- 1State Key Laboratory of Genetic Engineering, School of Life Science, Institute of Genetics, Fudan University, Shanghai, China
- 2Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada
- 3Key Laboratory of Medical Molecular Virology of Ministries of Education and Health, Fudan University, Shanghai, China
- 4Shanghai Engineering Research Center of Industrial Microorganisms, Shanghai, China
One strategy to develop the next generation of tuberculosis vaccines is to construct subunit vaccines based on T cell antigens. In this study, we have evaluated the vaccine potential of a fusion protein combining EsxB, EsxD, EsxG, EsxU, and EsxM of Mycobacterium tuberculosis (M. tb). This recombinant protein, named BM, was expressed in and purified from Escherichia coli. Immunization of C57BL/6 mice with purified BM protein formulated in Freund's incomplete adjuvant induced the production of Th1 cytokines (IFN-γ, TNF, and IL-2) and multifunctional CD4+ T cells. Vaccination of BALB/c mice with BM protein followed by intravenous challenge with Mycobacterium bovis BCG resulted in better levels of protection than the two leading antigens, Ag85A and PPE18. Taken together, these results indicate that BM is a protective antigen. Future studies to combine BM with other antigens and evaluate its effectiveness as a booster of BCG or as a therapeutic vaccine are warranted.
Introduction
Tuberculosis (TB) ranks alongside HIV/AIDS as a major cause of mortality by infectious diseases worldwide. As much as one-third of the world's population is latently infected with M. tb and 1.5 million people died from TB in 2014. The situation has been further complicated with the advent of M. tb/HIV co-infection and the emergence of drug resistant TB. Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is the only vaccine available for TB control. However, BCG exhibits highly variable efficacy against adult pulmonary TB (Brewer, 2000) and can cause disseminated BCG disease in immunocompromised individuals (WHO, 2007). In light of these situations, a more effective vaccine is urgently needed, which is essential to reduce the estimated 8–10 million new TB infections that occur annually.
Currently, it is thought that the next generation of TB vaccines will use a heterologous prime-boost strategy to strengthen the immune response introduced by BCG (Skeiky and Sadoff, 2006; Kaufmann, 2011). This “prime-boost” strategy would include administration of BCG or recombinant BCG, the “prime,” followed by a “booster” inoculation with a subunit vaccine (protein/peptide or DNA) to infants and young children before they are exposed to TB. This subunit vaccine could also be administered as a separate booster to young adults or as an adjunct to chemotherapy (Skeiky and Sadoff, 2006; Kaufmann, 2011).
A key aspect of the strategy concerns the selection of antigens to be used in constructing these subunit vaccines. Protection against TB requires a cell-mediated immune response, which is not fully understood but involves multiple components including CD4+ and CD8+ T cells, as well as unconventional T cells such as γδ T cells and CD1-restricted αβ T cells (North and Jung, 2004; Cooper, 2009; Andersen and Kaufmann, 2014). Currently, there is no proven immunological correlate of protection or “biomarker” for efficacy (Mittrucker et al., 2007; Soares et al., 2008; Nunes-Alves et al., 2014). BCG has been shown to induce a T helper cell 1 (Th1) type response, mostly IFN-γ by CD4+ T cells (Black et al., 2002). IFN-γ plays a critical role in the control of TB in both mice (Cooper et al., 1993; Flynn et al., 1993) and humans (Jouanguy et al., 1996; Newport et al., 1996). As such, the identification of M. tb antigens that induce strong IFN-γ production has been the main strategy for uncovering candidates for construction of subunit vaccines. Some antigens that have been identified thus far include the Esx family proteins (EsxA, B, G, H, G, N), the antigen 85 family (Ag85A, B, C), and several PE/PPE family proteins (e.g., PPE18, PPE14) (Borremans et al., 1989; Bassey et al., 1996; Coler et al., 1998; Brandt et al., 2000; Brodin et al., 2004; Dietrich et al., 2005). Three fusion proteins H1 (Ag85B-EsxA), H4 (Ag85B-EsxH), and M72 (PPE18-Rv0125) were constructed and have since entered clinical trials (Andersen and Kaufmann, 2014).
To stimulate greater CD8+ T cell recognition of antigens, DNA-based subunit vaccines have also been exploited, using replication-deficient viral vectors such as adenovirus or vaccinia virus for delivery. This is exemplified by MVA85A, a vaccinia virus expressing Ag85A; and AERAS-402, an adenovirus-35 expressing Ag85A, Ag85B, and EsxH (Andersen and Kaufmann, 2014). MVA85A has recently completed phase IIb trial, the first candidate to reach efficacy testing in clinical trials. Unfortunately, the results are disappointing (Tameris et al., 2013). In BCG-vaccinated South African infants, MVA85A did not significantly improve on BCG alone in terms of protection, either against TB disease or M. tb infection (Tameris et al., 2013). As such, it is necessary to continue searching for new antigens or novel combination of antigens for the pipeline. Here, we describe the immunogenicity and protective efficacy of a fusion protein that combines five EsxB-like proteins.
