the tick's post-feeding molt cycle, perhaps due to antimicrobial effects of by-products of hemoglobin digestion, competition for nutrients and other unknown factors. Following molting to the nymphal stage, feeding by the recently molted nymph stimulates surviving spirochetes to begin prolific multiplication and attempt migration out of the midgut lumen (reviewed by Ogden et al., 2014) During this initial phase, the spirochetes form a complex network that moves between the epithelial cells toward their basolateral surfaces. OspA expression is reduced, allowing spirochetes to detach. Subsequently, they transition to the second phase of midgut migration, in which the spirochetes become motile, separate and escape through the basement membranes into the hemocoel (Dunham-Ems et al., 2009). Some host derived factors also play a role in the process, e.g., host-derived plasminogen, which protects the bacteria against phagocytosis and possibly even enhances their ability to penetrate the basement membrane (Coleman et al., 1997). Bacterial enolase was reported as the surface receptor that binds to the midgut receptor, Tre31, which facilitates migration out of the midgut (Zhang et al., 2011). It also binds host-derived plasminogen in the midgut and degrades it to plasmin (Noguiera et al., 2012). Borreliae upregulate OspC, which also binds and immobilizes plasmin, the enzymatically active form, which further enhances degrading intercellular matrices and other barriers, such as basement membranes (Önder et al., 2012). Nevertheless, the vast majority of spirochetes migrating into the hemolymph are destroyed, mostly by phagocytic hemocytes (Coleman et al., 1997). Upon contact with the salivary glands, borreliae bind to SALP15 (Ramamurthy et al., 2011), an immunosuppressive factor that protects these spirochetes from antibody-mediated killing, as well as other salivary gland proteins e.g., tick salivary lectin pathway (Schuijt et al., 2011) and tick histamine release factors (Dai et al., 2010) that also protect these spirochetes from host immune reactions (de Silva et al., 2009; Hajdušek et al., 2013). Exploitation of host-derived factors that enable B. burgdorferi to multiply and evade innate immune attack suggests that ticks tolerate these pathogens in the midgut but not in the hemolymph and other body tissues. Nevertheless, many questions remain, especially how the spirochetes are able to penetrate between the tightly bound midgut epithelial cells, avoid triggering expression or upregulation of antimicrobial peptides, and how they the penetrate salivary gland acini for dissemination into vertebrate hosts. Overarching factors directed by the tick microbiome (Narasimhan and Fikrig, 2015) will also be important when examining the infection of and transmission by ticks.
Little is known about the specific infection mechanisms of spotted fever group (SFG) Rickettsia and their tick hosts (Munderloh and Kurtti, 1995, compared with the mammalian host cell. These bacteria, as well as other species of the Rickettsiales, invade host cells by binding to cellular receptors by means of their outer surface cell antigens (sca0 or rOmpAa and sca5 or rOmpB) and are internalized by receptor-mediated endocytosis via clathrin-coated vesicles, whereupon the microbes are incorporated into phagosomes (Chan et al., 2010). A similar sca5-mediated invasion mechanism used for vertebrate cells is employed by rickettsiae for invasion of tick cells (Thepparit et al., 2010). Upon invasion, rickettsiae quickly lyse these inclusions to escape into the cytosol. Once in the cytosol, rickettsiae replicate and then hijack the host cell's actin cytoskeleton and attach to it via actin tails (Gouin et al., 2005). The actin protein complex Arp2/3 is essential for the internalization of R. rickettsii, as well as other known SFG rickettsiae (Petchampai et al., 2014). In the vertebrate host cell, the bacteria express rickA, which promotes the activation of the host cell actin complex. This enables these bacteria to be propelled throughout the host cells as well as into protrusions that mediate cell to cell infection, thereby spreading the infection throughout the surrounding tissues (Gouin et al., 2004; Jeng et al., 2004). These actions are also effected by other cell proteins—profilin, fimbrin/T-plastin, capping protein, and cofilin, essential to actin assembly (Serio et al., 2010). In contrast to R. rickettsii which spread by means of actin bridges, R. parkeri and perhaps other rickettsial species, manipulate the intercellular tensions and mechano-transduction between host cells to facilitate their spread (Lampson et al., 2016). The roles of specific Sca molecules in facilitating rickettsial dissemination within the vector are under investigation. However, the host cell is not without a defense, as it is appreciated that ticks respond to rickettsiae (Macaluso et al., 2003; Mulenga et al., 2003). Using a tick cell culture (ISE6), investigators observed that pathogen infection led to decreased glucose metabolism but increased subolesin and heat shock protein expression, limiting rickettsial infection (Gillespie et al., 2012).
