
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 07 April 2017
Sec. Fungal Pathogenesis
Volume 7 - 2017 | https://doi.org/10.3389/fcimb.2017.00124
In this study, we found that ambroxol hydrochloride (128 μg/mL) exhibits synergistic antifungal effects in combination with fluconazole (2 μg/mL) against resistant planktonic Candida albicans (C. albicans) cells. This combination also exhibited synergistic effects against resistant C. albicans biofilms in different stages (4, 8, and 12 h) according to the microdilution method. In vitro data were further confirmed by the success of this combination in treating Galleria mellonella infected by resistant C. albicans. With respect to the synergistic mechanism, our result revealed that ambroxol hydrochloride has an effect on the drug transporters of resistant C. albicans, increasing the uptake and decreasing the efflux of rhodamine 6G, a fluorescent alternate of fluconazole. This is the first study to investigate the in vitro and in vivo antifungal effects, as well as the possible synergistic mechanism of ambroxol hydrochloride in combination with fluconazole against resistant C. albicans. The results show the potential role for this drug combination as a therapeutic alternative to treat resistant C. albicans and provide insights into the development of antifungal targets and new antifungal agents.
In the past few decades, fungi have emerged as an important cause of severe and fatal infections, with a >200% increase in annual fungal sepsis cases recorded in the USA (Pfaller and Diekema, 2007). C. albicans is one of the most common opportunistic pathogens in humans, and the infections caused by this fungus are associated with a high mortality, often in excess of 30%, due to the limited types of available antifungal agents and the emergence of drug resistance (Wisplinghoff et al., 2004; Pfaller and Diekema, 2007). Only a few types of antifungal agents are currently available, such as azoles, echinocandins, polyenes, and allylamines (Prasad et al., 2016). Among them, fluconazole (FLC), one of the azoles, is the most commonly used agent to treat infections caused by C. albicans due to its high bioavailability and low toxicity (Fiori and Van Dijck, 2012; Han et al., 2013; Sarkar et al., 2014). However, the extensive and frequent use of FLC has led to the development of genetically acquired drug resistance (e.g., overexpression of drug efflux genes) in C. albicans and has introduced challenges in the treatment of fungal infections. In addition, C. albicans can easily form biofilms on medical devices, which is considered physiologically associated with the acquisition of drug resistance and results in the resistance of C. albicans to antifungal agents (Mathe and Van Dijck, 2013; Zhang et al., 2013; Akbari and Kjellerup, 2015). Therefore, drug resistance in C. albicans is becoming an increasingly important issue. Treatment approaches for infections caused by drug-resistant C. albicans are lacking, and patients must receive pharmacotherapy with toxic antifungals (Chen et al., 2013). Thus, the development of improved antifungal treatments to combat drug resistance is urgently needed. Combination treatment of FLC with non-antifungal agents has been one of the most successful approaches to combat the resistance of fungi (Liu et al., 2014, 2016; Gu et al., 2016). Utilizing synergistic drug combinations could potentially reduce the toxicity that may occur when patients are exposed to high doses of drugs.
Ambroxol hydrochloride (ABH) is widely used to treat chronic obstructive pulmonary disease, chronic bronchitis, bronchial asthma, spastic bronchitis, and bronchopulmonary complications after chest surgery. ABH is associated with potent expectorating action, improvement of respiratory function and low toxicity (Malerba and Ragnoli, 2008). Furthermore, ABH has been reported to exert antioxidant activity and relieve inflammation by reducing the release of inflammatory cytokines (Gillissen et al., 1997; Pfeifer et al., 1997; Gibbs et al., 1999). The efficacy, tolerability and safety of ABH have been clinically verified (Fischer et al., 2002; Malerba and Ragnoli, 2008; de Mey et al., 2015). Interestingly, ABH alone at high concentrations from 1 to 60 mg/mL has been shown to exhibit antimicrobial effects on C. albicans, Candida parapsilosis and Pseudomonas aeruginosa (Li et al., 2008; Pulcrano et al., 2012; Rene et al., 2014). However, no study has investigated the combined antifungal effects of ABH with FLC.
The present study was designed to determine the potential antifungal effects of FLC and ABH against Candida spp. and the effects of this drug combination on resistant C. albicans biofilms in different stages in vitro. A recently established infection model, Galleria mellonella (G. mellonella), was used to determine the antifungal effects of this drug combination in vivo. The potential synergistic mechanism associated with this drug combination was also detected by determining the activity of drug uptake and efflux of resistant C. albicans.