Materials and Methods
Bacterial Strains and Culture Conditions
Mycobacterium bovis BCG-Pasteur was grown at 37°C in Middlebrook 7H9 broth (Difco™) supplemented with 0.2% glycerol, 10% albumin-dextrose-catalase (ADC; BD BBL™), and 0.05% Tween 80 or on Middlebrook 7H11 agar (Difco™) supplemented with 0.5% glycerol and 10% oleic acid-albumin-dextrose-catalase (OADC; BD BBL™). Escherichia coli strain DH5α was used for routine manipulation and propagation of plasmid DNA and strain BL21 was used for expression and purification of recombinant proteins. E. coli strains were grown in LB broth or agar.
Molecular Cloning
The DNA fragment for expressing the BM fusion protein was constructed by linking esxB-esxD-esxG-esxU-esxM in tandem in the linear order. A 9-amino acid linker (GGACTAGTACCCCGAGGATCAACAGGA) was added between each two adjoining components of the polyprotein. The ORFs encoding esxB, esxD, esxG, esxU, and esxM were amplified by PCR using the primers in Table S1 and the genomic DNA of M. tb H37Rv as the template. These five fragments were linked together and cloned into pUC19 vector by ClonExpress MultiS One Step Cloning Kit (Vazyme). After extracting the recombinant plasmid, BM full length DNA fragment was amplified by PCR using the appropriate primers (Table S1). The fragment was then cloned into pET28a by restriction digestion and ligation. Genes encoding three other proteins, PPE18, Ag85A and EsxB, were cloned into pET28a or pET SUMO using the appropriate primers (Table S1). Recombinant plasmids were transformed into E. coli DH5α and plated on LB agar containing 50 μg/ml kanamycin. Single colonies were randomly picked and grown in LB broth. The plasmids were isolated from E. coli DH5α cultures and confirmed by DNA sequencing.
Expression and Purification of Recombinant Proteins
To express the recombinant protein, the pET28 or pET SUMO constructs were individually transformed into E. coli BL21 and plated on LB agar containing kanamycin (50 μg/ml). After overnight incubation at 37°C, single colonies were randomly picked and grown in LB broth and subcultured to 1 L. To induce the expression of protein, E. coli BL21 cultures were grown at 37°C to OD600 = 0.8 and added with 1 mM IPTG for 3 h. For Ag85A and EsxB proteins, which were purified from the soluble fraction, the cultures were collected by centrifugation at 12,000 rpm for 10 min at 4°C, and resuspended with BugBuster protein extraction reagent (Novagen). The cell lysates were then centrifuged at 12,000 rpm for 20 min at 4°C and the supernatant was collected. The collected supernatant was subjected to Ni-NTA His•Bind® Resin (Novagen) and purified following the protocol recommended by the manufacturer. The SUMO tag was digested from the purified proteins by ULP1. For BM and PPE18 proteins, which were purified from inclusion body, the cultures collected after IPTG induction were resuspended with PBS and sonicated to break the cells. The cell lysates were centrifuged at 12,000 rpm for 20 min at 4°C and the pellet was collected. The collected pellet was resuspended with 20 mM Tris, pH 8.0, 100 mM NaCl, and 8 M urea, which was then subjected to Ni-NTA His•Bind® Resin. Purified proteins were centrifuged using microfuge tubes with a low molecular weight cut off filter to remove urea and imidazole. The protein purity was verified by polyacrylamide gel electrophoresis, and the protein concentration was measured by the Bradford assay (Bio-Rad).
Immunogenicity Assays
All of the animal procedures were approved by the local animal care committees at Fudan University. To determine the induction of Th1 cytokines, C57BL/6 mice (4 per group) were immunized subcutaneously with a mixture containing 100 μl each of the purified protein (10 μg) and 100 μl of Freund's incomplete adjuvant (Sigma). The vaccination procedure was repeated two more times (2 weeks apart). At week 8 after the first vaccination, the mice were sacrificed and their spleens were collected. Splenocytes were prepared in 24-well plate, with each well containing 106 lymphocytes. The corresponding purified proteins were added to the wells at two different concentrations (5 and 10 μg/ml). The plates were incubated at 37°C with 5% CO2 for 60 h. The cell supernatants were harvested and the production of Th1 cytokines (IFN-γ, TNF, IL-2) was determined by ELISA using the OptEIATM ELISA Kit (BD Biosciences) with appropriate mAbs, according to the protocol recommended by the manufacturer.