These bacteria employ a novel strategy for invading their host cells, evading cellular killing actions, manipulating the host cell's molecular machinery, and creating protected enclosures for their development and multiplication. In the tick, as well as in their vertebrate hosts, these bacteria avoid recognition by the host's innate immune system because they lack either peptidoglycans or lipopolysaccharides in their cell walls (Rikihisa, 2010). A. phagocytophilum bacteria also avoid the clathrin-receptor mediated endocytosis and phagolysosome typically used by host cells to capture and destroy invasive microbes. Instead, these bacteria are internalized via caveolae-mediated endocytosis: bacteria interact with caveolae and glycosylphosphatidylinositol-anchored proteins which enables them to bypass conventional phagolysosomes and form specialized endosomes. Bacterial outer surface protein MSP2 induces host intracellular signaling via an extracellular stimulation membrane receptor which induces recruitment of endocytic machinery at the binding site. This response leads to “zippering” around the pathogen and internalizing the microbe inside the host cell (Ireton, 2013). In tick cell cultures, A. phagocytophilum adhere to the cell membranes within 40 min post-infection. After binding, bacteria invade the host cells and form the specialized membrane-bound enclosures known as morulae. A mutant form of O-methyltransferase, identified as Msp4, also facilitates infection of the tick cells by A. phagocytophilum (Oliva Chávez et al., 2015). Within morulae, bacteria downregulate NADPH expression in enclosures, thereby minimizing reactive oxygen species (ROS) by suppressing expression of glutathione-s-transferase, superoxide dismutase and heat shock proteins (IJdo and Mueller, 2004). Like other rickettsiae, A. phagocytophilum alters cell gene expression (spectrin, fodrin) to control actin synthesis and remodel the host cell's cytoskeleton (de la Fuente et al., 2016). In mammalian cells, A. phagocytophilum hijacks host cholesterol to use in building the membrane surrounding the morulae (Xiong et al., 2009); however, whether this also occurs in tick cells is not known. In addition to these common strategies for infecting their hosts, A. phagocytophilum also exhibit more selective responses to different tissues. To infect the tick's midgut epithelial cells, these bacteria express genes that upregulate the JAK/STAT pathway, thereby inhibiting cell apoptosis (Ayllón et al., 2015). In addition, Cabezas-Cruz et al. (2016) suggest that A. phagocytophilum manipulates the host cell's epigenetics, increasing expression of histone deacetylase, Sirtuin and other molecules, which inhibits apoptosis and facilitates the microbe's multiplication. In addition, A. phagocytophilum infection can increase the levels of the tick's histone-modifying enzymes which makes it possible to regulate transcription and apoptosis selectively in different tissues, thereby not only facilitating both the pathogen's and the tick's survival. After escaping from the midgut, a salivary protein, P11, enables the microbes to infect circulating hemocytes, thereby enabling their migration to the salivary glands (Liu et al., 2011). Upon contact of infected hemocytes with the salivary gland cells, they induce expression of the salivary gland gene salp16 (Sukumaran et al., 2006), facilitating binding to the target cells. Following invasion of these host cells, the bacteria suppress the apoptotic mechanism by downregulating host cell porin expression, resulting in inhibition of cytochrome C release and thereby enabling their survival in the salivary glands. By upregulating this gene, the bacteria are able to selectively regulate transcription of this gene in association with RNAPII and the TATA-binding protein. However, the tick host is not without defenses. Recent work by Shaw et al. (2017), demonstrates a role for the tick IMD pathway in restricting A. phagocytophilum colonization of I. scapularis. Likewise, to control infection, tick salivary gland cells may limit A. phagocytophilum infection by inducing apoptosis via the extrinsic apoptosis pathway. Thus, in contrast to the midgut, these bacteria had considerably lower impact on salivary gland cells, presumably because they do not develop or multiply in that organ (Ayllón et al., 2015). Understanding the immune-related factors coordinating the balance between restriction and colonization of the vector is central to understanding vector competence. An illustration of how these pathogenic bacteria invade the tick host cells, multiply and prepare for transmission to their vertebrate hosts is shown in Figure 1.