Twelve Candida spp. isolates with different susceptibilities were used in this study, including four C. albicans isolates (CA4, CA8, CA10, and CA16), four Candida glabrata isolates (CG1, CG2, CG3, and CG8) and four Candida krusei isolates (CK2, CK3, CK9, and CK10). Both C. glabrata and C. krusei are non-C. albicans types. Each Candida spp. included two resistant and two susceptible isolates, as shown in Table 1. Candida albicans ATCC 10231 was used as a quality control isolate. All isolates were refreshed from the frozen storage at −80°C and inoculated twice onto yeast–peptone–dextrose (YPD) solid medium (2% agar, 2% peptone, 2% glucose, and 1% yeast extract) for 18 h at 35°C. YPD liquid medium consisted of 2% peptone, 2% glucose, and 1% yeast extract. Egg yolk agar medium consisted of 5% NaCl, 1% peptone, 3% glucose, 0.0006% CaCl2, 10% egg yolk, and 2% agar.
All drugs, including FLC, ABH, and ampicillin, were purchased from Dalian Meilun Biotech Co., Ltd. (Dalian, China). Stock solutions of FLC (2,560 μg/mL), ABH (5,120 μg/mL), and ampicillin (2,560 μg/mL) were prepared in sterile distilled water and stored at −20°C until use. The larvae of G. mellonella were purchased from Tianjin Huiyude Biotech Co., Ltd. (Tianjin, China).
The MICs of FLC and ABH against 12 Candida spp. isolates were determined with a broth microdilution method using two-fold serial dilutions in RPMI 1640 medium as described by the Clinical and Laboratory Standards Institute method M27-A3 document. The tests were performed in 96-well flat-bottomed microtiter plates. Plates were incubated at 35°C for 24 h. After incubation, an XTT reduction assay was used to determine the MIC, which was read at the lowest concentration that showed 80% inhibition of growth compared with that of the drug-free control (Li et al., 2015). The fractional inhibitory concentration index (FICI) model was used to interpret the antifungal effects on planktonic cells In vitro. The FICI model is as follows (Guo et al., 2008): FICI = FICFLC + FICABH = (MIC80 of FLC in combination/MIC80 of FLC alone) + (MIC80 of ABH in combination/MIC80 of ABH alone). The results were defined as FICI ≤ 0.5 for synergism, FICI > 4.0 for antagonism and 0.5 < FICI ≤ 4.0 for no interaction (Odds, 2003).
The SMICs of FLC and ABH against resistant C. albicans biofilms were tested as described previously (Ramage and Lopez-Ribot, 2005). As described in a previous study, 4, 8, and 12 h biofilms of CA10 were formed in 96-well flat-bottomed microtiter plates (Gu et al., 2016). Then, drugs were added to the plates and the plates were incubated for an additional 24 h at 37°C. XTT reduction assays were performed to determine the SMICs. SMICs were defined as the lowest antifungal concentrations where there was an 80% (SMIC80) reduction in the XTT-colorimetric readings in comparison to the drug-free control. Colorimetric changes were measured at 492 nm with a microtiter plate reader (SpectraMax190, USA). The FICI model was used to interpret the antifungal effects for biofilms as described above.
Batches of G. mellonella larvae in the final instar stage were stored in the dark at 25°C, and larvae weighing approximately 250 mg were selected for use in the experiments. The doses (1.6 μg/larva for FLC and 3.2 μg/larva for ABH) used to treat larvae mimicked those used to treat humans. Each group contained 16 randomly chosen larvae. The preliminary steps for each in vivo experiment were the same. In detail, larvae were inoculated with 10 μL of the cell suspension (5 × 108 CFU/mL) into the prolegs using 50- μL syringes (Shanghai Gaoge Biotech Co., Ltd. Shanghai, China). After 2 h, treatment with the drugs separately or in combination was administered using the 50- μL syringes, while a control group of infected larvae received PBS only.
For survival assays, a preliminary experiment was first carried out to test whether there are harmful effects of PBS and drugs on the uninfected G. mellonella larvae. Only 10 μL of PBS, FLC, ABH, and drug combination were inoculated into the G. mellonella larvae in each group, without treatment of the CA10 cell suspension. For the formal survival assay, the larvae were pretreated as described above. The number of surviving larvae in each group was recorded every day for up to 4 days. Survival data were plotted according to the Kaplan-Meier method using the Statistical Product and Service Solutions 19 software.