To determine the induction of multifunctional T cells, C57BL/6 mice (4 per group) were immunized as the above. Lymphocytes (106 cells per well) were stimulated with the corresponding purified proteins (5 μg/mL) and incubated at 37°C 5% CO2 for 12 h. Brefeldin A was used to block the Golgi apparatus 4 h before staining. Fluorescent monoclonal antibodies (1 μL) for CD3 and CD4 (BD Biosciences) were added to each wells for the staining of surface markers. The cells were then permeabilized and fixed with CytoFix/CytoPerm (BD Biosciences). Fluorescent antibodies (1 μL) for IFN-γ, TNF and IL-2 (BD Biosciences) were added for intracellular staining. The cells were then analyzed on a Gallios flow cytometer (Beckman Coulter).
Animal Protection Assays
BALB/c mice (5 per group) were immunized as described above in the “immunogenicity assays.” Eight weeks after the first vaccination, the mice were infected intravenously via a lateral tail with 107 CFU (colony forming unit) of Mycobacterium bovis BCG-Pasteur. After 3 weeks of infection, the mice were sacrificed and their lungs and spleens were collected. The CFUs of BCG in the lungs and spleen were determined by serial dilution and platting on 7H11 agar plates (OADC).
Results
Generation of BM5 Fusion Protein
The M. tb genome contains 23 esx genes (esxA-W), which are arranged in tandem pairs in 11 genomic loci, including five ESX loci flanked by components of type VII secretion (T7S) systems (ESX-1 to -5) (Cole et al., 1998; Abdallah et al., 2007; Bitter et al., 2009). The Esx family proteins are small (~100 amino acids) secreted proteins that are substrates of the T7S systems. The first Esx protein that was discovered was the EsxA (ESAT-6) protein (Sorensen et al., 1995). EsxA and its protein partner EsxB (CFP-10) form a heterodimer, which is exported by the ESX-1 system encoded by genes flanking esxA-esxB (Renshaw et al., 2002; Abdallah et al., 2007). Similar genetic organizations were found in the other four ESX loci (Abdallah et al., 2007). Many of the Esx family proteins are immunodominant T cell antigens (Skjot et al., 2000; Pallen, 2002), and three of them (EsxA, EsxB, and EsxH) have been used to construct subunit vaccines that have entered clinical trials (Andersen and Kaufmann, 2014). The Esx family proteins are also called the WxG100 proteins, based on a short conserved motif in the middle of the protein, tryptophan-X-glycine (WxG), which forms the turn that connects two helices (Pallen, 2002; Renshaw et al., 2005; Poulsen et al., 2014). Interestingly, despite their similarity in genetic organization and structural fold, the Esx family proteins have low sequence homology. For example, the EsxB-like proteins in the five ESX loci (EsxB, D, G, U, and M) (Bitter et al., 2009) share <32% sequence identity by pairwise comparisons (Figures 1A,B). Five other EsxB-like proteins elsewhere in the genome do share high levels of sequence identity to their homologs in the five ESX loci. EsxJ, K, P, and W proteins are nearly identical to EsxM in the ESX-5 locus, and EsxS is highly homologous to EsxG in the ESX-3 locus. A similar result was found if we compare the EsxA-like proteins in the five ESX loci and elsewhere in the genome.
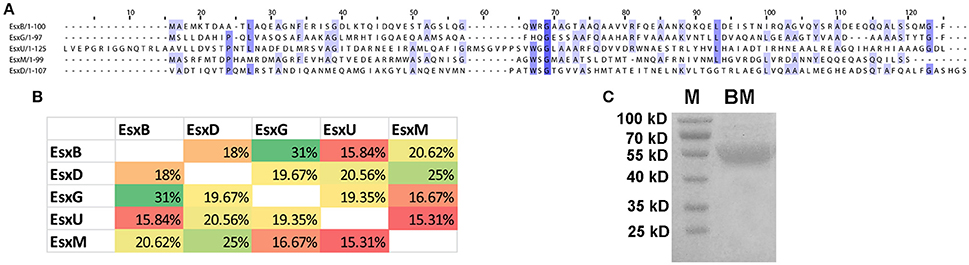
Figure 1. Sequence alignment of five EsxB-like proteins. (A) ClustalWS alignment of EsxB, EsxD, EsxG, EsxU, and EsxM. (B) Pairwise comparison of the sequence identity of the five EsxB-like proteins. (C) Purified recombinant BM fusion protein. M, molecular weight marker.