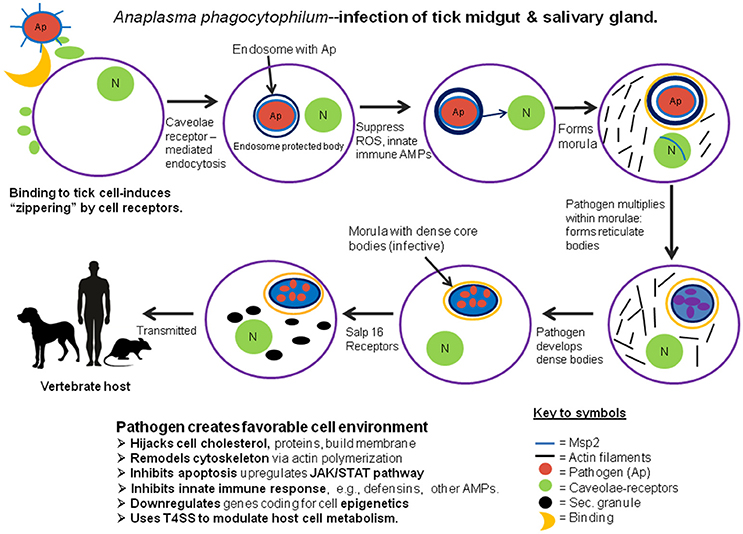
Figure 1. Diagrammatic representation of the process of invasion and development of the pathogenic microbe, Anaplasma phagocytophilum in the midgut and salivary glands of its tick host.
This eukaryotic microbe is the causative agent of human babesiosis, and is closely related to similar protozoans that cause deadly febrile disease in cattle and other livestock throughout the world. B. microti parasites are transmitted to humans during blood feeding of its vector, I. scapularis. After inoculation into the human host, the parasites (in the form of sporozoites) invade and undergo their development in the erythrocytes. These apicomplexan parasites express a membrane protein, apical membrane antigen 1 (AMA1), located near its apical end of its cell body that binds to the surfaces of the red cells, facilitating invasion (Moitra et al., 2015). Within the erythrocytes, the parasites transform into trophozoites (vegetative stage), divide into 2–4 merozoites. The merozoites lyse the host cells, escape into the blood plasma and invade other red blood cells. In their new host red blood cells, some mature into gametocytes. During tick blood feeding, the ingested erythrocytes are lysed in the tick's midgut lumen, liberating the B. microti gametocytes. The latter give rise to gametes, some of which develop a spike-like arrowhead organelle. These arrowhead-bearing gametes are known as strahlenkorpers. These gametes fuse to become zygotes. Zygotes metamorphose into elongated, motile 8–10 μm parasites, which proceed to invade the tick's midgut epithelial cells. To penetrate the peritrophic membrane, B. microti use their spike-like arrowhead organelles to rupture the membrane and allow these microbes to cross the membrane and access the lining epithelial cells. Upon contact with the midgut cells, contact by the arrowheads induces the membranes to invaginate around the babesias and allows them to invade the host cells (Rudzinska et al., 1979). Once inside the midgut cells, the arrowhead organelle is lysed and disappears (Rudzinska et al., 1983). Little is known about the life cycle of the parasite within the tick tissues, especially how they suppress or evade recognition by the tick's immune system in order to develop within the midgut cells. Ultimately, the parasites emerge from the midgut epithelium, transform into motile kinetes that escape into the hemolymph and invade the tick's salivary glands. Following invasion of the salivary glands, the microbes transform into sporoblasts. Development is arrested until the tick, usually a nymph, feeds again, whereupon thousands of sporozoites are produced from each sporoblast. The sporozoites are the infectious stage for the vertebrate host. Sporozoites are transmitted to the new host during tick feeding, whereupon they invade and develop within the host's erythrocytes.