For determination of the fungal burden, the larvae were pretreated as described above. Three larvae from each group were randomly culled every day over 4 days. C. albicans burdens were determined by inoculating dilutions of the homogenized larvae onto YPD solid medium plates as previously described (Gu et al., 2016). These plates were incubated overnight at 35°C. Fungal burden data were recorded using a CFU counting method.
For histological study, the larvae were pretreated as described above. One infected larva from each group was randomly selected at 3 days post-infection and cut into 7-μm tissue slices. The tissue slices of infected larvae were stained with Periodic acid Schiff reagent (PAS) and observed under an Olympus FSX100 fluorescence microscope with 4.2 × 10 objectives.
CA10 cells at a concentration of 1 × 106 CFU/mL were allowed to grow aerobically in 5 mL YPD liquid medium at 35°C for 18 h. The cells were harvested, washed twice with glucose-free PBS, and resuspended in the same buffer to obtain a concentration of 1 × 107 CFU/mL. The cells were then de-energized for 1 h in glucose-free PBS. The de-energized cells were harvested, washed, and then resuspended again to obtain a concentration of 1 × 107 CFU/mL.
To assess the uptake of Rh6G, Rh6G, and ABH were added to the exhausted cells at a final concentration of 10 μM and 128 μg/mL, respectively. Cells without ABH treatment served as the control. Samples were incubated for 50 min at 35°C and then washed with glucose-free PBS. A BD FACSAria II flow cytometer (Becton Dickinson, USA) with excitation at 488 nm and emission at 530 nm was used to determine the mean fluorescent intensity (MFI) of each sample at specific time intervals (0, 10, 20, 30, 40, and 50 min).
To assess the efflux of Rh6G, Rh6G was added to the exhausted cells at a final concentration of 10 μM. Cells were then washed with glucose-free PBS, and ABH was added to the cells at a final concentration of 128 μg/mL. Cells without ABH treatment served as the control. Samples were incubated for 50 min at 35°C and then washed with glucose-free PBS. After the addition of 5 mL of 5% glucose-PBS to each sample, the MFI was immediately determined using the flow cytometer as described above at specific time intervals (0, 30, 60, 90, 120, and 150 min).
To assess the in vitro interactions between FLC and ABH against Candida spp., the data were obtained using the broth microdilution method and interpreted using the FICI model. The MICs of FLC and ABH alone and in combination against the 12 Candida spp. isolates in vitro are listed in Table 1. The results show that ABH combined with FLC has synergistic effects on resistant C. albicans in vitro. The addition of ABH resulted in a decrease in the MIC of FLC for resistant C. albicans CA10 and CA16 from >512 to 2 μg/mL, while both of the FICI values were lower than 0.5 (Table 1, Table S1). However, no synergism was observed when this drug combination was used to inhibit two susceptible C. albicans isolates and eight non-C. albicans isolates in vitro, with the FICIs distributed from 0.625 to 1.500 (Table 1).
To assess interactions between FLC and ABH against C. albicans biofilms at different stages, the data obtained by the broth microdilution method were analyzed using the FICI model described above. The results for the tested drugs alone and in combination in biofilms of resistant C. albicans at 4, 8, and 12 h are summarized in Table 2. There was close agreement between these two models for the FLC-ABH combination treatment on C. albicans biofilms. As shown in Table 2, ABH combined with FLC showed synergistic antifungal effects against 4, 8, and 12 h biofilms, all with FICI = 0.252. In addition, the MIC of ABH for 12 h biofilms (256 μg/mL) was higher than that of 4 and 8 h biofilms (128 μg/mL).
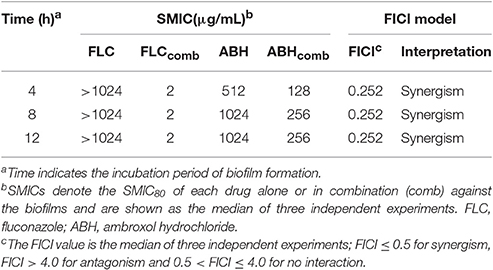
Table 2. Synergistic effects of FLC alone and in combination with ABH against biofilms of resistant C. albicans.