We hypothesized that a fusion protein combing Esx proteins with diverse sequences would have more T cell epitopes and would be more immunogenic and protective. To test this, we generated a fusion protein combining five EsxB-like proteins in the ESX loci, namely, EsxB, D, G, U, and M. These genes were cloned in tandem with a linker sequence between them, and the DNA fragment was inserted into pET28a and expressed in E. coli. A recombinant protein of ~55 KD was purified by affinity chromatography, which is in agreement with its predicted size (Figure 1C).
BM Induces the Production of Th1 Cytokines
To assess immunogenicity, C57BL/6 mice (4 per group) were immunized three times (2 weeks apart) with 10 μg purified BM protein formulated with Freund's incomplete adjuvant. Four weeks after the last vaccination, the mice were sacrificed and their spleens were collected. Splenocytes were prepared and cultured with or without the corresponding protein at two different concentrations (5 and 10 μg/ml). Three days after antigen stimulation, the cell supernatants were harvested and the production of Th1 cytokines (IFN-γ, TNF, IL-2) were determined by ELISA. Purified Ag85A was also included in the parallel experiment for comparison and as a positive control.
Results showed that BM was highly immunogenic. It induced significant levels of Th1 cytokines including IFN-γ, TNF, and IL-2, which were comparable to the levels of these cytokines induced by Ag85A (Figure 2). Both proteins induced equally high levels of IFN-γ and TNF but lower levels of IL-2.
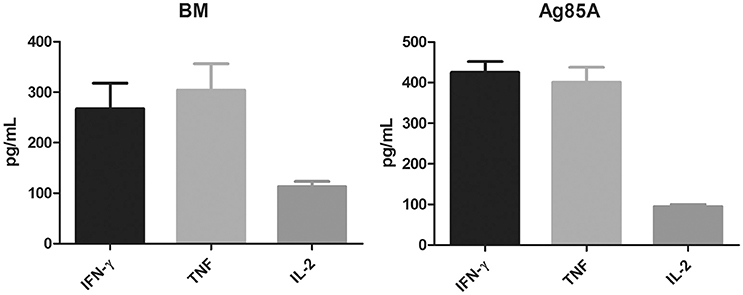
Figure 2. BM induces the secretion of Th1 cytokines. C57BL/6 mice were immunized three times (2 weeks apart) with 10 μg purified BM or Ag85A protein formulated with Freund's incomplete adjuvant. At 8 weeks post-vaccination, splenocytes were harvested and incubated with purified BM or Ag85A at two different concentrations (5 and 10 μg/ml) for 60 h and the productions of Th1 cytokines was analyzed by ELISA. Data were plotted as mean ± SEM (n = 4 mice).
BM Induces Multifunctional CD4+ T Cells
Recent reports from a number of different disease models have shown that multifunctional T cells are superior to their single-positive counterparts (Seder et al., 2008). In mice, vaccine-induced multifunctional CD4+ T cells have been shown to correlate with protection against Leishmania major infection (Darrah et al., 2007). Inductions of multifunctional CD4+ T cells were also reported for TB subunit vaccines MVA85A and H1 (Darrah et al., 2007; Lindenstrom et al., 2009).
To determine if BM induces multifunctional T cells, we repeated the immunization of CL57/BL6 mice with BM and then measured the production of CD4+ T cells expressing multiple effectors including IFN-γ, TNF, and IL-2. Ag85A and PPE18 were included in this experiment for comparison. Consistent with the above result, BM induced CD4+ T cells with the concomitant production of IFN-γ, TNF, and IL-2, and the level of induction was comparable to that of Ag85A and PPE18 (Figure 3).
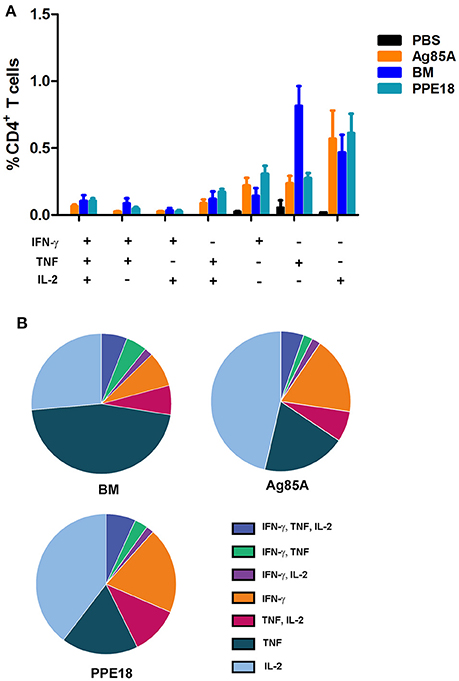
Figure 3. BM induces multiple functional CD4+ T cells. (A) C57BL/6 mice were immunized three times (2 weeks apart) with the indicated proteins (10 μg) formulated with Freund's incomplete adjuvant for 8 weeks. Splenocytes were isolated and stimulated with the corresponding purified protein (5 μg/ml) for 12 h and were then stained for surface markers and intracellular cytokines, followed by FACS analysis. Data were plotted as mean ± SEM (n = 4 mice). (B) Pie chart of the data.