DS wrote the preliminary draft. KM edited the draft and contributed additional information and related references. DS designed the original figure. KM revised the figure to emphasize clarity of the infection process and progress toward transmission, as well as improving the image quality. Both authors edited and accepted the final draft.
Parts of this review were done with support from a grant R01 NIH AI077784.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ayllón, N., Villar, M., Galindo, R. C., Kocan, K. M., Šíma, R., López, J. A., et al. (2015). Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis. PLoS Genet. 11:e1005120. doi: 10.1371/journal.pgen.1005120
Barker, S. C., and Murrell, A. (2008). “Systematics and evolution of ticks with a list of valid genus and species names,”in Ticks. Biology, Disease and Control, eds A. S. Bowman and P. A. Nuttall (Cambridge: Cambridge University Press), 1–39.
Cabezas-Cruz, A., Albedo, P., Ayllón, N., Valdés, J. J., Pierce, R., Villar, M., et al. (2016). Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 11, 303–319. doi: 10.1080/15592294.2016.1163460
Chan, Y. G. Y., Cardwell, M. M., Hermanas, M., Uchiyama, T., and Martinez, J. J. (2010). Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, cCbl clathrin and caveolin 2-dependent manner. Cell. Microbiol. 11, 629–644. doi: 10.1111/j.1462-5822.2008.01279.x
Coleman, J. L., Gebbia, J. A., Pressman, J., Degen, J. L., Buggy, T. H., and Benaco, J. L. (1997). Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89, 1111–1119. doi: 10.1016/S0092-8674(00)80298-6
Dai, J., Narasimhan, S., Zhang, L., Liu, L., Wang, P., and Fikrig, E. (2010). Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the Lyme disease agent. PLoS Pathog. 6:e1001205. doi: 10.1371/journal.ppat.1001205
de la Fuente, J., Villar, M., Cabezas-Cruz, A., Estrada-Peña, A., Ayllón, N., and Alberdi, P. (2016). Tick-host-pathogen interactions: conflict and cooperation. PLoS Pathog. 12:e1005488. doi: 10.1371/journal.ppat.1005488
de Silva, A. M., Tyson, K. R., and Pal, U. (2009). Molecular characterization of the tick-Borrelia interface. Front. Biosci. (Landmark Ed). 14, 3051–3063. doi: 10.2741/3434
Dunham-Ems, S. M., Caimano, M. J., Pal, U., Wolgemuth, C. W., Eggers, C. H., Basic, A., et al. (2009). Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 119:3652–3665. doi: 10.1172/JCI39401
Gillespie, J. J., Joardar, V., Williams, K. P., Driscoll, T., Hostetler, J. B., Nordberg, E., et al. (2012). A Rickettsia genome overrun by mobile genetic elements provides insight into the acquisition of genes characteristic of an obligate intracellular lifestyle. J. Bacteriol. 194, 376–394. doi: 10.1128/JB.06244-11
Gouin, E., Egile, C., Dehoux, P., Villiers, V., Adams, J., Gertler, F., et al. (2004). The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427, 457–461. doi: 10.1038/nature02318
Gouin, E., Welch, M. D., and Cossart, P. (2005). Actin-based motility of intracellular pathogens. Curr. Opin. Microbiol. 8, 35–45. doi: 10.1016/j.mib.2004.12.013