Following the identification of the synergism between FLC and ABH in vitro, experiments were designed to identify whether this effect would be replicated in vivo. In the preliminary survival assay, all uninfected G. mellonella larvae in four groups (PBS, FLC, ABH, and drug combination) were alive after 4 days, indicating that PBS and the drugs have no harmful effect on G. mellonella. The effect of the drugs alone or in combination on the survival rates, fungal burden and histopathology of infected larvae is shown in Figures 1–3, respectively. When used alone, the drugs, FLC and ABH had no significant effect on the survival rates of infected larvae. However, their combination significantly improved the survival rates, confirming the efficacy of this drug combination in vivo (Figure 1). Encouragingly, the combination of FLC and ABH resulted in a greater reduction in fungal burden compared to the control and individual drug groups, as shown by the slower proliferation of C. albicans in the group receiving the drug combination over 4 days (Figure 2). Slices of infected larvae in four groups revealed a significant decrease in the number of melanized nodules in larvae treated with this drug combination, compared to no drug or larvae treated with individual drugs (Figure 3). The histopathology results are in agreement with the survival rates and fungal burden experiments, which further confirms the synergistic effects of FLC and ABH combination against resistant C. albicans in vivo.
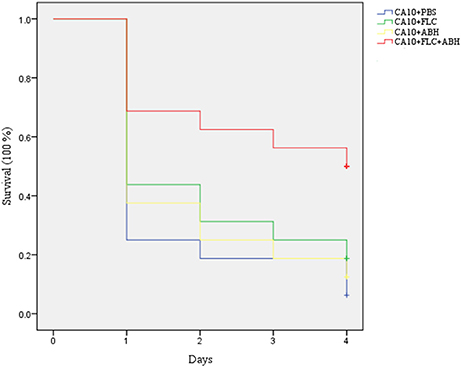
Figure 1. Effect of FLC alone or in combination with ABH on the survival of infected G. mellonella over 4 days. The concentration of yeast cells was 5 × 106 CFU/larva. Treatments consisted of PBS, FLC (1.6 μg/larva) alone, ABH (3.2 μg/larva) alone, or a combination of FLC (1.6 μg/larva) with ABH (3.2 μg/larva). The Statistical Product and Service Solutions 20 software was used to analyze the data. The experiments were performed three times on different days.
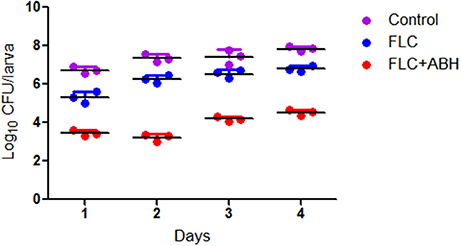
Figure 2. Effect of FLC alone or in combination with ABH on the fungal burden of infected G. mellonella over 4 days. The concentration of yeast cells was 5 × 106 CFU/larva. Treatments consisted of PBS, FLC (1.6 μg/larva) alone, ABH (1.6 μg/larva) alone, or a combination of FLC (1.6 μg/larva) with ABH (1.6 μg/larva). For clarity, data for the treatment with ABH are not shown because the data closely followed those of the control group. GraphPad Prism 6 software was used to analyze the data. The experiments were performed three times on different days.