Protective Efficacy of BM Fusion Protein
To determine the protective efficacy of BM, BALB/c mice (5 per group) were immunized subcutaneously three times (2 weeks apart) with purified BM, PPE18, or EsxB formulated with Freund's incomplete adjuvant for 8 weeks. Mice were then intravenously challenged with 107 CFUs of M. bovis BCG-Pasteur. Three weeks after injection, the mice were sacrificed and bacterial burdens in the lungs and spleen were determined. Results showed that mice immunized with BM had significantly lower BCG counts in the spleen than the unvaccinated PBS control (p <0.01, One-Way ANOVA) (Figure 4A). The mean BCG burden in the BM group (4.29 log10 CFU) was ~0.5 log10 lower than in the PBS control group (4.79 log10 CFU). Lower BCG burden (3.33 log10) was also found in the lungs of mice immunized with BM, compared to the PBS group (3.65 log10 CFU), and the difference was statistically significant (Figure 4B).
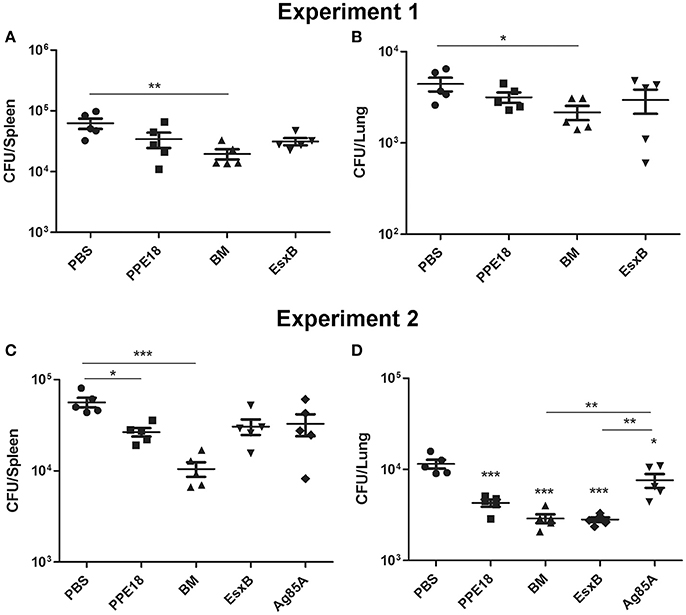
Figure 4. Protective efficacy of BM in intravenous infection model. BALB/c mice (5 per group) were immunized with the indicated proteins (10 μg) formulated with Freund's incomplete adjuvant for 8 weeks. Mice were then intravenously challenged with 107 CFUs of M. bovis BCG-Pasteur. At week 3 after injection, mice were sacrificed and their lungs and spleens were collected and homogenization for BCG colony counting. Data from two independent experiments were presented. Data were plotted as mean ± SEM (n = 5 mice). In (A,B) One-Way ANOVA and Bonferroni comparison tests were performed to compare the BM and PBS groups in each dataset. *p <0.05, **p <0.01. In (C,D) One-Way ANOVA and Bonferroni comparison tests were performed to compare all groups. In (D) the groups of BM, EsxB, and PPE were significantly different to the PBS group (***p <0.001). The Ag85A group was also significantly different to the PBS group (*p <0.05).
We repeated this experiment and also included Ag85A for comparison. Consistently, among all the vaccine candidates tested, the BM group had the lowest bacterial burden in the spleen. The mean BCG burden in the spleen of the BM group was 4.02 log10, which was 0.73 log10, 0.49 log10, 0.46 log10, and 0.40 log10 lower than the PBS, Ag85A, EsxB, and PPE groups, respectively. The difference between the BM group and the PBS group was statistically different (Figure 4C). The bacterial burden in the lungs of the BM group was also significantly lower than the PBS (ΔCFU = 0.60 log10, p <0.0001) and Ag85A groups (ΔCFU = 0.42 log10, p <0.01) (Figure 4D).