Gulia-Nuss, M., Nuss, A. B., Meyer, J. M., Sonenshine, D. E., Roe, R. M., Waterhouse, R. M., et al. (2016). Genomic insights into the Ixodes scapularis tick, vector of Lyme disease. Nat. Commun. 7:10507. doi: 10.1038/ncomms10507
Hajdušek, O., Síma, R., Ayllón, N., Jalovecká, M., Perner, J., de la Fuente, J., et al. (2013). Interactions of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol. 3:26. doi: 10.3389/fcimb.2013.00026
Ireton, K. (2013). Molecular mechanisms of cell–cell spread of intracellular bacterial pathogens. Open Biol. 3:130079. doi: 10.1098/rsob.130079
Jeng, R. L., Goley, E. D., D'Alessio, J. A., Chaga, Y. O., Svitkina, T. M., Borisy, G. G., et al. (2004). A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell. Microbiol. 6, 761–769. doi: 10.1111/j.1462-5822.2004.00402.x
IJdo, J. W., and Mueller, A. C. (2004). Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect. Immun. 72, 5392–5401. doi: 10.1128/IAI.72.9.5392-5401.2004
Lampson, R. L., Bastounis, E., Kafai, N. M., Serrano, R., del A' lamo, J. C., Theriot, J. A., et al. (2016). Rickettsia Sca4 reduces vinculin-mediated intercellular tension to promote spread. Cell 167, 670.e10–683.e10. doi: 10.1016/j.cell.2016.09.023
Liu, L., Narasimhan, S., Dai, J., Zhang, L., Cheng, G., and Fikrig, E. (2011). Ixodes scapularis salivary gland protein P11 facilitates migration of Anaplasma phagocytophilum from the tick gut to salivary glands. EMBO Rep. 12, 1196–1203. doi: 10.1038/embor.2011.177
Macaluso, K. R., Mulenga, A., Simser, J. A., and Azad, A. F. (2003). Differential expression of genes in uninfected and Rickettsia-infected Dermacentor variabilis ticks as assessed by differential-display PCR. Infect. Immun. 71, 6165–6170.
Moitra, P., Zheng, H., Anantharaman, V., Banerjee, R., Takeda, K., Mozaki, Y., et al. (2015). Expression, purification, and biological characterization of babesia microti apical membrane antigen 1. Infect. Immun. 83, 3890–3901. doi: 10.1128/IAI.00168-15
Mulenga, A., Macaluso, K. R., Simser, J. A., and Azad, A. F. (2003). Dynamics of Rickettsia-tick interactions: identification and characterization of differentially expressed mRNAs in uninfected and infected Dermacentor variabilis. Insect Mol. Biol. 12, 185–193. doi: 10.1046/j.1365-2583.2003.00400.x
Munderloh, U. G., and Kurtti, T. J. (1995). Cellular and molecular interrelationships between ticks and prokaryotic tick-borne pathogens. Annu. Rev. Entomol. 40, 221–243. doi: 10.1146/annurev.en.40.010195.001253
Narasimhan, S., and Fikrig, E. (2015). Tick microbiome: the force within. Trends Parasitol. 31, 315–323. doi: 10.1016/j.pt.2015.03.010
Noguiera, S. V., Smith, A. A., Qin, J. H., and Pal, U. (2012). A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks. Infect. Immun. 80, 82–90. doi: 10.1128/IAI.05671-11
Ogden, N. H., Artsob, H., Marogs, G., and Tsao, J. (2014). “Non-rickettsial tick-borne bacteria and the diseases they cause,” in Biology of Ticks, Chapter 10, Vol. 2, 2nd Edn., eds D. E. Sonenshine and R. M. Roe (New York, NY: Oxford University Press), 278–312.