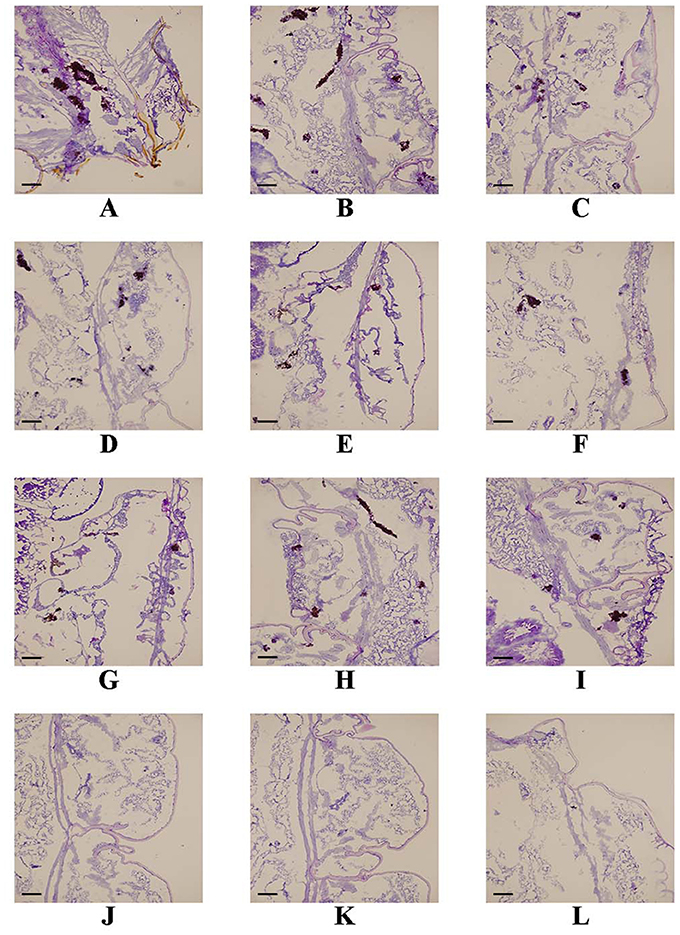
Figure 3. Effect of FLC alone or in combination with ABH on the histopathology of infected G. mellonella at day 2 post-infection. The concentration of yeast cells was 5 × 106 CFU/larva. Treatments consisted of PBS (A–C), FLC (1.6 μg/larva) alone (D–F), ABH (1.6 μg/larva) alone (G–I), or a combination (J–L) of FLC (1.6 μg/larva) with ABH (1.6 μg/larva). Melanized nodules consisted of mixtures of yeast cells and filaments. Bar = 200 μm. The experiments were performed three times on different days.
Both Rh6G and FLC are substrates of drug transporters in C. albicans. Rh6G was used as the alternate for FLC in the present study. We measured the uptake and efflux of Rh6G in resistant C. albicans in the absence and presence of ABH. Intracellular Rh6G uptake in the ABH-treated group increased over time, and that in the ABH group increased faster than that in the control group (Figure 4A). Cells treated with ABH pumped out lower concentrations of Rh6G than cells without drug treatment. This finding was evident by the sharp drop in the measured intracellular concentrations of Rh6G in the ABH-treated group (Figure 4B). Our results revealed that ABH can increase the uptake and decrease the efflux of rhodamine 6G.
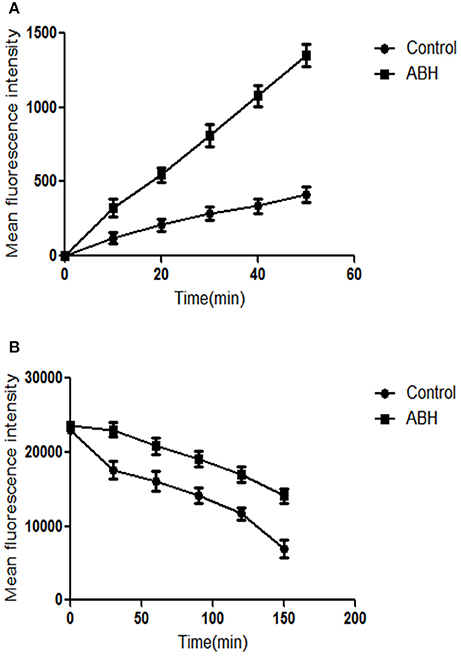
Figure 4. The effect of ABH on the (A) uptake and (B) efflux of Rh6G in resistant C. albicans. The uptake and efflux of Rh6G in the absence and presence of ABH (128 μg/mL) were determined by a flow cytometer. Ten thousand events were counted for each sample at specific time intervals. MFIs represent the intracellular Rh6G in C. albicans. GraphPad Prism 6 software was used to analyze the data. The experiments were performed three times on different days.
In this study, we first examined the effects of ABH combined with FLC against planktonic Candida spp. cells with different susceptibility and biofilms of resistant C. albicans in different stages. Our results showed that the combination of FLC at a low concentration (2 μg/mL) with ABH at a concentration of 128 μg/mL exerts synergistic effects against resistant C. albicans only, with no synergism on susceptible C. albicans and non-C. albicans. ABH combined with FLC also showed synergistic antifungal effects against 4, 8, and 12 h biofilms of resistant C. albicans. These findings indicate that ABH shows a potent antifungal effect against the planktonic cells and biofilms of FLC-resistant C. albicans in vitro when combined with FLC.