Discussion
TB vaccine research has gained momentum in the past decade, with a dozen candidates currently under clinical trial evaluations (Andersen and Kaufmann, 2014). However, there is no guarantee that the leading candidates will progress through phase III clinical trials and registration. As previously mentioned, MVA85A, the first vaccine candidate that reached efficacy testing in clinical trials, failed to demonstrate improved protection in BCG-immunized infants (Tameris et al., 2013). Several explanations have been proposed (Beverley, 2013; Andersen and Kaufmann, 2014), including the modest magnitude of immune response induced by MVA85A in children (Tameris et al., 2013) and that the Ag85 family antigens were downregulated to very low levels soon after M. tb infection and not preferentially recognized by the immune system (Aagaard et al., 2011; Commandeur et al., 2013). Given the failure of MVA85A, recent efforts have focused on identifying more effective antigens and novel combination of antigens. Several studies suggest that subunit vaccines that combine multistage antigens may be a better strategy to develop effective vaccines (Bertholet et al., 2010; Aagaard et al., 2011). For example, the fusion protein H56, which combines a latency associated antigen Rv2660c with Ag85B and EsxA, conferred better protection than H1 (Ag85B-EsxA) in mice at late-stage of M. tb infection (Aagaard et al., 2011).
In this study, we took advantage of the sequence diversity among some of the Esx proteins and their relative small size, and constructed a fusion protein that combines five different Esx proteins. The Esx family proteins are immunodominant T cell antigens (Skjot et al., 2000; Pallen, 2002) and therefore good candidates for constructing subunit vaccines. The diverging sequence among Esx proteins in the five ESX loci is consistent with the diverse function of the T7S systems. ESX-1 is required for mycobacterial virulence (Abdallah et al., 2007), ESX-3 plays a role in iron and zinc uptake (Serafini et al., 2009; Siegrist et al., 2014), and ESX-5 is involved in the export of PE/PPE proteins and pathogenicity (Abdallah et al., 2009). The functions of ESX-2 and ESX-4 remain unknown. ESX-4, which harbors a smaller number of genes than other ESX loci, appears to be the most ancestral T7S system in mycobacteria (Gey Van Pittius et al., 2001). Other ESX loci may have evolved later by gene duplication and diversification events, which results in sequence variation and function diversity.
Our results demonstrated that BM induces potent Th1 cytokines and CD4+ T cells, and confers protection against mycobacterial infection. Compared with Ag85A, PPE18, and EsxB, which have been used in subunit vaccines that are in clinical trial evaluation, BM exhibits better protection. The enhanced protection of BM could be attributed to more T cell epitopes in this fusion protein than other antigens of similar size. Our results suggest that BM is an effective antigen that could be included in the construction of novel TB vaccines. BM could be combined with latency associated antigens to construct multistage subunit vaccines, or to be expressed in BCG for the construction of live vaccines. Future studies to evaluate the effectiveness of BM as a booster of BCG or as a post-exposure vaccine will determine its full potential.
Author Contributions
JL conceived and designed the project. LZ supervised the experiments. ZhX, RS, CL, FC, JM, YL, ZyX performed the experiments. JL wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by grants from the China's 12th Five Year Programs for the prevention and cure of great infectious diseases (No. 2012ZX10003002-002), China's 13th Five Year Programs for the prevention and cure of great infectious diseases (No. 2017ZX10201301-005, 2017ZX10301301-001-005). Shanghai Municipal Natural Science Foundation (No. 16ZR1402800), International cooperation and exchange program of Shanghai Municipal (No. 16430724000) and Shanghai Science and Technology Commission (13DZ2252000), and a grant from Canadian Institutes of Health Research (MOP-106559).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fcimb.2017.00226/full#supplementary-material
References
Aagaard, C., Hoang, T., Dietrich, J., Cardona, P. J., Izzo, A., Dolganov, G., et al. (2011). A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17, 189–194. doi: 10.1038/nm.2285
Abdallah, A. M., Gey van Pittius, N. C., Champion, P. A., Cox, J., Luirink, J., Vandenbroucke-Grauls, C. M., et al. (2007). Type VII secretion–mycobacteria show the way. Nat. Rev. Microbiol. 5, 883–891. doi: 10.1038/nrmicro1773
Abdallah, A. M., Verboom, T., Weerdenburg, E. M., Gey van Pittius, N. C., Mahasha, P. W., Jimenez, C., et al. (2009). PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol. Microbiol. 73, 329–340. doi: 10.1111/j.1365-2958.2009.06783.x
Andersen, P., and Kaufmann, S. H. (2014). Novel vaccination strategies against tuberculosis. Cold Spring Harb. Perspect. Med. 4:a018523. doi: 10.1101/cshperspect.a018523
Bassey, E. O., Life, P. F., Catty, D., Gaston, J. S., and Kumararatne, D. S. (1996). T-cell response to mycobacterial proteins: a comparative study of tuberculous and control immunoblots of Mycobacterium tuberculosis and M. bovis BCG. Tuber. Lung Dis. 77, 146–153. doi: 10.1016/S0962-8479(96)90029-5
Bertholet, S., Ireton, G. C., Ordway, D. J., Windish, H. P., Pine, S. O., Kahn, M., et al. (2010). A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci. Transl. Med. 2:53ra74. doi: 10.1126/scitranslmed.3001094
Bitter, W., Houben, E. N., Bottai, D., Brodin, P., Brown, E. J., Cox, J. S., et al. (2009). Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 5:e1000507. doi: 10.1371/journal.ppat.1000507
Black, G. F., Weir, R. E., Floyd, S., Bliss, L., Warndorff, D. K., Crampin, A. C., et al. (2002). BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359, 1393–1401. doi: 10.1016/S0140-6736(02)08353-8
Borremans, M., de Wit, L., Volckaert, G., Ooms, J., de Bruyn, J., Huygen, K., et al. (1989). Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect. Immun. 57, 3123–3130.