Oliva Chávez, A. S., Fairman, J. W., Helheim, R. F., Nelson, C. M., Herron, M. J., Higgins, L., et al. (2015). An O-methyltransferase is required for infection of tick cells by Anaplasma phagocytophilum. PLoS Pathog. 11:e1005248. doi: 10.1371/journal.ppat.1005248
Önder, Ö., Humphrey, P. T., McOmber, B., Korobova, F., Francella, N., Greenbaum, D. C., et al. (2012). OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J. Biol. Chem. 287, 16860–16868. doi: 10.1074/jbc.M111.290775
Pal, U., Li, X., Wang, T., Montgomery, R. R., Ramamoorthi, N., Desilva, A. M., et al. (2004). TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119, 457–468. doi: 10.1016/j.cell.2004.10.027
Petchampai, N., Sunyakumthorn, P., Gillette, M. L., Verhoeven, V. I., Banerjee, K. H., Kearney, M. T., et al. (2014). Novel identification of Dermacentor variabilis Arp2/3 complex and its role in rickettsial infection of the arthropod vector. PLoS ONE 9:e93768. doi: 10.1371/journal.pone.0093768
Ramamurthy, N., Narasimhan, S., Pal, U., Bao, F., Yang, X. F., Fish, D., et al. (2011). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436, 573–577. doi: 10.1038/nature03812
Rikihisa, Y. (2010). Molecular events involved in cellular invasion by ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet. Parasitol. 167, 155–166. doi: 10.1016/j.vetpar.2009.09.017
Rudzinska, M. A., Spielman, A., Riek, R. F., Lewengrub, S. J. and Piesman, J. (1979). Intraerythrocytic ‘gametocytes' of Babesia microti and their maturation in ticks. Can. J. Zool. 57, 424–434. doi: 10.1139/z79-050
Rudzinska, M. A., Landgrab, S., Spielman, A., and Piesman, J. (1983). Invasion of Babesia microti into epithelial cells of the tick gut. J. Protozool. 30, 338–346. doi: 10.1111/j.1550-7408.1983.tb02927.x
Schuijt, T. J., Coumou, J., Narasimhan, S., Dai, J., Deponte, K., Wouters, D., et al. (2011). A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the Lyme disease agent. Cell Host Microbe 10, 136–146. doi: 10.1016/j.chom.2011.06.010
Serio, A. W., Jeng, R. L., Haglund, C. M., Reed, S. C., and Welch, M. D. (2010). Defining a core set of actin cytoskeletal proteins critical for actin-based motility of Rickettsia. Cell Host Microbe 7, 388–398. doi: 10.1016/j.chom.2010.04.008
Shaw, D. K., Wang, X., Brown, L. J., Oliva Chavez, A. S., Reif, K. E., Smith, A. A., et al. (2017). Infection–derived lipids elicit an immune deficiency circuit in arthropods. Nature Comm. 8:14401. doi: 10.1038/ncomms14401
Sukumaran, B., Narasimhan, S., Anderson, J. F., DePonte, K., Marcantonio, N., Krishnan, M. N., et al. (2006). An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J. Exp. Med. 203, 1507–1517. doi: 10.1084/jem.20060208
Thepparit, C., Bourchookam, A., Petchampai, N., Barker, S. A., and Macaluso, K. R. (2010). Interaction of Rickettsia files with histone H2B facilitates the infection of a tick cell line. Microbiology 156, 2855–2863. doi: 10.1099/mic.0.041400-0
Xiong, Q., Lin, M., and Rikihisa, Y. (2009). Cholesterol-dependent Anaplasma phagocytophilum exploits the low density lipoprotein uptake pathway. PLoS Pathog. 5:e1000329. doi: 10.1371/journal.ppat.1000329
Keywords: pathobiology, Arp 23, caveolae, clathrin, salp 16, P11, JAKSTAT
Citation: Sonenshine DE and Macaluso KR (2017) Microbial Invasion vs. Tick Immune Regulation. Front. Cell. Infect. Microbiol. 7:390. doi: 10.3389/fcimb.2017.00390
Received: 06 April 2017; Accepted: 21 August 2017;
Published: 05 September 2017.
Edited by:
Jose De La Fuente, Instituto de Investigación en Recursos Cinegéticos, SpainReviewed by:
Petr Kopacek, Institute of Parasitology (ASCR), CzechiaCopyright © 2017 Sonenshine and Macaluso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel E. Sonenshine, ZHNvbmVuc2hAb2R1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.