Although mouse models are considered a standard model to investigate fungal pathogenesis, their disadvantages, such as cost, logistical challenges, and the fact that they are time-consuming and ethically delicate, have limited their application for in vivo experimentation (Amorim-Vaz et al., 2015). Therefore, a number of simple insect models have recently been developed for use with fungal pathogens. For example, G. mellonella has been increasingly used to study fungal virulence and the antifungal activity of drug combinations (Mylonakis et al., 2005; Vu and Gelli, 2010). In the present study, treatment of G. mellonella larvae infected by C. albicans with a combination of FLC and ABH exhibited greater efficacy than FLC or ABH monotherapy, which was in accordance with the synergistic antifungal effects of this combination In vitro. In addition, this study demonstrated the utility of the G. mellonella larva model as an inexpensive, rapid in vivo model to prescreen potential novel antifungal therapies prior to more extensive mammalian animal models. Although, the concentration of ABH required to induced the in vivo effects may be higher than the usual doses that ABH achieves in humans, high doses of ABH are widely used in clinics, and a large number of clinical trials have proven that treatment with high doses of ABH (more than 15 mg/kg or 1,000 mg/day) appears to improve the condition of patients with acute or mild respiratory distress syndrome and acute lung injury (Wu et al., 2014). Therefore, the ABH concentration used in this study still has the potential to be used clinically.
Azole-resistant isolates commonly display reduced intracellular accumulation of antifungal agents due to their over-expression of drug transport pumps (Prasad and Rawal, 2014). Two main classes of drug transport pumps are responsible for the development of antifungal resistance: ATP-binding cassette transporters and major facilitator superfamily transporters (Holmes et al., 2016). Considering the importance of major antifungal transport pumps, one focus of recent research has been to understand the relationship between this synergism and drug transport pumps. Functional activities of drug transport pumps have traditionally been assayed with Rh6G, a known fluorescent substrate of several drug transport pumps responsible for multidrug resistance in yeasts and a FLC alternate (Ahmad et al., 2013). We employed this Rh6G-based assay to determine whether the synergistic effects were correlated with the inhibition of drug transport pumps. ABH was found to significantly increase the uptake of Rh6G and decrease the efflux of Rh6G, indicating that ABH improves the susceptibility of resistant C. albicans to FLC by increasing the intracellular concentration of FLC. Inhibiting the effects on drug transport pumps may be one of the synergism mechanisms of ABH and FLC in combination. Other potent mechanisms need to be further investigated.
We suggest that combinations of antifungal drugs with other, non-antifungal drugs, could be used as a useful approach for overcoming drug resistance in C. albicans. In this study, we found that combined FLC and ABH treatment results in synergistic effects against FLC-resistant C. albicans in vitro and in vivo. In addition, this combination also exhibited synergistic effects against resistant C. albicans biofilms in different stages. When looking for the mechanism of action of this drug combination, we could not rely on any known mechanism of ABH against C. albicans. Therefore, we first assayed the possibility that ABH acts on the most common FLC resistance mechanism in C. albicans, namely changes in drug uptake and efflux. Remarkably, we found that ABH can increase the uptake and decrease the efflux of a test compound, presumably by inhibiting drug transporters. This leads us to suggest that the synergy of ABH with FLC on drug-resistant C. albicans results from an increase in intracellular FLC concentration. Our results are thus the first to show that ABH, in combination with FLC, can be exploited to reverse the drug resistance of C. albicans. Therapeutic combination of ABH with FLC may constitute a new alternative in the treatment of infections by resistant C. albicans.
XL, YZ, XH, CY, YY and SS performed the experiments. XL and SS designed the research. XL analyzed the data and wrote the paper. All authors approved the manuscript for publication.
This work was supported by 2016GSF201187 from the Department of Science and Technology of Shandong Province of China. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the Translational Medicine Research Centre in Qianfoshan Hospital Affiliated to Shandong University, China, for laboratory assistance, and we wish to acknowledge all members who contributed to the anti-fungal resistance study group.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fcimb.2017.00124/full#supplementary-material
FLC, Fluconazole; ABH, Ambroxol hydrochloride; CA, C. albicans; YPD, Yeast–peptone–dextrose; G. Mellonella, Galleria mellonella; PAS, Periodic acid Schiff reagent; Rh6G, Rhodamine 6G; MFI, Mean fluorescent intensity.