Brandt, L., Elhay, M., Rosenkrands, I., Lindblad, E. B., and Andersen, P. (2000). ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68, 791–795. doi: 10.1128/IAI.68.2.791-795.2000
Brewer, T. F. (2000). Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31(Suppl. 3), S64–S67. doi: 10.1086/314072
Brodin, P., Majlessi, L., Brosch, R., Smith, D., Bancroft, G., Clark, S., et al. (2004). Enhanced protection against tuberculosis by vaccination with recombinant Mycobacterium microti vaccine that induces T cell immunity against region of difference 1 antigens. J. Infect. Dis. 190, 115–122. doi: 10.1086/421468
Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544. doi: 10.1038/31159
Coler, R. N., Skeiky, Y. A., Vedvick, T., Bement, T., Ovendale, P., Campos-Neto, A., et al. (1998). Molecular cloning and immunologic reactivity of a novel low molecular mass antigen of Mycobacterium tuberculosis. J. Immunol. 161, 2356–2364.
Commandeur, S., van Meijgaarden, K. E., Prins, C., Pichugin, A. V., Dijkman, K., van den Eeden, S. J., et al. (2013). An unbiased genome-wide Mycobacterium tuberculosis gene expression approach to discover antigens targeted by human T cells expressed during pulmonary infection. J. Immunol. 190, 1659–1671. doi: 10.4049/jimmunol.1201593
Cooper, A. M. (2009). Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27, 393–422. doi: 10.1146/annurev.immunol.021908.132703
Cooper, A. M., Dalton, D. K., Stewart, T. A., Griffin, J. P., Russell, D. G., and Orme, I. M. (1993). Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178, 2243–2247. doi: 10.1084/jem.178.6.2243
Darrah, P. A., Patel, D. T., De Luca, P. M., Lindsay, R. W., Davey, D. F., Flynn, B. J., et al. (2007). Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13, 843–850. doi: 10.1038/nm1592
Dietrich, J., Aagaard, C., Leah, R., Olsen, A. W., Stryhn, A., Doherty, T. M., et al. (2005). Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J. Immunol. 174, 6332–6339. doi: 10.4049/jimmunol.174.10.6332
Flynn, J. L., Chan, J., Triebold, K. J., Dalton, D. K., Stewart, T. A., and Bloom, B. R. (1993). An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178, 2249–2254. doi: 10.1084/jem.178.6.2249
Gey Van Pittius, N. C., Gamieldien, J., Hide, W., Brown, G. D., Siezen, R. J., and Beyers, A. D. (2001). The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:RESEARCH0044. doi: 10.1186/gb-2001-2-10-research0044
Jouanguy, E., Altare, F., Lamhamedi, S., Revy, P., Emile, J. F., Newport, M., et al. (1996). Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335, 1956–1961. doi: 10.1056/NEJM199612263352604
Kaufmann, S. H. (2011). Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect. Dis. 11, 633–640. doi: 10.1016/S1473-3099(11)70146-3
Lindenstrom, T., Agger, E. M., Korsholm, K. S., Darrah, P. A., Aagaard, C., Seder, R. A., et al. (2009). Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182, 8047–8055. doi: 10.4049/jimmunol.0801592
Mittrucker, H. W., Steinhoff, U., Kohler, A., Krause, M., Lazar, D., Mex, P., et al. (2007). Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 104, 12434–12439. doi: 10.1073/pnas.0703510104
Newport, M. J., Huxley, C. M., Huston, S., Hawrylowicz, C. M., Oostra, B. A., Williamson, R., et al. (1996). A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335, 1941–1949. doi: 10.1056/NEJM199612263352602
North, R. J., and Jung, Y. J. (2004). Immunity to tuberculosis. Annu. Rev. Immunol. 22, 599–623. doi: 10.1146/annurev.immunol.22.012703.104635