Ahmad, A., Khan, A., and Manzoor, N. (2013). Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 48, 80–86. doi: 10.1016/j.ejps.2012.09.016
Akbari, F., and Kjellerup, B. V. (2015). Elimination of bloodstream infections associated with Candida albicans biofilm in intravascular catheters. Pathogens 4, 457–469. doi: 10.3390/pathogens4030457
Amorim-Vaz, S., Delarze, E., Ischer, F., Sanglard, D., and Coste, A. T. (2015). Examining the virulence of Candida albicans transcription factor mutants using Galleria mellonella and mouse infection models. Front. Microbiol. 6:367. doi: 10.3389/fmicb.2015.00367
Chen, Y. L., Lehman, V. N., Averette, A. F., Perfect, J. R., and Heitman, J. (2013). Posaconazole exhibits In vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PLoS ONE 8:e57672. doi: 10.1371/journal.pone.0057672
de Mey, C., Patel, J., Lakha, D. R., Richter, E., and Koelsch, S. (2015). Efficacy and safety of an oral ambroxol spray in the treatment of acute uncomplicated sore throat. Drug Res. (Stuttg.) 65, 658–667. doi: 10.1055/s-0035-1547229
Fiori, A., and Van Dijck, P. (2012). Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob. Agents Chemother. 56, 3785–3796. doi: 10.1128/AAC.06017-11
Fischer, J., Pschorn, U., Vix, J. M., Peil, H., Aicher, B., Muller, A., et al. (2002). Efficacy and tolerability of ambroxol hydrochloride lozenges in sore throat. Randomised, double-blind, placebo-controlled trials regarding the local anaesthetic properties. Arzneimittelforschung 52, 256–263. doi: 10.1055/s-0031-1299889
Gibbs, B. F., Schmutzler, W., Vollrath, I. B., Brosthardt, P., Braam, U., Wolff, H. H., et al. (1999). Ambroxol inhibits the release of histamine, leukotrienes and cytokines from human leukocytes and mast cells. Inflamm. Res. 48, 86–93. doi: 10.1007/s000110050421
Gillissen, A., Scharling, B., Jaworska, M., Bartling, A., Rasche, K., and Schultze-Werninghaus, G. (1997). Oxidant scavenger function of ambroxol In vitro: a comparison with N-acetylcysteine. Res. Exp. Med. (Berl.) 196, 389–398. doi: 10.1007/s004330050049
Gu, W., Guo, D., Zhang, L., Xu, D., and Sun, S. (2016). The synergistic effect of azoles and fluoxetine against resistant Candida albicans strains is attributed to attenuating fungal virulence. Antimicrob. Agents Chemother. 60, 6179–6188. doi: 10.1128/AAC.03046-15
Guo, Q., Sun, S., Yu, J., Li, Y., and Cao, L. (2008). Synergistic activity of azoles with amiodarone against clinically resistant Candida albicans tested by chequerboard and time-kill methods. J. Med. Microbiol. 57, 457–462. doi: 10.1099/jmm.0.47651-0
Han, S., Kim, J., Yim, H., Hur, J., Song, W., Lee, J., et al. (2013). Population pharmacokinetic analysis of fluconazole to predict therapeutic outcome in burn patients with Candida infection. Antimicrob. Agents Chemother. 57, 1006–1011. doi: 10.1128/AAC.01372-12
Holmes, A. R., Cardno, T. S., Strouse, J. J., Ivnitski-Steele, I., Keniya, M. V., Lackovic, K., et al. (2016). Targeting efflux pumps to overcome antifungal drug resistance. Future Med. Chem. 8, 1485–1501. doi: 10.4155/fmc-2016-0050
Li, F., Yu, J., Yang, H., Wan, Z., and Bai, D. (2008). Effects of ambroxol on alginate of mature Pseudomonas aeruginosa biofilms. Curr. Microbiol. 57, 1–7. doi: 10.1007/s00284-008-9142-8
Li, H., Chen, Z., Zhang, C., Gao, Y., Zhang, X., and Sun, S. (2015). Resistance reversal induced by a combination of fluconazole and tacrolimus (FK506) in Candida glabrata. J. Med. Microbiol. 64, 44–52. doi: 10.1099/jmm.0.081760-0
Liu, S., Hou, Y., Chen, X., Gao, Y., Li, H., and Sun, S. (2014). Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int. J. Antimicrob. Agents 43, 395–402. doi: 10.1016/j.ijantimicag.2013.12.009
Liu, S., Yue, L., Gu, W., Li, X., Zhang, L., and Sun, S. (2016). Synergistic effect of fluconazole and calcium channel blockers against resistant Candida albicans. PLoS ONE 11:e0150859. doi: 10.1371/journal.pone.0168936
Malerba, M., and Ragnoli, B. (2008). Ambroxol in the 21st century: pharmacological and clinical update. Expert Opin. Drug Metab. Toxicol. 4, 1119–1129. doi: 10.1517/17425255.4.8.1119
Mathe, L., and Van Dijck, P. (2013). Recent insights into Candida albicans biofilm resistance mechanisms. Curr. Genet. 59, 251–264. doi: 10.1007/s00294-013-0400-3
Mylonakis, E., Moreno, R., El Khoury, J. B., Idnurm, A., Heitman, J., Calderwood, S. B., et al. (2005). Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73, 3842–3850. doi: 10.1128/IAI.73.7.3842-3850.2005
Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. doi: 10.1093/jac/dkg301
Pfaller, M. A., and Diekema, D. J. (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20, 133–163. doi: 10.1128/CMR.00029-06
Pfeifer, S., Zissel, G., Kienast, K., and Muller-Quernheim, J. (1997). Reduction of cytokine release of blood and bronchoalveolar mononuclear cells by ambroxol. Eur. J. Med. Res. 2, 129–132.
Prasad, R., and Rawal, M. K. (2014). Efflux pump proteins in antifungal resistance. Front. Pharmacol. 5:202. doi: 10.3389/fphar.2014.00202
Prasad, R., Shah, A. H., and Rawal, M. K. (2016). Antifungals: mechanism of action and drug resistance. Adv. Exp. Med. Biol. 892, 327–349. doi: 10.1007/978-3-319-25304-6_14
Pulcrano, G., Panellis, D., De Domenico, G., Rossano, F., and Catania, M. R. (2012). Ambroxol influences voriconazole resistance of Candida parapsilosis biofilm. FEMS Yeast Res. 12, 430–438. doi: 10.1111/j.1567-1364.2012.00792.x
Ramage, G., and Lopez-Ribot, J. L. (2005). Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol. Med. 118, 71–79. doi: 10.1385/1-59259-943-5:071
Rene, H. D., Jose, M. S., Isela, S. N., and Claudio, C. R. (2014). Effects of ambroxol on Candida albicans growth and biofilm formation. Mycoses 57, 228–232. doi: 10.1111/myc.12147
Sarkar, S., Uppuluri, P., Pierce, C. G., and Lopez-Ribot, J. L. (2014). In vitro study of sequential fluconazole and caspofungin treatment against Candida albicans biofilms. Antimicrob. Agents Chemother. 58, 1183–1186. doi: 10.1128/AAC.01745-13
Vu, K., and Gelli, A. (2010). Astemizole and an analogue promote fungicidal activity of fluconazole against Cryptococcus neoformans var. grubii and Cryptococcus gattii. Med. Mycol. 48, 255–262. doi: 10.3109/13693780903081968
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., and Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39, 309–317. doi: 10.1086/421946
Wu, X., Li, S., Zhang, J., Zhang, Y., Han, L., Deng, Q., et al. (2014). Meta-analysis of high doses of ambroxol treatment for acute lung injury/acute respiratory distress syndrome based on randomized controlled trials. J. Clin. Pharmacol. 54, 1199–1206. doi: 10.1002/jcph.389
Keywords: ambroxol hydrochloride, fluconazole, drug resistance, Candida albicans, drug uptake and efflux
Citation: Li X, Zhao Y, Huang X, Yu C, Yang Y and Sun S (2017) Ambroxol Hydrochloride Combined with Fluconazole Reverses the Resistance of Candida albicans to Fluconazole. Front. Cell. Infect. Microbiol. 7:124. doi: 10.3389/fcimb.2017.00124
Received: 03 January 2017; Accepted: 27 March 2017;
Published: 07 April 2017.
Edited by:
James Bernard Konopka, Stony Brook University, USAReviewed by:
Daniel Kornitzer, Technion–Israel Institute of Technology, IsraelCopyright © 2017 Li, Zhao, Huang, Yu, Yang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shujuan Sun, c3Vuc2h1anVhbjg4OEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.