Nunes-Alves, C., Booty, M. G., Carpenter, S. M., Jayaraman, P., Rothchild, A. C., and Behar, S. M. (2014). In search of a new paradigm for protective immunity to TB. Nat. Rev. Microbiol. 12, 289–299. doi: 10.1038/nrmicro3230
Pallen, M. J. (2002). The ESAT-6/WXG100 superfamily – and a new Gram-positive secretion system? Trends Microbiol. 10, 209–212. doi: 10.1016/S0966-842X(02)02345-4
Poulsen, C., Panjikar, S., Holton, S. J., Wilmanns, M., and Song, Y. H. (2014). WXG100 protein superfamily consists of three subfamilies and exhibits an alpha-helical C-terminal conserved residue pattern. PLoS ONE 9:e89313. doi: 10.1371/journal.pone.0089313
Renshaw, P. S., Lightbody, K. L., Veverka, V., Muskett, F. W., Kelly, G., Frenkiel, T. A., et al. (2005). Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 24, 2491–2498. doi: 10.1038/sj.emboj.7600732
Renshaw, P. S., Panagiotidou, P., Whelan, A., Gordon, S. V., Hewinson, R. G., Williamson, R. A., et al. (2002). Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277, 21598–21603. doi: 10.1074/jbc.M201625200
Seder, R. A., Darrah, P. A., and Roederer, M. (2008). T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8, 247–258. doi: 10.1038/nri2274
Serafini, A., Boldrin, F., Palu, G., and Manganelli, R. (2009). Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: essentiality and rescue by iron and zinc. J. Bacteriol. 191, 6340–6344. doi: 10.1128/JB.00756-09
Siegrist, M. S., Steigedal, M., Ahmad, R., Mehra, A., Dragset, M. S., Schuster, B. M., et al. (2014). Mycobacterial Esx-3 requires multiple components for iron acquisition. MBio 5, e01073–e01014. doi: 10.1128/mBio.01073-14
Skeiky, Y. A., and Sadoff, J. C. (2006). Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4, 469–476. doi: 10.1038/nrmicro1419
Skjot, R. L., Oettinger, T., Rosenkrands, I., Ravn, P., Brock, I., Jacobsen, S., et al. (2000). Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68, 214–220. doi: 10.1128/IAI.68.1.214-220.2000
Soares, A. P., Scriba, T. J., Joseph, S., Harbacheuski, R., Murray, R. A., Gelderbloem, S. J., et al. (2008). Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 180, 3569–3577. doi: 10.4049/jimmunol.180.5.3569
Sorensen, A. L., Nagai, S., Houen, G., Andersen, P., and Andersen, A. B. (1995). Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63, 1710–1717.
Tameris, M. D., Hatherill, M., Landry, B. S., Scriba, T. J., Snowden, M. A., Lockhart, S., et al. (2013). Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028. doi: 10.1016/S0140-6736(13)60177-4
Keywords: tuberculosis, vaccine, Esx family protein, subunit vaccine, fusion protein
Citation: Xiang Z-h, Sun R-f, Lin C, Chen F-z, Mai J-t, Liu Y-x, Xu Z-y, Zhang L and Liu J (2017) Immunogenicity and Protective Efficacy of a Fusion Protein Tuberculosis Vaccine Combining Five Esx Family Proteins. Front. Cell. Infect. Microbiol. 7:226. doi: 10.3389/fcimb.2017.00226
Received: 14 March 2017; Accepted: 16 May 2017;
Published: 31 May 2017.
Edited by:
Yinduo Ji, University of Minnesota, United StatesReviewed by:
Jianjun Sun, University of Texas at El Paso, United StatesSmitha Sasindran, Ohio State University at Columbus, United States
Copyright © 2017 Xiang, Sun, Lin, Chen, Mai, Liu, Xu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Zhang, emhhbmdsdTQwN0BmdWRhbi5lZHUuY24=
Jun Liu, anVuLmxpdUB1dG9yb250by5jYQ==
 Zhi-hao Xiang1
Zhi-hao Xiang1 Zi-yan Xu
Zi-yan Xu Jun Liu
Jun Liu